Abstract
Objective
To define modified Prenatal Growth Assessment Scores [mPGAS] for single and composite biometric parameters and determine their reference ranges in normal fetuses.
Methods
Nine anatomical parameters [ap] were measured and the weight estimated [EWTa, EWTb] in a longitudinal study of 119 fetuses with normal neonatal growth outcomes. Expected third trimester size trajectories, obtained from second trimester Rossavik size models, were used in calculating Percent Deviations [% Dev’s] and their age-specific reference ranges in each fetus. The components of individual % Dev’s values outside their reference ranges, designated +iapPGAS, −iapPGAS, were averaged to give +apPGAS and −apPGAS values for the 3rd trimester. The +iapPGAS and −iapPGAS values for different combinations of anatomical parameters [c1a (HC, AC, FDL, ThC, EWTa), c1b (HC, AC, FDL, ThC, EWTb), c2 (ThC, ArmC, AVol, TVol), c3 (HC, AC, FDL, EWTa)] were then averaged to give +icPGAS and −icPGAS values at different time points or at the end of the third trimester [+cPGAS, −cPGAS]. Values for iapPGAS, ic1bPGAS, and ic2PGAS were compared to their respective apPGAS or cPGAS reference ranges.
Results
All mPGAS values had one 95% range boundary at 0.0%. Upper boundaries of 1D +apPGAS values ranged from 0.0% [HC] to +0.49% [ThC] and were +0.06%, +2.3% and +1.8% for EWT, AVol and TVol, respectively. Comparable values for –apPGAS were 0.0% [BPD, FDL, HDL], to −0.58% [ArmC], −0.13% [EWT], −0.8% [AVol], and 0.0% [TVol]. The +cPGAS, 95% reference range upper boundaries varied from +0.36% [c1b] to +0.89% [c2]. Comparable values for –cPGAS lower boundaries were −0.17% [c1b] to −0.43% [c2].
Conclusions
The original PGAS concept has now been extended to individual biometric parameters and their combinations. With the standards provided, mPGAS values can now be tested to see if detection of different types of third trimester growth problems is improved.
Keywords: individualized growth assessment, pregnancy, Rossavik models, size standards
INTRODUCTION
In current clinical practice, fetal growth in the third trimester is most commonly evaluated by determining estimated fetal weight [EFW] [1–5]. Previous investigators have established weight estimation functions [6–18], developed EFW standards [19–43] and used EFW to predict neonatal outcomes [4, 44–79]. However, several studies using Individualized Growth Assessment [IGA] have reported that in different fetuses and neonates, growth abnormalities affect anatomical parameters other than EFW [80–94].
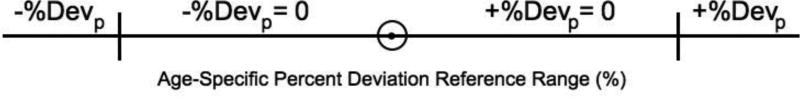
The Prenatal Growth Assessment Score (PGAS) was developed to solve this problem, utilizing a set of anatomical measurements {head circumference (HC), abdominal circumference (AC), femur diaphysis length (FDL), mid-thigh circumference (ThC) and estimated weight (EWT)} [95]. As originally defined, the PGAS was based on positive or negative pathological percent deviations {+%Devp, −% Devp (Figure 1)} [95]. All % Dev values below the upper boundary (+%Devp calculation), or above the lower boundary (−%Dev calculation), of a reference range were assigned a % Devp value of zero. The average of all available +%Devp or −% Devp values {up to the time in pregnancy being evaluated} was defined as either the +PGASAt or −PGASAt. The PGAS values for the entire third trimester were designated +PGASAT or −PGASAT.
Figure 1.
Definition of pathological percent deviations. Positive and negative parts of the age-specific reference range for a given anatomical parameter were subtracted from the Percent Deviation value to give the +% Devp and −%Devp values
The original PGAS was capable of detecting different types of fetal growth problems except those limited to only soft tissue abnormalities [82, 95]. Moreover, it is limited by reference ranges determined from all 3rd trimester data without regard for age-specific differences [96] and it was initially proposed for only one combination of anatomical parameters [95]. Equal weight is given to each component of this combination. In a recent IGA evaluation of fetal growth with normal neonatal growth outcomes, methods for calculating age-specific % Dev reference ranges were developed, allowing evaluation of % Dev values for an anatomical parameter at individual third trimester time points [94]. This new capability makes it possible to follow the development of growth problems in specific anatomical structures [e.g. HC in hydrocephalus] on an individualized basis [use of %Devp]. With the addition of new soft tissue measurements [88–90] and a new weight estimation procedure [97] to our biometric measurements, it is now possible to explore how new combinations of size parameters can be used to evaluate growth abnormalities. Growth studies in both fetuses [86] and neonates [87, 98], as well as the known biology of growth abnormalities [99–110], have shown that some anatomical parameters are more frequently affected in different pathological states and therefore are more important in detecting either IUGR or Macrosomia [93, 111–115]. A more suitable PGAS would be one that had a specific weighting factor for each anatomical parameter, reflecting its importance in identifying different types of abnormal growth.
The main objective of this investigation was to re-define the PGAS so that a variety of single and composite size parameters, could be utilized in evaluating fetal growth abnormalities. We also wanted to enable future use of different weighting factors for anatomical parameters and provide new mPGAS reference ranges for individual anatomical parameters and their combinations..
METHODS
Data
The data used for this investigation were obtained in a previous longitudinal study of size parameters in fetuses with normal neonatal growth outcomes, as determined from the modified Neonatal Growth Assessment Score (a composite neonatal size parameter [87, 116]) and a sample-specific reference range [94]. The m3NGAS51 values were calculated from predicted and measured neonatal head circumference [HC], abdominal circumference [AC], mid-thigh circumference [ThC], crown-heel length [CHL] and weight [WT] values obtained as previously described [87]. As specified previously [87], the m3NGAS51 is the third mNGAS studied, contains five size variables and would be the first of a set of mNGAS’s containing five variables if there were more than five variables.
Serial ultrasound examinations were carried out from approximately 18 weeks to 38 weeks, menstrual age [6–7 scans/fetus]. Measurements of the HC, AC, FDL, ThC, biparietal diameter [BPD], humerus diaphysis length [HDL] mid-arm circumference [ArmC], fractional arm volume [AVol] and fractional thigh volume [TVol] were obtained at each scan using previously described methods [94]. Head cubes [Hcube] and abdominal cubes [Acube] were calculated from respective profile diameter measurements [see Appendix for calculations and profile diameter estimation procedures]. Estimated weight was calculated from Hcube and Acube values (EWTa) [117] and from BPD, AC and TVol measurements (EWTb) [97]. Fetal age was primarily determined from first trimester crown-rump length [CRL] measurements [94].
In the second trimester, Rossavik size models [P = c(t)k+st] were specified by determining values for the coefficients c, k and s for each anatomical parameter in every fetus using the methods described by Deter et al [118]. The time variable t was defined as the menstrual age (MA) minus the start point [SP]: t = MA – SP where the SP was calculated from the coefficients of the linear function fit the data obtained before 28.2 weeks, MA [SP= −a0/a1] [118]. These models were used to generate predicted 3rd trimester size trajectories for all anatomical parameters in each fetus.
Third trimester size measurements were compared to those predicted and Percent Deviations calculated using the following equation [86]:
Data Analysis (see additional details in Supplementary File S1)
A. Generation of mPGAS values
Percent Deviation variance components, obtained using two-level random coefficient modeling [94], and the function of Royston [119] were used to generate age-specific variances (VarTi).
The age-specific 95% reference range (95 % r ri) is then given by the following function:
| [1] |
The positive and negative parts of the age-specific reference range for a given anatomical parameter [ap] were subtracted from the Percent Deviation value to give the +% Devp and −%Devp values (Figure 1).
| [2] |
| [3] |
If the +%Devpi had a negative value, it was set equal to zero, as were the positive −%Devpi values. The +%Devpi and −%Devpi values of a specific anatomical parameter [ap] for a given fetus (j) at individual time points [i] were designated +iapPGASj and –iapPGASj [definitions of all mPGAS’s are given in Table 1]. These two classes of iapPGASj values were averaged to give the +apPGASj and –apPGASj values for the specified fetus over the entire 3rd trimester [Table 1].
Table 1.
Definition of Modified Prenatal Growth Assessment Scores
| PGAS Parameter | Abbreviation | Definition |
|---|---|---|
|
| ||
| Individual anatomical parameter | iapPGAS | pathological Percent Deviation [% Devp] for a given anatomical parameter at a specified third trimester time point [can be positive or negative] |
|
| ||
| Anatomical parameter | apPGAS | mean value of the % Devp values obtained during the third trimester for a specific anatomical parameter [can be positive or negative] |
|
| ||
| Individual composite | icPGAS | mean value of the %Devp values obtained at a specified third trimester time point for a set of anatomical parameters [can be positive or negative] |
|
| ||
| Composite groups | cPGAS | mean value of the % Devp values obtained during the third trimester for a specified set of anatomical parameters [can be positive or negative] |
| c1aPGAS | ||
| HC, AC, ThC. FDL, EWTa [Hcube, Acube] | ||
| c1bPGAS | ||
| HC, AC, ThC. FDL, EWTb [BPD, AC, TVol] | ||
| c2PGAS | ||
| ArmC, AVol, ThC and TVol | ||
| c3PGAS | ||
| HC, AC, FDL, EWTa [Hcube, Acube] | ||
BPD = biparietal diameter, HC = head circumference, AC = abdominal circumference, FDL = femur diaphysis length; ThC = mid-thigh circumference; TVol = fractional thigh volume; EWT = estimated weight; Hcube = head short axis × head long axis)1.5; Acube = (abdominal short axis × abdominal long axis)1.5; +% Devp and −% Devp : component of % Deviation outside upper and lower limits of age-specific % Deviation reference range, respectively
To obtain +cPGASj and −cPGASj values for different combinations of anatomical parameters [Table 1: c1a, c1b, c2, c3], the apPGASj values for the specified parameters were averaged. During pregnancy, +cPGAS and −cPGAS were calculated at each time point [+icPGASj, −icPGASj] using iapPGASj values.
B. Reference Ranges
The 95% reference ranges for positive and negative apPGAS’s were determined for the 10 anatomical parameters studied. Since both types of apPGAS’s have one limit at zero, the other limit was defined by sorting positive and negative values by size and then identifying the boundary that eliminated the largest 5% [in absolute value] (Figure 1). The 95% reference ranges for four composite PGAS values, c1aPGAS, c1bPGAS, c2PGAS and c3PGAS (see Table 1 for definitions), were determined in a similar manner.
The relationships of iapPGAS values to menstrual age were evaluated with linear correlation. The use of reference ranges based on all 3rd trimester values [apPGAS] for evaluating iapPGAS’s at different time points was tested by determining how many values were considered abnormal with these reference ranges. Similar assessments of ic1bPGAS’s and ic2PGAS’s calculated at different time points in the 3rd trimester were carried out as these cPGAS values were considered to be the most and least reliable representatives of the cPGAS’s.
RESULTS
Anatomical Parameter Prenatal Growth Assessment Scores (apPGAS)
As shown in Table 2, the upper limits of the +apPGAS 95% reference ranges were between 0.0% [HC] and 0.49% [ThC] with the exceptions of AVol [2.30%] and TVol [1.76%]. For the −apPGAS, the lower boundaries of the 95% reference ranges varied from 0.0% [BPD, FDL, HDL, TVol] to −0.58% [ArmC], with boundary values for the 3D parameters that were similar to those for the 1D parameters.
Table 2.
Anatomical Parameter Prenatal Growth Assessment Score (apPGAS)
| 95% RANGE | PERCENT EXCLUDED | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Parameter | N | +apPGAS | −apPGAS | No. Scans | +iapPGAS | −iapPGAS |
|
| ||||||
| % | % | % | % | |||
| BPD | 117 | 0.0 to +0.38 | 0.0 to −0.00 | 400 | 2.8 | 1.0 |
| HC | 117 | 0.0 to +0.00 | 0.0 to −0.11 | 400 | 1.1 | 2.5 |
| AC | 118 | 0.0 to +0.02 | 0.0 to −0.05 | 403 | 2.0 | 2.5 |
| FDL | 117 | 0.0 to +0.27 | 0.0 to −0.00 | 401 | 3.7 | 1.0 |
| ThC | 113 | 0.0 to +0.49 | 0.0 to −0.04 | 388 | 2.3 | 1.8 |
| HDL | 118 | 0.0 to +0.08 | 0.0 to −0.00 | 402 | 2.2 | 1.7 |
| ArmC | 118 | 0.0 to +0.17 | 0.0 to −0.58 | 402 | 2.5 | 3.0 |
| AVol | 118 | 0.0 to +2.30 | 0.0 to −0.80 | 402 | 3.7 | 1.7 |
| TVol | 118 | 0.0 to +1.76 | 0.0 to −0.00 | 403 | 3.5 | 1.2 |
| EWT | 117 | 0.0 to +0.06 | 0.0 to −0.13 | 399 | 2.0 | 2.3 |
BPD = biparietal diameter, HC = head circumference, AC = abdominal circumference, FDL = femur diaphysis length; ThC = mid-thigh circumference; TVol = fractional thigh volume; EWT = estimated weight; N = number of subjects scanned; -apPGAS and +apPGAS refers to mean values of % Devp values for a specific anatomical parameter [positive or negative]; +iapPGAS and –iapPGAS refer to pathological Percent Deviation [% Devp] for a given anatomical parameter at a specified third trimester time point [positive or negative]; 95% reference ranges were determined by sorting.
Percent Excluded: proportion of iapPGAS values excluded from the reference range using apPGAS reference range values
Linear correlations of +iapPGAS values against MA were not statistically significant except for ArmC [R: −0.11]. For −apPGAS, only those for HC [R: −0.11] and ArmC [R: −0.12] were statistically significant. Table 2 presents the proportion excluded if the apPGAS reference ranges were used as the boundaries between normal and abnormal values at all menstrual ages. For +iapPGAS, the excluded proportion varied from 1.1% to 3.7%. Comparable values for −iapPGAS were 1% to 3%. A smaller proportion of –iapPGAS values were excluded for 6/10 anatomical parameters but these differences between +iapPGAS and –iapPGAS were small.
Composite Prenatal Growth Assessment Scores (cPGAS)
Table 3 presents the reference ranges for the four composite PGAS’s [c1aPGAS, c1bPGAS, c2PGAS, c3PGAS]. The first combination [HC, AC, ThC, FDL, EWT {Hcube, Acube}] is essentially the same as the original PGAS [95] and had 95% reference ranges of 0.0% to +0.39 % for +c1aPGAS and 0.0% to −0.18 % for –c1aPGAS. The second combination [the same as c1aPGAS except that EWT {Hcube, Acube} was replaced by EWT {BPD, AC, TVol}] had 95% reference ranges of 0.0% to +0.36% and 0.0% to −0.17% for +c1bPGAS and –c1bPGAS, respectively. For the soft tissue combination [c2PGAS], composed of ArmC, AVol, ThC and TVol, the corresponding 95% reference ranges were 0.0% to +0.94% and 0.0% to −0.43%. Finally, the conventional biometry composite PGAS [c3PGAS] had 95% reference ranges for +c3PGAS and –c3PGAS of 0.0% to +0.45% and 0.0% to −0.19%. As can be seen, all reference range boundaries were less than 1.0%
Table 3.
Composite Prenatal Growth Assessment Score (cPGAS)
| 95% RANGE | PERCENT EXCLUDED | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Parameter | N | +cPGAS % |
−cPGAS % |
No. Scans | +icPGAS % |
−icPGAS % |
| c1a | 112 | 0.0 to +0.39 | 0.0 to −0.18 | --- | --- | --- |
| c1b | 112 | 0.0 to +0.36 | 0.0 to −0.17 | 385 | 5.0 | 3.1 |
| c2 | 113 | 0.0 to +0.89 | 0.0 to −0.43 | 387 | 6.7 | 4.4 |
| c3 | 112 | 0.0 to +0.45 | 0.0 to −0.19 | -- | --- | --- |
BPD = biparietal diameter, HC = head circumference, AC = abdominal circumference, FDL = femur diaphysis length; ThC = mid-thigh circumference; TVol = fractional thigh volume; EWT = estimated weight; N = number of subjects scanned; c1a = HC, AC, ThC. FDL, EWTa [Hcube, Acube]; c1b = HC, AC, ThC. FDL, EWTb [BPD, AC, TVol]; c2 = ArmC, AVol, ThC and TVol; c3 = HC, AC, FDL, EWT [Hcube, Acube]; Hcube = (head short axis × head long axis)1.5; Acube = (abdominal short axis × abdominal long axis)1.5; 95% reference ranges were determined by sorting.
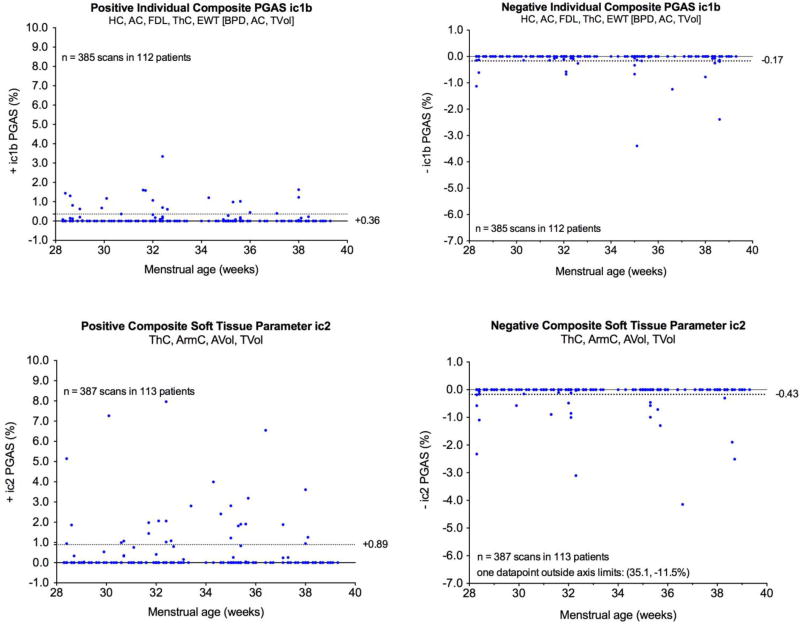
There were 385 time points in 112 fetuses that had complete sets of measurements for calculating ic1bPGAS values. For +ic1bPGAS, the upper boundary of the 95% reference range was +0.38% while for –ic1bPGAS, the lower boundary of the 95% reference range was −0.14%. In Figure 2, the ic1bPGAS values are plotted against fetal age at the time of scan. The upper boundary of the +c1bPGAS and lower boundary of the –c1PGAS are shown as interrupted lines. Linear correlations were not significant for either +ic1bPGAS or –ic1bPGAS and the c1bPGAS boundaries excluded 5.0% of the +ic1bPGAS values and 3.1% of the –ic1bPGAS values, respectively [Table 3].
Figure 2.
Evaluation of individual composite PGAS values for ic1bPGAS and ic2PGAS. These specific examples demonstrate composite parameters that include at least one soft tissue component such as fractional arm volume (AVol), fractional thigh volume (TVol), mid-arm circumference (ArmC), and mid-thigh circumference (ThC).
The ic2PGAS values could be calculated at 387 time points in 113 fetuses. For +ic2PGAS, the upper boundary of the 95% reference range was +1.3%. For –ic2PGAS, the lower boundary of the 95% reference range was −0.24%. In Figure 2, the ic2PGAS values are plotted as a function of fetal age at the time of scan. Linear correlations were not statistically significant for either +ic2PGAS or –ic2PGAS. Use of the upper boundary of the +c2PGAS excluded 6.7% of the +ic2PGAS values while use the lower boundary of –c2PGAS excluded 4.4% of the –ic2PGAS values [Table 3].
Table 4 gives examples of mPGAS values for fetuses with normal and abnormal 3rd trimester growth (pathological values given in red). The –iapPGAS values for different anatomical parameters are presented, together with the –ic1aPGAS values (far-right column) at specific third trimester time points. The last row of each table gives the –apPGAS values for different anatomical parameters at the end of the 3rd trimester. The average of all 3rd trimester –apPGAS values (−c1aPGAS) is presented in the lower, far-right corner. Differences between normal and abnormal growth are clearly illustrated, as is the evolution of growth abnormalities in different size measures.
Table 4.
Fetal Growth Evaluation Using mPGAS
| Fetus 1: Normal Growth in the Third Trimester | |||||||
|---|---|---|---|---|---|---|---|
| MA | −ihcPGAS1 | −iacPGAS | −ifdlPGAS | −ithcPGAS | −iewtPGAS | −ic1aPGAS2 | |
| wks | % | % | % | % | % | % | |
| 28.3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 32.3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 35.3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 38.1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| −apPGAS3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −c1aPGAS4 | 0.00 |
| Fetus 2: IUGR in the Third Trimester | |||||||
|---|---|---|---|---|---|---|---|
| MA | −ihcPGAS | −iacPGAS | −ifdlPGAS | −ithcPGAS | −iewtPGAS | −ic1aPGAS | |
| wks | % | % | % | % | % | % | |
| 27.7 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 30.7 | −1.40* | −4.76* | 0.00 | −0.63* | −1.30* | −1.62* | |
| 32.7 | 0.00 | 0.00 | 0.00 | −5.27* | 0.00 | −1.05* | |
| −apPGAS | −0.47* | −1.59* | 0.00 | −1.97* | −0.33* | −c1aPGAS | −0.87* |
−iapPGAS: negative individual Prenatal Growth Assessment Score for different anatomical parameters (ap)
−ic1aPGAS: average of the five −iapPGAS values at specific time points
−apPGAS: average of all −iapPGAS values for specified anatomical parameter
−c1aPGAS: average of the five −apPGAS values
Abnormal values displayed in
 with asterisk (*)
with asterisk (*)
DISCUSSION
Principal findings of the study
This investigation defines different types of modified Prenatal Growth Assessment Scores [mPGAS] for ten anatomical parameters and four combinations of these parameters. Using data from a previous study in fetuses with normal neonatal growth outcomes, reference ranges for the mPGAS at individual 3rd trimester time points [+iapPGAS, −iapPGAS], and at the end of the third trimester [+apPGAS, −apPGAS] were established for each anatomical parameter. Reference ranges for composite mPGAS’s, both at individual 3rd trimester time points [+icPGAS, −icPGAS] and at the end of the third trimester [+cPGAS, −cPGAS] were also determined. This mPGAS system has been partially implemented as part of a freely available software download [iGAP] that can be accessed at http://igap.research.bcm.edu.
Previous Studies of PGAS
Only two studies [82, 95] have provided boundary values for a cPGAS and only lower limit values for –c1aPGAS was defined. Our 95% lower boundary of −0.18% was in agreement with previous boundary values [−0.24%, −0.4%]. The 95% lower boundary of −c1bPGAS [−0.17%] observed in this investigation was also consistent with the –c1aPGAS lower boundary values. The upper boundaries of both +c1aPGA and +c1bPGAS [+0.39%, +0.36%] were similar. The 95% lower boundary of −c2PGAS [−0.38 %] was close to those for –c1aPGAS but the 95% upper boundary for +c2PGAS [+0.74 %] was somewhat higher. c3PGAS upper and lower boundaries were similar to those for c1aPGAS and c1bPGAS. All boundary values were less than 1%. These results suggest that cPGAS values will be sensitive indicators of growth abnormalities.
Modified PGAS’s for Individual Anatomical Parameters (apPGAS)
Originally, PGAS was designed for size assessments involving a combination of anatomical parameters [95]. Results from our current investigation demonstrate that the concept underlying PGAS [evaluation of pathological Percent Deviations [95] can now be extended to individual anatomical parameters. The use of apPGAS boundaries [more reliable since they based on means of iapPGAS values obtained during the 3rd trimester] excluded less than 5% of the iapPGAS values, indicating that they represent conservative criteria for detecting abnormal iapPGAS values. A quantitative, overall assessment of 3rd trimester growth is now provided for individual anatomical parameters by the apPGAS’s. Sequential calculation of +apPGAS and –apPGAS values after each time point studied provides a running summary of individual parameter growth processes during the third trimester.
Modified PGAS for Specified Sets of Anatomical Parameters (cPGAS)
Given the heterogeneity of growth abnormalities [86], using sets of anatomical parameters may be more effective in evaluating such problems since different anatomical parameters are sensitive to different types of growth abnormalities [86, 87, 99–101, 120–127] [Table 1]. The original, global PGAS [c1aPGAS] was effective in identifying twins with IUGR unless only soft tissue was affected [82, 95]. This latter type of growth problem may now be detected using our new soft tissue PGAS [c2PGAS]. Detection of early changes in soft tissue occurring in IUGR and Macrosomia [98, 99, 128–130] could be important in the prediction of subsequent metabolic and cognitive abnormalities in children and adults. A recent study has shown that term SGA neonates with normal umbilical Doppler findings have increased risks for axonal loss and cognitive deficits at 6 years of age [131].
Modified PGAS for Individual Sets of Anatomical Parameters (icPGAS)
The icPGAS provides a means for quantitatively assessing size at a specific time point using any set of anatomical parameters. By averaging the icPGAS values obtained at all-time points up to the current scan, one obtains a running summary of how the type of growth represented by the set of anatomical parameters is progressing. The use of icPGAS during the 3rd trimester requires definition of a boundary between normal and abnormal values. The use of the boundaries for the cPGAS reference ranges at the end of the 3rd trimester provide conservative criteria for detecting abnormal fetal growth based on individual composite PGAS values at different third trimester time points.
Novel aspects of this investigation
This evaluation of fetal growth is based on Individualized Growth Assessment [each fetus is its own control] that accounts for differences in growth potential and minimizes variability between fetuses. The primary measure, pathological Percent Deviations, takes into account the normal variation associated with each measurement of a Percent Deviation. As reference ranges are now provided for both single and combinations of ten anatomical parameters, at specific time points and at the end of the 3rd trimester, the mPGAS system gives new options for analyzing fetal growth patterns.
Study Limitations
This investigation was limited by the unavailability of pathological cases. These cases are needed to determine weighting factors for the components of composite mPGAS’s. Accordingly, the optimal mPGAS values for separating normally growing fetuses from those with growth abnormalities cannot be established.
Implications for research and clinical care
By adjusting for differences in growth potential and minimizing variability at individual 3rd trimester time points, the mPGAS provides a versatile tool for evaluating fetal growth abnormalities. The characterization of fetal growth abnormalities made possible by this approach could result in more specific links between growth abnormalities and perinatal complications. With such information, the clinician would have a better assessment of risk for fetuses with specific types of growth abnormalities. This should enhance clinical decision-making in these complicated pregnancies.
Conclusions
A modified Prenatal Growth Assessment Score has been used to evaluate third trimester pathological Percent Deviations values and the concept is now been extended to individual anatomical parameters and new combination of these parameters. This novel procedure permits the generation of scores for different sets of anatomical parameters at both specified time points and at the end of the third trimester. Third trimester reference ranges are also specified for both individual anatomical parameters and for four different sets of combined parameters. We hypothesize that the mPGAS will improve detection and monitoring of abnormal third trimester growth that is expressed differently in individual fetuses.
Supplementary Material
Acknowledgments
The Authors wish to acknowledge the technical assistance of Melissa Powell, RDMS and Beverley McNie, BS, CCRP
Declaration of interest:
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
APPENDIX
ESTIMATION OF HEAD AND ABDOMINAL PROFILE DIAMETERS FROM CONVENTIONAL FETAL ULTRASOUND MEASUREMENTS
The application of Individualized Growth Assessment [IGA] to estimated weight [EWT] requires special weight estimation procedures. At present, only the two methods described by Deter et al [117] and Lee et al [97] have been used in IGA analyses. The former is based on measurements of Head Cube and Abdominal Cube parameters. This requires Head and Abdominal Profile Diameter measurements as the cubes are calculated in the following manner [132]:
where HSA and ASA are the head and abdominal short axes, HLA and ALA are the head and abdominal long axes, and FOD is the fronto-occipital diameter. The method of Lee et al estimates fetal weight from measurements of the BPD, AC and TVol [97]. Both of these weight estimation procedures have similar reference ranges.
The Deter method is limited because profile diameters are usually not recorded, although available, when HC and AC measurements are made using an elliptical measurement tool. In the Lee method, a 3D ultrasound system must be used to measure TVol. To facilitate the use of IGA with EWT, a procedure for estimating profile diameters from conventional BPD, HC and AC measurements has been developed.
A study was carried out using 123 fetuses studied longitudinally [17–41 weeks, MA] as described previously [94]. The majority [68.3%] had normal neonatal growth outcomes and 993–994 measurements of BPD, HSA, HLA, FOD, HC, ASA, ALA, and AC [HC and AC measured using the elliptical tool] were available for analysis. Algebraic re-arrangement and regression analysis, as described previously [133], were used to obtain the following equations for generating estimates of the needed diameters:
| estimated HSA = 0.09071 + (1.03216 × BPD) | R2 =99.5% |
| estimated HLA = 0.79884 ((HC2 / 4.9298) – estimated HSA)1/2 | |
| estimated FOD = 0.29737 + (0.91567 × estimated HLA) | R2 = 98.3% |
| estimated abdominal mean diameter [AMD] = AC / 3.14 | |
| estimated ASA = 0.13993 + (0.920513 × estimated AMD) | R2 = 96.5% |
| estimated ALA = 0.02208 + (1.01794 × estimated AMD) | R2 =97.7% |
These head and abdominal diameter estimates are used to calculate Hcube and Acube parameters, which can then be used to estimate weight when direct diameter measurements are not available for this purpose.
References
- 1.Nyberg DA, Abuhamad A, Ville Y. Ultrasound assessment of abnormal fetal growth. Semin Perinatol. 2004;28:3–22. doi: 10.1053/j.semperi.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Dudley NJ. A systematic review of the ultrasound estimation of fetal weight. Ultrasound Obstet Gynecol. 2005;25:80–9. doi: 10.1002/uog.1751. [DOI] [PubMed] [Google Scholar]
- 3.Melamed N, Yogev Y, Meizner I, Mashiach R, Bardin R, Ben-Haroush A. Sonographic fetal weight estimation: which model should be used? J Ultrasound Med. 2009;28:617–29. doi: 10.7863/jum.2009.28.5.617. [DOI] [PubMed] [Google Scholar]
- 4.Mikolajczyk RT, Zhang J, Betran AP, Souza JP, Mori R, Gulmezoglu AM, Merialdi M. A global reference for fetal-weight and birthweight percentiles. Lancet. 2011;377:1855–61. doi: 10.1016/S0140-6736(11)60364-4. [DOI] [PubMed] [Google Scholar]
- 5.Mayer C, Joseph KS. Fetal growth: a review of terms, concepts and issues relevant to obstetrics. Ultrasound Obstet Gynecol. 2013;41:136–45. doi: 10.1002/uog.11204. [DOI] [PubMed] [Google Scholar]
- 6.Campbell S, Wilkin D. Ultrasonic measurement of fetal abdomen circumference in the estimation of fetal weight. Br J Obstet Gynaecol. 1975;82:689–97. doi: 10.1111/j.1471-0528.1975.tb00708.x. [DOI] [PubMed] [Google Scholar]
- 7.Shepard MJ, Richards VA, Berkowitz RL, Warsof SL, Hobbins JC. An evaluation of two equations for predicting fetal weight by ultrasound. Am J Obstet Gynecol. 1982;142:47–54. doi: 10.1016/s0002-9378(16)32283-9. [DOI] [PubMed] [Google Scholar]
- 8.Jordaan HV. Estimation of fetal weight by ultrasound. J Clin Ultrasound. 1983;11:59–66. doi: 10.1002/jcu.1870110202. [DOI] [PubMed] [Google Scholar]
- 9.Hadlock FP, Harrist RB, Carpenter RJ, Deter RL, Park SK. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology. 1984;150:535–40. doi: 10.1148/radiology.150.2.6691115. [DOI] [PubMed] [Google Scholar]
- 10.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am J Obstet Gynecol. 1985;151:333–7. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 11.Roberts AB, Lee AJ, James AG. Ultrasonic estimation of fetal weight: a new predictive model incorporating femur length for the low-birth-weight fetus. J Clin Ultrasound. 1985;13:555–9. doi: 10.1002/1097-0096(199010)13:8<555::aid-jcu1870130807>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Rose BI, McCallum WD. A simplified method for estimating fetal weight using ultrasound measurements. Obstet Gynecol. 1987;69:671–5. [PubMed] [Google Scholar]
- 13.Larsen T, Petersen S, Greisen G, Larsen JF. Normal fetal growth evaluated by longitudinal ultrasound examinations. Early Hum Dev. 1990;24:37–45. doi: 10.1016/0378-3782(90)90004-3. [DOI] [PubMed] [Google Scholar]
- 14.Combs CA, Jaekle RK, Rosenn B, Pope M, Miodovnik M, Siddiqi TA. Sonographic estimation of fetal weight based on a model of fetal volume. Obstet Gynecol. 1993;82:365–70. [PubMed] [Google Scholar]
- 15.Scott F, Beeby P, Abbott J, Edelman D, Boogert A. New formula for estimating fetal weight below 1000 g: comparison with existing formulas. J Ultrasound Med. 1996;15:669–72. doi: 10.7863/jum.1996.15.10.669. [DOI] [PubMed] [Google Scholar]
- 16.Ong S, Smith AP, Fitzmaurice A, Campbell D. Estimation of fetal weight in twins: a new mathematical model. Br J Obstet Gynaecol. 1999;106:924–8. doi: 10.1111/j.1471-0528.1999.tb08431.x. [DOI] [PubMed] [Google Scholar]
- 17.Smulian JC, Ranzini AC, Ananth CV, Rosenberg JC, Vintzileos AM. Comparison of three sonographic circumference measurement techniques to predict birth weight. Obstet Gynecol. 1999;93:692–6. doi: 10.1016/s0029-7844(98)00517-1. [DOI] [PubMed] [Google Scholar]
- 18.Benavides-Serralde A, Hernandez-Andrade E, Fernandez-Lara A, Figueras F, Moreno-Alvarez O, Camargo-Marin L, Acevedo-Gallegos S, Gallardo-Gaona J, Velazquez-Torres B, Guzman-Huerta M. Accuracy of different equations for estimating fetal weight. Gynecol Obstet Invest. 2011;72:264–8. doi: 10.1159/000328693. [DOI] [PubMed] [Google Scholar]
- 19.Jeanty P, Cantraine F, Romero R, Cousaert E, Hobbins JC. A longitudinal study of fetal weight growth. J Ultrasound Med. 1984;3:321–8. doi: 10.7863/jum.1984.3.7.321. [DOI] [PubMed] [Google Scholar]
- 20.Miller JM, Jr, Kissling GA, Brown HL, Gabert HA. Estimated fetal weight: applicability to small- and large-for-gestational-age fetus. J Clin Ultrasound. 1988;16:95–7. doi: 10.1002/jcu.1870160205. [DOI] [PubMed] [Google Scholar]
- 21.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181:129–33. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 22.Medchill MT, Peterson CM, Kreinick C, Garbaciak J. Prediction of estimated fetal weight in extremely low birth weight neonates (500–1000 g) Obstet Gynecol. 1991;78:286–90. [PubMed] [Google Scholar]
- 23.Mongelli M, Gardosi J. Reduction of false-positive diagnosis of fetal growth restriction by application of customized fetal growth standards. Obstet Gynecol. 1996;88:844–8. doi: 10.1016/0029-7844(96)00285-2. [DOI] [PubMed] [Google Scholar]
- 24.Mongelli M, Gardosi J. Gestation-adjusted projection of estimated fetal weight. Acta Obstet Gynecol Scand. 1996;75:28–31. doi: 10.3109/00016349609033279. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein IM, Mohs G, Rucquoi M, Badger GJ. Case for hybrid "fetal growth curves": a population-based estimation of normal fetal size across gestational age. J Matern Fetal Med. 1996;5:124–7. doi: 10.1002/(SICI)1520-6661(199605/06)5:3<124::AID-MFM5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 26.Gardosi J. Customized growth curves. Clin Obstet Gynecol. 1997;40:715–22. doi: 10.1097/00003081-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Nasrat HA. Use of ultrasound longitudinal data in the diagnosis of abnormal fetal growth. J Matern Fetal Med. 1997;6:209–14. doi: 10.1002/(SICI)1520-6661(199707/08)6:4<209::AID-MFM4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 28.Figueras F, Torrents M, Munoz A, Comas C, Antolin E, Echevarria M, Mallafre J, Carrera JM. References intervals for fetal biometrical parameters. Eur J Obstet Gynecol Reprod Biol. 2002;105:25–30. doi: 10.1016/s0301-2115(02)00141-0. [DOI] [PubMed] [Google Scholar]
- 29.Gardosi J. Customized fetal growth standards: rationale and clinical application. Semin Perinatol. 2004;28:33–40. doi: 10.1053/j.semperi.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 30.McCowan L, Stewart AW, Francis A, Gardosi J. A customised birthweight centile calculator developed for a New Zealand population. Aust N Z J Obstet Gynaecol. 2004;44:428–31. doi: 10.1111/j.1479-828X.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- 31.McCowan L, Stewart AW. Term birthweight centiles for babies from New Zealand's main ethnic groups. Aust N Z J Obstet Gynaecol. 2004;44:432–5. doi: 10.1111/j.1479-828X.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- 32.Ego A, Subtil D, Grange G, Thiebaugeorges O, Senat MV, Vayssiere C, Zeitlin J. Customized versus population-based birth weight standards for identifying growth restricted infants: a French multicenter study. Am J Obstet Gynecol. 2006;194:1042–9. doi: 10.1016/j.ajog.2005.10.816. [DOI] [PubMed] [Google Scholar]
- 33.Ego A, Blondel B, Zeitlin J. [Birthweight curves: a review of the literature] J Gynecol Obstet Biol Reprod (Paris) 2006;35:749–61. doi: 10.1016/s0368-2315(06)76475-4. [DOI] [PubMed] [Google Scholar]
- 34.Mongelli M, Figueras F, Francis A, Gardosi J. A customized birthweight centile calculator developed for an Australian population. Aust N Z J Obstet Gynaecol. 2007;47:128–31. doi: 10.1111/j.1479-828X.2007.00698.x. [DOI] [PubMed] [Google Scholar]
- 35.Bukowski R, Uchida T, Smith GC, Malone FD, Ball RH, Nyberg DA, Comstock CH, Hankins GD, Berkowitz RL, Gross SJ, Dugoff L, Craigo SD, Timor IE, Carr SR, Wolfe HM, D'Alton ME. Individualized norms of optimal fetal growth: fetal growth potential. Obstet Gynecol. 2008;111:1065–76. doi: 10.1097/AOG.0b013e3181704e48. [DOI] [PubMed] [Google Scholar]
- 36.Munim S, Figueras F, Shah SM, Khan F, Gardosi J. Ultrasound estimation of fetal weight: a formula for a Pakistani population. J Obstet Gynaecol Res. 2010;36:479–83. doi: 10.1111/j.1447-0756.2010.01169.x. [DOI] [PubMed] [Google Scholar]
- 37.Gardosi J, Figueras F, Clausson B, Francis A. The customised growth potential: an international research tool to study the epidemiology of fetal growth. Paediatr Perinat Epidemiol. 2011;25:2–10. doi: 10.1111/j.1365-3016.2010.01166.x. [DOI] [PubMed] [Google Scholar]
- 38.Kase BA, Carreno CA, Blackwell SC. Customized estimated fetal weight: a novel antenatal tool to diagnose abnormal fetal growth. Am J Obstet Gynecol. 2012;207:218, e1–5. doi: 10.1016/j.ajog.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 39.Harper LM, Roehl KA, Tuuli MG, Odibo AO, Cahill AG. Sonographic accuracy of estimated fetal weight in twins. J Ultrasound Med. 2013;32:625–30. doi: 10.7863/jum.2013.32.4.625. [DOI] [PubMed] [Google Scholar]
- 40.Landres IV, Clark A, Chasen ST. Improving antenatal prediction of small-for-gestational-age neonates by using customized versus population-based reference standards. J Ultrasound Med. 2013;32:1581–6. doi: 10.7863/ultra.32.9.1581. [DOI] [PubMed] [Google Scholar]
- 41.Villar J, Altman DG, Purwar M, Noble JA, Knight HE, Ruyan P, Cheikh Ismail L, Barros FC, Lambert A, Papageorghiou AT, Carvalho M, Jaffer YA, Bertino E, Gravett MG, Bhutta ZA, Kennedy SH. The objectives, design and implementation of the INTERGROWTH-21st Project. BJOG. 2013;120(Suppl 2):9–26. doi: 10.1111/1471-0528.12047. v. [DOI] [PubMed] [Google Scholar]
- 42.Papageorghiou AT, Sarris I, Ioannou C, Todros T, Carvalho M, Pilu G, Salomon LJ. Ultrasound methodology used to construct the fetal growth standards in the INTERGROWTH-21st Project. BJOG. 2013;120(Suppl 2):27–32. doi: 10.1111/1471-0528.12313. v. [DOI] [PubMed] [Google Scholar]
- 43.Unterscheider J, Geary MP, Daly S, McAuliffe FM, Kennelly MM, Dornan J, Morrison JJ, Burke G, Francis A, Gardosi J, Malone FD. The customized fetal growth potential: a standard for Ireland. Eur J Obstet Gynecol Reprod Biol. 2013;166:14–7. doi: 10.1016/j.ejogrb.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Eden RD, Jelovsek FR, Kodack LD, Killam AP, Gall SA. Accuracy of ultrasonic fetal weight prediction in preterm infants. Am J Obstet Gynecol. 1983;147:43–8. doi: 10.1016/0002-9378(83)90081-9. [DOI] [PubMed] [Google Scholar]
- 45.Villar J, Smeriglio V, Martorell R, Brown CH, Klein RE. Heterogeneous growth and mental development of intrauterine growth-retarded infants during the first 3 years of life. Pediatrics. 1984;74:783–91. [PubMed] [Google Scholar]
- 46.Weiner CP, Sabbagha RE, Vaisrub N, Socol ML. Ultrasonic fetal weight prediction: role of head circumference and femur length. Obstet Gynecol. 1985;65:812–7. [PubMed] [Google Scholar]
- 47.Simon NV, O'Connor TJ, 3rd, Shearer DM. Detection of intrauterine fetal growth retardation with abdominal circumference and estimated fetal weight using cross-sectional growth curves. J Clin Ultrasound. 1990;18:685–90. [PubMed] [Google Scholar]
- 48.Villar J, de Onis M, Kestler E, Bolanos F, Cerezo R, Bernedes H. The differential neonatal morbidity of the intrauterine growth retardation syndrome. Am J Obstet Gynecol. 1990;163:151–7. doi: 10.1016/s0002-9378(11)90690-5. [DOI] [PubMed] [Google Scholar]
- 49.Nzeh DA, Rimmer S, Moore WM, Hunt L. Prediction of birthweight by fetal ultrasound biometry. Br J Radiol. 1992;65:987–9. doi: 10.1259/0007-1285-65-779-987. [DOI] [PubMed] [Google Scholar]
- 50.Kaaij MW, Struijk PC, Lotgering FK. Accuracy of sonographic estimates of fetal weight in very small infants. Ultrasound Obstet Gynecol. 1999;13:99–102. doi: 10.1046/j.1469-0705.1999.13020099.x. [DOI] [PubMed] [Google Scholar]
- 51.Clausson B, Gardosi J, Francis A, Cnattingius S. Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. BJOG. 2001;108:830–4. doi: 10.1111/j.1471-0528.2001.00205.x. [DOI] [PubMed] [Google Scholar]
- 52.Lee W, Deter RL, Ebersole JD, Huang R, Blanckaert K, Romero R. Birth weight prediction by three-dimensional ultrasonography: fractional limb volume. J Ultrasound Med. 2001;20:1283–92. doi: 10.7863/jum.2001.20.12.1283. [DOI] [PubMed] [Google Scholar]
- 53.Lysikiewicz A, Bracero LA, Tejani N. Sonographically estimated fetal weight percentile as a predictor of preterm delivery. J Matern Fetal Med. 2001;10:44–7. doi: 10.1080/714052715. [DOI] [PubMed] [Google Scholar]
- 54.Stetzer BP, Thomas A, Amini SB, Catalano PM. Neonatal anthropometric measurements to predict birth weight by ultrasound. J Perinatol. 2002;22:397–402. doi: 10.1038/sj.jp.7210754. [DOI] [PubMed] [Google Scholar]
- 55.Smith-Bindman R, Chu PW, Ecker JL, Feldstein VA, Filly RA, Bacchetti P. US evaluation of fetal growth: prediction of neonatal outcomes. Radiology. 2002;223:153–61. doi: 10.1148/radiol.2231010876. [DOI] [PubMed] [Google Scholar]
- 56.Smith-Bindman R, Chu PW, Ecker J, Feldstein VA, Filly RA, Bacchetti P. Adverse birth outcomes in relation to prenatal sonographic measurements of fetal size. J Ultrasound Med. 2003;22:347–56. doi: 10.7863/jum.2003.22.4.347. quiz 57–8. [DOI] [PubMed] [Google Scholar]
- 57.Owen P, Ogah J, Bachmann LM, Khan KS. Prediction of intrauterine growth restriction with customised estimated fetal weight centiles. BJOG. 2003;110:411–5. [PubMed] [Google Scholar]
- 58.McCowan LM, Harding JE, Stewart AW. Customized birthweight centiles predict SGA pregnancies with perinatal morbidity. BJOG. 2005;112:1026–33. doi: 10.1111/j.1471-0528.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- 59.Figueras F, Figueras J, Meler E, Eixarch E, Coll O, Gratacos E, Gardosi J, Carbonell X. Customised birthweight standards accurately predict perinatal morbidity. Arch Dis Child Fetal Neonatal Ed. 2007;92:F277–80. doi: 10.1136/adc.2006.108621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hutcheon JA, Zhang X, Cnattingius S, Kramer MS, Platt RW. Customised birthweight percentiles: does adjusting for maternal characteristics matter? BJOG. 2008;115:1397–404. doi: 10.1111/j.1471-0528.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 61.Fox NS, Huang M, Chasen ST. Second-trimester fetal growth and the risk of poor obstetric and neonatal outcomes. Ultrasound Obstet Gynecol. 2008;32:61–5. doi: 10.1002/uog.5314. [DOI] [PubMed] [Google Scholar]
- 62.Iraola A, Gonzalez I, Eixarch E, Meler E, Illa M, Gardosi J, Gratacos E, Figueras F. Prediction of adverse perinatal outcome at term in small-for-gestational age fetuses: comparison of growth velocity vs. customized assessment. J Perinat Med. 2008;36:531–5. doi: 10.1515/JPM.2008.100. [DOI] [PubMed] [Google Scholar]
- 63.Pedersen NG, Figueras F, Wojdemann KR, Tabor A, Gardosi J. Early fetal size and growth as predictors of adverse outcome. Obstet Gynecol. 2008;112:765–71. doi: 10.1097/AOG.0b013e318187d034. [DOI] [PubMed] [Google Scholar]
- 64.Lee W, Balasubramaniam M, Deter RL, Hassan SS, Gotsch F, Kusanovic JP, Goncalves LF, Romero R. Fetal growth parameters and birth weight: their relationship to neonatal body composition. Ultrasound Obstet Gynecol. 2009;33:441–6. doi: 10.1002/uog.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Illa M, Coloma JL, Eixarch E, Meler E, Iraola A, Gardosi J, Gratacos E, Figueras F. Growth deficit in term small-for-gestational fetuses with normal umbilical artery Doppler is associated with adverse outcome. J Perinat Med. 2009;37:48–52. doi: 10.1515/JPM.2009.003. [DOI] [PubMed] [Google Scholar]
- 66.Zeitlin J, El Ayoubi M, Jarreau PH, Draper ES, Blondel B, Kunzel W, Cuttini M, Kaminski M, Gortner L, Van Reempts P, Kollee L, Papiernik E. Impact of fetal growth restriction on mortality and morbidity in a very preterm birth cohort. J Pediatr. 2010;157:733–9. e1. doi: 10.1016/j.jpeds.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 67.McCowan LM, Roberts CT, Dekker GA, Taylor RS, Chan EH, Kenny LC, Baker PN, Moss-Morris R, Chappell LC, North RA. Risk factors for small-for-gestational-age infants by customised birthweight centiles: data from an international prospective cohort study. BJOG. 2010;117:1599–607. doi: 10.1111/j.1471-0528.2010.02737.x. [DOI] [PubMed] [Google Scholar]
- 68.Melamed N, Yogev Y, Meizner I, Mashiach R, Pardo J, Ben-Haroush A. Prediction of fetal macrosomia: effect of sonographic fetal weight-estimation model and threshold used. Ultrasound Obstet Gynecol. 2011;38:74–81. doi: 10.1002/uog.8930. [DOI] [PubMed] [Google Scholar]
- 69.Odibo AO, Francis A, Cahill AG, Macones GA, Crane JP, Gardosi J. Association between pregnancy complications and small-for-gestational-age birth weight defined by customized fetal growth standard versus a population-based standard. J Matern Fetal Neonatal Med. 2011;24:411–7. doi: 10.3109/14767058.2010.506566. [DOI] [PubMed] [Google Scholar]
- 70.Figueras F, Cruz-Martinez R, Sanz-Cortes M, Arranz A, Illa M, Botet F, Costas-Moragas C, Gratacos E. Neurobehavioral outcomes in preterm, growth-restricted infants with and without prenatal advanced signs of brain-sparing. Ultrasound Obstet Gynecol. 2011;38:288–94. doi: 10.1002/uog.9041. [DOI] [PubMed] [Google Scholar]
- 71.Larkin JC, Hill LM, Speer PD, Simhan HN. Risk of morbid perinatal outcomes in small-for-gestational-age pregnancies: customized compared with conventional standards of fetal growth. Obstet Gynecol. 2012;119:21–7. doi: 10.1097/AOG.0b013e31823dc56e. [DOI] [PubMed] [Google Scholar]
- 72.Savchev S, Figueras F, Cruz-Martinez R, Illa M, Botet F, Gratacos E. Estimated weight centile as a predictor of perinatal outcome in small-for-gestational-age pregnancies with normal fetal and maternal Doppler indices. Ultrasound Obstet Gynecol. 2012;39:299–303. doi: 10.1002/uog.10150. [DOI] [PubMed] [Google Scholar]
- 73.Pasupathy D, McCowan LM, Poston L, Kenny LC, Dekker GA, North RA. Perinatal outcomes in large infants using customised birthweight centiles and conventional measures of high birthweight. Paediatr Perinat Epidemiol. 2012;26:543–52. doi: 10.1111/ppe.12002. [DOI] [PubMed] [Google Scholar]
- 74.Verlijsdonk JW, Winkens B, Boers K, Scherjon S, Roumen F. Suspected versus non-suspected small-for-gestational age fetuses at term: perinatal outcomes. J Matern Fetal Neonatal Med. 2012;25:938–43. doi: 10.3109/14767058.2011.600793. [DOI] [PubMed] [Google Scholar]
- 75.Charkaluk ML, Marchand-Martin L, Ego A, Zeitlin J, Arnaud C, Burguet A, Marret S, Roze JC, Vieux R, Kaminski M, Ancel PY, Pierrat V. The influence of fetal growth reference standards on assessment of cognitive and academic outcomes of very preterm children. J Pediatr. 2012;161:1053–8. doi: 10.1016/j.jpeds.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 76.Odibo AO, Cahill AG, Goetzinger KR, Harper LM, Tuuli MG, Macones GA. Customized growth charts for twin gestations to optimize identification of small-for-gestational age fetuses at risk of intrauterine fetal death. Ultrasound Obstet Gynecol. 2013;41:637–42. doi: 10.1002/uog.12404. [DOI] [PubMed] [Google Scholar]
- 77.Lindell G, Marsal K, Kallen K. Predicting risk for large-for-gestational age neonates at term: a population-based Bayesian theorem study. Ultrasound Obstet Gynecol. 2013;41:398–405. doi: 10.1002/uog.11218. [DOI] [PubMed] [Google Scholar]
- 78.Odibo AO, Goetzinger KR, Cahill AG, Odibo L, Macones GA. Combined Sonographic Testing Index and Prediction of Adverse Outcome in Preterm Fetal Growth Restriction. Am J Perinatol. 2013 doi: 10.1055/s-0033-1341574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Unterscheider J, Daly S, Geary MP, Kennelly MM, McAuliffe FM, O'Donoghue K, Hunter A, Morrison JJ, Burke G, Dicker P, Tully EC, Malone FD. Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO Study. Am J Obstet Gynecol. 2013;208:290, e1–6. doi: 10.1016/j.ajog.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 80.Deter RL, Harrist RB. Assessment of normal fetal growth. In: Chervenak FA, Isaacson GC, Campbell S, editors. Ultrasound in Obstetrics and Gynecology. Boston, MA: Litle, Brown and Company; 1993. pp. 361–86. [Google Scholar]
- 81.Ariyuki Y, Hata T, Kitao M. Evaluation of perinatal outcome using individualized growth assessment: comparison with conventional methods. Pediatrics. 1995;96:36–42. [PubMed] [Google Scholar]
- 82.Deter RL, Xu B, Milner LL. Prenatal prediction of neonatal growth status in twins using individualized growth assessment. J Clin Ultrasound. 1996;24:53–9. doi: 10.1002/(SICI)1097-0096(199602)24:2<53::AID-JCU1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 83.Deter RL. Individualized growth assessment predicts birth weight accurately. Ultrasound Obstet Gynecol. 1996;7:156–7. doi: 10.1046/j.1469-0705.1996.07020156.x. [DOI] [PubMed] [Google Scholar]
- 84.Gardosi J. Individualized fetal growth assessment and accuracy of prediction. Ultrasound Obstet Gynecol. 1996;7:462–3. doi: 10.1046/j.1469-0705.1996.07060461-2.x. [DOI] [PubMed] [Google Scholar]
- 85.Hata T, Kuno A, Akiyama M, Yanagihara T, Manabe A, Miyazaki K. Detection of small-for-gestational-age infants with poor perinatal outcomes using individualized growth assessment. Gynecol Obstet Invest. 1999;47:162–5. doi: 10.1159/000010085. [DOI] [PubMed] [Google Scholar]
- 86.Deter RL. Individualized growth assessment: evaluation of growth using each fetus as its own control. Semin Perinatol. 2004;28:23–32. doi: 10.1053/j.semperi.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 87.Deter RL, Spence LR. Identification of Macrosomic, normal and intrauterine growth retarded neonates using the modified Neonatal Growth Assessment Score. Fetal Diagn Ther. 2004;19:58–67. doi: 10.1159/000074262. [DOI] [PubMed] [Google Scholar]
- 88.Lee W, Deter RL, McNie B, Goncalves LF, Espinoza J, Chaiworapongsa T, Romero R. Individualized growth assessment of fetal soft tissue using fractional thigh volume. Ultrasound Obstet Gynecol. 2004;24:766–74. doi: 10.1002/uog.1779. [DOI] [PubMed] [Google Scholar]
- 89.Lee W, Deter RL, McNie B, Goncalves LF, Espinoza J, Chaiworapongsa T, Balasubramaniam M, Romero R. The fetal arm: individualized growth assessment in normal pregnancies. J Ultrasound Med. 2005;24:817–28. [PubMed] [Google Scholar]
- 90.Lee W, Deter RL, Sameera S, Espinoza J, Goncalves LF, Romero R. Individualized growth assessment of fetal thigh circumference using three-dimensional ultrasonography. Ultrasound Obstet Gynecol. 2008;31:520–8. doi: 10.1002/uog.5302. [DOI] [PubMed] [Google Scholar]
- 91.Figueras F, Gardosi J. Should we customize fetal growth standards? Fetal Diagn Ther. 2009;25:297–303. doi: 10.1159/000235875. [DOI] [PubMed] [Google Scholar]
- 92.Zhang J, Mikolajczyk R, Grewal J, Neta G, Klebanoff M. Prenatal application of the individualized fetal growth reference. Am J Epidemiol. 2011;173:539–43. doi: 10.1093/aje/kwq411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Figueras F, Gardosi J. Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstet Gynecol. 2011;204:288–300. doi: 10.1016/j.ajog.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 94.Deter RL, Lee W, Sangi-Haghpeykar H, Tarca AL, Yeo L, Romero R. Individualized fetal growth assessment: critical evaluation of key concepts in the specification of third trimester size trajectories. J Matern Fetal Neonatal Med. 2014;27:537–542. doi: 10.3109/14767058.2013.833904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Deter RL, Stefos T, Harrist RB, Hill RM. Detection of intrauterine growth retardation in twins using individualized growth assessment. II. Evaluation of third-trimester growth and prediction of growth outcome at birth. J Clin Ultrasound. 1992;20:579–85. doi: 10.1002/jcu.1870200903. [DOI] [PubMed] [Google Scholar]
- 96.Hata T, Deter RL, Hill RM. Individual growth curve standards in triplets: prediction of third-trimester growth and birth characteristics. Obstet Gynecol. 1991;78:379–84. [PubMed] [Google Scholar]
- 97.Lee W, Balasubramaniam M, Deter RL, Yeo L, Hassan SS, Gotsch F, Kusanovic JP, Goncalves LF, Romero R. New fetal weight estimation models using fractional limb volume. Ultrasound Obstet Gynecol. 2009;34:556–65. doi: 10.1002/uog.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu B, Deter RL, Milner LL, Hill RM. Evaluation of twin growth status at birth using individualized growth assessment: comparison with conventional methods. J Clin Ultrasound. 1995;23:277–86. doi: 10.1002/jcu.1870230502. [DOI] [PubMed] [Google Scholar]
- 99.Deter RL. Definition, Epidemiology and classification of Macrosomia. In: Divon MY, editor. Abnormal Fetal Growth: IUGR and Macrosomia. New York, NY: Elsevier Science Publishing Co; 1991. pp. 75–82. [Google Scholar]
- 100.Deter RL, Harrist RB. Detection of growth abnormalities. In: Chervenak FA, Isaacson GC, Campbell S, editors. Ultrasound in Obstetrics and Gynecology. Boston: Little, Brown and company; 1993. pp. 387–403. [Google Scholar]
- 101.Deter RL, Nazar R, Milner LL. Modified neonatal growth assessment score: a multivariate approach to the detection of intrauterine growth retardation in the neonate. Ultrasound Obstet Gynecol. 1995;6:400–10. doi: 10.1046/j.1469-0705.1995.06060400.x. [DOI] [PubMed] [Google Scholar]
- 102.Mitra SC, Seshan SV, Riachi LE. Placental vessel morphometry in growth retardation and increased resistance of the umbilical artery Doppler flow. J Matern Fetal Med. 2000;9:282–6. doi: 10.1002/1520-6661(200009/10)9:5<282::AID-MFM5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 103.Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92:35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 104.Redline RW, Shah D, Sakar H, Schluchter M, Salvator A. Placental lesions associated with abnormal growth in twins. Pediatr Dev Pathol. 2001;4:473–81. doi: 10.1007/s10024001-0044-z. [DOI] [PubMed] [Google Scholar]
- 105.Baschat AA. Fetal responses to placental insufficiency: an update. BJOG. 2004;111:1031–41. doi: 10.1111/j.1471-0528.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- 106.Baschat AA, Hecher K. Fetal growth restriction due to placental disease. Semin Perinatol. 2004;28:67–80. doi: 10.1053/j.semperi.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 107.Pringle PJ, Geary MP, Rodeck CH, Kingdom JC, Kayamba-Kay's S, Hindmarsh PC. The influence of cigarette smoking on antenatal growth, birth size, and the insulin-like growth factor axis. J Clin Endocrinol Metab. 2005;90:2556–62. doi: 10.1210/jc.2004-1674. [DOI] [PubMed] [Google Scholar]
- 108.Figueras F, Meler E, Eixarch E, Francis A, Coll O, Gratacos E, Gardosi J. Association of smoking during pregnancy and fetal growth restriction: subgroups of higher susceptibility. Eur J Obstet Gynecol Reprod Biol. 2008;138:171–5. doi: 10.1016/j.ejogrb.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 109.Hindmarsh PC, Geary MP, Rodeck CH, Kingdom JC, Cole TJ. Factors predicting ante- and postnatal growth. Pediatr Res. 2008;63:99–102. doi: 10.1203/PDR.0b013e31815b8e8f. [DOI] [PubMed] [Google Scholar]
- 110.Dessi A, Ottonello G, Fanos V. Physiopathology of intrauterine growth retardation: from classic data to metabolomics. J Matern Fetal Neonatal Med. 2012;25:13–8. doi: 10.3109/14767058.2012.714639. [DOI] [PubMed] [Google Scholar]
- 111.McFarland MB, Trylovich CG, Langer O. Anthropometric differences in macrosomic infants of diabetic and nondiabetic mothers. J Matern Fetal Med. 1998;7:292–5. doi: 10.1002/(SICI)1520-6661(199811/12)7:6<292::AID-MFM7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 112.Combs CA, Rosenn B, Miodovnik M, Siddiqi TA. Sonographic EFW and macrosomia: is there an optimum formula to predict diabetic fetal macrosomia? J Matern Fetal Med. 2000;9:55–61. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<55::AID-MFM12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 113.Landon MB. Prenatal diagnosis of macrosomia in pregnancy complicated by diabetes mellitus. J Matern Fetal Med. 2000;9:52–4. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<52::AID-MFM11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 114.Baschat AA. Pathophysiology of fetal growth restriction: implications for diagnosis and surveillance. Obstet Gynecol Surv. 2004;59:617–27. doi: 10.1097/01.ogx.0000133943.54530.76. [DOI] [PubMed] [Google Scholar]
- 115.Barker ED, McAuliffe FM, Alderdice F, Unterscheider J, Daly S, Geary MP, Kennelly MM, O'Donoghue K, Hunter A, Morrison JJ, Burke G, Dicker P, Tully EC, Malone FD. The role of growth trajectories in classifying fetal growth restriction. Obstet Gynecol. 2013;122:248–54. doi: 10.1097/AOG.0b013e31829ca9a7. [DOI] [PubMed] [Google Scholar]
- 116.Lee W, Riggs T, Koo W, Deter RL, Yeo L, Romero R. The relationship of newborn adiposity to fetal growth outcome based on birth weight or the modified neonatal growth assessment score. J Matern Fetal Neonatal Med. 2012;25:1933–40. doi: 10.3109/14767058.2012.683084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deter RL, Rossavik IK, Harrist RB. Development of individual growth curve standards for estimated fetal weight: I. Weight estimation procedure. J Clin Ultrasound. 1988;16:215–25. doi: 10.1002/jcu.1870160402. [DOI] [PubMed] [Google Scholar]
- 118.Deter RL, Rossavik IK, Harrist RB, Hadlock FP. Mathematic modeling of fetal growth: development of individual growth curve standards. Obstet Gynecol. 1986;68:156–61. [PubMed] [Google Scholar]
- 119.Royston P. Calculation of unconditional and conditional reference intervals for foetal size and growth from longitudinal measurements. Stat Med. 1995;14:1417–36. doi: 10.1002/sim.4780141303. [DOI] [PubMed] [Google Scholar]
- 120.Jeanty P, Romero R, Hobbins JC. Fetal limb volume: a new parameter to assess fetal growth and nutrition. J Ultrasound Med. 1985;4:273–82. doi: 10.7863/jum.1985.4.6.273. [DOI] [PubMed] [Google Scholar]
- 121.Vinkesteijn AS, Mulder PG, Wladimiroff JW. Fetal transverse cerebellar diameter measurements in normal and reduced fetal growth. Ultrasound Obstet Gynecol. 2000;15:47–51. doi: 10.1046/j.1469-0705.2000.00024.x. [DOI] [PubMed] [Google Scholar]
- 122.Niknafs P, Sibbald J. Accuracy of single ultrasound parameters in detection of fetal growth restriction. Am J Perinatol. 2001;18:325–34. doi: 10.1055/s-2001-17856. [DOI] [PubMed] [Google Scholar]
- 123.Ott WJ. Diagnosis of intrauterine growth restriction: comparison of ultrasound parameters. Am J Perinatol. 2002;19:133–7. doi: 10.1055/s-2002-25313. [DOI] [PubMed] [Google Scholar]
- 124.Larciprete G, Valensise H, Di Pierro G, Vasapollo B, Casalino B, Arduini D, Jarvis S, Cirese E. Intrauterine growth restriction and fetal body composition. Ultrasound Obstet Gynecol. 2005;26:258–62. doi: 10.1002/uog.1980. [DOI] [PubMed] [Google Scholar]
- 125.Breeze AC, Lees CC. Prediction and perinatal outcomes of fetal growth restriction. Semin Fetal Neonatal Med. 2007;12:383–97. doi: 10.1016/j.siny.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 126.al Riyami N, Walker MG, Proctor LK, Yinon Y, Windrim RC, Kingdom JC. Utility of head/abdomen circumference ratio in the evaluation of severe early-onset intrauterine growth restriction. J Obstet Gynaecol Can. 2011;33:715–9. doi: 10.1016/S1701-2163(16)34956-8. [DOI] [PubMed] [Google Scholar]
- 127.Talmor A, Daemen A, Murdoch E, Missfelder-Lobos H, Timmerman D, Bourne T, Giussani DA, Lees C. Defining the relationship between fetal Doppler indices, abdominal circumference and growth rate in severe fetal growth restriction using functional linear discriminant analysis. J R Soc Interface. 2013;10:20130376. doi: 10.1098/rsif.2013.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Simon NV, Deter RL, Grow DR, Kofinas AD. Detection of macrosomia by the individual fetal growth curve assessment method. Obstet Gynecol. 1991;77:793–7. [PubMed] [Google Scholar]
- 129.Farah N, Stuart B, Donnelly V, Rafferty G, Turner M. What is the value of ultrasound soft tissue measurements in the prediction of abnormal fetal growth? J Obstet Gynaecol. 2009;29:457–63. doi: 10.1080/01443610903003209. [DOI] [PubMed] [Google Scholar]
- 130.Garabedian C, Vambergue A, Salleron J, Deruelle P. Prediction of macrosomia by serial sonographic measurements of fetal soft-tissues and the liver in women with pregestational diabetes. Diabetes Metab. 2013;39:511–8. doi: 10.1016/j.diabet.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 131.Pueyo V, Oros D, Valle S, Tuquet H, Guerri N, Arguelles M, Ventura P. Axonal loss and cognitive deficits in term infants with normal umbilical artery Doppler born small-for-gestational age. Ultrasound Obstet Gynecol. 2012;40:297–303. doi: 10.1002/uog.11215. [DOI] [PubMed] [Google Scholar]
- 132.Deter RL, Rossavik IK. A simplified method for determining individual growth curve standards. Obstet Gynecol. 1987;70:801–6. [PubMed] [Google Scholar]
- 133.Deter RL, Harrist RB, Hill RM. Neonatal growth assessment score: a new approach to the detection of intrauterine growth retardation in the newborn. Am J Obstet Gynecol. 1990;162:1030–6. doi: 10.1016/0002-9378(90)91310-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.