Abstract
In pharmacological magnetic resonance imaging (phMRI) with anesthetized animals, there is usually only a single time window to observe the dynamic signal change to an acute drug administration since subsequent drug injections are likely to result in altered response properties (e.g. tolerance). Unlike the block-design experiments in which fMRI signal can be elicited with multiple repetitions of a task, these single-event experiments require stable baseline in order to reliably identify drug-induced signal changes. Such factors as subject motion, scanner instability and/or alterations in physiological conditions of the anesthetized animal could confound the baseline signal. The unique feature of such functional MRI (fMRI) studies necessitates a technique that is able to monitor MRI signal in a real-time fashion and to interactively control certain experimental procedures. In the present study, an approach for real-time MRI on a Bruker scanner is presented. The custom software runs on the console computer in parallel with the scanner imaging software, and no additional hardware is required. The utility of this technique is demonstrated in manganese-enhanced MRI (MEMRI) with acute cocaine challenge, in which temporary disruption of the blood brain barrier (BBB) is a critical step for MEMRI experiments. With the aid of real-time MRI, we were able to assess the outcome of BBB disruption following bolus injection of hyperosmolar mannitol in a near real-time fashion prior to drug administration, improving experimental success rate. It is also shown that this technique can be applied to monitor baseline physiological conditions in conventional fMRI experiments using blood oxygenation level-dependent (BOLD) contrast, further demonstrating the versatility of this technique.
Keywords: real-time MRI, manganese-enhanced MRI, functional MRI, phMR, cocaine
Introduction
Functional magnetic resonance imaging (fMRI) has been widely used to investigate the sensory, motor and cognitive functions of the brain (1). Most of these studies used the so called blood oxygenation level-dependent (BOLD) contrast (2). More recently, this technique has been applied to investigate the effects of pharmacological agents in the brain (3). The non-invasive nature of this technique, coupled with its high spatial and temporal resolution has made it an attractive tool for drug research. The application of fMRI techniques to neuropharmacology, termed pharmacological MRI (phMRI) (4), has been successfully used to identify regional brain responses to such pharmacological agents as cocaine and nicotine, providing information about neural substrates and pharmacodynamics of drug actions (5–9).
phMRI has been traditionally conducted by bolus administrations of drug and identification of brain regions that exhibit alterations in hemodynamic responses corresponding with the dynamic change of drug concentration in the brain. Due to such limitations as the pharmacokinetics of drug actions, neuronal adaptations and drug tolerance etc., subjects usually receive only one drug administration in a session. Such single-event experiments require stable baseline in order to reliably identify drug-induced fMRI responses. Subject motion, scanner instability as well as alterations in physiological conditions (e.g. variations in blood gases of the anesthetized animal) could confound the baseline signal. Real-time monitoring of baseline signals and interactive control of experiments are of great value for ensuring the success of phMRI data acquisition. This is particularly the case in pharmacological studies using manganese-enhance MRI (MEMRI) (10–12).
MEMRI is based on the fact that Mn2+ is a calcium analogue and it detects neuronal activity by measuring Mn2+ accumulation in activated neuronal cells, which is independent of the hemodynamic response. Psychostimulants such as cocaine could induce strong systemic vascular responses in addition to their powerful effects on the central nervous system (CNS) (13). MEMRI has the advantage of being able to detect neuronal activation without potential confounds from a drug’s vascular effects (14). However, Mn2+ has limited permeability through the blood-brain barrier (BBB) (15). Dynamic MEMRI requires temporal disruption of the BBB so that a sufficient amount of Mn2+ enters the extracellular space. The BBB disruption method of Lin et al. (10) requires catheterization of the carotid artery and bolus injection of hyperosmolar mannitol via the internal carotid artery, which is then redistributed to the anterior, middle, and posterior cerebral arteries via the circle of Willis. Such factors as the amount of mannitol, the speed of injection, and the temperature of the drug solution all can influence the outcome of BBB disruption (16). Further, since the circle of Willis in the rat does not provide complete vascular collateralization, it appears technically and anatomically difficult for mannitol to be distributed homogenously within both hemispheres of the rat brain with a single bolus injection. Thus, following a drug challenge, brain areas with intact BBB will have negligible Mn2+ accumulation into the parenchyma, leading to a false-negative outcome in a dynamic MEMRI experiment. Therefore, it is critical to monitor the outcome of BBB disruption following bolus injection of mannitol in a real-time fashion.
Recent development of real-time MRI (RTMRI) techniques on human scanners has proven to be useful for quality assurance purposes (17–25). Several studies have applied feedback of the fMRI signal as a means to manipulate task performance (23–25). However, most RTMRI implementation requires at least some changes in system hardware. Here, we report a RTMRI technique that was implemented on a Bruker 9.4T small bore scanner. The custom software runs on the console computer in parallel with the Bruker imaging software; no additional hardware is required. In combination with AFNI (26), it can be applied to situations where immediate feedback of imaging results is necessary. This paper describes technical details of this method, and its application in a neuropharmacological study of cocaine’s effect on the CNS of the rat brain using MEMRI. Given the fact that hypercanpia and hypoxia induce robust BOLD responses (27,28), we also investigated the feasibility of monitoring baseline physiological conditions in an fMRI experiment by introducing hapercapnia/hypoxia at variable levels, and examined RTMRI response using an echo planar imaging (EPI) sequence.
Methods
RTMRI on a Bruker Scanner
The AFNI software package has been widely used in the fMRI field. It is freely available from the National Institute of Mental Health, National Institutes of Health (http://afni.nimh.nih.gov/afni/download). In addition to its powerful data analysis capability in the offline mode, it can also run in the real-time mode. Our general strategy was to access and transfer image data from the imaging system into AFNI and to perform data processing in a near real-time fashion within the AFNI framework, while introducing minimal interference on data acquisition and image processing of the scanner.
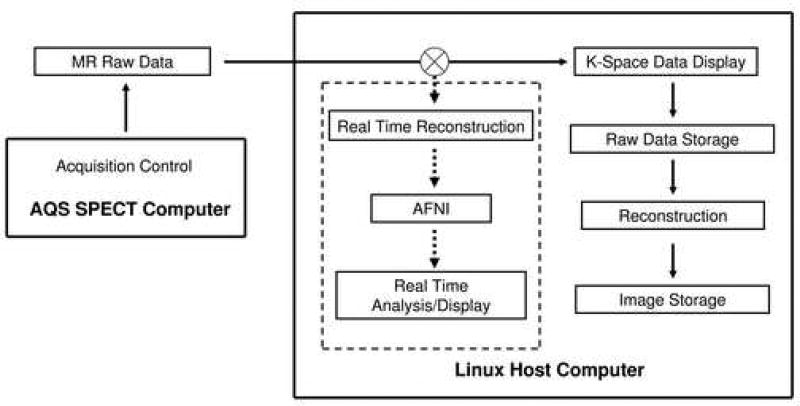
As shown in Fig. 1, the normal data flow of the Bruker Biospin scanner is as follows: Data acquisition is normally controlled by the SPECT computer. Digitized raw data are transferred to the console computer through an internal Ethernet line. Depending upon the options specified by the user, the raw data can be displayed on the monitor and/or saved to disk; they can also be reconstructed and stored to disk as desired.
Figure 1.
Schematic diagram showing the data flow of the proposed real-time MRI system on a Bruker scanner. Data acquisition is controlled by the AQS SPECT computer. Digitized MRI raw data are passed to a custom filter pipeline for real-time analysis in addition to the normal data processing within the console computer.
Potentially, there are two approaches to route MRI data to another process of the console computer: the first one is to wait until images have been reconstructed by the console computer and then send out image data to AFNI, in parallel to the image data storage process. In principle, this is a general approach and no custom image reconstruction program is required. But it depends upon how the system software handles the image reconstruction and image data storage processes. The delay time can be several seconds to minutes. For the purpose of real-time data feedback, one would ideally desire image data to be displayed with minimal time delay.
A second approach is to route k-space data from the SPECT computer to a custom program, conduct image reconstruction by the custom program and then send image data to AFNI running in real-time. The k-space data are also sent back to the console computer such that normal data acquisition processes are minimally disturbed. This approach interferes with data flow of both the SPECT computer and console computer, requiring careful process scheduling within both computers; it also requires the custom program to reconstruct images. However, this approach allows more flexibility for fast data display in AFNI, and is the approach that we have implemented.
A custom RTMRI program was developed on a Bruker 9.4T scanner running ParaVision 3.02 software. The host computer was composed of 1 GB physical memory, Intel Pentium 4 processor (2.8 GHz), running under Linux. Pulse generation, timing and data acquisition were controlled by the SPECT computer running under Unix. Communication between these two computers was through a high-speed local Ethernet link (100 MBPS). A customized pipeline filter program was written in C language. Raw k-space data were first routed to the pipeline filter and subsequently sent to Paravision. Image reconstruction, display and storage within Paravision framework were conducted within the Linux host computer as prescribed. Since AFNI receives only raw image dataset, custom image reconstruction programs were written for each pulse sequence. The reconstruction was performed within the pipeline filter program and subsequently sent to AFNI. Dataset header information, such as image matrix size, slice thickness, slice order etc., was acquired from the host computer automatically. Communication between Bruker Paravision reconstruction software and the user program was implemented through shared memory. Figure 1 shows the diagram of this method. As a proof of principle, we have implemented multi-slice gradient echo (FLASH), spin echo (MSME) and single-shot echo planar imaging (EPI) sequences on our scanner. Image reconstruction for MSME and FLASH sequences was virtually identical: k-space data were sorted based upon slice order and phase-encoding scheme, followed by Fourier transformation. No pulse sequence modification was required. For real-time EPI, a separate EPI sequence was developed. The central k-space line without phase-encoding gradient was scanned twice, but with opposite k-space traversal directions, which were used for phase corrections using the algorithm described by Jesmanowicz et al. (29). Images were formed by Fourier transformation of the phase-corrected data. Data points acquired during the gradient ramp periods were discarded.
Interactive MEMRI Experiments Using RTMRI
Twelve Sprague-Dawley rats (250–350g) were prepared as previously described (14,30). The procedures were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse-IRP. Briefly, the femoral artery and vein were catheterized under isoflurane anesthesia for physiological monitoring and drug delivery. The right external carotid artery (ECA) was catheterized in the direction of the common carotid artery, allowing blood flow through the internal carotid artery (ICA) to remain undisturbed. Rats were artificially ventilated, and end-tidal CO2 and O2 were continuously monitored using a computer. Core body temperature was maintained at 37.5 ± 0.5°C with a temperature-controlled water-circulating pad. After surgery, the animal’s head was secured with a bite bar and ear bars and positioned within the center of the magnet using a custom-built animal holder. Anesthesia was switched to α-chloralose during the MRI scan with an initial dose of 50 mg/kg (IV) followed by bolus injection of 50 mg/kg each hour. A neuromuscular blocking agent (gallamine triethiodide, 80 mg/kg IV) was administrated to further minimize motion artifacts.
MRI Scan
Slice localization for each animal was referenced to the anterior commissure. This was done by taking sagittal anatomical images using a Rapid Acquisition with Relaxation Enhancement (RARE) sequence (TR/TE: 2000/50 ms, RARE factor =8). The anterior commissure (−0.36 mm from bregma (31)) appears dark in T2-weighted images and can be readily identified. T1-weighted coronal images were acquired using a conventional spin echo sequence (MSME). Scan parameters were: TR = 450 ms, TE = 8 ms, slice thickness = 1 mm, spectral width = 50 kHz, FOV = 3.5 cm, matrix size = 128 × 128. The in-plane resolution was 0.27 mm. Each 2 min and 53 sec scan acquired thirteen slices. High resolution T2-weighted anatomical images were also acquired at the same slice locations, which were used for image registration.
Experimental Paradigm
Following anatomical localization scans, three baseline T1-weighted images were acquired. Continuous IV injection (1ml/hr) of 10% MnCl2 (Sigma, St. Louis, MO) was then initiated using an infusion pump. After injecting 30 mg/kg of MnCl2, a bolus injection (approx. 25 sec) of 25% mannitol (5–7 ml/kg, Sigma) was initiated through the ECA catheter to disrupt the BBB. Some animals received a second mannitol injection depending upon the outcome of BBB disruption as revealed by RTMRI (see below). MnCl2 infusion continued for another 25 min (preliminary studies showed that the MRI time course stabilized after about 25 min following BBB disruption). Since the intravascular half-life of Mn2+ is very short (16), continuous infusion of MnCl2 after BBB disruption was very important to allow the MRI signal to reach a stable plateau. Starting ten minutes before and lasting until 10 min after mannitol induced BBB disruption, 2% isoflurane was delivered to produce a deep anesthetic state. Preliminary experiments revealed that isoflurane reduced MEMRI signal resulting from non-specific stimuli (e.g. the loud noise of the scanner), and prevented brain swelling from the hyperosmolar mannitol injection. After the cessation of MnCl2 injection, either cocaine hydrochloride (2.0 mg/kg, n = 6) or the same amount of saline (n = 6) was injected IV, and T1-weighted images were continuously acquired for 45 min.
Data Analysis
To facilitate group comparisons, images were manually registered onto a common 3D space with AFNI. The datasets were spatially filtered using a Gaussian filter with a full width at half maximum (FWHM) of 0.4 mm. Time courses were de-trended as needed. Following bolus injection of hyperosmolar mannitol, the MR signal abruptly increased in regions where the BBB had been disrupted, and then stabilized within 25 min. The last three data points during the stabilization period were averaged to represent the new baseline signal (S1). The last three data points of the MEMRI scan were averaged to represent signal intensities after cocaine or saline injection (S2). Fractional signal changes resulting from cocaine or saline injection were calculated as: 100 × (S2 − S1)/S1, and were subject to a one-tailed t-test. p < 0.05 was considered significant.
Monitoring BOLD Signal Changes Resulting from Perturbations of Baseline Physiological Condition
Considering the highly variable pharmacokinetic profiles of different drugs, many phMRI studies rely on much faster data acquisition methods (such as EPI) than that used in the MEMRI experiment described above. In order to test whether the technique described is capable of detecting subtle signal changes resulting from perturbations of baseline physiological conditions, we systematically changed respirator tidal volume while keeping the respiration rate, inhale time and exhale time constant, and examined the fMRI time course in real-time using a single-shot, gradient echo EPI sequence. Experiments were conducted on three rats. Scan parameters were: FOV = 3.0 × 3.0 cm2, matrix size = 64 × 64, data acquisition bandwidth = 200 KHz, TR = 1 s, 5 slices with a slice thickness of 1.5 mm.
Arterial blood gases were perturbed by graded reduction of tidal volume to introduce transient hypercapnia/hypoxia for 20 sec followed by normal ventilation for 40 sec, and repeating the sequence 2–3 times. At each tidal volume level, arterial blood samples were collected for blood gas assay (GEM Premier 3000 Blood Gas Analyzer, Instrumentation Laboratory, MA). Fractional signal changes were calculated by 100 × (S(t) − S0)/S0, where S(t) and S0 represent BOLD time course and baseline signal, respectively. S0 was calculated by averaging 20 data points before gas challenge. Factional signal changes within individual time windows of gas challenge were calculated by averaging the data points within the full width at half minimum of each block, which were then averaged over blocks. To compare BOLD responses at three levels of hypercapnia/hypoxia, we also made BOLD response maps to gas challenges using the cross-correlation method (32). The reference function was generated in two steps: first, a function consisting of 1 and 0 representing “on” and “off” periods of gas challenge was used to generate BOLD activation maps. Voxels with the greatest BOLD responses (typically p<10−8) were averaged to generate an ideal reference function. This function was assumed to have the appropriate hemodynamic response delay, which was used to generate BOLD response maps. Only common voxels that showed significant BOLD responses at both the medium and high levels of hypercapnia/hypoxia were entered into a statistical comparison. We did not observe significant BOLD response at low levels of hypercapnia. To compare BOLD response at this level with two other levels, fractional signal changes were calculated from the same windows and the same voxels as at the medium level of hypercapnia. During the experiments, time courses were visualized qualitatively in real-time using the same software as for the MEMRI experiments. Quantifications were performed off-line. Data are presented as mean ± standard deviation unless otherwise specified.
Results
Monitoring the Effect of BBB Disruption Using RTMRI
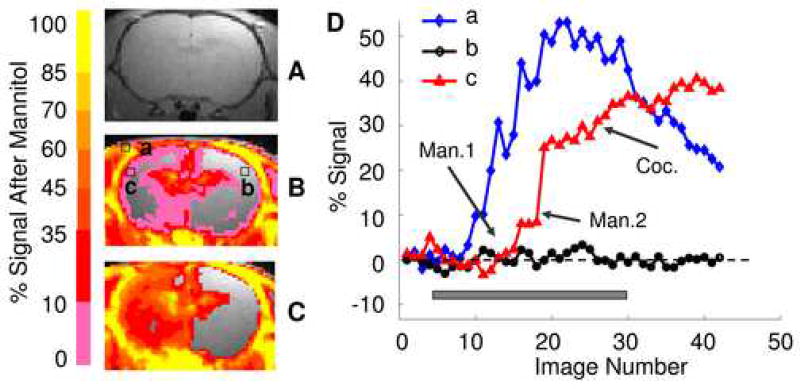
Figure 2B and C illustrate the effects of BBB disruption after mannitol injections with a raw T1-wighted image shown in Fig. 2A as a reference. Figure 2D shows voxel time courses from brain regions a, b and c as indicated in Fig. 2B. Following the first bolus injection of mannitol, signal enhancement was seen in some areas, including parts of the cerebral cortex and caudate putamen. However, it is apparent from Figs. 2B and D that signal enhancement was not sufficient after this injection, with less than 5% signal change in most areas. With the aid of RTMRI, we were able to readily assess the poor outcome of the mannitol injection. In this case, a second injection was initiated, whereupon an abrupt signal increase was seen in most brain areas. After the signal time courses reached a plateau following the second mannitol injection, cocaine was injected (2 mg/kg), which led to further activity-dependent signal enhancement in many brain areas (Fig. 2D). Without the RTMRI technique, we would have had to wait until the end of image acquisition to analyze the effect of BBB disruption. By then, cocaine would have been administered and, depending on the experimental protocol, it is likely that an unsuccessful experimental outcome would have resulted.
Figure 2.
Real time assessment of the outcome of BBB disruption following bolus injections of hyperosmolar mannitol. A is a traditional T1-weighted spin echo sequence anatomical image. B shows fractional signal changes following the first bolus injection of mannitol (thresholded at 5%). C shows fractional signal changes following the second bolus injection of mannitol (thresholded at 5%). D shows voxel time courses from regions a (skull muscle), b and c (right and left forelimb somatosensory cortex) indicated in B. Both B and D demonstrate insufficient signal enhancement following the first mannitol injection. In this case, an additional mannitol injection was initiated, which resulted in an abrupt signal increase on the ipsilateral side, while the signal on the contralateral side remained largely unchanged, as shown in C and D. Notice the distinct MEMRI signal patterns after the cessation of MnCl2 infusion: cocaine injection (Coc.) further increased MEMRI signal in c, while the signal decreased in a and remained unchanged in b. Gray box indicates continuous MnCl2 infusion. Man. 1 and 2 indicate first and second mannitol injection, respectively.
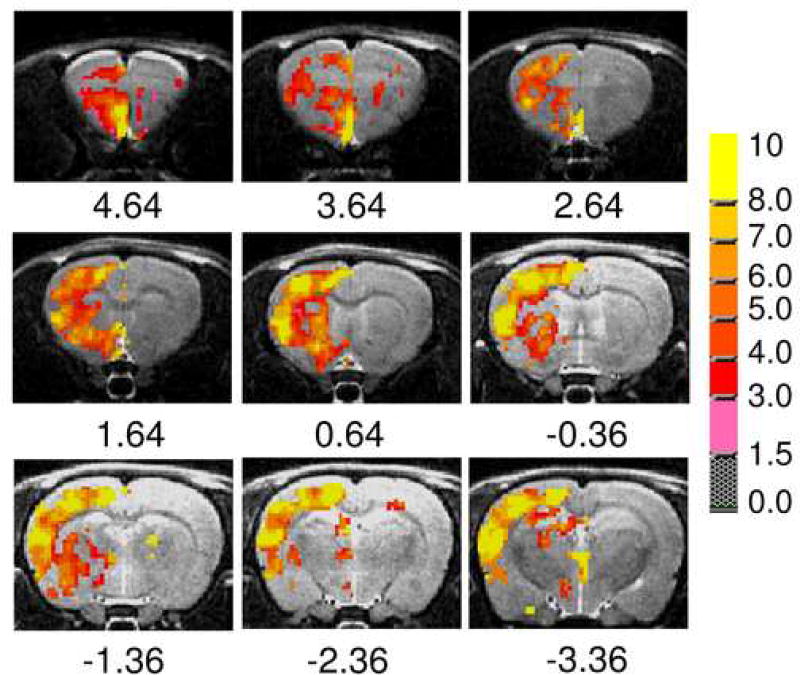
Percent signal change following cocaine and saline injections was calculated on a voxel-wise basis. Statistical comparison between the test (cocaine; n=6) and control (saline; n=6) groups was conducted. Figure 3 illustrates statistical activation maps superimposed onto high-resolution structural images. Regions significantly activated by cocaine were mostly cortical and subcortical limbic, and included olfactory tubercule (Olf), frontal, medial prefrontal (mPFC), prelimbic, cingulate and insular cortex, NAc, caudate-putamen and globus pallidus. Activation of these regions, all of which receive high density ventral tegmental area dopaminergic projections, once again supports a critical role for the mesocorticolimbic system in cocaine’s acute rewarding and drug-seeking properties (33). In most cases, only the BBB in the hemisphere ipsilateral to the carotid catheter was disrupted; uniform disruption of the entire brain was only observed occasionally as can be seen in Fig 3.
Figure 3.
Activation maps following acute cocaine administration. Regions significantly activated by cocaine were mostly limbic, sensory and motor cortices and subcortical limbic structures. The false negative outcome on the contralateral side was due to poor BBB disruption. The slice coverage ranged from −3.36 to +4.64 mm relative to bregma (31). Pseudo color bar represents t-values thresholded at p<0.05 following one-tailed t-test.
Monitoring BOLD Response Following Transient Blood Gas Perturbations Using RTMRI
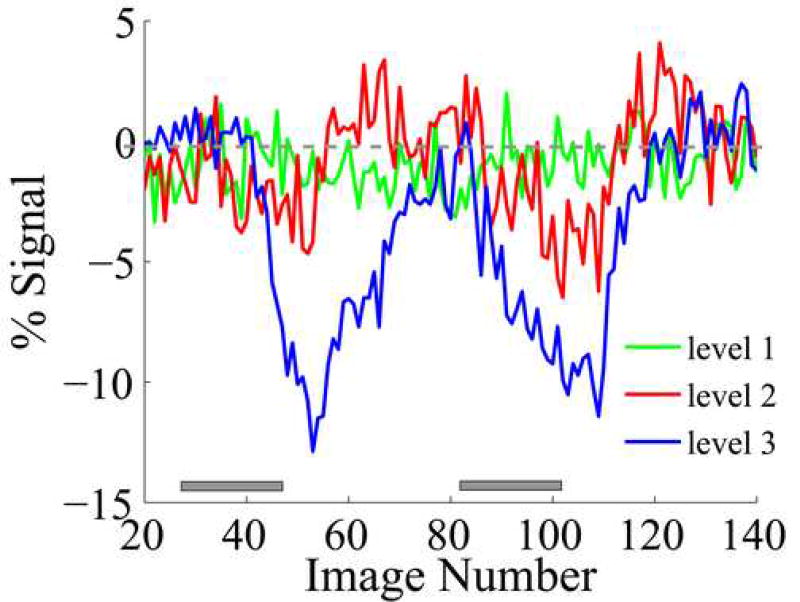
This experiment investigated the feasibility of monitoring baseline physiology with the aid of RTMRI. This was done by systematically changing tidal volume of the respirator to introduce different levels of hypercapnia/hypoxia and acquiring RTMRI data with a single-shot gradient echo EPI sequence. Voxel time courses were dynamically plotted on the screen with a time lag of about one TR (1 sec). As shown in Figure 4, when blood gases were adjusted from normal (PCO2 = 38 mmHg, PO2=148 mmHg) to minor hypercapnia (level 1: PCO2 = 44 mmHg, PO2=137 mmHg), there was no obvious BOLD response coupled to the gas challenge. However, when blood gases were adjusted to higher hypercapnic levels (level 2: PCO2 = 53 mmHg, PO2=117 mmHg; level 3: PCO2 = 64 mmHg, PO2=77 mmHg), apparent global BOLD responses were readily seen. Mean signal amplitude of −2.1 ± 0.4% and −6.6 ± 0.6% was observed at level 2 and 3, respectively. Average fractional signal changes following graded blood gas challenge from all animals (n = 3) were −0.1 ± 0.5%, −1.5 ± 0.8% and −4.4 ± 1.7%, at levels 1,2 and 3 respectively.
Figure 4.
Representative time courses from one animal during graded hypercapnia/hypoxia. Minimal BOLD alterations were seen when blood gases were adjusted from normal (PCO2 = 38 mmHg, PO2=148 mmHg) to minor hypercapnic levels (level 1: PCO2 = 44 mmHg, PO2=137 mmHg). However, a medium level of hypercapnia (level 2: PCO2 = 53 mmHg, PO2=117 mmHg) caused apparent global BOLD responses readily identified from the real-time plots. Further increases in BOLD response amplitude were observed when severe hypercapnia and hypoxia were introduced (level 3: PCO2 = 64 mmHg, PO2=77 mmHg). Gray boxes indicate blood gas challenge.
Discussion
In the present study, we demonstrated an approach for and application of real-time functional MRI in rat neuropharmacological studies. The custom software developed runs on the scanner console computer in parallel with the scanner imaging software; no additional hardware was required. Although we implemented this technique on a Bruker scanner, the principle is general and should also be applicable to other scanner systems.
The utility of this technique was demonstrated in interactive control of MEMRI experiments on rats. With the aid of RTMRI, we were able to assess the initially poor outcome of BBB disruption following bolus injection of hyperosmolar mannitol in a real-time fashion, allowing for a second mannitol injection to more completely open the BBB prior to cocaine administration, thus ensuring the success of the experiment. Without real-time monitoring, quality of the data can only be examined after the entire dataset has been collected.
In addition to interactive control of the timing of drug-administration, RTMRI can also be used to ensure a stable baseline during phMRI data acquisition. Most phMRI studies employ a single-event (i.e. drug administration) experimental design, and thus there is a single window for observing dynamic signal changes following acute drug administration. These single-event experiments require stable baseline to reliably identify drug-induced signal changes. In our experience, catheter failure and fluid blockade of tracheal tubing are common technical issues leading to experimental failure. Quite often these factors lead to perturbation of blood gases in artificially ventilated animals. With the aid of RTMRI, experimenters are alerted to such factors and thus can improve the success rate. The method described in this paper has been routinely applied to phMRI experiments in our lab. It is also generalizable to most animal MRI studies where interactive quality control procedures would significantly enhance experimental success.
In the present study, we quantified fractional MEMRI signal changes as a result of Mn2+ accumulation in groups of animals receiving either cocaine or saline, and conducted voxel-wise t-statistical analysis between these two groups. In principle, it is possible to quantify the MEMRI signal in terms of Mn2+ concentration rather than fractional signal changes, which would provide more insight into the underlying neuronal activity. However, several technical difficulties prevented such analysis. Mn2+ is positively charged. It interacts with proteins and other macromolecules and influences the spin properties of local water molecules. A recent study suggests that its spin relaxation effect appears to be region-specific (34), and may not be readily extrapolated from phantom data. More work is needed in order to model and quantify the MEMRI signal in terms of Mn2+ concentration.
Acknowledgments
The authors thank T. J. Ross at the National Institute on Drug Abuse, M. Mattingly of Bruker Biospin MRI, Inc. for valuable discussions. This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moonen CTW, Bandettini PA. Functional MRI. Springer-Verlag; 1999. [Google Scholar]
- 2.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–72. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein EA. fMRI: a new tool for the in vivo localization of drug actions in the brain. J Anal Toxicol. 2001;25:419–24. doi: 10.1093/jat/25.5.419. [DOI] [PubMed] [Google Scholar]
- 4.Chen YC, Galpern WR, Brownell AL, Matthews RT, Bogdanov M, Isacson O, Keltner JR, Beal MF, Rosen BR, Jenkins BG. Detection of dopaminergic neurotransmitter activity using pharmacologic MRI: correlation with PET, microdialysis, and behavioral data. Magn Reson Med. 1997;38:389–98. doi: 10.1002/mrm.1910380306. [DOI] [PubMed] [Google Scholar]
- 5.Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 6.Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155:1009–15. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- 7.Marota JJ, Mandeville JB, Weisskoff RM, Moskowitz MA, Rosen BR, Kosofsky BE. Cocaine activation discriminates dopaminergic projections by temporal response: an fMRI study in Rat. Neuroimage. 2000;11:13–23. doi: 10.1006/nimg.1999.0520. [DOI] [PubMed] [Google Scholar]
- 8.Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, Li SJ. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28:904–14. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 9.Febo M, Segarra AC, Nair G, Schmidt K, Duong TQ, Ferris CF. The neural consequences of repeated cocaine exposure revealed by functional MRI in awake rats. Neuropsychopharmacology. 2005;30:936–43. doi: 10.1038/sj.npp.1300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin YJ, Koretsky AP. Manganese ion enhances T1-weighted MRI during brain activation: an approach to direct imaging of brain function. Magn Reson Med. 1997;38:378–88. doi: 10.1002/mrm.1910380305. [DOI] [PubMed] [Google Scholar]
- 11.Duong TQ, Silva AC, Lee SP, Kim SG. Functional MRI of calcium-dependent synaptic activity: cross correlation with CBF and BOLD measurements. Magn Reson Med. 2000;43:383–92. doi: 10.1002/(sici)1522-2594(200003)43:3<383::aid-mrm10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Silva AC, Lee JH, Aoki I, Koretsky AP. Manganese-enhanced magnetic resonance imaging (MEMRI): methodological and practical considerations. NMR Biomed. 2004;17:532–43. doi: 10.1002/nbm.945. [DOI] [PubMed] [Google Scholar]
- 13.Kiritsy-Roy JA, Halter JB, Gordon SM, Smith MJ, Terry LC. Role of the central nervous system in hemodynamic and sympathoadrenal responses to cocaine in rats. J Pharmacol Exp Ther. 1990;255:154–60. [PubMed] [Google Scholar]
- 14.Lu H, Xi ZX, Gitajn L, Rea W, Yang Y, Stein EA. Cocaine-induced brain activation detected by dynamic manganese-enhanced magnetic resonance imaging (MEMRI) Proc Natl Acad Sci USA. 2007;104:2489–94. doi: 10.1073/pnas.0606983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitsanakis VA, Piccola G, Aschner JL, Aschner M. Manganese transport by rat brain endothelial (RBE4) cell-based transwell model in the presence of astrocyte conditioned media. J Neurosci Res. 2005;81:235–43. doi: 10.1002/jnr.20560. [DOI] [PubMed] [Google Scholar]
- 16.Aoki I, Naruse S, Tanaka C. Manganese-enhanced magnetic resonance imaging (MEMRI) of brain activity and applications to early detection of brain ischemia. NMR Biomed. 2004;17:569–80. doi: 10.1002/nbm.941. [DOI] [PubMed] [Google Scholar]
- 17.Cox RW, Jesmanowicz A, Hyde JS. Real-time functional magnetic resonance imaging. Magn Reson Med. 1995;33:230–6. doi: 10.1002/mrm.1910330213. [DOI] [PubMed] [Google Scholar]
- 18.Voyvodic JT. Real-time fMRI paradigm control, physiology, and behavior combined with near real-time statistical analysis. Neuroimage. 1999;10:91–106. doi: 10.1006/nimg.1999.0457. [DOI] [PubMed] [Google Scholar]
- 19.Frank JA, Ostuni JL, Yang Y, Shiferaw Y, Patel A, Qin J, Mattay VS, Lewis BK, Levin RL, Duyn JH. Technical solution for an interactive functional MR imaging examination: application to a physiologic interview and the study of cerebral physiology. Radiology. 1999;210:260–8. doi: 10.1148/radiology.210.1.r99ja23260. [DOI] [PubMed] [Google Scholar]
- 20.Cohen MS. Real-time functional magnetic resonance imaging. 2001;25:201–20. doi: 10.1006/meth.2001.1235. [DOI] [PubMed] [Google Scholar]
- 21.Mathiak K, Posse S. Evaluation of motion and realignment for functional magnetic resonance imaging in real time. Magn Reson Med. 2001;45:167–71. doi: 10.1002/1522-2594(200101)45:1<167::aid-mrm1023>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 22.Yoo SS, Guttmann CR, Zhao L, Panych LP. Real-time adaptive functional MRI. Neuroimage. 1999;10:596–606. doi: 10.1006/nimg.1999.0494. [DOI] [PubMed] [Google Scholar]
- 23.deCharms RC, Christoff K, Glover GH, Pauly JM, Whitfield S, Gabrieli JD. Learned regulation of spatially localized brain activation using real-time fMRI. Neuroimage. 2004;21:436–43. doi: 10.1016/j.neuroimage.2003.08.041. [DOI] [PubMed] [Google Scholar]
- 24.Yang S, Ross TJ, Zhang Y, Stein EA, Yang Y. Head motion suppression using real-time feedback of motion information and its effects on task performance in fMRI. Neuroimage. 2005;27:153–62. doi: 10.1016/j.neuroimage.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 25.Weiskopf N, Sitaram R, Josephs O, Veit R, Scharnowski F, Goebel R, Birbaumer N, et al. Real-time functional magnetic resonance imaging: methods and applications. Magn Reson Imaging. 2007;25:989–1003. doi: 10.1016/j.mri.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 27.Duong TQ. Cerebral blood flow and BOLD fMRI responses to hypoxia in awake and anesthetized rats. Brain Res. 2007;1135:186–94. doi: 10.1016/j.brainres.2006.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu G, Luo F, Li Z, Zhao X, Li SJ. Transient relationships among BOLD, CBV, CBF changes in rat brain as detected by functional MRI. Magn Reson Med. 2002;48:987–93. doi: 10.1002/mrm.10317. [DOI] [PubMed] [Google Scholar]
- 29.Jesmanowicz A, Wong EC, Hyde JS. Proc Soc Magn Reson 3rd Meeting. Nice: France; 1995. Self-correcting EPI reconstruction algorithm; p. 619. [Google Scholar]
- 30.Lu H, Patel S, Luo F, Li SJ, Hillard CJ, Ward BD, Hyde JS. Spatial correlations of laminar BOLD and CBV responses to rat whisker stimulation with neuronal activity localized by Fos expression. Magn Reson Med. 2004;52:1060–8. doi: 10.1002/mrm.20265. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Elsevier Academic Press; 2005. [Google Scholar]
- 32.Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for timecourse data sets in functional MRI of the human brain. Magn Reson Med. 30:161–73. doi: 10.1002/mrm.1910300204. 993. [DOI] [PubMed] [Google Scholar]
- 33.Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47:190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Zhang N, Fitsanakis VA, Aschner M, Avison MJ, Gore JC. Proc Intl Soc Magn Reson Med. Seattle, USA: 2006. Variations in relaxivity of manganese between regions in rat brain; p. 226. [Google Scholar]