Abstract
Here we report a facile and efficient method for site-directed glycosylation of peptide/protein. The method contains two sequential steps: generation of a GlcNAc-O-peptide/protein, and subsequent ligation of a eukaryotic N-glycan to the GlcNAc moiety. A pharmaceutical peptide, glucagon-like peptide-1 (GLP-1), and a model protein, bovine α-Crystallin, were successfully glycosylated using such an approach. It was shown that the GLP-1 with O-linked N-glycan maintained an unchanged secondary structure after glycosylation, suggesting the potential application of this approach for peptide/protein drug production. In summary, the coupled approach provides a general strategy to produce homogeneous glycopeptide/glycoprotein bearing eukaryotic N-glycans.
Graphical abstract
Glycans attached to proteins are closely related to protein stability,1 trafficking,2 signaling,3 and cell–cell interaction.4 N-linked glycosylation of proteins, one of the most prevalent post-translational modifications in eukaryotes, significantly affects protein folding, stability, and function.5,6 However, the heterogeneity of N-glycan in natural and recombinant glycoproteins greatly hampered the investigation of the roles of glycan in various biological processes. Therefore, access to homogeneous glycopeptides or glycoproteins is a prerequisite for their functional studies as well as biomedical application. To date, several strategies have been developed to produce uniform N-glycan modifications, including in vitro chemical and chemoenzymatic synthesis of glycoproteins7,8 and in vivo glycoengineering methods.9–11 Herein, we alternatively proposed a facile approach for site-directed glycosylation of peptide/protein (Figure 1). The method contains two sequential processes: (1) introduction of O-linked N-acetylglucosamine (O-GlcNAc) modification on a target peptide/protein by chemical or enzymatic approach, and (2) ligation of a eukaryotic N-glycan onto the GlcNAc moiety.
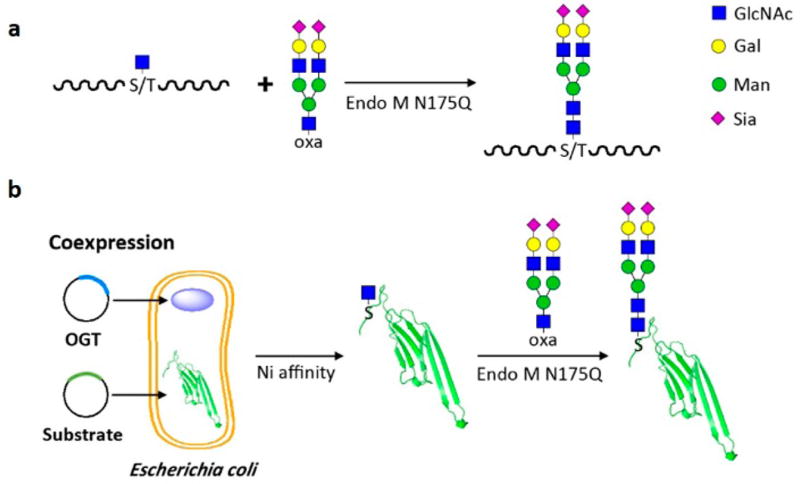
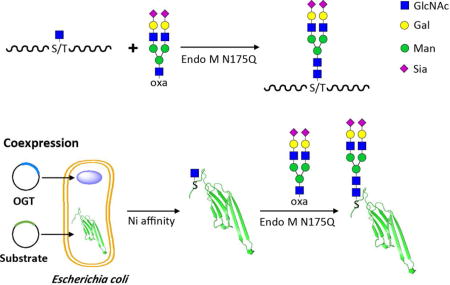
Figure 1.
Site-directed glycosylation of peptide/protein with uniform eukaryotic N-glycan. a. Chemically synthesized GlcNAc-O-peptide can be glycosylated by Endo M N175Q with complex-type glycan oxazoline (SCT-oxa) as a donor. b. GlcNAc-O-protein can be generated by coexpression with OGT and then linked with homogeneous eukaryotic N-glycan.
O-GlcNAc modification is a naturally existing protein modification which involves β-linked GlcNAc residue to a serine (Ser)/threonine (Thr) via the catalysis of O-linked GlcNAc transferase (OGT).12–14 To generate peptide/proteins with O-GlcNAc modification, two approaches were employed. The O-GlcNAcylated peptides (GlcNAc-O-peptides) were chemically synthesized, while the O-GlcNAcylated target protein was obtained by coexpression of OGT with the target protein, forming the O-GlcNAcylated protein (GlcNAc-O-protein) in vivo.
Then, we moved to the stage to transfer the N-glycan to the GlcNAc-O-peptide/protein. The N175Q mutant of endo-β-N-acetylglucosaminidase M (Endo M N175Q) is an enzyme that can efficiently glycosylate the GlcNAc-Asn-peptide/protein using N-glycan oxazolines as donor substrates.15–17 However, the feasibility of the Endo M N175Q-catalyzed trans-glycosylation reaction on GlcNAc-O-peptide/protein needs to be evaluated. Therefore, a series of O-GlcNAc modified peptide segments from natural O-GlcNAc-proteins (Table 1) (Database: dbOGAP) were synthesized (Figure S1), and Endo M N175Q was then applied to glycosylate the GlcNAc-O-peptides using the sialylated complex-type glycan oxazoline (SCT-oxa) as a sugar donor. Both HPLC and MALDI-TOF analysis illustrated that the remodeled products bear natural, full-size eukaryotic N-glycans (Figure S2). All target peaks (GlcNAc-O-peptide or Glycan-O-peptide) in HPLC chromatograms in Figure S1 and Figure S2 can be traced and the retention time of each target peak is listed in the text above the corresponding HPLC chromatogram. In addition, all target peaks were collected and further characterized by MS (as shown in Figure S1 and Figure S2). These results indicated that the Endo M N175Q mutant can fully tolerate GlcNAc-O-peptide although its natural substrate is GlcNAc-N-peptide, which will help to expand its applications for either natural or unnatural glycosylation of peptide/proteins to increase their stability and half-life. The percentage yields of the glycan-O-peptide varied from 21% to 73% for different peptide substrates (Table 1), implying that the reason for the difference in yield compared to that for GlcNAc-N-peptide (around 75%), the natural substrate for EndoM N175Q, may be that the substrates, GlcNAc-O-peptides, we used in this study are unnatural substrates for Endo M N175Q and the variant peptide sequences may result in the diverse yields.
Table 1.
N-Glycan Modification of Selected Peptides
| entry | peptide sequence | protein source | product yields (%) |
|---|---|---|---|
| Ser-01 | MVLSPADK | HBA_HUMAN | 61 |
| Ser-02 | PQFSYSA | AKT1_HUMAN | 73 |
| Ser-03 | PHTSGMNR | FOXO1_HUMAN | 30 |
| Ser-04 | KQVSQAQT | TAF4_HUMAN | 32 |
| Ser-05 | KIGSLDNI | TAU_HUMAN | 47 |
| Thr-01 | TKITGGSS | EMSY_HUMAN | 37 |
| Thr-02 | PKGTEITI | MLL5_HUMAN | 23 |
| Thr-03 | LLPTPPLS | MYC_HUMAN | 21 |
| Thr-04 | PTGTQATY | EMSY_HUMAN | 23 |
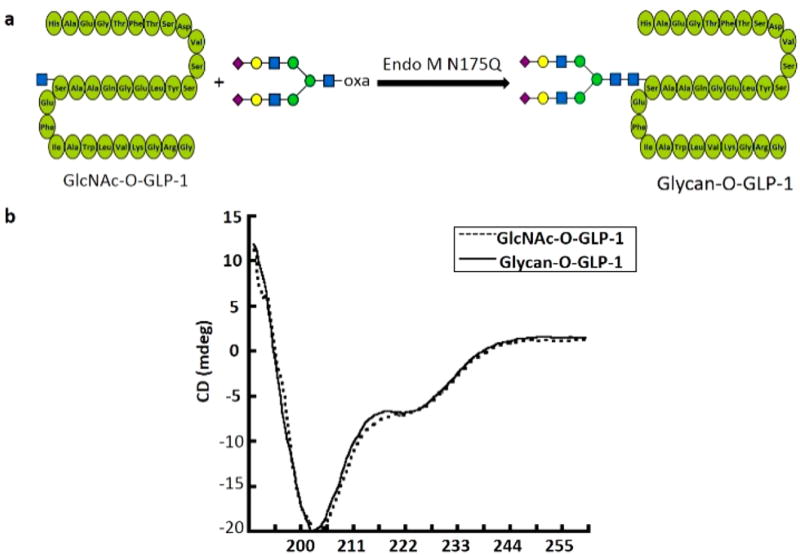
To investigate whether the O-linked N-glycan would influence the structural stability of peptides, we chose glucagon-like peptide-1 (GLP-1) as a candidate. GLP-1 is a well-known peptide drug for Type 2 diabetes and has an α-helix secondary structure.18 Liraglutide, a GLP-1 analogue modified with fatty acid at Lys26, is a long-acting GLP-1 agonist binding to receptors the same as the endogenous metabolic hormone GLP-1.19,20 Based on the structure of Liraglutide, we synthesized a GlcNAc-O-GLP-1 analogue with a GlcNAc attached to Ser26 to substitute the original Lys26 in natural GLP-1, and then applied Endo M N175Q to transfer a complex-type N-glycan onto it (Figure 2a). As expected, Endo M N175Q efficiently catalyzed the transglycosylation to form glycan-O-GLP-1 (Calculated: 5517.3776, Found: [M+4H]4+ = 1380.3643, [M+5H]5+ = 1104.4921, Figure S2). The secondary structure of GlcNAc-O-GLP-1 and glycan-O-GLP-1 were further examined by circular dichroism (CD) spectra in the far-UV (190–260 nm) range. The negligible difference of the CD spectra between GlcNAc-O-GLP-1 and glycan-O-GLP-1 demonstrates that the N-glycan modification has no effect on the α-helix secondary structure of GLP-1 (Figure 2b). Hence, by introducing a eukaryotic N-glycan into an O-GlcNAc site, the newly developed method can be potentially used to make glycosylated peptides with natural spatial structure, enhanced stability, and serum half-life.
Figure 2.
Glycosylation of GLP-1 and circular dichroism study. a. Scheme of glycosylation of GLP-1. b. Far-UV CD spectra of GlcNAc-O-GLP-1 and glycan-O-GLP-1.
With the site-directed glycopeptides in hand, we further explored their resistance to GlcNAc hydrolase (OGA), the hydrolase widely distributed in mammalian cells and highly efficient in removing the O-GlcNAc from diverse proteins.21,22 OGA was recently found to be very sensitive to a substitution of the N-acyl group of O-GlcNAc. The extension of this group in a substrate can markedly decrease the hydrolyzing efficiency of OGA.23 Several OGA inhibitors have been designed as GlcNAc analogues with an extended N-acyl group, such as PUGNAC or Thiamet-G.24 In light of this, N-glycan could be regarded as a GlcNAc with an extended N-acyl group. We thus assume that the glycopeptides we produced with O-linked N-glycans would be inert to OGA. To this end, we monitored the glycan loss on selected GlcNAc-peptides and N-glycan-O-peptides in the presence of OGA. As expected, OGA efficiently removed GlcNAc moiety from the GlcNAc-O-peptides (Table S1 and Figure S3), but cannot cleave glycan from N-glycan-Opeptides. This demonstrates that the O-linked N-glycan modification is capable of preventing the glycan-O-peptide or glycan-O-protein from OGA digestion in mammalian cell, which will benefit the maintaining of their stability in vivo. In addition, N-glycan tagged at O-GlcNAc site can also resist the hydrolysis of peptide-N-glycosidase F (PNGase F), which can exclusively remove all asparagine-linked complex, hybrid, or high mannose oligosaccharides. Therefore, our synthesized chimeric glycan-O-peptides may have improved stability and increased half-life.
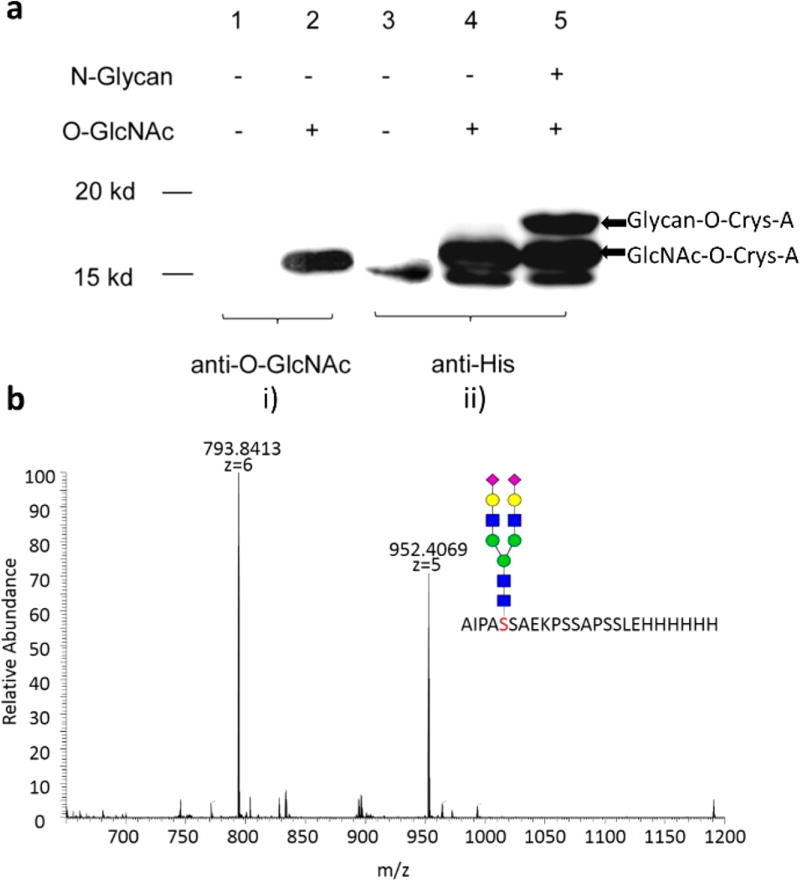
Next, we applied the glycosylation strategy on a model protein, a bovine α-Crystallin mutant (Crys-A) with a single O-GlcNAc modification site at Ser116. We have previously shown that Crys-A is prone to be modified by O-GlcNAc via its coexpression with OGT in Escherichia coli BL21 (DE3) cells,25 which is a convenient and effective approach to produce O-GlcNAcylated proteins. In this study, milligram-scale GlcNAc-O-Crys-A was produced in E. coli BL21 (DE3) and purified via Ni-NTA affinity chromatography, and then served as a substrate for Endo M N175Q catalyzed transglycosylation in the presence of SCT-oxa. Both substrate GlcNAc-O-Crys-A and product glycan-O-Crys-A were observed and identified by immunoblot and mass spectrometry (Figure 3). A total conversion yield of ~30% was observed as calculated by gray value analysis. These findings proved that glycan-Crys-A can be successfully produced by the combined approach involving O-GlcNAc modification in vivo and enzymatic glycan remodeling in vitro. Based on previous research, glycosylation can increase the activity and half-life of pharmaceutical proteins. For example, Avonex is a marketed glycosylated interferon-β and has a single N-linked complex carbohydrate moiety, the half-life and activity of which are longer and more increased than marketed Betaseron, the nonglycosylated interferon-β.26 According to this, a single glycan should be helpful for increasing the half-life and activity of pharmaceutical protein. Therefore, the strategy in this manuscript for producing homogeneous glycoprotein could be applied for increasing the stability of pharmaceutical proteins in the future.
Figure 3.
Glycosylation of bovine α-Crystallin-A (Crys-A) and identification by immunoblot and in-gel digestion plus mass spectrometry. a. Immunoblot analysis of GlcNAc-O-Crys-A and glycan-O-Crys-A. (i) Anti-O-GlcNAc antibody is a primary antibody to detect GlcNAc. (ii). Anti-His antibody is a primary antibody to detect His-tag in Crys-A. b. In-gel digestion by trypsin for mass spectrometric characterization of glycan-Crys-A, calculated 4754.9837, found [M+5H]5+ = 952.4069, [M+6H]6+ = 793.8413. MS/MS characterization can be found in Figure S4.
In summary, the approach provides a general platform for producing homogeneous peptides/glycoproteins with O-linked eukaryotic N-glycans in a site-directed manner, which may contribute to the enhancement of therapeutic efficiency of modified peptides/proteins. The proof-of-concept study can be extended to produce significant pharmaceutical peptides and proteins in the future.
Supplementary Material
Acknowledgments
This work was financially supported by the National Basic Research Program of China (973 Program, grant No. 2012CB910300 to Peng Wang), National Natural Science Foundation of China (30970644 to Peng Wang and 31000371 to Jing Li), and National Institute of Health (R01GM085267 to Peng George Wang).
Footnotes
ASSOCIATED CONTENT
- Materials, experimental procedures, and characterization data including HPLC chromatograms and MS (PDF)
The authors declare no competing financial interest.
References
- 1.Bager R, Johansen JS, Jensen JK, Stensballe A, Jendroszek A, Buxbom L, Sorensen HP, Andreasen PA. Protein conformational change delayed by steric hindrance from an N-linked glycan. J. Mol. Biol. 2013;425:2867–2877. doi: 10.1016/j.jmb.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Tsay HJ, Huang YC, Chen YJ, Lee YH, Hsu SM, Tsai KC, Yang CN, Huang FL, Shie FS, Lee LC, et al. Identifying N-linked glycan moiety and motifs in the cysteine-rich domain critical for N-glycosylation and intracellular trafficking of SR-AI and MARCO. J. Biomed. Sci. 2016;23:27. doi: 10.1186/s12929-016-0244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Streng-Ouwehand I, Ho NI, Litjens M, Kalay H, Boks MA, Cornelissen LAM, Singh SK, Saeland E, Garcia-Vallejo JJ, Ossendorp FA, et al. Glycan modification of antigen alters its intracellular routing in dendritic cells, promoting priming of T cells. eLife. 2016;5:e11765. doi: 10.7554/eLife.11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Moussodia RO, Vertesy S, Andre S, Klein ML, Gabius HJ, Percec V. Unraveling functional significance of natural variations of a human galectin by glycodendrimersomes with programmable glycan surface. Proc. Natl. Acad. Sci. U. S. A. 2015;112:5585–5590. doi: 10.1073/pnas.1506220112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satoh T, Yamaguchi T, Kato K. Emerging structural insights into glycoprotein quality control coupled with N-glycan processing in the endoplasmic reticulum. Molecules. 2015;20:2475–2491. doi: 10.3390/molecules20022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuen CT, Storring PL, Tiplady RJ, Izquierdo M, Wait R, Gee CK, Gerson P, Lloyd P, Cremata JA. Relationships between the N-glycan structures and biological activities of recombinant human erythropoietins produced using different culture conditions and purification procedures. Adv. Exp. Med. Biol. 2005;564:141–142. doi: 10.1007/0-387-25515-X_25. [DOI] [PubMed] [Google Scholar]
- 7.Wang LX, Amin MN. Chemical and chemoenzymatic synthesis of glycoproteins for deciphering functions. Chem. Biol. 2014;21:51–66. doi: 10.1016/j.chembiol.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giddens JP, Wang LX. Chemoenzymatic glycoengineering of monoclonal antibodies. Methods Mol. Biol. 2015;1321:375–387. doi: 10.1007/978-1-4939-2760-9_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anyaogu DC, Mortensen UH. Manipulating the glycosylation pathway in bacterial and lower eukaryotes for production of therapeutic proteins. Curr. Opin. Biotechnol. 2015;36:122–128. doi: 10.1016/j.copbio.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Loos A, Gruber C, Altmann F, Mehofer U, Hensel F, Grandits M, Oostenbrink C, Stadlmayr G, Furtmuller PG, Steinkellner H. Expression and glycoengineering of functionally active heteromultimeric IgM in plants. Proc. Natl. Acad. Sci. U. S. A. 2014;111:6263–6268. doi: 10.1073/pnas.1320544111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valderrama-Rincon JD, Fisher AC, Merritt JH, Fan YY, Reading CA, Chhiba K, Heiss C, Azadi P, Aebi M, DeLisa MP. An engineered eukaryotic protein glycosylation pathway in Escherichia coli. Nat. Chem. Biol. 2012;8:434–436. doi: 10.1038/nchembio.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaidani Y, Nomura T, Matsuura A, Ito M, Suzuki E, Murakami K, Nadano D, Matsuda T, Furukawa K, Okajima T. O-linked-N-acetylglucosamine on extracellular protein domains mediates epithelial cell-matrix interactions. Nat. Commun. 2011;2:583. doi: 10.1038/ncomms1591. [DOI] [PubMed] [Google Scholar]
- 13.Hart GW. Three decades of research on O-GlcNAcylation – a major nutrient sensor that regulates signaling, transcription and cellular metabolism. Front. Endocrinol. 2014;5:183. doi: 10.3389/fendo.2014.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart GW. Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu. Rev. Biochem. 1997;66:315–335. doi: 10.1146/annurev.biochem.66.1.315. [DOI] [PubMed] [Google Scholar]
- 15.Umekawa M, Li CS, Higashiyama T, Huang W, Ashida H, Yamamoto K, Wang LX. Efficient glycosynthase mutant derived from Mucor hiemalis Endo-β-N-acetylglucosaminidase capable of transferring oligosaccharide from both sugar oxazoline and natural N-glycan. J. Biol. Chem. 2010;285:511–521. doi: 10.1074/jbc.M109.059832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang W, Li C, Li B, Umekawa M, Yamamoto K, Zhang X, Wang LX. Glycosynthases enable a highly efficient chemoenzymatic synthesis of N-glycoproteins carrying intact natural N-glycans. J. Am. Chem. Soc. 2009;131:2214–2223. doi: 10.1021/ja8074677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umekawa M, Huang W, Li B, Fujita K, Ashida H, Wang LX, Yamamoto K. Mutants of Mucor hiemalis endo-β-N-acetylglucosaminidase show enhanced transglycosylation and glyco-synthase-like activities. J. Biol. Chem. 2008;283:4469–4479. doi: 10.1074/jbc.M707137200. [DOI] [PubMed] [Google Scholar]
- 18.Underwood CR, Garibay P, Knudsen LB, Hastrup S, Peters GH, Rudolph R, Reedtz-Runge S. Crystal structure of glucagon-like peptide-1 in complex with the extracellular domain of the glucagon-like peptide-1 receptor. J. Biol. Chem. 2010;285:723–730. doi: 10.1074/jbc.M109.033829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malm-Erjefalt M, Bjornsdottir I, Vanggaard J, Helleberg H, Larsen U, Oosterhuis B, van Lier JJ, Zdravkovic M, Olsen AK. Metabolism and excretion of the once-daily human glucagon-like peptide-1 analog Liraglutide in healthy male subjects and its in vitro degradation by dipeptidyl peptidase IV and neutral endopeptidase. Drug Metab. Dispos. 2010;38:1944–1953. doi: 10.1124/dmd.110.034066. [DOI] [PubMed] [Google Scholar]
- 20.Lund A, Knop FK, Vilsboll T. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: differences and similarities. Eur. J. Intern. Med. 2014;25:407–414. doi: 10.1016/j.ejim.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Alonso J, Schimpl M, van Aalten DM. O-GlcNAcase: promiscuous hexosaminidase or key regulator of O-GlcNAc signaling? J. Biol. Chem. 2014;289:34433–34439. doi: 10.1074/jbc.R114.609198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vocadlo DJ. O-GlcNAc processing enzymes: catalytic mechanisms, substrate specificity, and enzyme regulation. Curr. Opin. Chem. Biol. 2012;16:488–497. doi: 10.1016/j.cbpa.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez AC, Kohler JJ. Recognition of diazirine-modified O-GlcNAc by human O-GlcNAcase. MedChemComm. 2014;5:1227–1234. doi: 10.1039/C4MD00164H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gloster TM, Vocadlo DJ. Mechanism, structure, and inhibition of O-GlcNAc processing enzymes. Curr. Signal Transduction Ther. 2010;5:74–91. doi: 10.2174/157436210790226537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Li L, Wang Y, Yan H, Ma X, Wang PG, Zhang L. A peptide panel investigation reveals the acceptor specificity of O-GlcNAc transferase. FASEB J. 2014;28:3362–3372. doi: 10.1096/fj.13-246850. [DOI] [PubMed] [Google Scholar]
- 26.Runkel L, Meier W, Pepinsky RB, Karpusas M, Whitty A, Kimball K, Brickelmaier M, Muldowney C, Jones W, Goelz SE. Structural and functional differences between glycosylated and non-glycosylated forms of human interferon-beta (IFN-beta) Pharm. Res. 1998;15:641–649. doi: 10.1023/a:1011974512425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.