Abstract
Objective.
We evaluated the specific association between retinopathy and all-cause mortality among a national sample of the broader U.S. adult population.
Methods.
Data from the 2005–2008 National Health and Nutrition Examination Survey were used to identify 4,777 adults with complete data regarding screening for nonproliferative retinopathy using Early Treatment Diabetic Retinopathy Study grading criteria, as well as objective retinal imaging assessments using the Canon Non-Mydriatic Retinal Camera CR6-45NM. Participants were not included if they had been diagnosed with coronary artery disease, congestive heart failure, heart attack, or stroke at the baseline assessment.
Results.
Both mild and moderate/severe retinopathy were associated with increased all-cause mortality risk in unadjusted and adjusted models. In the adjusted model, and when compared to those with no retinopathy, those with mild and moderate/severe retinopathy, respectively, had 81% (hazard ratio [HR] 1.81, 95% CI 1.29–2.55) and 314% (HR 4.14, 95% CI 1.77–9.69) increased risks of all-cause mortality.
Conclusion.
In this nationally representative sample of adults, those with mild or moderate/severe retinopathy were at increased risk of all-cause mortality.
Previous research has demonstrated that retinopathy is positively associated with all-cause mortality among adults, especially those with diabetes (1–6). Retinopathy may be diagnosed by observable evidence of angiogenesis in close proximity to the optic nerve, formation of fibrous lesions, microaneurysms, hemorrhaging, edema, and intraretinal microvascular abnormalities, among an array of additional pathologies damaging to eye health and visual acuity (6). Retinopathy has also been shown to correlate with increased risk for cardiovascular events (2,5).
Although retinopathy may be an independent indicator of early death, meta-regression analyses have failed to support the hypothesis that confounding cardiovascular risk factors are fully explanative of the observed associations between retinopathy and all-cause mortality (2). According to recent work on this topic, <35% of deaths within the diabetes population are attributable to cardiovascular disease (1,7), providing plausibility for retinopathy to exert independent effects on patient health and longevity. A potential mechanism of action may be autonomically controlled neuropathy, wherein alterations in blood pressure, coupled with dysregulation of cardiac rhythm, may be perpetuated by retinopathic complications (8,9) not originating from preexisting cardiac morbidities.
In addition to examinations of diabetic retinopathy (1–6), studies have investigated the relationship between retinopathy and mortality in regional samples (1), international cohorts (3–5,10), and racially or ethnically diverse populations (7,11). To our knowledge, no study has explored the association between retinopathy and mortality risk within a nationally representative sample of U.S. citizens, with or without preexisting diabetes, which underscores the novelty of our research. To this end, the purpose of this article is to describe our analysis of the independent association of retinopathy to all-cause mortality within a nationally representative sample of the broader U.S. population.
Methods
Design
Data from the 2005–2008 National Health and Nutrition Examination Survey (NHANES) were used. Data from participants in these cycles were linked to death certificate data from the National Death Index via a probabilistic algorithm. Person-months of follow-up were calculated from the date of the interview until the date of death or censoring on 31 December 2011, whichever came first. Study procedures were approved by the National Center for Health Statistics ethics review board, with informed consent obtained prior to data collection.
The NHANES is an ongoing survey conducted by the Centers for Disease Control and Prevention that uses a representative sample of noninstitutionalized U.S. civilians selected by a complex, multistage, stratified, clustered probability design. The design consists of four stages, including the identification of counties and segments (city blocks) within the counties, random selection of households within the segments, and random selection of individuals within the households. Further information on NHANES methodology and data collection is available on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm).
Participants
The analyzed sample included 4,777 participants with complete data on the study variables who also did not have coronary artery disease, congestive heart failure, heart attack, or stroke at the baseline assessment.
Retinopathy
As we have described elsewhere (12,13), retinal imaging was performed using the Canon Non-Mydriatic Retinal Camera CR6-45NM (Canon, Tokyo, Japan). The presence of nonproliferative retinopathy (mild or moderate/severe retinopathy) was determined using the Early Treatment Diabetic Retinopathy Study (ETDRS) grading criteria (14).
Analysis
All statistical analyses, computed in Stata, version 12 (StataCorp, College Station, Tex.), accounted for the complex survey design employed in NHANES. A weighted multivariable Cox proportional hazards model was used, adjusting for age (years; continuous), sex, race/ethnicity (Mexican American, non-Hispanic white, non-Hispanic black, or other), self-reported smoking status (current, former, or never smoker), self-reported physical activity (metabolic equivalent [MET]-minutes/month; continuous), measured BMI (kg/m2; continuous), diabetes status (yes or no), physician-diagnosed hypertension (yes or no), and objectively measured visual acuity (15) (normal vision, uncorrected refractive effort, or vision impairment). With regard to diabetes status, participants were defined as having diabetes if they had a physician’s diagnosis, a fasting blood glucose ≥126 mg/dL, or an A1C ≥6.5% or were taking any diabetes medications. The proportional hazards assumption was checked and confirmed via Schoenfeld residuals. Significance was set at P <0.05.
Results
The duration of the unweighted median follow-up period was 55 months (interquartile range 45–68). For the entire sample, 270,379 person-months occurred. Table 1 displays the weighted characteristics of the study variables.
TABLE 1.
Weighted Characteristics of the Study Variables Across Retinopathy Status (n = 4,777)
| No Retinopathy | Mild Retinopathy | Moderate/Severe Retinopathy | |
|---|---|---|---|
| n | 4,274 | 433 | 70 |
| Age, mean (SE), years | 54.5 (0.3) | 57.8 (0.9) | 57.0 (1.1) |
| BMI, mean (SE), kg/m2 | 28.9 (0.1) | 29.0 (0.3) | 33.2 (1.8) |
| Sex, % female | 54.3 | 45.1 | 43.3 |
| Race/ethnicity, % | |||
| Non-Hispanic white | 77.8 | 68.6 | 50.3 |
| MVPA, mean (SE), MET-minutes/month | 1,986.9 (144.4) | 1,967.7 (437.5) | 1,595.9 (665.2) |
| Diabetes, % | 10.2 | 29.1 | 87.4 |
| Hypertension, % | 36.7 | 42.8 | 55.5 |
| Vision impairment, % | 0.7 | 1.5 | 3.0 |
| Smoker, % | 20.2 | 25.4 | 10.1 |
MVPA, moderate-to-vigorous physical activity.
Table 2 displays the weighted Cox proportional hazard model evaluating the independent associations of retinopathy status on all-cause mortality. Both mild and moderate/severe retinopathy were associated with increased all-cause mortality risk in unadjusted and adjusted models. In the adjusted model, which included diabetes status as a covariate, when compared to those with no retinopathy, those with mild and moderate/severe retinopathy, respectively, had 81% (hazard ratio [HR] 1.81, 95% CI 1.29–2.55) and 314% (HR 4.14, 95% CI 1.77–9.69) increased risks of all-cause mortality.
TABLE 2.
Weighted Cox Proportional Hazard Model Evaluating the Independent Associations of Retinopathy on All-Cause Mortality (n = 4,777)
| Unadjusted | Adjusted* | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Mild versus no retinopathy | 2.54 (1.83–3.52) | <0.001 | 1.81 (1.29–2.55) | 0.001 |
| Moderate/severe versus no retinopathy | 5.83 (3.11–10.93) | <0.001 | 4.14 (1.77–9.69) | 0.002 |
The adjusted model controlled for age (years; continuous), sex, race/ethnicity (Mexican American, non-Hispanic white, non-Hispanic black, or other), self-reported smoking status (current, former, or never smoker), self-reported physical activity (MET-minutes/month; continuous), measured BMI (kg/m2; continuous), diabetes status (yes or no), hypertension (yes or no), and objectively measured visual acuity (normal vision, uncorrected refractive effort, or vision impairment).
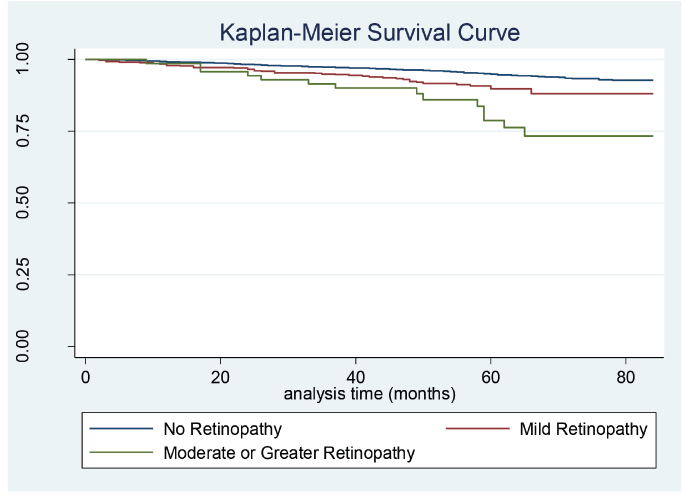
Notably, in a multiplicative interaction model, there was no interaction effect of diabetes status and retinopathy on mortality risk (P = 0.15). Similarly, there was no interaction effect of visual acuity and retinopathy on mortality risk (P = 0.80). The Kaplan-Meier survival curve depicting the probably of survival across retinopathy status is shown in Figure 1.
FIGURE 1.
Kaplan-Meier survival curve depicting the probability of survival based on retinopathy status (n = 4,777).
Discussion
Previous work on this topic in populations not representative of the entire United States has demonstrated that retinopathy status is an independent predictor of early mortality. The purpose of our analysis was to extend these findings by evaluating this topic in a representative cohort of Americans. The main finding of our study was that those with either mild or moderate/severe retinopathy had a substantially elevated risk of early death when compared to those with no retinopathy.
An important strength of this study is the use of objective retinal imaging to provide valid indications of retinopathy. Additionally, use of the ETDRS grading criteria has demonstrated adequate reliability and validity in previous research, although the clinical application of this measure may be limited (6,16). Administration of the ETDRS grading criteria requires specific comparison of retinal imaging to a standard photoset, as well as the use of multilevel scoring criteria (6), which could distort clear communication in clinical practice. This is an exigent concern for ophthalmologists and physicians, given that many patients with retinopathy may be of advanced age or lower education status or reside in rural communities with less proximal access to quality eye care (11).
In fact, the ETDRS grading criteria are not frequently used in treatment facilities because of their infeasibility and the aforementioned translational barriers (6). Effective screening tools and treatments should be more accessible to individuals susceptible to retinopathy because significant visual impairment may be decreased by as much as 90% (16,17) if early prevention efforts are successful. Regular eye exams are necessary to properly identify those who may be at risk, and prompt surgical or nonsurgical interventions must be offered to mitigate the negative complications associated with progressive retinopathy.
This study is a valuable extension of past research, which has identified associations between retinopathy and mortality within various populations. Our work supports previous publications suggesting that retinopathy is independently associated with all-cause mortality in adults but also adds to the growing body of evidence by considering retinopathy in a large, nationally representative U.S. cohort, as well as investigating retinopathy in participants with and without a diagnosis of diabetes. Older adults without diabetes may be at risk for developing ocular-related pathologies (18), including retinopathy, even with normal glucose metabolism. Elevated A1C concentration, high waist-to-hip ratio (WHR), lipid profile (19), and hypertension are all predictors of retinopathy (20). Specifically, an inflated WHR may be a strong determinant of incident retinopathy (21), independent of age, sex, A1C, and hypertension (20).
In the Hoorn Study (20), average hip measurements did not vary significantly between the 17 individuals who became retinopathic in the absence of diabetes or previous impairments in glucose metabolism. Therefore, waist circumference is, perhaps, a more instrumental factor in the genesis of retinopathy. Beyond early detection of retinopathy, early prevention of increasing abdominal adiposity, glucose intolerance, and high blood pressure should be promoted through the lens of regular physical activity and dietary management, especially among the elderly.
In conclusion, this study highlights the independent relationship between retinopathy and all-cause mortality among aging individuals within the larger U.S. population. Our findings, coupled with those of other work in different populations, underscore the importance of promoting health-enhancing strategies (22,23) to prolong survival among those with diabetes (24) and existing retinopathy.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
E.F. prepared the first draft of the manuscript. P.D.L. provided feedback on the manuscript draft and computed the analyses. P.D.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Klein R, Klein BE, Moss SE, Cruickshanks K. Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol 1999;117:1487–1495 [DOI] [PubMed] [Google Scholar]
- 2.Kramer CK, Rodrigues TC, Canani LH, Gross JL, Azevedo MJ. Diabetic retinopathy predicts all-cause mortality and cardiovascular events in both type 1 and 2 diabetes. Diabetes Care 2011;34:1238–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miettinen H, Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Retinopathy predicts coronary heart disease events in NIDDM patients. Diabetes Care 1996;19:1445–1448 [DOI] [PubMed] [Google Scholar]
- 4.Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH; EURODIAB Prospective Complications Study Group. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care 2008;31:1360–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Hecke MV, Dekker JM, Stehouwer CD, et al. ; EURODIAB Prospective Complications Study. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB Prospective Complications Study. Diabetes Care 2005;28:1383–1389 [DOI] [PubMed] [Google Scholar]
- 6.Wilkinson CP, Ferris FL 3rd, Klein RE, et al. ; Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–1682 [DOI] [PubMed] [Google Scholar]
- 7.Hanis CL, Chu HH, Lawson K, et al. Mortality of Mexican Americans with NIDDM: retinopathy and other predictors in Starr County, Texas. Diabetes Care 1993;16:82–89 [DOI] [PubMed] [Google Scholar]
- 8.Ayad F, Belhadj M, Pariés J, Attali JR, Valensi P. Association between cardiac autonomic neuropathy and hypertension and its potential influence on diabetic complications. Diabet Med 2010;27:804–811 [DOI] [PubMed] [Google Scholar]
- 9.Kramer CK, Leitão CB, Canani LH, et al. Late afternoon blood pressure increase is associated with diabetic retinopathy in normotensive type 2 diabetes mellitus patients. Diabetes Res Clin Pract 2009;84:e12–e14 [DOI] [PubMed] [Google Scholar]
- 10.Juutilainen A, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Retinopathy predicts cardiovascular mortality in type 2 diabetic men and women. Diabetes Care 2007;30:292–299 [DOI] [PubMed] [Google Scholar]
- 11.Xu L, Wang YX, Xie XW, Jonas JB. Retinopathy and mortality: the Beijing Eye Study. Graefes Arch Clin Exp Ophthalmol 2008;246:923–925 [DOI] [PubMed] [Google Scholar]
- 12.Loprinzi PD. Association of accelerometer-assessed sedentary behavior with diabetic retinopathy in the United States. JAMA Ophthalmol 2016;134:1197–1198 [DOI] [PubMed] [Google Scholar]
- 13.Loprinzi PD, Brodowicz GR, Sengupta S, Solomon SD, Ramulu PY. Accelerometer-assessed physical activity and diabetic retinopathy in the United States. JAMA Ophthalmol 2014;132:1017–1019 [DOI] [PubMed] [Google Scholar]
- 14.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology 1991;98(Suppl. 5):786–806 [PubMed] [Google Scholar]
- 15.Loprinzi PD, Pariser G, Ramulu PY. Accelerometer-assessed sedentary and physical activity behavior and its association with vision among U.S. adults with diabetes. J Phys Act Health 2014;11:1156–1161 [DOI] [PubMed] [Google Scholar]
- 16.Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy: ETDRS report number 9. Ophthalmology 1991;98:766–785 [PubMed] [Google Scholar]
- 17.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology 1978;85:82–106 [DOI] [PubMed] [Google Scholar]
- 18.Ehrlich R, Harris A, Ciulla TA, Kheradiya N, Winston DM, Wirostko B. Diabetic macular oedema: physical, physiological and molecular factors contribute to this pathological process. Acta Ophthalmol 2010;88:279–291 [DOI] [PubMed] [Google Scholar]
- 19.Chew EY, Klein ML, Ferris FL 3rd, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy: Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol 1996;114:1079–1084 [DOI] [PubMed] [Google Scholar]
- 20.van Leiden HA, Dekker JM, Moll AC, et al. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn Study. Arch Ophthalmol 2003;121:245–251 [DOI] [PubMed] [Google Scholar]
- 21.Porta M, Sjoelie AK, Chaturvedi N, et al. Risk factors for progression to proliferative diabetic retinopathy in the EURODIAB Prospective Complications Study. Diabetologia 2001;44:2203–2209 [DOI] [PubMed] [Google Scholar]
- 22.Richardson JK, Sandman D, Vela S. A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch Phys Med Rehabil 2001;82:205–209 [DOI] [PubMed] [Google Scholar]
- 23.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 2006;29:1433–1438 [DOI] [PubMed] [Google Scholar]
- 24.Armstrong MJ, Sigal RJ, Arena R, et al. Cardiac rehabilitation completion is associated with reduced mortality in patients with diabetes and coronary artery disease. Diabetologia 2015;58:691–698 [DOI] [PubMed] [Google Scholar]