Desmin, the intermediate filament protein in striated muscle, is an essential component of the striated muscle cell cytoskeleton. In muscle, the cytoskeleton is a multifunctional, dynamic and complex structure that serves as a scaffold to maintain the structural integrity and architecture of the cardiomyocyte (CM). However, it serves more than a passive, structural purpose as the cytoskeleton functions as a platform for inter- and intra-cellular signaling, linking the contractile apparatus so that sarcomeres may function efficiently as a syncytium, and linking those structures in turn to the sarcolemma, cell-cell junctions, the mitochondria and nucleus. It is not surprising then, that mutations in the gene encoding desmin can cause myopathies in general and cardiomyopathy in particular.1
As a cell is subjected to normal and abnormal stresses, proteins can misfold and mutations in desmin or its chaperone, αB-crystallin, can increase the likelihood of this occurring. In addition to having their normal function compromised, these misfolded proteins can assume a proteotoxic conformation, leading to a gain-of-function pathogenic potential, as is seen in many of the neurodegenerative diseases such as Alzheimer's disease with the Aβ-amyloid fragment.2 In silico modeling has indicated that hundreds if not thousands of peptide domains have some degree of amyloidogenic potential.3 Amyloid formation is a common theme in many neurodegenerative diseases and disorders in which protein conformation is affected, but true amyloid formation is somewhat rarer and characterized by formation of unbranched, 10 nm fibrils with β-pleated sheets and a distinct apple green birefringence and Congo-red positive staining.4 True amyloidoses are also associated with the formation of extracellular plaques or intracellular inclusion bodies. However, these amyloid-positive deposits may not be the primary proteotoxic entity but rather represent the terminal step in a prolonged pathogenic process in which an early intermediate step, the formation of toxic, pre-amyloid oligomers (PAO) occurs (Figure).5 Multiple peptide sequences can assume a PAO, amyloid-like conformation and development of antibodies that detect PAO conformers.6 revealed their presence in proteotoxic cardiac environments in both animal models and in human disease.7 A majority of the current data strongly indicate that it is the PAOs that are the primary cytotoxic elements in developing and possibly even mature proteotoxic cardiac disease.8 CM-specific expression of an exogenous peptide capable of assuming a PAO conformation was sufficient to cause heart failure and led to 100% early mortality in a transgenic mouse model.9
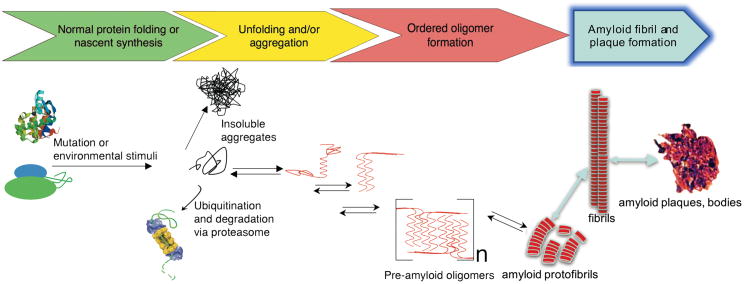
Figure. PAO formation and the amyloid pathway.
Shown is a schematic diagram of the temporal consequences of protein misfolding. Nascent proteins interact with chaperones and fold correctly. However, due to genetic mutation or environmental stimuli such as mechanical stress, hypoxia, ischemia or detrimental post-translational modifications, misfolded proteins or peptide fragments accumulate. These can aggregate into kinetically stable, insoluble entities or be recognized by the protein quality control machinery, ubiquitinated and degraded by the proteasome. Cleaved protein domains are able to assume a β-pleated sheet structure (red) and interact with one another to form a series of intermediate but stable structures, resulting in soluble pre-amyloid oligomers, which are cytotoxic. These entities can, under certain circumstances, go on to form protofibrils and may coalesce, resulting in the classic amyloid fibrils, plaques and tangles. However, there are no data confirming the presence of classic amyloids in the model used in this manuscript so the steps represented by the blue arrows are hypothetical at present for the models under discussion in Rainer et al. The reversibility of many of the processes leading to mature amyloid fibril deposition are emphasized by the bi-directional arrows. (Drawn after reference 8)
As we now know that CM-based PAO's can cause cardiac disease directly, at least in genetically modified mice, understanding the factors that lead to their accumulation, being able to detect them and determining the critical factors for their maintenance and removal become important. The data presented in Rainer et al in this issue of Circulation Research touch on PAO accumulation and detection, two of the critical parameters for understanding this important cardiopathic stimulus.
Rainer et al explore how desmin's post translational modification might impact on PAO formation in a heart failure murine model induced by transverse aortic constriction (TAC).10 They extend their data to human heart failure by observing PAO accumulations in samples derived from both ischemic and non-ischemic hearts. Importantly, they show that minimally invasive imaging using Positron Emission Tomography-Computed Tomography (PET/CT) is able to detect these PAO accumulations in vivo, opening the door not only to modulating post translation modification as a therapeutic modality but also the diagnostic imaging of PAOs in acquired heart failure.
PAO Accumulation in Acquired Heart Failure
They initiated the current study using a mouse model of acquired heart disease induced by TAC in which they documented concentric hypertrophy. PAO accumulation occurred and, upon Western blotting and subsequent visualization using infrared fluorescent imaging, they confirmed its colocalization with both native and cleaved desmin. Confirming the relevance of their observations to human heart disease, they showed that desmin-PAO was also present in heart biopsies derived from non-ischemic, dilated cardiomyopathic patients. Biopsies from ischemic cardiomyopathies were also examined and, while these samples also showed PAO accumulation and increased desmin cleavage, the size of the PAO-positive species differed between the two populations, suggesting that while PAO accumulation is shared between them, the mechanisms and interacting protein species may be disease-type specific. In any case, the data are consistent with previous studies showing CM-autonomous accumulations of PAO in samples derived from human heart failure patients.7 Rainer et al show the accumulation of PAO for the first time in acquired heart failure in a mouse TAC model and in ischemic cardiomyopathy patients, which suggests that PAO can accumulate in cardiac disease independent of the presence of a mutant protein.
Desmin Phosphorylation and PAO Formation
In the search for mechanisms that might increase the amyloidogenic potential of wild type desmin, the authors extended their previous data showing that a post translational modification of the protein, phosphorylation of serine residues 27 and 31 in canine and human heart failure, was mediated by GSK3β kinase and this enzyme is inhibited in some heart failure models.11 The authors then tested the hypothesis that the bi-phosphorylated form is the physiologically healthy protein by expressing bi- and mono-phosphate desmin mimetics in neonatal rat ventricular CMs. Expression of the bi-phosphomimetic led to increases in the number of actively contracting cells and desmin levels at the Z line, while expression of a mono-phosphorylated mimetic led to decreased Z line incorporation as well as increased desmin-positive protein aggregates. The authors propose that wild-type desmin can serve as a seed for fibrils if it is mono-phosphorylated at Ser31 and cleaved in response to stressors, indicating a role for posttranslational modifications in PAO formation.
Robust Detection Methods for Cardiac PAO
Detection of a pathogenic component such as PAO is essential for studying the pathogenic consequences but PAO has been notoriously difficult to detect and quantitate. Building upon the amyloid-like qualities of the pathogenic conformer, the authors developed an effective and convenient detection method, using a histological stain for amyloid, Thioflavin T, in SDS-PAGE-displayed protein extracts. Using a well-characterized, murine proteotoxic model of cardiac disease, occurring as a result of CM-restricted of a mutant αB crystallin that causes human cardiomyopathy,12 the authors validated their in-gel staining methodology. They were able to show that Thioflavin T staining coincided with normal desmin protein, indicating that under general proteotoxic conditions, normal proteins with amyloidogenic potential can undergo the initial stages of folding that generate PAO (Figure). Although the authors could not rigorously rule out other interpretations such as the partial loss of desmin function in the hearts due to the protein's cleavage or other factors, staining did show a 9-fold increase in PAO in the TAC hearts as well as approximately a 28-fold increase in a cleaved desmin fragment relative to intact protein.
It becomes critical to be able to detect and diagnose these proteotoxic processes, particularly in the pre-clinical stages as it has been shown that clearance of these aggregates and PAO can significantly affect the course of developing cardiac disease in animal models.13 PET/CT subsequent to the injection of the radiopharmaceutical Amyvid (Florbetapir F-18), may be a useful tool for minimally-invasive detection of β-amyloid plaque density in patients suspected of having Alzheimer's disease.14 The authors tested the novel application of this imaging modality for detecting PAO accumulations in the mouse TAC induced model of heart failure and found significantly elevated uptake of the diagnostic dye in these hearts 4 weeks post-TAC. Although not confirmed, the authors hypothesize that the detected signal corresponded to the same PAO-positive desmin species seen in the SDS PAGE studies.
Together with the in-gel staining methods outlined in the manuscript, Rainer et al. describe relatively simple and robust methods to detect PAO in vitro and, more importantly, in vivo for research purposes. A number of animal models are thought to generate proteotoxic species, but potentially low levels of PAOs are difficult to detect in cardiac tissues using the available labprepared and commercial antibodies. With the application of Thioflavin T in SDS-PAGE, it may now be possible to detect even low levels of PAOs in cardiac tissue samples. Moreover, the authors establish PET/CT, which is already used to detect cardiac amyloidosis in patients, as a method to visualize PAO in mouse hearts and potentially in other animal models as well. PET/CT in combination with echocardiography will allow PAO content and cardiac function correlations using minimally-invasive imaging and ultrasound. This will significantly advance our ability to study PAO formation and its relation to developing cardiac disease and the attendant morbidities.
Unresolved Issues
Work remains to be done. Although in vitro data and data from a few animal models are compelling, it is not clear yet if PAOs significantly contribute to cardiac disease in acquired human heart failure or are merely a byproduct. PAOs represent an intermediate step in amyloid fibril formation that begins when a native soluble protein becomes misfolded (Figure) but desmin related myopathies do not appear to develop a true amyloidosis as characterized by established criteria, including Congo red staining, a characteristic “apple green” color upon visualization with birefringence microscopy, formation of 10nm unbranched, fibrils and eventually, the appearance of inclusion bodies or plaques containing amyloid as defined by the criteria above.15 PAO formation precedes the actual formation of amyloids but amyloidosis, as rigorously defined 4,15, does not necessarily follow from it. For example, it remains formally inaccurate to call mutant αB crystallin-induced disease an amyloidosis as it is more accurately characterized as amyloid-like and similar caveats exist for the TAC-induced model until otherwise documented The present study shows that epigallocatechin gallate, which can disrupt amyloid-like and prion deposits,11,16 , de-aggregates wild type desmin PAOs in proteotoxic heart failure in vitro, but it will be important to know whether the compound or other treatments reduce PAOs in vivo and if this benefits cardiac function in human acquired heart failure. The amyloid and protein aggregate field is littered with disappointments in large clinical trials when the supposed toxic entities are targeted.17 Furthermore, it is of great interest to evaluate if monophosphorylated and cleaved desmin can seed PAO formation in other cardiomyopathies and thus represent a general pathogenic mechanism. If PAOs are a general feature found in some classes of human heart disease and there are preliminary data indicating this is the case,7 many cardiomyopathies might benefit from a therapy that targets them. And if PAOs are indeed crucial to disease progression, it will be very important to determine whether their late-stage dissolution is able to reverse heart failure or at least prevent further decline in cardiac function. Although this and other studies provide promising and exciting data, one should not neglect that disruption and clearance of these pre-existing amyloid-associated toxic species in clinical trials have failed so far. It will be essential to determine in PAO dissolution effectively alters the course of human cardiac disease in the affected patient populations.
Acknowledgments
Sources of Funding: This work was supported by National Institutes of Health grants P01HL69779, P01HL059408, R01HL05924, R011062927 and a Trans-Atlantic Network of Excellence grant from Le Fondation Leducq (JR).
Footnotes
Disclosures: None
References
- 1.Goldfarb LG, Dalakas MC. Tragedy in a heartbeat: Malfunctioning desmin causes skeletal and cardiac muscle disease. J Clin Invest. 2009;119:1806–1813. doi: 10.1172/JCI38027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iqbal K, Liu F, Gong CX. Alzheimer disease therapeutics: Focus on the disease and not just plaques and tangles. Biochem Pharmacol. 2014;88:631–639. doi: 10.1016/j.bcp.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galzitskaya OV, Garbuzynskiy SO, Lobanov MY. Prediction of amyloidogenic and disordered regions in protein chains. PLoS Comput Biol. 2006;2:e177. doi: 10.1371/journal.pcbi.0020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westermark P, Benson MD, Buxbaum JN, Cohen AS, Frangione B, Ikeda S, et al. Amyloid: Toward terminology clarification. Report from the nomenclature committee of the international society of amyloidosis. Amyloid. 2005;12:1–4. doi: 10.1080/13506120500032196. [DOI] [PubMed] [Google Scholar]
- 5.Glabe CG. Structural classification of toxic amyloid oligomers. J Biol Chem. 2008;283:29639–29643. doi: 10.1074/jbc.R800016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kayed R, Glabe CG. Conformation-dependent anti-amyloid oligomer antibodies. Methods Enzymol. 2006;413:326–344. doi: 10.1016/S0076-6879(06)13017-7. [DOI] [PubMed] [Google Scholar]
- 7.Sanbe A, Osinska H, Saffitz JE, Glabe CG, Kayed R, Maloyan A, et al. Desmin-related cardiomyopathy in transgenic mice: A cardiac amyloidosis. Proc Natl Acad Sci U S A. 2004;101:10132–10136. doi: 10.1073/pnas.0401900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Robbins J. Heart failure and protein quality control. Circ Res. 2006;99:1315–1328. doi: 10.1161/01.RES.0000252342.61447.a2. [DOI] [PubMed] [Google Scholar]
- 9.Pattison JS, Sanbe A, Maloyan A, Osinska H, Klevitsky R, Robbins J. Cardiomyocyte expression of a polyglutamine preamyloid oligomer causes heart failure. Circulation. 2008;117:2743–2751. doi: 10.1161/CIRCULATIONAHA.107.750232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rainer P, Dong P, Sorge M, Fert-Bober J, Holewinski R, Wang Y, et al. Desmin phosphorylation triggers preamyloid oligomers formation and myocyte dysfunction in acquired heart failure. Circ Res. 2018;122:XXX–XXX. doi: 10.1161/CIRCRESAHA.117.312082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agnetti G, Halperin VL, Kirk JA, Chakir K, Guo Y, Lund L, et al. Desmin modifications associate with amyloid-like oligomers deposition in heart failure. Cardiovasc Res. 2014;102:24–34. doi: 10.1093/cvr/cvu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Osinska H, Klevitsky R, Gerdes AM, Nieman M, Lorenz J, et al. Expression of R120G-alphaB-crystallin causes aberrant desmin and alphaB-crystallin aggregation and cardiomyopathy in mice. Circ Res. 2001;89:84–91. doi: 10.1161/hh1301.092688. [DOI] [PubMed] [Google Scholar]
- 13.Bhuiyan MS, Pattison JS, Osinska H, James J, Gulick J, McLendon PM, et al. Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest. 2013;123:5284–5297. doi: 10.1172/JCI70877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilebro B, Arvidsson S, Lindqvist P, Sundstrom T, Westermark P, Antoni G, et al. Positron emission tomography (PET) utilizing Pittsburgh compound b (pib) for detection of amyloid heart deposits in hereditary transthyretin amyloidosis. J Nucl Cardiol. 2018;25:240–248. doi: 10.1007/s12350-016-0638-5. [DOI] [PubMed] [Google Scholar]
- 15.Budson AE, Solomon PR. New criteria for alzheimer disease and mild cognitive impairment: Implications for the practicing clinician. Neurologist. 2012;18:356–363. doi: 10.1097/NRL.0b013e31826a998d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts BE, Duennwald ML, Wang H, Chung C, Lopreiato NP, Sweeny EA, et al. A synergistic small-molecule combination directly eradicates diverse prion strain structures. Nat Chem Biol. 2009;5:936–946. doi: 10.1038/nchembio.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, et al. Trial of solanezumab for mild dementia due to Alzheimer's disease. N Engl J Med. 2018;378:321–330. doi: 10.1056/NEJMoa1705971. [DOI] [PubMed] [Google Scholar]