Abstract
Purpose
Pancreatic ductal adenocarcinoma (PDAC) is associated with an immunosuppressive milieu that supports immune system evasion and disease progression. Here, we interrogated genetic, stromal, and immunological features of PDAC to delineate impact on prognosis and means to more effectively employ immunotherapy.
Experimental design
A cohort of 109 PDAC cases annotated for overall survival was utilized as a primary discovery cohort. Gene expression analysis defined immunological subtypes of PDAC that were confirmed in the Cancer Genome Atlas data set. Stromal and metabolic characteristics of PDAC cases were evaluated by histological analysis and immunostaining. Enumeration of lymphocytes, as well as staining for CD8, FOXP3, CD68, CD163, PDL1, and CTLA4 characterized immune infiltrate. Neo-antigens were determined by analysis of whole exome sequencing data. Random-forest clustering was employed to define multi-marker subtypes, with univariate and multivariate analyses interrogating prognostic significance.
Results
PDAC cases exhibited distinct stromal phenotypes that were associated with prognosis, glycolytic and hypoxic biomarkers and immune infiltrate composition. Immune infiltrate was diverse among PDAC cases and enrichment for M2 macrophages and select immune checkpoints regulators were specifically associated with survival. Composite analysis with neo-antigen burden, immunological, and stromal features defined novel subtypes of PDAC that could have bearing on sensitivity to immunological therapy approaches. Additionally, a subtype with low levels of neo-antigens and minimal lymphocyte infiltrate was associated with improved overall survival.
Conclusions
The mutational burden of PDAC is associated with distinct immunosuppressive mechanisms that are conditioned by the tumor stromal environment. The defined subtypes have significance for utilizing immunotherapy in the treatment of PDAC.
BACKGROUND
Pancreatic ductal adenocarcinoma (PDAC) is therapy recalcitrant and has yet to see significant improvement in long-term survival beyond a few weeks with recent chemotherapy regimens(1–3). Therefore, there is a major opportunity to provide precision approaches to PDAC treatment. Genetic analyses of pancreatic cancer revealed few currently actionable targets for therapeutic intervention(4–6). However, these analyses demonstrated that a subset of PDAC is highly chromosomally unstable or associated with mutator-like phenotypes that could serve as the basis for targeted intervention(4, 6).
A promising treatment modality that has yielded long-term clinical benefit in historically therapeutically recalcitrant cancers is the use of immunotherapy(7–9). This approach takes multiple different forms, including tumor specific vaccines, activated T-cell therapy, or immune checkpoint inhibitors(10–13). Each of these modalities has been interrogated in preclinical models and demonstrated enthusiasm for action in the context of PDAC. However, to date, single agent immunotherapy trials yielded no clinical benefit in pancreatic cancer. Most notably, a large Phase III trial of Algenpantucel-L vaccine recently failed to demonstrate clinical improvement, as did multiple single agent checkpoint inhibitor trials(14, 15). These findings suggest a need for thoughtful and targeted approach to the use of immunotherapy in pancreatic cancer that will require combinatorial treatments overcoming complex immunosuppressive mechanism and/or increase recruitment of immune cells to the tumor site.
Cancer microenvironment was postulated to limit immune cell infiltration and impair their function in the tumors (16, 17). A unique feature of PDAC is presence of a desmoplastic stroma that accounts for majority of the tumor volume (18–22). The stromal compartment, also referred to as tumor microenvironment (TME), consists of cancer associated fibroblasts (CAFs) and immune cells that are embedded in an extracellular matrix rich in cytokines and soluble growth factors. The role of the stromal compartment in pancreatic cancer progression is complex with studies supporting both tumor-promoting and tumor-restrictive roles. Desmoplastic PDAC stroma has been proposed to limit the access of drugs, impinge on tumor metabolic features, seed metastasis, and alter the immune milieu relevant to immunotherapy(23–26). However, depletion of the stroma did not yield benefit in clinical studies, and resulted in more aggressive form of PDAC in mouse models(27, 28). Importantly, from the analysis of clinical PDAC specimens it is evident that the stroma is highly heterogeneous (29); therefore, wholesale targeting of stromal compartment without consideration of its complexity could have deleterious impact on clinical outcomes. Similarly, it will be critical to understand the inter-relationship between stroma, neoplastic cells, and immune cells in making immunotherapy effective for patients with PDAC(21, 22).
Here, we used a genetically characterized PDAC cohort to evaluate the association between tumor microenvironment, immune features of the tumor, and tumor genetics. These data reinforce the concept that PDAC is highly diverse, and also provides insight into subtypes of PDAC that could be amenable to specific immunotherapies.
MATERIALS AND METHODS
Patient Cohort, sequencing, and tissue microarray
Patients with a diagnosis of PDAC were consented for tissue collection and exome sequencing analysis under an IRB approved protocol at the University of Texas Southwestern. The 109 patient cohort and sequencing has been previously described although followup was extended. Formalin fixed paraffin embedded PDAC tissue was evaluated by an anatomic pathologist for the construction of tissue microarrays. The arrays were constructed using standard approaches.
Immunohistochemical analysis and staining
Hematoxylin staining and evaluation of the tissue architecture was used to define the stromal type. The TMAs were stained with the following antibodies: PD-L1 (Cell Signaling, dilution 1:600), CD163 (Cell Marque, prediluted), FOXP3 (Abcam, dilution 1:200), CD8 (). The tissues were also stained for CA9 indicative of hypoxia antibody (Cell Marque, dilution of 1:500). MCT4 was employed as a marker of glycolytic preference (Santa Cruz, dilution 1:250). Expression of IHC markers was categorized semi-quantitatively using published criteria. Stromal tumor infiltrating lymphocytes (TILs) were assessed based on criteria developed by Denkert and colleagues (30).
Oncoimmunology panel and neoantigen determination
The HTG EdgeSeq Immuno-Oncology Panel was run on pancreatic FFPE samples as described previously(31). This assay contains probes to measure the expression of 549 RNAs. Briefly, the area of interest was identified on 109 pancreatic FFPE sections (5 microns on glass slides), and was scraped and lysed in HTG’s lysis buffer. Depending on the size of the section, either 7.5 mm2 or 15 mm2 tisuse was used in the assay. Following nuclease protection, each sample was tagged individually with molecular barcodes; tagged samples were pooled and sequenced on an Illumina MiSeq or NextSeq sequencer. Fastq files from the individual samples were processed and the expression data reported by the HTG EdgeSeq parser software. Data from two samples did not pass QC metrics and were excluded from the analysis. Neoantigen analysis was performed as previously described on exome sequencing data(32).
Bioinformatic analysis
Unsupervised hierarchical clustering and Pearson correlation analysis were performed using R. TCGA data was obtained from the TCGA web portal. Gene ontologies were determined using DAVID Functional Analysis tool for enrichment analysis. Network diagrams were generated using ReactomeFIViz (33). Unsupervised random forest clustering, survival analysis and multivariate Cox proportional hazards statistics were also performed using R.
RESULTS
Immunological expression features of pancreatic cancer
To probe the intersection between tumor genetics, immune system, and stromal features of disease, we interrogated a cohort of 109 surgically resected PDAC cases. The median follow-up was 560 days and the 2 year overall survival was 59%, which is consistent with historical clinical presentation (Fig S1). None of the cases had received neo-adjuvant chemotherapy or radiation therapy. Initially a subset of this cohort was interrogated using HTG EdgeSeq system that applied novel target capture and library preparation chemistry enabling RNA sequencing from FFPE samples (Fig 1). A panel of 549 genes implicated in host immune response to the tumor was employed to measure tumor immune cell composition, chemokines, and other immunomodulatory soluble factors using a single section of FFPE tissue where highly cellular portions of the tumor were obtained. These data demonstrated that two principle gene expression clusters could be delineated in PDAC cases (Fig 1A). The first gene expression cluster that was expressed at a higher level in approximately 50% of cases (demarcated with yellow color-bar) was enriched for 191 genes implicated in modulating lymphocyte activation and proliferation (Fig 1B). Highly expressed genes in this cluster also included cytokine/chemokine and interleukin signaling (Fig 1C). The second cluster was characterized by lower expression of multiple immune pathways genes; however, it was enriched for adhesion molecules and proteasomes (Fig 1D). To further characterize the composition of immune pathways within the two clusters, a series of previously published signatures were employed (Fig S2 and 1E). These analyses demonstrated that the cases segregated based on enrichment for T-cell and B-cell receptor signaling pathways (yellow cluster) vs. macrophage, MHC class 1 and type I interferon response (purple cluster). Additional analysis that included genes associated with T-cell function (i.e. IL2, Il3, IL4, IL5, IL6, IL10, CCL2, CXCL12, CXCR4) and macrophages (i.e. CD68), as well as immune checkpoint modulators (i.e. CTLA4, PDCD1, CD274) reinforced existence of distinct subtypes of PDAC (Fig 1F). The macrophage-rich (purple cluster) subset was characterized by high expression of CXCL12/CXCR4 signaling that could result in lymphocyte exclusion and overall lower cytolytic activity (26). These data suggest that there is an intrinsic diversity within PDAC cases in terms of immune milieu in tissue. Importantly, analysis of an additional 183 cases from TCGA confirmed existence of these distinct immunological subtypes of PDAC (Fig S2).
Figure 1. Onco-immune gene expression analysis.
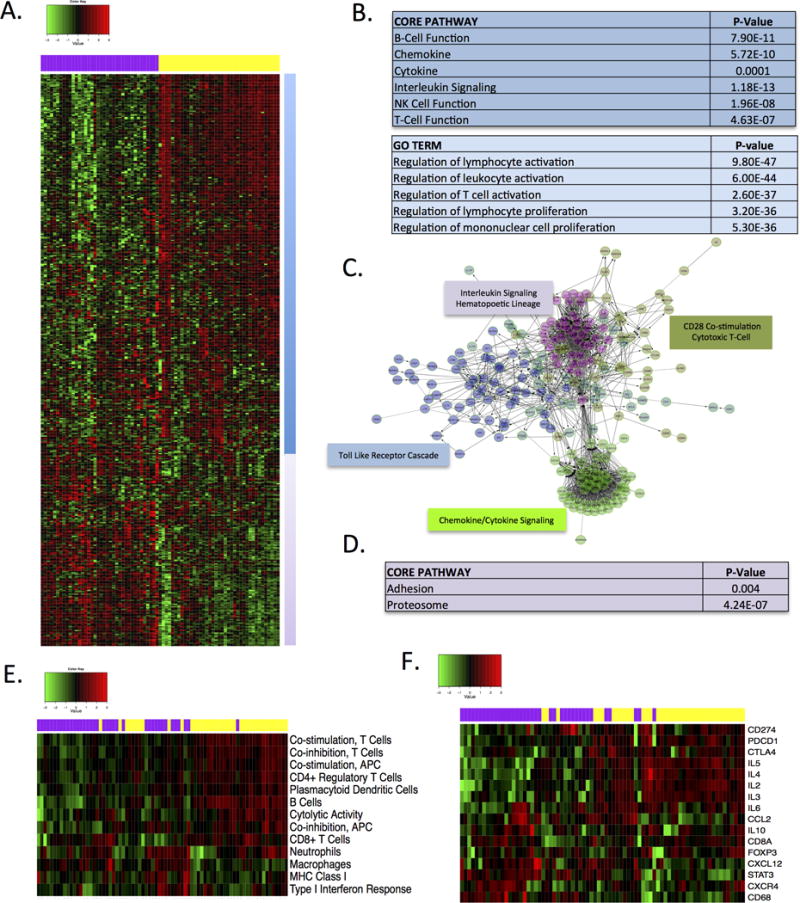
(A) Heatmap demonstrating two principle expression behaviors of genes within the HTG oncoimmunology panel. (B) The yellow cluster is significantly enriched for multiple genes involved in T- and C-cell activation. (C) Example of gene networks significantly enriched within the yellow cluster. (D) The purple cluster is enriched for genes involved in adhesion and proteasome function. (E) Heatmap of gene expression signatures associated with specific branches of the immune system. Color-bar denotes canonical cluster from unsupervised analysis. (F) Expression of select genes involved in immune activation and evasion are shown. Coloar-bar denotes the canonical cluster from the unsupervised analysis.
Distinct stromal compartments compartments in pancreatic cancer are associated with prognosis
While innate and adaptive immune responses are active during initial stages of PDAC precursor development, immune evasion is a common feature of established PDAC tumors. Previous studies demonstrated that stromal compartment played important role in modulating and dampening immunological responses in PDAC(23). PDAC stromal compartment can impact immune responses via several mechanisms including limiting access of tumor infiltrating lymphocytes (TILs) to tumor cells and creating hypoxic immunosuppressive environment modulating function of recruited immune cells. To investigate impact of PDAC microenvironment on immune composition of the tumor, we initially evaluated stromal volume using whole tumor hematoxylin and eosin-stained sections. When cases were dichotomized around the median stromal volume, there was a statistically significant association of low stromal volume with poor overall survival (Fig 2A and S3) as previously reported (34, 35). Next, we evaluated morphologic features of the stromal compartment consistent with the reported biological diversity of stroma (29). When evaluating the number of cancer associated fibroblasts, presence of mature collagen fibers and loose stromal matrix, PDAC cases could be divided into three stromal subtypes. These included cases with dense collagenous stroma and low number of CAFs (called “mature”), a highly cellular and collagen-poor stroma (“immature”), and an intermediate form of the stroma (“intermediate”) (Fig 2B). The morphological characteristics of PDAC stromal compartment were strongly associated with prognosis, wherein the immature stroma correlated with shorter overall survival (Fig 2B).
Figure 2. Stromal and metabolic features are associated with prognosis.
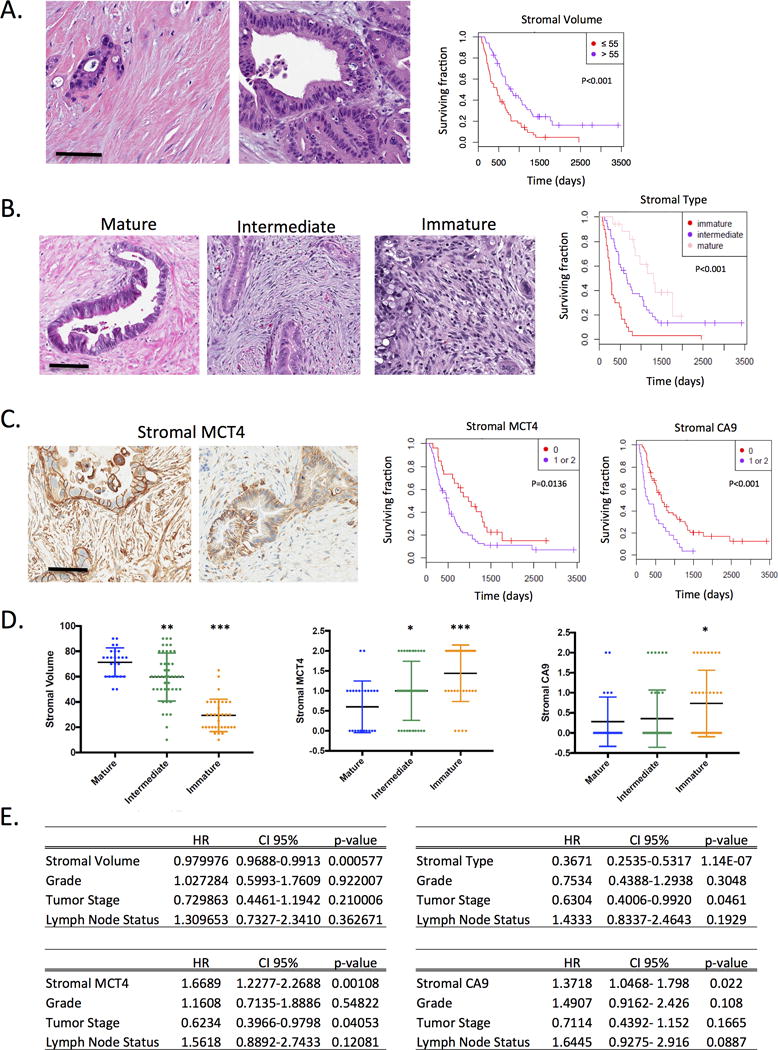
(A) PDAC cases exhibit distinct stromal volume as shown in the representative images. PDAC were stratified based on stromal volume and the association with survival is shown. (B) PDAC cases exhibit three distinct stromal subtypes as shown in the representative images. Cases were stratified based on stromal type and the immature form of PDAC stroma was significantly associated with poor prognosis as determined by Kaplan-Meier analysis. (C) The expression of MCT4 and CA9 are markers of glycolytic and hypoxic environments respectively. The high expression of each marker was significantly associated with poor prognosis as determined by Kaplan-Meier analysis. (D) The stromal volume, stromal MCT4 expression, or stromal CA9 expression were evaluated dependent on the stromal type. Statistical association was determined by t-test (*p<0.05,**p<0.01,***p<0.001). (E) Multivariate analysis of the prognostic significance of stromal volume, stromal type, stromal MCT4, or stromal CA9 were determined against the clinical variable grade, tumor stage, and nodal status. Each marker remains significant in the multivariate model.
Metabolic and hypoxic features of pancreatic tumors are related to stromal features of disease
The hypoxia and aberrant production of metabolites in the tumor microenvironment can result in a multitude of effects ranging from preferential recruitment of specific immune cell subtypes to impacting directly on innate and adaptive effector cell functions. MCT4 and CA9 were employed as markers for glycolysis and hypoxia respectively due to their well-characterized functions in tumor biology(36), and established conditions for immune-staining to delineate compartment specific relationship to prognosis. We observed that glycolytic metabolic preference (characterized by high expression of lactate monocarboxylate transporter 4-MCT4) and hypoxia (characterized by high expression of carbonic anhydrase 9-CA9) were associated with poor outcome in this cohort (Fig 2C, 2E, and S3). These findings are consistent with previously published work in independent patient cohorts (37, 38). To probe the inter-relationship between hypoxia, metabolic features, and stromal volume, the stromal type was used to stratify these parameters (Fig 2D). These data indicated that the immature stromal type was associated with low stromal volume, and high levels of stromal CA9 and MCT4. Correlation analysis indicated that the stromal variables are related (not shown); however, they all harbor prognostic value above standard pathological features (e.g. tumor grade, nodal status, and tumor stage) (Fig 2E).
Tumor infiltrating lymphocytes are conditioned by the tumor stroma
The composite features of stromal architecture and metabolism are expected to influence the immune milieu within the tumor. As a first step in analyzing immune features of PDAC we evaluated the number of tumor infiltrating lymphocytes (TILs) in the tumor and in the areas adjacent to tumor (Peri-T lymphocytes). Higher levels of lymphocytes around the tumor were associated with lower overall survival (Fig 3A). Interestingly, the number of TILs within the stromal tumor compartment was not a prognostic feature (Fig 3B). Similarly, number of CD8+ T-cells, although highly variable across cases, was not associated with survival (Fig 3C). To evaluate how stromal biology may impact on the infiltrate, the levels of TILs and CD8+ T-cells, cases were stratified based on stromal type and stromal volume (Fig 3D). Stromal type influenced the abundance of TILs, wherein collagenous mature stroma had lower number of infiltrating lymphocytes. In contrast, stromal type had no impact on the number of CD8+ T-cells, suggesting that other subsets of lymphocytes account for a differences in TILs.
Figure 3. Tumor infiltrating cells and prognosis.
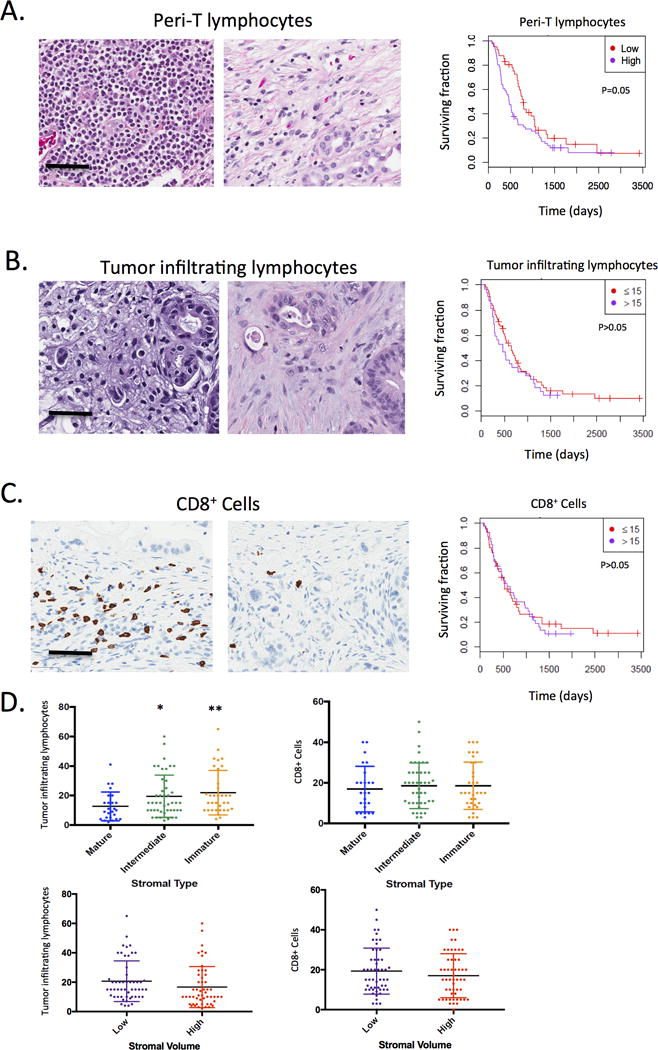
(A) Tumor infiltrating lymphocytes in the periphery of the tumor were scored on tissue sections using established criteria by a surgical pathologist with extensive experience with PDAC histology. The association with survival was determined by Kaplan-Meier analysis. (B) The level of tumor infiltrating lymphocytes was determined using established criteria by a surgical pathologist with extensive experience in PDAC histology. The level of tumor infiltrating lymphocytes were not significantly associated with overall survival as determined by Kaplan-Meier analysis. (C) CD8+ cells within the tumor were quantified and exhibited diverse levels within the tumor. The level of CD8+ cells was not significantly associated with overall survival as determined by Kaplan-Meier analysis. (D) The stromal tumor infiltrating lymphocytes and CD8+ cells were evaluated dependent on the stromal volume and stromal type. Statistical association was determined by t-test (*p<0.05,**p<0.01).
Multiple immune-suppressive mechanisms are engaged in pancreatic cancer
Differential immune cell recruitment could be reflective of distinct immunosuppressive mechanisms in the tumor microenvironment. Tumor associated macrophages (TAMs) can limit immune engagement locally and have become an exciting target in the treatment of PDAC (39–41). The total number of macrophages in the tumor environment was determined by CD68 staining. The presence of high number of macrophages was associated with poor prognosis (Fig 4A). The expression of CD163, indicative of suppressive M2 macrophages was also associated with decreased survival consistent with other studies (Fig 4A) (42). Importantly, the number of TAMs was strongly correlated with stromal type, where immature stroma exhibited significantly increased macrophage content. The expression of CTLA4 was determined in lymphocytes and we observed that the presence of CTLA4 positive cells was associated with poor outcome (Fig 4B). In contrast, FOXP3, which is a marker of T-regulatory cells had no relationship to prognosis (Fig 4B). While there was no association between stromal type and number of FOXP3+ cells, CTLA4 lymphocytes were enriched in the immature stromal type (Fig 4B). Lastly, the expression of PDL1 was evaluated in the tumor cells (PDL1-T), in the immune cells along the invading edge of the tumor (PDL1-Front), and in the tumor microenvironment (PDL1-TME). Only in the tumor microenvironment PDL1 levels were associated with survival (Fig 4C and S3). These findings illustrate the complexity of PDAC immune milieu and suggest that in any given tumor multiple and diverse immune suppressive mechanisms could be at play to impact immunotherapeutic strategies.
Figure 4. Differential engagement of immune suppressive features in PDAC.
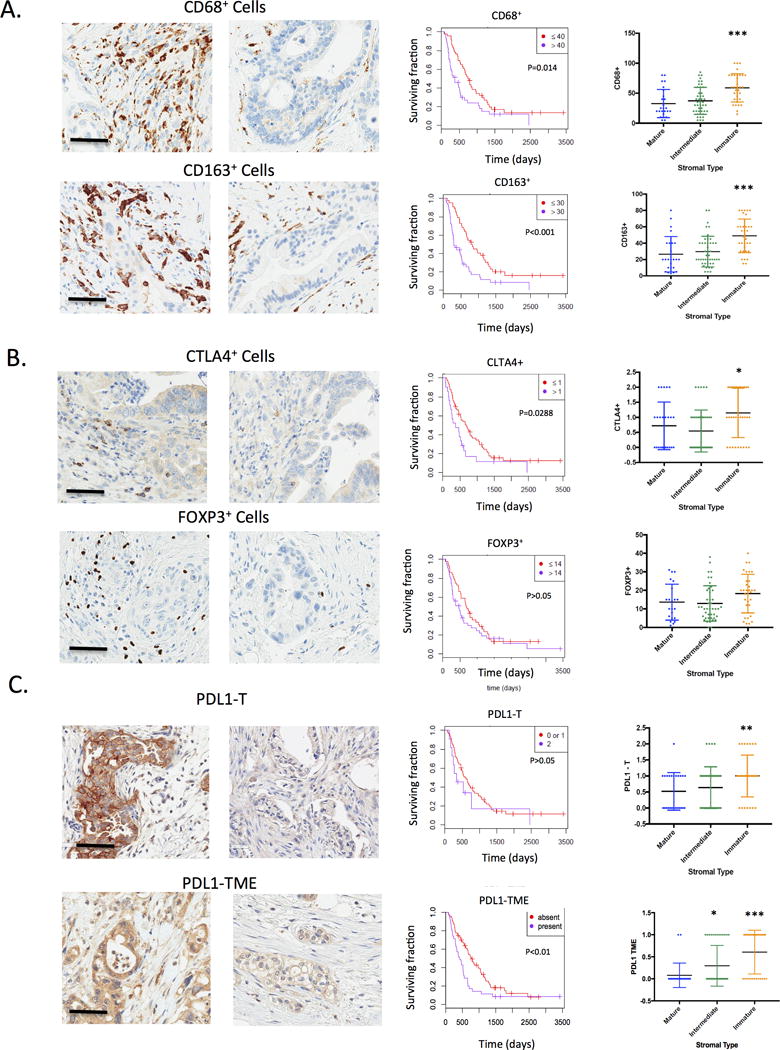
(A) The presence of macrophages or type II macrophages was determined by staining for CD68 and CD163 respectively. The overall presence of macrophages within the tumor microenvironment was significantly associated with overall survival as determined by Kaplan-Meier analysis. The level of CD68+ and CD163+ cells was associated with an immature stromal type (***p<0.001). (B) The presence of FOXP3 positive cells (indicative of T-regulatory cells) or CTLA4+ lymphocytes was determined within the PDAC tumors. CTLA4+ lymphocytes were significantly associated with overall survival, while FOXP3 was not significantly associated with overall survival as determined by Kaplan-Meier analysis. The level of CTLA4+ and FOXP3+ cells was determined as a function of stromal type (*p<0.05) (C) The expression of PDL1 was determined by immunostaining both in tumor cores (PDL1-T) and in the tumor micro-environment (PDL1-TME). The association with overall survival was determined by Kaplan-Meier Analysis. The level of PDL1 in various tumor comparments was analyzed as a function of stromal-type (*p<0.05,**p<0.01,***p<0.001).
Composite relationship of neo-antigens and stromal features with immune milieu defines prognostic subtypes of pancreatic cancer
The burden of tumor specific antigens (neo-antigens) that emerge as a product of mutational processes in the tumor was shown to impact responses to immune checkpoint therapy (32, 43). Since all cases in the cohort were exome sequenced, the presence of neo-antigens was determined using an established computational method(32). PDAC exhibited diverse levels of neo-antigens (Fig 5A), however the number of neo-antigens per tumor was generally lower than that observed in melanoma or lung cancer wherein immunotherapy with checkpoint inhibitors has been most effective (4, 6). Next, correlation analysis that integrated stromal type, immune infiltrate, and neo-antigen burden was employed to define landscape features of PDAC. These data revealed the presence of multiple interdependent processes that were generally inversely associated with stromal volume and longer survival (Fig 4B). We also employed the TCGA data to determine if observed patterns of expression were preserved in an independent cohort. In the TCGA cohort, gene expression of immunological markers including PDL1 (CD274), FOXP3, CTLA4, CD8, CD68 and CD163 were positively correlated, and inversely related to survival times (Fig S4).
Figure 5. Composite analysis of tumor genetics, microenvironment, and immune milieu.
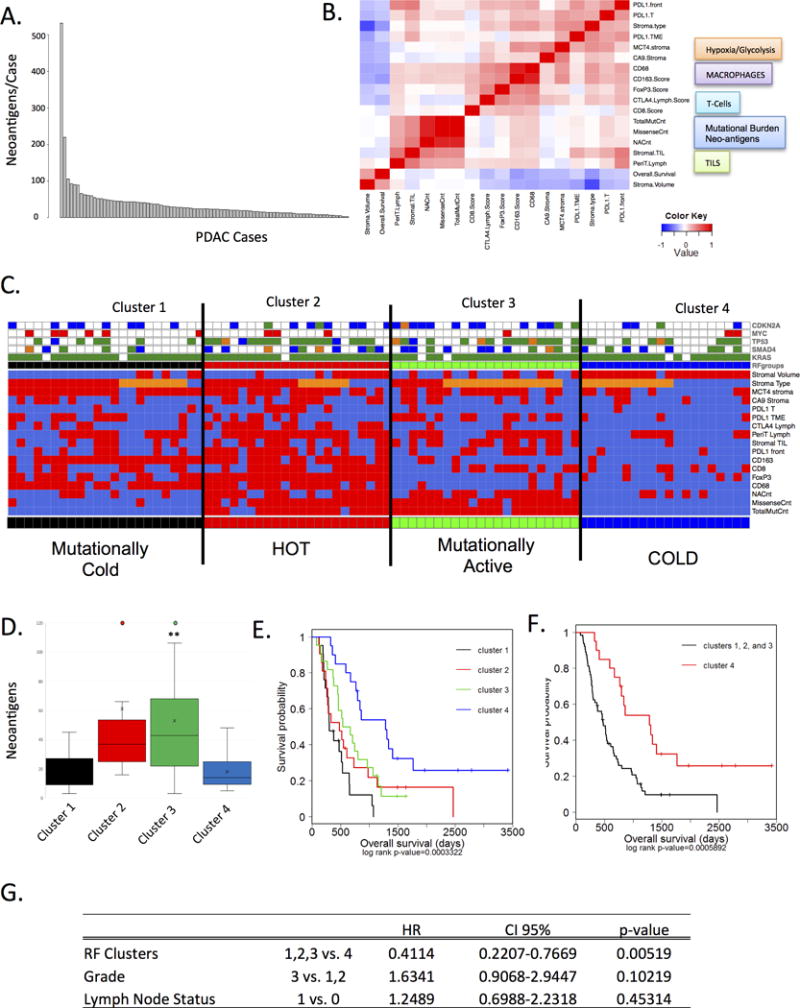
(A) The presence of neoantigens in the tumor cohort was determined from whole exome sequencing. Graph demonstrates the number of neoantigens per case. (B) Correlation analysis between histological features, the number of neoantigens, the number of mutations, immunologoical/metabolic markers, and overall survival is summarized in the heatmap. (C) Random Forest clustering was employed on all of the markers summarized in the heatmap to define four clusters (red=high, organge=intermediate, blue=low). The presence of hallmark genetic alterations targeting KRAS, CDKN2A, SMAD4, TP53, and MYC are shown in the color-bar (green=mutation, orange=INDEL, red=amplification, blue=deletion). (D) quantification of the number of neoantigens in each of the clusters is shown (**p<0.01) (E) The association of the Random Forest clusters with survival was determined by Kaplan-Meier analysis and statistical significance was assessed by log-rank analysis. (F) The analysis of Cluster 4 vs. all other cases was determined by Kaplan-Meier analysis and statistical significance was determined by log-rank analysis. (G) The significance of the Random Forest clusters was evaluated by multivariate analysis relative to grade and lymph-node (LN) status in the cohort. Cluster 1 remained significant relative to improved outcome.
Unsupervised random-forest clustering using neo-antigens, stromal, and immune infiltrate features yielded four distinct “immuno-subtypes” of PDAC (Fig 4C and S5). Cluster 4 exhibited low levels of veritably all immune and stromal markers, harbored a mature stromal type, high stromal volume and low number of neo-antigens (Fig 5C and D). While these tumors exhibited the ubiquitous activation of KRAS, they were underrepresented for mutations targeting other canonical genetic events in PDAC (e.g. CDKN2A, MYC, and TP53). This configuration ostensibly represents a “cold” tumor. Cluster 1 also harbored low number of mutations, however it exhibited high-levels of MCT4, low stromal volume and immature stromal type (Fig 5C). This finding indicates that glycolytic tumor microenvironment is not universally a feature of high-mutational burden in PDAC. The immune infiltrate in Cluster 1 was dominated by macrophages, likely induced by glycolytic and acidic microenvironment. Cluster 3, harbored a high mutational burden and intermediate morphological stromal type, higher numbers of TILs and peritumoral lymphocytes but exhibited relatively low levels of CD68 and CD163+ macrophages. Cluster 2, demonstrated high levels of veritably all immune cell subsets and was also mutationally active. Of the clusters, Cluster 4 was associated with increased overall survival that was significant in univariate analysis with each of the other immune subtypes (Fig 4E, 4F). Cluster 4 was also associated with improved outcome when considering tumor grade and lymph node status in multivariate analysis (Fig 4G). Interestingly KRAS Q61 mutations were enriched for in this cluster (Fig S5) and could contribute to longer survival, as KRAS Q61 mutation was shown to be predictor of better prognosis (4).
DISCUSSION
Immunotherapy holds substantial promise for tumors that are recalcitrant to standard therapies and for which disease recurrence is a major clinical problem. Here, we explored features of the immune system and microenvironment to delineate subtypes of PDAC that may be expected to be responsive to distinct forms of immunotherapy. These data illustrate that there is a profound diversity in the nature of immune response in PDAC that is not solely governed by neo-antigen burden, and that select features of the tumor microenvironment are associated with distinct immunosuppressive mechanisms.
The role of the immune milieu of PDAC as a prognostic feature is only starting to emerge. Here, we analyzed multiple different subsets of tumor infiltrating immune cells. The overall burden of TILs or CD8+ T cells was not associated with overall survival consistent with the notion that PDAC represents “non-immunogenic” tumor (44, 45) and fibroblast activation protein α-expressing CAFs contribute to lymphocyte exclusion in PDAC (26). These findings contrast with “immunogenic” cancers that are characterized by naturally occurring high number of TILs and respond to immunotherapy(30, 46). We did not observe prognostic significance of FoxP3+ regulatory T-cells in agreement with recent meta-analysis study (47). In contrast, the presence of macrophages (CD68 positive) and in particular M2 macrophages (CD163 positive) had negative effect on survival. These data are consistent with an emerging literature that the presence of tumor-associated macrophages (TAMs) is associated with more aggressive form of disease(40, 48). In addition to supporting tumor invasiveness and metastatic spread, TAMs inhibit T cell responses by production of indoleamine dioxygenase metabolites and reactive oxygen species and indirectly by recruiting regulatory T-cells to the tumor (49, 50). Regulatory T-cells in turn inhibit T-cell production of IFN-γ and IL-2 in response to tumor specific antigens, as well as their cytotoxic function with resulting impediment to naturally occurring anti-tumor immunity. Expression of the immunosuppressive proteins CTLA4 and PDL1 was observed in a subset of PDAC with PDL1 expression noted in several compartments. CTLA4 expression on immune cells and PDL1 positivity in tumor microenvironment were significantly associated with poor overall survival. In contrast to melanoma and non-small cell lung cancer, there was no prognostic significance for PDL1 expression in tumor cells or in the immune cells at the tumor invasive front. That is consistent with the concept that PDL1 expression on tumor cells correlates with number of tumor neo-antigens and TILs (51). Interestingly, PDL1 expression in the TME was a predictor of poor survival and associated with immature stromal type and glycolytic metabolic preference. Recent study has demonstrated that depletion of glucose from TME and resultant lactate production can become limiting for T-cell effector functions, as this subset of immune cell is dependent on aerobic glycolysis (52). In contrast, regulatory T-cells and macrophages are capable of utilizing fatty acid oxidation to survive in low-glucose environment (53, 54). Together, these data illustrate the diversity of the immune system in PDAC that would ostensibly condition any approaches to immunotherapy.
PDAC is somewhat unique among other solid tumors in having a particularly prominent stromal component that has been proposed to limit vascularization of the tumor and provide a mechanical barrier limiting recruitment of immune cells. The volume of the stromal compartment in PDAC, as well as its histomorphologic characteristics, is highly variable across cases. We observed that immature stromal type (dominated by cancer associated fibroblasts and poor in mature collagen) was associated with a higher number of TILs and diversity of immune repertoires than collagen-rich mature stroma. This finding supports the hypothesis that in some cases dense desmoplastic stroma may provide a mechanical impediment to recruitment of immune cells. Interestingly, immature stroma was also associated with hypoxia and a more glycolytic metabolism. The acidic pH has been shown to suppress CD8+ T-cell effector activity and recruit macrophages and in our cohort we could observe the association between increased expression of lactate exporter MCT4 and higher number of CD68+ macrophages and CD163+ M2 macrophages (55).
The engagement of anti-tumor immunity is currently believed to be conditioned by the number of neo-antigens that represent mutated peptides that are shed from tumor cells and considered as non-self (43). Consistent with this overall hypothesis tumors that have very high mutational burdens have been found to elicit more of an anti-tumor immune response and represent disease for which immune checkpoint inhibitors appear to be effective (e.g. melanoma, non small cell lung carcinoma)(32). Here, we have shown that although the level of neo-epitopes is in general lower in PDAC compared to highly immunogenic tumors, a subset of pancreatic cancer cases harbors a significant neo-antigens number. As expected, the load of neo-antigens was most elevated in cases with microsatellite instability (MSI). The MSI cases were also characterized by higher number of peritumoral and tumor infiltrating lymphocytes and may represent a minor subset of PDAC that could show response to immune checkpoints blockade. However, we have also observed that PDAC with a higher number of cancer specific epitopes are characterized by prominent immunosuppressive infiltrate and harbor a microenvironment hostile to T-cell function. Composite analysis with neo-antigen burden, immunological, and stromal features delineated four subtypes of PDAC. Low mutational burden and low levels of immune effector and suppressive cells characterized one of the subtypes. This “cold” subtype harbored KRAS mutations; however it was underrepresented for many of the canonical PDAC related genetic events (e.g. SMAD4 or CDKN2A loss). Based on the absence of MCT4 and CA9 expression, this subtype was likely predominantly utilizing oxidative phosphorylation, which may correspond to low levels of immunosuppression. Higher stromal volume and presence of collagen-rich stroma could contribute to limited recruitment of immune cells and also result in paucity of immune suppressive cells. This subtype could be therefore amenable to approaches activating immune system, such as anti-cancer vaccines (e.g., MUC1, GVAX) or use of chimeric antigen receptor T-cell therapy with immune modulating agents to off-set immunosuppressive mechanisms that may emerge with increased immune infiltration post-therapy. The “mutationally cold” subtype also harbored a low number of mutations, however it exhibited low stromal volume and immature stromal type with high-levels of MCT4 indicating a glycolytic and acidic microenvironment. The immune infiltrate in this subtype was dominated by macrophages and could benefit from therapies targeting macrophages, such as CD40 agonists that induce tumoricidal macrophage function and induce T-cell infiltrate (56). The two subsets termed “hot” and “mutationally active” harbored a relatively high mutational burden, higher numbers of TILs and peritumoral lymphocytes as well as immune checkpoints (CTLA-4 and PDL-1) and regulatory T-cells but exhibited variable levels of tumor associated macrophages. Notably, immunosuppressive features of M2 macrophages would ostensibly persist in the face of CTLA4 and PD1 inhibitors. Similarly, a high concentration of lactate could similarly weaken immune responses and lead to therapeutic resistance(57, 58). Therefore, normalizing TME metabolism or biologic features of tumor stroma and/or combination therapies targeting multiple immunosupressive mechanism may be required to successfully implement immunotherapy in PDAC. Interestingly, in spite of substantial genomic sequencing efforts it is not possible to predict tumor/stroma features that are of clear relevance to immune engagement. However, potentially with greater number of cases sequenced it is possible that specific correlations will begin to emerge.
Together, these data suggest that due to the genetic, stromal and immunological diversity of PDAC, \ it will be important to apply immunotherapy in a targeted fashion. Our data also indicates a need for combinatorial approaches (e.g. simultaneously targeting distinct immunosuppressive mechanisms, applying immunotherapy in concert with modifying metabolic reprograming in tumor microenvironment, activating immune responses via adaptive cell transfer) and provides potential explanations to why single agent trials with immune checkpoint inhibitors have not been as promising as hoped.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Pancreatic cancer is therapy recalcitrant disease for which targeted interventions are needed. Although successful in several solid tumor types, to date, single agent immunotherapy trials yielded no clinical benefit in pancreatic cancer. This is because mutational burden of PDAC is associated with distinct immunosuppressive mechanisms that are conditioned by the tumor stromal environment. Composite analysis with neo-antigen burden, immunological, and stromal features defined novel subtypes of PDAC that could have bearing on sensitivity to immunological therapy approaches. These findings suggest a need for thoughtful and targeted approach to the use of immunotherapy in pancreatic cancer that will require combinatorial treatments overcoming complex immunosuppressive mechanism and/or increase recruitment of immune cells to the tumor site.
Footnotes
Disclosures: Debrah Thompson and Ihab Botros are employees of HTG Molecular Diagnostics that markets an immunoncology panel used in this study. No other authors have any disclsosures.
References
- 1.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 2.Tempero MA, Klimstra D, Berlin J, Hollingsworth T, Kim P, Merchant N, et al. Changing the way we do business: recommendations to accelerate biomarker development in pancreatic cancer. Clin Cancer Res. 2013;19:538–40. doi: 10.1158/1078-0432.CCR-12-2745. [DOI] [PubMed] [Google Scholar]
- 3.Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–26. doi: 10.1053/j.gastro.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 4.Witkiewicz AK, McMillan EA, Balaji U, Baek G, Lin WC, Mansour J, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nature communications. 2015;6:6744. doi: 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 6.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 8.Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65:185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 9.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18:206–13. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–84. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–8. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 13.Thomas S, Prendergast GC. Cancer Vaccines: A Brief Overview. Methods in molecular biology. 2016;1403:755–61. doi: 10.1007/978-1-4939-3387-7_43. [DOI] [PubMed] [Google Scholar]
- 14.McCormick KA, Coveler AL, Rossi GR, Vahanian NN, Link C, Chiorean EG. Pancreatic cancer: Update on immunotherapies and algenpantucel-L. Hum Vaccin Immunother. 2016;12:563–75. doi: 10.1080/21645515.2015.1093264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coveler AL, Rossi GR, Vahanian NN, Link C, Chiorean EG. Algenpantucel-L immunotherapy in pancreatic adenocarcinoma. Immunotherapy. 2016;8:117–25. doi: 10.2217/imt.15.113. [DOI] [PubMed] [Google Scholar]
- 16.Johansson A, Hamzah J, Payne CJ, Ganss R. Tumor-targeted TNFalpha stabilizes tumor vessels and enhances active immunotherapy. Proc Natl Acad Sci U S A. 2012;109:7841–6. doi: 10.1073/pnas.1118296109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calcinotto A, Grioni M, Jachetti E, Curnis F, Mondino A, Parmiani G, et al. Targeting TNF-alpha to neoangiogenic vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy. J Immunol. 2012;188:2687–94. doi: 10.4049/jimmunol.1101877. [DOI] [PubMed] [Google Scholar]
- 18.Korc M. Pancreatic cancer-associated stroma production. Am J Surg. 2007;194:S84–6. doi: 10.1016/j.amjsurg.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleeff J, Beckhove P, Esposito I, Herzig S, Huber PE, Lohr JM, et al. Pancreatic cancer microenvironment. Int J Cancer. 2007;121:699–705. doi: 10.1002/ijc.22871. [DOI] [PubMed] [Google Scholar]
- 20.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–97. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 21.Whatcott CJ, Han H, Von Hoff DD. Orchestrating the Tumor Microenvironment to Improve Survival for Patients With Pancreatic Cancer: Normalization, Not Destruction. Cancer journal. 2015;21:299–306. doi: 10.1097/PPO.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whatcott CJ, Diep CH, Jiang P, Watanabe A, LoBello J, Sima C, et al. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin Cancer Res. 2015;21:3561–8. doi: 10.1158/1078-0432.CCR-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 24.Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2010;60:861–8. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 25.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212–7. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–47. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catenacci DV, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, et al. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer. J Clin Oncol. 2015;33:4284–92. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nature genetics. 2015 doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014;25:1544–50. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 31.Girard L, Rodriguez-Canales J, Behrens C, Thompson DM, Botros IW, Tang H, et al. An Expression Signature as an Aid to the Histologic Classification of Non-Small Cell Lung Cancer. Clin Cancer Res. 2016;22:4880–9. doi: 10.1158/1078-0432.CCR-15-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. The New England journal of medicine. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fabregat A, Sidiropoulos K, Garapati P, Gillespie M, Hausmann K, Haw R, et al. The Reactome pathway Knowledgebase. Nucleic Acids Res. 2016;44:D481–7. doi: 10.1093/nar/gkv1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang LM, Silva MA, D’Costa Z, Bockelmann R, Soonawalla Z, Liu S, et al. The prognostic role of desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget. 2016;7:4183–94. doi: 10.18632/oncotarget.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bever KM, Sugar EA, Bigelow E, Sharma R, Laheru D, Wolfgang CL, et al. The prognostic value of stroma in pancreatic cancer in patients receiving adjuvant therapy. HPB (Oxford) 2015;17:292–8. doi: 10.1111/hpb.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parks SK, Chiche J, Pouyssegur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13:611–23. doi: 10.1038/nrc3579. [DOI] [PubMed] [Google Scholar]
- 37.Knudsen ES, Balaji U, Freinkman E, McCue P, Witkiewicz AK. Unique metabolic features of pancreatic cancer stroma: relevance to the tumor compartment, prognosis, and invasive potential. Oncotarget. 2016 doi: 10.18632/oncotarget.11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baek G, Tse YF, Hu Z, Cox D, Buboltz N, McCue P, et al. MCT4 Defines a Glycolytic Subtype of Pancreatic Cancer with Poor Prognosis and Unique Metabolic Dependencies. Cell reports. 2014;9:2233–49. doi: 10.1016/j.celrep.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 39.Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–21. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 40.Habtezion A, Edderkaoui M, Pandol SJ. Macrophages and pancreatic ductal adenocarcinoma. Cancer Lett. 2016;381:211–6. doi: 10.1016/j.canlet.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–41. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu H, Hang JJ, Han T, Zhuo M, Jiao F, Wang LW. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumour Biol. 2016;37:8657–64. doi: 10.1007/s13277-015-4741-z. [DOI] [PubMed] [Google Scholar]
- 43.Mandal R, Chan TA. Personalized Oncology Meets Immunology: The Path toward Precision Immunotherapy. Cancer Discov. 2016 doi: 10.1158/2159-8290.CD-16-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark CE, Beatty GL, Vonderheide RH. Immunosurveillance of pancreatic adenocarcinoma: insights from genetically engineered mouse models of cancer. Cancer Lett. 2009;279:1–7. doi: 10.1016/j.canlet.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 45.Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616–31. doi: 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–66. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Scientific reports. 2015;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutcheson J, Balaji U, Porembka MR, Wachsmann MB, McCue PA, Knudsen ES, et al. Immunologic and Metabolic Features of Pancreatic Ductal Adenocarcinoma Define Prognostic Subtypes of Disease. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–72. [PubMed] [Google Scholar]
- 51.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75:2139–45. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–41. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang SC, Everts B, Ivanova Y, O’Sullivan D, Nascimento M, Smith AM, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:846–55. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191:1486–95. doi: 10.4049/jimmunol.1202702. [DOI] [PubMed] [Google Scholar]
- 56.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell metabolism. 2016 doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 58.Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, et al. Neutralization of Tumor Acidity Improves Antitumor Responses to Immunotherapy. Cancer Res. 2016;76:1381–90. doi: 10.1158/0008-5472.CAN-15-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


