Abstract
Purpose:
Socioeconomic status (SES) influences health care outcomes, but the influence of primary payer on cancer-associated wasting is unknown. We hypothesized that primary payer as an indicator of SES would influence pretreatment cancer-associated weight loss and treatment outcomes.
Materials and Methods:
Retrospective review of medical records identified 1,366 patients with non–small-cell lung cancer (NSCLC) consecutively treated at a tertiary care health system between January 1, 2006 and December 31, 2013. Insurance status was obtained from an institutional tumor registry. Cancer-associated weight loss was based on the validated international consensus definition of cachexia. Multivariable regression analyses were used to identify prognostic factors of pretreatment cancer-associated weight loss and survival.
Results:
The cohort included a representative group of patients with a median age at diagnosis of 64 years, 47% females, and 33% patients of nonwhite race. Pretreatment cancer-associated weight loss was present at the time of NSCLC diagnosis in 17%, 14%, 32%, and 38% of patients with stage I, II, III, and IV disease, respectively. Pretreatment cancer-associated weight loss was associated with increasing age at diagnosis, black race, single marital status, tobacco use, and disease stage. Compared with private insurance, Medicaid insurance (odds ratio, 2.17; 95% CI, 1.42 to 3.30) and lack of insurance (odds ratio, 2.32; 95% CI, 1.50 to 3.58) were associated with pretreatment cancer-associated weight loss. Among cachectic patients, comorbidity, histology, tumor grade, and disease stage were prognostic of survival on multivariable analysis; however, primary payer was not.
Conclusion:
Pretreatment cancer-associated weight loss is common in patients with NSCLC, and its presence is significantly associated with lower SES. However, among patients with pretreatment cancer-associated weight loss, SES was not predictive of survival. Early use of cancer cachexia–directed therapies may improve outcomes, and further study on the biologic mechanisms of cancer cachexia will provide novel therapeutic avenues.
INTRODUCTION
Cancer cachexia is a multifactorial syndrome characterized by ongoing, unintentional weight loss that cannot be fully reversed by nutritional support and leads to progressive functional impairment.1 Moreover, it is associated with decreases in tolerance to anticancer therapy, quality of life, and survival.2-4 Importantly, weight loss occurring before the initiation of cancer therapies is also associated with these detrimental effects.4 Therefore, identifying factors predictive of pretreatment cancer-associated weight loss is critical to providing optimal cancer therapy.
Socioeconomic status (SES) influences health care outcomes in various settings, including non–small-cell lung cancer (NSCLC).5-9 Association of lower SES and diminished health outcomes has been proposed to be related to decreased access to medical care, which could further extend to anticachexia interventions.5,6 However, the interaction of SES and cancer-associated weight loss is poorly described. To our knowledge, our report is the first to explore the impact of SES on pretreatment cancer-associated weight loss at the time of NSCLC diagnosis and the prognostic significance of SES in patients with cancer-associated weight loss.
MATERIALS AND METHODS
Population Cohort and Data Acquisition
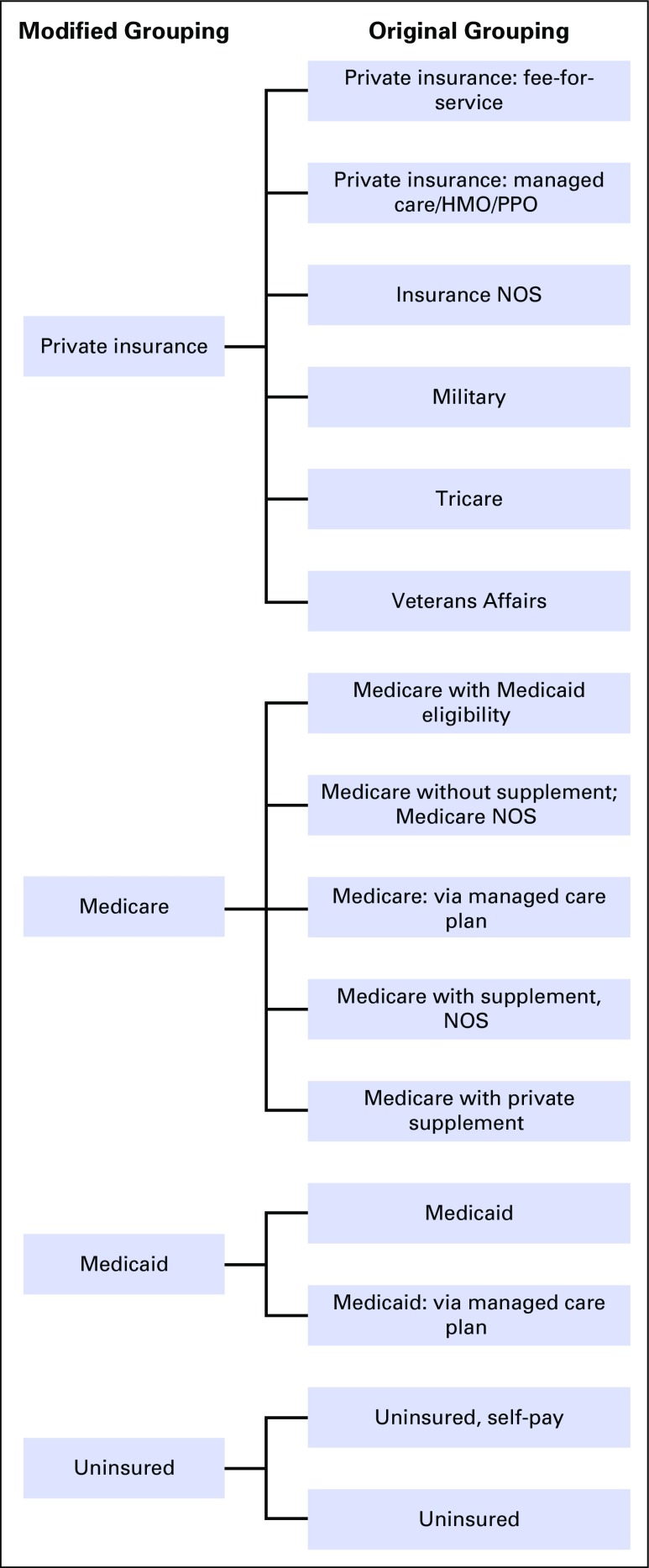
Institutional review board approval was obtained for this study. A prospectively maintained institutional tumor registry identified 1,678 adult patients with a primary lung malignancy consecutively treated within a single tertiary care health system between January 1, 2006 and December 31, 2013. Patients with carcinoma in situ, small-cell lung cancer, carcinoid tumor, synchronous or metachronous malignancies, and incomplete electronic medical records were excluded. After these exclusions, 1,366 patients with NSCLC were eligible for further analysis. Patient and tumor characteristics were obtained from the registry. Primary payer was also obtained from the registry but was categorized as private insurance, Medicare, Medicaid, or uninsured (Appendix Fig A1, online only).
Assessment of Cancer-Associated Weight Loss
Medical records were systematically reviewed by a single author (B.S.G.) to assess for documented weight loss at the time of cancer diagnosis but before any therapeutic measure. Cancer-associated weight loss was defined using the well-accepted and validated international consensus definition of cachexia.1 This is summarized as > 5% weight loss over 6 months preceding the date of cancer diagnosis for patients with body mass index ≥ 20 kg/m2 or unintentional weight loss > 2% for patients with body mass index < 20 kg/m2. Patients with stable weight, weight gain, or purposeful weight loss were classified as not having weight loss. A second author (S.K.M.L.) independently reviewed the medical records of a random sample of patients and verified the results for these patients in a blinded manner.
Statistics
Inter-rater reliability was assessed using a κ coefficient. Descriptive statistics were used to summarize patient characteristics at baseline. Time to oncologist and time to therapy were compared using analysis of variance. Stepwise logistic regression was used to identify factors associated with the presence of cancer-associated weight loss at the time of cancer diagnosis. Stepwise Cox regression analysis was used to identify significant independent factors associated with overall survival among patients with weight loss. Factors with a P value < .2 on univariable analysis were entered as variables in stepwise regression analyses. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Population Cohort
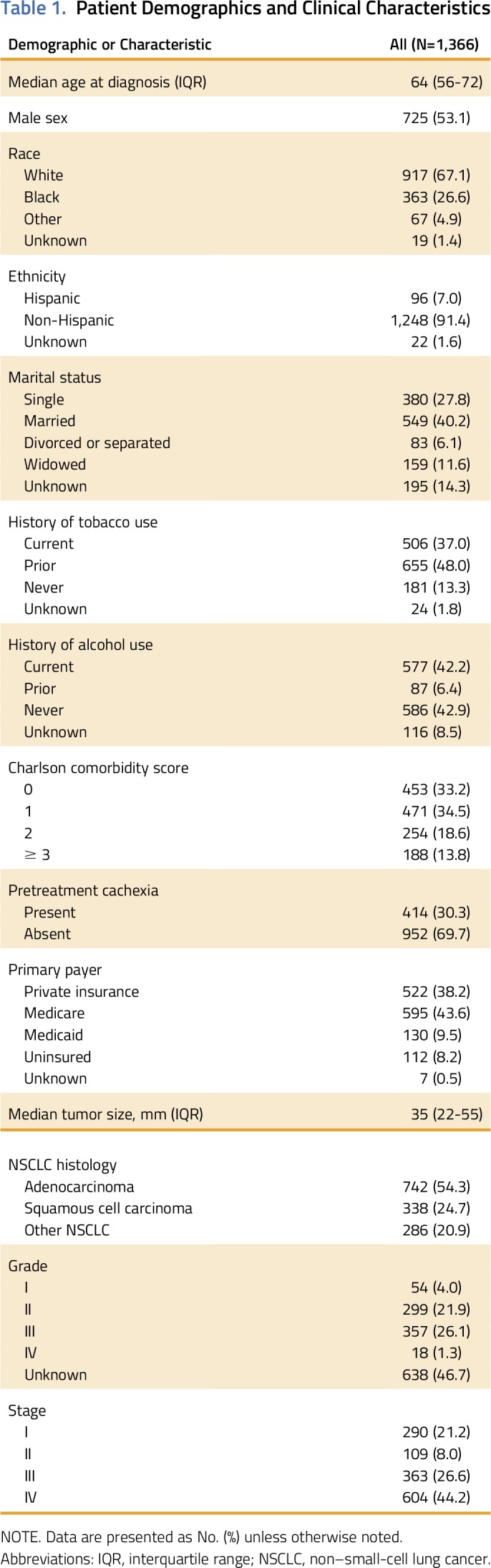
A total of 1,366 adult patients with NSCLC were eligible for this study. Patient characteristics are presented in Table 1. The cohort was representative, with a median age at diagnosis of 64 years (range, 24 to 96 years), 725 (53.1%) men, and 449 (32.9%) patients of nonwhite race. The most common histology was adenocarcinoma (n = 742; 54.3%), with squamous cell carcinoma (n = 338; 24.7%) the second most common. Cancer-associated weight loss was present at the time of NSCLC diagnosis in 414 (30.3%) patients. Inter-rater κ coefficient was 0.71, indicating high concordance between raters.10 A majority of patients had private or government-subsidized insurance: 522 (38.2%) patients had private insurance, 595 (43.6%) had Medicare, and 130 (9.5%) had Medicaid. In total, 112 (8.2%) patients were uninsured, and insurance status was unknown for only seven (0.5%) patients.
Table 1.
Patient Demographics and Clinical Characteristics
Delay to oncologist, defined as elapsed days from biopsy confirmation of malignancy to consultation with an oncology specialist, differed by insurance status (P < .001). Patients with private insurance had intermediate delay to oncologist (mean, 31 days), whereas patients with Medicare had significantly shorter delay to oncologist (mean, 24 days). Patients with Medicaid (mean, 37 days) and uninsured patients (mean, 48 days) had longer delays to oncologist. In contrast, delay to therapy, defined as elapsed days from biopsy to the first date of cancer-directed therapy, was similar regardless of insurance status (P = .063). Surgery and radiotherapy were used at similar rates by NSCLC stage, with the exception of surgery in stage III disease (20% of patients without pretreatment weight loss compared with 6% in its presence; P < .001).
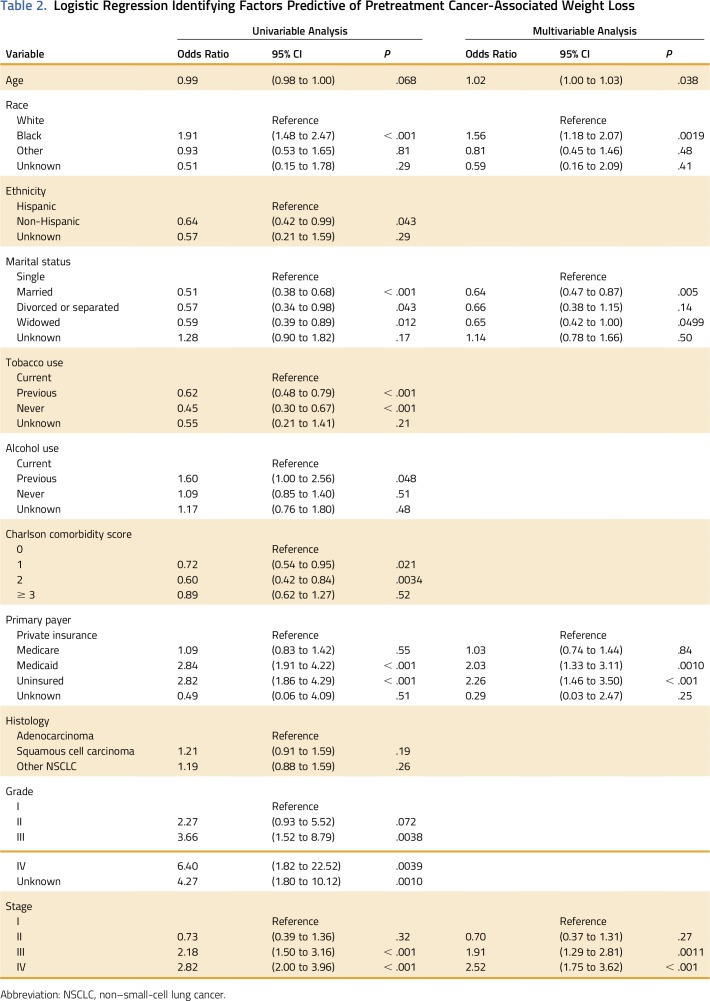
Factors Associated With Pretreatment Weight Loss at NSCLC Diagnosis
Race, ethnicity, marital status, tobacco use, alcohol use, primary payer, Charlson comorbidity score, tumor stage, and tumor grade influenced pretreatment cancer-associated weight loss on univariable logistic regression. Age and histology did not influence pretreatment cancer-associated weight loss on univariable logistic regression (Table 2). Specifically, patients with Medicaid (odds ratio [OR], 2.84; 95% CI, 1.91 to 4.22) and the uninsured (OR, 2.82; 95% CI, 1.86 to 4.29) were more likely to have pretreatment weight loss. This was on a similar magnitude as black race compared with white race (OR, 1.91; 95% CI, 1.48 to 2.47) or stage IV disease compared with stage I disease (OR, 2.82; 95% CI, 2.00 to 3.98). Married and widower statuses were protective for pretreatment weight loss. (OR, 0.51; 95% CI, 0.38 to 0.68).
Table 2.
Logistic Regression Identifying Factors Predictive of Pretreatment Cancer-Associated Weight Loss
Stepwise multivariable logistic regression was used to further identify factors independently associated with pretreatment wasting (Table 2). Increasing age and tumor stage were positively correlated with pretreatment weight loss at NSCLC diagnosis, as was black race. Married status was protective for pretreatment weight loss. After accounting for other factors, primary payer remained an independent predictor of pretreatment cancer-associated weight loss. Compared with patients with private insurance, those with Medicaid (OR, 2.03; 95% CI, 1.33 to 3.11) and the uninsured (OR, 2.26; 95% CI, 1.46 to 3.50) were more likely to have pretreatment weight loss.
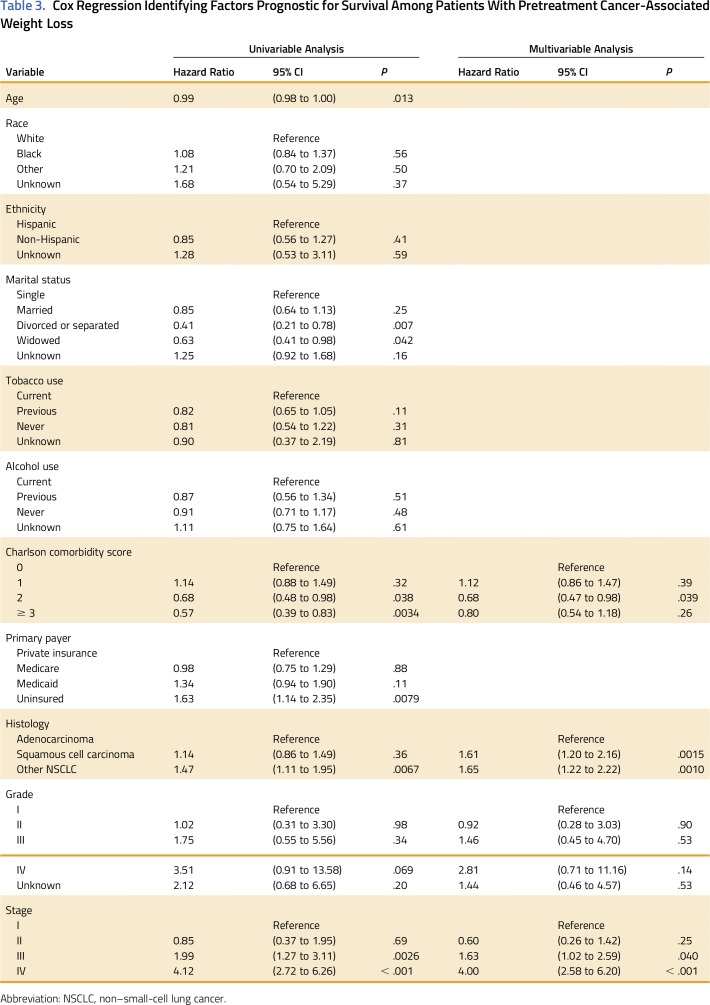
Factors Associated With Survival in Patients With NSCLC With Weight Loss
Median survival for all patients was 17.0 months. Cox regression analysis was performed to test the hypothesis that lower SES is associated with worse survival for patients with cancer-associated weight loss. Among the 414 patients with pretreatment weight loss at NSCLC diagnosis, patient age, marital status, Charlson comorbidity score, primary payer, tumor histology, tumor grade, and tumor stage were associated with survival on univariable Cox regression (Table 3). Specifically, divorced or legally separated (hazard ratio [HR], 0.41; 95% CI, 0.21 to 0.78) and widowed marital status (HR, 0.63; 95% CI, 0.41 to 0.98) were associated with increased survival compared with single status. There was a paradoxical association of Charlson comorbidity score 2 with survival compared with patients with a score of 0 (HR, 0.68; 95% CI, 0.48 to 0.98). Among patients with pretreatment weight loss, uninsured patients had diminished survival compared with those with private insurance (HR, 1.63; 95% CI, 1.14 to 2.35). Insurance status was not associated with survival for patients without pretreatment weight loss (data not shown). Race, ethnicity, tobacco use, and alcohol use were not prognostic of survival on univariable analysis.
Table 3.
Cox Regression Identifying Factors Prognostic for Survival Among Patients With Pretreatment Cancer-Associated Weight Loss
Stepwise multivariable Cox regression was used to further identify predictors of overall survival among patients with NSCLC with pretreatment weight loss (Table 3). As expected, increased tumor stage was prognostic of diminished survival. Squamous cell (HR, 1.61; 95% CI, 1.20 to 2.16) and other NSCLC histology (HR, 1.65; 95% CI, 1.22 to 2.22) were also associated with worse survival compared with adenocarcinoma. SES as represented by primary payer was not independently prognostic of survival on multivariable analysis for patients with NSCLC with pretreatment weight loss.
DISCUSSION
SES is prognostic of various health care outcomes, and the association of lower SES with worse outcomes has been attributed to diminished access to medical care.5-7 Cancer cachexia is clearly associated with functional impairment and poor prognosis.2,3 Moreover, weight loss occurring before any cancer therapy is prognostic of diminished survival for patients with advanced NSCLC.4 However, the influence of SES on cancer cachexia is unknown. To our knowledge, this study is the first to assess the impact of SES on pretreatment cancer-associated weight loss and survival among cachectic patients with NSCLC. For the first time, we demonstrate primary payer as an independent predictor of pretreatment cancer-associated weight loss in a diverse cohort of patients with NSCLC (Table 2). Interestingly, among patients with weight loss at the time of cancer diagnosis, SES as measured by primary payer was not independently prognostic of survival (Table 3).
The association of primary payer with pretreatment cancer-associated weight loss in our cohort is provocative. Prior studies have focused on an association of lower SES with more advanced malignancy at the time of diagnosis.8,9 Halpern et al8 reviewed > 3.7 million patients, including 693,697 patients with lung cancer, registered to the National Cancer Database. Medicaid insurance and lack of insurance were associated with more advanced stage at presentation compared with private insurance.8 Lower SES was also correlated with more advanced NSCLC stage at diagnosis in our cohort (data not shown). Interestingly, primary payer remained predictive of pretreatment cancer-associated weight loss after controlling for stage. Together, these findings suggest SES influences multiple aspects of oncogenesis and disease presentation.
Although lower SES was prognostic for survival when all patients in our cohort were included (data not shown), SES as represented by primary payer was not independently prognostic among patients with pretreatment weight loss. Previous studies have revealed an association of lower SES with diminished survival.5,6,11,12 Cella et al5 reviewed 2,089 patients, including 961 patients with lung cancer, enrolled in eight Cancer and Leukemia Group B protocols. Lower income and lower educational attainment were prognostic of worse survival even after controlling for stage at presentation.5 Interestingly, SES remains a powerful prognostic indicator of cancer survival in universal health care systems designed with equal access in mind.13,14 Together, these findings suggest pretreatment cancer-associated weight loss has a stronger influence on survival than SES. A complementary possibility is that currently available anticachexia therapies, which may be more readily available to patients of higher SES, do not provide durable benefit or are not used early enough before patients progress to refractory cachexia.15
Among those with pretreatment weight loss, the paradoxical association of Charlson comorbidity score 2 with overall survival has several possible explanations. The group may be heterogeneous, because comorbidities of varying severity may lead to a Charlson score of 2. Similarly, the increasing early diagnosis of comorbid conditions, such as diabetes mellitus, may alter the prognostic significance of them. Finally, could a comorbidity or its treatments, such as diabetes and metformin, actually be protective against the negative prognostic effects of weight loss? This raises interesting points that will be further explored.
This analysis has limitations inherent to all retrospective studies. Although patients were identified using a prospectively maintained database, the presence of cancer-associated weight loss was retrospectively assessed. Although cancer-associated weight loss was based on the consensus definition of cachexia, the diagnostic criterion of sarcopenia was omitted because muscle mass measurements are not routinely performed at our institution.1 Moreover, primary payer was the sole indicator of SES because educational attainment and household income on an individual patient level are not routinely documented at our institution. Finally, because all patients in our cohort had NSCLC, the generalization of these findings to malignancies other than NSCLC may be limited.
In conclusion, patients with NSCLC of lower SES as measured by primary payer are disproportionately affected by cancer-associated weight loss, which in turn is prognostic of diminished survival. Among patients with pretreatment weight loss, lower SES is not independently prognostic of survival. These findings suggest early recognition and management of cachexia, even at the time of cancer diagnosis, could result in improved survival.
ACKNOWLEDGMENT
Supported in part by National Center for Advancing Translational Sciences Grants No. TL1TR001104 and UL1TR001105 (B.S.G.). S.K.M.L. and B.S.G. contributed equally to this work. R.I. and P.I. contributed equally to this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Alejandra Madrigales and Philip Reeder of the University of Texas Southwestern Medical Center Tumor Registry for their contributions to this work.
Appendix
Fig A1.
Classification schema for insurance status. HMO, health maintenance organization; NOS, not otherwise specified; PPO, preferred provider organization.
AUTHOR CONTRIBUTIONS
Conception and design: Steven K.M. Lau, Bhavani S. Gannavarapu, Jeffrey J. Meyer, Rodney Infante, Puneeth Iyengar
Collection and assembly of data: Steven K.M. Lau, Bhavani S. Gannavarapu, Kristen Carter, Puneeth Iyengar
Data analysis and interpretation: Steven K.M. Lau, Bhavani S. Gannavarapu, Ang Gao, Chul Ahn, Jeffrey J. Meyer, David J. Sher, Aminah Jatoi, Rodney Infante, Puneeth Iyengar
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of Socioeconomic Status on Pretreatment Weight Loss and Survival in Non–Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Steven K.M. Lau
Employment: LabCorp (I)
Bhavani S. Gannavarapu
No relationship to disclose
Kristen Carter
No relationship to disclose
Ang Gao
No relationship to disclose
Chul Ahn
Honoraria: Advenchen Laboratories
Travel, Accommodations, Expenses: Macrogen
Jeffrey J. Meyer
Honoraria: UpToDate
Research Funding: Peregrine Pharmaceuticals (Inst), DFINE (Inst)
David J. Sher
No relationship to disclose
Aminah Jatoi
No relationship to disclose
Rodney Infante
No relationship to disclose
Puneeth Iyengar
Consulting or Advisory Role: Helsinn Therapeutics
REFERENCES
- 1.Fearon K, Strasser F, Anker SD, et al. : Definition and classification of cancer cachexia: An international consensus. Lancet Oncol 12:489-495, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Dewys WD, Begg C, Lavin PT, et al. : Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am J Med 69:491-497, 1980 [DOI] [PubMed] [Google Scholar]
- 3.van der Meij BS, Schoonbeek CP, Smit EF, et al. : Pre-cachexia and cachexia at diagnosis of stage III non-small-cell lung carcinoma: An exploratory study comparing two consensus-based frameworks. Br J Nutr 109:2231-2239, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Ross PJ, Ashley S, Norton A, et al. : Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 90:1905-1911, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cella DF, Orav EJ, Kornblith AB, et al. : Socioeconomic status and cancer survival. J Clin Oncol 9:1500-1509, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Sorlie P, Rogot E, Anderson R, et al. : Black-white mortality differences by family income. Lancet 340:346-350, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Albain KS, Unger JM, Crowley JJ, et al. : Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst 101:984-992, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halpern MT, Ward EM, Pavluck AL, et al. : Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: A retrospective analysis. Lancet Oncol 9:222-231, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Ayanian JZ, Kohler BA, Abe T, et al. : The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med 329:326-331, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Landis JR, Koch GG: The measurement of observer agreement for categorical data. Biometrics 33:159-174, 1977 [PubMed] [Google Scholar]
- 11.Du XL, Lin CC, Johnson NJ, et al. : Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival: Findings from the National Longitudinal Mortality Study, 1979-2003. Cancer 117:3242-3251, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erhunmwunsee L, Joshi MB, Conlon DH, et al. : Neighborhood-level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer 118:5117-5123, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Mackillop WJ, Zhang-Salomons J, Groome PA, et al. : Socioeconomic status and cancer survival in Ontario. J Clin Oncol 15:1680-1689, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Booth CM, Li G, Zhang-Salomons J, et al. : The impact of socioeconomic status on stage of cancer at diagnosis and survival: A population-based study in Ontario, Canada. Cancer 116:4160-4167, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Baracos VE: Clinical trials of cancer cachexia therapy, now and hereafter. J Clin Oncol 31:1257-1258, 2013 [DOI] [PubMed] [Google Scholar]