Abstract
Purpose:
Cancer-associated weight loss is associated with poor prognosis in advanced malignancy; however, its pretreatment prevalence and survival impact are inadequately described in large cohorts. Such data, stratified by tumor type and stage, may facilitate the optimal and timely allocation of complementary care, leading to improvements in patient survival and quality of life.
Methods:
We performed a retrospective cohort study of 3,180 consecutively treated adult patients with lung or GI (including colorectal, liver, and pancreatic) cancer. Pretreatment cancer-associated weight loss was based on the international consensus definition of cachexia. Prevalence and survival impact of pretreatment cancer-associated weight loss were evaluated using the Kaplan-Meier method and compared using log-rank test.
Results:
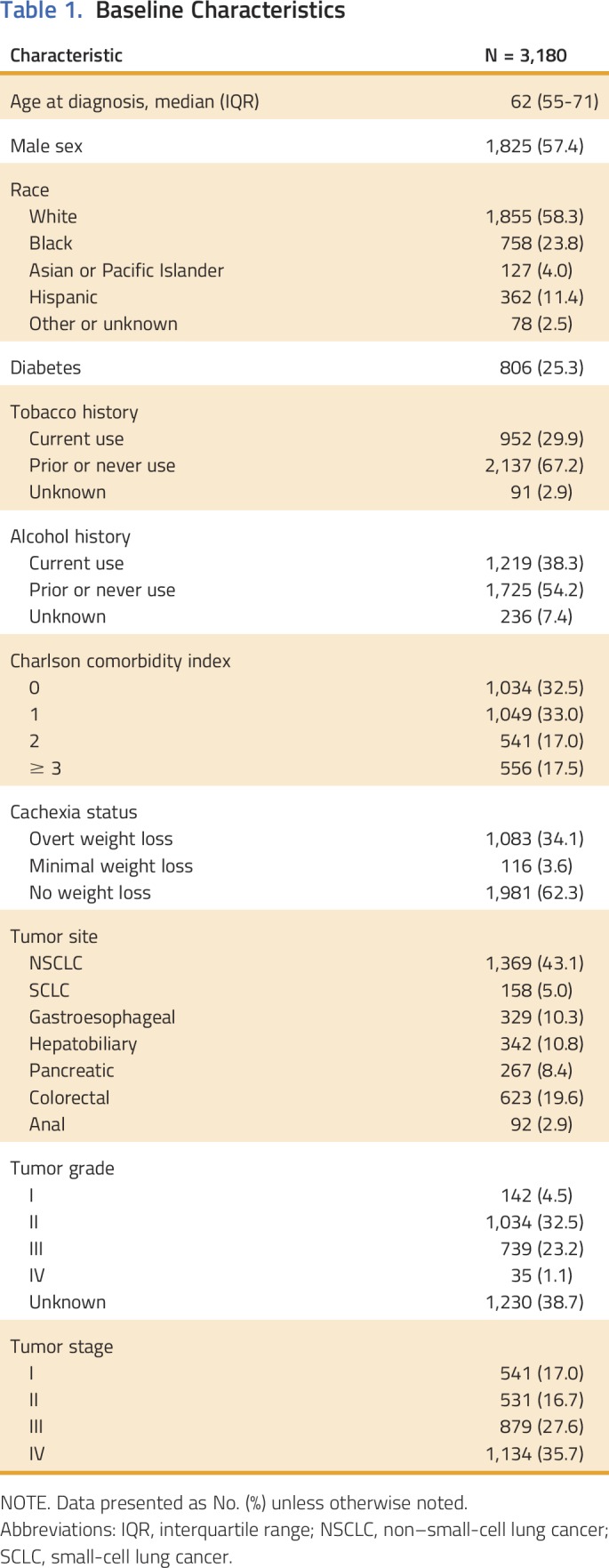
Cancer-associated weight loss was observed at the time of cancer diagnosis in 34.1% of patients. Pretreatment weight loss was documented in 17.6%, 25.8%, 36.6%, and 43.3% of stage I, II, III, and IV cancers, respectively. Wasting was common regardless of tumor type, with prevalence at diagnosis ranging from 27.3% in patients with colorectal cancer to 53.4% in patients with gastroesophageal cancer. Pretreatment weight loss was associated with reduced overall survival after adjusting for stage, size, grade, comorbidity, age, sex, and tobacco history (hazard ratio, 1.26; 95% CI, 1.13 to 1.39).
Conclusion:
Pretreatment cancer-associated weight loss is common, even in early-stage disease, and is independently associated with reduced survival. Minimal weight loss represents a clinically distinct entity with an associated overall survival intermediate to that of no weight loss and overt wasting. Early diagnosis and treatment of cancer-associated wasting offers a novel therapeutic avenue for reducing cancer mortality.
INTRODUCTION
Cancer cachexia is a multifactorial syndrome characterized by ongoing, unintentional weight loss that cannot be fully reversed by nutritional support and leading to progressive functional impairment.1,2 The syndrome, a spectrum of disease with three clinically relevant phases (precachexia, cachexia, and refractory cachexia), is associated with declines in tolerance to anticancer therapy, quality of life, and survival.3-7 Traditionally, successful treatment of the underlying cancer was believed to adequately address cancer-associated cachexia; however, recent efforts have shifted to specifically manage cachexia. More than 100 randomized clinical trials of anticachexia therapies have been reported, but none have identified a durable survival benefit with treatment of cachexia. Proposed explanations for the limited success of anticachexia strategies include inclusion of patients with refractory cachexia who may be unresponsive to any treatment, and interventions failing to address the basic mechanisms driving cachexia.8 These limitations, coupled with an underappreciation of the effects of cachexia on morbidity and mortality, have contributed to inadequate treatment outcomes in patients with cancer cachexia.5,7,9 Therefore, further characterization of cancer-associated weight loss is necessary.
Early recognition of patients with cancer cachexia could facilitate the optimal and timely allocation of complementary care, including nutrition, physical therapy, and palliative care.10 In fact, early implementation of palliative care alongside standard oncologic care has already been shown to improve quality of life and overall survival compared with standard oncologic care alone in the setting of incurable thoracic or GI cancers.11,12 Unfortunately, the prevalence of cancer cachexia and its impact on survival are poorly described across a broad range of pathologies and stages. Herein, we describe the largest cohort analysis of weight loss in patients with cancer. Furthermore, we report for the first time a survival detriment associated with minimal weight loss at the time of cancer diagnosis, which demonstrates clinical significance for even the earliest sign of cancer-associated weight loss.
METHODS
Population Cohort
Using a prospectively maintained tumor registry at a single tertiary care center, we identified 3,802 consecutive adult patients with a lung or GI malignancy treated between January 1, 2006 and December 31, 2013. Exclusion criteria for this study included synchronous or metachronous malignancy and incomplete data. Patients were also excluded if their tumor histology was classified as carcinoma in situ, carcinoid, neuroendocrine, lymphoma, melanoma, or sarcoma. After these exclusions, 3,180 patients were eligible for further analysis. Patient and tumor characteristics, including age, sex, race, date of death, comorbidities, primary tumor site, tumor grade, and tumor stage, were recorded. In 2009, 7th edition American Joint Committee on Cancer staging was adopted, but patient stage was not adjusted for the purpose of this study. This study was approved by our Institutional Review Board.
Assessment of Cancer-Associated Weight Loss
A single author (B.S.G.) reviewed medical records, including vital signs, physician notes, and dietician notes at the time of cancer diagnosis but before any therapeutic measure, for documented weight loss and cachexia-associated symptoms. At our health system, patients are routinely weighed as a part of each office visit, and this measurement is documented in the electronic medical record. Patients missing data on weight before the initiation of therapy were excluded. Cancer-associated weight loss was based on the validated international consensus definition of cancer cachexia.1,2 Overt cancer-associated weight loss was defined as unintentional weight loss > 5% within 6 months preceding cancer diagnosis in patients with body mass index ≥ 20 kg/m2 or unintentional weight loss > 2% in patients with body mass index < 20 kg/m2. Minimal weight loss was defined as unintentional weight loss not meeting this threshold for overt weight loss.1 Patients with stable weight, weight gain, or purposeful weight loss were classified as not having weight loss. Categorization is summarized in Appendix Table A1 (online only). When multiple measures of weight were available in the pretreatment period, a consistent weight decrease was required for a patient to be classified as having weight loss. Survival was calculated from time of cancer diagnosis to death with patients censored at the time of last clinic visit (if lost to follow up) or end of study period (December 31, 2013).
Statistics
Descriptive statistics were used to summarize patient and tumor characteristics. The Kaplan-Meier approach was used to estimate overall survival, and the log-rank test was used to compare survival outcomes between groups. Stepwise Cox regression analysis was used to identify significant independent factors associated with overall survival. All tests were two-sided and performed at the 5% significance level. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and SPSS Statistics version 24.0 (International Business Machines, Armonk, NY).
RESULTS
Patient Cohort
Patient characteristics are summarized in Table 1. The cohort was representative, with a median age at diagnosis of 62 years (interquartile range, 55-71 years) and 1,355 (42.6%) women. A broad sample of cancer sites and stages was represented, including non–small-cell lung cancer (NSCLC; n = 1,369), colorectal (n = 623), and pancreatic (n = 267) primaries (Table 1).
Table 1.
Baseline Characteristics
Prevalence of Pretreatment Cancer-Associated Weight Loss
Cancer-associated weight loss classification and cachexia-associated symptoms are detailed in Table 1. Overt pretreatment weight loss was observed in 1,083 (34.1%) patients at cancer diagnosis. Minimal weight loss was identified in 116 (3.6%) patients. Prevalence of cancer-associated weight loss at diagnosis stratified by primary tumor site, histology, and stage is reported in Table 2. Wasting was identified in 30.4% (n = 416) of patients diagnosed with NSCLC, 38.0% (n = 60) with small-cell lung cancer, 56.5% (n = 186) with gastroesophageal cancers, 24.3% (n = 83) with hepatobiliary cancers, 53.2% (n = 142) with pancreatic cancer, 27.6% (n = 172) with colorectal cancer, and 26.1% (n = 24) with anal cancer. In addition, after excluding patients with ascites or evidence of volume overload, which may interfere with the assessment of weight loss,10 overt weight loss was documented in 34.6% (45 of 130) of patients with hepatobiliary cancers. Pretreatment cancer-associated weight loss was commonly observed even with early-stage disease, including 17.6% of stage I and 25.8% of stage II disease in addition to 36.6% of stage III and 43.3% of stage IV disease.
Table 2.
Overall Survival Stratified by Primary Site, Stage, and Pretreatment Weight Loss Status
Prognostic Significance of Pretreatment Cancer-Associated Weight Loss
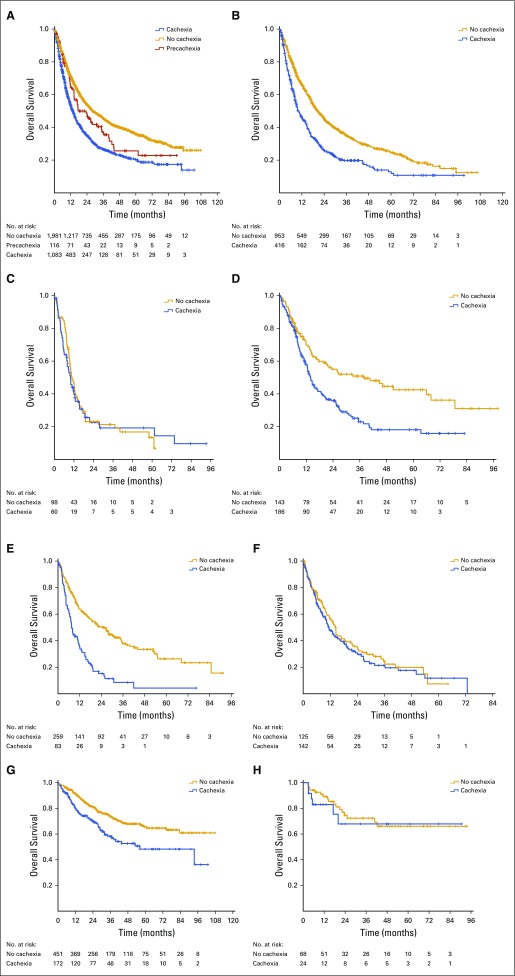
Median survival of the overall cohort was 21.5 months (95% CI, 19.8 to 23.4). Across the entire cohort, weight loss at the time of cancer diagnosis was predictive of reduced survival, even after controlling for age, sex, comorbidities, tobacco history, stage, size, and grade (hazard ratio, 1.26; 95% CI, 1.13 to 1.39; Appendix Table A2). The median survival of patients without weight loss, with minimal weight loss, and with overt weight loss at diagnosis was 28.2, 17.5, and 13.6 months, respectively (P < .001; Fig 1A). Survival of patients with no weight loss and minimal weight loss was similar in the first year after diagnosis but diverged thereafter (Fig 1A).
Fig 1.
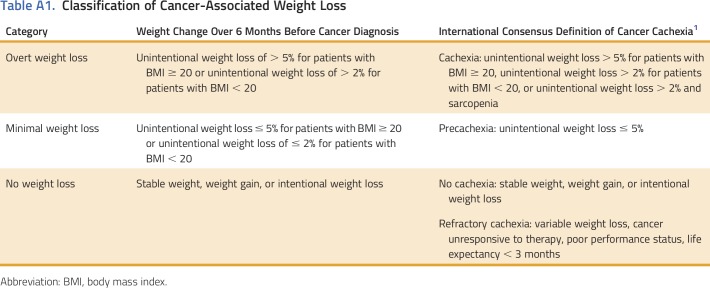
Pretreatment cancer-associated weight loss is a powerful prognostic factor. (A) Overall survival of all eligible patients with thoracic and GI malignancies. Patients with no weight loss, minimal weight loss, and overt weight loss had median survival of 28.2, 17.5, and 13.6 months, respectively (P < .001). (B) Non–small-cell lung cancer. Patients with no weight loss and overt weight loss had median survival of 20.5 and 9.9 months, respectively (P < .001). (C) Small-cell lung cancer. Patients with no weight loss and overt weight loss had median survival of 10.6 and 9.9 months, respectively (P = .75). (D) Gastroesophageal cancer. Patients with no weight loss and overt weight loss had respective median survival of 37.9 and 13.9 months (P < .001). (E) Hepatobiliary cancer. Patients with no weight loss and overt weight loss had respective median survival of 25.1 and 7.6 months (P < .001). (F) Pancreatic cancer. Patients with no weight loss and overt weight loss had respective median survival of 14.4 and 11.4 months (P = .40). (G) Colorectal cancer. Median survival was not reached for patients without pretreatment weight loss, whereas median survival of those with overt weight loss was 56.3 months (P < .001). (H) Anal cancer. Median survival was not reached for patients with or without cachexia (P = .61).
The association between cancer-associated wasting at diagnosis and reduced survival was consistent across disease sites (Table 2; Figs 1B-1H). However, the prognostic impact was particularly evident in NSCLC, gastroesophageal cancer, and hepatobiliary cancer: 20.5 versus 9.9 months (P < .001), 37.9 versus 13.9 months (P < .001), and 25.1 versus 7.6 months (P < .001), respectively. Overall survival stratified by primary tumor site, stage, and pretreatment weight loss classification is presented in Table 2.
A slightly higher proportion of patients with stage I to III disease and overt weight loss at diagnosis did not receive any therapeutic intervention for malignancy compared with those without weight loss (7.0% v 4.7%; P = .001). In contrast, a similar number of patients with stage IV disease did not receive therapy regardless of weight loss (4.9% in both groups; P = .31). In the subset of patients who received anticancer therapy, pretreatment weight loss remained a significant prognostic factor. Median survival was 32.4, 24.0, and 15.1 months for patients who received anticancer therapy and had no pretreatment weight loss, minimal weight loss, and overt weight loss, respectively.
DISCUSSION
Cachexia is recognized as a significant contributor to morbidity and mortality in patients with cancer. However, its prevalence—specifically at the time of cancer diagnosis—and prognostic significance are not well characterized. We comprehensively reviewed medical records to present, to our knowledge, the largest cohort study of cancer-associated wasting to date, including a broad range of thoracic and GI primary tumor sites and stages. Pretreatment cancer-associated weight loss was observed in 34.1% of all patients with cancer in this cohort, including 17.6% of patients with stage I disease at diagnosis. We found that weight loss at the time of cancer diagnosis was predictive of reduced survival even after adjusting for age, sex, Charlson comorbidity, stage, and other prognostic factors.
For the first time, we report that minimal weight loss (approximating the consensus definition of precachexia)1 at the time of cancer diagnosis is associated with intermediate survival compared with patients with no weight loss and overt weight loss. This suggests minimal cancer-associated weight loss represents a distinct clinical entity and demonstrates provocative clinical significance for even minimal weight loss. The difference in survival between patients with minimal weight loss and no weight loss did not manifest until approximately 1 year after cancer diagnosis. We hypothesize this finding is related to two factors: (1) the time to transition from minimal weight loss to overt wasting and (2) the time to deplete the adipose reserve in our patient population. If true, this would suggest that cachexia-directed interventions may have their greatest impact on survival in the early, minimal weight-loss state before frank muscle and fat atrophy associated with overt cachexia. Unfortunately, early cancer-associated weight loss is under-diagnosed.1 Our data suggest that recognition of minimal weight loss and early use of anticachexia interventions may ensure optimal treatment and survival outcomes. This may include referral for multimodality care, including evaluation by dieticians, physical therapists, and palliative care specialists.10 A landmark randomized trial demonstrated that early use of palliative care improves quality of life and, remarkably, overall survival for patients with incurable thoracic or GI cancer.11,12 In our view, an application of this principle for patients with precachexia has the greatest potential to improve patient outcomes. Similar guidelines have been adopted by other groups, including the European School of Oncology.9
Our study has several key advantages over previous studies. Dewys and colleagues have described the impact of cancer-associated weight loss on survival, but they focused on patients with advanced cancers.2,4,13-17 In contrast, our cohort includes many patients with early-stage disease, and the presence of cancer-associated weight loss was determined at the time of initial cancer diagnosis using a validated consensus definition. Moreover, for the first time to our knowledge, our study reveals significant differences in survival across the clinical spectrum of cachexia across multiple tumor types. Unlike with other cancers, in SCLC and pancreatic cancers, early survival is dictated by the metastatic and aggressive nature of tumor progression. Therefore, survival early after diagnosis is less influenced by the presence or absence of cachexia.
This analysis has limitations inherent to all retrospective studies. For example, cachexia was retrospectively assessed from medical records rather than via a formal prospective protocol. Although cancer-associated weight loss was based on the consensus definition of cancer cachexia,1 the diagnostic criterion of sarcopenia was omitted, because muscle mass measurements are not routinely performed. Additionally, our cohort includes a relatively small subset of patients with minimal weight loss, but its true prevalence is likely to be higher because patients and clinicians may not report or be sensitive to small changes in weight, muscle mass, or subtle symptoms—a challenge exacerbated by epidemic obesity.18,19 This latter concession again reinforces the need for improved recognition of the precachexia state.
In conclusion, the results of this study affirm the detrimental effect of cancer-associated weight loss on survival across a range of cancer pathology, even in early-stage disease. The presence of early, minimal weight loss alone is predictive of worse outcomes and highlights the need for early detection and intervention of cancer cachexia in hopes of improvement in survival. Further clinical and preclinical research is needed to elucidate the molecular mechanisms underlying cancer cachexia and to generate novel therapies directed at managing cancer-associated weight loss.
ACKNOWLEDGMENT
B.S.G. and S.K.M.L. contributed equally to this work. R.I. and P.I. contributed equally to this work. Supported in part by the National Center for Advancing Translational Sciences Grants No. TL1TR001104 and UL1TR001105. Presented in part at the Association for Clinical and Translational Science 2015 Meeting, Washington, DC, April 16-18, 2015 and the 16th World Conference on Lung Cancer, Denver, CO, September 6-9, 2015. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank Alejandra Madrigales and Philip Reeder of the University of Texas Southwestern Tumor Registry for their contributions to this research.
Appendix
Table A1.
Classification of Cancer-Associated Weight Loss
Table A2.
Stepwise Cox Regression Model for Overall Survival
AUTHOR CONTRIBUTIONS
Conception and design: Bhavani S. Gannavarapu, Nathan A. Cannon, Jeffrey J. Meyer, David J. Sher, Puneeth Iyengar
Collection and assembly of data: Bhavani S. Gannavarapu, Steven K.M. Lau, Kristen Carter
Data analysis and interpretation: Bhavani S. Gannavarapu, Steven K.M. Lau, Ang Gao, Chul Ahn, Jeffrey J. Meyer, David J. Sher, Aminah Jatoi, Puneeth Iyengar
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prevalence and Survival Impact of Pretreatment Cancer-Associated Weight Loss: A Tool for Guiding Early Palliative Care
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Bhavani S. Gannavarapu
No relationship to disclose
Steven K.M. Lau
Employment: LabCorp (I)
Kristen Carter
No relationship to disclose
Nathan A. Cannon
Employment: 21st Century Oncology
Ang Gao
No relationship to disclose
Chul Ahn
No relationship to disclose
Jeffrey J. Meyer
Honoraria: UpToDate
Research Funding: Peregrine Pharmaceuticals (Inst), DFINE (Inst)
David J. Sher
No relationship to disclose
Aminah Jatoi
Research Funding: Entera Health, Boston Biologics
Rodney Infante
No relationship to disclose
Puneeth Iyengar
Consulting or Advisory Role: Helsinn Therapeutics
REFERENCES
- 1.Fearon K, Strasser F, Anker SD, et al. : Definition and classification of cancer cachexia: An international consensus. Lancet Oncol 12:489-495, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Blum D, Stene GB, Solheim TS, et al. : Validation of the consensus-definition for cancer cachexia and evaluation of a classification model: A study based on data from an international multicentre project (EPCRC-CSA). Ann Oncol 25:1635-1642, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Ross PJ, Ashley S, Norton A, et al. : Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 90:1905-1911, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dewys WD, Begg C, Lavin PT, et al. : Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am J Med 69:491-497, 1980 [DOI] [PubMed] [Google Scholar]
- 5.Mariani L, Lo Vullo S, Bozzetti F: Weight loss in cancer patients: A plea for a better awareness of the issue. Support Care Cancer 20:301-309, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Arrieta O, Michel Ortega RM, Villanueva-Rodríguez G, et al. : Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: A prospective study. BMC Cancer 10:50, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Haehling S, Anker SD: Cachexia as a major underestimated and unmet medical need: Facts and numbers. J Cachexia Sarcopenia Muscle 1:1-5, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baracos VE: Clinical trials of cancer cachexia therapy, now and hereafter. J Clin Oncol 31:1257-1258, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Aapro M, Arends J, Bozzetti F, et al. : Early recognition of malnutrition and cachexia in the cancer patient: A position paper of a European School of Oncology Task Force. Ann Oncol 25:1492-1499, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Bruggeman AR, Kamal AH, LeBlanc TW, et al. : Cancer cachexia: Beyond weight loss. J Oncol Pract 12:1163-1171, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363:733-742, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Temel JS, Greer JA, El-Jawahri A, et al. : Effects of early integrated palliative care in patients with lung and GI cancer: A randomized clinical trial. J Clin Oncol 35:834-841, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeBlanc TW, Nipp RD, Rushing CN, et al. : Correlation between the international consensus definition of the cancer anorexia-cachexia syndrome (CACS) and patient-centered outcomes in advanced non-small cell lung cancer. J Pain Symptom Manage 49:680-689, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Bachmann J, Heiligensetzer M, Krakowski-Roosen H, et al. : Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg 12:1193-1201, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Vigano A, Del Fabbro E, Bruera E, et al. : The cachexia clinic: From staging to managing nutritional and functional problems in advanced cancer patients. Crit Rev Oncog 17:293-303, 2012 [DOI] [PubMed] [Google Scholar]
- 16.van der Meij BS, Schoonbeek CP, Smit EF, et al. : Pre-cachexia and cachexia at diagnosis of stage III non-small-cell lung carcinoma: An exploratory study comparing two consensus-based frameworks. Br J Nutr 109:2231-2239, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Martin L, Watanabe S, Fainsinger R, et al. : Prognostic factors in patients with advanced cancer: Use of the patient-generated subjective global assessment in survival prediction. J Clin Oncol 28:4376-4383, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Tan BH, Birdsell LA, Martin L, et al. : Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 15:6973-6979, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Prado CM, Lieffers JR, McCargar LJ, et al. : Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol 9:629-635, 2008 [DOI] [PubMed] [Google Scholar]