Abstract
OBJECTIVE
This study aimed to investigate the neural correlates of psychotic-like experiences in youth on measures of inhibitory control, reward anticipation and emotion processing. A secondary aim was to test whether these neuro-functional correlates of risk were predictive of psychotic symptoms 2 years later.
METHOD
Functional imaging response to three paradigms: the Stop-Signal, Monetary Incentive Delay, and Faces tasks was collected in youth at age 14, as part of the IMAGEN study. At baseline, youth from London and Dublin sites were assessed on psychotic-like experiences and those reporting significant experiences were compared with matched controls. Significant brain activity differences between the groups were used to predict, with cross-validation, the presence of psychotic symptoms in the context of mood fluctuation at age 16, assessed in the full sample. These prediction analyses were conducted with the London-Dublin subsample (N=246) and the full sample (N=1196).
RESULTS
Youth reporting psychotic-like experiences showed increased hippocampus/amygdala activity during neutral faces processing and reduced dorsolateral prefrontal activity during failed inhibition relative to controls. The most prominent region for classifying 16-year olds with mood fluctuation and psychotic symptoms relative to the control groups (those with mood fluctuations but no psychotic symptoms and those with no mood symptoms) included hyperactivation of the hippocampus/amygdala, when controlling for baseline psychotic-like experiences and cannabis use.
CONCLUSIONS
The results stress the importance of the limbic network’s increased response to neutral facial stimuli as a marker of the extended psychosis phenotype. These findings might help to guide early intervention strategies for at-risk youth.
INTRODUCTION
There is evidence of a continuity between clinical and subclinical phenotypes of psychosis that is measurable in the general population (1) and in individuals with a psychiatric diagnosis (2). On a clinical level, individual differences in psychosis proneness are expressed across a number of psychiatric conditions besides schizophrenia, namely mood, anxiety, eating, impulse control, and substance use disorders (2). At a subclinical level this liability is characterized by “attenuated” or “brief” psychotic symptoms that might not co-exist with other diagnostic criteria (frequency and intensity) to meet full diagnosis, yet sufficient impairment is observed to motivate treatment seeking (3). This clinical high-risk state has been shown to be a robust risk factor for progression to clinically-significant psychiatric disorders (4), but does not necessarily predict to one specific disorder and instead is predictive of a number of psychopathologies that include psychotic symptoms (5).
At the far end of the extended psychosis continuum are children/adolescents from the community reporting psychotic-like experiences (i.e., perceptual abnormalities and delusional thoughts) prior to the onset of more impairing psychotic symptoms. These preclinical experiences, even though they are common in children/young adolescents (7 to 23% (6)), are associated with increased risk for psychotic or Axis I disorders over the longer term (6, 7). Studying young adolescents prone to such experiences will help to identify etiologic processes implicated in psychosis proneness, without the confounds of diverse risk factors and iatrogenic effects, such as substance misuse, medication and social impairment (8). Investigating the neural correlates of this preclinical psychosis proneness on cognitive functioning can shed light on early altered neural processes prior to significant cognitive impairments.
However, the vast majority of functional magnetic resonance imaging (fMRI) studies have focused on adults with a clinical risk to psychosis, not on young adolescents reporting psychotic-like experiences. These studies have mostly investigated the neural circuits implicated in executive functioning, social cognition and reinforcement learning. Recent fMRI studies in individuals with psychosis-spectrum symptoms have shown significant reduced activation in the dorso-lateral prefrontal cortex (dlPFC) during executive functioning (e.g., working memory, inhibitory control) relative to low-risk controls (9, 10). These results are consistent with findings of dlPFC hypoactivation in patients with clinical diagnoses of psychosis or bipolar disorder on tasks of working memory and response inhibition (11, 12), suggesting that the brain markers associated with psychosis proneness cross diagnostic boundaries.
Social cognition which encompasses emotion processing and theory of mind processes has also been identified as a domain which might differentiate individuals at clinical risk for psychosis from low-risk individuals (13). Neuroimaging studies show that the experience of high arousal negative emotions is associated, in individuals at clinical risk relative to healthy controls, with both reduced (14) and increased activation of fronto-limbic areas (9), depending on the contrast used (15), while viewing neutral material is more consistently associated with increased activation of this network (14, 16).
Another core feature of psychosis, dysfunctional reinforcement learning, was shown to be shared with distinct diagnostic categories such as major depressive and bipolar disorders (17). A recent meta-analysis of fMRI studies has demonstrated that psychosis spectrum disorders are associated with a blunted response from the ventral striatum during anticipation of reward, which might explain why patients demonstrate impaired learning of stimulus-reinforcement associations (18). Functional MRI studies with clinically at-risk individuals have shown modest reduced activity in fronto-striatal regions during reward anticipation, relative to controls (19, 20).
Among the very few neuroimaging studies investigating the early neural correlates of preclinical psychosis proneness prior to the onset of more impairing psychotic symptoms, Modinos et al. (21, 22) found that community youth self-reporting psychotic-like experiences had reduced activation of the medial prefrontal cortex, insula and amygdala during passive viewing and reappraisal of negative pictures relative to low-risk youth. In a quite young sample of 11- to 13-year olds reporting these experiences, Jacobson et al. (23) observed reduced activity in prefrontal and temporal regions during a response inhibition task. However, the sample was small (11 in the at-risk group). Consequently, we intended to extend these findings in another community sample of young adolescents with psychotic-like experiences. Of note, considering that psychotic-like experiences are, for most individuals, transient and not persistent (1), it is crucial to understand to what extent these early neural abnormalities relate to a subsequent psychosis vulnerability in terms of clinically validated symptoms.
The primary aim of the present exploratory study was to identify brain correlates of psychotic-like experiences in youth prior to exposure to regular substance use on fMRI measures of emotion processing, inhibitory control and reward anticipation using data from the IMAGEN study, in which two sites, London and Dublin, assessed these preclinical experiences to participants when they were 14. The secondary aim was to validate whether these brain correlates predicted emergence of psychotic symptoms in the context of mood fluctuation symptoms at age 16 in the full IMAGEN sample. We hypothesized that psychotic-like experiences would be associated with reduced activity in the executive network during response inhibition, altered activity in fronto-limbic regions during processing of emotional and non-emotional stimuli as well as modest reduced ventral striatum activity during anticipation of reward.
METHODS
Participants
2257 14-year old adolescents were recruited through high schools in the large European multicenter IMAGEN study from 8 sites across the United Kingdom, Ireland, France and Germany. Parents and adolescents gave written informed consent to the study procedures. All procedures were approved by each local institutional ethics committee. A detailed description of the study recruitment and assessment procedure, exclusion criteria, data storage and safety, as well as imaging acquisition protocol may be found elsewhere (24).
Measures
For a more detailed description of the study measures, see the online data supplement.
Psychotic-like experiences
At baseline, 14-year olds from London and Dublin completed the self-report Adolescent Psychotic-Like Symptoms Screener (25), which contains 7 items evaluating perceptual abnormalities and delusional thoughts in the past 6 months. Participants were asked to rate their responses to different statements on a 1-point scale (0=not true, 0.5=somewhat true, 1=certainly true). Based on Cannon’s team (23, 25) previous studies, to identify youth with significant psychotic-like experiences, we used the following criteria: a total score ≥2 and a score ≥0.5 on the auditory hallucination (this item revealed 88% probability of predicting which individuals would be classified as “at-risk”, as determined by consensus ratings from the Structured Interview for Prodromal Syndromes).
Among 410 adolescents [mean (SD), 14.3 (0.4) years old; 51.7% girls] from London and Dublin sites, 300 had complete fMRI and behavioral information. Among them, 27 were classified as having significant psychotic-like experiences. None had yet started using cannabis and they reported minimal alcohol and cigarette use (<3–5 times in the previous year). Using an in-house groupwise matching script designed by the IMAGEN consortium, the group was matched (on sex, handedness, imaging site, general IQ and puberty development) to a control group 5 times larger (135 adolescents) which included those with a total score ≤ 1 and a score of 0 on the auditory hallucination question.
Psychotic symptoms at age 16
For the secondary objective of the study, psychotic symptoms were evaluated with the Self-Report Development and Well-Being Assessment interview (www.dawba.com) (26), a computer-based package of questionnaires designed to generate DSM-IV-TR psychiatric diagnoses for 5- to 16-year olds. While the schizophrenia module was not administered to participants at age 16, the bipolar module was more developmentally appropriate for this age group, and therefore all participants answered initial screening questions assessing mood dysregulation (“rapid mood changes” and “abnormally high mood”), and if positive, they were then asked three specific items assessing the presence of visual and auditory hallucinations and delusional beliefs. Among the 300 individuals from London and Dublin with complete baseline assessments, 246 (82.0%) completed the bipolar module at age 16 and were further divided into three groups: those who endorsed mood dysregulation plus hallucinatory/delusional symptoms (i.e., group with mood and psychotic symptoms, N=12), those reporting mood dysregulation without hallucinatory/delusional symptoms (i.e., group with mood symptoms only, N=80) and those who did not endorse the mood dysregulation criteria (i.e., no mood symptoms group, N=154).
Additionally, we conducted similar analyses on the full IMAGEN sample: among the 1602 participants re-assessed at 16 years old, 1196 had complete fMRI and behavioral information and were divided into three groups: those with mood and psychotic symptoms (N=72), those with mood symptoms only (N=451), and those without any mood symptoms (N=673).
Neuroimaging tasks
We report results from three task-based fMRI paradigms: the Faces task to assess emotional processing, the Stop-Signal Task to evaluate motor inhibitory control and a modified version of the Monetary Incentive Delay Task to examine reward anticipation. The block design Faces task, known to elicit prefrontal and amygdala activations (27), uses video clips displaying a neutral expression progressively turning into angry or a second neutral expression. A control condition displays expanding/contracting circles. In the event-related adaptation of the Stop-Signal Task used to measure activation of the fronto-striatal network (28), a motor response to high frequency go signals (80% of trials) has to be inhibited when infrequently and unexpectedly (in randomised 20% of trials), a stop signal appears after the go signal. In the modified Monetary Incentive Delay task, participants had to respond to a target in order to win a previously indicated amount of points (3 trial types: no win, small win and large win). In the anticipation phase, which elicits striatal and medial prefrontal activity (29), participants were presented with cues signaling the amount of reward that could be won in a given trial.
Data analysis
fMRI
To test activity differences between the group reporting or not psychotic-like experiences on each of the contrasts of interests (Faces: angry vs neutral and neutral vs control; Stop-Signal: stop success vs baseline and stop failure vs baseline; Monetary Incentive Delay: anticipation of large reward vs no reward), we conducted two-sample t tests, using a whole-brain approach in SPM8 (Wellcome Trust Centre for Neuroimaging). Following Eklund et al. (30) recommendations for controlling type 1 error, significant voxels were required to be part of cluster of more than 24 contiguous voxels giving a 0.05% probability of a cluster surviving due to chance (AFNI’s 3dClustSim). For our secondary objective, we used a more liberal threshold: significant voxels were required to be part of cluster of more than 10 contiguous voxels. Then, we created regions-of-interest based on the regions’ coordinates and extracted the mean contrast value (betas) for each region of interest and for each subject.
Machine learning procedure
For our secondary objective, we aimed to classify youth according to 16-years old psychotic outcomes with fMRI information. We conducted cross-validated logistic regressions with elastic-net regularization to model this relationship. Cross-validation is used to evaluate how well a predictive model generalizes to out-of-sample observations. On one hand, leave-one-out cross-validations were used during classification of the groups within the smaller London-Dublin subsample; on the other hand, k(10)-fold cross-validations were used during classification of the groups within the full sample. Cross-validation analysis within the London-Dublin subsample allowed to test the predictive capacity of the brain markers while controlling for baseline psychotic-like experiences. Considering that the sample size of the groups was much larger in the full sample, we were able to control for more predictors such as developmental risk factors for psychotic symptoms (i.e., cannabis, alcohol and cigarette use, as well as internalizing and externalizing behaviors (31)), gathered at age 14.
Elastic-net regularization is used to achieve better prediction performance by penalizing the regression coefficients in an attempt to minimize overfit. Elastic-net regularization is an example of a sparse regression method, which imposes a hybrid of both L1- and L2-norm penalties (i.e., penalties on the absolute (L1-norm) and squared values of the regression coefficients (L2-norm)). Model performance was evaluated using the area under the curve (AUC) of the receiver-operating characteristic (ROC), which quantifies the predicted sensitivity (true positive rate) as a function of false positive rate (1-specificity).
RESULTS
Demographic and clinical information of the groups at age 14
Reported in Table 1 are the means of the variables used as matching parameters between the 27 adolescents with and the 135 matched controls without psychotic-like experiences (no significant differences between the groups). Furthermore, the groups were not different on age, as well as alcohol, cigarette and cannabis use in the previous year.
Table 1.
Demographic, substance use and clinical characteristics of the groups at 14 years old (baseline).
| Characteristics | PLEs group (N=27) |
Control group (N=135) |
P Valuea |
|---|---|---|---|
| Demographic | |||
|
| |||
| Sex: female, (%)b | 64.3% | 65.2% | .93 |
| Age at testing, mean (SD) | 14.26 (0.31) | 14.35 (0.38) | .24 |
| Imaging site: London, (%)b | 57.1% | 64.4% | .47 |
| Right handed, (%)b | 92.9% | 91.0% | .88 |
| Puberty status, mean (SD) | 3.73 (0.72) | 3.69 (0.69) | .77 |
| Cognition | |||
|
| |||
| Verbal IQ, mean (SD) | 107.28 (13.64) | 110.25 (13.43) | .31 |
| Abstract reasoning IQ, mean (SD) | 106.60 (16.70) | 107.34 (14.04) | .82 |
| Substance use | |||
|
| |||
| Cigarette use, mean (SD) | 0.43 (1.17) | 0.40 (1.18) | .92 |
| Alcohol use, mean (SD) | 2.25 (1.84) | 1.90 (1.84) | .37 |
| Cannabis use, mean (SD) | 0.00 (0.00) | 0.08 (0.48) | .36 |
Abbreviations: SD, standard deviation; PLEs, Psychotic-like experiences.
All p-values in the table are 2-tailed, uncorrected.
Unless specified byb, t-tests were used for comparing group means. When specified byb, Chi-squared tests were used to compare proportions for categorical variables.
Task activation differences between the groups
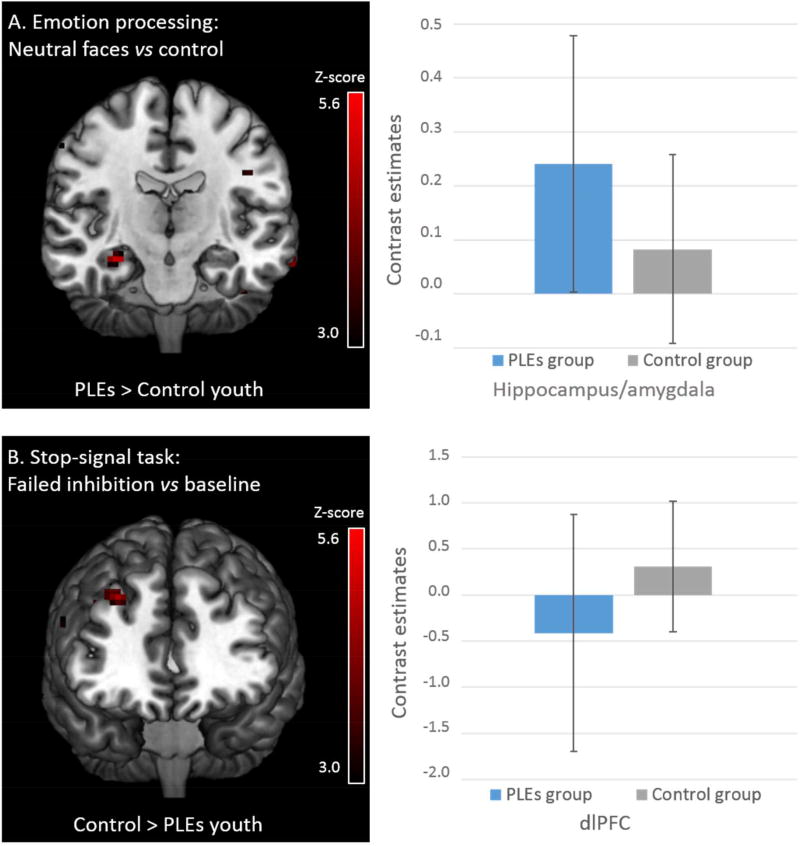
Between-group differences were present in small clusters in the three tasks (Table 2). Only two significant clusters of activity differences survived the cluster-corrected threshold of 24 contiguous voxels: a hyperactivation of the right anterior hippocampus/amygdala during passive viewing of neutral/ambiguous faces and a reduced activity in the right dlPFC during failure to inhibit a motor response in youth with psychotic-like experiences (Figure 1).
Table 2.
Regions showing group differences in the fMRI contrasts
| Regions | Direction | T-Value | MNI coordinates |
Voxels | Effect size (Cohen’s d) |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Faces Task, Angry > Neutral contrast | |||||||
|
| |||||||
| Cerebellum L | CT > PLEs | 3.58 | −3 | −79 | −38 | 10 | |
|
| |||||||
| Faces Task, Neutral > Reference | |||||||
|
| |||||||
| Hippocampus/amygdala R | PLEs > CT | 4.68 | 30 | −13 | −17 | 25* | 0.987 |
| Middle temporal gyrus R | Temporal | PLEs > CT | 4.19 | 54 | 5 | −20 | 10 | |
| Cerebellum L | PLEs > CT | 3.88 | −42 | −49 | −32 | 11 | |
| Inferior frontal gyrus, orbital part L | CT > PLEs | 4.18 | −30 | 32 | −20 | 13 | |
| Lingual gyrus R | CT > PLEs | 4.15 | 21 | −55 | −5 | 12 | |
| Fusiform gyrus L | CT > PLEs | 4.14 | −33 | −31 | −17 | 12 | |
|
| |||||||
| Stop-Signal Task, Stop success > Baseline | |||||||
|
| |||||||
| No significant activation difference | |||||||
|
| |||||||
| Stop-Signal Task, Stop failure > Baseline | |||||||
|
| |||||||
| Middle frontal gyrus R | CT > PLEs | 4.65 | 30 | 38 | 40 | 37* | 0.980 |
| Caudate nucleus L | CT > PLEs | 4.41 | −15 | 8 | 19 | 10 | |
|
| |||||||
| Monetary Incentive Delay Task, Anticipation large win > No win | |||||||
|
| |||||||
| Anterior/middle cingulate gyrus R | PLEs > CT | 3.77 | 3 | 29 | 31 | 13 | |
| Fusiform gyrus L | CT > PLEs | 4.40 | −27 | −37 | −23 | 15 | |
Abbreviations: MNI, Montreal Neurological Institute space; L, left; R, right; CT, Control group reporting no significant psychotic-like experiences; PLEs, Group reporting significant psychotic-like experiences.
Cluster presented are corrected at p<0.05 according to AFNI 3dClustSim. Other brain regions presented survived the more liberal cluster threshold of ≥ 10 contiguous voxels.
Figure 1.
Cluster-corrected activation differences between 14-year olds with (N=27) and without (N=135) psychotic-like experiences.
Abbreviations: PLEs, Psychotic-like experiences.
Only cluster-corrected activations are shown in the maps. Bar graphs refer to standard deviations (SD).
Prediction of psychotic-related symptoms at age 16
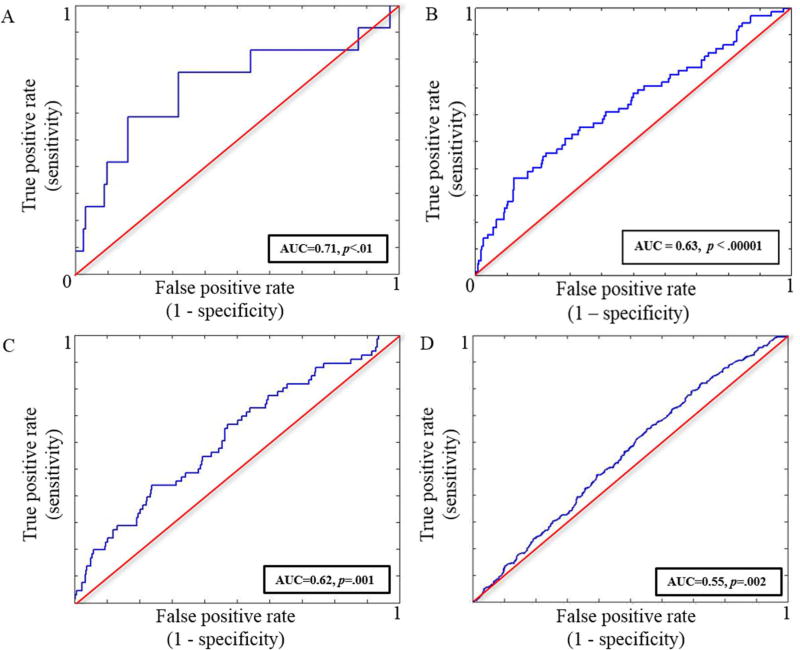
First, from the London-Dublin subsample, we classified N=12 youths reporting both mood- and psychotic-related symptoms at 16 from N=154 youths reporting no mood symptoms. The final models returned from this analysis had a mean AUC=.709 (95% CI=.706–.713, p<.01) (Figure 2A). This model included all brain regions that survived the more liberal threshold of 10 contiguous voxels (all regions reported in Table 2), and controlled for psychotic-like experiences’ score at age 14 as well as demographic information (i.e., age, sex, handedness and site). All features were present in at least 9 folds (out of 10) of the final model. In addition to psychotic-like experiences, the most robust brain classifiers were cerebellum activity during processing of angry faces and the hippocampus/amygdala activity during neutral faces processing (Table 3A). The performance of each domain on its own (i.e., brain activity vs psychotic-like experiences) is displayed in Fig S1. We could not significantly classify youth with mood only symptoms (N=80) from the other two groups (i.e. mood and psychotic symptoms group, no mood symptoms group) (AUC=.532, 95% CI=.525–.539, p=.36; AUC=.453, 95% CI=.450–.456, p=.90 respectively).
Figure 2.
Receiver-operating characteristics (ROC) curves
A, ROC of age 16 classification between youth from the London-Dublin subsample with mood and psychosis symptoms and those with no mood symptoms
B, ROC of age 16 classification between youth from the full sample with mood and psychosis symptoms and those with no mood symptoms
C, ROC of age 16 classification between youth from the full sample with mood and psychosis symptoms and those with mood symptoms only
D, ROC of age 16 classification between youth from the full sample with mood symptoms only and those with no mood symptoms
Table 3.
Beta weights for prediction of age 16 outcome
| A | |
|---|---|
|
| |
| Predictors | Mean Betas |
| Demographic information | |
|
| |
| Age | 0.101 |
| Sex (male) | −0.079 |
| Site | 0.105 |
| Handedness (right-handed) | −0.182 |
| Symptoms | |
|
| |
| Psychotic-like experiences at baseline | 0.577 |
| Brain ROIs | |
|
| |
| dlPFC during failed inhibition | 0.021 |
| Caudate during failed inhibition | −0.088 |
| Cerebellum during angry faces processing | 0.276 |
| Hippocampus/amygdala during neutral faces processing | 0.253 |
| Middle temporal during neutral faces processing | 0.070 |
| Cerebellum during neutral faces processing | −0.234 |
| Inferior frontal during neutral faces processing | −0.126 |
| Lingual gyrus during neutral faces processing | −0.142 |
| Fusiform gyrus during neutral faces processing | −0.077 |
| ACC/MCC during anticipation of reward | 0.066 |
| Fusiform gyrus during anticipation of reward | −0.216 |
| B | |
|---|---|
|
| |
| Predictors | Mean Betas |
| Demographic and substance use information | |
|
| |
| Cannabis use in the previous year | 0.137 |
| Lifetime cigarette use | 0.150 |
| Symptoms | |
|
| |
| Internalizing behaviors | 0.307 |
| Externalizing behaviors | 0.084 |
| Brain ROIs | |
|
| |
| Cerebellum during angry faces processing | 0.092 |
| Hippocampus/amygdala during neutral faces processing | 0.073 |
| Cerebellum during neutral faces processing | −0.090 |
| C | |
|---|---|
|
| |
| Predictors | Mean Betas |
| Demographic and substance use information | |
|
| |
| Age | 0.027 |
| Sex (male) | 0.188 |
| Site | 0.023 |
| Handedness (right-handed) | −0.020 |
| Puberty development score | 0.077 |
| Cannabis use in the previous year | 0.250 |
| Lifetime cigarette use | 0.268 |
| Alcohol use in the previous year | −0.171 |
| Symptoms | |
|
| |
| Internalizing behaviors | 0.297 |
| Externalizing behaviors | 0.098 |
| Brain ROIs | |
|
| |
| dlPFC during failed inhibition | 0.100 |
| Caudate during failed inhibition | −0.142 |
| Cerebellum during angry faces processing | 0.317 |
| Hippocampus/amygdala during neutral faces processing | 0.192 |
| Middle temporal during neutral faces processing | 0.100 |
| Cerebellum during neutral faces processing | −0.066 |
| Inferior frontal during neutral faces processing | −0.101 |
| Lingual gyrus during neutral faces processing | −0.158 |
| Fusiform gyrus during neutral faces processing | −0.135 |
| ACC/MCC during anticipation of reward | −0.005 |
| Fusiform gyrus during anticipation of reward | −0.306 |
| D | |
|---|---|
|
| |
| Predictors | Mean Betas |
| Demographic information | |
|
| |
| Age | −0.024 |
| Sex (male) | −0.053 |
| Site | −0.097 |
| Handedness (right-handed) | 0.041 |
| Puberty development score | 0.096 |
| Cannabis use in the previous year | 0.140 |
| Lifetime cigarette use | −0.057 |
| Alcohol use in the previous year | 0.058 |
| Symptoms | |
|
| |
| Internalizing behaviors | 0.154 |
| Externalizing behaviors | 0.100 |
| Brain ROIs | |
|
| |
| Caudate during failed inhibition | 0.062 |
| Cerebellum during angry faces processing | −0.083 |
| Hippocampus/amygdala during neutral faces processing | −0.029 |
| Middle temporal during neutral faces processing | −0.025 |
| Cerebellum during neutral faces processing | −0.132 |
| Inferior frontal during neutral faces processing | 0.036 |
| Lingual gyrus during neutral faces processing | 0.071 |
| Fusiform gyrus during neutral faces processing | 0.039 |
| ACC/MCC during anticipation of reward | 0.025 |
| Fusiform gyrus during anticipation of reward | 0.088 |
Abbreviations: dlPFC, dorsolateral prefrontal cortex; ACC/MCC, anterior/middle cingulate cortex.
A, Mean beta weights (averaged over 10 outer folds) of the features that were present in at least 9 folds (out of 10) of the final model for classification of the mood and psychotic symptoms group versus the no mood symptoms group in the London-Dublin subsample. B, Mean beta weights (averaged over 10 outer folds) of the features that were present in at least 9 folds (out of 10) of the final model for classification of the mood and psychotic symptoms group versus the no mood symptoms group in the full IMAGEN sample. C, Mean beta weights (averaged over 10 outer folds) of the features that were present in at least 9 folds (out of 10) of the final model for classification of the mood and psychotic symptoms group versus the mood symptoms only group in the full IMAGEN sample. D, Mean beta weights (averaged over 10 outer folds) of the features that were present in at least 9 folds (out of 10) of the final model for classification of the mood symptoms only group versus the no mood symptoms group in the full IMAGEN sample.
In the second prediction analyses, using the full IMAGEN sample, we classified N=72 youths reporting both mood- and psychotic-related symptoms at age 16 from N=673 youths reporting no mood symptoms and from N=451 youths with mood symptoms only. The final models returned from this analysis had a mean AUC=.633 (95% CI=.630–.636, p<.0001) and AUC=.615 (95% CI=.614–.617, p=.001) respectively (Figure 2B, 2C). This model included all brain regions that survived the more liberal threshold of 10 contiguous voxels, and controlled for internalizing and externalizing behaviors, cigarette, alcohol and cannabis use, as well as demographic information (i.e. puberty development index, handedness, age, sex and site). When classifying the mood and psychotic symptoms group relative to the no mood symptoms group, only the following features: internalizing and externalizing behaviors, cigarette and cannabis use, hippocampus/amygdala and cerebellum activity during neutral faces processing as well as cerebellum activity during angry faces processing were present in at least 9 folds of the final model (Table 3B). However, when classifying the mood and psychotic group relative to the mood only group, all features were present in at least 9 folds of the final model, with cerebellum activity during angry faces processing, fusiform activity during anticipation of reward, internalizing behaviors, cigarette and cannabis use, and hippocampus/amygdala activity during neutral face processing making the strongest contribution to group classification (Table 3C).
Finally, we classified N=451 individuals with mood symptoms only from N=673 individuals reporting no mood symptoms with a mean AUC=.553 (95% CI=.552–.553, p=.002) barely better than chance (Figure 2D). All features, except the dlPFC activity during failed response inhibition, were present in at least 9 folds of the final model. The most important classifiers were internalizing and externalizing behaviors, cannabis use, reduced activity from the cerebellum during neutral faces processing, puberty development scale, and site (Table 3D).
DISCUSSION
At age 14, across the brain networks implicated in emotion processing, response inhibition and reward anticipation, the cluster-corrected markers of psychotic-like experiences included an increased response from the hippocampus/amygdala during processing of neutral material as well as reduced activity from the dlPFC during failed inhibition. Of note, hyperactivity from the hippocampus/amygdala during the processing of neutral faces further discriminated at 2-year follow-up individuals with mood- and psychotic-related symptoms relative to the other groups in both the London-Dublin subsample and the full IMAGEN sample, even when controlling for baseline psychotic-like experiences as well as cannabis and cigarette use. The cross-validation models best discriminated the mood and psychotic group from the no mood symptoms group in comparison to the mood only group from the no mood symptoms group.
One of the most replicated neural markers of psychosis and high-risk states is hypofunctioning of PFC and dlPFC during executive functioning (32). Our results support findings from other community-based studies of youth reporting psychosis-spectrum symptoms showing reduced PFC activity during working memory and response inhibition tasks (9, 23). However, the activity of the dlPFC during the Stop-Signal task was a weak brain classifier for adolescents reporting both mood and psychotic symptoms relative to the other groups. A possible explanation might be that reduced dlPFC activation is not directly related to positive or mood symptoms, but more to disorganized symptoms or cognitive deficits (which were not assessed by our screening tools) (9, 33). Consequently, dlPFC alterations would appear to be a promising neuro-functional marker of the clinical risk for psychosis when, in addition to positive and negative symptoms, significant cognitive impairments are observed; not of youth reporting psychotic-like experiences prior to a cognitive decline. It is worth mentioning that the use of a working memory task instead of response inhibition could have yielded more significant dlPFC results considering that working memory paradigms, in comparison to Stroop tasks or Go-Nogos, consistently elicit a more widespread loci of significant activation in the dLPFC and anterior cingulate cortex in both healthy controls and schizophrenia patients (11).
The current exploratory study stresses the importance of an observed increased activity in the limbic network in the extended psychosis phenotype. Both fMRI and perfusion studies have highlighted increased hippocampal activity at rest and across cognitive tasks in clinically at-risk individuals (32, 34). Interestingly, Schobel et al. (35) demonstrated that baseline hypermetabolism of the hippocampus in clinical high-risk individuals is directly related to a subsequent volume loss (via a hyperglutamatergic state); thereby supporting the heightened hippocampus activity as a highly promising early vulnerability marker to psychotic disorders. In the context of emotion processing, a recent meta-analysis showed that the apparent deficit in amygdala activity observed in individuals with a psychotic disorder during the viewing of negative material may be explained by an elevated amygdala response to neutral material (15). These findings have led some authors to propose that abnormalities in salience attribution might be core to the extended psychosis phenotype, rather than stress reactivity, per se (36). Thus, the increased neural response to neutral information may reflect an atypical assignment of motivational salience to these stimuli (37). Results from other cognitive studies showing an impaired decoding of facial expressions in patients with psychosis and high-risk populations further suggest that the abnormal neural activity in the current study might be due to an erroneous identification of neutral faces specifically. For instance, children and adolescents reporting psychotic-like experiences over-attribute significance (i.e. negative valence) to neutral faces (38). Considering that impaired emotion recognition is linked to declining social functioning in high-risk populations (39), it represents a potential target for psychosis symptoms prevention strategies for at-risk youth, prior to subsequent impaired social functioning.
Considering that cerebellar activity significantly contributed to the classification of youth with mood- and psychotic-related symptoms relative to the other groups even in the absence of a marked alteration in individuals reporting psychotic-like experiences, its role in emotion processing in the psychosis-spectrum remains elusive but deserves to be clarified in the future.
No cluster-corrected activity difference between 14-year olds with and without psychotic-like experiences were observed during reward anticipation. Even when using a more liberal cluster threshold, significant activity related to reward anticipation did not robustly contribute to discriminate the groups at age 16. These findings are inconsistent with recent fMRI studies showing a blunted response from the ventral striatum during reward processing in psychosis and high-risk individuals (18, 40). A possible explanation for this negative result may be explained by Radua et al.’s findings of a negative correlation between striatal activity and the severity of negative symptoms in both patients and individuals at clinical risk for psychosis (18). Here, only positive experiences/symptoms were assessed.
Limitations
The use of an extended risk phenotype (i.e. youths self-reporting psychotic-like experiences) may constitute both a strength and weakness. While it might be too liberal to predict vulnerability to specific disorders, particularly those with very low prevalence, one advantage of this approach is that it might capture a dimension of vulnerability that is implicated in a number of different psychopathological outcomes. The current study also did not investigate interactions with family, substance misuse and genetic data, which might further clarify how this extended phenotype is implicated in future psychiatric outcomes. Another potential limitation to the study is that the use of the bipolar module, at age 16, may have under-estimated the emergence of psychotic symptoms in the no mood symptoms group. However, the prevalence of psychotic symptoms is low at the end of adolescence (e.g. 5–7%) (1). Finally, the timeframe for studying outcomes was relatively brief and might predate the typical age of onset of psychotic disorders, however, this might also be considered a strength, as we were able to detect relevant brain-related abnormalities before psychotic experiences begin to cause significant functional and cognitive impairment, substance misuse and require medical intervention.
Conclusion
The results of the present study suggest that an aberrant neural response to non-salient stimuli may be an important early vulnerability marker for psychosis, at least in the context of mood fluctuations. These findings might help to guide early intervention strategies for at-risk youth. It has yet to be determined whether individual differences in emotional reactivity to non-salient stimuli can be modified in young adolescents and whether such modifications have any clinical significance for high-risk youth.
Supplementary Material
Acknowledgments
Dr. Banaschewski has served as an advisor or consultant to Bristol-Myers Squibb, Desitin Arzneimittel, Eli Lilly, Medice, Novartis, Pfizer, Shire, UCB, and Vifor Pharma; he has received conference attendance support, conference support, or speaking fees from Eli Lilly, Janssen McNeil, Medice, Novartis, Shire, and UCB; and he is involved in clinical trials conducted by Eli Lilly, Novartis, and Shire; the present work is unrelated to these relationships. Dr. Gallinat has received research funding from the German Federal Ministry of Education and Research, AstraZeneca, Eli Lilly, Janssen-Cilag, and Bristol-Myers Squibb; he has received speaking fees from AstraZeneca, Janssen-Cilag, and Bristol-Myers Squibb. Dr Barker has received honoraria from General Electric for teaching on scanner programming courses.
This work received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behaviour in normal brain function and psychopathology) (LSHM-CT-2007-037286), the Horizon 2020 funded ERC Advanced Grant ‘STRATIFY’ (Brain network based stratification of reinforcement-related disorders) (695313), ERANID (Understanding the Interplay between Cultural, Biological and Subjective Factors in Drug Use Pathways) (PR-ST-0416-10004), BRIDGET (JPND: BRain Imaging, cognition Dementia and next generation GEnomics) (MR/N027558/1), the FP7 projects IMAGEMEND(602450; IMAging GEnetics for MENtal Disorders) and MATRICS (603016), the Innovative Medicine Initiative Project EU-AIMS (115300-2), the Medical Research Council Grant 'c-VEDA’ (Consortium on Vulnerability to Externalizing Disorders and Addictions) (MR/N000390/1), the Swedish Research Council FORMAS, the Medical Research Council, the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; eMED SysAlc01ZX1311A; Forschungsnetz AERIAL), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-1, SM 80/7-2, SFB 940/1). Further support was provided by grants from: ANR (project AF12-NEUR0008-01 - WM2NA, and ANR-12-SAMA-0004), the Fondation de France, the Fondation pour la Recherche Médicale, the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012; the National Institutes of Health, U.S.A. (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1), and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence.
Footnotes
Previous presentation: Portions of this study were presented as a poster presentation at the Organization for Human Brain Mapping (OHBM); June 26–30 2016, Geneva, Switzerland.
Disclosures: The other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 2.McGrath JJ, Saha S, Al-Hamzawi A, Andrade L, Benjet C, Bromet EJ, Browne MO, Caldas de Almeida JM, Chiu WT, Demyttenaere K, Fayyad J, Florescu S, de Girolamo G, Gureje O, Haro JM, Ten Have M, Hu C, Kovess-Masfety V, Lim CC, Navarro-Mateu F, Sampson N, Posada-Villa J, Kendler KS, Kessler RC. The Bidirectional Associations Between Psychotic Experiences and DSM-IV Mental Disorders. Am J Psychiatry. 2016;173:997–1006. doi: 10.1176/appi.ajp.2016.15101293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yung AR, Nelson B, Thompson AD, Wood SJ. Should a "Risk Syndrome for Psychosis" be included in the DSMV? Schizophr Res. 2010;120:7–15. doi: 10.1016/j.schres.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, Barale F, Caverzasi E, McGuire P. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 5.Lin A, Wood SJ, Nelson B, Beavan A, McGorry P, Yung AR. Outcomes of nontransitioned cases in a sample at ultra-high risk for psychosis. Am J Psychiatry. 2015;172:249–258. doi: 10.1176/appi.ajp.2014.13030418. [DOI] [PubMed] [Google Scholar]
- 6.Kelleher I, Keeley H, Corcoran P, Lynch F, Fitzpatrick C, Devlin N, Molloy C, Roddy S, Clarke MC, Harley M, Arseneault L, Wasserman C, Carli V, Sarchiapone M, Hoven C, Wasserman D, Cannon M. Clinicopathological significance of psychotic experiences in non-psychotic young people: evidence from four population-based studies. Br J Psychiatry. 2012;201:26–32. doi: 10.1192/bjp.bp.111.101543. [DOI] [PubMed] [Google Scholar]
- 7.Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children's self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57:1053–1058. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- 8.Mackie CJ, O'Leary-Barrett M, Al-Khudhairy N, Castellanos-Ryan N, Struve M, Topper L, Conrod P. Adolescent bullying, cannabis use and emerging psychotic experiences: a longitudinal general population study. Psychol Med. 2013;43:1033–1044. doi: 10.1017/S003329171200205X. [DOI] [PubMed] [Google Scholar]
- 9.Wolf DH, Satterthwaite TD, Calkins ME, Ruparel K, Elliott MA, Hopson RD, Jackson CT, Prabhakaran K, Bilker WB, Hakonarson H, Gur RC, Gur RE. Functional Neuroimaging Abnormalities in Youth With Psychosis Spectrum Symptoms. JAMA Psychiatry. 2015;72:456–465. doi: 10.1001/jamapsychiatry.2014.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colibazzi T, Horga G, Wang Z, Huo Y, Corcoran C, Klahr K, Brucato G, Girgis R, Gill K, Abi-Dargham A, Peterson BS. Neural Dysfunction in Cognitive Control Circuits in Persons at Clinical High-Risk for Psychosis. Neuropsychopharmacology. 2016;41:1241–1250. doi: 10.1038/npp.2015.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keener MT, Phillips ML. Neuroimaging in bipolar disorder: a critical review of current findings. Curr Psychiatry Rep. 2007;9:512–520. doi: 10.1007/s11920-007-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O, Stieglitz RD, Vita A, McGuire P, Borgwardt S. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69:562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- 14.Modinos G, Tseng HH, Falkenberg I, Samson C, McGuire P, Allen P. Neural correlates of aberrant emotional salience predict psychotic symptoms and global functioning in high-risk and first-episode psychosis. Soc Cogn Affect Neurosci. 2015;10:1429–1436. doi: 10.1093/scan/nsv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anticevic A, Van Snellenberg JX, Cohen RE, Repovs G, Dowd EC, Barch DM. Amygdala recruitment in schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging studies. Schizophr Bull. 2012;38:608–621. doi: 10.1093/schbul/sbq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seiferth NY, Pauly K, Habel U, Kellermann T, Shah NJ, Ruhrmann S, Klosterkotter J, Schneider F, Kircher T. Increased neural response related to neutral faces in individuals at risk for psychosis. Neuroimage. 2008;40:289–297. doi: 10.1016/j.neuroimage.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radua J, Schmidt A, Borgwardt S, Heinz A, Schlagenhauf F, McGuire P, Fusar-Poli P. Ventral Striatal Activation During Reward Processing in Psychosis: A Neurofunctional Meta-Analysis. JAMA Psychiatry. 2015;72:1243–1251. doi: 10.1001/jamapsychiatry.2015.2196. [DOI] [PubMed] [Google Scholar]
- 19.Wotruba D, Heekeren K, Michels L, Buechler R, Simon JJ, Theodoridou A, Kollias S, Rossler W, Kaiser S. Symptom dimensions are associated with reward processing in unmedicated persons at risk for psychosis. Front Behav Neurosci. 2014;8:382. doi: 10.3389/fnbeh.2014.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juckel G, Friedel E, Koslowski M, Witthaus H, Ozgurdal S, Gudlowski Y, Knutson B, Wrase J, Brune M, Heinz A, Schlagenhauf F. Ventral striatal activation during reward processing in subjects with ultra-high risk for schizophrenia. Neuropsychobiology. 2012;66:50–56. doi: 10.1159/000337130. [DOI] [PubMed] [Google Scholar]
- 21.Modinos G, Ormel J, Aleman A. Altered activation and functional connectivity of neural systems supporting cognitive control of emotion in psychosis proneness. Schizophr Res. 2010;118:88–97. doi: 10.1016/j.schres.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Modinos G, Pettersson-Yeo W, Allen P, McGuire PK, Aleman A, Mechelli A. Multivariate pattern classification reveals differential brain activation during emotional processing in individuals with psychosis proneness. Neuroimage. 2012;59:3033–3041. doi: 10.1016/j.neuroimage.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson S, Kelleher I, Harley M, Murtagh A, Clarke M, Blanchard M, Connolly C, O'Hanlon E, Garavan H, Cannon M. Structural and functional brain correlates of subclinical psychotic symptoms in 11-13 year old schoolchildren. Neuroimage. 2010;49:1875–1885. doi: 10.1016/j.neuroimage.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Buchel C, Conrod PJ, Dalley JW, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Mallik C, Mann K, Martinot JL, Paus T, Poline JB, Robbins TW, Rietschel M, Reed L, Smolka M, Spanagel R, Speiser C, Stephens DN, Strohle A, Struve M, consortium I. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15:1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- 25.Kelleher I, Harley M, Murtagh A, Cannon M. Are screening instruments valid for psychotic-like experiences? A validation study of screening questions for psychotic-like experiences using in-depth clinical interview. Schizophr Bull. 2011;37:362–369. doi: 10.1093/schbul/sbp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000;41:645–655. [PubMed] [Google Scholar]
- 27.Grosbras MH, Paus T. Brain networks involved in viewing angry hands or faces. Cereb Cortex. 2006;16:1087–1096. doi: 10.1093/cercor/bhj050. [DOI] [PubMed] [Google Scholar]
- 28.Chevrier AD, Noseworthy MD, Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Hum Brain Mapp. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman R, Renfrew D, Mullick M. Predicting type of psychiatric disorder from Strengths and Difficulties Questionnaire (SDQ) scores in child mental health clinics in London and Dhaka. Eur Child Adolesc Psychiatry. 2000;9:129–134. doi: 10.1007/s007870050008. [DOI] [PubMed] [Google Scholar]
- 32.Fusar-Poli P, Perez J, Broome M, Borgwardt S, Placentino A, Caverzasi E, Cortesi M, Veggiotti P, Politi P, Barale F, McGuire P. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2007;31:465– 484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Goghari VM, Sponheim SR, MacDonald AW., 3rd The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci Biobehav Rev. 2010;34:468–486. doi: 10.1016/j.neubiorev.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen P, Chaddock CA, Egerton A, Howes OD, Bonoldi I, Zelaya F, Bhattacharyya S, Murray R, McGuire P. Resting Hyperperfusion of the Hippocampus, Midbrain, and Basal Ganglia in People at High Risk for Psychosis. Am J Psychiatry. 2016;173:392–399. doi: 10.1176/appi.ajp.2015.15040485. [DOI] [PubMed] [Google Scholar]
- 35.Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, Inbar BP, Corcoran CM, Lieberman JA, Moore H, Small SA. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reininghaus U, Kempton MJ, Valmaggia L, Craig TK, Garety P, Onyejiaka A, Gayer-Anderson C, So SH, Hubbard K, Beards S, Dazzan P, Pariante C, Mondelli V, Fisher HL, Mills JG, Viechtbauer W, McGuire P, van Os J, Murray RM, Wykes T, Myin-Germeys I, Morgan C. Stress Sensitivity, Aberrant Salience, and Threat Anticipation in Early Psychosis: An Experience Sampling Study. Schizophr Bull. 2016;42:712–722. doi: 10.1093/schbul/sbv190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 38.Dickson H, Calkins ME, Kohler CG, Hodgins S, Laurens KR. Misperceptions of facial emotions among youth aged 9-14 years who present multiple antecedents of schizophrenia. Schizophr Bull. 2014;40:460–468. doi: 10.1093/schbul/sbs193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Rijn S, Aleman A, de Sonneville L, Sprong M, Ziermans T, Schothorst P, van Engeland H, Swaab H. Misattribution of facial expressions of emotion in adolescents at increased risk of psychosis: the role of inhibitory control. Psychol Med. 2011;41:499–508. doi: 10.1017/S0033291710000929. [DOI] [PubMed] [Google Scholar]
- 40.Lancaster TM, Linden DE, Tansey KE, Banaschewski T, Bokde AL, Bromberg U, Buchel C, Cattrell A, Conrod PJ, Flor H, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Martinot JL, Paillere Martinot ML, Artiges E, Lemaitre H, Nees F, Orfanos DP, Paus T, Poustka L, Smolka MN, Vetter NC, Jurk S, Mennigen E, Walter H, Whelan R, Schumann G, Consortium I. Polygenic Risk of Psychosis and Ventral Striatal Activation During Reward Processing in Healthy Adolescents. JAMA Psychiatry. 2016;73:852–861. doi: 10.1001/jamapsychiatry.2016.1135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.