Abstract
Placement of transjugular intrahepatic portosystemic shunt (TIPS) is necessary in children with portal hypertension complicated by variceal bleeding or ascites. However, placement of adult-sized endografts may be problematic due to the smaller anatomy of pediatric patients. On the other hand, placement of fixed diameter smaller stents have the corresponding problem of not accommodating future growth of the child. We describe a novel method to create an adjustable diameter TIPS as a technical solution to these problems. In this technique, a balloon expandable bare metal stent is placed concentrically around the ePTFE TIPS endograft, creating an intentional narrowing in the shunt diameter than can be expanded with balloon dilation at future procedures as needed. This allows for optimal calibration of shunt hemodynamics according to the child’s growth and prevents the potential need for placement of additional shunts or technically challenging TIPS reduction procedures.
In children with complicated portal hypertension, transjugular intrahepatic portosystemic shunt (TIPS) placement is an accepted option for the management of variceal bleeding refractory to endoscopic and medical therapy and ascites refractory to medical therapy (1–3). TIPS in children has been shown to be effective, with similar rates of technical success as in adults (4, 5). Initial reports for pediatric TIPS described the use of the Wallstent (Boston Scientific) and other self-expanding bare metal stents (1, 4, 5). However, recently there has been a shift toward the use of the VIATORR expanded polytetrafluoroethylene (e-PTFE) endograft (W.L. Gore and Associates) in children, aligning with its standard use in adults (3). Multiple studies, including a recent randomized control trial (6), have shown superiority of covered stents for shunt patency in adults.
A challenge in pediatric TIPS placement is selection of an appropriately sized shunt diameter (3). Under- or overshunting can occur at the time of initial placement when the fixed stent diameter is too small or large. Undershunting can also occur over time, if the patient outgrows the shunt. This report describes the creation of a “constrained” shunt, using concentric deployment of an outer balloon-expandable stainless steel stent around the VIATORR endograft. This results in an intentional narrowing of the construct whose nominal diameter is smaller than that of the endograft. Because the outer stent can be easily overdilated, shunt diameter can be increased at a later date, thereby accommodating interval growth of the patient or hemodynamic changes.
Technique
Three pediatric patients (mean age, 10 years; range, 6–15 years) underwent constrained TIPS procedures between January 2014 and June 2014. The clinical indications included portal hypertension and cirrhosis secondary to biliary atresia with massive gastroesophageal bleeding (case 1), portal hypertension and portal vein thrombosis with gastroesophageal variceal bleeding (case 2), and cavernous transformation of the portal vein with gastroesophageal variceal bleeding (case 3). Written informed consent was obtained from the patients’ parents after a discussion of risks, benefits, and alternatives of the procedure. All three procedures were performed under general anesthesia by a single pediatric interventional radiologist. The safety and effectiveness of the VIATORR endoprosthesis has not been established in patients younger than 18 years of age and was used off-label.
TIPS placement proceeded in usual fashion, from a right internal jugular approach using a Rosch-Uchida liver access set (Cook Medical) and standard exchanges. Pressure measurements in all cases demonstrated elevated initial portal vein to right atrium gradients of greater than 12 mmHg. Right hepatic vein to right portal vein punctures were successful.
A wire was advanced into the superior mesenteric vein and the TIPS tract dilated with a 6×40 mm Mustang balloon (Boston Scientific). A constraining 6×27 mm balloon-expandable Express LD stent (Boston Scientific) was deployed in the hepatic parenchymal tract (Fig. 1). Following this, a 10×80 mm (60 mm covered and 20 mm uncovered) VIATORR stent was placed through the Express LD stent, extending from the portal vein to the hepatic vein (Fig. 2). The entire stented tract was dilated with a 6×30 mm Mustang balloon.
Figure 1.
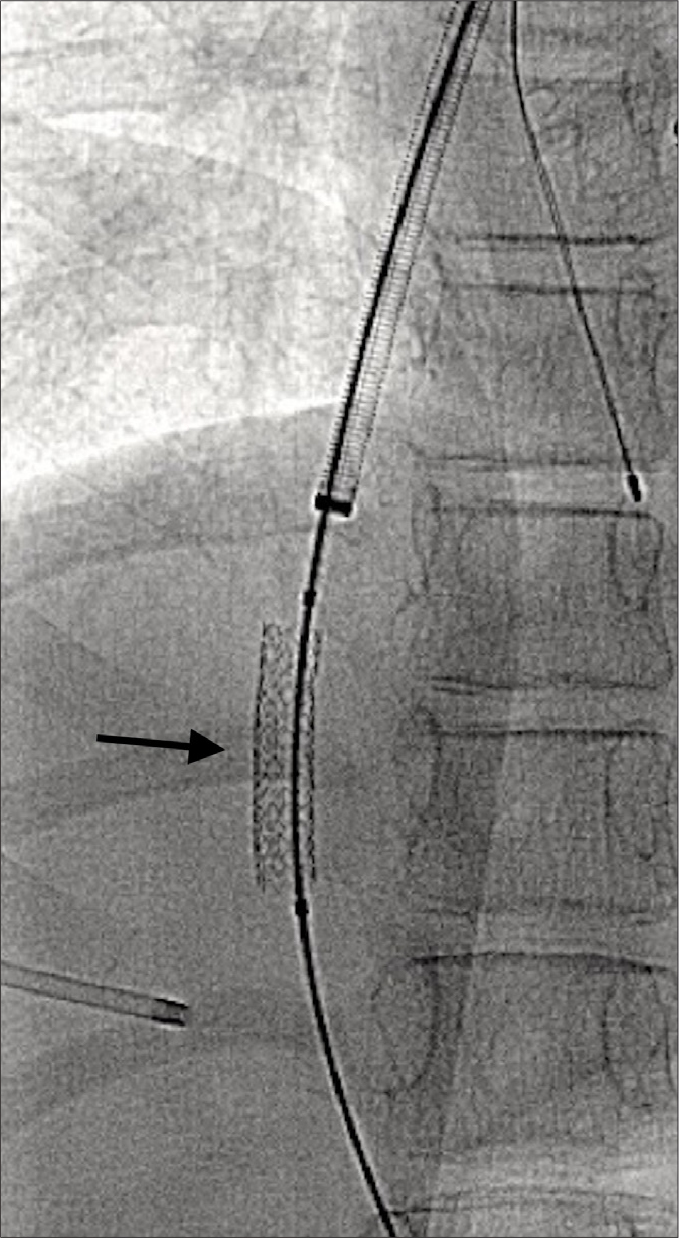
Following transhepatic puncture into the right portal vein, a 6×27 mm Express LD balloon expandable stent (arrow) was deployed in the hepatic parenchymal tract.
Figure 2. a, b.
A constrained shunt (a) was created using concentric placement of a 10 mm diameter VIATORR endograft (white arrows) within the 6 mm balloon expandable stent (black arrow). In this case, the entire shunt was dilated to 8 mm (b) because of a persistently elevated portosystemic gradient. Gastric variceal embolization coils are also noted.
In two of the three cases, the entire TIPS tract, including the constraining 6 mm stent, was then dilated with an 8×40 mm Mustang balloon due gradients greater than 10 mmHg. In one case the constrained portion remained at 6 mm. Repeat pressure measurements showed the final gradient reduced to less than 10 mmHg in all three cases.
Case 1
Following TIPS placement the patient’s acute gastroesophageal bleeding ceased and she remained hemodynamically stable. Three days following TIPS placement, the patient underwent liver transplantation. The stents were easily extricated from the vein at surgery and the patient was discharged home approximately 6 weeks following transplant. 13 months following transplant, she has normal liver function and no recurrent variceal bleeding.
Case 2
A follow-up ultrasound at one, two, and five months at our institution following TIPS placement demonstrated a widely patent shunt with expected velocities. Ultrasound at an outside facility, eight months after placement, demonstrated decreased flow and a stenosis within the TIPS. At an outside facility, the patient underwent dilation of the entire TIPS to 7 mm. Further ultrasound follow-up after revision is not currently available; however, by report the patient has had no evidence of hepatic encephalopathy, recurrent thrombosis, or recurrence of gastroesophageal variceal bleeding.
Case 3
The patient’s gastroesophageal variceal bleeding resolved following TIPS placement and she was discharged two days later. Follow-up ultrasound at ten days, two months, three months, and six months demonstrated a widely patent shunt with expected velocities. The patient has had no hepatic encephalopathy or recurrent gastroesophageal bleeding.
Discussion
TIPS placement in children with variceal bleeding or ascites from portal hypertension is effective when endoscopic or medical treatment fails (1, 3–5). The covered ePTFE VIATORR endograft is now standard for adult TIPS placement, due to improved shunt durability and patency rates (6). VIATORR placement in children is less widely reported, with less long-term outcome data (1, 3).
TIPS devices specific for pediatric use are not commercially available. The VIATORR endograft is available in fixed diameters of 8, 10, and 12 mm and cannot be significantly altered by under- or over-dilation. Recent studies have shown that although underdilation of endografts is a common practice, stents expand to nominal caliber over time (7, 8). An important consideration in pediatric TIPS placement, especially in younger children, is the effect of the child’s growth on shunt hemodynamics. As the child grows, insufficient shunting may occur. However, initial placement of a larger shunt may lead to overshunting and complications such as hepatic encephalopathy and hepatic insufficiency.
Due to pediatric patients’ smaller anatomy, the risk of overshunting may be increased by use of fixed diameter stents originally developed for adults. To address this problem, concentric placement of an outer, bare metal balloon expandable stent and inner, ePTFE VIATORR endograft can be performed (Fig. 3). This “constrained” TIPS technique creates an intentional narrowing of the shunt, which can be overdilated at a future procedure. The problem of overshunting is not specific to the pediatric population. There are various reported techniques for shunt reduction reported throughout the literature. Techniques using an hour-glass stent deployed within the TIPS (9) are similar to the constrained technique. The main important distinction and advantage of the constrained technique is that the integrity of the ePTFE stent is preserved. Other techniques for shunt reduction involve deployment of a device within a well-functioning TIPS.
Figure 3. a–c.
On-table image of deployed 10 mm diameter VIATORR stents (8 cm graft-lined and 2 cm unlined length) constrained by 6 × 27 mm Express LD balloon expandable stents dilated to (a) 6 mm, (b) 8 mm, and (c) 10 mm.
The impact of shunt reduction is not well known due to the low number of patients and variety of techniques used. The constrained technique could be a useful approach to consider in patients when there is increased concern of overshunting. It is not known if the constrained technique results in reduction of hepatic encephalopathy and further investigation is needed. Additionally, the current report is limited by the small sample size and absence of long-term patency data.
In conclusion, TIPS placement in children can be technically difficult due to patients’ smaller anatomy and potential for future growth. Placement of ePTFE VIATORR endografts constrained by balloon-expandable stents may allow for more favorable calibration of shunt diameter and permits increases in shunt diameter at future procedures to accommodate patient growth or hemodynamic changes. The constrained TIPS technique may be particularly useful in the pediatric patient.
Main points.
Adjustable diameter transjugular intrahepatic portosystemic shunt (TIPS) can be created for pediatric patients by using an outer constraining stent.
Overshunting can be a problem for pediatric TIPS given patients’ smaller size and different hemodynamics compared with adult patients.
Placement of a constrained TIPS diameter can obviate the need for technically challenging TIPS reduction procedures.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Huppert PE, Goffette P, Astfalk W, et al. Transjugular intrahepatic portosystemic shunts in children with biliary atresia. Cardiovasc Intervent Radiol. 2002;25:484–493. doi: 10.1007/s00270-002-1913-1. https://doi.org/10.1007/s00270-002-1913-1. [DOI] [PubMed] [Google Scholar]
- 2.Lillegard JB, Hanna AM, McKenzie TJ, et al. A single-institution review of portosystemic shunts in children: an ongoing discussion. HPB Surg. 2010;2010:964597. doi: 10.1155/2010/964597. https://doi.org/10.1155/2010/964597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vo NJ, Shivaram G, Andrews RT, et al. Midterm follow-up of transjugular intrahepatic portosystemic shunts using polytetrafluoroethylene endografts in children. J Vasc Interv Radiol. 2012;23:919–924. doi: 10.1016/j.jvir.2012.04.004. https://doi.org/10.1016/j.jvir.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Hackworth CA, Leef JA, Rosenblum JD, et al. Transjugular intrahepatic portosystemic shunt creation in children: initial clinical experience. Radiology. 1998;206:109–114. doi: 10.1148/radiology.206.1.9423659. https://doi.org/10.1148/radiology.206.1.9423659. [DOI] [PubMed] [Google Scholar]
- 5.Heyman MB, LaBerge JM, Somberg KA, et al. Transjugular intrahepatic portosystemic shunts (TIPS) in children. Pediatrics. 1997;131:914–919. doi: 10.1016/s0022-3476(97)70043-x. https://doi.org/10.1016/S0022-3476(97)70043-X. [DOI] [PubMed] [Google Scholar]
- 6.Perarnau JM, Gouge AL, Nicolas C, et al. Covered vs uncovered stents for transjugular intrahepatic portosystemic shunt: a randomized controlled trial. J Hepatol. 2014;60:962–968. doi: 10.1016/j.jhep.2014.01.015. https://doi.org/10.1016/j.jhep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Gaba RC, Parvinian A, Minocha J, et al. Should transjugular intrahepatic portosystemic shunt stent grafts be underdilated? J Vasc Interv Radiol. 2015;26:382–387. doi: 10.1016/j.jvir.2014.08.012. https://doi.org/10.1016/j.jvir.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Pieper CC, Sprinkart AM, Nadal J, et al. Postinterventional passive expansion of partially dilated transjugular intrahepatic portosystemic shunt stents. J Vasc Interv Radiol. 2015;26:388–394. doi: 10.1016/j.jvir.2014.10.021. https://doi.org/10.1016/j.jvir.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Kroma G, Lopera J, Cura M, et al. Transjugular intrahepatic portosystemic shunt flow reduction with adjustable polytetrafluoroethylene-covered balloon-expandable stents. J Vasc Interv Radiol. 2009;20:981–986. doi: 10.1016/j.jvir.2009.03.042. https://doi.org/10.1016/j.jvir.2009.03.042. [DOI] [PubMed] [Google Scholar]




