Abstract
Background
Youth with chronic conditions may engage in risky behaviour to the same, if not higher, degree as their healthy peers.
Objectives
To determine the prevalence of alcohol, tobacco, cannabis and illicit substance use in adolescents with type 1 diabetes (T1DM) compared to a general adolescent population.
Methods
Cross-sectional survey of adolescents with T1DM (13 to 18 years). A published contemporary Canadian youth survey on use of alcohol, tobacco and illicit drugs was used as data representative of the general adolescent population. Outcome measures between the T1DM and general group were compared using Chi-square and Fisher’s exact test where appropriate.
Results
One hundred and sixty-four adolescents with T1DM (mean 15.6 years [SD 1.5]; 51.3% male) were participated. The proportions of adolescents with T1DM who have tried substances were: alcohol 51.8%, tobacco 27.4%, cannabis 22.6% and other illicit substances 7.3%. Compared to the general population (n=3469), there were no significant differences in the proportion of adolescents that reported ever consuming alcohol, tobacco or cannabis. Reported illicit substance use was significantly lower in adolescents with T1DM compared to general population (7.3% versus 36.0%, P<0.0001).
Conclusions
Proportions reporting having ever consumed alcohol, tobacco or cannabis were not significantly different between the two groups. However, the proportion of adolescents with T1DM who reported ever consuming an illicit substance was different from the comparison group. It is important to explore risky behaviours with adolescents with T1DM and focus on prevention and education during routine clinic visits.
Keywords: Adolescent, Alcohol, Cannabis, Substance use, Tobacco, Type 1 diabetes
Adolescents with chronic health conditions face many challenges in addition to the various stresses of normal teenage development. Adolescence is known to be a time when experimental behaviours such as substance abuse occur (1). Previously, having a chronic condition was thought to be a protective factor against risky behaviours (1). However, recent evidence suggests that youth with chronic conditions are likely to engage in risky behaviour to the same, if not higher, degree as their healthy peers (2). This is concerning as youth with a chronic condition have an increased potential for adverse health outcomes or complications from participating in a risky behaviour (1,3).
For adolescents with Type 1 Diabetes (T1DM), this time period is often associated with worsening glycemic control for various reasons including pubertal changes and nonadherence (4). Participating in risky behaviours can contribute to the poor glycemic control seen in T1DM during adolescence; alcohol, tobacco or other illicit substance use can contribute to both acute and chronic complications of T1DM (3). This underscores the importance of exploring risky behaviours with teens and focusing on prevention and education in clinic visits.
The aim of this study is to assess the prevalence of alcohol, tobacco, cannabis and other illicit substance use in adolescents with T1DM followed at the Alberta Children’s Hospital (Calgary, Alberta, Canada), and to compare the rates to the general adolescent population in Alberta, Canada. This work is important for clinical practice to inform anticipatory guidance, education of clinicians, families and patients, as well as for further research assessing the effects of targeted education and trends overtime.
METHODS
Design
This study was a cross-sectional survey of adolescents with T1DM being followed in a tertiary centre paediatric diabetes clinic.
Subjects
Participants were recruited from the Alberta Children’s Hospital Diabetes Clinic (Calgary, Alberta, Canada) from July 2014 to November 2014. Adolescents were included if they were between the ages of 13 to 18 years, diagnosed with T1DM for at least 1 year duration, and were being followed in the paediatric diabetes clinic at the Alberta Children’s Hospital. Exclusion criteria included children with intellectual disability precluding ability to understand and complete the survey and any language barriers that could limit the understanding of survey questions. Participants were invited to complete the survey during clinic visits and the survey was advertised with posters.
Ethics
Consent was obtained from all participants 15 years of age and older. For participants 13 to 14 years of age, consent was obtained from parents and assent was obtained from participants. This study was approved by the Conjoint Health Research Ethics Board at the University of Calgary (Calgary, Alberta, Canada).
Data collection
Published data from The Alberta Youth Experience Survey (TAYES) was used for the comparison group, which was generated from a survey done in 2008 of the general adolescent population in Alberta (5). TAYES was administered to students in public, separate and charter schools in Alberta in grades 7 to 12. Of the 20 school districts contacted, 100% gave consent for the survey to be done in their district. Of the 57 individual schools invited to participate, 29 consented to participate (51%). A total of 9066 students were invited to participate, but only 3515 of the students obtained parental or guardian consent to complete the survey (39%). Some surveys were excluded (i.e., missing data, invalid responses) and 3469 surveys were included in the analysis (38%) (5).
TAYES 2008 technical report (5) was also used to help generate questions for the survey of adolescents with T1DM so that the responses could be comparable. The survey questions for adolescents with T1DM consisted of demographic information (age, sex), participation in risky behaviours (alcohol, smoking tobacco, cannabis and other illicit substance use) and frequency and amount of use. The initial version of the survey was piloted on adolescent volunteers with and without diabetes to assess for flow and reading level as well as time needed to complete the survey. The surveys were completed on a touch screen tablet computer by the adolescent in a confidential clinic room setting without the presence of their family or usual health care provider. A research assistant was present to answer any questions and provide instructions on how to complete the survey.
Statistical analysis
The participant demographics were expressed as means with standard deviation. For comparison purposes, the same categories for substances used in TAYES were utilized: alcohol, tobacco, cannabis and other illicit substances. Other illicit substances were defined as an illicit drug excluding cannabis. The difference in proportions between adolescents with T1DM and control data from the TAYES (4) study was assessed using a Chi-square test. Fisher’s exact test was used when numbers were less than 5 in a group. Given the multiple comparisons, a Bonferroni correction was applied and a significance level α = 0.001 was used for statistical tests.
RESULTS
Participants
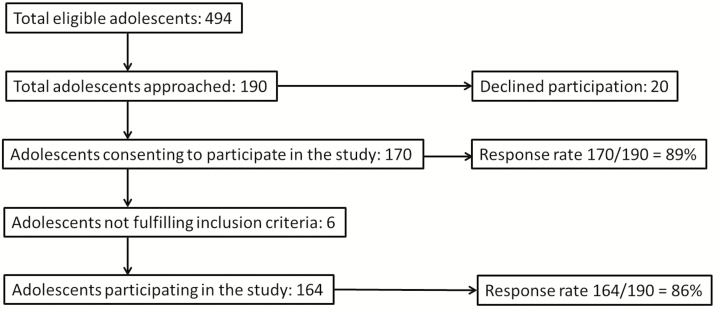
A total of 190 patients were approached and 164 were included in the analysis (Figure 1). Recruitment for the survey was carried out in the diabetes clinic from July 2014 to November 2014, so not all eligible adolescents were approached in this time frame if they did not have a regularly scheduled diabetes clinic appointment. For the 494 eligible adolescents between 13 and 18 years old in the diabetes clinic, the mean age was 15.8 years (SD = 1.7) and 52.6% were male. For the 164 adolescents that participated in the study, the mean age was 15.6 years (SD = 1.5) and 51.3% were male. Baseline demographics for TAYES and T1DM are shown in Table 1.
Figure 1.
Flow chart representation of study population.
Table 1.
Demographic information for adolescents surveyed in TAYES and for adolescents surveyed with T1DM
| TAYES N (%) | T1DM N (%) | ||
|---|---|---|---|
| Total | 3469 | 164 | |
| Sex | Male | 1587 (45.8%) | 84 (51.3%) |
| Female | 1882 (54.2%) | 80 (48.7%) | |
| Grade Level | Grade 7 | 518 (14.9%) | 21 (12.8%) |
| Grade 8 | 606 (17.5%) | 36 (21.9%) | |
| Grade 9 | 651 (18.8%) | 26 (15.8%) | |
| Grade 10 | 708 (20.4%) | 36 (21.9%) | |
| Grade 11 | 586 (16.9%) | 32 (19.5%) | |
| Grade 12 | 400 (11.5%) | 13 (7.9%) |
TAYES The Alberta Youth Experience Survey 2008; T1DM Type 1 diabetes mellitus
Prevalence
There were no significant differences in the proportions of adolescents that reported having ever consumed each alcohol, tobacco or cannabis between the TAYES and T1DM population (Table 2). In contrast to this, the proportion of adolescents that reported having ever consumed other illicit substances was significantly lower in the T1DM adolescents (Table 2).
Table 2.
Comparison of prevalence of consumption of alcohol, tobacco, cannabis and other illicit substances for adolescents surveyed in TAYES (n=3469) compared to adolescents surveyed with T1DM (n=164)
| Alcohol | Tobacco | Cannabis | Other Illicit Substance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAYES N (%) | T1DM N (%) | P value | TAYES N (%) | T1DM N (%) | P value | TAYES N (%) | T1DM N (%) | P value | TAYES N (%) | T1DM N (%) | P value | |
| Have consumed in lifetime | 1800/3469 (51.9%) | 85/164 (51.8%) | 1.00 | 985/3469 (28.4%) | 45/164 (27.4%) | 0.79 | 715/3469 (20.6%) | 37/164 (22.6%) | 0.55 | 1248/3469 (36.0%) | 12/164 (7.3%) | <0.0001 |
| Male | 811/1587 (51.1%) | 47/84 (55.9%) | 0.39 | 446/1587 (28.1%) | 25/84 (29.8%) | 0.74 | 340/1587 (21.4%) | 23/84 (27.4%) | 0.20 | 628/1587 (39.6%) | 5/84 (6.0%) | <0.0001 |
| Female | 991/1882 (52.7%) | 38/80 (47.5%) | 0.36 | 540/1882 (28.7%) | 20/80 (25.0%) | 0.47 | 373/1882 (19.8%) | 14/80 (17.5%) | 0.61 | 785/1882 (41.7%) | 7/80 (8.8%) | <0.0001 |
| Grade 7 | 83/461 (18.0%) | 2/21 (9.5%) | 0.40 | 46/461 (10.0%) | 1/21 (4.8%) | 0.51 | 0/461 (0%) | 0/21 (0%) | N/A | 120/461 (26.0%) | 0/21 (0%) | 0.007 |
| Grade 8 | 182/543 (33.5%) | 13/36 (36.1%) | 0.75 | 71/543 (13.1%) | 8/36 (22.2%) | 0.13 | 31/543 (5.7%) | 4/36 (11.1%) | 0.26 | 143/543 (26.3%) | 2/36 (5.6%) | 0.004 |
| Grade 9 | 267/514 (52.0%) | 15/26 (57.7%) | 0.56 | 129/514 (25.1%) | 6/26 (23.1%) | 0.82 | 86/514 (16.7%) | 7/26 (26.9%) | 0.18 | 190/514 (37.0%) | 3/26 (11.5%) | 0.01 |
| Grade 10 | 420/686 (61.2%) | 23/36 (63.9%) | 0.75 | 240/686 (35.0%) | 9/36 (25.0%) | 0.22 | 183/686 (26.7%) | 8/36 (22.2%) | 0.55 | 335/686 (48.8%) | 2/36 (5.6%) | <0.0001 |
| Grade 11 | 446/618 (72.1%) | 20/32 (62.5%) | 0.24 | 265/618 (42.9%) | 12/32 (37.5%) | 0.55 | 216/618 (35.0%) | 10/32 (31.3%) | 0.67 | 321/618 (52.0%) | 3/32 (9.4%) | <0.0001 |
| Grade 12 | 500/647 (77.3%) | 12/13 (92.3%) | 0.32 | 309/647 (47.8%) | 9/13 (69.2%) | 0.16 | 256/647 (39.8%) | 8/13 (61.5%) | 0.15 | 362/647 (55.9%) | 2/11 (18.2%) | 0.015 |
TAYES The Alberta Youth Experience Survey 2008; T1DM Type 1 diabetes mellitus
Frequency and amount
Adolescents that reported drinking alcohol had no significant differences in the frequency of alcohol consumption between TAYES and T1DM (Table 3). However, the proportion of adolescents with T1DM who reported consuming fewer drinks was significantly higher compared to the TAYES (Table 3). Of those adolescents that reported smoking tobacco, there were no significant differences between the TAYES and T1DM in the frequency and amount of consumption. There were no comparison data available from TAYES for cannabis consumption around frequency and amount.
Table 3.
Comparison of the frequency and amount of consumption of alcohol, tobacco, cannabis and other illicit substances for adolescents surveyed in TAYES compared to adolescents surveyed with T1DM
| TAYES N (%) | T1DM N (%) | P value | |
|---|---|---|---|
| Alcohol Frequency (in the last month) | N=1957 | N=85 | 0.13 |
| Did not drink in the last month | 624 (31.9%) | 32 (37.6%) | |
| Once or twice in the last month | 918 (46.9%) | 45 (52.9%) | |
| Once or twice a week in the last month | 260 (13.3%) | 6 (7.1%) | |
| Three or more times a week in the last month | 102 (5.2%) | 2 (2.4%) | |
| No response | 53 (2.7%) | 0 (0%) | |
| Alcohol Amount (number of drinks consumed during a typical day of drinking in the last month) | N=1384 | N=85 | <0.0001 |
| 1–2 drinks | 505 (36.5%) | 56 (65.9%) | |
| 3–4 drinks | 295 (21.3%) | 14 (16.4%) | |
| 5–9 drinks | 396 (28.6%) | 7 (8.2%) | |
| Over 10 drinks | 115 (8.3%) | 1 (1.2%) | |
| No response | 73 (5.3%) | 7 (8.3%) | |
| Smoking Frequency (number of days in the last month) | N=754 | N=45 | 0.08 |
| None | 295 (39.1%) | 26 (57.8%) | |
| 1–5 days | 181 (24.0%) | 13 (28.9%) | |
| 6–10 days | 35 (4.6%) | 0 (0.0%) | |
| 11–20 days | 39 (5.2%) | 2 (4.4%) | |
| 21–29 days | 48 (6.3%) | 1 (2.2%) | |
| 30 days | 78 (10.2%) | 2 (4.4%) | |
| No response | 78 (10.3%) | 1 (2.2%) | |
| Smoking Amount (amount during a typical day of smoking in the last month) | N=754 | N=45 | 0.004 |
| None | 294 (39.0%) | 30 (66.7%) | |
| A few puffs to a whole cigarette | 154 (20.3%) | 6 (13.3%) | |
| Two or more cigarettes | 227 (30.0%) | 8 (17.8%) | |
| No response | 79 (10.4%) | 1 (0.2%) |
TAYES The Alberta Youth Experience Survey 2008; T1DM Type 1 diabetes mellitus
DISCUSSION
This is the first Canadian study that examined the prevalence of alcohol, tobacco, cannabis and other illicit substance use in adolescents with T1DM compared to a general adolescent population. We found that teens with T1DM were not different regarding their engagement in alcohol, tobacco and cannabis use compared to their peers. We also found that a much smaller proportion of adolescents with T1DM reported ever consuming other illicit substances. Of those who consumed alcohol, they reported consuming smaller amounts than those reported by controls. This study contributes to the growing body of evidence that adolescents with chronic conditions are as likely, or in some studies more likely, to participate in certain risky behaviours compared to their peers, but this does not apply to all substances.
Similar to our results, Scaramuzza et al. found that, compared with controls, male adolescents with T1DM in Italy had similar proportions of alcohol and cannabis use compared to a control group (2). However, in contrast to our results, they also had a similar proportion of other illicit drug use (2). They also found that males with T1DM had a higher proportion of tobacco use than the control group (2). Females with T1DM also showed higher proportions for alcohol, cannabis and other drug use than controls, and a similar proportion for tobacco consumption (2).
Adolescents with T1DM in Chile consumed alcohol at a similar proportion to a control group during the last year (49.5% and 51.5%, respectively), but reported a lower 30-day and lifetime consumption (3). For tobacco use, they reported a lower life, annual and 30-day prevalence of tobacco use compared to controls (3). They also had a lower lifetime use of illicit drugs, including marijuana (9.6% versus 22.2%) (3). When the results were grouped by grade, the authors found that youth in grades 8, 9 and 10 had a lower prevalence of tobacco, alcohol and illicit drug use, but by grade 11 and 12, had a similar frequency of consumption as their peers (3).
Ng and colleagues focused on street drug use among youth with T1DM (6). They found that 29% of youth ages 16 to 30 years who responded to an anonymous confidential questionnaire reported street drug use including cannabis, cocaine, amphetamine and ecstasy, comparable to the prevalence reported in the USA of 25% (6). Sixty-eight per cent of UK youth reported using drugs at least once a month (6).
In contrast, of 209 Polish adolescents with T1DM, this group had a significantly lower lifetime prevalence of illicit drug use than 12,000 healthy controls (28% versus 46%) (7). Cannabis was the most commonly used illicit drug among teens in both groups (18.3% of teens with T1DM versus 33.1% of healthy controls) (7).
These studies demonstrate the mixed results of prevalence of substance use in teens with T1DM, likely due to methodological differences in each study. Despite this, authors agree that teens with T1DM who participate in risky behaviour are at a disadvantage in that substance use can potentially lead to greater adverse health outcomes (2,7). Alcohol ingestion, for example, can cause an altered level of consciousness and result in reduced diabetes self-management (e.g., blood glucose monitoring and insulin adjustment) (8). This can lead to labile blood sugars, hypoglycemia for up to 24 hours after drinking alcohol and diabetic ketoacidosis (DKA) (8,9).
Smoking has been shown to increase cardiovascular risk factors in T1DM by worsening glucose metabolism, lipids and endothelial function (10). Illicit drugs such as cannabis can impair judgement and affect intake by either causing food cravings or loss of appetite (7). Long-term use of cannabis may affect motivation for diabetes self-care and may increase the risk of psychiatric disorders (7). Other illicit drugs, such as ecstasy or cocaine, can increase the release of hormones that enhance glucose production, and their use has been associated with episodes of DKA (7).
Participation in risky behaviours can also lead to diabetes mismanagement and poor glycemic control. Scaramuzza et al. found that patients with T1DM who engaged in risky behaviour (drug use as well as sexual behaviour) showed higher rates of treatment mismanagement (76% versus 34%) including purposely missing insulin boluses, reporting false blood sugar readings or urinary stick results and missing a meal/snacks (2). Further, HbA1C values were higher in the patients who reported participating in one or more risky behaviour versus those who did not (8.4% versus 7.8%) (2). Hogendorf and colleagues did not find a difference in HbA1C in patients based on a lifetime use of illicit drugs, however, a lifetime and past year use of marijuana was associated with a HbA1C ≥8% (7). A contributing factor could be health literacy of adolescents who are engaging in substance use. For example, Barnard et al. found that young adults with T1DM had low alcohol health literacy and only 7.3% were able to correctly identify the alcohol content of ≥6 of 10 drinks in a survey (9). Even more concerning was that none of the respondents were able to identify the carbohydrate content of ≥6 of 10 drinks in this survey (9). Only 66.5% of youth reported they take precautions to drink safely and 5% reported they did not take precautions; some of the reported precautions were possibly harmful or ineffective (9). Of UK youth, only 28% of street drug users reported being aware of the negative effects of the drugs (6). This highlights the need for clinicians to provide supportive guidance on lower-risk substance use during clinic visits. This is a routine part of our diabetes clinic visits, and may explain why our patients reported consuming a smaller amount of alcohol compared to their peers, as a risk-reduction strategy.
Limitations of this study include the smaller sample size of our patient population compared to the general adolescent comparison group. The survey was administered by a research assistant and was confidential, however, answers may have been biased. It is possible that there was selection bias with those that chose to consent and respond to the questions as they were approached during a clinic visit and may be less likely to participate in risky behaviours compared to those that do not routinely come to clinic. Strengths include a large contemporary comparison group and use of the similar questions for both the T1DM and general adolescent group.
CONCLUSION
In this current survey of adolescents with T1DM, the proportion reporting having ever consumed each alcohol, tobacco or cannabis was not different from a previous survey of adolescents in Alberta. However, a significantly smaller proportion of adolescents with T1DM reported ever consuming other illicit substances. Of the adolescents with T1DM who consumed alcohol, the proportion that reported consuming only 1 to 2 drinks was greater than the general adolescent population. Participating in risky behaviours can lead to diabetes mismanagement and acute and chronic complications of T1DM, and our results demonstrate the importance of education and guidance on substance use to teens with T1DM. We would recommend routinely asking adolescents in diabetes clinic about alcohol, tobacco, cannabis and other illicit substances in order to identify those at risk. In addition, education and anticipatory guidance about the effects of these substances on diabetes management should be routinely provided to all adolescents regardless of whether they report use or not.
Conflict of interest
None of the authors has any conflicts of interest to disclose.
Author Contributions
KP, PL, DP, HV, ANA, LK and JH all contributed substantially to the conception and interpretation of the data for this paper. KP, JH and PL drafted the article. KP, PL, DP, HV, ANA, LK and JH critically appraised this paper for important intellectual content. All authors approved the final version of the article to be published.
Acknowledgements
We are grateful for the participation of our adolescents with T1DM and the support of the diabetes clinic at the Alberta Children’s Hospital.
Institution: University of Calgary
References
- 1. Sawyer SM, Drew S, Yeo MS, Britto MT. Adolescents with a chronic condition: Challenges living, challenges treating. Lancet 2007;369(9571):1481–9. [DOI] [PubMed] [Google Scholar]
- 2. Scaramuzza A, De Palma A, Mameli C, Spiri D, Santoro L, Zuccotti GV. Adolescents with type 1 diabetes and risky behaviour. Acta Paediatr 2010;99(8):1237–41. [DOI] [PubMed] [Google Scholar]
- 3. Martínez-Aguayo A, Araneda JC, Fernandez D, Gleisner A, Perez V, Codner E. Tobacco, alcohol, and illicit drug use in adolescents with diabetes mellitus. Pediatr Diabetes 2007;8(5):265–71. [DOI] [PubMed] [Google Scholar]
- 4. Bryden KS, Peveler RC, Stein A, Neil A, Mayou RA, Dunger DB. Clinical and psychological course of diabetes from adolescence to young adulthood: A longitudinal cohort study. Diabetes Care 2001;24(9):1536–40. [DOI] [PubMed] [Google Scholar]
- 5. Alberta Health Services Addiction and Mental Health. The Alberta Youth Experience Survey 2008: Technical Report. Alberta, Canada: Alberta Health Service, 2009. [Google Scholar]
- 6. Ng RS, Darko DA, Hillson RM. Street drug use among young patients with type 1 diabetes in the UK. Diabet Med 2004;21(3):295–6. [DOI] [PubMed] [Google Scholar]
- 7. Hogendorf AM, Fendler W, Sieroslawski J, Bobeff K, Wegrewicz K, Malewska KI et al. Breaking the taboo: Illicit drug use among adolescents with type 1 diabetes mellitus. J Diabetes Res. 2016;2016:4153278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnard K, Sinclair JM, Lawton J, Young AJ, Holt RI. Alcohol-associated risks for young adults with type 1 diabetes: A narrative review. Diabet Med 2012;29(4):434–40. [DOI] [PubMed] [Google Scholar]
- 9. Barnard KD, Dyson P, Sinclair JM et al. Alcohol health literacy in young adults with type 1 diabetes and its impact on diabetes management. Diabet Med 2014;31(12):1625–30. [DOI] [PubMed] [Google Scholar]
- 10. Schwab KO, Doerfer J, Hallermann K et al. Marked smoking-associated increase of cardiovascular risk in childhood type 1 diabetes. Int J Adolesc Med Health 2008;20(3):285–92. [DOI] [PubMed] [Google Scholar]