Abstract
Objective
To determine if maternal plasma concentrations of angiogenic and anti-angiogenic factors measured at 24–28 weeks of gestation can predict subsequent fetal death.
Methods
A case-cohort study was designed to include 1000 randomly selected subjects and all remaining fetal deaths (cases) from a cohort of 4006 women with a singleton pregnancy, enrolled at 6–22 weeks of gestation, in a pregnancy biomarker cohort study. The placentas of all fetal deaths were histologically examined by pathologists who used a standardized protocol and were blinded to patient outcomes. Placental growth factor (PlGF), soluble endoglin (sEng), and soluble vascular endothelial growth factor receptor-1 (sVEGFR-1) concentrations were measured by ELISA assays. Quantiles of the analyte concentrations (or concentration ratios) were estimated as a function of gestational age among women who delivered live neonates but did not develop preeclampsia or deliver small-for-gestational–age (SGA) newborns. A positive test was defined as analyte concentrations (or ratios) <2.5th and 10th centiles [PlGF, PlGF/sVEGFR-1 (angiogenic index-1), and PlGF/sEng)] or >90th and 97.5th centiles (sVEGFR-1 and sEng). Inverse probability weighting was used to reflect the parent cohort when estimating the relative risk.
Results
There were 11 fetal deaths and 829 controls with samples available for analysis between 24–28 weeks of gestation. Three fetal deaths occurred prior to 28 weeks and eight at or after 28 weeks of gestation. The rate of placental lesions consistent with maternal vascular underperfusion was 33.3% (1/3) among those who had a fetal death before 28 weeks and 87.5% (7/8) of those who had this complication at or after 28 weeks of gestation.
The maternal plasma angiogenic index-1 value was below the 10th centile in 63.6% (7/11) of the fetal death group and in 11.1% (92/829) of the controls. The angiogenic index-1 value was <2.5th centile in 54.5% (6/11) of the fetal death group and in 3.7% (31/829) of the controls. An angiogenic index-1 value <2.5th centile had the largest positive likelihood ratio for predicting fetal death >24 weeks (14.6; 95% CI, 7.7–27.7) and a relative risk of 29.1 (95% CI, 8.8–97.1), followed by sEng >97.5thcentile and PlGF/sEng <2.5th, both with a positive likelihood ratio of 13.7 (95% CI, 7.3–25.8) and a relative risk of 27.4 (95% CI, 8.2–91.2).
Among women without a fetal death whose plasma angiogenic index-1 concentration ratio was below the 2.5th centile, 61% (19/31) developed preeclampsia or delivered an SGA neonate; when the 10th centile was used as the cut-off, 37% (34/92) of women had these adverse outcomes.
Conclusions
1) A maternal plasma angiogenic index-1 value below the 2.5th centile (0.126) at 24–28 weeks of gestation carries a 29-fold increase in the risk of subsequent fetal death and identifies 55% of subsequent fetal deaths with a false-positive rate of 3.5%; and 2) 61% of women who have a false-positive test result will subsequently experience adverse pregnancy outcomes.
Keywords: Endoglin, maternal vascular underperfusion, preeclampsia, preterm delivery, placenta, placental growth factor (PlGF), small for gestational age (SGA), soluble vascular endothelial growth factor receptor-1 (sVEGFR-1), soluble fms-like tyrosine kinase-1 (sFLT-1)
Introduction
Fetal death is an obstetrical syndrome 1, 2 caused by multiple etiologies rather than the end stage of a single disease process 3–6. This syndrome affected 23,595 pregnancies in the United States 7 in 2013, and according to the World Health Organization (WHO), an estimated 2.6 million fetal deaths occurred globally during the third trimester 8, 9. The most common type of fetal death is unexplained stillbirth 10–13, which comprises a progressively larger proportion of all fetal deaths as pregnancy advances. This category of fetal death accounts for approximately 20% of all cases just after 20 weeks of gestation and for about 40% of all cases at term10–15, while infection and congenital anomalies cause most early fetal deaths (<28 weeks of gestation) 16–19, placental causes leading to fetal death are more frequent after 26 weeks of gestation 14 including placental vascular underperfusion, resulting in an impaired supply of nutrients to the fetus, 20–35 abruptio placentae 36–38, and placental senescence that has been implicated as a mechanism of fetal death at term. 22
The term “placental malperfusion” (formerly called “maternal vascular lesions of underperfusion”) refers to a group of vascular lesions, including villous infarcts, syncytial knots, villous agglutination, increased intervillous fibrin deposition, villous hypoplasia, persistent muscularization of the basal plate arteries, and mural hypertrophy of the decidual arterioles as well as acute atherosis of the basal plate and decidual arteries 39–43. The frequency and burden of placental vascular lesions of malperfusion is reflected in the maternal plasma by an imbalance between the concentration of angiogenic [placental growth factor (PlGF)] and anti-angiogenic factors [soluble vascular endothelial growth factor receptor-1 (sVEGFR-1) and soluble endoglin (sEng)]44.
Several investigators 45–47 have characterized the changes in the plasma concentrations of angiogenic and anti-angiogenic factors in women who subsequently had a fetal death, demonstrating that, from 20 weeks of gestation onward, these patients had a lower maternal plasma PlGF and higher sEng and sVEGFR-1 concentrations than women with a normal pregnancy 45. Additionally, a maternal plasma PIGF/sVEGFR-1 concentration ratio (angiogenic index-1) of less than 0.12 multiples of the median at 30–34 weeks of gestation in normal pregnancy—which corresponds to the 5th–6th centile of the distribution—identified 4 of 5 fetal deaths that occurred later in pregnancy 48. Given that an imbalance in angiogenic and anti-angiogenic factors reflects the presence and burden of placental vascular lesions44 of malperfusion that have been proposed to be the leading cause of fetal death in the late second and third trimesters until term14, we sought to validate and extend these findings by determining whether plasma angiogenic index-1 at 24–28 weeks of gestation could be used as a biomarker to identify patients at risk for a subsequent fetal death.
Methods
Study Design & Participants
This was a case-cohort study. We randomly selected 1,000 patients from a cohort of 4,006 pregnant women enrolled in a longitudinal study previously reported by our group.44 The remaining women in the original cohort who had a fetal death, but were not selected in the random sample of 1,000 women, were subsequently added to the case-cohort. Women who had multiple gestations or any of the following conditions at the time of enrollment were excluded from this study: active vaginal bleeding; severe maternal morbidity (i.e., renal insufficiency, congestive heart disease, and chronic respiratory insufficiency); chronic hypertension requiring medication; asthma requiring systemic steroids; requirement of anti-platelet or non-steroidal anti-inflammatory drugs; active hepatitis; or fetal chromosomal abnormalities and congenital anomalies. All study participants provided written informed consent and were followed until delivery. The use of clinical data and biological specimens obtained from these women for research purposes was approved by the Institutional Review Boards of Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS).
Clinical Definitions
The following definitions were used in this study:
Fetal death: diagnosed as the death of the fetus after 20 weeks of gestation and confirmed by ultrasound examination 49;
Preeclampsia: diagnosed by the presence of systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg on at least two occasions, 4 hours to 1 week apart, and of proteinuria ≥300 mg in a 24-hour urine collection or by one dipstick with ≥1+ 50, 51. We have used this definition after analysis from the Collaborative Perinatal Project, which was reported in detail by Friedman and Neff52, and the reasons described by one of the authors53. Moreover, the definition outlined above was the one used in our Center to collect outcome information when the patients were recruited and delivered;
Small-for-gestational-age (SGA) neonate: a birth weight <10th centile for gestational age at delivery according to a U.S. reference population54; and
Preterm delivery: a delivery occurring prior to the 37th week of gestation.
Sample collection and immunoassays
Patients were scheduled to donate maternal plasma in EDTA tubes at enrollment, then every four weeks until the 24th week of gestation, and bi-weekly thereafter until delivery. Samples were centrifuged and stored at −700 C. Maternal plasma concentrations of sVEGFR-1, PlGF, and sEng were measured by immunoassays (R&D Systems, Minneapolis, MN, USA) as previously described 55. The inter- and intra-assay coefficients of variation of the assays were 1.4% and 3.9% for sVEGFR-1, 2.3% and 4.6% for sEng, and 6.02% and 4.8% for PlGF, respectively. The sensitivity of each assay was 16.97 pg/ml for sVEGFR-1, 0.08 ng/ml for sEng, and 9.52 pg/ml for PlGF. Laboratory personnel performing the assays were blinded to the clinical information.
Histologic Placental Examination
Placentas were examined according to standardized protocols by perinatal pathologists blinded to clinical diagnoses and obstetrical outcomes. Placental lesions consistent with maternal vascular lesions of underperfusion (now known as placental malperfusion) were diagnosed using criteria established by the Perinatal Section of the Society for Pediatric Pathology56 and were classified as the following: 1) villous changes, which are further subdivided into abrupt onset (remote villous infarcts, recent villous infarcts), gradual onset with intermediate duration (increased syncytial knots, villous agglutination, increased intervillous fibrin), or gradual onset with prolonged duration (decreased placental weight/increased feto-placental weight ratio, distal villous hypoplasia); and 2) vascular lesions (persistent muscularization of the basal plate arteries, mural hypertrophy of the decidual arterioles, acute atherosis of the basal plate arteries and/or the decidual arterioles).40
Statistical Analysis
Using quantile regression, which does not assume data normality, we estimated the percentiles of maternal plasma concentration of analytes and their ratios in a subset of controls (i.e. women who delivered live babies at term and did not have preeclampsia or an SGA neonate). Data was first log-transformed, a standard approach to improve normality of distribution57 and to reduce the non-linearity of the relation between the response and gestational age at sample collection. Linear quantile regression was then used iteratively to estimate the quantiles at discrete values of gestation, in narrow windows of gestational age, over which the linear assumption holds. The resulting estimated percentiles were not smooth because they were obtained from different models (fitted to the different narrow gestational-age windows); therefore, we fitted a 5th degree polynomial function to smooth the estimated percentiles48, 58. The quantile regression was performed using the quantreg package of the R open source statistical software. 59, 60
Positive tests were defined as analyte concentrations (or their ratios) below the 2.5th and 10th centiles (PlGF, PlGF/sVEGFR-1, and PlGF/sEng) or above the 90th and 97.5th centiles (sVEGFR-1 and sEng). Data from the 11 fetal deaths and 829 controls with available measurements were used to construct Receiver Operating Characteristic (ROC) curves and to determine the sensitivity, specificity, and likelihood ratios (positive and negative). Likelihood ratios for a positive test result above 10 and likelihood ratios for a negative test result below 0.1 were taken as strong predictive evidence under most circumstances. Moderate prediction could be achieved with likelihood ratios of 5–10 and 0.1–0.2, whereas ratios <5 and >0.2 were only minimally predictive.61 Modified Poisson regression was used to estimate relative risk (RR) with 95% confidence intervals (CI)62. To estimate positive predictive values (PPV), negative predictive values (NPV), and RR, we weighted the data by the inverse probability of selection to reflect the parent cohort 63.
For demographic data analysis, we used a Chi-square or Fisher’s exact test and reported proportions for categorical variables, and medians and interquartile ranges (IQR) for continuous variables.
Results
There were 24 fetal deaths (0.6%, 24/4,006): six were initially selected with 994 controls in the random sample of 1,000 women. Following this selection process, we added the remaining 18 patients with a fetal death from the parent cohort to the cases group, reaching a total of 24 patients. Of the 24 fetal deaths, 12 were diagnosed < 28 weeks, and 12 ≥ 28 weeks of gestation.
Table 1 shows the characteristics of the cases and controls.. Cases delivered at a lower median gestational age (p < 0.0001) had lower median birth weights (p < 0.0001). There were no significant differences between cases and controls in median maternal age and the proportion of smokers, nulliparity, and African-American ethnicity.
Table 1.
Descriptive characteristics of THE study participants
| Characteristic | No Fetal Death (n=994) | Fetal Death (n=24) | P |
|---|---|---|---|
| Maternal age, years | 23 (20–27) | 22.5 (20–29.5) | 0.59 |
| Smoker | 206 (20.8) | 5 (20.8) | 1 |
| Nulliparity | 381 (38.7) | 8 (33.3) | 0.64 |
| African Americans | 921 (92.7) | 23 (95.8) | 1 |
| Pre-Pregnancy BMI | 26.6 (22.5–32.5) | 26.8 (23.2–33.3) | 0.54 |
| GA at delivery | 39.1 (37.9–40.1) | 28.3 (23–31.5) | <0.0001 |
| Birthweight | 3172.5 (2800–3485) | 924.5 (493.5–1400) | <0.0001 |
Data presented as median (interquartile range) for continuous variables and number (%) for categorical variables.
Patients with a fetal death had a higher rate of placental lesions consistent with maternal vascular underperfusion than the remainder of the study cohort [fetal death, 58.3% (14/24) versus the remainder of the cohort, 21.3% (206/968), and an odds ratio of 5.2 (2.1–13.2), p=0.0001]. Among women with a fetal death, the rate of placental lesions consistent with maternal vascular underperfusion was 42% (5/12) in those diagnosed prior to 28 weeks of gestation and 75% (9/12) in those diagnosed ≥28 weeks of gestation (Fisher’s exact test, p=0.2).
The Changes in Angiogenic and Anti-Angiogenic Profiles between 24 and 28 Weeks of Gestation in Women with a Subsequent Fetal Death
This analysis included only patients whose blood samples were collected between 24 and 28 weeks of gestation. Seven women had fetal deaths before 24 weeks of gestation and were excluded from further analysis. Additionally, six patients were excluded, as samples were not available at the desired gestational-age interval.
Eleven women with a fetal death were available for further analysis; eight had a fetal death ≥28 weeks and three of these women had a fetal death <28 weeks of gestation. From the remaining case-cohort, blood samples collected at 24–28 weeks of gestation were available from 829 controls.
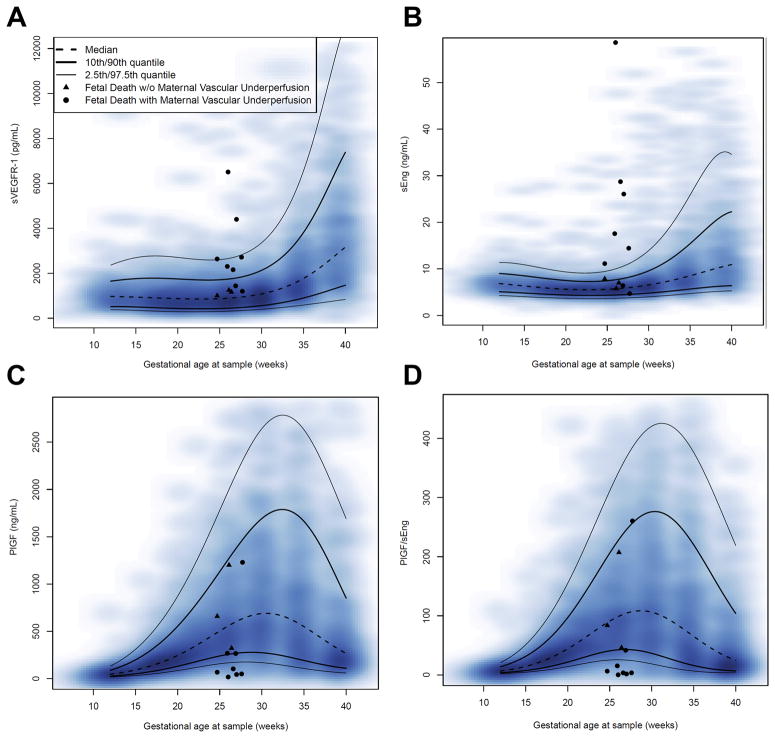
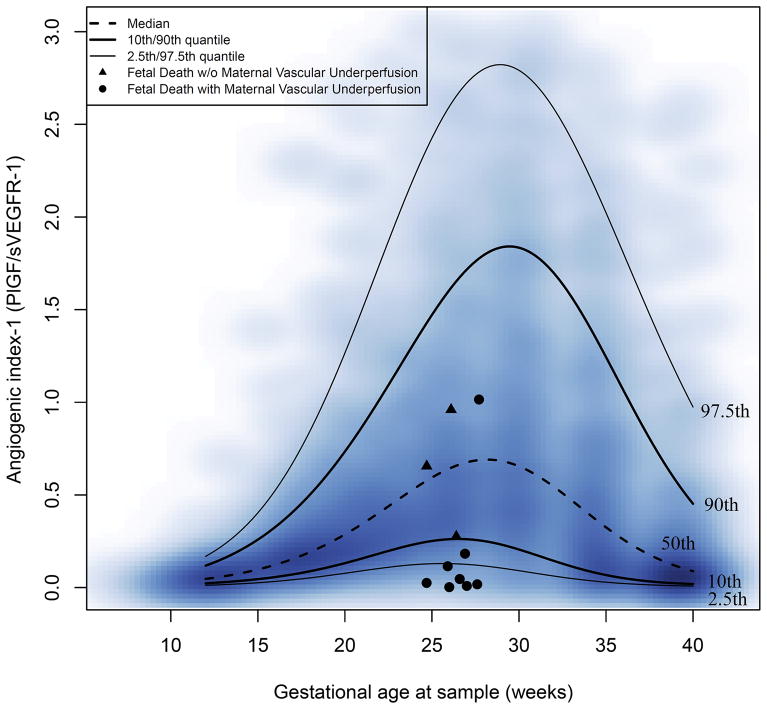
Figure 1(a–d) shows the changes in the maternal plasma soluble VEGFR-1, Eng, PlGF concentrations and the PlGF/ sEng ratio for fetal death cases superimposed on the estimated quantiles for the controls (after excluding patients with preeclampsia and those with an SGA neonate; see Table 2 for quantile values of all analytes and ratios). Similar data for maternal plasma angiogenic index-1 are shown in Figure 2. The value of the 2.5th centile of angiogenic index-1 was constant (0.126) between 24 and 28 weeks of gestation and corresponds to 0.2 multiples of the median (MOM).
Figure 1. Concentrations of maternal plasma angiogenic and anti-angiogenic factors in patients with fetal death.
For each fetal death case, data for one sample collected at 24–28 weeks are shown; triangles identify cases without placental lesions of maternal vascular underperfusion (MVU) and filled circles represent cases with MVU. The lines correspond with the percentiles of the analytes (or ratios) (2.5th, 10th, 50th, 90th, and 97.5th centiles). The distribution of the raw longitudinal data used to derive the percentiles is shown using a heat map; the color intensity denotes the frequency of the data points for the corresponding gestational age: A) soluble vascular endothelial growth factor receptor-1, B) soluble endoglin, C) placental growth factor and D) placental growth factor/soluble endoglin ratio.
TABLE 2.
the 2.5th, 10th, 50th, 90th, and 97.5th centiles of the concentrations of PlGF, sVEGFR-1, and sEng, and the ratios of PlGF/sVEGFR-1 and PlGF/sEng, according to gestational age at sample collection.
| PlGF (pg/mL) | sVEGFR1 (pg/mL) | sEng (ng/mL) | PlGF/sVEGFR1 | (PlGF/sEng) x 1000 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GA(weeks) | 2.5th | 10th | 50th | 90th | 97.5th | GA(weeks) | 2.5th | 10th | 50th | 90th | 97.5th | GA(weeks) | 2.5th | 10th | 50th | 90th | 97.5th | GA(weeks) | 2.5th | 10th | 50th | 90th | 97.5th | GA(weeks) | 2.5th | 10th | 50th | 90th | 97.5th |
| 12 | 17.3 | 25.3 | 45.4 | 88.4 | 132.4 | 12 | 384.8 | 513.9 | 970.8 | 1650.2 | 2364.6 | 12 | 4.28 | 5.09 | 6.84 | 9.01 | 11.37 | 12 | 0.012 | 0.023 | 0.047 | 0.118 | 0.169 | 12 | 2.2 | 3.6 | 6.6 | 13.9 | 20.9 |
| 13 | 21.5 | 32.3 | 58.0 | 117.8 | 180.2 | 13 | 376.2 | 516.6 | 962.1 | 1695.5 | 2501.5 | 13 | 4.21 | 5.01 | 6.68 | 8.88 | 11.32 | 13 | 0.016 | 0.029 | 0.060 | 0.156 | 0.228 | 13 | 2.8 | 4.6 | 8.6 | 18.4 | 28.7 |
| 14 | 26.6 | 40.8 | 73.3 | 154.1 | 238.9 | 14 | 363.8 | 511.9 | 951.9 | 1733.5 | 2613.8 | 14 | 4.12 | 4.91 | 6.50 | 8.70 | 11.14 | 14 | 0.020 | 0.036 | 0.077 | 0.202 | 0.306 | 14 | 3.6 | 5.9 | 11.2 | 24.2 | 38.7 |
| 15 | 32.8 | 50.9 | 91.6 | 198.0 | 309.5 | 15 | 349.6 | 502.0 | 940.1 | 1761.7 | 2696.7 | 15 | 4.03 | 4.82 | 6.32 | 8.50 | 10.88 | 15 | 0.026 | 0.046 | 0.098 | 0.259 | 0.404 | 15 | 4.6 | 7.5 | 14.4 | 31.4 | 51.1 |
| 16 | 40.3 | 62.8 | 113.5 | 250.3 | 392.9 | 16 | 335.2 | 488.9 | 926.8 | 1778.5 | 2748.6 | 16 | 3.95 | 4.72 | 6.15 | 8.29 | 10.56 | 16 | 0.033 | 0.057 | 0.123 | 0.327 | 0.525 | 16 | 5.9 | 9.4 | 18.4 | 40.3 | 66.1 |
| 17 | 49.3 | 76.7 | 139.4 | 311.6 | 490.0 | 17 | 321.7 | 474.4 | 912.6 | 1783.9 | 2770.6 | 17 | 3.87 | 4.62 | 5.99 | 8.07 | 10.22 | 17 | 0.042 | 0.071 | 0.154 | 0.408 | 0.673 | 17 | 7.5 | 11.8 | 23.3 | 51.2 | 84.0 |
| 18 | 59.8 | 92.8 | 169.8 | 382.4 | 601.8 | 18 | 309.9 | 460.1 | 898.2 | 1779.2 | 2766.8 | 18 | 3.79 | 4.54 | 5.85 | 7.86 | 9.88 | 18 | 0.053 | 0.088 | 0.192 | 0.502 | 0.846 | 18 | 9.4 | 14.6 | 29.2 | 64.3 | 104.8 |
| 19 | 71.9 | 110.8 | 205.0 | 463.1 | 728.6 | 19 | 300.5 | 446.9 | 884.3 | 1766.5 | 2743.0 | 19 | 3.73 | 4.46 | 5.73 | 7.66 | 9.58 | 19 | 0.064 | 0.109 | 0.236 | 0.611 | 1.045 | 19 | 11.6 | 18.0 | 36.1 | 79.5 | 128.5 |
| 20 | 85.4 | 130.8 | 245.2 | 553.7 | 871.0 | 20 | 293.6 | 436.0 | 872.1 | 1748.8 | 2706.7 | 20 | 3.68 | 4.40 | 5.63 | 7.50 | 9.34 | 20 | 0.077 | 0.132 | 0.287 | 0.734 | 1.266 | 20 | 14.0 | 21.7 | 44.1 | 97.0 | 155.0 |
| 21 | 100.0 | 152.4 | 290.2 | 654.0 | 1028.5 | 21 | 289.5 | 427.8 | 862.3 | 1729.5 | 2665.5 | 21 | 3.64 | 4.36 | 5.56 | 7.38 | 9.17 | 21 | 0.091 | 0.158 | 0.344 | 0.870 | 1.503 | 21 | 16.7 | 25.8 | 52.9 | 116.6 | 184.0 |
| 22 | 115.2 | 174.9 | 339.8 | 763.3 | 1200.5 | 22 | 288.3 | 423.0 | 856.2 | 1712.4 | 2627.5 | 22 | 3.62 | 4.33 | 5.53 | 7.30 | 9.09 | 22 | 0.104 | 0.185 | 0.406 | 1.016 | 1.748 | 22 | 19.4 | 30.0 | 62.5 | 137.9 | 214.9 |
| 23 | 130.4 | 197.6 | 392.9 | 880.5 | 1385.0 | 23 | 290.2 | 422.0 | 854.7 | 1701.2 | 2600.3 | 23 | 3.61 | 4.32 | 5.52 | 7.29 | 9.12 | 23 | 0.115 | 0.211 | 0.471 | 1.169 | 1.991 | 23 | 21.9 | 34.1 | 72.3 | 160.4 | 247.0 |
| 24 | 144.7 | 219.6 | 448.2 | 1004.0 | 1579.4 | 24 | 295.2 | 425.2 | 859.0 | 1699.7 | 2591.1 | 24 | 3.61 | 4.33 | 5.55 | 7.33 | 9.26 | 24 | 0.124 | 0.234 | 0.535 | 1.324 | 2.220 | 24 | 23.9 | 37.8 | 82.0 | 183.5 | 279.5 |
| 25 | 157.2 | 239.6 | 503.7 | 1131.4 | 1779.6 | 25 | 303.5 | 433.1 | 870.2 | 1711.7 | 2607.2 | 25 | 3.64 | 4.35 | 5.62 | 7.46 | 9.55 | 25 | 0.129 | 0.252 | 0.594 | 1.472 | 2.426 | 25 | 25.2 | 40.7 | 91.0 | 206.1 | 311.4 |
| 26 | 167.0 | 256.4 | 556.8 | 1259.6 | 1980.3 | 26 | 315.1 | 446.0 | 889.7 | 1741.3 | 2655.8 | 26 | 3.68 | 4.40 | 5.73 | 7.66 | 9.99 | 26 | 0.129 | 0.261 | 0.642 | 1.605 | 2.596 | 26 | 25.6 | 42.5 | 98.7 | 227.3 | 341.4 |
| 27 | 173.4 | 268.9 | 604.7 | 1384.7 | 2174.8 | 27 | 330.2 | 464.6 | 918.8 | 1792.7 | 2744.9 | 27 | 3.73 | 4.47 | 5.87 | 7.96 | 10.62 | 27 | 0.125 | 0.261 | 0.676 | 1.716 | 2.723 | 27 | 25.1 | 43.0 | 104.5 | 245.9 | 368.5 |
| 28 | 175.9 | 276.3 | 644.4 | 1502.1 | 2355.7 | 28 | 349.0 | 489.3 | 959.4 | 1871.0 | 2883.9 | 28 | 3.81 | 4.56 | 6.05 | 8.37 | 11.46 | 28 | 0.117 | 0.251 | 0.690 | 1.796 | 2.799 | 28 | 23.6 | 42.0 | 107.8 | 260.8 | 391.4 |
| 29 | 174.3 | 278.0 | 672.9 | 1606.6 | 2514.5 | 29 | 371.6 | 520.9 | 1013.3 | 1981.9 | 3083.9 | 29 | 3.90 | 4.67 | 6.28 | 8.91 | 12.56 | 29 | 0.105 | 0.232 | 0.683 | 1.837 | 2.822 | 29 | 21.3 | 39.8 | 108.4 | 271.1 | 409.1 |
| 30 | 168.7 | 273.9 | 688.0 | 1692.5 | 2643.0 | 30 | 398.0 | 560.3 | 1082.9 | 2132.4 | 3358.8 | 30 | 4.00 | 4.80 | 6.55 | 9.58 | 13.93 | 30 | 0.091 | 0.206 | 0.655 | 1.834 | 2.792 | 30 | 18.6 | 36.4 | 106.1 | 276.0 | 420.7 |
| 31 | 159.7 | 264.3 | 688.1 | 1753.8 | 2733.6 | 31 | 428.4 | 608.2 | 1171.0 | 2330.8 | 3725.7 | 31 | 4.12 | 4.95 | 6.86 | 10.40 | 15.63 | 31 | 0.077 | 0.175 | 0.607 | 1.787 | 2.711 | 31 | 15.7 | 32.3 | 101.2 | 275.2 | 425.5 |
| 32 | 148.2 | 250.2 | 672.9 | 1785.2 | 2780.1 | 32 | 462.8 | 665.5 | 1280.5 | 2587.1 | 4205.1 | 32 | 4.25 | 5.11 | 7.22 | 11.40 | 17.69 | 32 | 0.062 | 0.144 | 0.545 | 1.698 | 2.585 | 32 | 12.9 | 27.9 | 94.0 | 268.7 | 423.3 |
| 33 | 135.0 | 232.5 | 643.3 | 1782.1 | 2778.6 | 33 | 501.0 | 733.1 | 1415.0 | 2912.5 | 4820.9 | 33 | 4.40 | 5.29 | 7.62 | 12.57 | 20.11 | 33 | 0.049 | 0.115 | 0.473 | 1.571 | 2.423 | 33 | 10.3 | 23.5 | 85.2 | 256.8 | 414.0 |
| 34 | 121.1 | 212.6 | 601.2 | 1741.4 | 2728.1 | 34 | 542.7 | 811.6 | 1577.7 | 3318.7 | 5597.7 | 34 | 4.55 | 5.48 | 8.06 | 13.91 | 22.87 | 34 | 0.038 | 0.089 | 0.398 | 1.416 | 2.233 | 34 | 8.2 | 19.4 | 75.3 | 240.2 | 398.1 |
| 35 | 107.4 | 191.9 | 549.7 | 1662.3 | 2630.7 | 35 | 587.7 | 901.2 | 1771.9 | 3816.6 | 6557.3 | 35 | 4.70 | 5.67 | 8.53 | 15.42 | 25.87 | 35 | 0.029 | 0.068 | 0.325 | 1.244 | 2.023 | 35 | 6.5 | 15.8 | 65.1 | 219.9 | 376.3 |
| 36 | 94.5 | 171.5 | 492.2 | 1546.7 | 2491.3 | 36 | 635.3 | 1001.8 | 1999.4 | 4412.4 | 7709.5 | 36 | 4.86 | 5.86 | 9.02 | 17.03 | 28.95 | 36 | 0.022 | 0.051 | 0.259 | 1.065 | 1.804 | 36 | 5.2 | 12.9 | 55.1 | 197.2 | 349.6 |
| 37 | 83.1 | 152.4 | 432.1 | 1399.3 | 2317.9 | 37 | 684.7 | 1112.1 | 2260.1 | 5102.6 | 9038.7 | 37 | 5.00 | 6.04 | 9.53 | 18.68 | 31.80 | 37 | 0.017 | 0.039 | 0.202 | 0.889 | 1.584 | 37 | 4.3 | 10.5 | 45.8 | 173.1 | 319.2 |
| 38 | 73.3 | 135.4 | 372.8 | 1227.6 | 2119.9 | 38 | 735.3 | 1229.7 | 2549.7 | 5866.0 | 10483.9 | 38 | 5.13 | 6.20 | 10.03 | 20.23 | 34.01 | 38 | 0.013 | 0.030 | 0.155 | 0.725 | 1.368 | 38 | 3.8 | 8.7 | 37.6 | 148.9 | 286.4 |
| 39 | 65.5 | 120.9 | 317.0 | 1041.4 | 1907.6 | 39 | 786.1 | 1350.7 | 2858.3 | 6654.4 | 11917.8 | 39 | 5.23 | 6.32 | 10.50 | 21.49 | 35.09 | 39 | 0.010 | 0.023 | 0.117 | 0.578 | 1.164 | 39 | 3.5 | 7.4 | 30.4 | 125.6 | 252.6 |
| 40 | 59.6 | 109.4 | 266.4 | 851.6 | 1691.3 | 40 | 836.3 | 1469.3 | 3167.7 | 7383.6 | 13132.3 | 40 | 5.29 | 6.40 | 10.91 | 22.26 | 34.55 | 40 | 0.008 | 0.019 | 0.089 | 0.452 | 0.975 | 40 | 3.7 | 6.6 | 24.5 | 103.9 | 219.0 |
Concentrations are given in pg/mL for PlGF and sVEGFR-1 and ng/mL for sENG. Values for the PlGF/sENG ratio were multiplied by 1,000. Of note, by dividing the percentile values to the corresponding median value (50th percentile), one can obtain multiple of median (MOM) cut-offs.
Figure 2. Angiogenic index-1 in patients with a fetal death.
For each fetal death case, data for one sample collected at 24–28 weeks are shown; triangles identify cases without placental lesions of maternal vascular underperfusion (MVU) and filled circles represent cases with MVU. The lines correspond with the percentiles of angiogenic index-1 as described by the labels in the graph (2.5th, 10th, 50th, 90th, and 97.5th centiles). The distribution of the raw longitudinal data used to derive the percentiles is shown using a heat map; the color intensity denotes the frequency of the data points for the corresponding gestational age and angiogenic index-1 value.
Table 3 shows the prediction performance for fetal death >24 weeks when defining a positive test based on abnormal angiogenic and anti-angiogenic factor concentrations and ratios measured at 24–28 weeks of gestation. An angiogenic index-1 value <2.5th centile had the largest positive likelihood ratio (14.6; 95% CI, 7.7–27.7) and relative risk (29.1; 95% CI, 8.8–97.1), followed by sEng concentrations >97.5thcentile and PlGF/sEng ratios <2.5th, both of which had a positive likelihood ratio of 13.7 (95% CI, 7.3–25.8) and a relative risk of 27.4 (95% CI, 8.2–91.2).
Table 3.
Prediction performance of maternal plasma angiogenic and anti-angiogenic factors concentrations and ratios (at 24–28 weeks of gestation) for subsequent fetal death (from 24–37.6 weeks of gestation).
| Predictor | Cutoff* | Relative Risk | AUC | Sensitivity | Specificity | Likelihood ratio (+) | Likelihood ratio (−) |
|---|---|---|---|---|---|---|---|
| sVEGFR1 | >90 | 9.4 (2.9–31.1) |
0.85 | 0.55 (0.23–0.83) |
0.89 (0.87–0.91) |
4.9 (2.8–8.7) |
0.51 (0.27–0.98) |
| sVEGFR1 | >97.5 | 10.8 (2.8–41.4) |
0.85 | 0.27 (0.06–0.61) |
0.97 (0.95–0.98) |
8.4 (3–23.5) |
0.75 (0.52–1.08) |
| sEng | >90 | 14 (4.1–48.3) |
0.82 | 0.64 (0.31–0.89) |
0.89 (0.87–0.91) |
5.9 (3.6–9.5) |
0.41 (0.19–0.89) |
| sEng | >97.5 | 27.4 (8.2–91.2) |
0.82 | 0.55 (0.23–0.83) |
0.96 (0.94–0.97) |
13.7 (7.3–25.8) |
0.47 (0.25–0.9) |
| PlGF | <10 | 7.7 (2.3–25.5) |
0.73 | 0.55 (0.23–0.83) |
0.87 (0.84–0.89) |
4.1 (2.3–7.2) |
0.52 (0.27–1) |
| PlGF | <2.5 | 16.7 (5–55.4) |
0.73 | 0.45 (0.17–0.77) |
0.95 (0.94–0.97) |
9.9 (4.8–20.3) |
0.57 (0.33–0.98) |
| PlGF/sVEGFR1 | <10 | 13.7 (4–47.1) |
0.79 | 0.64 (0.31–0.89) |
0.89 (0.87–0.91) |
5.7 (3.5–9.3) |
0.41 (0.19–0.89) |
| PlGF/sVEGFR1 | <2.5 | 29.1 (8.8–97.1) |
0.79 | 0.55 (0.23–0.83) |
0.96 (0.95–0.97) |
14.6 (7.7–27.7) |
0.47 (0.25–0.9) |
| PlGF/sEng | <10 | 12 (3.5–41.1) |
0.77 | 0.64 (0.31–0.89) |
0.87 (0.85–0.9) |
5.1 (3.1–8.2) |
0.42 (0.19–0.91) |
| PlGF/sEng | <2.5 | 27.4 (8.2–91.2) |
0.77 | 0.55 (0.23–0.83) |
0.96 (0.94–0.97) |
13.7 (7.3–25.8) |
0.47 (0.25–0.9) |
Data is presented as pecentile for cutoff values; as number( 95% confidence interval) for sensitivity, specificity and likelihood ratios. sVEGFR-1- soluble vascular endothelial growth factor receptor -1;sEng- soluble endoglin; PlGF- placental growth factor; AUC: the Area Under the Receiver Operating Characteristic curve.
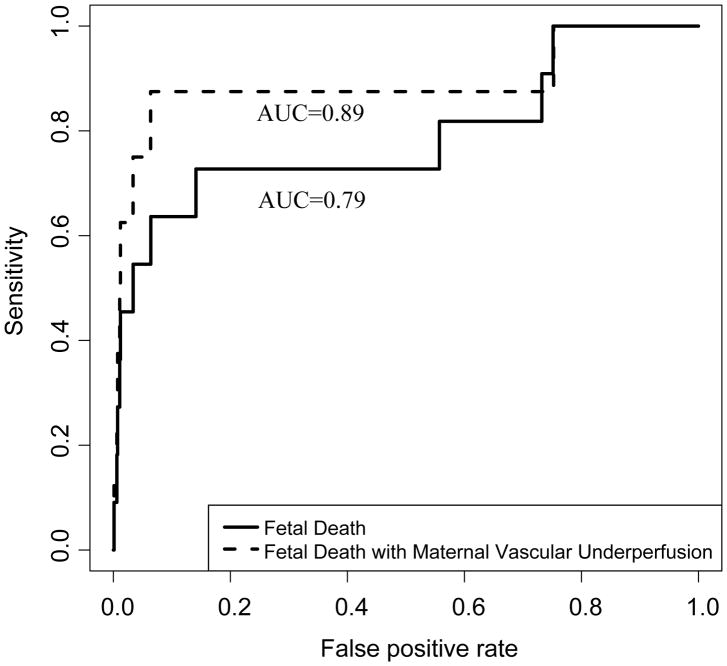
Table 4 presents the number of cases and controls with maternal plasma angiogenic index-1 values below the 10th and 2.5th centiles. Using the 10th percentile cut-off, maternal plasma angiogenic index-1 had a sensitivity of 63.6% (7/11), a false-positive rate of 11.1% (92/829), and a positive likelihood ratio (LR+) of 5.7. Using the 2.5th centile cut-off, maternal plasma angiogenic index-1 had a sensitivity of 54.5% (6/11), a false-positive rate of 3.7% (31/829), and a LR+ of 14.6 (Tables 2 and 3). The whole range of sensitivities and corresponding false-positive rates of angiogenic index-1 is shown by the Receiver Operating Characteristic (ROC) curve in Figure 3 (AUC= 0.79, 95% confidence interval (CI) 0.61–0.97, p=0.0005.).
Table 4.
Contingency table for the association between an abnormal maternal plasma angiogenic index-1 and fetal death at>24 weeks.
| Angiogenic index-1 | Fetal death>24 weeks | Controls |
|---|---|---|
| < 10th centile | 7 | 92 |
| ≥ 10th centile | 4 | 737 |
| Total | 11 | 829 |
| < 2.5th centile | 6 | 31 |
| ≥ 2.5th centile | 5 | 798 |
| Total | 11 | 829 |
Figure 3. The Receiver Operating Characteristic (ROC) curves of the performance of angiogenic index-1 in the detection of patients who will subsequently have a fetal death.
The area under the curve (AUC) of the ROC curve for the detection of all patients with a subsequent fetal death (Bold line) is 0.79, 95% confidence interval (CI) 0.61–0.97, p=0.0005. The area under the curve (AUC) of the ROC curve for the detection of subsequent fetal death of patients who also had placental lesions consistent with maternal vascular under perfusion (broken line) is 0.89, 95% CI 0.71–1.00, p=0.00007.
Eight of the 11 cases had placental findings consistent with maternal vascular lesions of underperfusion. The positive likelihood ratio of angiogenic index-1 in these 8 cases increased from 5.7 to 7.9 at the 10th centile cut-off, and from 14.6 to 20.0 at the 2.5th centile cut-off, as three of the four cases missed by a positive angiogenic index-1 at the 10th centile did not have placental lesions consistent with maternal vascular underperfusion. In other words, angiogenic index-1 identified 7 of 8 (87.58%) subsequent fetal deaths associated with placental lesions consistent with maternal underperfusion (Figure 2). The performance of the test in the detection of fetal death in patients with maternal vascular lesions of underperfusion is shown by the ROC curve in Figure 3 (AUC=0.89, 95% CI 0.71–1.00, p=0.00007).
The risk of fetal death in patients with abnormal angiogenic index-1
In the tested cohort in which the prevalence of fetal death after 24 weeks was 1.3% (11/840), 7% of women who had a ‘positive’ angiogenic index-1 value at the 10th centile had a subsequent fetal death, and 16% [6/(6+31)] of those who had a ‘positive’ angiogenic index-1 value at the 2.5th centile had a subsequent fetal death (Table 4). When weighting of cases and controls in the tested cohort was performed so that the weight of cases was the same as the prevalence of fetal death in the full cohort (0.4%, 17/4006), the risk of fetal death >24 weeks was determined to be 2% (PPV) for a ‘positive’ angiogenic index-1 value at the 10th centile, and 6% [6*1.55/(6*1.55+31*4.81)] (PPV) for a ‘positive’ angiogenic index-1 value at the 2.5th centile.
Pregnancy outcomes of women with abnormal angiogenic index-1 values who did not have a fetal death?
Of the 31 women who had “false-positive” test results at the 2.5th centile cut-off, 48% (15/31) developed preeclampsia, 39% (12/31) delivered an SGA neonate, and 61% (19/31) had at least one of these outcomes. Of the 92 women who had “false-positive” test results at the 10th centile cut-off, 23% (21/92) developed preeclampsia, 24% (22/92) delivered an SGA neonate, and 37% (34/92) had at least one of these outcomes.
Discussion
Principal findings of the study
1) The maternal plasma angiogenic index-1 value (PlGF/sVEGFR-1 maternal plasma concentration ratio) at 24–28 weeks can identify women who subsequently have a fetal death; 2) an angiogenic index-1 < 2.5th centile had a positive likelihood ratio of 14.6 for identifying a subsequent fetal death and can detect one-half of all fetal deaths (sensitivity, 54.5%) in the late second and third trimesters of pregnancy; 3) in the subset of fetal deaths associated with placental lesions consistent with maternal vascular underperfusion, the positive likelihood ratio of angiogenic index-1 < 2.5th centile increased to 20.0; and 4) 61% of women whose angiogenic index-1 at 24–28 weeks was < 2.5th centile, who did not have a subsequent fetal death, developed preeclampsia or delivered an SGA neonate.
Placental lesions and fetal death
The placenta is considered to be a record of fetal life64, and by examining it histologically, one can often glean the intrauterine pathological processes that may have led to obstetrical complications such as fetal death32. The pathologic processes implicated in fetal death include: infection65–84, placental abruption,5, 37, 70, 85–92 vascular lesions of the placenta, 28, 35, 93–100 preeclampsia,41, 101–105 fetal growth restriction9, 106–114, maternal anti-fetal rejection, 35, 115–120 metabolic disorders,121–133 genetic disorders,80, 131, 134–137 umbilical cord accident,97, 138–145 trauma146–148, and placental senescence22; however, most of the 47,000 stillbirths reported from developed countries in 2015149 were classified as unknown etiology. It is currently believed that the causes of fetal loss change with gestational age: chromosomal abnormalities150–153 and infection75 are the most common causes during the first half of pregnancy, placental causes (abruption or vascular abnormalities)154–156, and maternal anti-fetal rejection118 become the most common causes after 26 weeks until term14, after which the etiology of in most cases, especially after 40 weeks, is unknown14. In this study, the frequency of placental lesions consistent with maternal vascular underperfusion was significantly higher in cases than controls, supporting the view that the most common cause of fetal death in the late second and third trimesters of pregnancy is placental in origin.
What is the association between fetal death and maternal plasma angiogenic index-1?
The placenta is a network of blood vessels surrounded by trophoblast 64, and the processes of vasculogenesis and angiogenesis are crucial for the success of pregnancy. Indeed, deletion of a single VEGF allele in an embryo (haploinsufficiency) is lethal 157. An imbalance in the maternal plasma angiogenic and anti-angiogenic factors has been reported in women with preeclampsia48, 55, 58, 158–172, SGA neonates 166, 169, 170, 173, a subset of those who had a spontaneous preterm parturition174, mirror syndrome 175–177, twin-to-twin transfusion syndrome 178, and fetal death 45, 46. These alterations in the maternal plasma concentrations of PlGF and sVEGFR-1 can be detected at the time of disease and at several weeks prior to the onset of the different obstetrical syndromes.
What are the clinical implications of a low maternal plasma angiogenic index-1?
In previous studies44, we reported that maternal plasma angiogenic index-1 measured at 30–34 weeks of gestation identified 80% of subsequent late fetal deaths. Among those who had a fetal death, identified by the maternal plasma angiogenic index-1 value, 80% had maternal vascular underperfusion.44 This result, coupled with our findings that women who subsequently had a fetal death have higher maternal plasma sVEGFR-1 and lower PIGF concentrations, as early as 20 weeks of gestation, compared to those with a normal pregnancy, led us to explore whether the ratio between these two analytes was predictive of fetal death as early as 24 weeks of gestation. This study found that a maternal plasma angiogenic index-1 below the 2.5th centile at 24–28 weeks of gestation (i.e., below 0.126) could identify 54.5% of subsequent fetal deaths A patient whose plasma angiogenic index-1 at 24–28 weeks of gestation is < 0.126 has a 6% chance of a fetal death, while a patient whose plasma angiogenic index-1 is > 0.126 only a 0.2% chance of a fetal death.
Two factors support choosing the 2.5th centile over the 10th centile as the cut-off to define a ‘positive’ angiogenic index-1. First, angiogenic index-1 is constant below the 2.5th centile but not below the 10th centile, so a single value can be used to determine whether a test is ‘positive’ or ‘negative’ using the 2.5th centile as the cut-off. Second, the proportion of false-positives associated with preeclampsia or SGA neonates was higher for false positives below the 2.5th centile (61%) than for the 10th centile (37%), making a positive result below the 2.5th centile more clinically significant than a positive result below the 10th centile, even if the latter cut-off is slightly more predictive of a subsequent fetal death.
A preliminary report indicating that statins may improve pregnancy outcome by reducing the anti-angiogenic state in pregnancy179 has increased the potential value of screening pregnancies for the PIGF/sVEGFR-1 concentration. We previously reported a case in which a patient with recurrent fetal losses due to maternal perivillous fibrin deposition was successfully treated with pravastatin, and the treatment improved her PIGF/sVEGFR-1 concentration ratio, leading to a successful pregnancy. Maternal perivillous fibrin deposition has a stereotypic pattern for angiogenic index-1180, and we have provided evidence that this may reflect (at least, in some cases) maternal anti-fetal rejection120. Since treatment with pravastatin can reverse low plasma PIGF/sVEGFR-1 concentration ratios, therapeutic trials of pravastatin in women who have low plasma PIGF/sVEGFR-1 concentration ratios are warranted, especially given the negligible risks resulting from a false-positive screening test result. Other interventions that could be effective include metformin181 and proton pump inhibitors.182, 183
Thus, the maternal plasma angiogenic index-1 measured at 24–28 weeks of gestation can serve as a biomarker to identify women at risk of having a subsequent fetal death or developing other obstetrical complications. The availability of potentially effective treatments makes the formulation of practical screening programs using plasma angiogenic index-1 a clinical priority.
Strengths and limitations of this study
The strengths of this study are as follows: 1) the use of a case-cohort study design included a random sample of about one-quarter of the full cohort of 4,006 women and all remaining women with a fetal death in the parent cohort. Unlike a nested case-control study, this case-cohort design allowed us to estimate relative risk and to account for other complications that may involve a common pathway with fetal death; 2) the demonstration that angiogenic index-1 below the 2.5th centile has a higher positive likelihood ratio for patients whose placenta had histologic lesions of maternal vascular underperfusion, emphasizing that this risk-assessment method is also related to the underlying mechanism of disease leading to fetal death; and 3) the proportion of placental-associated fetal deaths in this study is comparable to that reported in another study34.
Limitations of this study include: 1) In the study design, the sub-cohort of 1000 random individuals was not selected completely at random from the full cohort but rather from all patients who had at least three blood samples available for analysis. Such longitudinal data are best-suited for establishing reference intervals in the control group; 2) the fact that not all of the patients selected for inclusion in the case-cohort had a sample during the 24–28 week interval for analysis; 3) the small number of late fetal deaths included in the analysis; however, this reflects the prevalence of the disease in the population, and, in spite of this limitation, we demonstrated a highly positive result; and 4) although an abnormal angiogenic index-1 had the highest relative risk and positive likelihood ratio for fetal death, a cost-effectiveness analysis is still warranted to determine the optimal screening method, since sEng (Figure 1b) alone had a similar prediction performance for this outcome.
Conclusions
1) A maternal plasma angiogenic index-1 value below the 2.5th centile at 24–28 weeks of gestation carries a 29-fold increase for the risk of subsequent fetal death and identifies 55% of these patients at a very low (3.7%) false-positive rate; and 2) 61% of the women who had a false-positive result subsequently had adverse pregnancy outcomes (preeclampsia or an SGA neonate).
Supplementary Material
Acknowledgments
Funding: This research was supported, in part, by the Perinatology Research Branch, Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
References
- 1.Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern Fetal Neonatal Med. 2009;22:636–9. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 2.Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neonatal Med. 2009;22:633–5. doi: 10.1080/14767050902866804. [DOI] [PubMed] [Google Scholar]
- 3.Silver RM, Saade GR, Thorsten V, Parker CB, Reddy UM, Drews-Botsch C, et al. Factor V Leiden, prothrombin G20210A, and methylene tetrahydrofolate reductase mutations and stillbirth: the Stillbirth Collaborative Research Network. Am J Obstet Gynecol. 2016;215:468 e1–68 e17. doi: 10.1016/j.ajog.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Takita H, Hasegawa J, Nakamura M, Arakaki T, Oba T, Matsuoka R, et al. Causes of intrauterine fetal death are changing in recent years. J Perinat Med. 2017 doi: 10.1515/jpm-2016-0337. [DOI] [PubMed] [Google Scholar]
- 5.Reddy UM, Goldenberg R, Silver R, Smith GC, Pauli RM, Wapner RJ, et al. Stillbirth classification--developing an international consensus for research: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;114:901–14. doi: 10.1097/AOG.0b013e3181b8f6e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoyert DL, Gregory EC. Cause of Fetal Death: Data From the Fetal Death Report, 2014. Natl Vital Stat Rep. 2016;65:1–25. [PubMed] [Google Scholar]
- 7.Macdorman MF, Gregory EC. Fetal and Perinatal Mortality: United States, 2013. Natl Vital Stat Rep. 2015;64:1–24. [PubMed] [Google Scholar]
- 8.Lawn JE, Blencowe H, Waiswa P, Amouzou A, Mathers C, Hogan D, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016;387:587–603. doi: 10.1016/S0140-6736(15)00837-5. [DOI] [PubMed] [Google Scholar]
- 9.Lawn JE, Blencowe H, Pattinson R, Cousens S, Kumar R, Ibiebele I, et al. Stillbirths: Where? When? Why? How to make the data count? Lancet. 2011;377:1448–63. doi: 10.1016/S0140-6736(10)62187-3. [DOI] [PubMed] [Google Scholar]
- 10.Man J, Hutchinson JC, Heazell AE, Ashworth M, Levine S, Sebire NJ. Stillbirth and intrauterine fetal death: factors affecting determination of cause of death at autopsy. Ultrasound Obstet Gynecol. 2016;48:566–73. doi: 10.1002/uog.16016. [DOI] [PubMed] [Google Scholar]
- 11.Gardosi J, Kady SM, Mcgeown P, Francis A, Tonks A. Classification of stillbirth by relevant condition at death (ReCoDe): population based cohort study. BMJ. 2005;331:1113–7. doi: 10.1136/bmj.38629.587639.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Froen JF, Arnestad M, Frey K, Vege A, Saugstad OD, Stray-Pedersen B. Risk factors for sudden intrauterine unexplained death: epidemiologic characteristics of singleton cases in Oslo, Norway, 1986–1995. Am J Obstet Gynecol. 2001;184:694–702. doi: 10.1067/mob.2001.110697. [DOI] [PubMed] [Google Scholar]
- 13.Huang DY, Usher RH, Kramer MS, Yang H, Morin L, Fretts RC. Determinants of unexplained antepartum fetal deaths. Obstet Gynecol. 2000;95:215–21. doi: 10.1016/s0029-7844(99)00536-0. [DOI] [PubMed] [Google Scholar]
- 14.Smith GC, Yu CK, Papageorghiou AT, Cacho AM, Nicolaides KH. Maternal uterine artery Doppler flow velocimetry and the risk of stillbirth. Obstet Gynecol. 2007;109:144–51. doi: 10.1097/01.AOG.0000248536.94919.e3. [DOI] [PubMed] [Google Scholar]
- 15.Stillbirth Collaborative Research Network Writing Group. Causes of death among stillbirths. Jama. 2011;306:2459–68. doi: 10.1001/jama.2011.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petitti DB. The epidemiology of fetal death. Clin Obstet Gynecol. 1987;30:253–8. doi: 10.1097/00003081-198706000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Fretts RC, Usher RH. Causes of fetal death in women of advanced maternal age. Obstet Gynecol. 1997;89:40–5. doi: 10.1016/s0029-7844(96)00427-9. [DOI] [PubMed] [Google Scholar]
- 18.Ahlenius I, Floberg J, Thomassen P. Sixty-six cases of intrauterine fetal death. A prospective study with an extensive test protocol. Acta Obstet Gynecol Scand. 1995;74:109–17. doi: 10.3109/00016349509008917. [DOI] [PubMed] [Google Scholar]
- 19.Goldenberg RL, Mcclure EM, Saleem S, Reddy UM. Infection-related stillbirths. Lancet. 2010;375:1482–90. doi: 10.1016/S0140-6736(09)61712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flenady V, Middleton P, Smith GC, Duke W, Erwich JJ, Khong TY, et al. Stillbirths: the way forward in high-income countries. Lancet. 2011;377:1703–17. doi: 10.1016/S0140-6736(11)60064-0. [DOI] [PubMed] [Google Scholar]
- 21.Horn LC, Langner A, Stiehl P, Wittekind C, Faber R. Identification of the causes of intrauterine death during 310 consecutive autopsies. Eur J Obstet Gynecol Reprod Biol. 2004;113:134–8. doi: 10.1016/S0301-2115(03)00371-3. [DOI] [PubMed] [Google Scholar]
- 22.Maiti K, Sultana Z, Aitken RJ, Morris J, Park F, Andrew B, et al. Evidence that fetal death is associated with placental aging. Am J Obstet Gynecol. 2017 doi: 10.1016/j.ajog.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Hovatta O, Lipasti A, Rapola J, Karjalainen O. Causes of stillbirth: a clinicopathological study of 243 patients. Br J Obstet Gynaecol. 1983;90:691–6. doi: 10.1111/j.1471-0528.1983.tb09296.x. [DOI] [PubMed] [Google Scholar]
- 24.Kidron D, Bernheim J, Aviram R. Placental Findings Contributing to Fetal Death, a Study of 120 Stillbirths between 23 and 40 Weeks Gestation. Placenta. 2009;30:700–04. doi: 10.1016/j.placenta.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Korteweg FJ, Erwich JJ, Holm JP, Ravise JM, Van Der Meer J, Veeger NJ, et al. Diverse placental pathologies as the main causes of fetal death. Obstet Gynecol. 2009;114:809–17. doi: 10.1097/AOG.0b013e3181b72ebe. [DOI] [PubMed] [Google Scholar]
- 26.Ptacek I, Sebire NJ, Man JA, Brownbill P, Heazell AEP. Systematic review of placental pathology reported in association with stillbirth. Placenta. 2014;35:552–62. doi: 10.1016/j.placenta.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Varli IH, Petersson K, Bottinga R, Bremme K, Hofsjo A, Holm M, et al. The Stockholm classification of stillbirth. Acta Obstet Gynecol Scand. 2008;87:1202–12. doi: 10.1080/00016340802460271. [DOI] [PubMed] [Google Scholar]
- 28.Labarrere CA, Dicarlo HL, Bammerlin E, Hardin JW, Kim YM, Chaemsaithong P, et al. Failure of physiologic transformation of spiral arteries, endothelial and trophoblast cell activation, and acute atherosis in the basal plate of the placenta. Am J Obstet Gynecol. 2017;216:287 e1–87.e16. doi: 10.1016/j.ajog.2016.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amir H, Weintraub A, Aricha-Tamir B, Apel-Sarid L, Holcberg G, Sheiner E. A piece in the puzzle of intrauterine fetal death: pathological findings in placentas from term and preterm intrauterine fetal death pregnancies. J Matern Fetal Neonatal Med. 2009;22:759–64. doi: 10.3109/14767050902929396. [DOI] [PubMed] [Google Scholar]
- 30.Tellefsen CH, Vogt C. How important is placental examination in cases of perinatal deaths? Pediatr Dev Pathol. 2011;14:99–104. doi: 10.2350/10-07-0870-OA.1. [DOI] [PubMed] [Google Scholar]
- 31.Bonetti LR, Ferrari P, Trani N, Maccio L, Laura S, Giuliana S, et al. The role of fetal autopsy and placental examination in the causes of fetal death: a retrospective study of 132 cases of stillbirths. Arch Gynecol Obstet. 2011;283:231–41. doi: 10.1007/s00404-009-1317-4. [DOI] [PubMed] [Google Scholar]
- 32.Miller ES, Minturn L, Linn R, Weese-Mayer DE, Ernst LM. Stillbirth evaluation: a stepwise assessment of placental pathology and autopsy. Am J Obstet Gynecol. 2016;214:115 e1–6. doi: 10.1016/j.ajog.2015.08.049. [DOI] [PubMed] [Google Scholar]
- 33.Heazell AE, Martindale EA. Can post-mortem examination of the placenta help determine the cause of stillbirth? J Obstet Gynaecol. 2009;29:225–8. doi: 10.1080/01443610802716042. [DOI] [PubMed] [Google Scholar]
- 34.Helgadottir LB, Turowski G, Skjeldestad FE, Jacobsen AF, Sandset PM, Roald B, et al. Classification of stillbirths and risk factors by cause of death--a case-control study. Acta Obstet Gynecol Scand. 2013;92:325–33. doi: 10.1111/aogs.12044. [DOI] [PubMed] [Google Scholar]
- 35.Pinar H, Goldenberg RL, Koch MA, Heim-Hall J, Hawkins HK, Shehata B, et al. Placental findings in singleton stillbirths. Obstet Gynecol. 2014;123:325–36. doi: 10.1097/AOG.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ananth CV, Berkowitz GS, Savitz DA, Lapinski RH. Placental abruption and adverse perinatal outcomes. Jama. 1999;282:1646–51. doi: 10.1001/jama.282.17.1646. [DOI] [PubMed] [Google Scholar]
- 37.Getahun D, Ananth CV, Kinzler WL. Risk factors for antepartum and intrapartum stillbirth: a population-based study. Am J Obstet Gynecol. 2007;196:499–507. doi: 10.1016/j.ajog.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Raisanen S, Gissler M, Nielsen HS, Kramer MR, Williams MA, Heinonen S. Social disparity affects the incidence of placental abruption among multiparous but not nulliparous women: a register-based analysis of 1,162,126 singleton births. Eur J Obstet Gynecol Reprod Biol. 2013;171:246–51. doi: 10.1016/j.ejogrb.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Redline RW, Boyd T, Campbell V, Hyde S, Kaplan C, Khong TY, et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7:237–49. doi: 10.1007/s10024-003-8083-2. [DOI] [PubMed] [Google Scholar]
- 40.Redline R. Classification of placental lesions. Am J Obstet Gynecol. 2015;213:S21–S28. doi: 10.1016/j.ajog.2015.05.056. [DOI] [PubMed] [Google Scholar]
- 41.Aurioles-Garibay A, Hernandez-Andrade E, Romero R, Qureshi F, Ahn H, Jacques SM, et al. Prenatal diagnosis of a placental infarction hematoma associated with fetal growth restriction, preeclampsia and fetal death: clinicopathological correlation. Fetal Diagn Ther. 2014;36:154–61. doi: 10.1159/000357841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YM, Chaemsaithong P, Romero R, Shaman M, Kim CJ, Kim JS, et al. The frequency of acute atherosis in normal pregnancy and preterm labor, preeclampsia, small-for-gestational age, fetal death and midtrimester spontaneous abortion. J Matern Fetal Neonatal Med. 2015;28:2001–9. doi: 10.3109/14767058.2014.976198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redline R. Placenta and Cord. In: Spong C, editor. Stillbirth: prediction, prevention and managment. Wiley-Blackwell; 2011. [Google Scholar]
- 44.Korzeniewski SJ, Romero R, Chaiworapongsa T, Chaemsaithong P, Kim CJ, Kim YM, et al. Maternal plasma angiogenic index-1 (placental growth factor/soluble vascular endothelial growth factor receptor-1) is a biomarker for the burden of placental lesions consistent with uteroplacental underperfusion: a longitudinal case-cohort study. Am J Obstet Gynecol. 2016;214:629 e1–29.e17. doi: 10.1016/j.ajog.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero R, Chaiworapongsa T, Erez O, Tarca AL, Gervasi MT, Kusanovic JP, et al. An imbalance between angiogenic and anti-angiogenic factors precedes fetal death in a subset of patients: results of a longitudinal study. J Matern Fetal Neonatal Med. 2010;23:1384–99. doi: 10.3109/14767051003681121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Espinoza J, Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Nien JK, et al. Unexplained fetal death: another anti-angiogenic state. J Matern Fetal Neonatal Med. 2007;20:495–507. doi: 10.1080/14767050701413022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith GC, Crossley JA, Aitken DA, Jenkins N, Lyall F, Cameron AD, et al. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstet Gynecol. 2007;109:1316–24. doi: 10.1097/01.AOG.0000265804.09161.0d. [DOI] [PubMed] [Google Scholar]
- 48.Chaiworapongsa T, Romero R, Korzeniewski SJ, Kusanovic JP, Soto E, Lam J, et al. Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. Am J Obstet Gynecol. 2013;208:287 e1–87 e15. doi: 10.1016/j.ajog.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macdorman MF, Kirmeyer S. The challenge of fetal mortality. NCHS Data Brief. 2009:1–8. [PubMed] [Google Scholar]
- 50.Von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22:143–8. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 51.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 52.Friedman EA, Neff RK. Pregnancy hypertension: a systematic evaluation of clinical diagnostic criteria. PSG Pub. Co; Number of pages. [Google Scholar]
- 53.Romero R, Lockwood C, Oyarzun E, Hobbins JC. Toxemia: new concepts in an old disease. Semin Perinatol. 1988;12:302–23. [PubMed] [Google Scholar]
- 54.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 55.Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–38. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation--a workshop report. Placenta. 2005;26(Suppl A):S114–7. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Royston P, Dg A. Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. J R Stat Soc Series C Appl Stat. 1994;43:429–67. [Google Scholar]
- 58.Chaiworapongsa T, Romero R, Savasan ZA, Kusanovic JP, Ogge G, Soto E, et al. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187–207. doi: 10.3109/14767058.2011.589932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koenker R, Bassett G., Jr Regression quantiles. Econometrica. 1978:33–50. [Google Scholar]
- 60.Koenker R. Quantile regression. Cambridge university press; Number of pages. [Google Scholar]
- 61.Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA. 1994;271:703–7. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 62.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 63.Breslow NE, Lumley T, Ballantyne CM, Chambless LE, Kulich M. Using the whole cohort in the analysis of case-cohort data. Am J Epidemiol. 2009;169:1398–405. doi: 10.1093/aje/kwp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burton GJ, Jauniaux E. What is the placenta? Am J Obstet Gynecol. 2015;213:S6e1, S6–8. doi: 10.1016/j.ajog.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 65.Arnesen L, Serruya S, Duran P. Gestational syphilis and stillbirth in the Americas: a systematic review and meta-analysis. Rev Panam Salud Publica. 2015;37:422–9. [PubMed] [Google Scholar]
- 66.Blackwell C. The Role of Infection and Inflammation in Stillbirths: Parallels with SIDS? Front Immunol. 2015;6:248. doi: 10.3389/fimmu.2015.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blackwell S, Romero R, Chaiworapongsa T, Kim YM, Bujold E, Espinoza J, et al. Maternal and fetal inflammatory responses in unexplained fetal death. J Matern Fetal Neonatal Med. 2003;14:151–7. doi: 10.1080/jmf.14.3.151.157. [DOI] [PubMed] [Google Scholar]
- 68.Blackwell S, Romero R, Chaiworapongsa T, Refuerzo J, Gervasi MT, Yoshimatsu J, et al. Unexplained fetal death is associated with changes in the adaptive limb of the maternal immune response consistent with prior antigenic exposure. J Matern Fetal Neonatal Med. 2003;14:241–6. doi: 10.1080/jmf.14.4.241.246. [DOI] [PubMed] [Google Scholar]
- 69.Goldenberg RL, Andrews WW, Faye-Petersen OM, Cliver SP, Goepfert AR, Hauth JC. The Alabama preterm birth study: corticosteroids and neonatal outcomes in 23- to 32-week newborns with various markers of intrauterine infection. Am J Obstet Gynecol. 2006;195:1020–4. doi: 10.1016/j.ajog.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 70.Bodnar LM, Parks WT, Perkins K, Pugh SJ, Platt RW, Feghali M, et al. Maternal prepregnancy obesity and cause-specific stillbirth. Am J Clin Nutr. 2015;102:858–64. doi: 10.3945/ajcn.115.112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caluwaerts S, Fautsch T, Lagrou D, Moreau M, Modet Camara A, Gunther S, et al. Dilemmas in Managing Pregnant Women With Ebola: 2 Case Reports. Clin Infect Dis. 2016;62:903–05. doi: 10.1093/cid/civ1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chalouhi GE, Benedetti S, Alby C, Benzina N, Ville Y. Cause of fetal demise in first-trimester parvovirus infection: anemia, placentitis or myocarditis? Ultrasound Obstet Gynecol. 2014;44:618–9. doi: 10.1002/uog.13416. [DOI] [PubMed] [Google Scholar]
- 73.Feitoza HaC, Koifman S, Koifman RJ, Saraceni V. Dengue infection during pregnancy and adverse maternal, fetal, and infant health outcomes in Rio Branco, Acre State, Brazil, 2007–2012. Cad Saude Publica. 2017;33:e00178915. doi: 10.1590/0102-311X00178915. [DOI] [PubMed] [Google Scholar]
- 74.Goldenberg RL, Saleem S, Pasha O, Harrison MS, Mcclure EM. Reducing stillbirths in low-income countries. Acta Obstet Gynecol Scand. 2016;95:135–43. doi: 10.1111/aogs.12817. [DOI] [PubMed] [Google Scholar]
- 75.Goldenberg RL, Thompson C. The infectious origins of stillbirth. Am J Obstet Gynecol. 2003;189:861–73. doi: 10.1067/s0002-9378(03)00470-8. [DOI] [PubMed] [Google Scholar]
- 76.Mcclure EM, Bose CL, Garces A, Esamai F, Goudar SS, Patel A, et al. Global network for women’s and children’s health research: a system for low-resource areas to determine probable causes of stillbirth, neonatal, and maternal death. Matern Health Neonatol Perinatol. 2015;1:11. doi: 10.1186/s40748-015-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016;165:1081–91. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paixao ES, Costa M, Teixeira MG, Harron K, De Almeida MF, Barreto ML, et al. Symptomatic dengue infection during pregnancy and the risk of stillbirth in Brazil, 2006–12: a matched case-control study. Lancet Infect Dis. 2017;17:957–64. doi: 10.1016/S1473-3099(17)30366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwartz DA. Autopsy and Postmortem Studies Are Concordant: Pathology of Zika Virus Infection Is Neurotropic in Fetuses and Infants With Microcephaly Following Transplacental Transmission. Arch Pathol Lab Med. 2017;141:68–72. doi: 10.5858/arpa.2016-0343-OA. [DOI] [PubMed] [Google Scholar]
- 80.Silver RM, Varner MW, Reddy U, Goldenberg R, Pinar H, Conway D, et al. Work-up of stillbirth: a review of the evidence. Am J Obstet Gynecol. 2007;196:433–44. doi: 10.1016/j.ajog.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goldenberg RL, Mcclure EM. Dengue and stillbirth. Lancet Infect Dis. 2017;17:886–88. doi: 10.1016/S1473-3099(17)30455-3. [DOI] [PubMed] [Google Scholar]
- 82.Gibbs RS. The origins of stillbirth: infectious diseases. Semin Perinatol. 2002;26:75–8. doi: 10.1053/sper.2002.29839. [DOI] [PubMed] [Google Scholar]
- 83.Nunes PC, Paes MV, De Oliveira CA, Soares AC, De Filippis AM, da Lima MR, et al. Detection of dengue NS1 and NS3 proteins in placenta and umbilical cord in fetal and maternal death. J Med Virol. 2016;88:1448–52. doi: 10.1002/jmv.24479. [DOI] [PubMed] [Google Scholar]
- 84.Oduyebo T, Pineda D, Lamin M, Leung A, Corbett C, Jamieson DJ. A Pregnant Patient With Ebola Virus Disease. Obstet Gynecol. 2015;126:1273–5. doi: 10.1097/AOG.0000000000001092. [DOI] [PubMed] [Google Scholar]
- 85.Faiz AS, Demissie K, Rich DQ, Kruse L, Rhoads GG. Trends and risk factors of stillbirth in New Jersey 1997–2005. J Matern Fetal Neonatal Med. 2012;25:699–705. doi: 10.3109/14767058.2011.596593. [DOI] [PubMed] [Google Scholar]
- 86.Flenady V, Koopmans L, Middleton P, Froen JF, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377:1331–40. doi: 10.1016/S0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]
- 87.Goldenberg RL, Harrison MS, Mcclure EM. Stillbirths: The Hidden Birth Asphyxia - US and Global Perspectives. Clin Perinatol. 2016;43:439–53. doi: 10.1016/j.clp.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 88.Nkwabong E, Tiomela Goula G. Placenta abruption surface and perinatal outcome. J Matern Fetal Neonatal Med. 2017;30:1456–59. doi: 10.1080/14767058.2016.1219988. [DOI] [PubMed] [Google Scholar]
- 89.Smith GC. Predicting antepartum stillbirth. Clin Obstet Gynecol. 2010;53:597–606. doi: 10.1097/GRF.0b013e3181eb64a6. [DOI] [PubMed] [Google Scholar]
- 90.Stanek J, Biesiada J. Relation of placental diagnosis in stillbirth to fetal maceration and gestational age at delivery. J Perinat Med. 2014;42:457–71. doi: 10.1515/jpm-2013-0219. [DOI] [PubMed] [Google Scholar]
- 91.Stormdal Bring H, Hulthen Varli IA, Kublickas M, Papadogiannakis N, Pettersson K. Causes of stillbirth at different gestational ages in singleton pregnancies. Acta Obstet Gynecol Scand. 2014;93:86–92. doi: 10.1111/aogs.12278. [DOI] [PubMed] [Google Scholar]
- 92.Ananth CV, Lavery JA, Vintzileos AM, Skupski DW, Varner M, Saade G, et al. Severe placental abruption: clinical definition and associations with maternal complications. Am J Obstet Gynecol. 2016;214:21. doi: 10.1016/j.ajog.2015.09.069. [DOI] [PubMed] [Google Scholar]
- 93.Kim YM, Chaemsaithong P, Romero R, Shaman M, Kim CJ, Kim JS, et al. Placental lesions associated with acute atherosis. J Matern Fetal Neonatal Med. 2015;28:1554–62. doi: 10.3109/14767058.2014.960835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Labarrere CA, Catoggio LJ, Mullen EG, Althabe OH. Placental lesions in maternal autoimmune diseases. Am J Reprod Immunol Microbiol. 1986;12:78–86. doi: 10.1111/j.1600-0897.1986.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 95.Man J, Hutchinson JC, Heazell AE, Ashworth M, Jeffrey I, Sebire NJ. Stillbirth and intrauterine fetal death: role of routine histopathological placental findings to determine cause of death. Ultrasound Obstet Gynecol. 2016;48:579–84. doi: 10.1002/uog.16019. [DOI] [PubMed] [Google Scholar]
- 96.Ornoy A, Salamon-Arnon J, Ben-Zur Z, Kohn G. Placental findings in spontaneous abortions and stillbirths. Teratology. 1981;24:243–52. doi: 10.1002/tera.1420240302. [DOI] [PubMed] [Google Scholar]
- 97.Ptacek I, Smith A, Garrod A, Bullough S, Bradley N, Batra G, et al. Quantitative assessment of placental morphology may identify specific causes of stillbirth. BMC Clin Pathol. 2016;16:016–0023. doi: 10.1186/s12907-016-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roescher AM, Timmer A, Erwich JJ, Bos AF. Placental pathology, perinatal death, neonatal outcome, and neurological development: a systematic review. PLoS One. 2014:9. doi: 10.1371/journal.pone.0089419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stanek J. Placental examination in nonmacerated stillbirth versus neonatal mortality. J Perinat Med. 2017 doi: 10.1515/jpm-2017-0198. [DOI] [PubMed] [Google Scholar]
- 100.Ernst LM, Rand CM, Bao R, Andrade J, Linn RL, Minturn L, et al. Stillbirth: Genome-wide copy number variation profiling in archived placental umbilical cord samples with pathologic and clinical correlation. Placenta. 2015;36:783–9. doi: 10.1016/j.placenta.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 101.Baltajian K, Bajracharya S, Salahuddin S, Berg AH, Geahchan C, Wenger JB, et al. Sequential plasma angiogenic factors levels in women with suspected preeclampsia. Am J Obstet Gynecol. 2016;215:89 e1–89 e10. doi: 10.1016/j.ajog.2016.01.168. [DOI] [PubMed] [Google Scholar]
- 102.Chaiworapongsa T, Kusanovic JP, Savasan ZA, Mazaki-Tovi S, Kim SK, Vaisbuch E, et al. Fetal death: a condition with a dissociation in the concentrations of soluble vascular endothelial growth factor receptor-2 between the maternal and fetal compartments. J Matern Fetal Neonatal Med. 2010;23:960–72. doi: 10.3109/14767050903410664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544 e1–44 e12. doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 104.Erez O, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Kim CJ, et al. Evidence of maternal platelet activation, excessive thrombin generation, and high amniotic fluid tissue factor immunoreactivity and functional activity in patients with fetal death. J Matern Fetal Neonatal Med. 2009;22:672–87. doi: 10.1080/14767050902853117. [DOI] [PubMed] [Google Scholar]
- 105.Schoen CN, Moreno SC, Saccone G, Graham NM, Hand LC, Maruotti GM, et al. Outpatient versus inpatient management for superimposed preeclampsia without severe features: a retrospective, multicenter study. J Matern Fetal Neonatal Med. 2017:1–7. doi: 10.1080/14767058.2017.1333101. [DOI] [PubMed] [Google Scholar]
- 106.Khalil AA, Khan N, Bowe S, Familiari A, Papageorghiou A, Bhide A, et al. Discordance in fetal biometry and Doppler are independent predictors of the risk of perinatal loss in twin pregnancies. Am J Obstet Gynecol. 2015;213:28. doi: 10.1016/j.ajog.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 107.Gardosi J, Giddings S, Buller S, Southam M, Williams M. Preventing stillbirths through improved antenatal recognition of pregnancies at risk due to fetal growth restriction. Public Health. 2014;128:698–702. doi: 10.1016/j.puhe.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 108.Monteith C, Flood K, Mullers S, Unterscheider J, Breathnach F, Daly S, et al. Evaluation of normalization of cerebro-placental ratio as a potential predictor for adverse outcome in SGA fetuses. Am J Obstet Gynecol. 2017;216:11. doi: 10.1016/j.ajog.2016.11.1008. [DOI] [PubMed] [Google Scholar]
- 109.Serena C, Marchetti G, Rambaldi MP, Ottanelli S, Di Tommaso M, Avagliano L, et al. Stillbirth and fetal growth restriction. J Matern Fetal Neonatal Med. 2013;26:16–20. doi: 10.3109/14767058.2012.718389. [DOI] [PubMed] [Google Scholar]
- 110.Gudmundsson S, Flo K, Ghosh G, Wilsgaard T, Acharya G. Placental pulsatility index: a new, more sensitive parameter for predicting adverse outcome in pregnancies suspected of fetal growth restriction. Acta Obstet Gynecol Scand. 2017;96:216–22. doi: 10.1111/aogs.13060. [DOI] [PubMed] [Google Scholar]
- 111.Mecacci F, Serena C, Avagliano L, Cozzolino M, Baroni E, Rambaldi MP, et al. Stillbirths at Term: Case Control Study of Risk Factors, Growth Status and Placental Histology. PLoS One. 2016;11:e0166514. doi: 10.1371/journal.pone.0166514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saccone G, Berghella V, Maruotti GM, Ghi T, Rizzo G, Simonazzi G, et al. Antiphospholipid antibody profile based obstetric outcomes of primary antiphospholipid syndrome: the PREGNANTS study. Am J Obstet Gynecol. 2017;216:30. doi: 10.1016/j.ajog.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 113.Premru-Srsen T, Verdenik I, Ponikvar BM, Steblovnik L, Gersak K, Cerar LK. Infant mortality and causes of death by birth weight for gestational age in non-malformed singleton infants: a 2002–2012 population-based study. J Perinat Med. 2017 doi: 10.1515/jpm-2017-0103. [DOI] [PubMed] [Google Scholar]
- 114.Bukowski R, Hansen NI, Pinar H, Willinger M, Reddy UM, Parker CB, et al. Altered fetal growth, placental abnormalities, and stillbirth. PLoS One. 2017;12:e0182874. doi: 10.1371/journal.pone.0182874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chaturvedi V, Ertelt JM, Jiang TT, Kinder JM, Xin L, Owens KJ, et al. CXCR3 blockade protects against Listeria monocytogenes infection-induced fetal wastage. J Clin Invest. 2015;125:1713–25. doi: 10.1172/JCI78578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kanellopoulos-Langevin C, Caucheteux SM, Verbeke P, Ojcius DM. Tolerance of the fetus by the maternal immune system: role of inflammatory mediators at the feto-maternal interface. Reprod Biol Endocrinol. 2003;1:121. doi: 10.1186/1477-7827-1-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol. 2015;213:041. doi: 10.1016/j.ajog.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lannaman K, Romero R, Chaiworapongsa T, Kim YM, Korzeniewski SJ, Maymon E, et al. Fetal death: an extreme manifestation of maternal anti-fetal rejection. J Perinat Med. 2017;1:2017–0073. doi: 10.1515/jpm-2017-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee J, Romero R, Dong Z, Xu Y, Qureshi F, Jacques S, et al. Unexplained fetal death has a biological signature of maternal anti-fetal rejection: chronic chorioamnionitis and alloimmune anti-human leucocyte antigen antibodies. Histopathology. 2011;59:928–38. doi: 10.1111/j.1365-2559.2011.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Romero R, Whitten A, Korzeniewski SJ, Than NG, Chaemsaithong P, Miranda J, et al. Maternal floor infarction/massive perivillous fibrin deposition: a manifestation of maternal antifetal rejection? Am J Reprod Immunol. 2013;70:285–98. doi: 10.1111/aji.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berger H, Gagnon R, Sermer M, Basso M, Bos H, Brown RN, et al. Diabetes in Pregnancy. J Obstet Gynaecol Can. 2016;38:667–79. doi: 10.1016/j.jogc.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 122.Castro MA, Fassett MJ, Reynolds TB, Shaw KJ, Goodwin TM. Reversible peripartum liver failure: a new perspective on the diagnosis, treatment, and cause of acute fatty liver of pregnancy, based on 28 consecutive cases. Am J Obstet Gynecol. 1999;181:389–95. doi: 10.1016/s0002-9378(99)70567-3. [DOI] [PubMed] [Google Scholar]
- 123.Hadi T, Bardou M, Mace G, Sicard P, Wendremaire M, Barrichon M, et al. Glutathione prevents preterm parturition and fetal death by targeting macrophage-induced reactive oxygen species production in the myometrium. Faseb J. 2015;29:2653–66. doi: 10.1096/fj.14-266783. [DOI] [PubMed] [Google Scholar]
- 124.Starikov R, Dudley D, Reddy UM. Stillbirth in the pregnancy complicated by diabetes. Curr Diab Rep. 2015;15:015–0580. doi: 10.1007/s11892-015-0580-y. [DOI] [PubMed] [Google Scholar]
- 125.Edwards A, Springett A, Padfield J, Dorling J, Bugg G, Mansell P. Differences in post-mortem findings after stillbirth in women with and without diabetes. Diabet Med. 2013;30:1219–24. doi: 10.1111/dme.12272. [DOI] [PubMed] [Google Scholar]
- 126.Hutcheon JA, Kuret V, Joseph KS, Sabr Y, Lim K. Immortal time bias in the study of stillbirth risk factors: the example of gestational diabetes. Epidemiology. 2013;24:787–90. doi: 10.1097/EDE.0b013e3182a6d9aa. [DOI] [PubMed] [Google Scholar]
- 127.Hod M, Damm P, Kaaja R, Visser GH, Dunne F, Demidova I, et al. Fetal and perinatal outcomes in type 1 diabetes pregnancy: a randomized study comparing insulin aspart with human insulin in 322 subjects. Am J Obstet Gynecol. 2008;198:186 e1–7. doi: 10.1016/j.ajog.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 128.Lamminpaa R, Vehvilainen-Julkunen K, Gissler M, Selander T, Heinonen S. Pregnancy outcomes in women aged 35 years or older with gestational diabetes - a registry-based study in Finland. J Matern Fetal Neonatal Med. 2016;29:55–9. doi: 10.3109/14767058.2014.986450. [DOI] [PubMed] [Google Scholar]
- 129.Langer O. Obesity or diabetes: which is more hazardous to the health of the offspring? J Matern Fetal Neonatal Med. 2016;29:186–90. doi: 10.3109/14767058.2014.995084. [DOI] [PubMed] [Google Scholar]
- 130.Melville A, Jerrett I, Gallaher J, Adeyemo A, Yoong W. Intrauterine fetal death associated with maternal ketoacidosis as a first presentation of diabetes in an African woman. J Obstet Gynaecol. 2014;34:196–7. doi: 10.3109/01443615.2013.840565. [DOI] [PubMed] [Google Scholar]
- 131.Perkins RP. Hydrops fetalis and stillbirth in a male glucose-6-phosphate dehydrogenase-deficient fetus possibly due to maternal ingestion of sulfisoxazole; a case report. Am J Obstet Gynecol. 1971;111:379–81. doi: 10.1016/0002-9378(71)90781-2. [DOI] [PubMed] [Google Scholar]
- 132.Price JT, Schwartz N. Maternal rhabdomyolysis and twin fetal death associated with gestational diabetes insipidus. Obstet Gynecol. 2013;122:493–5. doi: 10.1097/AOG.0b013e3182918565. [DOI] [PubMed] [Google Scholar]
- 133.Tennant PW, Glinianaia SV, Bilous RW, Rankin J, Bell R. Pre-existing diabetes, maternal glycated haemoglobin, and the risks of fetal and infant death: a population-based study. Diabetologia. 2014;57:285–94. doi: 10.1007/s00125-013-3108-5. [DOI] [PubMed] [Google Scholar]
- 134.Gerber S, Vardhana S, Meagher-Villemure K, Vial Y, Hohlfeld P, Witkin SS. Association between fetal interleukin-1 receptor antagonist gene polymorphism and unexplained fetal death. Am J Obstet Gynecol. 2005;193:1472–7. doi: 10.1016/j.ajog.2005.02.112. [DOI] [PubMed] [Google Scholar]
- 135.Basu MN, Johnsen IBG, Wehberg S, Sorensen RG, Barington T, Norgard BM. Causes of death among full term stillbirths and early neonatal deaths in the Region of Southern Denmark. J Perinat Med. 2017 doi: 10.1515/jpm-2017-0171. [DOI] [PubMed] [Google Scholar]
- 136.Olech EM, Zemojtel T, Sowinska-Seidler A, Mundlos S, Robinson PN, Karczewski M, et al. Identification of a molecular defect in a stillborn fetus with perinatal lethal hypophosphatasia using a disease-associated genome sequencing approach. Pol J Pathol. 2016;67:78–83. doi: 10.5114/pjp.2016.59480. [DOI] [PubMed] [Google Scholar]
- 137.Sahlin E, Gustavsson P, Lieden A, Papadogiannakis N, Bjareborn L, Pettersson K, et al. Molecular and cytogenetic analysis in stillbirth: results from 481 consecutive cases. Fetal Diagn Ther. 2014;36:326–32. doi: 10.1159/000361017. [DOI] [PubMed] [Google Scholar]
- 138.Collins JH. Umbilical cord accidents: human studies. Semin Perinatol. 2002;26:79–82. doi: 10.1053/sper.2002.29860. [DOI] [PubMed] [Google Scholar]
- 139.Sinkey RG, Odibo AO, Dashe JS. #37: Diagnosis and management of vasa previa. Am J Obstet Gynecol. 2015;213:615–9. doi: 10.1016/j.ajog.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 140.Baergen RN. Cord abnormalities, structural lesions, and cord “accidents”. Semin Diagn Pathol. 2007;24:23–32. doi: 10.1053/j.semdp.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 141.Chen KH, Seow KM, Chen LR. The role of preterm placental calcification on assessing risks of stillbirth. Placenta. 2015;36:1039–44. doi: 10.1016/j.placenta.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 142.Engmann C, Garces A, Jehan I, Ditekemena J, Phiri M, Mazariegos M, et al. Causes of community stillbirths and early neonatal deaths in low-income countries using verbal autopsy: an International, Multicenter Study. J Perinatol. 2012;32:585–92. doi: 10.1038/jp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ryan WD, Trivedi N, Benirschke K, Lacoursiere DY, Parast MM. Placental histologic criteria for diagnosis of cord accident: sensitivity and specificity. Pediatr Dev Pathol. 2012;15:275–80. doi: 10.2350/11-12-1127-OA.1. [DOI] [PubMed] [Google Scholar]
- 144.Collins JH. Umbilical cord accidents--time of death? Am J Obstet Gynecol. 1997;177:1566. doi: 10.1016/s0002-9378(97)70131-5. [DOI] [PubMed] [Google Scholar]
- 145.Meganathan HS, Rajeshwaran R, Bhuvana S. MRI findings in a fetus with a tight cord around the neck. Neurol India. 2017;65:1130–31. doi: 10.4103/neuroindia.NI_671_16. [DOI] [PubMed] [Google Scholar]
- 146.Battaloglu E, Mcdonnell D, Chu J, Lecky F, Porter K. Epidemiology and outcomes of pregnancy and obstetric complications in trauma in the United Kingdom. Injury. 2016;47:184–7. doi: 10.1016/j.injury.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 147.Lucia A, Dantoni SE. Trauma Management of the Pregnant Patient. Crit Care Clin. 2016;32:109–17. doi: 10.1016/j.ccc.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 148.Van Der Knoop BJ, Zonnenberg IA, Otten VM, Van Weissenbruch MM, De Vries JI. Trauma in pregnancy, obstetrical outcome in a tertiary centre in the Netherlands. J Matern Fetal Neonatal Med. 2017:1–11. doi: 10.1080/14767058.2017.1285891. [DOI] [PubMed] [Google Scholar]
- 149.Blencowe H, Cousens S, Jassir FB, Say L, Chou D, Mathers C, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. 2016;4:e98–e108. doi: 10.1016/S2214-109X(15)00275-2. [DOI] [PubMed] [Google Scholar]
- 150.Plachot M. Chromosome analysis of spontaneous abortions after IVF. A European survey. Hum Reprod. 1989;4:425–9. doi: 10.1093/oxfordjournals.humrep.a136921. [DOI] [PubMed] [Google Scholar]
- 151.Guerneri S, Bettio D, Simoni G, Brambati B, Lanzani A, Fraccaro M. Prevalence and distribution of chromosome abnormalities in a sample of first trimester internal abortions. Hum Reprod. 1987;2:735–9. doi: 10.1093/oxfordjournals.humrep.a136623. [DOI] [PubMed] [Google Scholar]
- 152.Be C, Velasquez P, Youlton R. Spontaneous abortion: cytogenetic study of 609 cases. Rev Med Chil. 1997;125:317–22. [PubMed] [Google Scholar]
- 153.Angell RR, Sandison A, Bain AD. Chromosome variation in perinatal mortality: a survey of 500 cases. J Med Genet. 1984;21:39–44. doi: 10.1136/jmg.21.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smith GC, Crossley JA, Aitken DA, Pell JP, Cameron AD, Connor JM, et al. First-trimester placentation and the risk of antepartum stillbirth. JAMA. 2004;292:2249–54. doi: 10.1001/jama.292.18.2249. [DOI] [PubMed] [Google Scholar]
- 155.Smith GC, Fretts RC. Stillbirth. Lancet. 2007;370:1715–25. doi: 10.1016/S0140-6736(07)61723-1. [DOI] [PubMed] [Google Scholar]
- 156.Smith GC, Shah I, White IR, Pell JP, Crossley JA, Dobbie R. Maternal and biochemical predictors of antepartum stillbirth among nulliparous women in relation to gestational age of fetal death. BJOG. 2007;114:705–14. doi: 10.1111/j.1471-0528.2007.01343.x. [DOI] [PubMed] [Google Scholar]
- 157.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–42. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 158.Chaiworapongsa T, Romero R, Korzeniewski SJ, Cortez JM, Pappas A, Tarca AL, et al. Plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med. 2014;27:132–44. doi: 10.3109/14767058.2013.806905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Soto E, Romero R, Kusanovic JP, Ogge G, Hussein Y, Yeo L, et al. Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. J Matern Fetal Neonatal Med. 2012;25:498–507. doi: 10.3109/14767058.2011.591461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vaisbuch E, Whitty JE, Hassan SS, Romero R, Kusanovic JP, Cotton DB, et al. Circulating angiogenic and antiangiogenic factors in women with eclampsia. Am J Obstet Gynecol. 2011;204:152 e1–9. doi: 10.1016/j.ajog.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Levine RJ, Karumanchi SA. Circulating angiogenic factors in preeclampsia. Clin Obstet Gynecol. 2005;48:372–86. doi: 10.1097/01.grf.0000160313.82606.d7. [DOI] [PubMed] [Google Scholar]
- 162.Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res. 2005;57:6. doi: 10.1203/01.PDR.0000159567.85157.B7. [DOI] [PubMed] [Google Scholar]
- 163.Powers RW, Jeyabalan A, Clifton RG, Van Dorsten P, Hauth JC, Klebanoff MA, et al. Soluble fms-Like tyrosine kinase 1 (sFlt1), endoglin and placental growth factor (PlGF) in preeclampsia among high risk pregnancies. PLoS One. 2010;5:0013263. doi: 10.1371/journal.pone.0013263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reslan OM, Khalil RA. Molecular and vascular targets in the pathogenesis and management of the hypertension associated with preeclampsia. Cardiovasc Hematol Agents Med Chem. 2010;8:204–26. doi: 10.2174/187152510792481234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Silasi M, Cohen B, Karumanchi SA, Rana S. Abnormal placentation, angiogenic factors, and the pathogenesis of preeclampsia. Obstet Gynecol Clin North Am. 2010;37:239–53. doi: 10.1016/j.ogc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 166.Chaiworapongsa T, Romero R, Whitten AE, Korzeniewski SJ, Chaemsaithong P, Hernandez-Andrade E, et al. The use of angiogenic biomarkers in maternal blood to identify which SGA fetuses will require a preterm delivery and mothers who will develop pre-eclampsia. J Matern Fetal Neonatal Med. 2015:1–15. doi: 10.3109/14767058.2015.1048431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10:466–80. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, Yeo L, Romero R. Pre-eclampsia part 2: prediction, prevention and management. Nat Rev Nephrol. 2014;10:531–40. doi: 10.1038/nrneph.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–87. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee Kim Y, Goncalves LF, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award Am J Obstet Gynecol. 2004;190:1541–7. doi: 10.1016/j.ajog.2004.03.043. discussion 47–50. [DOI] [PubMed] [Google Scholar]
- 172.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 173.Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med. 2008;21:25–40. doi: 10.1080/14767050701832833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chaiworapongsa T, Romero R, Tarca A, Kusanovic JP, Mittal P, Kim SK, et al. A subset of patients destined to develop spontaneous preterm labor has an abnormal angiogenic/anti-angiogenic profile in maternal plasma: evidence in support of pathophysiologic heterogeneity of preterm labor derived from a longitudinal study. J Matern Fetal Neonatal Med. 2009;22:1122–39. doi: 10.3109/14767050902994838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Espinoza J, Romero R, Nien JK, Kusanovic JP, Richani K, Gomez R, et al. A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome’ (Ballantyne’s syndrome) J Matern Fetal Neonatal Med. 2006;19:607–13. doi: 10.1080/14767050600922677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Stepan H, Faber R. Cytomegalovirus-induced mirror syndrome associated with elevated levels of angiogenic factors. Obstet Gynecol. 2007;109:1205–6. doi: 10.1097/01.AOG.0000263776.46071.d1. author reply 06. [DOI] [PubMed] [Google Scholar]
- 177.Llurba E, Marsal G, Sanchez O, Dominguez C, Alijotas-Reig J, Carreras E, et al. Angiogenic and antiangiogenic factors before and after resolution of maternal mirror syndrome. Ultrasound Obstet Gynecol. 2012;40:367–9. doi: 10.1002/uog.10136. [DOI] [PubMed] [Google Scholar]
- 178.Kusanovic JP, Romero R, Espinoza J, Nien JK, Kim CJ, Mittal P, et al. Twin-to-twin transfusion syndrome: an antiangiogenic state? Am J Obstet Gynecol. 2008;198:382 e1–8. doi: 10.1016/j.ajog.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chaiworapongsa T, Romero R, Korzeniewski SJ, Chaemsaithong P, Hernandez-Andrade E, Segars JH, et al. Pravastatin to prevent recurrent fetal death in massive perivillous fibrin deposition of the placenta (MPFD) J Matern Fetal Neonatal Med. 2016;29:855–62. doi: 10.3109/14767058.2015.1022864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Whitten AE, Romero R, Korzeniewski SJ, Tarca AL, Schwartz AG, Yeo L, et al. Evidence of an imbalance of angiogenic/antiangiogenic factors in massive perivillous fibrin deposition (maternal floor infarction): a placental lesion associated with recurrent miscarriage and fetal death. Am J Obstet Gynecol. 2013;208:310 e1–10 e11. doi: 10.1016/j.ajog.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Brownfoot FC, Hastie R, Hannan NJ, Cannon P, Tuohey L, Parry LJ, et al. Metformin as a prevention and treatment for preeclampsia: effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am J Obstet Gynecol. 2016;214:356 e1–56 e15. doi: 10.1016/j.ajog.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 182.Onda K, Tong S, Beard S, Binder N, Muto M, Senadheera SN, et al. Proton Pump Inhibitors Decrease Soluble fms-Like Tyrosine Kinase-1 and Soluble Endoglin Secretion, Decrease Hypertension, and Rescue Endothelial Dysfunction. Hypertension. 2017;69:457–68. doi: 10.1161/HYPERTENSIONAHA.116.08408. [DOI] [PubMed] [Google Scholar]
- 183.Cluver CA, Walker SP, Mol BW, Theron GB, Hall DR, Hiscock R, et al. Double blind, randomised, placebo-controlled trial to evaluate the efficacy of esomeprazole to treat early onset pre-eclampsia (PIE Trial): a study protocol. BMJ Open. 2015;5:e008211. doi: 10.1136/bmjopen-2015-008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.