Abstract
PURPOSE
The Mini-Mental State Examination (MMSE) is one of the most widely used instruments to screen for cognitive deficits; however, this instrument alone is not sensitive enough to detect early symptoms of dementia. We aimed to investigate whether additionally using the Visual Association Test (VAT) improves the predictive value of the MMSE score for development of dementia.
METHODS
Analyses were based on data from 2,690 primary care patients aged 70 to 78 years who participated in the Prevention of Dementia by Intensive Vascular Care (preDIVA) trial. We assessed change in the 30-point MMSE score over 2 years and the VAT score at 2 years—dichotomized as perfect (6 points) or imperfect (≤5 points)—and evaluated the predictive values of these tests for a diagnosis of dementia in the subsequent 4 to 6 years. Data were analyzed with logistic regression analysis.
RESULTS
Patients having a decline of 2 points or more in total MMSE score over 2 years had an odds ratio of 3.55 (95% CI, 2.51–5.00) for developing dementia. Patients having the same decline in MMSE score plus an imperfect VAT score had an odds ratio of 9.55 (95% CI, 5.89–15.41) for developing dementia. A 1-point decline in MMSE score increased odds of dementia only when the VAT score was imperfect. Dementia risk for patients with a 2- or 3-point decrease in MMSE score and a perfect VAT score did not differ significantly from the average risk of the cohort as a whole.
CONCLUSIONS
Administering the VAT in patients with a small decline on the MMSE over a 2-year period has substantial incremental value for identifying those at elevated risk for developing dementia. This simple test may help distinguish older adults who need further cognitive examination from those in whom a watchful waiting policy is justified.
Keywords: cognition, dementia, neuropsychological tests, family practice, primary care, practice-based research
INTRODUCTION
Timely diagnosis of dementia is important as it will allow for tailored counseling and care of patients and caregivers.1–3 Clinicians use various instruments to screen for cognitive impairment. The Mini-Mental State Examination (MMSE)4 is widely used in clinical practice and research5 in spite of its limitations, including limited sensitivity for early stages of cognitive impairment.6
When screening for dementia, the implications of a decline of only 1 or 2 points on the MMSE are unclear.7–9 Clinicians are then faced with the question of whether such a modest decrease heralds incipient cognitive decline, which would warrant further investigation.
The Visual Association Test (VAT)10 might be useful for this purpose. It is a very brief (3-minute) and easy-to-administer test of associative memory that is highly sensitive for detecting impaired anterograde memory, without bias based on language skills. It has particularly good test characteristics for the detection of early signs of Alzheimer disease, and it has a higher specificity and positive predictive value for the recognition of dementia than other cued recall tasks.11
In this study, we investigated the predictive value of changes in MMSE score over the course of 2 years for the development of dementia during the 4 to 6 years thereafter and whether adding the VAT improves the overall predictive value.
METHODS
Patients
Our study sample was drawn from the Prevention of Dementia by Intensive Vascular Care (preDIVA) trial.12 This cluster-randomized controlled trial assessed the efficacy of nurse-led intensive vascular care on the prevention of dementia in a primary care population of 3,526 older adults 70 to 78 years old who had a mean follow-up of 6.7 years. Exclusion criteria were prevalent dementia (or MMSE score ≤24 and possible dementia). Carefully instructed practice nurses carried out all assessments. A detailed description of the pre- DIVA study design and procedures has been published elsewhere.12,13 Dementia incidence was not affected by the intervention, so we analyzed the trial population as a single cohort. Patients were excluded if they received a dementia diagnosis before the 2-year assessment or within 3 months thereafter, because the purpose of this study was not to diagnose dementia, but to predict incident dementia on long-term follow-up.
Dementia Diagnosis
All patients had assessments of cognitive status during follow-up at 2-year intervals, supplemented by available clinical information from general practitioners’ electronic health records. An independent and blinded outcome adjudication committee, including neurologists, geriatric psychiatrists, geriatricians, cardiologists, and family physicians, evaluated dementia diagnoses. To minimize the risk of false-positive diagnoses, all dementia diagnoses were reevaluated based on additional information after 1 more year of follow-up.12 For this analysis, patients were classified as having incident dementia if they developed dementia at more than 2 years, 3 months from baseline.
MMSE and VAT
We used the Dutch version of the MMSE,14 which has a maximum obtainable score of 30 points, with higher scores indicating more normal cognition. Change over time in MMSE score was ascertained by comparing the scores at baseline and at the 2-year follow-up assessment.
The VAT test (version A)10 consisted of 6 cue cards (eg, showing an ape) and 6 target cards showing an unexpected visual association such as the ape holding an umbrella (Figure 1). The maximum score is 6 points, with 1 point given for each correctly recalled target. To analyze its added value, we used the VAT score at the 2-year follow-up assessment.
Figure 1.
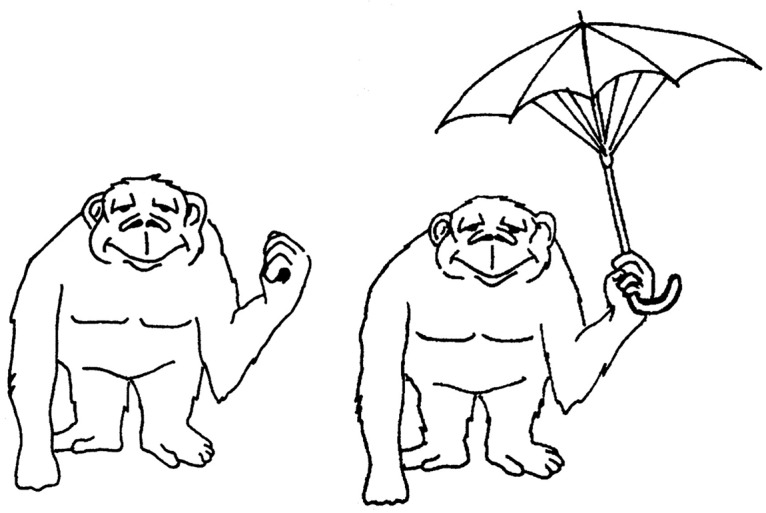
Example of cue and target cards used in the Visual Association Test.
Note: Cue card on the left and target card on the right. First, patients are shown the cue cards one at a time and asked to name the object(s) (eg, “an ape”). Next, patients are shown the target cards and again asked to name the object(s) (eg, “an ape holding an umbrella”). Finally, patients are shown the cue cards again one at a time and asked to name the missing object (eg, “an umbrella”). Patients are not told to remember any objects, so the test requires incidental learning.
Statistical Analysis
Patients were included in analyses if they had available MMSE scores at both baseline and 2-year follow-up, and a VAT score at 2-year follow-up. We performed logistic regression analysis with a diagnosis of dementia as the dependent variable and with dichotomized change in MMSE score—stable or improved (change of −1 point or less) vs decline (change of −2 points or more) and dichotomized VAT score—perfect (score of 6) vs imperfect (score of 5 or lower)—as independent variables separately. This strict cutoff for the VAT score was chosen because the study concerned a cognitively healthy (older) population. We adjusted all analyses for age and educational level, as both factors are known possible confounders for the relation between MMSE score and dementia. Logistic regression analysis allowed us to calculate the cumulative risk over time.
In addition, we performed logistic regression analysis for dementia predicted by combining MMSE score change (stable or improved vs decline) and VAT score (perfect vs imperfect), which created 4 groups of patients. Finally, we assessed the percentage of dementia cases per category of MMSE change score (from −3 points or less, to 3 points or greater), both overall and separately for patients with a perfect VAT score and for patients with an imperfect VAT score.
We used SPSS software, version 23 (IBM Corp) for all analyses.
RESULTS
Study Population
In total, 2,690 (76.3%) of the 3,526 preDIVA trial patients without dementia completed baseline and 2-year follow-up assessments and were included in our analyses. Their sociodemographic and cognitive characteristics are summarized in Table 1. We excluded 14 patients who received a dementia diagnosis before or shortly after the 2-year follow-up visit.
Table 1.
Patients’ Characteristics (N = 2,690)
| Characteristic | Value |
|---|---|
| Age, mean (SD), y | 73.7 (2.4) |
| Male, No. (%) | 1,212 (45.1) |
| Educational levela | |
| Low (<7 y), No. (%) | 591 (22.0) |
| Intermediate (7–12 y), No. (%) | 1,672 (62.2) |
| High (>12 y), No. (%) | 368 (13.7) |
| White, No. (%)b | 2,555 (95.0) |
| MMSE scorec | |
| At baseline, median (IQR) | 29 (27–29) |
| At 2 y, median (IQR) | 29 (27–29) |
| VAT scored | |
| At baseline, median (IQR)e | 6 (5–6) |
| At 2 y, median (IQR) | 6 (5–6) |
Note: Characteristics at baseline unless otherwise noted.
IQR = interquartile range; MMSE = Mini-Mental State Examination; VAT = Visual Association Test.
Data missing for 59 patients.
Data missing for 40 patients.
Scores range from 0 to 30, with higher scores indicating more normal cognitive function.
Scores range from 0 to 6, with higher scores indicating more normal cognitive function.
Data missing for 14 patients.
Overall, 2,648 (98.4%) of the 2,690 patients were evaluated for dementia after a median follow-up time of 6.7 years from baseline. Dementia was diagnosed in 157 patients (5.9%; 95% CI, 5.0%–6.8%) in this group.
Dementia Prediction
The odds ratio for dementia was 3.55 (95% CI, 2.51–5.00) among patients having a 2-year decline in MMSE (change of −2 or greater) and 3.28 (95% CI, 2.35–4.58) among patients having an imperfect VAT score at 2 years (Supplemental Table 1, available at http://www.annfammed.org/content/16/3/206/suppl/DC1/). Compared with the group of patients having better performance on both measures (a stable or improved MMSE score plus perfect VAT score), the group having poorer performance on both measures (a decline in MMSE score plus an imperfect VAT score) had highest risk for incident dementia, with an odds ratio of 9.55 (95% CI, 5.89–15.41).
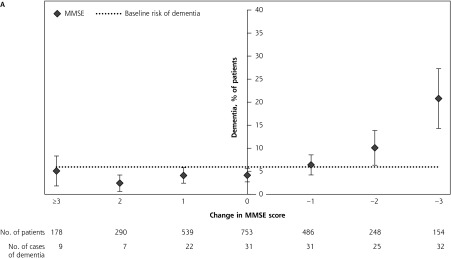
The percentage of patients in whom dementia was diagnosed for each category of change in MMSE score is shown in Figure 2A. Of those who had a stable or improved MMSE score, the risk of developing dementia varied (2.4%–6.4%) around the average risk of 5.9%. A 2-point decline and a 3-point decline in MMSE score, however, were associated with elevated risks of developing dementia of 10.1% and 20.8%, respectively, significantly higher than the average risk.
Figure 2.
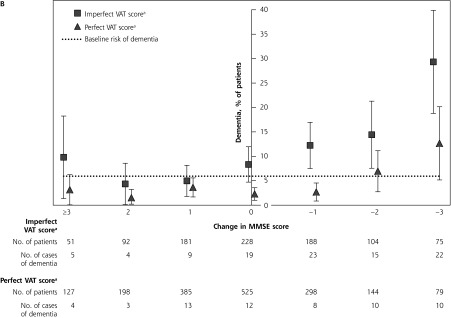
Incident dementia according to change in MMSE score alone (A) and according to change in MMSE score combined with VAT score (B).
MMSE = Mini-Mental State Examination; VAT = Visual Association Test.
Note: Left of center, patients who improved in total MMSE change score over 2 years; right of center, patients who had decline in MMSE change score over 2 years.
a VAT score dichotomized as perfect (6 points) or imperfect (≤5 points).
When comparing dichotomized VAT scores at the 2-year assessment per category of change in MMSE score (Figure 2B), groups with imperfect VAT scores (≤5 points) all had substantially higher rates of incident dementia (Figure 2A). An imperfect VAT score increased the predictive value of a 2- or 3-point decrease on the MMSE substantially, from 10.1% to 14.4% and from 20.8% to 29.3%, respectively. Even among patients who had a decline of 1 point on the MMSE score, an imperfect score on the VAT doubled the risk to 12.2% (95% CI, 7.5%–17.0%). In contrast, the risk of developing dementia for patients with a 2- or 3-point decrease on the MMSE score and a perfect VAT score was not significantly different from the average risk of the cohort as a whole (Figure 2B).
DISCUSSION
Key Findings
Our findings show that the VAT score has incremental value in discriminating between older adults with and without increased risk of dementia, especially among those with a (minor) decline in MMSE score. The risk of developing dementia for older adults with a decrease of 2 points or more on the MMSE and a perfect VAT score was not significantly different from the average risk of the entire cohort.
There have been no studies analyzing the additional value of the VAT after performing the MMSE, even though the MMSE seems unreliable in predicting and detecting (early) dementia.6 The VAT was developed specifically for this purpose, and it has a higher specificity and positive predictive value for detecting dementia when compared with other cognitive tests.11 In our analyses, an imperfect VAT in patients with a decline of only 1 or 2 points in MMSE score was associated with a significant and clinically meaningful increased risk of dementia.
Strengths and Limitations
The strengths of this study are the large sample size, the long follow-up period, the blinded and careful adjudication of dementia diagnoses (including a 1-year follow-up after the initial diagnosis), and completeness of follow-up on all-cause dementia.12 In addition, we used a clinical perspective in assessing instruments that can be administered easily in daily practice.
A study limitation was that patients were included in the analysis only if they had MMSE assessments at both baseline and 2-year follow-up, and a VAT assessment at the 2-year follow-up. This requirement led to a smaller sample in our study than in the original study and possibly to selection bias. In addition, only version A of the VAT was mandatory and could thus be used for analysis.
Conclusion
Among older adults with a minor decline on the MMSE, the VAT—a 3-minute easily administered test of associative memory—can help to distinguish those at increased risk of developing dementia (requiring counseling, additional examination, or both) from those in whom watchful waiting is justified.
Acknowledgments
The authors want to thank the preDIVA participants and nurses for their work and participation. Also, we want to thank Dr M. Hoevenaar-Blom for her statistical advice.
Footnotes
Conflicts of interest: authors report none.
Author contributions: W.A.vG. designed the study. S.J. conducted the statistical analysis and literature search and wrote the article. All authors contributed intellectually to the writing or revising of the manuscript, and approved the final version.
Trial registration: The preDIVA trial is registered with ISRCTN, number ISRCTN29711771.
Supplementary materials: Available at http://www.AnnFamMed.org/content/16/3/206/suppl/DC1/.
Funding support: The preDIVA study was funded by the Dutch Ministry of Health, Welfare and Sport; Dutch Innovation Fund of Collaborative Health Insurances; and Netherlands Organisation for Health Research and Development.
References
- 1.Cordell CB, Borson S, Boustani M, et al. Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement. 2013; 9(2): 141–150. [DOI] [PubMed] [Google Scholar]
- 2.Baruch N, Allan CL, Cundell M, Clark S, Murray B. Promoting early dementia diagnosis: a video designed by patients, for patients. Int Psychogeriatr. 2017; 29(5): 863–867. [DOI] [PubMed] [Google Scholar]
- 3.Khanassov V, Vedel I. Family physician-case manager collaboration and needs of patients with dementia and their caregivers: A systematic mixed studies review. Ann Fam Med. 2016;14(2):166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12(3): 189–198. [DOI] [PubMed] [Google Scholar]
- 5.Lin JS, O’Connor E, Rossom RC, et al. US Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Screening for Cognitive Impairment in Older Adults: An Evidence Update for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 6.Pendlebury ST, Cuthbertson FC, Welch SJ, Mehta Z, Rothwell PM. Underestimation of cognitive impairment by Mini-Mental State Examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: a population-based study. Stroke. 2010; 41(6): 1290–1293. [DOI] [PubMed] [Google Scholar]
- 7.Kopecek M, Bezdicek O, Sulc Z, Lukavsky J, Stepankova H. Montreal Cognitive Assessment and Mini-Mental State Examination reliable change indices in healthy older adults. Int J Geriatr Psychiatry. 2017; 32(8): 868–875. [DOI] [PubMed] [Google Scholar]
- 8.Hensel A, Luck T, Luppa M, Glaesmer H, Angermeyer MC, Riedel-Heller SG. Does a reliable decline in Mini Mental State Examination total score predict dementia? Diagnostic accuracy of two reliable change indices. Dement Geriatr Cogn Disord. 2009; 27(1): 50–58. [DOI] [PubMed] [Google Scholar]
- 9.Stein J, Luppa M, Maier W, et al. ; AgeCoDe Study Group. Assessing cognitive changes in the elderly: reliable change indices for the Mini-Mental State Examination. Acta Psychiatr Scand. 2012; 126(3): 208–218. [DOI] [PubMed] [Google Scholar]
- 10.Lindeboom J, Schmand B, Tulner L, Walstra G, Jonker C. Visual association test to detect early dementia of the Alzheimer type. J Neurol Neurosurg Psychiatry. 2002; 73(2): 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs A, Wiese B, Altiner A, Wollny A, Pentzek M. Cued recall and other cognitive tasks to facilitate dementia recognition in primary care. J Am Geriatr Soc. 2012; 60(1): 130–135. [DOI] [PubMed] [Google Scholar]
- 12.Moll van Charante EP, Richard E, Eurelings LS, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet. 2016; 388(10046): 797–805. [DOI] [PubMed] [Google Scholar]
- 13.Richard E, Van den Heuvel E, Moll van Charante EP, et al. Prevention of dementia by intensive vascular care (PreDIVA): a cluster-randomized trial in progress. Alzheimer Dis Assoc Disord. 2009; 23(3): 198–204. [DOI] [PubMed] [Google Scholar]
- 14.Kok RM, Verhey FRJ. Dutch translation of the Mini Mental State Examination. http://www.tijdschriftvoorpsychiatrie.nl/assets/measuringinstruments/meetinstrumenten_115pdf.pdf Published 2002. Accessed May 22, 2017.