Abstract
Myocarditis is an uncommon, potentially life-threatening disease that presents with a wide range of symptoms in children and adults. Viral infection is the most common cause of myocarditis in developed countries, but other etiologies include bacterial and protozoal infections, toxins, drug reactions, autoimmune diseases, giant cell myocarditis, and sarcoidosis. Acute injury leads to myocyte damage, which in turn activates the innate and humeral immune system, leading to severe inflammation. In most patients, the immune reaction is eventually down-regulated and the myocardium recovers. In select cases, however, persistent myocardial inflammation leads to ongoing myocyte damage and relentless symptomatic heart failure or even death. The diagnosis is usually made based on clinical presentation and noninvasive imaging findings. Most patients respond well to standard heart failure therapy, although in severe cases, mechanical circulatory support or heart transplantation is indicated. Prognosis in acute myocarditis is generally good except in patients with giant cell myocarditis. Persistent, chronic myocarditis usually has a progressive course but may respond to immunosuppression.
Keywords: Myocarditis, Dilated cardiomyopathy, Endomyocardial biopsy, Heart failure
Myocarditis presents with a spectrum of symptoms ranging from mild dyspnea or chest pain that spontaneously resolves without treatment to cardiogenic shock and sudden death. The major long-term consequence is dilated cardiomyopathy (DCM) with chronic heart failure. Common viral infections are the most frequent cause of myocarditis, but other pathogens, hypersensitivity reactions, and systemic and autoimmune diseases have also been implicated. The diagnosis can usually be made based on clinical presentation and noninvasive imaging findings, but endomyocardial biopsy (EMB) may be indicated in select cases. Treatment and prognosis vary according to etiology and may include mechanical cardiac assist devices or heart transplant for severe cases. The aim of this review was to provide a concise and practical approach to the evaluation and treatment of suspected myocarditis.
Definition
The Dallas criteria were proposed in 1986 to provide a histopathologic classification for the diagnosis of myocarditis. These criteria require that an inflammatory cellular infiltrate with or without associated myocyte necrosis be present on conventionally stained myocardial tissue sections. Although these criteria have been used for more than 20 years, they are limited by variability in expert interpretation, lack of prognostic value, discrepancy with other markers of viral infection and immune activation in the myocardium, and low sensitivity that is at least partly due to sampling error.1,2 Newer histologic criteria rely on cell-specific immunoperoxidase stains for surface antigens such as anti-CD3, anti-CD4, anti-CD20, anti-CD28, and antihuman leukocyte antigen.3,4 Recent research has shown that criteria based on this type of staining has greater sensitivity than the Dallas criteria and may have prognostic value.5
Although EMB has long been the gold standard for confirming the diagnosis of myocarditis, preliminary studies have shown that cardiac magnetic resonance imaging (MRI) has become an important tool for noninvasive assessment of patients with suspected myocarditis.6–8 Cardiac MRI diagnostic targets include functional and morphological abnormalities as well as tissue pathology as characteristic of myocardial inflammation. Given the lack of consensus regarding the role of EMB and the excellent prognosis of patients with mild acute DCM who have suspected myocarditis, a recent consensus statement recommends that EMB be reserved for patients who are likely to have specific myocardial disorders with unique prognoses and specific treatment recommendations.9
Incidence and natural history of myocarditis
The true incidence of myocarditis has been difficult to determine because clinical presentations vary widely, and EMB is rarely used due to perceived risks and lack of a widely accepted and sensitive histologic standard. Autopsy reports have revealed varying estimates of the incidence of myocarditis according to the population studied, with estimates ranging from 0.12% to 12%.10–14 The Myocarditis Treatment Trial reported the incidence of biopsy-documented myocarditis in patients with unexplained heart failure to be 9.6%.15 The observation that there is a high prevalence of viral genomes in patients with DCM16 and that viral genomes are more common in patients with chronic DCM than in patients with ischemic heart disease suggests that viral myocarditis may lead to a substantial disease burden in the community.
Most studies of acute myocarditis report a slight predominance of men,17–19 which may be partly mediated by sex hormones. In female mice, estrogenic hormones have been shown to protect against viremia and viral infectivity of cardiomyocytes,20 while also decreasing the potentially harmful myocardial inflammatory response.21 In contrast, testosterone has been shown to have a detrimental effect through inhibition of antiinflammatory responses in male mice.22
Young adults are most commonly affected. The mean age of patients with giant cell myocarditis (GCM) is 42 years,23 whereas the mean age of adult patients with other forms of myocarditis has been reported to range from 20 to 51 years.24,25 The consequences are sometimes devastating in this population, as acute myocarditis has been shown to be the cause of sudden death in up to 12% of cases in autopsy studies of patients less than 40 years of age,14,26,27 military recruits,28 and young athletes.29
Myocarditis is also an important cause of sudden death in children30–32 as well as childhood cardiomyopathy.33,34 The risk of death and heart transplantation persists up to 12 years after diagnosis of acute myocarditis in the pediatric population.35
Occasionally, myocarditis occurs concurrently with other cardiomyopathies and may adversely affect outcomes. For instance, mortality is higher in patients with cardiac amyloidosis if histologic evidence of myocarditis is present.36 Similarly, patients with hypertrophic cardiomyopathy deteriorate more quickly if myocarditis is present.37 Although myocarditis may mimic arrhythmogenic right ventricular cardiomyopathy (dysplasia) in clinical presentation and the presence and severity of structural and functional right ventricular abnormalities,38 the 2 diseases may also occur simultaneously.39
Clinical presentation
Adults
The clinical presentation of acute myocarditis in adults is highly variable, ranging from subclinical disease to fulminant heart failure. A viral prodrome including fever, rash, myalgias, arthralgias, fatigue, and respiratory or gastrointestinal symptoms frequently, but not always, precedes the onset of myocarditis by several days to a few weeks. Patients may present with chest pain, dyspnea, palpitations, fatigue, decreased exercise tolerance, or syncope. Chest pain in acute myocarditis may mimic typical angina and be associated with electrocardiographic changes, including ST-segment elevation. Rarely, chest pain associated with coronary artery vasospasm may occur in patients with myocarditis.40 Alternatively, chest pain may be more typical for pericarditis, suggesting pericardial involvement. Cardiac rhythm disturbances are not uncommon and may include new-onset atrial or ventricular arrhythmias or high-grade atrioventricular (AV) block. Of the 3055 adult patients with suspected acute or chronic myocarditis who were screened in the European Study of the Epidemiology and Treatment of inflammatory Heart Disease,41 72% had dyspnea, 32% had chest pain, and 18% had arrhythmias. Patients with fulminant myocarditis typically present with severe heart failure symptoms that may rapidly lead to cardiogenic shock, whereas patients with GCM commonly present with heart failure symptoms that relentlessly progress to probable early death despite optimal treatment.
Children
The clinical presentation in children varies according to age. Infants may have nonspecific symptoms including anxiousness, malaise, fever, poor appetite, tachypnea, tachycardia, and cyanosis. Symptoms in children greater than 2 years of age may also include chest pain, abdominal pain, myalgias, fatigue, cough, and edema. The severity of symptoms is dependent on the age of the child, as newborns or infants are often more severely affected. In a study of 31 children found to have definite myocarditis (n = 16) or probable myocarditis (n = 15), the most common findings during initial presentation to the emergency department were respiratory distress (68%); tachycardia (58%); lethargy (39%); hepatomegaly (36%); abnormal heart sounds, including murmur (32%); and fever (30%).42 Children, particularly infants, often have a more fulminant presentation than adults and may require advanced circulatory and respiratory support in early stages of their disease.43
Diagnosis
Electrocardiogram
Although widely used as a screening tool, the sensitivity of electrocardiogram (ECG) for myocarditis is only 47%.44 The most common ECG findings are nonspecific T-wave changes. Occasionally, the ECG changes may mimic acute myocardial infarction or pericarditis with ST-segment elevation, ST-segment depression, PR depression, and pathologic Q waves.45,46 In referral populations, new-onset supraventricular or ventricular arrhythmias occur in up to 55% of patients.47 These tachyarrhythmias are often nonsustained and rarely cause hemodynamic compromise. The presence of abnormal QRS complexes, northwest axis deviation, or new left bundle branch block is associated with higher rates of death or cardiac transplantation.19,44,48,49
Chest x-ray
Chest x-ray may show cardiomegaly due to chamber dilatation, pericardial effusion, or both. Additional findings may include pulmonary venous congestion, interstitial infiltrates, and pleural effusions.
Laboratory
Nonspecific serum markers of inflammation, including erythrocyte sedimentation rate, C-reactive protein, and leukocyte count, are often elevated but are not used to make the diagnosis of acute myocarditis. Elevated levels of serum Fas and Fas ligand on initial presentation are associated with increased mortality in patients with acute myocarditis,50 whereas elevated serum levels of interleukin-10 predict a poor prognosis in patients with fulminant myocarditis.51
Cardiac biomarkers of myocardial injury are not elevated in most patients with myocarditis52 but, if elevated, may be helpful to confirm the diagnosis. Increased serum concentrations of troponin I (TnI) or troponin T (TnT) are more common than increased levels of creatinine kinase or creatinine kinase-MB in both adults and children with acute myocarditis.52–55 Higher levels of TnT have been shown to be a prognostic marker for poor outcome in adult patients presenting with acute myocarditis.51 Troponin I has high specificity (89%) and low sensitivity (34%) in adults presenting with acute myocarditis,52 whereas in children, TnT has been reported to have a specificity of 83% and a sensitivity of 71%.55
Echocardiography
Echocardiography is useful for evaluating cardiac chamber size, wall thickness, systolic and diastolic functions, and the presence of intracavitary thrombi. Its most prominent role is to rule out other causes of heart failure. There are no specific echocardiographic features of myocarditis, as patterns consistent with dilated, hypertrophic, and ischemic cardiomyopathies have all been described in patients with histologically proven myocarditis.56 Left ventricular systolic dysfunction is common,57 whereas right ventricular dysfunction is relatively uncommon. The presence of right ventricular dysfunction is important, however, as it was the most powerful predictor of death or need for cardiac transplantation in a series of 23 patients with biopsy-confirmed myocarditis.58 Segmental or global wall motion abnormalities are often present and may mimic myocardial infarction.59
Diastolic filling patterns are abnormal in most patients, with a restrictive pattern frequently present.60 Ventricular thrombi have been noted in up to 25% of patients.61 Pericardial effusion, typically small, is not uncommon. Patients with fulminant myocarditis tend to present with small cardiac chambers and thickened walls, whereas those with acute myocarditis have marked left ventricular dilation and normal wall thickness.62
Cardiac MRI
Cardiac MRI is being used with increasing frequency for noninvasive assessment of patients with suspected myocarditis.8,63–68 With a unique potential for tissue characterization, particularly with the use of T1- and T2-weighted images,66 cardiac MRI can evaluate 3 markers of tissue injury, that is, intracellular and interstitial edema, hyperemia and capillary leakage, and necrosis and fibrosis.6
A recent white paper written by the International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis states that a cardiac MRI study should be performed in symptomatic patients with clinical suspicion of myocarditis in whom the MRI results will affect clinical management.6 Three imaging criteria for confirming the diagnosis of myocarditis (the “Lake Louise Criteria”) by cardiac MRI have been proposed (Table 1). When 2 or more of the 3 criteria are positive, myocardial inflammation can be predicted with a diagnostic accuracy of 78%; if only delayed, postgadolinium enhancement imaging is performed, the diagnostic accuracy drops to 68%.6
Table 1.
Proposed cardiac MRI diagnostic criteria for myocarditis6
|
Contrast cardiac MRI may be used to direct EMB. In a study by Mahrholdt et al,7 histopathologic evaluation of biopsy specimens directed by contrast cardiac MRI with delayed enhancement revealed active myocarditis in 19 of 21 patients. In contrast, when biopsy could not be obtained from the region of contrast enhancement, active myocarditis was found in only 1 of 11 patients.
Endomyocardial biopsy
The role of EMB in the evaluation of suspected myocarditis was recently addressed in a scientific statement by the American College of Cardiology and the European Society of Cardiology.9 Fourteen scenarios are described, only 2 of which received a class I recommendation for EMB. The first of these 2 scenarios describes the classic presentation of fulminant myocarditis, that is, unexplained new-onset heart failure symptoms less than 2 weeks in duration associated with a normal-sized or dilated left ventricle and hemodynamic compromise. The second scenario describes the distinctive clinical picture associated with GCM, that is, unexplained new-onset heart failure symptoms 2 weeks to 3 months in duration associated with a dilated left ventricle and new ventricular arrhythmias, high-degree AV heart block, or failure to respond to usual care within 1 to 2 weeks.
The role of EMB in patients who do not present with these clinical scenarios is not well established.69 Patients with 1 of the 2 indications described above should undergo EMB at a medical center with special expertise in this procedure. Although the complication rate is low, death may occur in even the most experienced hands.70
Etiology
Infectious
A variety of infectious and noninfectious diseases can cause myocarditis (Table 2). Viral infection is the most common cause in Western Europe and North America, with adenovirus and enterovirus (including coxsackie-virus) historically being the most frequently identified viruses.16,67,71 Recently, the most commonly detected viral genomes in EMB samples were parvovirus B-19 and human herpesvirus-6. Serologic studies, predominantly in Japan, have linked the hepatitis C virus to myocarditis and DCM.72 Other viruses associated with myocarditis less frequently include cytomegalovirus, herpes simplex virus, and Epstein-Barr virus.71,73 Coinfection with 2 more viruses has been found in more than 25% of myocarditis patients.16
Table 2.
Etiologies
| Infectious | Toxins |
|---|---|
| Viral: adenovirus, arborvirus, Chikungunya virus, Cytomegalovirus, echovirus, Enterovirus (Coxsackie B), Epstein-Barr virus, Flavivirus (dengue fever and yellow fever), hepatitis B virus, hepatitis C virus, herpes viruses (human herpesvirus-6), HIV/AIDS, influenza A and B viruses, Parvovirus (parvovirus B-19), mumps virus, poliovirus, rabies virus, respiratory syncytial virus, rubeola virus, rubella virus, varicella virus, variola virus (smallpox) | Drugs: aminophylline, amphetamines, anthracyclines, catecholamines, chloramphenicol, cocaine, cyclophosphamide, doxorubicin, ethanol, 5-flurouracil, imatimib mesylate, interleukin-2, methysergide, phenytoin, trastuzumab, zidovudine |
| Bacterial: Burkholderia pseudomallei (melioidosis), Brucella, Chlamydia (especially Chlamydia pneumonia and Chlamydia psittacosis), Corynebacterium diphtheriae (diphtheria), Francisella tularensis (tularemia), Haemophilus influenzae, gonococcus, Clostridium, Legionella pneumophila (Legionnaire disease), Mycobacterium (tuberculosis), Neisseria meningitidis, Salmonella, Staphylococcus, Streptococcus A (rheumatic fever), Streptococcus pneumoniae, syphilis, tetanus, tularemia, Vibrio cholera | Environmental: arsenic, carbon monoxide, copper, iron, lead |
| Spirochetal: Borrelia burgdorferi (Lyme disease), Borrelia recurrentis (relapsing fever), leptospira, Treponema pallidum (syphilis) | Hypersensitivity reactions Drugs: azithromycin, benzodiazepines, clozapine, cephalosporins, dapsone, dobutamine, gefitinib, lithium, loop diuretics, methyldopa, mexiletine, nonsteroidal antiinflammatory drugs, penicillins, phenobarbital, smallpox vaccination, streptomycin, sulfonamides, tetanus toxoid, tetracycline, thiazide diuretics, tricyclic antidepressants |
| Rickettsial: Coxiella burnetii (Q fever), Orientia tsutsugamushi (scrub typhus), Rickettsia prowazekii (typhus), Rickettsia rickettsii (Rocky Mountain spotted fever) | Other: bee venom, wasp venom, black widow spider venom, scorpion venom, snake venom |
| Fungal: Actinomyces, Aspergillus, Blastomyces, Candida, Coccidioides, Cryptococcus, Histoplasma, Mucor species, Nocardia, Sporothrix schenckii, Strongyloides stercoralis | |
| Protozoal: Balantidium, Entamoeba histolytica (amebiasis), Leishmania, Plasmodium falciparum (malaria), Sarcocystis, Trypanosoma cruzi (Chagas disease), Trypanosoma brucei (African sleeping sickness), Toxoplasma gondii (toxoplasmosis) | Autoimmune diseases Dermatomyositis, GCM, inflammatory bowel disease, rheumatoid arthritis, Sjögren syndrome, systemic lupus erythematosus, Takayasu’s arteritis, Wegener’s granulomatosis |
| Helminthic: Ascaris, Echinococcus granulosus, Heterophyes, Paragonimus westermani, Schistosoma, Strongyloides stercoralis, Taenia solium (cysticercosis), Toxocara canis (visceral larva migrans), Trichinella spiralis, Wuchereria bancrofti (filariasis) | Systemic diseases Celiac disease, Churg-Strauss syndrome, collagen-vascular diseases, hypereosinophilic syndrome with eosinophilic endomyocardial disease, Kawasaki disease, sarcoidosis (idiopathic granulomatous myocarditis), scleroderma |
| Other Heart stroke, hypothermia, rejection of the posttransplant heart, radiation therapy |
Most common etiologies are rendered in bold.
Human immunodeficiency virus (HIV) has been associated with myocarditis and DCM. Although direct myocardial damage by the HIV virus is infrequent, cardiomyopathy in HIV-infected patients may be caused by coinfections, antiretroviral medications,74 or inhibition of cardiac contractility by HIV type 1 glycoprotein 120.75 In studies performed before the highly active antiretroviral therapy (HAART) era, myocarditis was identified in more than 50% of patients.76,77 Since the introduction of HAART, the incidence of myocarditis in HIV-infected patients living in developed countries has significantly decreased.78 In developing countries, however, where the availability of HAART is limited, the incidence of HIV-associated myocarditis continues to increase.79
Although numerous bacterial infections can cause myocarditis, bacterial-induced myocarditis is far less common than viral-induced myocarditis. Toxin-producing bacteria, including clostridium and diphtheria, can cause severe myocardial damage. Bacteremia from any source may result in myocarditis, with the most common pathogens being meningococcus, streptococcus, and Listeria.
The spirochete Borrelia burgdorferi causes Lyme disease, which can result in both acute and chronic myocarditis. A recent study of 207 children with early disseminated Lyme disease found that 33 (16%) had mild to fulminant myocarditis, 14 (42%) of whom had advanced AV heart block.80 Although full recovery is the norm, Lyme carditis sometimes persists and may lead to chronic heart failure.81
Infection with the protozoa Trypanosoma cruzi (Chagas disease), common in Central and South America and occasionally seen in the United States, can present as acute myocarditis or chronic cardiomyopathy. The mechanism is thought to be immune activation after infection.82 Pericardial effusion is common in the acute phase, whereas the chronic phase is often characterized by DCM, segmental wall motion abnormalities, and left ventricular apical aneurysm.83 Electrocardiographic changes include right bundle branch block, left anterior fascicular block, and AV block.84
Toxins and hypersensitivity reactions
Drugs can cause myocardial inflammation by either a direct toxic effect on the heart or by inducing hypersensitivity reactions. Anthracycline toxicity is relatively common,85 and cocaine has been increasingly implicated in acute myocarditis,86 but numerous other medications can cause cardiotoxicity including cyclophosphamide, phenytoin, zidovudine, and amphetamines. Drug-induced hypersensitivity reactions can cause an eosinophilic myocarditis that often responds to withdrawal of the offending medication, though adjuvant corticosteroid therapy is often necessary.87 Medications implicated in hypersensitivity myocarditis include several anticonvulsants and antipsychotics as well as a number of antibiotics. The possibility of drug-induced hypersensitivity myocarditis should be considered in any patient taking either prescription or over the counter medications, particularly if eosinophilia or eosinophilic myocardial infiltration is present.
Autoimmune and systemic diseases
Systemic diseases, particularly Churg-Strauss syndrome, cancer, and hypereosinophilic syndrome, 88–90 as well as certain protozoal, helminthic, and parasitic infections,91 may also induce eosinophilic myocarditis. Eosinophilic myocarditis has been reported after vaccination for several diseases including tetanus and smallpox.92,93 Clinical manifestations of eosinophilic myocarditis may include congestive heart failure, constitutional symptoms, rash, cough, endocardial and valvular fibrosis, and endocardial thrombi. Necrotizing eosinophilic myocarditis is a rare aggressive form of eosinophilic myocarditis with a short-term onset and high death rate.
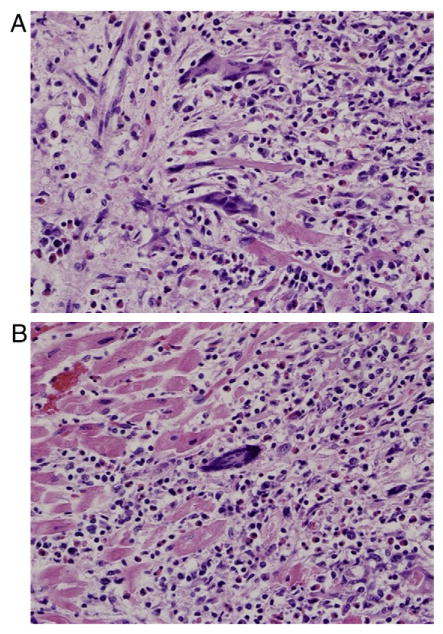
Idiopathic GCM is a rare, virulent, autoimmune form of myocarditis histologically defined by the presence of multinucleated giant cells, a lymphocytic inflammatory infiltrate, and myocyte necrosis (Fig 1A and B).94 This disease usually occurs in young adults and carries a high risk of death unless cardiac transplantation is performed. It is considered to be autoimmune because of its association with other autoimmune disorders,23 thymoma,95 and drug hypersensitivity.96 Rare in adults, GCM is rarer still in children and is associated with immune-mediated disease in other organs.23 Cardiac sarcoidosis is another unusual form of idiopathic myocarditis that is distinct from GCM in that it is characterized histologically by interstitial granulomas without myocyte necrosis97 and has a lower fatality rate.98 Patients tend to present with either chronic DCM with new ventricular arrhythmias or high-grade AV block or do not respond to optimal therapy.99
Fig 1.
Giant cell myocarditis. A and B. Endomyocardial biopsy specimen demonstrates widespread lymphocytic infiltrate, myocyte necrosis, numerous eosinophils, and several giant cells (hematoxylin and eosin). Images provided courtesy of Dr. Wendy Gunther.
Pathogenic mechanisms
Host factors
Factors that determine susceptibility to viral myocarditis are not fully known, although a variety of factors such as malnutrition, pregnancy, sex hormones,21 and age have been implicated. Genetic host factors, including major histocompatibility haplotype,100 HLA-DQ locus,101 and CD45 polymorphisms,102 may be important determinants of early viral infection. Other host factors including selenium deficiency,103 vitamin E deficiency,103 and mercury exposure104,105 have been reported to increase viral virulence. Viral factors, including genome phenotype, have been shown to affect cardiovirulence as well.106
Viral entry into cardiomyocytes
Most of our understanding of the pathophysiology of viral and autoimmune myocarditis comes from studies in rodent models in which susceptible strains of mice are infected with a cardiotropic virus such as coxsackievirus B. The virus is taken up in the cell by endothelial receptors, most notably the coxsackie-adenovirus receptor (CAR).107 In addition to CAR, coxsackievirus serotypes B1, B3, and B5 use decay-accelerating factor108 and adenoviruses uses αv integrins109 as coreceptors for viral entry.110,111 Differential binding to decay-accelerating factor increases viral virulence in coxsackievirus B infections.112
Coxsackie-adenovirus receptor is highly expressed in the brain and heart, peaking in the perinatal period with subsequent overall levels decreasing with age.113 In immature hearts, CAR is detected on the entire surface of cardiac myocytes, whereas in the adult heart, CAR is predominantly found in the intercalated disks.114 The expression level and the location of CAR in neonates and infants may help to explain the susceptibility of this population to coxsackievirus B3 (CVB3)-mediated myocarditis. A recent study involving inducible CAR knockout mice provided the first genetic evidence that CAR is the receptor for coxsackievirus in vivo while also demonstrating that the elimination of CAR blocked virus entry into myocytes and prevented signs of inflammatory cardiomyopathy.115 Previously, it was thought that myocarditis was primarily an autoimmune-mediated disease due to the presence of autoantibodies directed against cardiac myocyte proteins in patients with myocarditis,116 but the lack of myocardial damage noted in the CAR knockout mice suggests that the primary mechanism in viral myocarditis may be viral mediated, at least in the acute phase.
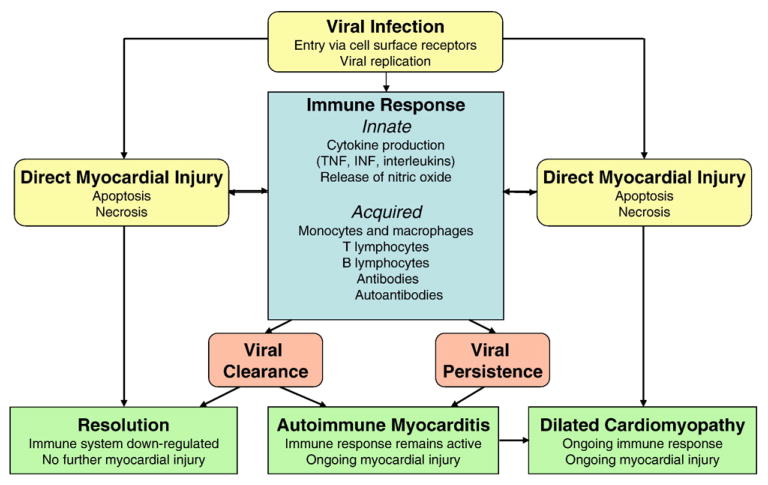
Innate immune response
The duration and degree of the innate immune response to viral infection has a crucial role in the development of myocarditis (Fig 2). A variety of inflammatory mediators, including cytokines such as tumor necrosis factor (TNF), nitric oxide, toll-like receptors, and complement are upregulated. Studies in mice have shown that these mediators may play a dual role in the development of viral-induced myocarditis. For instance, one murine model demonstrated increased TNF levels not only decreased viral load but also led to an exaggerated immune response and late mortality.117 Nitric oxide not only inhibits viral replication118 but also contributes to the development of cardiomyopathy by enhancing ongoing myocardial injury.119 Toll-like receptors and myeloid differentiation factor-88 (MyD88), an adapter molecule for toll-like receptors, minimize viral replication in the heart,120 but MyD88 appears to significantly affect the severity of myocardial inflammation.121 Complement amplifies not only the innate immune response but also the adaptive immune response, increasing susceptibility to autoimmune myocarditis and progression to chronic DCM.122
Fig 2.
Pathogenesis of viral myocarditis.
Direct viral injury
Viruses that evade the innate immune system replicate, producing viral proteins that cause direct myocardial injury. Coxsackievirus B3 infection in mice with severe combined immune deficiency induces myocardial injury.123 Picornavirus protease 2A has been shown to inhibit host cell protein synthesis,124 and CVB3 protease 2A cleaves the host protein dystrophin, which may induce cardiomyopathy.100,125 In addition to their proteolytic activity in myocytes, CVB3 proteases 2A and 3C can induce apoptosis, causing further cardiomyocyte injury.126 Inhibition of these viral proteases could be a novel target in the treatment of viral-induced myocarditis.
Acquired immune response
Cell-mediated immunity plays a critical role in the development of both viral and autoimmune myocarditis (Fig 2). The inflammatory infiltrate observed in myocardial lesions of myocarditis consists of more than 70% mononuclear cells,127 primarily monocytes, macrophages, and T lymphocytes. Inhibition of monocyte chemoattractant protein 1 and macrophage inflammatory protein 1α reduces the severity and prevalence of myocarditis in experimental autoimmune myocarditis (EAM) mice.128 Helper T cell types 1 and 2 (Th1 and Th2) secrete cytokines such as TNF and interleukins, which are associated with the development of autoimmune cardiomyopathy, within 6 to 12 hours of viral infection in susceptible mice. Helper T cell type 17 (TH17) produces interleukin-17, which drives the development of severe autoimmune myocarditis in interferon-γ–deficient EAM mice.129 Inhibition of T lymphocyte proliferation and activation in EAM mice attenuates the immune response and decreases the severity of myocarditis.130
Acute cardiac injury activates the adaptive immune response that further mediates cardiac damage. CD4+ T lymphocytes play a critical in the induction of autoimmune myocarditis, not only due to their production of key cytokines but also due their production of antibodies and autoantibodies131–133 Animals developing autoimmune myocarditis after CVB3 infection develop antibodies to multiple cardiac antigens, including epitopes in the S2 hinge region of cardiac myosin.134 Autoantibodies to numerous cardiac antigens are common in patients with myocarditis and DCM135,136 and may actually precede the development of disease.137 In particular, antibodies against cardiac β-1 adrenergic receptors have been found in the sera of patients with myocarditis and DCM,138 and removal of the circulating β-1 adrenergic receptor autoantibody in patients with DCM by immunoabsorption improved cardiac function.139,140 Antibodies against TnI lead to severe inflammation and fibrosis in the myocardium in experimental mice, leading to cardiac dilatation and reduced survival,141 although antibodies against TnT have not produced a similar immunologic response.142,143
In most patients with viral myocarditis, the pathogen is cleared and the immune system is down-regulated with no further adverse effects. However, in a minority of patients, the virus is not cleared, resulting in persistent myocyte damage and myocardial inflammation due the immune response to cardiac autoantibodies (Fig 2).
Treatment
Heart failure therapy
Most patients with acute myocarditis presenting with DCM respond well to standard heart failure therapy including diuretics, angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists, and the introduction of β-blockers such as bisoprolol, metoprolol succinate, or carvedilol once they are clinically stable. Digoxin should be used with caution and only in low doses in patients with viral myocarditis because administration of digoxin in high doses to mice with viral-induced myocarditis increased mortality.144 Amlodipine improved survival, ratio of heart weight to body weight, and the histopathologic grades of myocardial lesions in one study in a murine model of viral myocarditis,145 whereas nifedipine decreased the activation of proinflammatory cytokines in another study in mice with viral myocarditis146; however, no data on use of calcium channel blockers in humans with viral myocarditis has been published. Patients with high filling pressures may require support with intravenous vasodilators, whereas patients who present with severe symptoms may require intravenous inotropic medications. Offending agents, including cardiotoxic drugs and alcohol, should be discontinued.
Nonsteroidal antiinflammatory drugs
Treatment with nonsteroidal antiinflammatory drugs has not proven to be effective in several murine models and should actually be avoided, as use of these medications in murine models of acute viral myocarditis resulted in increased inflammation and higher mortality.147–149
Mechanical circulatory support and transplantation
Mechanical circulatory support may be effective in patients presenting with cardiogenic shock. Extracorporeal membrane oxygenation support may be especially beneficial for adult and pediatric patients with fulminant myocarditis with profound shock, as short-term recovery is expected.150,151 For patients with cardiogenic shock due to acute myocarditis who deteriorate despite optimal medical management, recovery is more likely to be prolonged and implantation of a ventricular assist device as a bridge to transplantation or recovery may be effective.152,153 Cardiac transplantation is reserved for those patients who are refractory to optimal medial therapy and mechanical circulatory support.
Antiviral treatment
As viral infection is the most common cause of myocarditis, it would seem plausible that treatment with antiviral medications or antiviral vaccines would be beneficial. It is clear that viral genomes are present in a subset of patients with acute myocarditis,154 but it remains uncertain whether this affects mortality or the need for cardiac transplantation.5,47 Data regarding antiviral treatment in myocarditis are limited to murine models and a few case series in humans, but results have been promising. Antiviral therapy with ribavirin or interferon in murine viral myocarditis prevented onset of cardiomyopathy, reduced the severity of the disease, and decreased mortality.155–158 In the single case series of antiviral therapy use in humans with fulminant myocarditis, ribavirin therapy did not prove effective.159 This is not surprising, as most patients with acute myocarditis are diagnosed several weeks after viral infection, so it is unlikely that antiviral therapy administered once myocarditis has been confirmed would provide much benefit. In patients with chronic DCM and persistent viral genomes, however, treatment with interferon resulted in the elimination of the viral genomes and improved left ventricular function.160
Immunosuppressive treatment
Numerous studies have investigated the use of immunosuppressants for treatment of acute myocarditis and DCM (Table 3). Early results of randomized controlled trials enrolling adult patients with acute myocarditis and idiopathic DCM were disappointing, as treatment with immunosuppressants such as prednisone, cyclosporin, and azathioprine showed little or no treatment effect.17,168 In patients with GCM, however, long-term survival is improved with treatment with cyclosporin and corticosteroids, and in fact, withdrawal of immunosuppression in this population has at times resulted in increased risk of recurrent, occasionally fatal, GCM.23,165 Several case series and a small trial in children have demonstrated that immunosuppressive treatment improved left ventricular function and arrhythmias,166,173,174 but there have been no large randomized controlled trials in children to validate these outcomes. In any case, caution must be used in interpreting these data, as the improvement seen may at least partially be due to the natural history of the disease in which spontaneous recovery frequently occurs.
Table 3.
Myocarditis and DCM treatment trials
| Treatment Trial | Trial Type | Disease | No. of Patients | Agent(s) | Primary Outcome Measure | Result |
|---|---|---|---|---|---|---|
| Adults: Acute Myocarditis | ||||||
| Jones,161 1991 | Prospective | Acute lymphocytic myocarditis | 9 | Prednisone plus azathioprine | Improvement in LVEF | No treatment benefit |
| Maisch,162 1995 | RCT | Acute lymphocytic myocarditis | 17 | Prednisone plus either cyclosporine or azathioprine | Improvement in LVEF at 3 mo | Significant treatment benefit |
| Mason,17 1995: The Myocarditis Treatment Trial | RCT | Acute lymphocytic myocarditis | 111 | Prednisone plus cyclosporine | Improvement in LVEF at 6 mo | No treatment benefit |
| McNamara,163 1997 | Prospective | Acute lymphocytic myocarditis | 10 | IVIG | Improvement in LVEF at 1 y | Treatment benefit |
| McNamara,164 1999: Immune Modulation for Acute Cardiomyopathy | RCT | Acute lymphocytic myocarditis | 62 | IVIG | Improvement in LVEF at 6 mo | No treatment benefit |
| Cooper,165 2008: Giant Cell Myocarditis Treatment Trial | Prospective | GCM | 11 | Prednisone plus cyclosporine | Survival at 1 y | Treatment benefit |
| Children: Acute Myocarditis | ||||||
| Chan,166 1991 | Retrospective | Acute myocarditis | 13 | Prednisone (1 patient also received azathioprine) | Clinical improvement (ECG changes, heart size, ejection fraction) | Small treatment benefit |
| Drucker,167 1994 | RCT | Acute myocarditis | 21 | IVIG | Survival and improvement in LVEF at 1 y | Treatment benefit |
| Chronic Myocarditis/DCM | ||||||
| Parrillo,168 1989 | RCT | Idiopathic DCM | 102 | Prednisone | Improvement in LVEF at 3 mo | Small treatment benefit |
| Wojnicz,169 2001 | RCT | DCM | 84 | Prednisone plus azathioprine | Composite of death, heart transplantation, and hospital readmission at 2 y | No treatment benefit (secondary outcome benefit) |
| Frustaci,170 2003 | Prospective | Active lymphocytic myocarditis with chronic heart failure | 41 | Prednisone and azathioprine | Improvement in LVEF at 1 y | Treatment benefit for patients with no viral genome in the myocardium |
| Gullestad,171 2001 | RCT | DCM | 40 | IVIG | Improvement in LVEF at 26 wk | Treatment benefit |
| Kuhl,160 2003 | Prospective | Chronic virus-positive DCM | 22 | Interferon-β | Viral clearance and improvement in LV size and LVEF at 6 mo | Treatment benefit for both outcomes |
| Frustaci,172 2009: Therapy in Inflammatory Dilated Cardiomyopathy | RCT | Chronic virus-negative DCM | 85 | Prednisone and azathioprine | Improvement in LVEF at 6 mo | Significant treatment benefit |
Abbreviations: LVEF indicates left ventricular ejection fraction; RCT, randomized controlled trial.
In contrast, immunosuppression may be beneficial in treating patients with chronic DCM unresponsive to standard heart failure therapy. Several studies evaluating immunosuppressive treatment of chronic virus-negative DCM, including the recently completed Therapy in Inflammatory Dilated Cardiomyopathy trial, have shown that the use of azathioprine and prednisone results in significant improvement in left ventricular ejection fraction and New York Heart Association class.169,170,172 These data suggest that immunosuppression may prove beneficial in patients with chronic virus-negative DCM that persists despite optimal medical treatment.
Intravenous immunoglobulin
Intravenous immunoglobulin (IVIG) has both antiviral and immunomodulating effects, suggesting that it might be an effective therapy in acute viral myocarditis. The recent Immune Modulation for Acute Cardiomyopathy trial, however, demonstrated that adult patients with recent onset of myocarditis or DCM who were treated with IVIG fared no better than similar patients treated with placebo.175 There are no randomized controlled trials investigating the use of IVIG to treat children with acute myocarditis, but several case series have shown that use of high-dose IVIG to treat acute myocarditis in this population leads to improved recovery of left ventricular function and better survival.43,167 Routine use of IVIG is therefore not recommended in adults but may be considered in select pediatric cases with acute myocarditis.
Antiarrhythmic treatment
High-grade AV block and tachyarrhythmias may be treated with appropriate medications and placement of a temporary or permanent pacemaker, as needed. Arrhythmias usually resolve after several weeks. Patients with symptomatic or sustained ventricular tachycardia may require antiarrhythmic therapy or possibly an implantable cardioverter-defibrillator or cardiac transplantation if arrhythmias persist after the acute inflammatory phase.
Physical activity
Animal studies have shown that sustained aerobic exercise during acute viral myocarditis leads to increased mortality.176 Based on these results and the knowledge that myocarditis is a cause of sudden death in young athletes,177 patients with acute myocarditis are advised to withdraw from competitive sports and other vigorous exercise for up to 6 months or longer after onset of symptoms, with length of recuperation required based on recovery of left ventricular function.178
Prognosis
Prognosis is excellent for adult patients with acute lymphocytic myocarditis with mild symptoms and preserved left ventricular ejection fraction, as most of them spontaneously improve without residual sequelae. In contrast, the Myocarditis Treatment Trial demonstrated that symptomatic adult patients who present with heart failure symptoms and a left ventricular ejection fraction less than 45% at baseline have a 4-year mortality of 56%.17 These results were obtained before the routine use of β-blockers, however, so prognosis in the current era has likely improved. Predictors of increased likelihood of death or need for cardiac transplantation include syncope,179 right ventricular systolic dysfunction,58 elevated pulmonary artery pressure,180 and advanced New York Heart Association functional class.5 Elevated levels of Fas, Fas ligand, TNF, and IL-10 50,51,181,182 as well as immunohistologic signs of inflammation (CD3 and/or CD68),5 are also predictors of increased risk of death.
Less information is available on the natural history of myocarditis in children. One study of 70 children with acute myocarditis reported that 73% of patients had histologic resolution of myocarditis at 6 months and 96% of patients survived to 1 year. Another study of 41 children with acute myocarditis who were treated with immunosuppression reported that at 5 years, most patients had complete recovery, whereas a quarter of patients died or required cardiac transplantation.183 Children who do not fully recover may develop chronic DCM that may result in death or cardiac transplantation up to 12 years after diagnosis.35
Patients with fulminant myocarditis who survive the acute phase have an excellent long-term prognosis and are more likely to experience complete recovery of left ventricular function compared with patients with acute myocarditis.180 Children and adults with GCM, on the other hand, have a median survival of less than 6 months and usually require cardiac transplantation. Transplant-free survival is better in patients with cardiac sarcoidosis than for patients with GCM, with a 70% 5-year survival rate.98 Most patients with Lyme carditis experience complete recovery.
Survival among patients undergoing heart transplant for treatment of acute myocarditis is similar to that of patients undergoing transplant for other cardiac diseases.184 Patients with GCM may be successfully treated with heart transplantation even though GCM recurs in up to 25% of transplanted adult hearts.23 Transplanted hearts with recurrent GCM may be effectively treated with augmented immunosuppression in most cases. Recurrent GCM with or without immunosuppressive treatment may be more virulent in children than in adults.185
Prevention
Specific strategies for the prevention of myocarditis have yet to be determined. To the extent that they have eliminated the diseases, vaccination against measles, mumps, rubella, poliomyelitis, and influenza has made myocarditis secondary to these diseases quite rare and raises the question of whether vaccinations against other cardiotropic viruses may prevent myocarditis in the future. Murine models have demonstrated that vaccination is protective against viral infection and prevents myocardial damage,186,187 but there have been no vaccination trials in humans. Given the low incidence of myocarditis and the cost of developing these vaccines, it is doubtful that antiviral vaccines to combat this disease will be developed in the near future.
Summary and future directions
Myocarditis is a potentially life-threatening disease that primarily affects children and young adults with sometimes devastating consequences, including sudden death. The primary long-term consequences are DCM and chronic heart failure. Although much progress has been made in recent years in the diagnosis, pathophysiology, and treatment of this disease, numerous questions remain and indicate the need for further investigation. Noninvasive strategies for confirming the diagnosis of myocarditis, including cardiac MRI, are promising but require additional validation. The identification of novel biomarkers of cardiac inflammation in peripheral blood, including analysis of messenger RNA and specific proteins, is underway.188 If a panel of blood-based biomarkers with high sensitivity and specificity can be developed as a noninvasive diagnostic tool, it may decrease the need for EMB. Although the pathophysiology of myocarditis has been studied in great detail in experimental animal models, research on the cellular processes contributing to myocardial damage in human myocarditis patients remains limited. Ongoing research efforts in this area that may lead to novel treatment strategies directed toward pathway-specific targets include the identification of genomic profiles that affect individual susceptibility to myocarditis or predict the likelihood of recovery.
Abbreviations and Acronyms
- AV
atrioventricular
- CAR
coxsackie-adenovirus receptor
- CVB3
coxsackievirus B3
- DCM
dilated cardiomyopathy
- EAM
experimental autoimmune myocarditis
- ECG
electrocardiogram
- EMB
endomyocardial biopsy
- GCM
giant cell myocarditis
- HAART
highly active antiretroviral therapy
- HIV
human immunodeficiency virus
- IL
interleukin
- IVIG
intravenous immunoglobulin
- MRI
magnetic resonance imaging
- TNF
tumor necrosis factor
- Tnt
troponin T
Footnotes
Author’s note: Substantial content in this review article is drawn from Cooper LT Jr. Myocarditis. N Engl J Med 2009;9;360(15):1526–38.
Statement of Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Chow LH, Radio SJ, Sears TD, et al. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol. 1989;14:915–920. doi: 10.1016/0735-1097(89)90465-8. [DOI] [PubMed] [Google Scholar]
- 2.Baughman KL. Diagnosis of myocarditis: death of Dallas criteria. Circulation. 2006;113:593–595. doi: 10.1161/CIRCULATIONAHA.105.589663. [DOI] [PubMed] [Google Scholar]
- 3.Herskowitz A, Ahmed-Ansari A, Neumann DA, et al. Induction of major histocompatibility complex antigens within the myocardium of patients with active myocarditis: a nonhistologic marker of myocarditis. J Am Coll Cardiol. 1990;15:624–632. doi: 10.1016/0735-1097(90)90637-5. [DOI] [PubMed] [Google Scholar]
- 4.Maisch B, Portig I, Ristic A, et al. Definition of inflammatory cardiomyopathy (myocarditis): on the way to consensus. A status report. Herz. 2000;25:200–209. doi: 10.1007/s000590050007. [DOI] [PubMed] [Google Scholar]
- 5.Kindermann I, Kindermann M, Kandolf R, et al. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–648. doi: 10.1161/CIRCULATIONAHA.108.769489. [DOI] [PubMed] [Google Scholar]
- 6.Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahrholdt H, Goedecke C, Wagner A, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–1258. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 8.Gutberlet M, Spors B, Thoma T, et al. Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology. 2008;246:401–409. doi: 10.1148/radiol.2461062179. [DOI] [PubMed] [Google Scholar]
- 9.Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–2233. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 10.Wakafuji S, Okada R. Twenty year autopsy statistics of myocarditis incidence in Japan. Jpn Circ J. 1986;50:1288–1293. doi: 10.1253/jcj.50.1288. [DOI] [PubMed] [Google Scholar]
- 11.Carniel E, Sinagra G, Bussani R, et al. Fatal myocarditis: morphologic and clinical features. Ital Heart J. 2004;5:702–706. [PubMed] [Google Scholar]
- 12.Kyto V, Saraste A, Voipio-Pulkki LM, et al. Incidence of fatal myocarditis: a population-based study in Finland. Am J Epidemiol. 2007;165:570–574. doi: 10.1093/aje/kwk076. [DOI] [PubMed] [Google Scholar]
- 13.Gravanis MB, Sternby NH. Incidence of myocarditis. A 10-year autopsy study from Malmo, Sweden. Arch Pathol Lab Med. 1991;115:390–392. [PubMed] [Google Scholar]
- 14.Doolan A, Langlois N, Semsarian C. Causes of sudden cardiac death in young Australians. Med J Aust. 2004;180:110–112. doi: 10.5694/j.1326-5377.2004.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 15.Hahn EA, Hartz VL, Moon TE, et al. The Myocarditis Treatment Trial: design, methods and patients enrollment. Eur Heart J. 1995;16(Suppl O):162–167. doi: 10.1093/eurheartj/16.suppl_o.162. [DOI] [PubMed] [Google Scholar]
- 16.Kuhl U, Pauschinger M, Noutsias M, et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111:887–893. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 17.Mason JW, O’Connell JB, Herskowitz A, et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 18.Caforio AL, Calabrese F, Angelini A, et al. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetio-pathogenetic features at diagnosis. Eur Heart J. 2007;28:1326–1333. doi: 10.1093/eurheartj/ehm076. [DOI] [PubMed] [Google Scholar]
- 19.Magnani JW, Danik HJ, Dec GW, Jr, et al. Survival in biopsy-proven myocarditis: a long-term retrospective analysis of the histopathologic, clinical, and hemodynamic predictors. Am Heart J. 2006;151:463–470. doi: 10.1016/j.ahj.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 20.Lyden DC, Olszewski J, Feran M, et al. Coxsackievirus B-3-induced myocarditis. Effect of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol. 1987;126:432–438. [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz J, Sartini D, Huber S. Myocarditis susceptibility in female mice depends upon ovarian cycle phase at infection. Virology. 2004;330:16–23. doi: 10.1016/j.virol.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 22.Frisancho-Kiss S, Coronado MJ, Frisancho JA, et al. Gonadectomy of male BALB/c mice increases Tim-3(+) alternatively activated M2 macrophages, Tim-3(+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav Immun. 2009;23:649–657. doi: 10.1016/j.bbi.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper LT, Jr, Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis—natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med. 1997;336:1860–1866. doi: 10.1056/NEJM199706263362603. [DOI] [PubMed] [Google Scholar]
- 24.Fenoglio JJ, Jr, Ursell PC, Kellogg CF, et al. Diagnosis and classification of myocarditis by endomyocardial biopsy. N Engl J Med. 1983;308:12–18. doi: 10.1056/NEJM198301063080103. [DOI] [PubMed] [Google Scholar]
- 25.Kyto V, Saukko P, Lignitz E, et al. Diagnosis and presentation of fatal myocarditis. Hum Pathol. 2005;36:1003–1007. doi: 10.1016/j.humpath.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Shen WK, Edwards WD, Hammill SC, et al. Sudden unexpected nontraumatic death in 54 young adults: a 30-year population-based study. Am J Cardiol. 1995;76:148–152. doi: 10.1016/s0002-9149(99)80047-2. [DOI] [PubMed] [Google Scholar]
- 27.Passarino G, Burlo P, Ciccone G, et al. Prevalence of myocarditis at autopsy in Turin. Italy Arch Pathol Lab Med. 1997;121:619–622. [PubMed] [Google Scholar]
- 28.Eckart RE, Scoville SL, Campbell CL, et al. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–834. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- 29.Maron BJ, Doerer JJ, Haas TS, et al. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation. 2009;119:1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 30.Rasten-Almqvist P, Eksborg S, Rajs J. Myocarditis and sudden infant death syndrome. Apmis. 2002;110:469–480. doi: 10.1034/j.1600-0463.2002.100605.x. [DOI] [PubMed] [Google Scholar]
- 31.Topaz O, Edwards JE. Pathologic features of sudden death in children, adolescents, and young adults. Chest. 1985;87:476–482. doi: 10.1378/chest.87.4.476. [DOI] [PubMed] [Google Scholar]
- 32.Neuspiel DR, Kuller LH. Sudden and unexpected natural death in childhood and adolescence. JAMA. 1985;254:1321–1325. [PubMed] [Google Scholar]
- 33.Nugent AW, Daubeney PE, Chondros P, et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003;348:1639–1646. doi: 10.1056/NEJMoa021737. [DOI] [PubMed] [Google Scholar]
- 34.Weber MA, Ashworth MT, Risdon RA, et al. Clinicopathological features of paediatric deaths due to myocarditis: an autopsy series. Arch Dis Child. 2008;93:594–598. doi: 10.1136/adc.2007.128686. [DOI] [PubMed] [Google Scholar]
- 35.Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 36.Rahman JE, Helou EF, Gelzer-Bell R, et al. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004;43:410–415. doi: 10.1016/j.jacc.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 37.Frustaci A, Verardo R, Caldarulo M, et al. Myocarditis in hypertrophic cardiomyopathy patients presenting acute clinical deterioration. Eur Heart J. 2007;28:733–740. doi: 10.1093/eurheartj/ehl525. [DOI] [PubMed] [Google Scholar]
- 38.Pieroni M, Dello Russo A, Marzo F, et al. High prevalence of myocarditis mimicking arrhythmogenic right ventricular cardiomyopathy differential diagnosis by electroanatomic mapping-guided endomyocardial biopsy. J Am Coll Cardiol. 2009;53:681–689. doi: 10.1016/j.jacc.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Basso C, Corrado D, Marcus FI, et al. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–1300. doi: 10.1016/S0140-6736(09)60256-7. [DOI] [PubMed] [Google Scholar]
- 40.McCully RB, Cooper LT, Schreiter S. Coronary artery spasm in lymphocytic myocarditis: a rare cause of acute myocardial infarction. Heart (British Cardiac Society) 2005;91:202. doi: 10.1136/hrt.2004.035675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hufnagel G, Pankuweit S, Richter A, et al. The European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID). First epidemiological results. Herz. 2000;25:279–285. doi: 10.1007/s000590050021. [DOI] [PubMed] [Google Scholar]
- 42.Freedman SB, Haladyn JK, Floh A, et al. Pediatric myocarditis: emergency department clinical findings and diagnostic evaluation. Pediatrics. 2007;120:1278–1285. doi: 10.1542/peds.2007-1073. [DOI] [PubMed] [Google Scholar]
- 43.Amabile N, Fraisse A, Bouvenot J, et al. Outcome of acute fulminant myocarditis in children. Heart (British Cardiac Society) 2006;92:1269–1273. doi: 10.1136/hrt.2005.078402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgera T, Di Lenarda A, Dreas L, et al. Electrocardiography of myocarditis revisited: clinical and prognostic significance of electrocardiographic changes. Am Heart J. 1992;124:455–467. doi: 10.1016/0002-8703(92)90613-z. [DOI] [PubMed] [Google Scholar]
- 45.Vignola PA, Aonuma K, Swaye PS, et al. Lymphocytic myocarditis presenting as unexplained ventricular arrhythmias: diagnosis with endomyocardial biopsy and response to immunosuppression. J Am Coll Cardiol. 1984;4:812–819. doi: 10.1016/s0735-1097(84)80411-8. [DOI] [PubMed] [Google Scholar]
- 46.Dec GW, Jr, Waldman H, Southern J, et al. Viral myocarditis mimicking acute myocardial infarction. J Am Coll Cardiol. 1992;20:85–89. doi: 10.1016/0735-1097(92)90141-9. [DOI] [PubMed] [Google Scholar]
- 47.Kuhl U, Pauschinger M, Seeberg B, et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112:1965–1970. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- 48.Nakashima H, Katayama T, Ishizaki M, et al. Q wave and non-Q wave myocarditis with special reference to clinical significance. Jpn Heart J. 1998;39:763–774. doi: 10.1536/ihj.39.763. [DOI] [PubMed] [Google Scholar]
- 49.Greenwood RD, Nadas AS, Fyler DC. The clinical course of primary myocardial disease in infants and children. Am Heart J. 1976;92:549–560. doi: 10.1016/s0002-8703(76)80074-9. [DOI] [PubMed] [Google Scholar]
- 50.Fuse K, Kodama M, Okura Y, et al. Predictors of disease course in patients with acute myocarditis. Circulation. 2000;102:2829–2835. doi: 10.1161/01.cir.102.23.2829. [DOI] [PubMed] [Google Scholar]
- 51.Nishii M, Inomata T, Takehana H, et al. Serum levels of interleukin-10 on admission as a prognostic predictor of human fulminant myocarditis. J Am Coll Cardiol. 2004;44:1292–1297. doi: 10.1016/j.jacc.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 52.Smith SC, Ladenson JH, Mason JW, et al. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation. 1997;95:163–168. [PubMed] [Google Scholar]
- 53.Franz WM, Remppis A, Kandolf R, et al. Serum troponin T: diagnostic marker for acute myocarditis. Clin Chem. 1996;42:340–341. [PubMed] [Google Scholar]
- 54.Lauer B, Niederau C, Kuhl U, et al. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol. 1997;30:1354–1359. doi: 10.1016/s0735-1097(97)00317-3. [DOI] [PubMed] [Google Scholar]
- 55.Soongswang J, Durongpisitkul K, Nana A, et al. Cardiac troponin T: a marker in the diagnosis of acute myocarditis in children. Pediatr Cardiol. 2005;26:45–49. doi: 10.1007/s00246-004-0677-6. [DOI] [PubMed] [Google Scholar]
- 56.Pinamonti B, Alberti E, Cigalotto A, et al. Echocardiographic findings in myocarditis. Am J Cardiol. 1988;62:285–291. doi: 10.1016/0002-9149(88)90226-3. [DOI] [PubMed] [Google Scholar]
- 57.Nieminen MS, Heikkila J, Karjalainen J. Echocardiography in acute infectious myocarditis: relation to clinical and electrocardiographic findings. Am J Cardiol. 1984;53:1331–1337. doi: 10.1016/0002-9149(84)90089-4. [DOI] [PubMed] [Google Scholar]
- 58.Mendes LA, Dec GW, Picard MH, et al. Right ventricular dysfunction: an independent predictor of adverse outcome in patients with myocarditis. Am Heart J. 1994;128:301–307. doi: 10.1016/0002-8703(94)90483-9. [DOI] [PubMed] [Google Scholar]
- 59.Angelini A, Calzolari V, Calabrese F, et al. Myocarditis mimicking acute myocardial infarction: role of endomyocardial biopsy in the differential diagnosis. Heart (British Cardiac Society) 2000;84:245–250. doi: 10.1136/heart.84.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.James KB, Lee K, Thomas JD, et al. Left ventricular diastolic dysfunction in lymphocytic myocarditis as assessed by Doppler echocardiography. Am J Cardiol. 1994;73:282–285. doi: 10.1016/0002-9149(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 61.Daly K, Monaghan M, Richardson P, et al. Significant incidence of mural thrombi in acute myocarditis—indications for early anticoagulation (abstract) J Am Coll Cardiol. 1983;1:584. [Google Scholar]
- 62.Felker GM, Boehmer JP, Hruban RH, et al. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36:227–232. doi: 10.1016/s0735-1097(00)00690-2. [DOI] [PubMed] [Google Scholar]
- 63.Friedrich MG, Strohm O, Schulz-Menger J, et al. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802–1809. doi: 10.1161/01.cir.97.18.1802. [DOI] [PubMed] [Google Scholar]
- 64.Laissy JP, Messin B, Varenne O, et al. MRI of acute myocarditis: a comprehensive approach based on various imaging sequences. Chest. 2002;122:1638–1648. doi: 10.1378/chest.122.5.1638. [DOI] [PubMed] [Google Scholar]
- 65.Laissy JP, Hyafil F, Feldman LJ, et al. Differentiating acute myocardial infarction from myocarditis: diagnostic value of early- and delayed-perfusion cardiac MR imaging. Radiology. 2005;237:75–82. doi: 10.1148/radiol.2371041322. [DOI] [PubMed] [Google Scholar]
- 66.Abdel-Aty H, Boye P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–1822. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 67.Mahrholdt H, Wagner A, Deluigi CC, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–1590. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 68.Yilmaz A, Mahrholdt H, Athanasiadis A, et al. Coronary vasospasm as the underlying cause for chest pain in patients with PVB19 myocarditis. Heart (British Cardiac Society) 2008;94:1456–1463. doi: 10.1136/hrt.2007.131383. [DOI] [PubMed] [Google Scholar]
- 69.Asimaki A, Tandri H, Huang H, et al. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360:1075–1084. doi: 10.1056/NEJMoa0808138. [DOI] [PubMed] [Google Scholar]
- 70.Holzmann M, Nicko A, Kuhl U, et al. Complication rate of right ventricular endomyocardial biopsy via the femoral approach: a retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation. 2008;118:1722–1728. doi: 10.1161/CIRCULATIONAHA.107.743427. [DOI] [PubMed] [Google Scholar]
- 71.Matsumori A, Shimada T, Chapman NM, et al. Myocarditis and heart failure associated with hepatitis C virus infection. J Card Fail. 2006;12:293–298. doi: 10.1016/j.cardfail.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 72.Matsumori A. Hepatitis C virus infection and cardiomyopathies. Circ Res. 2005;96:144–147. doi: 10.1161/01.RES.0000156077.54903.67. [DOI] [PubMed] [Google Scholar]
- 73.Herskowitz A, Wu TC, Willoughby SB, et al. Myocarditis and cardiotropic viral infection associated with severe left ventricular dysfunction in late-stage infection with human immunodeficiency virus. J Am Coll Cardiol. 1994;24:1025–1032. doi: 10.1016/0735-1097(94)90865-6. [DOI] [PubMed] [Google Scholar]
- 74.Breuckmann F, Neumann T, Kondratieva J, et al. Dilated cardiomyopathy in two adult human immunodeficiency positive (HIV+) patients possibly related to highly active antiretroviral therapy (HAART) Eur J Med Res. 2005;10:395–399. [PubMed] [Google Scholar]
- 75.Chen F, Shannon K, Ding S, et al. HIV type 1 glycoprotein 120 inhibits cardiac myocyte contraction. AIDS Res Hum Retrovir. 2002;18:777–784. doi: 10.1089/08892220260139512. [DOI] [PubMed] [Google Scholar]
- 76.Lanjewar DN, Katdare GA, Jain PP, et al. Pathology of the heart in acquired immunodeficiency syndrome. Indian Heart J. 1998;50:321–325. [PubMed] [Google Scholar]
- 77.Hofman P, Drici MD, Gibelin P, et al. Prevalence of toxoplasma myocarditis in patients with the acquired immunodeficiency syndrome. Br Heart J. 1993;70:376–381. doi: 10.1136/hrt.70.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pugliese A, Isnardi D, Saini A, et al. Impact of highly active antiretroviral therapy in HIV-positive patients with cardiac involvement. J Infect. 2000;40:282–284. doi: 10.1053/jinf.2000.0672. [DOI] [PubMed] [Google Scholar]
- 79.Barbaro G. Reviewing the cardiovascular complications of HIV infection after the introduction of highly active antiretroviral therapy. Curr Drug Targets. 2005;5:337–343. doi: 10.2174/1568006054553444. [DOI] [PubMed] [Google Scholar]
- 80.Costello JM, Alexander ME, Greco KM, et al. Lyme carditis in children: presentation, predictive factors, and clinical course. Pediatrics. 2009;123:e835–e841. doi: 10.1542/peds.2008-3058. [DOI] [PubMed] [Google Scholar]
- 81.Stanek G, Klein J, Bittner R, et al. Borrelia burgdorferi as an etiologic agent in chronic heart failure. Scand J Infect Dis. 1991;77:85–87. [PubMed] [Google Scholar]
- 82.Higuchi MD, Ries MM, Aiello VD, et al. Association of an increase in CD8+ T cells with the presence of Trypanosoma cruzi antigens in chronic, human, chagasic myocarditis. Am J Trop Med Hyg. 1997;56:485–489. doi: 10.4269/ajtmh.1997.56.485. [DOI] [PubMed] [Google Scholar]
- 83.Acquatella H. Echocardiography in Chagas heart disease. Circulation. 2007;115:1124–1131. doi: 10.1161/CIRCULATIONAHA.106.627323. [DOI] [PubMed] [Google Scholar]
- 84.Maguire JH, Hoff R, Sherlock I, et al. Cardiac morbidity and mortality due to Chagas’ disease: prospective electrocardiographic study of a Brazilian community. Circulation. 1987;75:1140–1145. doi: 10.1161/01.cir.75.6.1140. [DOI] [PubMed] [Google Scholar]
- 85.Simmons A, Vacek JL, Meyers D. Anthracycline-induced cardiomyopathy. Postgraduate medicine. 2008;120:67–72. doi: 10.3810/pgm.2008.11.1940. [DOI] [PubMed] [Google Scholar]
- 86.Phillips K, Luk A, Soor GS, et al. Cocaine cardiotoxicity: a review of the pathophysiology, pathology, and treatment options. Am J Cardiovasc Drugs. 2009;9:177–196. doi: 10.2165/00129784-200909030-00005. [DOI] [PubMed] [Google Scholar]
- 87.Taliercio CP, Olney BA, Lie JT. Myocarditis related to drug hypersensitivity. Mayo Clin Proc. 1985;60:463–468. doi: 10.1016/s0025-6196(12)60870-2. [DOI] [PubMed] [Google Scholar]
- 88.Hervier B, Masseau A, Bossard C, et al. Vasa-vasoritis of the aorta and fatal myocarditis in fulminant Churg-Strauss syndrome. Rheumatology (Oxford) 2008;47:1728–1729. doi: 10.1093/rheumatology/ken329. [DOI] [PubMed] [Google Scholar]
- 89.Frustaci A, Cuoco L, Chimenti C, et al. Celiac disease associated with autoimmune myocarditis. Circulation. 2002;105:2611–2618. doi: 10.1161/01.cir.0000017880.86166.87. [DOI] [PubMed] [Google Scholar]
- 90.Corssmit EP, Trip MD, Durrer JD. Loffler’s endomyocarditis in the idiopathic hypereosinophilic syndrome. Cardiology. 1999;91:272–276. doi: 10.1159/000006923. [DOI] [PubMed] [Google Scholar]
- 91.Kirchhoff LV, Weiss LM, Wittner M, et al. Parasitic diseases of the heart. Front Biosci. 2004;9:706–723. doi: 10.2741/1255. [DOI] [PubMed] [Google Scholar]
- 92.Dilber E, Karagoz T, Aytemir K, et al. Acute myocarditis associated with tetanus vaccination. Mayo Clin Proc. 2003;78:1431–1433. doi: 10.4065/78.11.1431-a. [DOI] [PubMed] [Google Scholar]
- 93.Cassimatis DC, Atwood JE, Engler RM, et al. Smallpox vaccination and myopericarditis: a clinical review. J Am Coll Cardiol. 2004;43:1503–1510. doi: 10.1016/j.jacc.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 94.Davies MJ, Pomerance A, Teare RD. Idiopathic giant cell myocarditis—a distinctive clinico-pathological entity. Br Heart J. 1975;37:192–195. doi: 10.1136/hrt.37.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kilgallen CM, Jackson E, Bankoff M, et al. A case of giant cell myocarditis and malignant thymoma: a postmortem diagnosis by needle biopsy. Clin Cardiol. 1998;21:48–51. doi: 10.1002/clc.4960210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Daniels PR, Berry GJ, Tazelaar HD, et al. Giant cell myocarditis as a manifestation of drug hypersensitivity. Cardiovasc Pathol. 2000;9:287–291. doi: 10.1016/s1054-8807(00)00049-1. [DOI] [PubMed] [Google Scholar]
- 97.Litovsky SH, Burke AP, Virmani R. Giant cell myocarditis: an entity distinct from sarcoidosis characterized by multiphasic myocyte destruction by cytotoxic T cells and histiocytic giant cells. Mod Pathol. 1996;9:1126–1134. [PubMed] [Google Scholar]
- 98.Okura Y, Dec GW, Hare JM, et al. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol. 2003;41:322–329. doi: 10.1016/s0735-1097(02)02715-8. [DOI] [PubMed] [Google Scholar]
- 99.Yazaki Y, Isobe M, Hiramitsu S, et al. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;82:537–540. doi: 10.1016/s0002-9149(98)00377-4. [DOI] [PubMed] [Google Scholar]
- 100.Badorff C, Lee GH, Lamphear BJ, et al. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999;5:320–326. doi: 10.1038/6543. [DOI] [PubMed] [Google Scholar]
- 101.Lozano MD, Rubocki RJ, Wilson JE, et al. Human leukocyte antigen class II associations in patients with idiopathic dilated cardiomyopathy. Myocarditis Treatment Trial Investigators. J Card Fail. 1997;3:97–103. doi: 10.1016/s1071-9164(97)90041-5. [DOI] [PubMed] [Google Scholar]
- 102.Tchilian EZ, Gil J, Navarro ML, et al. Unusual case presentations associated with the CD45 C77G polymorphism. Clin Exp Immunol. 2006;146:448–454. doi: 10.1111/j.1365-2249.2006.03230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beck MA, Levander OA, Handy J. Selenium deficiency and viral infection. J Nutr. 2003;133(5 Suppl 1):1463S–1467S. doi: 10.1093/jn/133.5.1463S. [DOI] [PubMed] [Google Scholar]
- 104.Ilback NG, Wesslen L, Fohlman J, et al. Effects of methyl mercury on cytokines, inflammation and virus clearance in a common infection (coxsackie B3 myocarditis) Toxicol Lett. 1996;89:19–28. doi: 10.1016/s0378-4274(96)03777-0. [DOI] [PubMed] [Google Scholar]
- 105.Cooper LT, Rader V, Ralston NV. The roles of selenium and mercury in the pathogenesis of viral cardiomyopathy. Congest Heart Fail (Greenwich, Conn) 2007;13:193–199. doi: 10.1111/j.1527-5299.2007.06410.x. [DOI] [PubMed] [Google Scholar]
- 106.Tracy S, Hofling K, Pirruccello S, et al. Group B coxsackievirus myocarditis and pancreatitis: connection between viral virulence phenotypes in mice. Journal of medical virology. 2000;62:70–81. doi: 10.1002/1096-9071(200009)62:1<70::aid-jmv11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 107.Bergelson JM, Cunningham JA, Droguett G, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science (New York, NY) 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 108.Shafren DR, Bates RC, Agrez MV, et al. Coxsackieviruses B1, B3, and B5 use decay accelerating factor as a receptor for cell attachment. J Virol. 1995;69:3873–3877. doi: 10.1128/jvi.69.6.3873-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wickham TJ, Filardo EJ, Cheresh DA, et al. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127:257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wickham TJ, Mathias P, Cheresh DA, et al. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 111.Salone B, Martina Y, Piersanti S, et al. Integrin alpha3beta1 is an alternative cellular receptor for adenovirus serotype 5. J Virol. 2003;77:13448–13454. doi: 10.1128/JVI.77.24.13448-13454.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martino TA, Petric M, Brown M, et al. Cardiovirulent coxsackieviruses and the decay-accelerating factor (CD55) receptor. Virology. 1998;244:302–314. doi: 10.1006/viro.1998.9122. [DOI] [PubMed] [Google Scholar]
- 113.Fechner H, Noutsias M, Tschoepe C, et al. Induction of coxsackievirus-adenovirus-receptor expression during myocardial tissue formation and remodeling: identification of a cell-to-cell contact-dependent regulatory mechanism. Circulation. 2003;107:876–882. doi: 10.1161/01.cir.0000050150.27478.c5. [DOI] [PubMed] [Google Scholar]
- 114.Kashimura T, Kodama M, Hotta Y, et al. Spatiotemporal changes of coxsackievirus and adenovirus receptor in rat hearts during postnatal development and in cultured cardiomyocytes of neonatal rat. Virchows Arch. 2004;444:283–292. doi: 10.1007/s00428-003-0925-9. [DOI] [PubMed] [Google Scholar]
- 115.Shi Y, Chen C, Lisewski U, et al. Cardiac deletion of the Coxsackievirus-adenovirus receptor abolishes Coxsackievirus B3 infection and prevents myocarditis in vivo. J Am Coll Cardiol. 2009;53:1219–1226. doi: 10.1016/j.jacc.2008.10.064. [DOI] [PubMed] [Google Scholar]
- 116.Huber SA. Autoimmunity in coxsackievirus B3 induced myocarditis. Autoimmunity. 2006;39:55–61. doi: 10.1080/08916930500484906. [DOI] [PubMed] [Google Scholar]
- 117.Huang CH, Vallejo JG, Kollias G, et al. Role of the innate immune system in acute viral myocarditis. Basic Res Cardiol. 2009;104:228–237. doi: 10.1007/s00395-008-0765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lowenstein CJ, Hill SL, Lafond-Walker A, et al. Nitric oxide inhibits viral replication in murine myocarditis. J Clin Invest. 1996;97:1837–1843. doi: 10.1172/JCI118613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Szalay G, Sauter M, Hald J, et al. Sustained nitric oxide synthesis contributes to immunopathology in ongoing myocarditis attributable to interleukin-10 disorders. Am J Pathol. 2006;169:2085–2093. doi: 10.2353/ajpath.2006.060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hardarson HS, Baker JS, Yang Z, et al. Toll-like receptor 3 is an essential component of the innate stress response in virus-induced cardiac injury. Am J Physiol. 2007;292:H251–H258. doi: 10.1152/ajpheart.00398.2006. [DOI] [PubMed] [Google Scholar]
- 121.Fuse K, Chan G, Liu Y, et al. Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of Coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation. 2005;112:2276–2285. doi: 10.1161/CIRCULATIONAHA.105.536433. [DOI] [PubMed] [Google Scholar]
- 122.Fairweather D, Frisancho-Kiss S, Njoku DB, et al. Complement receptor 1 and 2 deficiency increases coxsackievirus B3-induced myocarditis, dilated cardiomyopathy, and heart failure by increasing macrophages, IL-1beta, and immune complex deposition in the heart. J Immunol. 2006;176:3516–3524. doi: 10.4049/jimmunol.176.6.3516. [DOI] [PubMed] [Google Scholar]
- 123.Chow LH, Beisel KW, McManus BM. Enteroviral infection of mice with severe combined immunodeficiency. Evidence for direct viral pathogenesis of myocardial injury. Lab Invest. 1992;66:24–31. [PubMed] [Google Scholar]
- 124.Lamphear BJ, Yan R, Yang F, et al. Mapping the cleavage site in protein synthesis initiation factor eIF-4 gamma of the 2A proteases from human Coxsackievirus and rhinovirus. J Biol Chem. 1993;268:19200–19203. [PubMed] [Google Scholar]
- 125.Xiong D, Yajima T, Lim BK, et al. Inducible cardiac-restricted expression of enteroviral protease 2A is sufficient to induce dilated cardiomyopathy. Circulation. 2007;115:94–102. doi: 10.1161/CIRCULATIONAHA.106.631093. [DOI] [PubMed] [Google Scholar]
- 126.Chau DH, Yuan J, Zhang H, et al. Coxsackievirus B3 proteases 2A and 3C induce apoptotic cell death through mitochondrial injury and cleavage of eIF4GI but not DAP5/p97/NAT1. Apoptosis. 2007;12:513–524. doi: 10.1007/s10495-006-0013-0. [DOI] [PubMed] [Google Scholar]
- 127.Pummerer C, Berger P, Fruhwirth M, et al. Cellular infiltrate, major histocompatibility antigen expression and immunopathogenic mechanisms in cardiac myosin-induced myocarditis. Lab Invest. 1991;65:538–547. [PubMed] [Google Scholar]
- 128.Goser S, Ottl R, Brodner A, et al. Critical role for monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha in induction of experimental autoimmune myocarditis and effective anti-monocyte chemoattractant protein-1 gene therapy. Circulation. 2005;112:3400–3407. doi: 10.1161/CIRCULATIONAHA.105.572396. [DOI] [PubMed] [Google Scholar]
- 129.Rangachari M, Mauermann N, Marty RR, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Haga T, Suzuki J, Kosuge H, et al. Attenuation of experimental autoimmune myocarditis by blocking T cell activation through 4-1BB pathway. J Mol Cell Cardiol. 2009;46:719–727. doi: 10.1016/j.yjmcc.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 131.Afanasyeva M, Wang Y, Kaya Z, et al. Experimental autoimmune myocarditis in A/J mice is an interleukin-4-dependent disease with a Th2 phenotype. Autoimmunity. 2001;159:193–203. doi: 10.1016/S0002-9440(10)61685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eriksson U, Ricci R, Hunziker L, et al. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat Med. 2003;9:1484–1490. doi: 10.1038/nm960. [DOI] [PubMed] [Google Scholar]
- 133.Hayward SL, Bautista-Lopez N, Suzuki K, et al. CD4 T cells play major effector role and CD8 T cells initiating role in spontaneous autoimmune myocarditis of HLA-DQ8 transgenic IAb knockout nonobese diabetic mice. J Immunol. 2006;176:7715–7725. doi: 10.4049/jimmunol.176.12.7715. [DOI] [PubMed] [Google Scholar]
- 134.Neumann DA, Rose NR, Ansari AA, et al. Induction of multiple heart autoantibodies in mice with coxsackievirus B3- and cardiac myosin-induced autoimmune myocarditis. J Immunol. 1994;152:343–350. [PubMed] [Google Scholar]
- 135.Caforio AL, Mahon NJ, Tona F, et al. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 2002;4:411–417. doi: 10.1016/s1388-9842(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 136.Neumann DA, Burek CL, Baughman KL, et al. Circulating heart-reactive antibodies in patients with myocarditis or cardiomyopathy. J Am Coll Cardiol. 1990;16:839–846. doi: 10.1016/s0735-1097(10)80331-6. [DOI] [PubMed] [Google Scholar]
- 137.Caforio AL, Mahon NJ, McKenna WJ. Cardiac autoantibodies to myosin and other heart-specific autoantigens in myocarditis and dilated cardiomyopathy. Autoimmunity. 2001;34:199–204. doi: 10.3109/08916930109007385. [DOI] [PubMed] [Google Scholar]
- 138.Wallukat G, Morwinski M, Kowal K, et al. Autoantibodies against the beta-adrenergic receptor in human myocarditis and dilated cardiomyopathy: beta-adrenergic agonism without desensitization. Eur Heart J. 1991;12(Suppl D):178–181. doi: 10.1093/eurheartj/12.suppl_d.178. [DOI] [PubMed] [Google Scholar]
- 139.Staudt A, Dorr M, Staudt Y, et al. Role of immunoglobulin G3 subclass in dilated cardiomyopathy: results from protein A immunoadsorption. Am Heart J. 2005;150:729–736. doi: 10.1016/j.ahj.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 140.Warraich RS, Noutsias M, Kazak I, et al. Immunoglobulin G3 cardiac myosin autoantibodies correlate with left ventricular dysfunction in patients with dilated cardiomyopathy: immunoglobulin G3 and clinical correlates. Am Heart J. 2002;143:1076–1084. doi: 10.1067/mhj.2002.124406. [DOI] [PubMed] [Google Scholar]
- 141.Latva-Hirvela J, Kyto V, Saraste A, et al. Development of troponin autoantibodies in experimental coxsackievirus B3 myocarditis. Eur J Clin Invest. 2009;39:457–462. doi: 10.1111/j.1365-2362.2009.02113.x. [DOI] [PubMed] [Google Scholar]
- 142.Kaya Z, Katus HA, Rose NR. Cardiac troponins and autoimmunity: Their role in the pathogenesis of myocarditis and of heart failure. Clin Immunol (Orlando, Fla) 2009 doi: 10.1016/j.clim.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Goser S, Andrassy M, Buss SJ, et al. Cardiac troponin I but not cardiac troponin T induces severe autoimmune inflammation in the myocardium. Circulation. 2006;114:1693–1702. doi: 10.1161/CIRCULATIONAHA.106.635664. [DOI] [PubMed] [Google Scholar]
- 144.Matsumori A, Igata H, Ono K, et al. High doses of digitalis increase the myocardial production of proinflammatory cytokines and worsen myocardial injury in viral myocarditis: a possible mechanism of digitalis toxicity. Jpn Circ J. 1999;63:934–940. doi: 10.1253/jcj.63.934. [DOI] [PubMed] [Google Scholar]
- 145.Wang WZ, Matsumori A, Yamada T, et al. Beneficial effects of amlodipine in a murine model of Congest Heart Fail induced by viral myocarditis. A possible mechanism through inhibition of nitric oxide production. Circulation. 1997;95:245–251. doi: 10.1161/01.cir.95.1.245. [DOI] [PubMed] [Google Scholar]
- 146.Liu W, Shimada M, Xiao J, et al. Nifedipine inhibits the activation of inflammatory and immune reactions in viral myocarditis. Life Sci. 2009;85:235–240. doi: 10.1016/j.lfs.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 147.Khatib R, Reyes MP, Smith FE. Enhancement of Coxsackievirus B3 replication in Vero cells by indomethacin. J Infect Dis. 1990;162:997–998. doi: 10.1093/infdis/162.4.997-a. [DOI] [PubMed] [Google Scholar]
- 148.Khatib R, Reyes MP, Smith F, et al. Enhancement of coxsackievirus B4 virulence by indomethacin. J Lab Clin Med. 1990;116:116–120. [PubMed] [Google Scholar]
- 149.Costanzo-Nordin MR, Reap EA, O’Connell JB, et al. A nonsteroid anti-inflammatory drug exacerbates Coxsackie B3 murine myocarditis. J Am Coll Cardiol. 1985;6:1078–1082. doi: 10.1016/s0735-1097(85)80312-0. [DOI] [PubMed] [Google Scholar]
- 150.Pages ON, Aubert S, Combes A, et al. Paracorporeal pulsatile biventricular assist device versus extracorporal membrane oxygenation-extracorporal life support in adult fulminant myocarditis. J Thorac Cardiovasc Surg. 2009;137:194–197. doi: 10.1016/j.jtcvs.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 151.Duncan BW, Bohn DJ, Atz AM, et al. Mechanical circulatory support for the treatment of children with acute fulminant myocarditis. J Thorac Cardiovasc Surg. 2001;122:440–448. doi: 10.1067/mtc.2001.115243. [DOI] [PubMed] [Google Scholar]
- 152.Reinhartz O, Hill JD, Al-Khaldi A, et al. Thoratec ventricular assist devices in pediatric patients: update on clinical results. Asaio J. 2005;51:501–503. doi: 10.1097/01.mat.0000178214.95010.c0. [DOI] [PubMed] [Google Scholar]
- 153.Topkara VK, Dang NC, Barili F, et al. Ventricular assist device use for the treatment of acute viral myocarditis. J Thorac Cardiovasc Surg. 2006;131:1190–1191. doi: 10.1016/j.jtcvs.2005.08.073. [DOI] [PubMed] [Google Scholar]
- 154.Bowles NE, Ni J, Kearney DL, et al. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–472. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- 155.Wang YX, da Cunha V, Vincelette J, et al. Antiviral and myocyte protective effects of murine interferon-beta and -{alpha}2 in coxsackievirus B3-induced myocarditis and epicarditis in Balb/c mice. Am J Physiol. 2007;293:H69–H76. doi: 10.1152/ajpheart.00154.2007. [DOI] [PubMed] [Google Scholar]
- 156.Horwitz MS, La Cava A, Fine C, et al. Pancreatic expression of interferon-gamma protects mice from lethal coxsackievirus B3 infection and subsequent myocarditis. Nat Med. 2000;6:693–697. doi: 10.1038/76277. [DOI] [PubMed] [Google Scholar]
- 157.Okada I, Matsumori A, Matoba Y, et al. Combination treatment with ribavirin and interferon for coxsackievirus B3 replication. J Lab Clin Med. 1992;120:569–573. [PubMed] [Google Scholar]
- 158.Matsumori A, Crumpacker CS, Abelmann WH. Prevention of viral myocarditis with recombinant human leukocyte interferon alpha A/D in a murine model. J Am Coll Cardiol. 1987;9:1320–1325. doi: 10.1016/s0735-1097(87)80472-2. [DOI] [PubMed] [Google Scholar]
- 159.Ray CG, Icenogle TB, Minnich LL, et al. The use of intravenous ribavirin to treat influenza virus-associated acute myocarditis. J Infect Dis. 1989;159:829–836. doi: 10.1093/infdis/159.5.829. [DOI] [PubMed] [Google Scholar]
- 160.Kuhl U, Pauschinger M, Schwimmbeck PL, et al. Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation. 2003;107:2793–2798. doi: 10.1161/01.CIR.0000072766.67150.51. [DOI] [PubMed] [Google Scholar]
- 161.Jones SR, Herskowitz A, Hutchins GM, et al. Effects of immunosuppressive therapy in biopsy-proved myocarditis and borderline myocarditis on left ventricular function. Am J Cardiol. 1991;68:370–376. doi: 10.1016/0002-9149(91)90834-8. [DOI] [PubMed] [Google Scholar]
- 162.Maisch B, Camerini F, Schultheiss HP. Immunosuppressive therapy for myocarditis. N Engl J Med. 1995;333:1713. author reply 4. [PubMed] [Google Scholar]
- 163.McNamara DM, Rosenblum WD, Janosko KM, et al. Intravenous immune globulin in the therapy of myocarditis and acute cardiomyopathy. Circulation. 1997;95:2476–2478. doi: 10.1161/01.cir.95.11.2476. [DOI] [PubMed] [Google Scholar]
- 164.McNamara DM, Starling RC, Dec GW. Intervention in myocarditis and acute cardiomyopathy with immune globulin: results from the randomized placebo controlled IMAC trial. Circulation. 1999;100(Suppl I):I-21. [Abstract] [Google Scholar]
- 165.Cooper LT, Jr, Hare JM, Tazelaar HD, et al. Usefulness of immunosuppression for giant cell myocarditis. Am J Cardiol. 2008;102:1535–1539. doi: 10.1016/j.amjcard.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chan KY, Iwahara M, Benson LN, et al. Immunosuppressive therapy in the management of acute myocarditis in children: a clinical trial. J Am Coll Cardiol. 1991;17:458–460. doi: 10.1016/s0735-1097(10)80115-9. [DOI] [PubMed] [Google Scholar]
- 167.Drucker NA, Colan SD, Lewis AB, et al. Gamma-globulin treatment of acute myocarditis in the pediatric population. Circulation. 1994;89:252–257. doi: 10.1161/01.cir.89.1.252. [DOI] [PubMed] [Google Scholar]
- 168.Parrillo JE, Cunnion RE, Epstein SE, et al. A prospective, randomized, controlled trial of prednisone for dilated cardiomyopathy. N Engl J Med. 1989;321:1061–1068. doi: 10.1056/NEJM198910193211601. [DOI] [PubMed] [Google Scholar]
- 169.Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, et al. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: two-year follow-up results. Circulation. 2001;104:39–45. doi: 10.1161/01.cir.104.1.39. [DOI] [PubMed] [Google Scholar]
- 170.Frustaci A, Chimenti C, Calabrese F, et al. Immunosuppressive therapy for active lymphocytic myocarditis: virological and immunologic profile of responders versus nonresponders. Circulation. 2003;107:857–863. doi: 10.1161/01.cir.0000048147.15962.31. [DOI] [PubMed] [Google Scholar]
- 171.Gullestad L, Aass H, Fjeld JG, et al. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation. 2001;103:220–225. doi: 10.1161/01.cir.103.2.220. [DOI] [PubMed] [Google Scholar]
- 172.Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J. 2009 doi: 10.1093/eurheartj/ehp249. [DOI] [PubMed] [Google Scholar]
- 173.Camargo PR, Snitcowsky R, da Luz PL, et al. Favorable effects of immunosuppressive therapy in children with dilated cardiomyopathy and active myocarditis. Pediatr Cardiol. 1995;16:61–68. doi: 10.1007/BF00796819. [DOI] [PubMed] [Google Scholar]
- 174.Balaji S, Wiles HB, Sens MA, et al. Immunosuppressive treatment for myocarditis and borderline myocarditis in children with ventricular ectopic rhythm. Br Heart J. 1994;72:354–359. doi: 10.1136/hrt.72.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McNamara DM, Holubkov R, Starling RC, et al. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation. 2001;103:2254–2259. doi: 10.1161/01.cir.103.18.2254. [DOI] [PubMed] [Google Scholar]
- 176.Cabinian AE, Kiel RJ, Smith F, et al. Modification of exercise-aggravated coxsackievirus B3 murine myocarditis by T lymphocyte suppression in an inbred model. J Lab Clin Med. 1990;115:454–462. [PubMed] [Google Scholar]
- 177.Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349:1064–1075. doi: 10.1056/NEJMra022783. [DOI] [PubMed] [Google Scholar]
- 178.Maron BJ, Ackerman MJ, Nishimura RA, et al. Task Force 4: HCM and other cardiomyopathies, mitral valve prolapse, myocarditis, and Marfan syndrome. J Am Coll Cardiol. 2005;45:1340–1345. doi: 10.1016/j.jacc.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 179.Magnani JW, Dec GW. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006;113:876–890. doi: 10.1161/CIRCULATIONAHA.105.584532. [DOI] [PubMed] [Google Scholar]
- 180.McCarthy RE, III, Boehmer JP, Hruban RH, et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342:690–695. doi: 10.1056/NEJM200003093421003. [DOI] [PubMed] [Google Scholar]
- 181.Sheppard R, Bedi M, Kubota T, et al. Myocardial expression of fas and recovery of left ventricular function in patients with recent-onset cardiomyopathy. J Am Coll Cardiol. 2005;46:1036–1042. doi: 10.1016/j.jacc.2005.05.067. [DOI] [PubMed] [Google Scholar]
- 182.Fuse K, Kodama M, Okura Y, et al. Short-term prognostic value of initial serum levels of interleukin-10 in patients with acute myocarditis. Eur J Heart Fail. 2005;7:109–112. doi: 10.1016/j.ejheart.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 183.Gagliardi MG, Bevilacqua M, Bassano C, et al. Long term follow up of children with myocarditis treated by immunosuppression and of children with dilated cardiomyopathy. Heart (British Cardiac Society) 2004;90:1167–1171. doi: 10.1136/hrt.2003.026641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cooper DK, Schlesinger RG, Shrago S, et al. Heart transplantation for giant cell myocarditis. J Heart Lung Transplant. 1994;13:555. [PubMed] [Google Scholar]
- 185.Singh TP, Rabah R, Cooper LT, et al. Total lymphoid irradiation: new therapeutic option for refractory giant cell myocarditis. J Heart Lung Transplant. 2004;23:492–495. doi: 10.1016/S1053-2498(03)00214-6. [DOI] [PubMed] [Google Scholar]
- 186.Fontanella GH, De Vusser K, Laroy W, et al. Immunization with an engineered mutant trans-sialidase highly protects mice from experimental Trypanosoma cruzi infection: a vaccine candidate. Vaccine. 2008;26:2322–2334. doi: 10.1016/j.vaccine.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 187.Fohlman J, Ilback NG, Friman G, et al. Vaccination of Balb/c mice against enteroviral mediated myocarditis. Vaccine. 1990;8:381–384. doi: 10.1016/0264-410x(90)90098-7. [DOI] [PubMed] [Google Scholar]
- 188.Oberg AL, Mahoney DW, Eckel-Passow JE, et al. Statistical analysis of relative labeled mass spectrometry data from complex samples using ANOVA. J Proteome Res. 2008;7:225–233. doi: 10.1021/pr700734f. [DOI] [PMC free article] [PubMed] [Google Scholar]