Abstract
Allergic bronchial asthma (BA) is characterized by chronic airway inflammation, development of airway hyperreactivity and recurrent reversible airway obstruction. T-helper 2 cells and their products have been shown to play an important role in this process. In contrast, the mechanisms by which immune cells interact with the cells residing in lung and airways, such as neurons, epithelial or smooth muscle cells, still remains uncertain. Sensory and motor neurons innervating the lung exhibit a great degree of functional plasticity in BA defined as 'neuronal plasticity'. These neurons control development of airway hyperresponsiveness and acute inflammatory responses, resulting in the concept of 'neurogenic inflammation'. Such quantitative and/or qualitative changes in neuronal functions are mediated to a great extent by a family of cytokines, the neurotrophins, which in turn are produced by activated immune cells, among others in BA. We have therefore developed the concept that neurotrophins such as nerve growth factor and brain-derived neurotrophic factor link pathogenic events in BA to dysfunctions of the immune and nervous system.
Keywords: bronchial asthma, neurogenic inflammation, neuronal plasticity, neurotrophins
Introduction
BA is a complex disease with several clinically well-defined pathogenic components, including recurrent reversible airway obstruction, chronic airway inflammation and development of airway hyperresponsiveness [1]. The constituents of the inflammatory component have, in recent years, been relatively well characterized and defined. There is now overwhelming evidence that T cells play a central role in, particularly, allergic BA. Strong evidence supports the notion that T-helper 2 cells orchestrate allergic inflammation and control many important aspects of the effector phase response, including recruitment, activation and survival of eosinophils, activation of mast cells and IgE production. Reversible airway obstruction is pathogenetically related to mucus hypersecretion, development of local tissue edema as a consequence of acute inflammatory responses, and constriction of airway smooth muscle. Nonspecific bronchial hyperresponsiveness may be defined as an increase in the ease and degree of airway narrowing in response to a wide range of bronchoconstrictor stimuli. The development of airway hyperresponsiveness is mediated by multiple independent and additive pathways working in concert, which can be clinically tested using stimuli such as methacholine, histamine, exercise, cold air, capsaicin, and so on. Constriction of airway smooth muscle is largely controlled by sensory and motor neurons innervating the airways and the lung. The autonomic nerves that regulate many aspects of airway function, including airway smooth muscle tone, mucus secretion and bronchial microcirculation, can be functionally subdivided into cholinergic, adrenergic and nonadrenergic noncholinergic pathways.
Neurogenic inflammation and neuronal plasticity in BA
Sensory and motor neurons exhibit drastic functional changes in BA. These changes are defined by the term 'neuronal plasticity' [2]. Increased levels of neuropeptides including substance P have been detected in the lungs of asthmatic patients [3,4]. Increased levels of Neurokinin A have been detected in bronchoalveolar lavage fluids of asthmatic patients following airway allergen challenge. Since cholinergic nerves represent the dominant bronchoconstrictor pathway, anticholinergic drugs are very effective bronchodilators in asthma therapy. This further underlines the importance of cholinergic mechanisms in the development of BA and airway hyperresponsiveness. The underlying mechanisms include enhanced reflex activity, increased mediator release, enhanced sensitivity of smooth muscle to neuropeptides and tachykinins, and increased density of receptor expression of both airway smooth muscle cells and neurons. In addition to qualitative changes in neuronal functions, debate still continues regarding whether quantitative changes in sensory and/or motor neurons also occur in this disease.
Neuropeptides and tachykinins are involved in several key features of BA, including airway smooth muscle constriction, vascular dilatation, increased vascular permeability, mucus hypersecretion and acceleration of airway inflammation. These effects attributed to the function of neuropeptides and tachykinins lead to the concept of 'neurogenic inflammation' in BA [5,6,7].
Neurotrophins
The functional plasticity of sensory and motor neurons is under close control of neurotrophins. The neurotrophins nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 and neurotrophin-4/neurotrophin-5 belong to a family of homologous proteins that exert their effects primarily as target-derived paracrine or autocrine neurotrophic factors. The role of the neurotrophins in survival, differentiation and maintenance of neurons is defined well [8]. They exhibit partially overlapping but distinct patterns of expression and cellular targets. In addition to the effects in the central nervous system, neurotrophins also effect peripheral afferent and efferent neurons. The biological effects of neurotrophins are mediated by binding either to the high affinity (KD10-11) tyrosine kinese receptors (trkA, trkB, trkC) or the low affinity (KD10-9) pan-neurotrophin receptor p75NTR. Substantial biological effects of neurotrophins are mediated by the high affinity kinese receptors. The high affinity effector for NGF is trkA, that for BDNF and neurotrophin-4 is trkB, and that for neurotrophin-3 is trkC. Neurotrophin receptors are widely expressed on the neurons of the peripheral and the central nervous system, both during development and in adults. However, trk receptors as well as p75NTR are also expressed on non-neuronal cells, including immune cells, muscle cells and epithelial cells.
The traditional cellular sources of neurotrophins under physiological conditions are primarily nerve-associated cells such as glia cells, Schwann cells or fibroblasts and neurons themselves [8,9]. NGF is also produced in inflammatory processes by a wide range of hematopoetic cells, including mast cells [10], macrophages [11], T cells [12] and B cells [13]. This has been shown in a well-characterized animal model system of allergic airway inflammation and airway hyperresponsiveness [11,14,15]. In addition, airway epiphilium constitutively expresses BDNF but not NGF, and BDNF production is further enhanced during inflammatory responses. Further to animal model systems, enhanced neurotrophin production has also been shown in patients with several allergic conditions. The initial report of enhanced NGF production was provided by Bonini et al, indicating that patients with severe allergic BA display high serum levels of NGF [16]. Furthermore, increases in NGF serum levels have been demonstrated in patients with vernal ceratonconjunctivities and allergic rhinoconjunctivities [17]. Together with the group of Virchow et al, we have more recently shown that neurotrophin production is increased in bronchoalveolar lavage fluids from patients undergoing segmental allergen provocation. BDNF and NGF levels were particularly increased 18 hours after provocation, whereas no increases were detected 20 min after provocation [18]. These data again indicate local production and release of neurotrophins on stimulation, and increased levels of neurotrophins are associated with late-phase allergic responses, but not with the early-phase response.
What are the functional effects of increased neurotrophin production during the allergic response? Based on the data provided by other workers and our group, we propose the concept that neurotrophins play an important role in the pathophysiology of asthma in several ways (Fig. 1). The predominant effect on peripheral nerves is described by the term 'neuronal plasticity', which is defined as qualitative and/or quantitative changes in the functional activity and capacity of peripheral neurons. Examples include increased production of neuropeptides and tachykinins, increased receptor expression, increases in the number of nerves producing certain neuropeptides and tachykinins, and lowering of the firing threshold of nerves. For all these effects, there are ample examples provided by studies conducted either in animal model systems or using human cell cultures [19,20,21,22,23,24]. Initial studies were carried out by Undem et al, demonstrating allergen-induced sensory neuroplasticity in guinea pig airways [25] and NGF-induced phenotypic switch in airway sensory neurons [26]. One result of these functional alterations is the development of airway hyperresponsiveness in BA.
Figure 1.
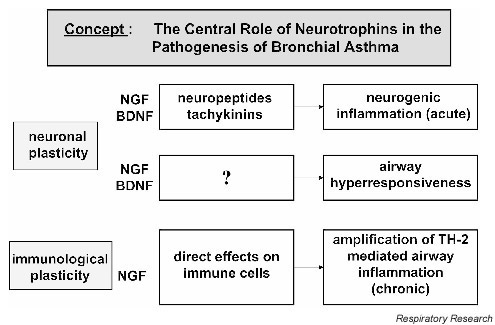
Concept for the role of neurotrophins in bronchial asthma. BDNF, brain-derived neurotrophic factor; NGF, nerve growth factor; TH-2, T-helper 2 cells.
In parallel, neurotrophins also exhibit profound effects on immune cells residing in airways and lung tissue. These effects are described by the term 'immunological plasticity'. In this regard, neurotrophins act as amplifiers of the locally occurring immune dysbalance. This effect has so far exclusively been demonstrated for NGF, but not for BDNF. NGF augments the production of IL-4 and IL-5 but not IFN-γ on activation of lymphocytes with allergen. Furthermore, these increases result in enhanced levels of IgE and IgG1 but not IgG2a antibodies [11].
Conclusion
It is important to note that the effects of neurotrophins are not immediate, but rather long acting. In this context, we propose the concept that neurotrophins act as intermediate or long-acting modulators of neuronal and immune functions in the pathogenesis of BA. Data on the kinetics of local neurotrophin production (manuscript in preparation) support this concept because peak levels of neurotrophin content in bronchoalveolar lavage fluid have been detected 7–10 days following local allergen challenges, and these levels return to baseline level no sooner than 3 weeks after allergen provocation.
Further experiments are certainly required to further evaluate this concept. Treatment modalities particularly need to be explored, aimed to locally antagonize increased neurotrophin production. Neurotrophins may, however, represent the 'common' trunk of immune and nerve cell modulators. This may lead to similar clinical signs and symptoms (e.g. airway hyperresponsiveness and airway obstruction owing to enhanced airway smooth muscle contractility, mucus hypersecretion and edema) observed in asthma patients, regardless of the underlying cause (e.g. allergen-induced asthma, airway hyperresponsiveness in association with viral infections, exercise-induced asthma, etc.).
Abbreviations
BA = bronchial asthma; BDNF = brain-derived neurotrophic factor; IFN = interferon; IL = interleukin; NGF = nerve growth factor.
References
- Kay AB. Pathology of mild, severe, and fatal asthma. Am J Respir Crit Care Med. 1996;154:S66–S69. doi: 10.1164/ajrccm/154.2_Pt_2.S66. [DOI] [PubMed] [Google Scholar]
- Lundberg JM. Tachykinins, sensory nerves, and asthma - an overview. Can J Physiol Pharmacol. 1995;73:908–914. doi: 10.1139/y95-125. [DOI] [PubMed] [Google Scholar]
- Baumgarten CR, Witzel A, Kleine-Tebbe J, Kunkel G. Substance P enhances antigen-evoked mediator release from human nasal mucosa. Peptides. 1996;17:25–30. doi: 10.1016/0196-9781(95)02057-8. [DOI] [PubMed] [Google Scholar]
- Kaltreider HB, Ichikawa S, Byrd PK, Ingram DA, Kishiyama JL, Sreedharan SP, Warnock ML, Beck JM, Goetzl EJ. Upregulation of neuropeptides and neuropeptide receptors in a murine model of immune inflammation in lung parenchyma. Am J Respir Cell Mol Biol. 1997;16:133–144. doi: 10.1165/ajrcmb.16.2.9032120. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Neuroeffector mechanisms: the interface between inflammation and neuronal responses [discussion S81-73]. J Allergy Clin Immunol. 1996;98:S73–S81. [PubMed] [Google Scholar]
- Cheung D, van der Veen H, den Hartigh J, Dijkman JH, Sterk PJ. Effects of inhaled substance P on airway responsiveness to methacholine in asthmatic subjects in vivo. J Appl Physiol. 1994;77:1325–1332. doi: 10.1152/jappl.1994.77.3.1325. [DOI] [PubMed] [Google Scholar]
- Cheung D, Timmers MC, Zwinderman AH, den Hartigh J, Dijkman JH, Sterk PJ. Neutral endopeptidase activity and airway hyper-responsiveness to neurokinin A in asthmatic subjects in vivo. Am Rev Respir Dis. 1993;148:1467–1473. doi: 10.1164/ajrccm/148.6_Pt_1.1467. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Dal Toso R, della Valle F, Skaper SD, Leon A. Update of the NGF saga. J Neurol Sci. 1995;130:119–127. doi: 10.1016/0022-510X(95)00007-O. [DOI] [PubMed] [Google Scholar]
- Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci USA. 1994;91:3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Appel E, Baruch R, Herz U, Botchkarev V, Paus R, Brodie C, Renz H. Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur J Immunol. 1998;28:3240–3251. doi: 10.1002/(SICI)1521-4141(199810)28:10<3240::AID-IMMU3240>3.3.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Ehrhard PB, Erb P, Graumann U, Otten U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc Natl Acad Sci USA. 1993;90:10984–10988. doi: 10.1073/pnas.90.23.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torcia M, Bracci-Laudiero L, Lucibello M, Nencioni L, Labardi D, Rubartelli A, Cozzolino F, Aloe L, Garaci E. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 1996;85:345–356. doi: 10.1016/s0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- Braun A, Lommatzsch M, Mannsfeldt A, Neuhaus-Steinmetz U, Fischer A, Schnoy N, Lewin GR, Renz H. Cellular sources of enhanced brain-derived neurotrophic factor production in a mouse model of allergic inflammation. Am J Respir Cell Mol Biol. 1999;21:537–546. doi: 10.1165/ajrcmb.21.4.3670. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Braun A, Mannsfeldt A, Botchkarev VA, Botchkareva NV, Paus R, Fischer A, Lewin GR, Renz H. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived Neurotrophic functions. Am J Pathol. 1999;155:1183–1193. doi: 10.1016/S0002-9440(10)65221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini S, Lambiase A, Angelucci F, Magrini L, Manni L, Aloe L. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc Natl Acad Sci USA. 1996;93:10955–10960. doi: 10.1073/pnas.93.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Bonini S, Micera A, Magrini L, Bracci-Laudiero L, Aloe L. Increased plasma levels of nerve growth factor in vernal keratoconjunctivitis and relationship to conjunctival mast cells. Invest Ophthalmol Vis Sci. 1995;36:2127–2132. [PubMed] [Google Scholar]
- Virchow JC, Julius P, Lommatzsch M, Luttmann W, Renz H, Braun A. Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am J Respir Crit Care Med. 1998;158:2002–2005. doi: 10.1164/ajrccm.158.6.9803023. [DOI] [PubMed] [Google Scholar]
- Hoyle GW, Graham RM, Finkelstein JB, Nguyen KP, Gozal D, Friedman M. Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol. 1998;18:149–157. doi: 10.1165/ajrcmb.18.2.2803m. [DOI] [PubMed] [Google Scholar]
- Albers KM, Wright DE, Davis BM. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J Neurosci. 1994;14:1422–1432. doi: 10.1523/JNEUROSCI.14-03-01422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean DB, Lewis SF, Wheeler FB. Substance P content in cultured neonatal rat vagal sensory neurons: the effect of nerve growth factor. Brain Res. 1988;457:53–62. doi: 10.1016/0006-8993(88)90056-X. [DOI] [PubMed] [Google Scholar]
- Vedder H, Affolter HU, Otten U. Nerve growth factor (NGF) regulates tachykinin gene expression and biosynthesis in rat sensory neurons during early postnatal development. Neuro-peptides. 1993;24:351–357. doi: 10.1016/0143-4179(93)90006-v. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337:362–364. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- Mandelzys A, Cooper E, Verge VM, Richardson PM. Nerve growth factor induces functional nicotinic acetylcholine receptors on rat sensory neurons in culture. Neuroscience. 1990;37:523–530. doi: 10.1016/0306-4522(90)90420-9. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Hunter DD, Liu M, Haak-Frendscho M, Oakragly A, Fischer A. Allergen-induced sensory neuroplasticity in airways. Int Arch Allergy Immunol. 1999;118:150–153. doi: 10.1159/000024053. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Myers AC, Undem BJ. Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med. 2000;161:1985–1990. doi: 10.1164/ajrccm.161.6.9908051. [DOI] [PubMed] [Google Scholar]


