Abstract
The varying topography and environment that resulted from paleoorogeny and climate fluctuations of the Himalaya–Hengduan Mountains (HHM) areas had a considerable impact on the evolution of biota during the Quaternary. To understand the phylogeographic pattern and historical dynamics of Triosteum himalayanum (Caprifoliaceae), we sequenced three chloroplast DNA fragments (rbcL-accD, rps15-ycf1, and trnH-psbA) from 238 individuals representing 20 populations. Nineteen haplotypes (H1–H19) were identified based on 23 single-site mutations and eight indels. Most haplotypes were restricted to a single population or neighboring populations. Analysis of molecular variance revealed that variations among populations were much higher than that within populations for the overall gene pool, as well as for the East Himalayan group (EH group) and the North Hengduan group (NHM group), but not for the Hengduan Mountains group (HM group). Ecoregions representing relatively high genetic diversity or high frequencies of private haplotypes were discovered, suggesting that this alpine herbaceous plant underwent enhanced allopatric divergence in isolated and fragmented locations during the Quaternary glaciations. The current phylogeographic structure of T. himalayanum might be due to heterogeneous habitats and Quaternary climatic oscillations. Based on the phylogeographic structure of T. himalayanum populations, the phylogenetic relationship of identified haplotypes and palaeodistributional reconstruction, we postulated both westwards and northwards expansion from the HM group for this species. The westwards dispersal corridor could be long, narrow mountain areas and/or the Yarlung Zangbo Valley, while the northwards movement path could be south–north oriented mountains and low-elevation valleys.
Keywords: Chloroplast DNA, Palaeodistributional reconstruction, Himalaya–Hengduan Mountains, Phylogeography, Range expansion
Introduction
The present population genetic structure of species carries signals of past dynamics (Hewitt, 2000). Exploration of the interaction between environmental heterogenization, climate changes, and biota evolution can help us understand how organisms have been influenced by various environmental and climatic events. The Himalayas and Hengduan Mountains are both biodiversity hotspots of the Northern Hemisphere. The Himalaya–Hengduan Mountains (HHM) region, which extends along the southern frontier to the southeastern rim of the Qinghai–Tibetan Plateau (QTP), contains more than 20,000 species of vascular plants and harbors most plant families and genera of Eurasian flora with a high frequency of endemics (Wu, 1988; Li & Li, 1993). The HHM is also considered to be the core area of the Sino-Himalayan flora (Wu & Wu, 1996) as well as the distribution and diversification center for many alpine plants (Sun, 2002). Moreover, this region has been regarded as the origin center of boreal-temperate plants (Li & Li, 1993; Gao et al., 2012a; Jia et al., 2012; Lu et al., 2014; Favre et al., 2015).
In the early Eocene, the Indian Plate collided with the Eurasian Plate, directly resulting in subsequent uplift of the QTP and the Himalayas and Hengduan Mountains (Zheng & Yao, 2004; Chatterjee, Goswami & Scotese, 2013). The significant orogeny of Himalayas likely occurred during the Miocene (Wang et al., 2008), although the onset of Himalayan orogeny may have started slightly earlier (Chatterjee, Goswami & Scotese, 2013; Gébelin et al., 2013). The elevation history of the QTP suggests that the orogeny of the Hengduan Mountains occurred as a final phase of the uplift after 10 Ma (Mulch & Chamberlain, 2006). Sun et al. (2011) concludes that the Hengduan Mountains underwent a major uplift only after the Miocene, attaining its peak elevation shortly before the Late Pliocene according to paleobotanical and paleoclimatic data. Consequently, long, narrow east–west oriented mountainous areas and valleys (e.g., the Yarlung Zangbo Valley) of the Himalayan Mountains were formed, while north–south oriented valleys surrounded by high peaks of the Hengduan Mountains appeared.
Although geological records show that no unified ice sheets were developed in QTP and adjacent regions during the Quaternary period (Li & Pan, 2002), arid-cold glacial and wet-warm interglacial periods greatly affected the distribution range and diversification of plant species in this region. In fact, regional expansions and intraspecific divergences during the Quaternary climatic oscillations are very common in most studied alpine and subalpine species of the HHM (Liu et al., 2012; Yu et al., 2017, and references therein).
Thus, the unique, abundant, and diverse flora of the HHM, resulting from topographical diversity and climate fluctuation, have made this area one of the most prevalent regions for phylogeographic survey. Numerous hypotheses have been provided based on different phylogeographic patterns. However, previous studies mainly focused on the tree (Opgenoorth et al., 2010; Li et al., 2013; Liu et al., 2013; Sun et al., 2014) and shrub species (Wang et al., 2009; 2010; Jian, Tang & Sun, 2015; Gao et al., 2015), while only a few studies have focused on herbaceous plant species (Zhang, Volis & Sun, 2010; Gao et al., 2012b), especially those that grow in the humid habitats of the HHM region.
Triosteum himalayanum Wallich (Caprifoliaceae) is a perennial herb with a distribution in the eastern Himalayas, Hengduan Mountains, and central China (including Tibet, Yunnan, Sichuan, Hubei, Shaanxi, and Henan) at elevations between 1,800 and 4,100 m a.s.l. This species shows a fragmented distribution, occupying relatively wet habitats on mountain slopes, coniferous forests, streamsides, and grasslands (Yang et al., 2011). Gould & Donoghue (2000) conducted a phylogenetic and biogeographic study on the genus Triosteum L. based on morphological features and sequence polymorphism of the nuclear DNA internal transcribed spacer and granule-bound starch synthase gene (GBSSI or waxy), which provided the biogeographic pattern of Triosteum across North America and eastern Asia. However, they presented no details of the intraspecific variation and biogeography of T. himalayanum in the HHM and adjacent regions. A phylogeographic study of T. himalayanum would give us a better understanding of how fragmented populations have evolved since species diversification in the context of environmental heterogenization and Quaternary glaciation. Furthermore, a phylogeographic survey of multiple co-distributed species (e.g., the coniferous trees Taxus wallichiana, Picea likiangensis, and Juniperus tibetica) would be greatly useful for providing a detailed picture of plant diversity and endemism of regional hotspots (Qiu, Fu & Comes, 2011). Here, we employed three chloroplast DNA (cpDNA) sequences, rbcL-accD, rps15-ycf1, and trnH-psbA, combined with palaeodistributional reconstruction modeling analysis to infer phylogeography and demography dynamics of T. himalayanum. The detailed aims of this study are: (1) to reveal the genetic structure of this species with fragmented distribution and (2) to infer the historical dynamics, especially the dispersal direction between ecogroups and pathways in association with habitat differentiation and Quaternary climate fluctuations. In order to better interpret these issues, we divided all the sampled populations into three groups: the East Himalayan group (EH group, including populations P1–P7), the Hengduan Mountains group (HM group, P8–P12), and the north Hengduan Mountains group (NHM group, including P13–P20).
Materials and Methods
Material sampling
Field expeditions were conducted between 2006 and 2015 to cover all of the possible distribution localities of T. himalayanum according to specimen records in the herbaria. However, populations of T. himalayanum that were previously distributed in central China (according to the specimen study) were difficult to collect due to land use changes. Fresh leaves of 4–25 individuals were collected for each population, separated at least 20 m apart. A total of 238 individuals from 20 populations were collected. Among these populations, 19 were from the HHM, and only one population (P20) was not from this region (Table 1; Fig. 1). Leaf materials were dried in silica gel and stored at room temperature. Voucher specimens of all populations are deposited in the Herbarium of Northwest Institute of Plateau Biology (HNWP), Xining, Qinghai, China.
Table 1. Details of sample locations and sample sizes of 20 T. himalayanum populations.
| POP | Locality | Voucher no. | Latitude (N) | Longitude (E) | Altitude (m) | Sample size | Haplotypes (no. of individuals) |
|---|---|---|---|---|---|---|---|
| P1 | Yadong, T | Chen2013450 | 27°45′ | 88°58′ | 3,065 | 6 | H10(6) |
| P2 | Yadong, T | Chen2014519 | 27°38′ | 89°02′ | 4,200 | 5 | H16(5) |
| P3 | Yadong, T | Chen2014520 | 27°26′ | 88°55′ | 2,915 | 16 | H16(16) |
| P4 | Yadong, T | Chen2013464 | 27°37′ | 89°07′ | 4,771 | 12 | H16(12) |
| P5 | Luozha, T | Chen2013390 | 28°05′ | 91°05′ | 4,249 | 8 | H12(8) |
| P6 | Cuona, T | Chen2014443 | 27°48′ | 91°59′ | 3,720 | 4 | H16(4) |
| P7 | Luozha, T | Chen2014305 | 29°42′ | 94°44′ | 3,500 | 19 | H5(9) H6(10) |
| P8 | Xianggelila, YN | Chen2012111 | 27°54′ | 99°38′ | 3,380 | 18 | H2(13) H7(2) H8(1) H9(1) H14(1) |
| P9 | Xianggelila, YN | Chensl1849 | 27°38′ | 99°40′ | 3,580 | 12 | H7(1) H8(3) H15(8) |
| P10 | Yulong, YN | Chensl1846 | 27°03′ | 100°12′ | 3,420 | 15 | H1(2) H4(11) H11(1) H13(1) |
| P11 | Xianggelila, YN | Chen2013578 | 27°54′ | 99°44′ | 3,331 | 20 | H2(15) H3(1) H7(2) H14(2) |
| P12 | Yanyuan, SC | Chen2013519 | 27°41′ | 101°13′ | 3,240 | 4 | H7(2) H14(1) H17(1) |
| P13 | Luhuo, SC | Chen2013129 | 31°10′ | 100°53′ | 3,080 | 7 | H14(7) |
| P14 | Luhuo, SC | Chen06310 | 31°09′ | 100°54′ | 3,060 | 10 | H14(10) |
| P15 | Daofu, SC | Chensl1879 | 30°52′ | 101°15′ | 3,380 | 16 | H14(16) |
| P16 | Danba, SC | Chensl1881 | 30°32′ | 101°35′ | 3,810 | 25 | H14(24) H18(1) |
| P17 | Danba, SC | Chen06127 | 30°33′ | 101°38′ | 3,410 | 17 | H14(11) H18(6) |
| P18 | Hongyuan, SC | Chensl1840 | 31°50′ | 102°41′ | 3,370 | 12 | H14(12) |
| P19 | Maoxian, SC | Chen06084 | 31°40′ | 103°56′ | 3,500 | 4 | H19(4) |
| P20 | Langao, SX | Zhang2015014 | 32°17′ | 109°04′ | 2,130 | 8 | H19(8) |
Notes:
For each population, haplotype composition (H1–H19) is indicated.
YN, Yunnan; SC, Sichuan; SX, Shaanxi; T, Tibet.
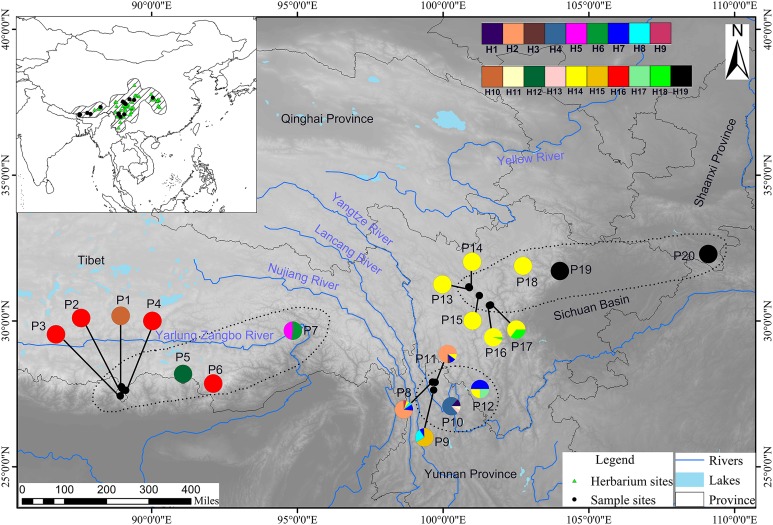
Figure 1. Geographic distribution of cpDNA haplotypes (H1–H19) detected among 20 populations (for population codes see Table 1) and herbarium sites of T. himalayanum.
Pie charts show the proportion of haplotypes within each population.
Lonicera trichosantha Bureau & Franchet (Caprifoliaceae), has been chosen as out group for haplotype phylogenetic analyses.
DNA extraction, polymerase chain reaction amplification, and sequencing
Total DNA was extracted from the silica-gel dried leaf materials following a modified CTAB (Cetyltrimethyl Ammonium Bromide; Doyle & Doyle, 1987). Samples were genetically screened using the universal cpDNA primers described by Hamilton (1999) and Dong et al. (2012). After preliminary screening (pilot screening) of more than 10 intergenic spacers, rbcL-accD, rps15-ycf1, and trnH-psbA were chosen for further analysis, since these three intergenic spacers revealed sequence polymorphism. Polymerase chain reactions (PCRs) were performed in 25 μL volume containing 0.6 μL of template DNA (approximately 20 ng), 2.5 μL of 10 × PCR buffer (with Mg2+), 1.0 μL of 10 mM dNTPs, 0.5 μL of 5 pM of each primer, and 0.2 μL (1.5 units) of Taq polymerase (Takara, Dalian, China). Amplification conditions were: 5 min at 95 °C; followed by 35 cycles of 50 s at 95 °C, 1 min at 58 °C, and 1 min at 72 °C; with a final extension of 6 min at 72 °C. PCR products were purified using a CASpure PCR Purification Kit (CASarry, Shanghai, China) following the manufacturer’s protocols. Sequencing reactions and analysis were performed using an ABI 3730xl DNA sequencer (Applied Biosystems, Foster City, CA, USA). Primers used for sequencing were the same as those used for amplification.
Population genetic and phylogeographic analysis
The chromatograms of each sequence were checked visually using Chromas ver. 2.33 (http://www.technelysium.com.au). DNA sequences were aligned in Clustal_X (Thompson et al., 1997) and then checked by visual inspection with minor adjustment. The cpDNA haplotypes were identified by DnaSP ver. 5.0 (Librado & Rozas, 2009). All newly generated sequences from T. himalayanum were submitted to GenBank, and the access numbers are MH122760–MH122783. Three cpDNA fragments, rbcL-accD, rps15-ycf1, and trnH-psbA, were then concatenated for the subsequent analyses. In the following analysis, we did not code the indels; each indel was considered as a one-step mutation.
The spatial genetic structure of cpDNA haplotypes was analyzed to define groups of populations that were geographically homogeneous and maximally differentiated from each other using the program SAMOVA ver. 1.0 (Dupanloup, Schneider & Excoffier, 2002); the number of initial conditions was set to 100, and pairwise difference (DNA) was chosen as the molecular distance.
Estimates of average gene diversity within populations (HS), total gene diversity (HT), GST (Nei, 1987), and NST (Grivet & Petit, 2002) were calculated for the overall populations and for each regional group using PERMUT ver. 2.0 (Pons & Petit, 1996). GST and NST were used to estimate population differentiations; GST only takes haplotype frequency into account, whereas NST also takes sequence similarities between haplotypes into account. A permutation test with 1,000 permutations was used to compare GST and NST. Significantly higher values of NST than the estimated GST indicated the presence of a phylogeographical structure (Pons & Petit, 1996).
Analysis of molecular variance (AMOVA; Excoffier, Smouse & Quattro, 1992) for overall populations and each regional group of T. himalayanum was conducted using ARLEQUIN version 3.11 (Excoffier, Laval & Schneider, 2005). The F-statistic (FST) was calculated, and significance was tested.
Phylogenetic analyses
The appropriate model of DNA substitution for maximum likelihood (ML) analysis was determined using the AIC in jModelTest ver. 2.1.6 (Darriba et al., 2012; Guindon & Gascuel, 2003). Best-fit models for the cpDNA datasets were determined to be HKY+G. ML trees of cpDNA haplotypes of T. himalayanum were generated in PhyML ver. 3.1 (Guindon & Gascuel, 2003) using L. trichosantha as the outgroup. We selected BioNJ as the distance-based tree reconstruction method during tree searching. Subtree pruning and regrafting (SPR) moves were relied on to explore the space of tree topologies. The number of random starting trees was five. The tree branch support was estimated by non-parametric bootstrap analysis, and the number of bootstrap replicates was 100. Bayesian inference was conducted using MrBayes ver. 3.2 (Ronquist et al., 2012). The MCMC algorithm was run for 10,000,000 generations starting from random trees, and one in every 100 generations was sampled. The first 25% of the trees was discarded as burn-in followed by reconstruction of the majority consensus tree. Internodes with posterior probabilities >95% were considered to be statistically significant. When the average standard deviation of split frequencies between the two independent runs was smaller than 0.01, the analysis was stopped. Maximum parsimony (MP) analysis was implemented using Phylip ver. 3.69 (Felsenstein, 1985, 1988; Margush & McMorris, 1981). The number of bootstrap replicates was set to 1,000, and the random number seed was five.
To further detect genealogical relationships, a haplotype network was constructed among cpDNA haplotypes using the median‐joining method and MP calculations as implemented in Network ver. 5.0.0.0 (Bandelt, Forster & Röhl, 1999; Polzin & Daneschmand, 2003; http://www.fluxus-engineering.com).
Demographic analyses
For the overall populations and three regional groups, Tajima’s D and Fu’s Fs of neutrality tests with 1,000 simulated samples were performed using ARLEQUIN ver. 3.11 (Fu, 1997). Negative values are expected when there have been recent population expansions or population bottlenecks (Tajima, 1989). Pairwise mismatch distribution analysis (Rogers & Harpending, 1992) for overall populations and each group based on the cpDNA dataset was performed assuming historical population dynamics using DnaSP ver. 5.0 (Librado & Rozas, 2009). Unimodal distributions reflect rapid growth from small populations, while multimodal distributions reflect long-term population stability (Slatkin & Hudson, 1991; Rogers & Harpending, 1992).
Palaeodistributional reconstruction
Potential geographic ranges and the effects of past climatic oscillations for T. himalayanum has computed across current, Mid Holocene (6 ka), last glacial maximum (LGM; 22 ka), and last interglacial maximum (LIG; 130 ka, Otto-Bliesner et al., 2006). We simulated all the species distribution models, i.e., GBM (generalized boosted models; Ridgeway, 2006), SRE (surface range envelop; Busby, 1991), GLM (generalized linear models; Peter & John, 1989), CTA (classification tree analysis; Breiman, 2001, 2017), ANN (artificial neural network; Ripley, 1996), FDA (flexible discriminant analysis; Hastie, Tibshirani & Buja, 1994), MARS (multivariate adaptive regression splines; Friedman, 1991), RF (random forests; Breiman, 2001), and MAXENT (maximum entropy; Phillips, Dudík & Schapire, 2004) in R packages “Biomod 2” (Thuiller, Georges & Engler, 2014), supported with rworld map, rgdal, dismo, SDMTools. The effectiveness of all the models were evaluated using TSS (true skill statistics) and AUC (area under curve; curve-receiver operating characteristic curve) values >0.7. A total of nine bioclimatic variables (BIO4 = temperature seasonality, BIO5 = max temperature of warmest month, BIO6 = min temperature of coldest month, BIO7 = temperature annual range, BIO8 = mean temperature of wettest quarter, BIO9 = mean temperature of driest quarter, BIO10 = mean temperature of warmest quarter, BIO11 = mean temperature of coldest quarter, and BIO15 = precipitation seasonality) showed low correlation and high informativeness after a jackknife procedure on the 19 BIOCLIM variables downloaded from the WorldClim data set (Hijmans et al., 2005).
Results
Haplotype variation and distribution
The combined cpDNA sequences of rbcL-accD, rps15-ycf1, and trnH-psbA of T. himalayanum ranged from 1,698 to 1,718 bp in length, with an alignment length of 1,727 bp. Nineteen haplotypes (H1–H19) were identified based on polymorphic sites across the concatenated cpDNA sequence data (Table 2). Among those polymorphic sites, we found 23 single-site mutations and eight indels. The rbcL-accD region was the most variable fragment (14 substitutions; three indels), followed by rps15–ycf1 (six substitutions; five indels), and trnH-psbA (three substitutions).
Table 2. Sequence polymorphisms detected in three chloroplast DNA regions of T. himalayanum identifying 19 haplotypes (H1–H19).
| Nucleotide position | rbcL-accD | rps15-ycf1 | trnH-psbA | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 4 | 5 | 5 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 2 | 2 | 3 | 3 | 4 | 4 | 4 | 4 | 1 | 1 | 2 | ||||||
| 1 | 5 | 0 | 3 | 5 | 6 | 4 | 6 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 5 | 2 | 7 | 9 | 4 | 9 | 0 | 8 | 2 | 4 | 4 | 4 | 2 | 7 | 1 | |
| 3 | 2 | 5 | 9 | 0 | 7 | 9 | 9 | 3 | 4 | 5 | 1 | 2 | 4 | 5 | 6 | 5 | 4 | 9 | 8 | 6 | 8 | 9 | 4 | 3 | 5 | 8 | 9 | 8 | 7 | 1 | |
| H1 | A | G | G | C | G | C | – | G | T | A | A | A | A | – | T | T | * | C | d | – | A | A | T | T | – | G | – | – | G | G | C |
| H2 | · | · | · | · | · | · | – | · | · | · | · | · | · | $ | · | · | · | A | · | – | · | · | · | · | – | · | – | – | · | · | · |
| H3 | · | · | · | · | · | · | – | · | · | · | · | · | · | $ | · | · | · | A | · | – | · | · | · | · | – | · | – | – | · | T | · |
| H4 | · | · | · | · | · | · | – | · | · | · | · | · | · | – | · | · | · | A | · | – | · | · | · | · | – | · | – | – | · | · | · |
| H5 | T | · | A | · | A | · | – | · | · | · | · | · | · | $ | A | · | – | A | · | – | · | C | · | G | – | · | ψ | – | T | · | · |
| H6 | T | · | · | · | A | · | – | · | · | · | · | · | · | $ | A | · | – | A | · | – | · | C | · | G | – | · | ψ | – | T | · | · |
| H7 | · | · | · | · | · | · | – | · | · | · | · | · | · | $ | A | · | · | A | · | – | · | · | · | · | – | · | – | – | · | · | · |
| H8 | · | · | · | · | · | · | – | · | · | · | · | · | · | $ | A | A | · | A | · | – | · | · | · | · | – | · | – | – | · | · | · |
| H9 | · | A | · | · | · | · | – | · | · | · | · | · | · | $ | A | · | · | A | · | – | · | · | · | · | – | · | – | – | · | · | · |
| H10 | · | · | · | · | · | · | – | · | A | · | · | · | · | $ | A | · | · | A | § | – | · | · | · | · | – | · | – | – | · | · | · |
| H11 | · | · | · | · | · | · | # | · | A | · | · | T | · | $ | A | · | · | A | · | – | · | · | · | · | – | · | – | – | · | · | · |
| H12 | · | · | · | · | · | · | – | · | A | · | · | · | T | $ | A | · | · | A | · | – | · | · | · | · | – | · | – | – | · | · | · |
| H13 | · | · | · | · | · | · | – | · | · | · | · | · | – | $ | A | · | · | A | · | – | · | · | · | · | – | · | – | – | · | · | · |
| H14 | · | · | · | · | · | · | – | · | · | · | · | · | T | $ | A | · | · | A | · | – | · | · | · | · | – | · | – | – | · | · | · |
| H15 | · | · | · | · | · | · | – | · | · | · | · | · | T | $ | A | · | · | A | · | – | · | · | · | · | – | · | – | – | · | T | · |
| H16 | · | · | · | · | · | T | – | · | · | · | · | · | T | $ | A | · | · | A | · | – | · | · | · | · | – | · | – | – | · | · | · |
| H17 | · | · | · | · | · | · | – | · | · | · | · | · | T | $ | A | · | · | A | · | – | · | · | · | · | ξ | · | – | – | · | · | · |
| H18 | · | · | · | · | · | · | – | · | · | · | · | · | T | $ | A | · | · | A | – | – | · | · | · | · | – | · | · | – | · | · | · |
| H19 | · | · | · | A | · | · | # | A | T | T | T | · | · | $ | A | · | · | A | – | ж | G | · | G | · | – | A | ψ | & | T | · | A |
Notes:
Sequences are numbered from the 5′ to the 3′ end in each region.
# = AGGTCATGTGCCAC, $ = A, * = TCTC, § = CGAATT, ж = T, ξ = ATATC, ψ = G, & = GG.
Twelve haplotypes (accounting for 63.2% of the total haplotypes) were relatively rare, each was restricted to a single population (H1, H3–H6, H9–H13, H15, and H17). Moreover, all of the twelve haplotypes were distributed in the HM group of five populations, indicating a very high level of molecular diversity (Table 1). Another seven haplotypes were shared by at least two populations, suggesting a substantial amount of population isolation at the cpDNA level.
The most common haplotype was H14, which occurred in nine populations with variable locations in the HM group (three populations) and NHM group (six populations). Haplotypes H7 and H16 both occurred in four populations but with locations not far from each other (Fig. 1).
Population variation and phylogeographic structure
Twelve populations (P1–P6, P13–P15, and P18–P20) were fixed for single haplotype, showing no variation within the population. However, the remaining eight populations (P7–P12, P16, and P17) in the HM and NHM groups were particularly diverse, with two to five haplotypes in each population (Table 1; Fig. 1). Populations P1–P6 were found to belong to the EH group; each contained only one haplotype, and all three haplotypes present in these six populations were endemic.
Population P19 was located far from P20, isolated by the Sichuan Basin. However, these two populations shared the sole haplotype H19.
SAMOVA failed to detect any meaningful geographical groups based on cpDNA haplotypes. K values increased from two to seven while FCT values changed irregularly from 0.897 to 0.781. However, each newly defined group was represented by a single population. On the basis of the distribution ecoregions of the populations and the genetic structure of the T. himalayanum populations, we divided the populations into three groups: the East Himalayan group (EH group, including populations P1–P7), the Hengduan Mountains group (HM group, P8–P12), and the North Hengduan Mountains group (NHM group, including P13–P20). The division backgrounds which can be considered as temporal and biota differences between these three eco-regions are illustrated as follows: (1) distinctions between the Hengduan Mountains and the Himalaya. Mulch & Chamberlain (2006) suggests that the orogeny of the Hengduan Mountains occurred as a final propagation of the palaeoelevation history of the QTP uplift after 10 Ma, reflecting its much younger age than the Himalaya. In addition, the HM is more species-rich compared with the Himalaya, and biota diversification process of HM is distinguishing from that of Himalaya (Xing & Ree, 2017); (2) differences between the NHM group and HM group-even the NHM group occupies part of the Hengduan Mountains and adjoining highland, the genetic diversity of T. himalayanum and some other species (Du et al., 2017) in there is quite lower than that of core area of Hengduan Mountains (HM group). A similar grouping strategy can be found in many studies of plant species that are sympatric with T. himalayanum (Sun & Zhou, 1996; Zou et al., 2012; Liu et al., 2013; Yu et al., 2015; Gao et al., 2016; Du et al., 2017). The EH group was distributed along the Yarlung Zangbo River or Himalayan Mountains, ranging from the big bend gorge of Yalutsangpu to the Yadong valley. The HM group is located in the core region of the Hengduan Mountains with rich molecular diversity. The location of the NHM group is the northern part of the Hengduan Mountains region, which is also the eastern edge of the QTP.
For the overall populations, the NST value was 0.929, while the GST value was 0.781, and the difference between them was significant (p < 0.001). A significant difference between NST and GST was also found in the EH and NHM groups but not in the HM group. The values of total genetic diversity (HT) were much higher than those of gene diversity within populations (HS) for T. himalayanum in the overall populations (HT = 0.878, HS = 0.192), EH group (HT = 0.714, HS = 0.075), HM group (HT = 0.922, HS = 0.550), and NHM group (HT = 0.498, HS = 0.071, Table 3). AMOVA for the overall populations revealed that 89.43% of the total genetic variation occurred among populations, compared with 10.57% within populations; the pairwise FST value was 0.8943. Similar AMOVA results were obtained for both the EH group and NHM group. However, the HM group exhibited a higher level of within-population variation compared with among-population variation (Table 4).
Table 3. Estimates of average gene diversity within populations (HS), total gene diversity (HT), interpopulation differentiation (GST), and number of substitution types (NST).
| HS | HT | GST | NST | |
|---|---|---|---|---|
| Overall populations | 0.192 | 0.878 | 0.781 | 0.929* |
| EH group | 0.075 | 0.714 | 0.895 | 0.983* |
| HM group | 0.550 | 0.922 | 0.404 | 0.253 |
| NHM group | 0.071 | 0.498 | 0.858 | 0.966* |
Note:
Indicates that NST is significantly different from GST (p < 0.05).
Table 4. Analysis of molecular variance (AMOVA) of chlorotypes for populations and population groups of T. himalayanum.
| Regions | Source variation | d.f. | SS | VC | Variation (%) | FST |
|---|---|---|---|---|---|---|
| Whole distribution | Among populations | 19 | 695.940 | 3.089 | 89.43 | |
| Within populations | 218 | 79.585 | 0.365 | 10.57 | ||
| Total | 237 | 775.525 | 3.453 | 0.894* | ||
| EH group | Among populations | 6 | 186.249 | 3.253 | 97.74 | |
| Within populations | 63 | 4.737 | 0.075 | 2.26 | ||
| Total | 69 | 190.986 | 3.328 | 0.977* | ||
| HM group | Among populations | 4 | 27.756 | 0.470 | 39.66 | |
| Within populations | 64 | 45.794 | 0.716 | 60.34 | ||
| Total | 68 | 73.551 | 1.186 | 0.397* | ||
| NHM group | Among populations | 7 | 358.340 | 4.269 | 93.04 | |
| Within populations | 91 | 29.054 | 0.319 | 3.96 | ||
| Total | 98 | 387.394 | 4.588 | 0.930* | ||
| EH group, HM group versus NHM group | Among groups | 1 | 59.032 | 0.446 | 16.20 | |
| NHM group | Among populations within groups | 10 | 214.005 | 1.907 | 69.35 | |
| Within populations | 127 | 50.531 | 0.398 | 14.46 | ||
| Total | 138 | 323.568 | 2.752 | 0.855* |
Notes:
p < 0.001.
d.f., degree of freedom; SS, sum of squares; VC, variance component.
Phylogenetic relationship
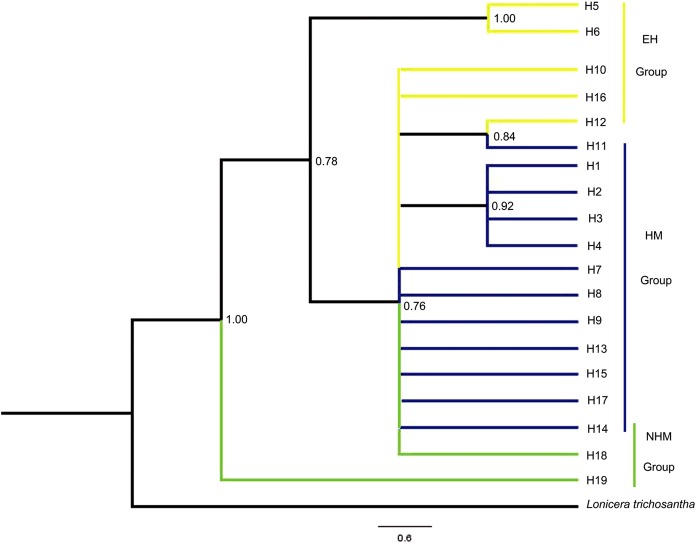
The relationships of the 19 haplotypes are shown in Figs. 2 and 3. Phylogenetic trees constructed using MP, ML, and Bayesian methods are not consistent with each other. They do not show clear relationships between cpDNA haplotypes. However, H19 was observed to occupy the radical position in all trees, and H5 and H6 clustered as one clade in the basal position but only in the Bayesian inference and ML trees. The brush structure observed in the Bayesian tree may be due to the rapid diversification of this species. In the network phylogenetic analysis, the number of mutations between H6 and H7 was seven, illustrating considerable differentiation between the EH group and HM group.
Figure 2. Phylogenetic relationships inferred among the cpDNA haplotypes of T. himalayanum based on the sequences of the combined cpDNA from Bayesian inference.
Posterior probabilities are given at the nodes.
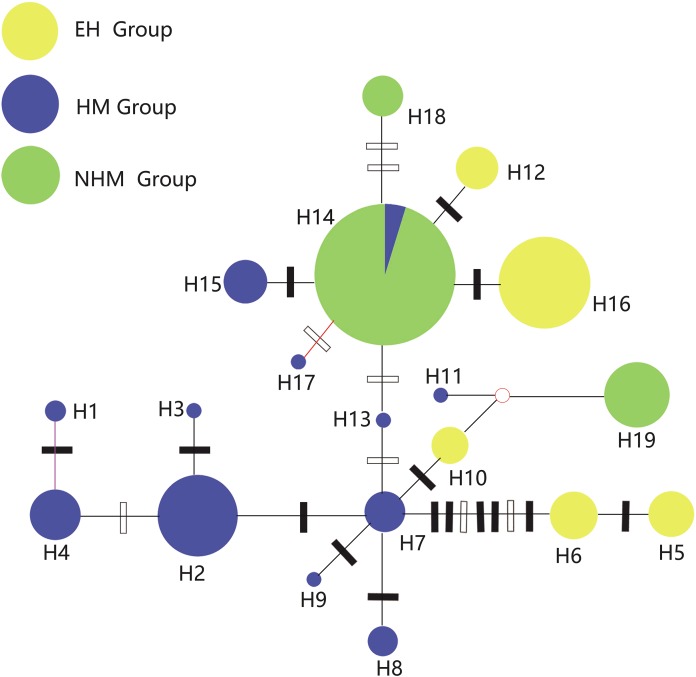
Figure 3. Network of 19 cpDNA haplotypes of T. himalayanum (identified by numbers: H1–H19).
The size of circles corresponds to the frequency of each haplotype. The small open circles indicate inferred intermediate haplotypes not detected in this investigation. Solid and open bars represent nucleotide substitutions and indels, respectively.
Demographic history
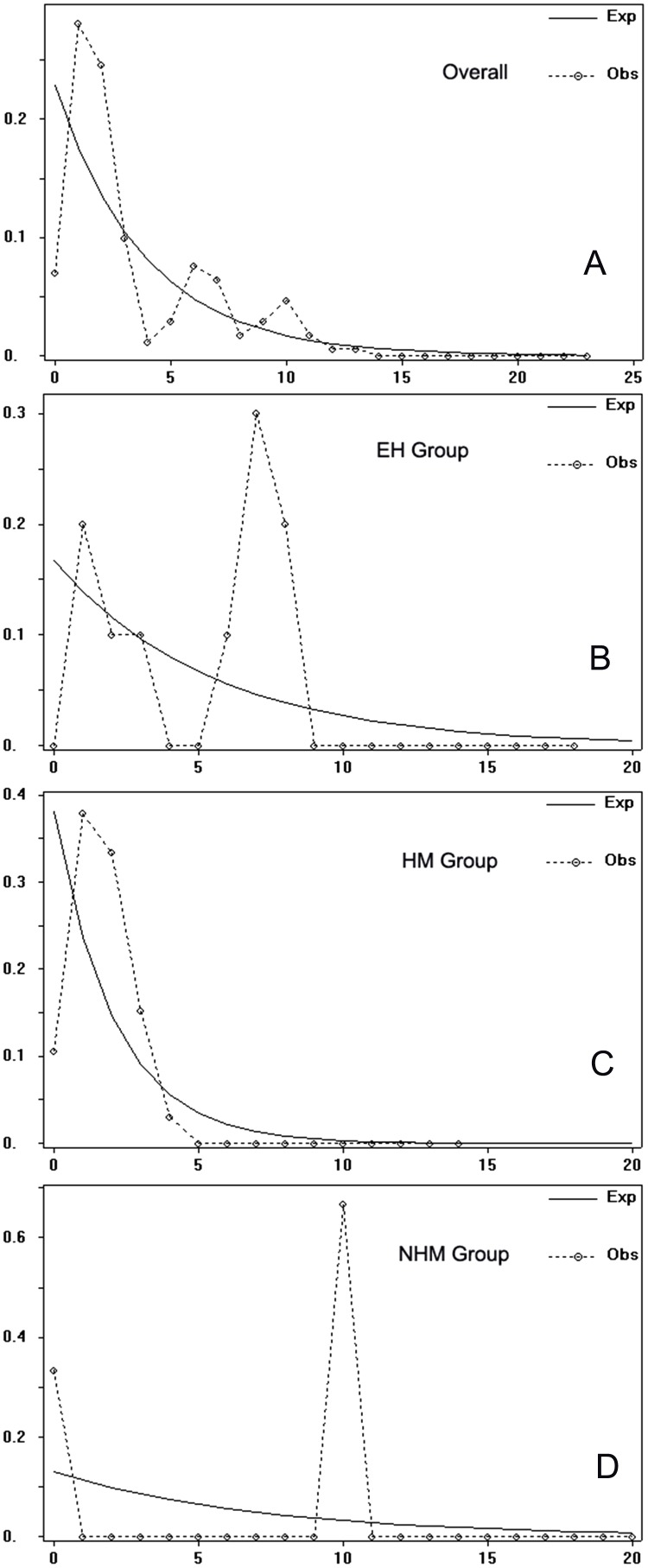
Tajima’s D values of the overall populations and the HM group were negative but not significant (p > 0.05), suggesting slight range expansion. Fu’s Fs statistics showed positive values or not significant negative values for the whole gene pool and three groups (Table 5). Mismatch distributions of the overall populations, the EH group and the NHM group were multimodal, indicating a demographic equilibrium; the HM was unimodal (Fig. 4). A star-like pattern network is considered to indicate a demographic expansion (Hudson, 1990), thus supporting the findings of the Tajima’s D. H7 and H14 are more likely to be the ancestral haplotypes because they occupy a relative central position in the network (Carbone & Kohn, 2001).
Table 5. Results of neutrality tests and mismatch distribution analysis for the overall populations and three regional groups of T. himalayanum based on the cpDNA dataset.
| Tajima’s D test | Fu’s Fs test | |||
|---|---|---|---|---|
| Populations | D | p value | Fs | p value |
| Overall | −0.60499 | 0.30700 | 2.42674 | 0.78800 |
| EH group | 2.34795 | 0.99000 | 9.98669 | 0.98600 |
| HM group | −0.60620 | 0.31700 | −2.49952 | 0.16000 |
| NHM group | 0.28687 | 0.66000 | 22.46008 | 0.99800 |
Figure 4. Mismatch distribution in the overall populations and the three groups for chloroplast DNA sequence data of T. himalayanum.
(A) Mismatch distribution for overall populations. (B) Mismatch distribution for EH group. (C) Mismatch distribution for HM group. (D) Mismatch distribution for NHM group. Solid lines represent expected differences among sequences, whereas dashed lines are drawn from the observed differences.
Paleaodistributional reconstruction
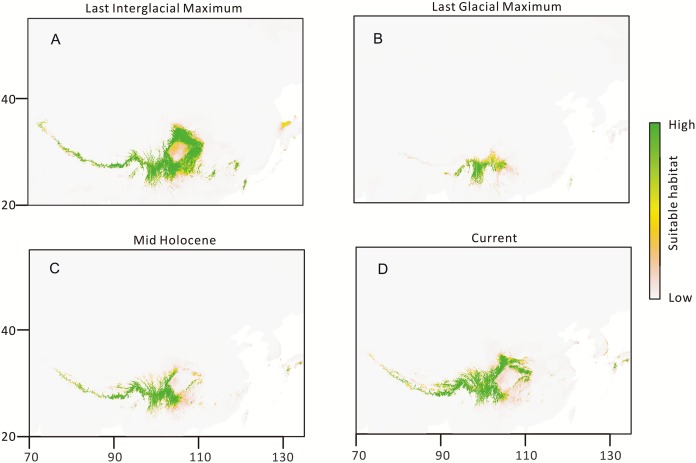
The Palaeodistributional reconstruction showed that during the last interglacial maximum T. himalayanum occupied large scope of Himalaya, almost the whole of Hengduan Mountains area, and adjoining region arounding the Sichuan Basin (Fig. 5). During the last glacial maximum, the species was restricted southwards with sharp reduction of distribution, and there were few distribution in East Himalaya and North Hengduan Mountains. From Mid Holocene up to the current the species is expanding Northwards and Westwards again. Climatic niche during three different times suggesting that this alpine herb went through repeated retreat and expansion, and affected by the severe climatic conditions of the QTP.
Figure 5. Estimated climatic niche models for T. himalayanum distribute at QTP.
Maps are shown for each of the four periods tested. (A) Suitable areas for LIG. (B) Suitable areas for LGM. (C) Suitable areas for Mid-Holocene. (D) Suitable areas for Current.
Discussion
Paleoorogeny has dramatically changed the topography and geology of the HHM, leading to complex land conditions, such as high mountains and plains incised deeply by numerous valleys and rivers. Thus, this region presents drastic altitudinal variations ranging from 1,000 m a.s.l. (deep valley floors) to numerous peaks above 6,000 m a.s.l., and it is one of the most ecologically diverse areas in the QTP region (López-Pujol et al., 2011). Furthermore, this region is also influenced by both the Indian and East Asian summer monsoons (Zhang et al., 2012a). During the Quaternary, climate changes, especially glacial-interglacial cycles, greatly affected the HHM environment (Zheng, Xu & Shen, 2002). Topographic diversity and climatic circumstances provide heterogeneous habitats for the biota of this region. Several biomes are present (e.g., alpine tundra, meadow, montane forests, and rainforests), and different biomes can be found on the same mountain simultaneously (Ni & Herzschuh, 2011; Zheng & Chen, 1981). To adapt to environmental and climate modifications, organisms are forced to change their habitats and life cycles or to develop new physical traits (Berteaux et al., 2010). Therefore, species differentiation and dispersal are common in the HHM area (Xu, Li & Sun, 2014; Xing & Ree, 2017). Differentiation usually appears between isolated populations, and dispersal usually occurs between regions with broad physiographic similarities (Gao et al., 2015; Xing & Ree, 2017).
Phylogeographic structure of T. himalayanum and its correlation with heterogeneous habitats and Quaternary climatic oscillation
One characteristic of the geographic genetic structure of T. himalayanum is a high level of private haplotypes. Twelve out of 19 detected cpDNA haplotypes were private. High total diversity versus a relatively low within-population diversity is another feature of T. himalayanum, and this type of geographic genetic structure is found in many studied alpine species located in the HHM and adjoining regions (Yang et al., 2008; Wang et al., 2009; Zhang, Meng & Rao, 2014; Gao et al., 2016; Wan et al., 2016). The comparisons between the values of HT and those of HS in the overall populations and the three regional groups suggest high genetic diversity between populations. The AMOVA results also showed high levels of genetic differentiation between populations. These findings indicate a clear phylogeographic structure (Avise, 2004) except for the HM group (with an among-population variation of 39.66%). Factors promoting the among-population differentiation of T. himalayanum are likely to be complicated. Among those, fragmented distribution and population isolation caused by historical orogenesis and climatic oscillations could be the main factors. In heterogeneous habitats, diverse elevations, precipitation, temperatures, edaphic factors, wind systems, and ecological selection resulting from competitors, predators, parasites, and perhaps mutualists that might restrict other species’ ranges, could accelerate species differentiation (Renner, 2016; Chozas et al., 2017).
Orogenic activities cause a large geomorphologic adjustment, and hydrologic, and tectonic configurations are produced (Li & Fang, 1999). Studies on palaeoaltimetry of the QTP show a progressive uplift from south to north which is likely to have started during or shortly after the beginning of the collision (about 45–40 Ma; Li & Fang, 1999; Mulch & Chamberlain, 2006). Since then, the QTP has experienced shortening on its north–south axis accompanied by the formation of rough surface features and clockwise rotation in its eastern part (Favre et al., 2015). These result in the formation of north–south oriented valleys/rivers in the southeast plateau (including the Yangtze River, the Langcang River, and the Nujiang valleys), as well as west–east oriented valleys/rivers in the south (e.g., the Yarlung Zangbo River) surrounded by high peaks (Favre et al., 2015), which provide pathways for south–north and east–west migration (Wu, 1979; Yu et al., 2017). Due to its immense size and altitude, the QTP and adjacent highland is closely linked to development of the Asian monsoon system and consequently contributed to climatic changes in this region (Ruddiman & Kutzbach, 1991; Liu & Yin, 2002; Tang et al., 2013). During the glacial-interglacial cycle, many species experienced divergent or “contact” evolution during glacial contractions and postglacial expansions (Avise, 2000; Yu et al., 2015). Based on our survey, the distribution of diverse haplotypes in the HM group may have resulted from several contraction/expansion cycles following the repeated advance/retreat of glaciations. The contraction/expansion process may also result in the accumulation of rich haplotypes within the population. This region may play a diversification role and may even be the origin center of T. himalayanum. Successive founder events during postglacial recolonization would have led to reduced genomic variability and areas of genetic homogeneity along the recolonization route (Hewitt, 2004). This phenomenon has been frequently observed in the HHM biota (Opgenoorth et al., 2010; Gao et al., 2016; Yu et al., 2017, and references therein). Thus, the decline of gene diversity from the HM group (HT = 0.922) to the NHM group (HT = 0.498) could result from the south–north direction postglacial recolonization. This hypothesis was also best supported by the palaeodistributional reconstruction (Fig. 5). Furthermore, complicated topography produced by tectonic movement and Quaternary glacial-interglacial cycles could result in range fragmentation of T. himalayanum, leading to reduced gene flow and the initiation of intra-species allopatric divergence (Coyne, 1992; Rice & Hostert, 1993; Hewitt, 2000, 2004). This may explain that eight of the 12 haplotypes in the HM group and four of the five haplotypes in the EH group are private. Most studies on the alpine flora of the HHM and adjacent regions have highlighted the effect of the Quaternary period on intraspecific diversification (Zhang, Volis & Sun, 2010; Gao et al., 2012b; Zhang et al., 2012b; Wang et al., 2013; Li et al., 2013; Khan et al., 2015). Therefore, the high genetic variations among populations of T. himalayanum could result from strong bottlenecks and founder effects/genetic drift to fix different alleles in isolated populations of fragmented regions (Birky & William, 2001).
Demographic history of triosteum himalayanum in the HHM area
Even the values of Fu’s Fs and mismatch distributions for the overall populations (Table 5) reject the model of current extensive expansion, the star-like network together with the negative values of Tajima’s D for the overall populations indicate a demographic expansion. Additionally, the palaeodistributional reconstruction revealed a clear pattern of recolonization from the Mid Holocene up to current. Therefore, we prefer the indication that T. himalayanum did experience range expansion from the core region of Hengduan Mountains (HM group) to both the East Himalaya area (EH group) and the North Hengduan Mountains (NHM group) from Mid Holocene.
The phylogenic relationship of the star‐like network indicates that haplotypes of the EH group originated from Hengduan Mountains areas, and there was also a decline of gene diversity from the HM group (HT = 0.922) to the EH group (HT = 0.714). One of the migration corridors may be the Yarlung Zangbo Valley, which is the largest vapor channel of the QTP (Lin & Wu, 1990). Lower elevation and intense airflow create ideal circumstances for the dispersal of seed and pollen of T. himalayanum. The other important dispersal corridors could be the long, narrow mountain areas. Since the Himalayas are linked to the Hengduan Mountains, exchanges along the mountain ridges or slopes occur easily. This hypothesis has been assumed in many studies and reviews (Cun & Wang, 2010; Luebert & Weigend, 2014; Yu et al., 2015, 2017). As discussed above, the south–north direction of the Hengduan Mountains and the river valley provide channels for glacial contractions and postglacial expansions for plant and animals and facilitate communication between different ecoregions of the HM and NHM areas. Therefore, haplotype H14 is not only shared by both the HM group and the NHM group, but it is also the most dominant and widely distributed haplotype.
However, anthropogenic activities that reduce the distribution range and genetic diversity could be other factors responsible for lower gene diversity and within-population variation of the NHM group in addition to bottleneck or founder effects. Many previous habitats of T. himalayanum recorded in documents are now surrounded by folk houses, and we were not able to locate them during the field work conducted two years ago. We should pay more attention to this phenomenon and more effectively preserve this alpine herb for future study and utilization.
Conclusion
The hypothesis that HHM is both the distribution and diversification center for alpine plants was supported by molecular diversity of T. himalayanum. The south–north and east–west migration from core area of HM was inferred from the phylogeographic structure, including the decline of gene diversity from the HM group to both the NHM group and EH group, phylogenetic relationship, and feasible topography and environment for dispersal. The palaeodistributional reconstruction result also showed an extremely clear pattern of retreat and recolonization during last episodes of interglacial and glacial periods.
However, more integrative multidimensional analyses of morphology, geography, ecology, and phylogeny should shed light on this fascinating research region, since the understanding of interactions between varying topography, environment, and the evolution of biota during the Quaternary are far from sufficient.
Supplemental Information
Haplotypes (H1–19) detected among 20 populations of Triosteum himalayanum..
Acknowledgments
We thank Wen-hui Liu of the Northwest Institute of Ecology and Environmental Resources, Chinese Academy of Sciences, for providing image processing support.
Funding Statement
This study was financially supported by the National Natural Science Foundation of China (31110103911), the Science and Technology Basic Work, Project of the Ministry of Science and Technology, China (2013FY112100 and 2015FY110500), the Applied Basic Research Programs of Qinghai Province, China (2016-ZJ-761), the Youth Innovation Promotion Association CAS (2016378), the CAS “Light of West China” Program, and the Construction Project for Innovation Platform of Qinghai province (2017-ZJ-Y14). There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Qing-bo Gao, Email: qbgao@nwipb.cas.cn.
Shi-long Chen, Email: slchen@nwipb.cas.cn.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Hai-rui Liu conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, approved the final draft.
Qing-bo Gao conceived and designed the experiments, authored or reviewed drafts of the paper.
Fa-qi Zhang contributed reagents/materials/analysis tools.
Gulzar Khan contributed reagents/materials/analysis tools.
Shi-long Chen conceived and designed the experiments.
Data Availability
References
- Avise (2000).Avise JC. Phylogeography: The History and Formation of Species. London: Harvard University Press; 2000. [Google Scholar]
- Avise (2004).Avise JC. Molecular Markers, Natural History and Evolution. Second Edition. Sunderland: Sinauer Associates; 2004. [Google Scholar]
- Bandelt, Forster & Röhl (1999).Bandelt HJ, Forster P, Röhl A. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution. 1999;16(1):37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- Berteaux et al. (2010).Berteaux D, de Blois S, Angers JF, Bonin J, Casajus N, Darveau M, Fournier F, Humphries MM, McGill B, Larivée J, Logan T, Nantel P, Périé C, Poisson F, Rodrigue D, Rouleau S, Siron R, Thuiller W, Vescovi L. The CC-bio project: studying the effects of climate change on Quebec biodiversity. Diversity. 2010;2(11):1181–1204. doi: 10.3390/d2111181. [DOI] [Google Scholar]
- Birky & William (2001).Birky JR, William C. The inheritance of genes in mitochondria and chloroplasts: laws, mechanisms, and models. Annual Review of Genetics. 2001;35(1):125–148. doi: 10.1146/annurev.genet.35.102401.090231. [DOI] [PubMed] [Google Scholar]
- Breiman (2001).Breiman L. Random forests. Machine Learning. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- Breiman (2017).Breiman L. Manual on setting up, using, and understanding random forests V3. 1. 2017. http://www.stat.berkeley.edu/~breiman http://www.stat.berkeley.edu/~breiman
- Busby (1991).Busby JR. BIOCLIM—a bioclimate analysis and prediction system. In: Margules CR, Austin MP, editors. Nature Conservation: Cost Effective Biological Surveys and Data Analysis. Canberra: CSIRO; 1991. pp. 64–68. [Google Scholar]
- Carbone & Kohn (2001).Carbone I, Kohn LM. A microbial population-species interface: nested cladistic and coalescence inference with multilocus data. Molecular Ecology. 2001;10(4):947–964. doi: 10.1046/j.1365-294x.2001.01244.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee, Goswami & Scotese (2013).Chatterjee S, Goswami A, Scotese CR. The longest voyage: tectonic, magmatic, and paleoclimatic evolution of the Indian plate during its northward flight from Gondwana to Asia. Gondwana Research. 2013;23(1):238–267. doi: 10.1016/j.gr.2012.07.001. [DOI] [Google Scholar]
- Chozas et al. (2017).Chozas S, Chefaoui RM, Correia O, Bonal R, Hortal J. Environmental niche divergence among three dune shrub sister species with parapatric distributions. Annals of Botany. 2017;119(7):1157–1167. doi: 10.1093/aob/mcx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne (1992).Coyne JA. Genetics and speciation. Nature. 1992;355(6360):511–515. doi: 10.1038/355511a0. [DOI] [PubMed] [Google Scholar]
- Cun & Wang (2010).Cun YZ, Wang XQ. Plant recolonization in the Himalaya from the southeastern Qinghai-Tibetan Plateau: geographical isolation contributed to high population differentiation. Molecular Phylogenetics and Evolution. 2010;56(3):972–982. doi: 10.1016/j.ympev.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Darriba et al. (2012).Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods. 2012;9(8):772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong et al. (2012).Dong WP, Liu J, Yu J, Wang L, Zhou SL. Highly variable chloroplast markers for evaluating plant phylogeny at low taxonomic levels and for DNA barcoding. PLOS ONE. 2012;7(4):e35071. doi: 10.1371/journal.pone.0035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle & Doyle (1987).Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf material. Phytochemical Bulletin. 1987;19:11–15. [Google Scholar]
- Du et al. (2017).Du FK, Hou M, Wang WT, Mao KS, Hampe A. Phylogeography of Quercus aquifolioides provides novel insights into the Neogene history of a major global hotspot of plant diversity in south–west China. Journal of Biogeography. 2017;44(2):294–307. doi: 10.1111/jbi.12836. [DOI] [Google Scholar]
- Dupanloup, Schneider & Excoffier (2002).Dupanloup I, Schneider S, Excoffier L. A simulated annealing approach to define the genetic structure of populations. Molecular Ecology. 2002;11(12):2571–2581. doi: 10.1046/j.1365-294X.2002.01650.x. [DOI] [PubMed] [Google Scholar]
- Excoffier, Laval & Schneider (2005).Excoffier L, Laval G, Schneider S. Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online. 2005;1:47–50. doi: 10.1177/117693430500100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier, Smouse & Quattro (1992).Excoffier LP, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 1992;131(2):479–491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre et al. (2015).Favre A, Päckert M, Pauls SU, Jähnig SC, Uhl D, Michalak I, Muellner-Riehl AN. The role of the uplift of the Qinghai-Tibetan Plateau for the evolution of Tibetan biotas. Biological Reviews. 2015;90:236–253. doi: 10.1111/brv.12107. [DOI] [PubMed] [Google Scholar]
- Felsenstein (1985).Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Felsenstein (1988).Felsenstein J. Phylogenies from molecular sequences: inference and reliability. Annual Review of Genetics. 1988;22(1):521–565. doi: 10.1146/annurev.genet.22.1.521. [DOI] [PubMed] [Google Scholar]
- Friedman (1991).Friedman JH. Multivariate adaptive regression splines. Annals of Statistics. 1991;19(1):1–67. doi: 10.1214/aos/1176347963. [DOI] [Google Scholar]
- Fu (1997).Fu YX. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics. 1997;147(2):915–925. doi: 10.1093/genetics/147.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al. (2012a).Gao J, Wang BS, MAO JF, Ingvarsson P, Zeng QY, Wang XR. Demography and speciation history of the homoploid hybrid pine Pinus densata on the Tibetan Plateau. Molecular Ecology. 2012a;21(19):4811–4827. doi: 10.1111/j.1365-294X.2012.05712.x. [DOI] [PubMed] [Google Scholar]
- Gao et al. (2012b).Gao QB, Zhang DJ, Duan YZ, Zhang FQ, Li YH, Fu PC, Chen SL. Intraspecific divergences of Rhodiola alsia (Crassulaceae) based on plastid DNA and internal transcribed spacer fragments. Botanical Journal of the Linnean Society. 2012b;168(2):204–215. doi: 10.1111/j.1095-8339.2011.01193.x. [DOI] [Google Scholar]
- Gao et al. (2015).Gao YD, Zhang Y, Gao XF, Zhu ZM. Pleistocene glaciations, demographic expansion and subsequent isolation promoted morphological heterogeneity: a phylogeographic study of the alpine Rosa sericea complex (Rosaceae) Scientific Reports. 2015;5(1):11698. doi: 10.1038/srep11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al. (2016).Gao QB, Zhang FQ, Xing R, Gornall RJ, Fu PC, Li Y, Gengji ZM, Chen SL. Phylogographic study revealed microrefugia for an endemic species on the Qinghai–Tibetan Plateau: Rhodiola chrysanthemifolia (Crassulaceae) Plant Systematics Evolution. 2016;302(9):1179–1193. doi: 10.1007/s00606-016-1324-4. [DOI] [Google Scholar]
- Gébelin et al. (2013).Gébelin A, Mulch A, Teyssier C, Jessup MJ, Law RD, Brunel M. The Miocene elevation of Mount Everest. Geology. 2013;41(7):799–802. doi: 10.1130/G34331.1. [DOI] [Google Scholar]
- Gould & Donoghue (2000).Gould KR, Donoghue MJ. Phylogeny and biogeography of Triosteum (Caprifoliaceae) Harvard Papers in Botany. 2000;5:157–166. [Google Scholar]
- Grivet & Petit (2002).Grivet D, Petit RJ. Phylogeography of the common ivy (Hedera sp.) in Europe: genetic differentiation through space and time. Molecular Ecology. 2002;11(8):1351–1362. doi: 10.1046/j.1365-294X.2002.01522.x. [DOI] [PubMed] [Google Scholar]
- Guindon & Gascuel (2003).Guindon S, Gascuel O. A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology. 2003;52(5):696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Hamilton (1999).Hamilton MB. Four primer pairs for the amplification of chloroplast intergenic regions with intraspecific variation. Molecular Ecology. 1999;8(3):521–523. [PubMed] [Google Scholar]
- Hastie, Tibshirani & Buja (1994).Hastie T, Tibshirani R, Buja A. Flexible disriminant analysis by optimal scoring. Journal of the American Statistical Association. 1994;89(428):1255–1270. doi: 10.1080/01621459.1994.10476866. [DOI] [Google Scholar]
- Hewitt (2000).Hewitt GM. The genetic legacy of the Quaternary ice ages. Nature. 2000;405(6789):907–913. doi: 10.1038/35016000. [DOI] [PubMed] [Google Scholar]
- Hewitt (2004).Hewitt GM. Genetic consequences of climate oscillations in the Quaternary. Philosophical Transactions of the Royal Society B. 2004;359(1442):183–195. doi: 10.1098/rstb.2003.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans et al. (2005).Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25(15):1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- Hudson (1990).Hudson RR. Gene genealogies and the coalescent process. In: Futuyma D, Antonovics J, editors. Oxford Surveys in Evolutionary Biology. Oxford: Oxford University Press; 1990. pp. 1–44. [Google Scholar]
- Jia et al. (2012).Jia DR, Abbott RJ, Liu TL, Mao KS, Bartish IV, Liu JQ. Out of the Qinghai–Tibet Plateau: evidence for the origin and dispersal of Eurasian temperate plants from a phylogeographic study of Hippophaë rhamnoides (Elaeagnaceae) New Phytologist. 2012;194(4):1123–1133. doi: 10.1111/j.1469-8137.2012.04115.x. [DOI] [PubMed] [Google Scholar]
- Jian, Tang & Sun (2015).Jian HY, Tang KX, Sun H. Phylogeography of Rosa soulieana (Rosaceae) in the Hengduan Mountains: refugia and ‘melting’ pots in the Quaternary climate oscillations. Plant Systematics and Evolution. 2015;301(7):1819–1830. doi: 10.1007/s00606-015-1195-0. [DOI] [Google Scholar]
- Khan et al. (2015).Khan G, Zhang FQ, Gao QB, Fu PC, Xing R, Wang JL, Liu HR, Chen SL. Phylogenetic analyses of Spiraea (Rosaceae) distributed in the Qinghai-Tibetan Plateau and adjacent regions: insights from molecular data. Plant Systematics Evolution. 2015;302(1):11–21. doi: 10.1007/s00606-015-1238-6. [DOI] [Google Scholar]
- Li et al. (2013).Li L, Abbott RJ, Liu BB, Sun YS, Li LL, Zou JB, Wang X, Miehe G, Liu JQ. Pliocene intraspecific divergence and Plio-Pleistocene range expansions within Picea likiangensis (Lijiang spruce), a dominant forest tree of the Qinghai-Tibet Plateau. Molecular Ecology. 2013;22(20):5237–5255. doi: 10.1111/mec.12466. [DOI] [PubMed] [Google Scholar]
- Li & Fang (1999).Li JJ, Fang XM. Uplift of the Tibetan Plateau and environmental changes. Chinese Science Bulletin. 1999;44(23):2117–2124. doi: 10.1007/bf03182692. [DOI] [Google Scholar]
- Li & Li (1993).Li XW, Li J. A preliminary floristic study on the seed plants from the region of Hengduan Mountain. Acta Botanica Yunnanica. 1993;15:217–231. [Google Scholar]
- Li & Pan (2002).Li BY, Pan BT. Progress in paleogeographic study of the Tibetan Plateau. Geographical Research. 2002;21:61–70. [Google Scholar]
- Librado & Rozas (2009).Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics Applications Note. 2009;25(11):1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Lin & Wu (1990).Lin Z, Wu X. A preliminary analysis about the tracks of moisture transportation on the Qinghai-Xizang Plateau. Geographical Research. 1990;9:33–40. [Google Scholar]
- Liu et al. (2013).Liu J, Möller M, Provan J, Gao LM, Poudel RC, Li DZ. Geological and ecological factors drive cryptic speciation of yews in a biodiversity hotspot. New Phytologist. 2013;199(4):1093–1108. doi: 10.1111/nph.12336. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2012).Liu JQ, Sun YS, Ge XJ, Gao LM, Qiu YX. Phylogeographic studies of plants in China: advances in the past and directions in the future. Journal of Systematics and Evolution. 2012;50(4):267–275. doi: 10.1111/j.1759-6831.2012.00214.x. [DOI] [Google Scholar]
- Liu & Yin (2002).Liu XD, Yin ZY. Sensitivity of East Asian monsoon climate to the uplift of the Tibetan Plateau. Palaeogeography, Palaeoclimatology, Palaeoecology. 2002;183(3–4):223–245. doi: 10.1016/s0031-0182(01)00488-6. [DOI] [Google Scholar]
- López-Pujol et al. (2011).López-Pujol J, Zhang FM, Sun HQ, Ying TS, Ge S. Centres of plant endemism in China: places for survival or for speciation? Journal of Biogeography. 2011;38(7):1267–1280. doi: 10.1111/j.1365-2699.2011.02504.x. [DOI] [Google Scholar]
- Lu et al. (2014).Lu ZQ, Tian B, Liu BB, Yang C, Liu JQ. Origin of Ostryopsis intermedia (Betulaceae) in the southeast Qinghai-Tibet Plateau through hybrid speciation. Journal of Systematics and Evolution. 2014;52(3):250–259. doi: 10.1111/jse.12091. [DOI] [Google Scholar]
- Luebert & Weigend (2014).Luebert F, Weigend M. Phylogenetic insights into Andean plant diversification. Frontiers in Ecology and Evolution. 2014;2:27. doi: 10.3389/fevo.2014.00027. [DOI] [Google Scholar]
- Margush & McMorris (1981).Margush T, McMorris FR. Consensus n-trees. Bulletin of Mathematical Biology. 1981;43(2):239–244. doi: 10.1016/s0092-8240(81)90019-7. [DOI] [Google Scholar]
- Mulch & Chamberlain (2006).Mulch A, Chamberlain CP. The rise and growth of Tibet. Nature. 2006;439(7077):670–671. doi: 10.1038/439670a. [DOI] [PubMed] [Google Scholar]
- Nei (1987).Nei M. Molecular Evolutionary Genetics. New York: Columbia University Press; 1987. [Google Scholar]
- Ni & Herzschuh (2011).Ni J, Herzschuh U. Simulating biome distribution on the Tibetan Plateau using a modified global vegetation model. Arctic, Antarctic, and Alpine Research. 2011;43(3):429–441. doi: 10.1657/1938-4246-43.3.429. [DOI] [Google Scholar]
- Opgenoorth et al. (2010).Opgenoorth L, Vendramin GG, Mao KS, Miehe G, Miehe S, Liepelt S, Liu JQ, Ziegenhagen B. Tree endurance on the Tibetan Plateau marks the world’s highest known tree line of the Last Glacial Maximum. New Phytologist. 2010;185(1):332–342. doi: 10.1111/j.1469-8137.2009.03007.x. [DOI] [PubMed] [Google Scholar]
- Otto-Bliesner et al. (2006).Otto-Bliesner BL, Marshall SJ, Overpeck JT, Miller GH, Hu A, CAPE Last Interglacial Project Members Simulating arctic climate warmth and ice field retreat in the last interglaciation. Science. 2006;311(5768):1751–1753. doi: 10.1126/science.1120808. [DOI] [PubMed] [Google Scholar]
- Peter & John (1989).Peter MC, John N. Generalized Linear Models. Second Edition. Boca Raton: Chapman and Hall/CRC; 1989. [Google Scholar]
- Phillips, Dudík & Schapire (2004).Phillips SJ, Dudík M, Schapire RE. A maximum entropy approach to species distribution modeling. Proceedings of the Twenty-First International Conference on Machine Learning, Banff, Alberta, Canada; 2004. pp. 655–662. [Google Scholar]
- Polzin & Daneschmand (2003).Polzin T, Daneschmand SV. On Steiner trees and minimum spanning trees in hypergraphs. Operations Research Letters. 2003;31(1):12–20. doi: 10.1016/s0167-6377(02)00185-2. [DOI] [Google Scholar]
- Pons & Petit (1996).Pons O, Petit RJ. Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics. 1996;144:1237–1245. doi: 10.1093/genetics/144.3.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, Fu & Comes (2011).Qiu YX, Fu CX, Comes HP. Plant molecular phylogeography in China and adjacent regions: tracing the genetic imprints of Quaternary climate and environmental change in the world’s most diverse temperate flora. Molecular Phylogenetics and Evolution. 2011;59(1):225–244. doi: 10.1016/j.ympev.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Renner (2016).Renner SS. Available data point to a 4-km-high Tibetan Plateau by 40 Ma, but 100 molecular-clock papers have linked supposed recent uplift to young node ages. Journal of Biogeography. 2016;43(8):1479–1487. doi: 10.1111/jbi.12755. [DOI] [Google Scholar]
- Rice & Hostert (1993).Rice WR, Hostert EE. Laboratory experiments on speciation: what have we learned in 40 years. Evolution. 1993;47(6):1637–1653. doi: 10.1111/j.1558-5646.1993.tb01257.x. [DOI] [PubMed] [Google Scholar]
- Ridgeway (2006).Ridgeway G. GBM: generalized boosted regression models. Documentation on the R package ‘gbm’. Version 1.5–7https://cran.r-project.org/web/packages/gbm/index.html 2006
- Ripley (1996).Ripley BD. Pattern Recognition and Neural Networks. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Rogers & Harpending (1992).Rogers AR, Harpending H. Population growth makes waves in the distribution of pairwise genetic differences. Molecular Biology and Evolution. 1992;9(3):552–569. doi: 10.1093/oxfordjournals.molbev.a040727. [DOI] [PubMed] [Google Scholar]
- Ronquist et al. (2012).Ronquist F, Teslenko M, Mark PVD, Ayres D, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddiman & Kutzbach (1991).Ruddiman WF, Kutzbach JE. Plateau uplift and climatic change. Scientific American. 1991;264(3):66–75. doi: 10.1038/scientificamerican0391-66. [DOI] [Google Scholar]
- Slatkin & Hudson (1991).Slatkin M, Hudson RR. Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics. 1991;129(2):555–562. doi: 10.1093/genetics/129.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun (2002).Sun H. Evolution of Arctic-Tertiary flora in Himalayan–Hengduan Mountains. Acta Botanica Yunnanica. 2002;24:671–688. [Google Scholar]
- Sun et al. (2014).Sun YS, Abbott RJ, Li LL, Li L, Zou JB, Liu JQ. Evolutionary history of Purple cone spruce (Picea purpurea) in the Qinghai-Tibet Plateau: homoploid hybrid origin and Pleistocene expansion. Molecular Ecology. 2014;23(2):343–359. doi: 10.1111/mec.12599. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2011).Sun BN, Wu JY, Liu YS(C), Ding ST, Li XC, Xie SP, Yan DF, Lin ZC. Reconstructing Neogene vegetation and climates to infer tectonic uplift in western Yunnan, China. Palaeogeography, Palaeoclimatology, Palaeoecology. 2011;304(3–4):328–336. doi: 10.1016/j.palaeo.2010.09.023. [DOI] [Google Scholar]
- Sun & Zhou (1996).Sun H, Zhou ZK. The characters and origin of the flora from the big bend gorge of Yalutsanpu (Brahmabutra) river, eastern Himalayas. Acta Botanica Yunnanica. 1996;18:185–204. [Google Scholar]
- Tajima (1989).Tajima F. The effect of change in population size on DNA polymorphism. Genetics. 1989;123:597–601. doi: 10.1093/genetics/123.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang et al. (2013).Tang H, Micheels A, Eronen J T, Ahrens B, Fortelius M. Asynchronous responses of East Asian and Indian summer monsoons to mountain uplift shown by regional climate modelling experiments. Climate Dynamics. 2013;40(5–6):1531–1549. doi: 10.1007/s00382-012-1603-x. [DOI] [Google Scholar]
- Thompson et al. (1997).Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller, Georges & Engler (2014).Thuiller W, Georges D, Engler R. biomod2: ensemble platform for species distribution modeling. R package version 3http://CRAN.R-project.org/package=biomod2 2014:1–64.
- Wan et al. (2016).Wan DS, Feng JJ, Jiang DC, Mao KS, Duan YW, Miehe G, Opgenoorth L. The Quaternary evolutionary history, potential distribution dynamics, and conservation implications for a Qinghai-Tibet Plateau endemic herbaceous perennial, Anisodus tanguticus (Solanaceae) Ecology and Evolution. 2016;6(7):1977–1995. doi: 10.1002/ece3.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2013).Wang Q, Abbott RJ, Yu QS, Lin K, Liu JQ. Pleistocene climate change and the origin of two desert plant species, Pugionium cornutum and Pugionium dolabratum (Brassicaceae), in northwest China. New Phytologist. 2013;199(1):277–287. doi: 10.1111/nph.12241. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2009).Wang LY, Ikeda H, Liu TL, Wang YJ, Liu JQ. Repeated range expansion and glacial endurance of Potentilla glabra (Rosaceae) in the Qinghai-Tibetan Plateau. Journal of Integrative Plant Biology. 2009;51(7):698–706. doi: 10.1111/j.1744-7909.2009.00818.x. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2010).Wang H, La Q, Sun K, Lu F, Wang YG, Song ZP, Wu QH, Chen JK, Zhang WJ. Phylogeographic structure of Hippophae tibetana (Elaeagnaceae) highlights the highest microrefugia and the rapid uplift of the Qinghai–Tibetan Plateau. Molecular Ecology. 2010;19(14):2964–2979. doi: 10.1111/j.1365-294X.2010.04729.x. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2008).Wang CS, Zhao XX, Liu ZF, Lippert PC, Graham SA, Coe RS, Yi HS, Zhu LD, Liu S, Li YL. Constraints on the early uplift history of the Tibetan Plateau. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(13):4987–4992. doi: 10.1073/pnas.0703595105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu (1979).Wu ZY. On zonal problem of flora in China. Acta Botanica Yunnaica. 1979;1:1–20. [Google Scholar]
- Wu (1988).Wu ZY. Hengduan Mountain flora and her significance. Journal of Japanese Botany. 1988;63:297–311. [Google Scholar]
- Wu & Wu (1996).Wu ZY, Wu SG. A proposal for new floristic kingdom (realm) In: Zhang AL, Wu SG, editors. Floristic Characteristics and Diversity of East Asian Plants. Hong Kong: Springer-Verlag; 1996. pp. 3–42. [Google Scholar]
- Xing & Ree (2017).Xing YW, Ree RH. Uplift-driven diversification in the Hengduan Mountains, a temperate biodiversity hotspot. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(17):E3444–E3451. doi: 10.1073/pnas.1616063114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Li & Sun (2014).Xu B, Li ZM, Sun H. Plant diversity and floristic characters of the alpine subnival belt flora in the Hengduan Mountains, SW China. Journal of Systematics and Evolution. 2014;52(3):271–279. doi: 10.1111/jse.12037. [DOI] [Google Scholar]
- Yang et al. (2011).Yang QE, Landrein S, Osborne J, Borosova R. Caprifoliaceae. In: Wu ZY, Raven PH, editors. Flora of China. Vol. 19. St. Louis, Beijing: Science Press and Missouri Botanical Garden Press; 2011. pp. 616–617. [Google Scholar]
- Yang et al. (2008).Yang FS, Li YF, Ding X, Wang XQ. Extensive population expansion of Pedicularis longiflora (Orobanchaceae) on the Qinghai-Tibetan Plateau and its correlation with the Quaternary climate change. Molecular Ecology. 2008;17(23):5135–5145. doi: 10.1111/j.1365-294X.2008.03976.x. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2015).Yu HB, Zhang YL, Liu LS, Qi W, Li SC, Hu ZJ. Combining the least cost path method with population genetic data and species distribution models to identify landscape connectivity during the late Quaternary in Himalayan hemlock. Ecology and Evolution. 2015;5(24):5781–5791. doi: 10.1002/ece3.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al. (2017).Yu HB, Zhang YL, Wang ZF, Liu LS, Chen Z, Qi W. Diverse range dynamics and dispersal routes of plants on the Tibetan Plateau during the late Quaternary. PLOS ONE. 2017;12(5):e0177101. doi: 10.1371/journal.pone.0177101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2012a).Zhang QQ, Ferguson DK, Mosbrugger V, Wang YF, Li CS. Vegetation and climatic changes of SW China in response to the uplift of Tibetan Plateau. Palaeogeography, Palaeoclimatology, Palaeoecology. 2012a;363–364:23–36. doi: 10.1016/j.palaeo.2012.08.009. [DOI] [Google Scholar]
- Zhang et al. (2012b).Zhang FQ, Gao QB, Zhang DJ, Duan YZ, Li YH, Fu PC, Xing R, Gulzar K, Chen SL. Phylogeography of Spiraea alpina (Rosaceae) in the Qinghai–Tibetan Plateau inferred from chloroplast DNA sequence variations. Journal of Systematics and Evolution. 2012b;50(4):276–283. doi: 10.1111/j.1759-6831.2012.00194.x. [DOI] [Google Scholar]
- Zhang, Meng & Rao (2014).Zhang JQ, Meng SY, Rao GY. Phylogeography of Rhodiola kirilowii (Crassulaceae): a story of miocene divergence and quaternary expansion. PLOS ONE. 2014;9(11):e112923. doi: 10.1371/journal.pone.0112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Volis & Sun (2010).Zhang YH, Volis S, Sun H. Chloroplast phylogeny and phylogeography of Stellera chamaejasme on the Qinghai-Tibet Plateau and in adjacent regions. Molecular Phylogenetics and Evolution. 2010;57(3):1162–1172. doi: 10.1016/j.ympev.2010.08.033. [DOI] [PubMed] [Google Scholar]
- Zheng & Chen (1981).Zheng D, Chen WL. A preliminary study on the vertical belts of vegetation of the Eastern Himalayas. Acta Botanica Sinica. 1981;23:228–234. [Google Scholar]
- Zheng, Xu & Shen (2002).Zheng BX, Xu QQ, Shen YP. The relationship between climate change and Quaternary glacial cycles on the Qinghai–Tibetan plateau: review and speculation. Quaternary International. 2002;97–98:93–101. doi: 10.1016/s1040-6182(02)00054-x. [DOI] [Google Scholar]
- Zheng & Yao (2004).Zheng D, Yao TD. Progress in research on formation and evolution of Tibetan Plateau with its environment and resource effects. China Basic Science. 2004;2:15–21. [In Chinese with English abstract] [Google Scholar]
- Zou et al. (2012).Zou JB, Peng XL, Li L, Liu JQ, Miehe G, Opgenoorth L. Molecular phylogeography and evolutionary history of Picea likiangensis in the Qinghai–Tibetan Plateau inferred from mitochondrial and chloroplast DNA sequence variation. Journal of Systematics and Evolution. 2012;50(4):341–350. doi: 10.1111/j.1759-6831.2012.00207.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Haplotypes (H1–19) detected among 20 populations of Triosteum himalayanum..
Data Availability Statement
The following information was supplied regarding data availability: