Abstract
Background
Exercise mitigates many cardiovascular risk factors associated with atrial fibrillation (AF). Endurance training has been associated with atrial structural changes which can increase the risk for AF. The dose of exercise training required for these changes is uncertain. We sought to evaluate the impact of exercise on left atrial (LA) mechanical and electrical function in healthy, sedentary, middle-aged adults.
Methods and Results
61 adults (52 +/− 5 years) were randomized to either 10 months of high intensity exercise training or yoga. At baseline and post training, all participants underwent maximal exercise stress testing to assess cardiorespiratory fitness, P-wave signal averaged electrocardiography (P-SAECG) for filtered p-wave duration (FPD) and atrial late potentials (RMS20), and echocardiography for LA volume, LV end diastolic volume (LVEDV) and mitral inflow for assessment of LA active emptying. Post-training data were compared to 14 healthy age-matched Masters athletes. LA volume, VO2max and LVEDV increased in the exercise group (15%, 17%, 16%, respectively) with no change in control p<0.0001). LA active emptying decreased post exercise versus controls (5%, p=0.03). No significant changes in FPD or RMS20 occurred after exercise training. LA and LV volumes remained below Masters athletes’. The athletes had longer FPD but no difference in the frequency of atrial arrhythmia.
Conclusions
Changes in LA structure, LA mechanical function, and LV remodeling occurred after 10 months of exercise but without significant change in atrial electrical activity. A longer duration of training may be required to induce electrical changes thought to cause AF in middle aged endurance athletes.
Clinical Trial Registration
https://clinicaltrials.gov; Unique Identifier: NCT02039154
Journal Subject Terms: Atrial Fibrillation, Arrhythmias, Exercise, Risk Factors, Remodeling
Keywords: atrial fibrillation, left atrium, left ventricle, electrocardiography, exercise training, left atrial remodelling, athletes
INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia in the United States. Its prevalence in the general population increases significantly with age; it is as low as 0.1% in adults younger than 55 years of age and as high as 9% after the age of 80.1 While exercise mitigates many of the cardiovascular risk factors for AF such as hypertension, diabetes and obesity, 2 there is growing evidence, 3–5 that participation in long term endurance sports is associated with an increased risk of developing AF in middle aged adults. Notably, a systematic review and meta-analysis of studies evaluating the incidence of AF in athletes found that the overall risk is significantly higher in athletes involved in endurance sports compared to non-athletes 6. In one of the first studies to suggest an increased risk for AF in healthy middle-aged endurance athletes, AF was observed in 5.3% of athletes compared to 0.9% of healthy sedentary aged matched controls.4 Additionally, a cross-sectional study of non-elite runners, stratified according to self-reported number of lifetime training hours, found that the athletes with the most cumulative training time (> 4500 hours) had the highest risk.7 A recent meta- analysis on AF in athletes found that the overall risk is significantly higher by ~5 fold in athletes involved in any type of endurance sport compared to non- athletes 6.
Indeed, endurance athletes with a long history of training have been found to have increased left atrial (LA) volumes, prolonged p-wave durations on signal averaged electrocardiography (P-SAECG), and frequent premature atrial contractions (PACs), which have been associated with an increased risk for the development of AF. 7 However, whether these changes reflect a positive physiologic response for improved athletic performance (which may nevertheless increase the risk of AF) or a pathologic response to a harmful dose of exercise is uncertain. While the risk of AF has been demonstrated to increase in competitive endurance athletes, the dose of exercise that is required to increase the risk of AF is currently unknown.
The purpose of this study was to determine the impact of high-intensity exercise training on LA mechanical and electrical function in healthy, sedentary middle-aged adults. To put these data in context, the longitudinal data acquired before and after training were compared in a cross sectional design to a group of competitive endurance athletes, some of whom had paroxysmal atrial fibrillation. We hypothesized that 10 months of vigorous exercise training would increase cardiorespiratory fitness and result in LA structural and electrical changes different than controls and similar to long term endurance athletes.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects
Sixty-one healthy, middle-aged, sedentary adults aged 45 to 64 years (29 men, 32 women; average age 52.1 ± 5 years) were randomized to either an aerobic exercise interval training program or yoga (active control group). Participants were excluded if they were exercising more than 90 minutes per week or if they had any history of AF. To limit the possibility of silent AF, all participants underwent extended telemetry monitoring for up to 14 days at baseline via a 5″ × 2″ bipolar electrode patch (Zio Patch, iRhythm technologies, CA, USA) 8 which provided a qualitative and quantitative burden of atrial arrhythmias (PACs and AF). Atrial fibrillation was defined as irregularly irregular atrial activity lasting > 30 seconds with the absence of P waves.
All participants signed an informed consent form approved by the institutional review board of the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital Dallas. All participants were screened for comorbidities and were excluded if any of the following conditions were present: hypertension, diabetes mellitus, body mass index ≥ 30 kg/m2, untreated hypo- or hyperthyroidism, obstructive sleep apnea, chronic obstructive pulmonary disease, tobacco use during the past 10 years, coronary artery disease, or structural heart disease. A complete medical history was obtained, and physical examination performed, by a study physician. A 12-lead electrocardiogram (ECG) was obtained for each participant to evaluate for arrhythmias, conduction abnormalities, or evidence of ischemia. All participants were fitted with a 24-hour ambulatory blood pressure (BP) monitor (SunTech Oscar 2, USA) for baseline readings and circadian variability.9 Participants with mean BP > 140/90 mm Hg were excluded.
All measures described below were obtained before and after 10 months of exercise training or yoga.
Exercise Testing
Body composition was measured by underwater weighing. 10, 11 cardiorespiratory fitness (peak V̇O2) was assessed using a modified Astrand-Saltin incremental treadmill protocol. 12 Ventilatory gas exchange was measured using the Douglas bag technique; gas fractions were analyzed by mass spectrometer (Marquette Electronics, model 1100) 13 and ventilatory volume was measured with a 200L Tissot spirometer. Peak V̇O2 was defined as the highest oxygen uptake obtained from at least a 30 second Douglas bag. Cardiac output was measured non-invasively by the acetylene rebreathing method which has been previously validated in healthy participants at rest and during exercise. 14, 15 Blood pressure measurements were obtained on the left arm (Tango, SunTech Medical) and heart rate (HR) and rhythm were assessed with a 12 lead EKG (Mortara, USA). Stroke volume was calculated by dividing cardiac output by HR and the Fick equation was used to calculate the arteriovenous oxygen difference at peak exercise (a-vO2diff = peakV̇O2/Qc). PeakV̇O2 was indexed to both total body and fat free mass. 16
Echocardiography
Assessment of LA and left ventricular (LV) structure and function were obtained using both 2D and 3D echocardiography (IE33 Phillips Medical Systems, Andover, USA). For 3D LA volumes, images were obtained with a matrix array transducer and the dataset was transferred to Q-LAB (Phillips Medical Systems, Andover, USA) for offline analysis.17 Pulsed wave Doppler, positioned at the tips of the mitral valve leaflets with a sample volume of 2.0 mm, was used to assess mitral inflow velocities.18, 19
Left ventricular filling patterns were assessed by mitral inflow velocities where early filling (E wave) and late filling (A wave) peak velocities were recorded. The area under the curve 20 was subsequently traced for the E wave, diastasis time (D) and A wave. The active emptying percentage of the total LA emptying phase21 was calculated as follows:
In the above formula, A represents active emptying during the atrial contractile phase, E represents passive emptying after mitral valve opening and D represents the diastasis time. AUC denotes area under the curve.
Left ventricular end-systolic and end-diastolic volume (LVESV and LVEDV), Left atrial end-systolic and end-diastolic volume (LAESV and LAEDV) were measured using 3D echocardiography, with identification of 5 points at the apex and mitral annulus in the 2- and 4 -chamber views, and analysed using QLAB. 22 The computer generated outline in each view was then reviewed and modified by an experienced analyst to better outline the specified cardiac chamber and to obtain a more accurate measure.
P-wave Signal Average Electrocardiogram (P-SAECG)
Continuous supine 12-lead ECGs were obtained using a Mortara XScribe system (Mortara Instruments, USA) at a 1 kHz sample rate. Digital ECG files were analysed using MATLAB custom software (Mathworks, Natick, MA). QRS detection was performed by template matching algorithm of median QRS complexes generated from a 10-second segment for each of the ECG leads. A segment of the ECG, free of artifact and with stable resting heart rate, was selected for P-wave analysis. An initial average P-wave was calculated for each lead based on QRS triggering. This averaged P-wave was then cross-correlated with each raw P-wave for temporal alignment. A total of 200 P-waves per patient were examined. Individual P-waves that were more than two standard deviations from the mean P-wave were excluded in the final calculation of the averaged P-wave. A blinded operator measured the P-wave duration of the 12-lead averaged P-wave.
Filtered signal-averaged P-wave analysis was done by an orthogonal lead ECG approximation of the averaged 12-lead P-wave derived using the inverse Dower transformation. The XYZ signals were then highpass filtered at 40 Hz then combined into a vector magnitude, ((x2 + y2 + z2)1/2). The filtered P-wave duration (FPD) in milliseconds was then manually determined by a blinded operator and the root mean square voltage of the last 20 milliseconds (RMS20) of the averaged P wave was computed. 23
Exercise Training
Exercise participants trained either at home, at their local gymnasium, or at our hospital based fitness center. The exercise training program was designed to increase in duration and intensity over time. See Table 1 for a detailed summary of the exercise prescription program. Maximal steady state (MSS) was obtained from ventilatory and lactate thresholds as previously described. 24, 25 Based on MSS HR (HRMSS) and peak HR (HRpeak), 4 training zones were established for each participant randomized to the exercise group: MSS, base pace (20 beats below MSS), interval, and recovery. The majority of the training in the early phase was base pace training. As participants acclimated to the training, MSS training was added starting with 2 sessions per week during the second month and increasing to 3 sessions per week from the third month forward. Thirty-minute strength training sessions were added twice per week to compliment aerobic exercise beginning during the second month. From the third month on, aerobic interval training consisting of “4 × 4” interval sessions (4 minutes of exercise at 95% peak HR followed by 3 minutes of active recovery at 60–75% peak HR, repeated four times) were incorporated 26. A recovery day consisting of 20–30 minutes of walking followed each interval day to maximize performance gains. By the sixth month, participants were training 5–6 hours per week including at least 2 aerobic intervals and one long session (at least an hour) each week. This dose of exercise was then maintained for an additional four months after which post training measurements are obtained.
Table 1.
Exercise Prescribed over a 10 month program
| Month | Base Pace | Maximal Steady State | Intervals | Recovery | Strength Training |
|---|---|---|---|---|---|
| 1 | 12@ 30 min | None | None | None | None |
| 2 | 12@ 30 min | 2 @ 30 min | None | None | 8/month |
| 3 | 8 @ 30 min | 4 @ 30 min | 4 sessions of 4 min “on” 3 min “off” | 4 @ 30 min | 8/month |
| 4 | 2 @ 30 min/2 @ 45 min/2 @ 60 min | 4 @ 30 min | 4 sessions of 4 min “on” 3 min “off” | 4 @ 30 min | 8/month |
| 5 | 2 @ 30 min/4 @ 60 min | 4 @ 30 min | 6 sessions of 4 min “on” 3 min “off” | 4 @ 30 min | 8/month |
| 6 to 9 | 4 @ 30 min/4 @ 60 min | None | 8 sessions of 4 min “on” 3 min “off” | 4 @ 30 min | 8/month |
| 10 to 24 | 4 @ 30 min/4 @ 60 min | 2 @ 30 min | 4 sessions of 4 min “on” 3 min “off” | 4 @ 30 min | 8/month |
Base pace: 1–20bpm < MSSzone, MSS: VTHR ±5bpm, ~ 85–90% of HRmax, Intervals: ≥ 95% of PeakHR, Recovery: < Base Pace)
Yoga
Yoga participants performed one hour of yoga at least 3 times per week either at home using an instructional video or in a group class in the community. All participants were assigned an exercise physiologist who provided close follow-up and monitored training at least twice per month at our institute or at their place of training. All participants also kept logs of their training activities and wore HR monitors (Polar, Kempele, Finland) to record exercise duration, training HR, and to document adherence to the exercise prescription.
Endurance Athletes
A group of competitive endurance athletes considered at risk for training-induced AF was added in a cross-sectional study design to examine the long-term effects of endurance exercise on cardiac structure, function, and atrial arrhythmias, and to provide context for the amount of exercise training typically associated with AF in endurance athletes. Fourteen middle-aged competitive athletes were enrolled (10 men and 4 women; average age 52 ± 4 years, minimum training duration of 10 years). Four athletes had a history of paroxysmal AF, but all were in sinus rhythm at the time of the study. Athletes with AF were excluded only if they had a history of catheter ablation or were taking anti-arrhythmic drugs at the time of enrolment. Inclusion criteria for the endurance athletes were (i) participation in competitive swimming, cycling, marathon running, or triathlons for a minimum of 5 years, (ii) participation in at least 2 competitive events per year, (iii) current active training for a competitive event.
As in the longitudinal study, body composition, exercise testing, echocardiography, P-SAECG, and extended ambulatory telemetry monitoring were performed on all participants.
Statistical Analysis
Data were analysed using GraphPad Prism 7 for Windows. Data were expressed as means ± SD unless stated otherwise. Student’s T-test was used to detect differences after 10 months of exercise training as compared to yoga. A two-way ANOVA was used to examine within-group and between-group differences only when there was a significant group by time interaction. A P value < 0.05 was considered statistically significant.
For the cross sectional comparison, a one-way ANOVA with post hoc testing (i.e. Bonferroni for homogenous variables or Dunnett T3 for non-homogenous variables) was used to detect differences between the athlete, exercise, and yoga groups. Kruskal Wallis test was used to assess differences in the frequency of PACs between the athletes and longitudinal study participants prior to commencement of training; ambulatory telemetry monitoring was only available at this time point. Relationships between variables were assessed with Pearson Correlation coefficients.
RESULTS
Participant Demographics
Table 2 shows baseline participant characteristics. Fifty-seven of 61 participants completed the 10 month training. One participant dropped out of the yoga program and 3 participants dropped out of the exercise training program (one man because of time constraints, a second man due to injuries not related to the study, and one woman dropped out before the start of training). Data were analysed for 57 participants in the longitudinal comparison (n= 26 for yoga, n=31 for exercise). The adherence rate to the 10 month exercise training program was 86%. The exercise, yoga, and athlete groups were well-matched for age and body mass index. As expected, the athletes had a significantly lower percentage of body fat compared to sedentary participants.
Table 2.
Baseline Characteristics
| Characteristics | Yoga Group | Exercise Group | Athletes |
|---|---|---|---|
| n | 27 | 34 | 14 |
| Age, years | 51 ± 6 | 53 ± 5 | 52 ± 4 |
| Men | 13 | 16 | 11 |
| Women | 14 | 18 | 3 |
| Height, cm | 169.5 ± 9.5 | 169.5 ± 10 | 174.8 ± 8.2 |
| Weight, kg | 75.6 ± 14.9 | 75 ± 14 | 76 ± 10.4 |
| BMI, kg/m2 | 26.1 ± 3.5 | 25.9 ± 3 | 24.7 ± 2.3 |
| Body Fat, % | 32.9 ± 7.2* | 32.2 ± 7* | 27 ± 4 |
| Lean Body Mass, kg | 51 ± 11 | 51 ± 11 | 55 ± 9.9 |
| SBP, mmHg | 123 ± 8 | 119 ± 7* | 127 ± 9 |
| DBP, mmHg | 74 ± 6 | 72 ± 5 | 72 ± 6 |
| MAP, mmHg | 90 ± 6 | 87 ± 5 | 90 ± 6 |
| Pulse Pressure, mmHg | 49 ± 5 | 48 ± 6 | 54 ± 7 |
| PACs/24 hours | 14 ± 13 | 30 ± 46 | 66 ± 127 |
| VO2 Max, mL/kg/min | 30 ± 4.9* | 29 ± 4.7* | 50 ± 4.5 |
P value < 0.05 vs. Athletes
Body composition measures derived by Hydrostatic testing
Atrial arrhythmias
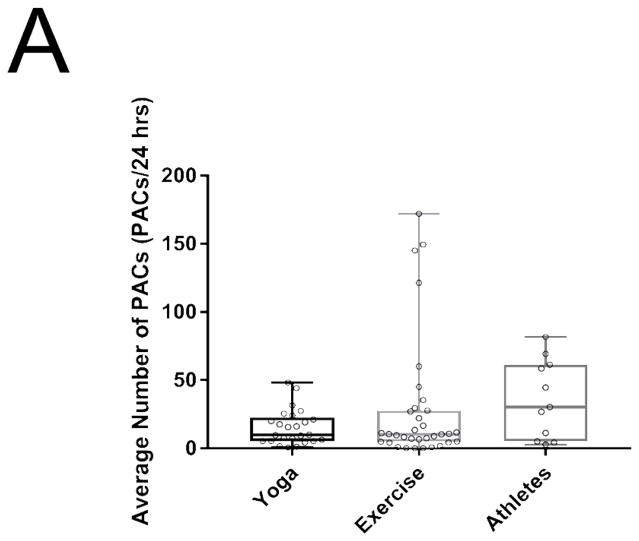
Athletes appeared to have an increased burden of PACs per 24 hours compared to the baseline sedentary adults though there was substantial variability in PAC burden so that this difference did not achieve levels of statistical significance (sedentary adults 24 ± 37 vs athletes 71 ± 125 PACs/24Hr, respectively; P = 0.21 ; Figure 1A). In athletes with a history of AF there was no significant difference in frequency of PACs per 24 hrs compared to those athletes without a history of AF. However, when excluding an outlier athlete with a 10 fold higher PAC burden than remaining athletes, there was a less compellingly higher burden of PACs in athletes with AF compared to those without (55 ± 5 vs 29 ± 11 PACs/24Hr, respectively; P = 0.67).
Figure 1.
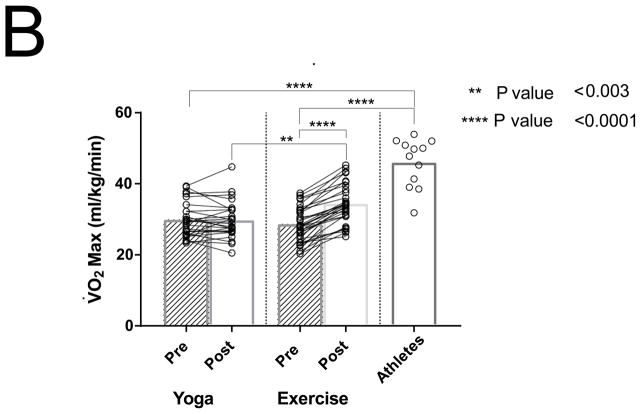
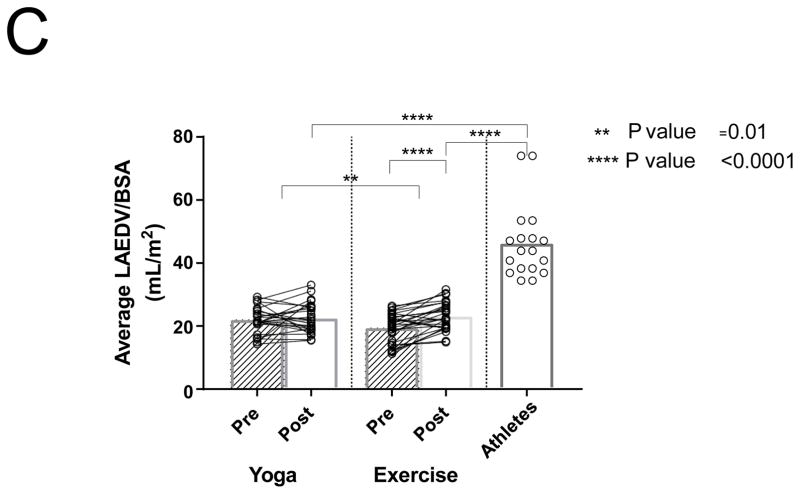
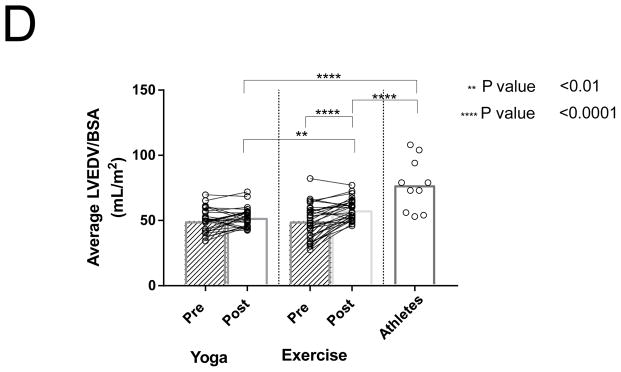
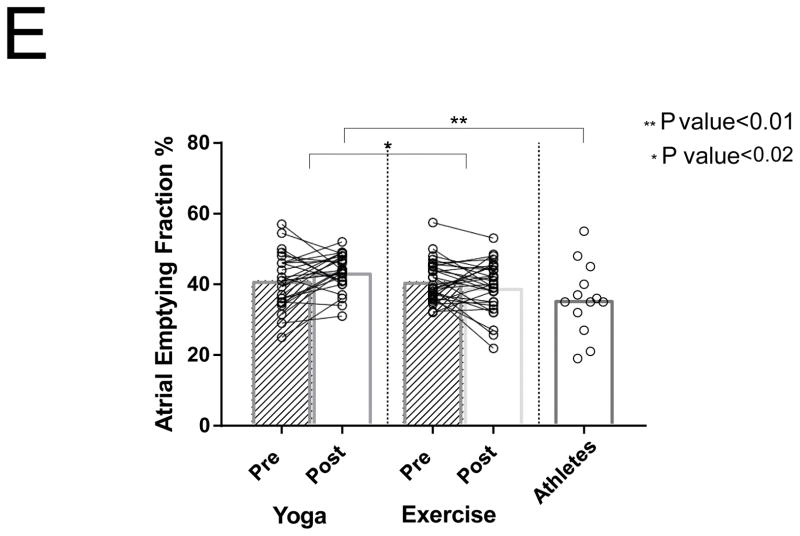
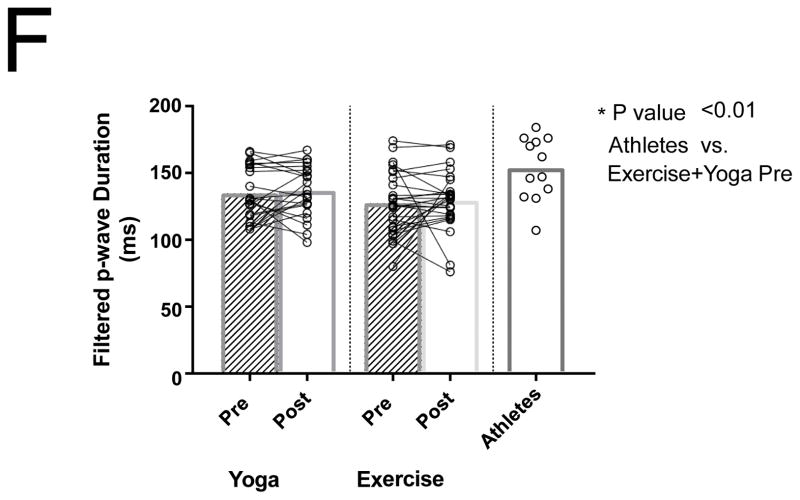
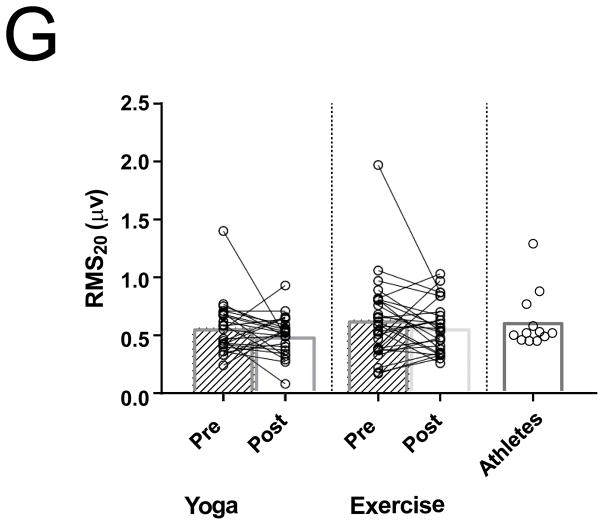
Differences between PACs/24Hrs in combined yoga/exercise pre and athletes (A). Differences between the three groups with changes in individual values Pre and Post Yoga and Exercise training for (B) V̇O2 max, (C) LA volume index, (D) LV end-diastolic volume index, (E) LA active contribution to LV diastolic filling, (F) Filtered P- wave duration, (G) RMS20 Pre and Post values shown for Yoga and Exercise training groups.
Cardiorespiratory Fitness
Ten months of aerobic exercise training significantly increased peak V̇O2 by 20% (P < 0.0001) without a change in the yoga group (P = 0.77; Figure 1B). Despite this marked improvement, the peak V̇O2 in the subjects after training remained substantially below that of the competitive athletes (P< 0.0001).
Echocardiography
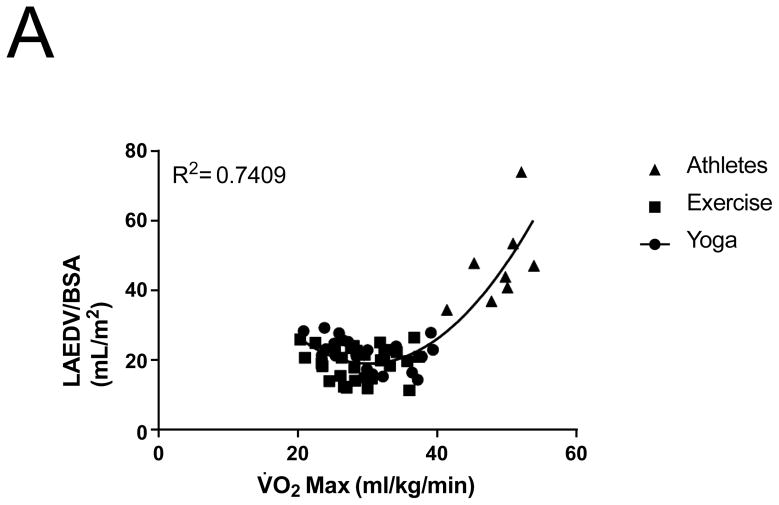
3D Echocardiographic data was unavailable or of insufficient quality to measure volumes in 4 patients in the yoga group, and 4 patients in the exercise group. Maximal LA volume indexed to body surface area (BSA) increased 18.5% in the exercise group, with no changes noted after yoga training (19.5 ± 4.6 to 23.1 ± 4.5 ml/m2 vs yoga 21.8 ± 4.4 to 22.6 ± 4.8 ml/m2, P <0.0001; P interaction = 0.03; Figure 1C). The athletes had significantly higher LA volumes compared to both post-yoga and post-exercise training groups (46.3 ± 12 vs to 22.6 ± 4.8 vs 23.1 ± 4.5ml/m2 P < 0.0001, P interaction = 0.03; Figure 1C). Left atrial volume correlated with V̇O2 max for all subjects with a curvilinear relationship (Figure 2A). LA ejection fraction increased by 7.6% in the exercise group (50.4% ± 11.4 to 58.0% ± 10.9, P=0.005) with no change noted following yoga training (53.2% ± 8.6 to 52.8% ± 6.4, P=0.91).
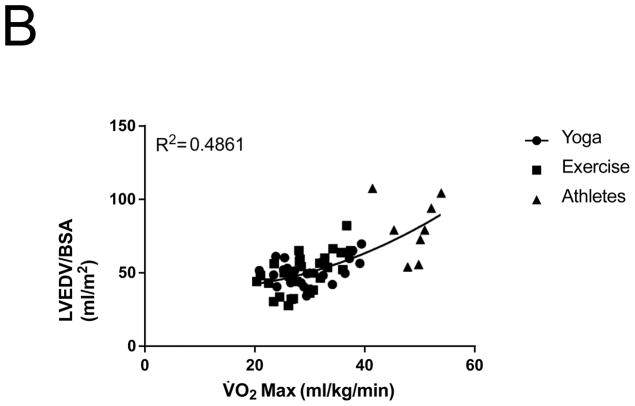
Figure 2.
Relationship between V̇O2 max and (A) LA Volume index, (B) LV end-diastolic volume index
Similar to changes noted in LA volumes, LVEDV indexed to BSA increased after exercise training by 16% (P=0.0001; Figure 1D). Athletes had significantly larger indexed LVEDVs compared to the post-exercise and post-yoga groups (P < 0.0001; Figure 1D). Indexed LVEDV also correlated with V̇O2 max for all subjects (Figure 2B).
After exercise training there was an 5% reduction in the contribution of the LA contractile phase to diastole, compared to no significant change after yoga training (41 ± 5.8 to 39 ± 7 % vs 41 ± 7.6 to 43 ± 5%, respectively; P = 0.03) (Figure 1E). Athletes had significantly lower LA active emptying percentages compared to post-intervention groups (P < 0.01; Figure 1E).
P-SAECG
Filtered p-wave duration did not change significantly after exercise or yoga training (P = 0.99 Figure 1F). There was also no significant change in RMS20 after exercise or yoga training (P = 0.97; Figure 1G). The athlete group had a measured FPD that was significantly longer than the combined baseline sedentary adults (154 ± 24 vs 133 ± 21, P < 0.01; Figure 1F). The measured RMS20 in the athlete group, however, was not different than the baseline sedentary adults (Figure 1G). There was no significant difference in FPD or RMS20 in the athletes with AF compared to those without AF.
DISCUSSION
The primary findings from this study were: a) Ten months of exercise training incorporating regular aerobic intervals in sedentary, healthy, middle aged adults that was sufficient to improve cardiorespiratory fitness led to commensurate left atrial and left ventricular remodeling; b) Despite participation in training regimens designed to be similar to those practiced by endurance athletes shown to be at risk for atrial fibrillation, the degree of cardiac remodeling remained considerably below that observed in well trained, age matched endurance athletes; c) There was no detectable change in LA electrical function after 10 months of exercise training; d) Both FPD and RMS20 in endurance athletes were not significantly different from the exercise group. Collectively these data suggest that 10 months of intensive aerobic exercise training changes LA structural and mechanical function without a concomitant change in LA electrical function.
The apparent increased risk of AF in athletes presents a paradox. For example, the benefits of exercise in preventing cardiovascular risk factors for AF such as HTN, diabetes and obesity are well known 27. But there is growing evidence that long term endurance training is associated with an increased risk for AF in otherwise healthy adults especially by middle age. 3,4, 28–32 It has been proposed that LA enlargement, LV hypertrophy, increased vagal tone, chronic inflammation, and fibrosis, factors that encompass the “athlete’s heart”, contribute to the development of atrial arrhythmias in otherwise healthy adults. 31, 33–36 However, the studies that have examined this idea are retrospective, and cannot separate cause from effect.
Effects of High Intensity Aerobic Exercise Training on Left Atrial Structure and Mechanical Function
As expected, aerobic exercise training with high intensity intervals increased V̇O2 max substantially in these healthy but previously sedentary middle aged adults.37 This increase in aerobic power was accompanied by a balanced increase in LA and LV volumes consistent with the eccentric hypertrophy seen in a similar high intensity training program in younger individuals 25.
LA dilatation is well documented in long term endurance athletes as a physiological adaptation to exercise and is an independent risk factor for AF 7, 33, 38, 39. Although there are a number of studies examining the change in LV morphology longitudinally with training, [45, 40, 41 the data regarding longitudinal changes in LA morphology with an exercise program are limited. In the present study, we used 3DE to most accurately assess atrial remodeling as it is less prone to variability given its automated border detection system, lower test-retest variability, and close agreement in accuracy with cardiac magnetic resonance imaging.42, 43.
We observed an increased maximal LA volume of 18.5% following a 10-month exercise program. Long term enlargement of the LA is due to intermittent hemodynamic stretch of the myocardium due to increased pressure and volume load during exercise 44. As described elegantly by La Gerche, during exercise, the time the heart spends in diastole is reduced by half, meaning that the AV valves are closed for a proportionally longer period of time. This results in an increase in atrial pressure and contractility which leads to more atrial deformation during intense exercise to meet the requirement of increased circulatory flow 44. An increase in total blood volume and LA active filling, combined with increased LA pressure due to the proportionally longer time the AV valves are closed lead to increased LA distension during exercise and atrial remodeling. Left atrial dilatation without concomitant wall thickening may lead to increased wall stress and a decrease in active emptying percentage. Several studies suggest a dose-response effect with more cumulative years of training associated with increasing LA volumes 7, 29, 39. The finding of higher LA volumes in our triathlete group with an average training duration of 10 years supports this idea.
Of particular interest regarding the observed and well documented LA enlargement in endurance athletes is the “dose” (duration and intensity) of training required for the structural remodeling to begin. A study of already competitive adolescent soccer players enrolled in an 8 month training program demonstrated a LA volume increase of 8.95 ± 4.47 mL/m2.45 We observed an increase of 3.6 ± 3.7 mL/m2 in previously sedentary middle aged adults with much lower baseline cardiorespiratory fitness following 10 months of exercise. Our study therefore suggests that LA enlargement is an early adaptation to endurance training, influenced both by duration and intensity of training.
In this study, LA ejection fraction was noted to increase in response to exercise, likely as a function of an increased maximal LA volume (reservoir function) without a change in minimal LA volume. However, there was also a significant change in the conduit and contractile phases of LA emptying. In the present study, exercise training resulted in augmented LA passive emptying (conduit phase) and decreased reliance on active emptying (contractile phase). These findings are in agreement with other studies that endurance training results in decreased LA active emptying, likely due to diastolic suction. 21 More importantly, we also demonstrate that 10 months of aerobic exercise with high intensity intervals is sufficient to decrease LA active emptying similar to long term endurance competitive athletes. Improved LA compliance and LV relaxation are factors known to contribute to decreased LA contractile contribution.43 LV diastolic compliance is also a determinant of LA contractile function. Although LV diastolic compliance was not directly assessed in this study, previous studies in our laboratory have demonstrated that masters athletes have LV diastolic compliance similar to the young.46 LV morphologic changes associated with exercise begin with concentric hypertrophy followed by eccentric hypertrophy, with significant changes in LVEDV being observed at around 6 months after the start of exercise training.25 The enlarged LV in endurance athletes is also more compliant and therefore able to adapt to the increased preload associated with endurance exercise.47 Our findings of decreased contribution of active LA emptying during diastole suggest a balanced atrioventricular remodeling due to endurance training that influences both the LA reservoir function (LA volume) and the emptying function.
Left Atrial Electrical Function after Exercise Training
Despite the LA enlargement, filtered p-wave duration did not increase with exercise training contrary to our hypothesis. After 10 months of aerobic exercise training there was no difference in FPD between the exercise and yoga group before and after training.
Altered LA substrate with the formation of LA fibrosis leads to intra-atrial conduction delay with subsequent prolongation of FPD 48. In addition, autonomic remodeling with increased vagal tone has been shown to begin early after the initiation of a moderate endurance training program. 49 Increased vagal tone due to long term endurance training is associated with frequent PACs, which may then trigger atrial fibrillation in some athletes. There seems to be a threshold in cumulative training hours in order for changes in LA electrical function to be manifested. A recent study found a U-shaped dose response between cumulative lifetime endurance training hours and the risk for AF (>2000 hrs associated with increased risk), though LA electrical function was not evaluated.50 A study on marathon runners found that cumulative lifetime training hours > 4500 hrs is associated with a significantly increased vagal tone, prolonged FPD and more frequent PACs not evident in lesser cumulative training hours. 7 Compared to our study, these endurance athletes had a longer mean duration of training (22 years).
It is therefore possible that while our exercise training intensity and duration of 10 months was sufficient to initiate a process of autonomic remodeling, it may have been below the threshold necessary for the manifestation of increased atrial ectopy and alteration of LA substrate evident by a prolonged FPD.
STUDY LIMITATIONS
Our study had a number of limitations. First, the small sample size of endurance athletes may have limited our ability to detect changes in atrial ectopy associated with long term endurance training. Previous studies have reported prolonged filtered p-wave duration and frequent PACs in endurance athletes. While our study did show a significantly increased FPD between athletes and baseline sedentary adults, there was only a trend towards significance for increased PAC burden in athletes compared to baseline sedentary adults, and no difference between athletes with and without AF. However, we were only able to recruit 14 athletes, and just 4 athletes with atrial fibrillation as part of the study. We powered our study based on a recent study evaluating LA volumes, P-SAECG and frequency of PACs in middle aged long term marathon runners.7 We estimated that in order to detect a 6 ml/m2 difference in LA volume between the previously sedentary cohort and the endurance athletes, with a standard deviation of 6 ml (type 1 error of 0.05, power 0.80), we needed to study 10 middle aged endurance athletes. In our present study, we did in fact find a significant difference in LA volume between the previously sedentary adults and the endurance athletes.
Second, in our inclusion criteria we recruited endurance athletes with a minimum of 5 years of endurance sports training and competition. Increased PAC burden may require a longer duration of cumulative training years prior to manifestation; additionally, frequent PACs may not occur in all endurance athletes due to influence from other factors, including genetics.
Third, the population of our endurance athletes was a heterogeneous mixture. A larger sample size of athletes participating only in marathon running, cycling, or swimming might have improved the study’s ability to detect changes PAC burden. However, this population did include a significant number or triathletes with a high volume of training, including swimming, which may increase LA pressure from water immersion, thereby maximizing LA remodeling.
CONCLUSIONS
Ten months of aerobic exercise training with high intensity aerobic intervals causes changes in left atrial structure and function in previously sedentary middle aged adults. The contribution of left atrial active emptying in diastole decreases after 10 months of exercise training, similar to that of long term endurance athletes, and is coexistent with the development of left ventricular remodeling. Despite changes in left atrial structure and mechanical function after only 10 months of exercise training, there were no clear changes in atrial electrophysiology; a longer duration or dose of training may be required to induce left atrial electrical changes thought to be causative of the increased risk of atrial fibrillation in endurance athletes.
Supplementary Material
What is known?
Exercise mitigates many cardiovascular risk factors for atrial fibrillation such as hypertension, diabetes mellitus, and obesity.
There is however an increasing amount of literature suggesting an association between endurance training and an increased risk for atrial fibrillation.
What the study adds?
Very few prospective studies employing an aerobic exercise training program with high intensity aerobic intervals have been done to assess the progressive LA structural, mechanical and electrical changes that lead to atrial fibrillation associated with endurance training.
Regular high intensity aerobic exercise may lead to LA structural and mechanical changes such as increased LA volumes during the 10-months study period, but it may take longer duration of high intensity endurance training to develop LA remodeling and electrical changes that increase the risk for AF in athletes with structurally normal hearts
Acknowledgments
Special gratitude to Mitchel Samels, MS, Braden Everding, MS and Kara Boyd, MS for training the participants and assisting with testing, Jamie Kowal for her strong efforts in recruiting the athletes, and to Sheryl Livingston, MS, RN for providing excellent nursing care to the participants during testing.
Sources of Funding:
This study was supported by the National Institutes of Health grants 3R01AG017479 and 3R01AG017479-11S1
Footnotes
Disclosures: None
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. Jama. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Pathak RK, Elliott A, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Hendriks JM, Twomey D, Kalman JM, Abhayaratna WP. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: The CARDIO-FIT study. Journal of the American College of Cardiology. 2015;66:985–996. doi: 10.1016/j.jacc.2015.06.488. [DOI] [PubMed] [Google Scholar]
- 3.Mont L, Tamborero D, Elosua R, Molina I, Coll-Vinent B, Sitges M, Vidal B, Scalise A, Tejeira A, Berruezo A. Physical activity, height, and left atrial size are independent risk factors for lone atrial fibrillation in middle-aged healthy individuals. Europace. 2008;10:15–20. doi: 10.1093/europace/eum263. [DOI] [PubMed] [Google Scholar]
- 4.Karjalainen J, Kujala UM, Kaprio J, Sarna S, Viitasalo M. Lone atrial fibrillation in vigorously exercising middle aged men: case-control study. Bmj. 1998;316:1784–1785. doi: 10.1136/bmj.316.7147.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molina L, Mont L, Marrugat J, Berruezo A, Brugada J, Bruguera J, Rebato C, Elosua R. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: a follow-up study. Europace. 2008;10:618–623. doi: 10.1093/europace/eun071. [DOI] [PubMed] [Google Scholar]
- 6.Abdulla J, Nielsen JR. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace. 2009;11:1156–1159. doi: 10.1093/europace/eup197. [DOI] [PubMed] [Google Scholar]
- 7.Wilhelm M, Roten L, Tanner H, Wilhelm I, Schmid J-P, Saner H. Atrial remodeling, autonomic tone, and lifetime training hours in nonelite athletes. The American journal of cardiology. 2011;108:580–585. doi: 10.1016/j.amjcard.2011.03.086. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg MA, Samuel M, Thosani A, Zimetbaum PJ. Use of a noninvasive continuous monitoring device in the management of atrial fibrillation: a pilot study. Pacing and Clinical Electrophysiology. 2013;36:328–333. doi: 10.1111/pace.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. New England Journal of Medicine. 2006;354:2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- 10.Roche AF, Heymsfield SB, Lohman TG. Human body composition. Human Kinetics Publishers; 1996. [Google Scholar]
- 11.Carrick-Ranson G, Hastings JL, Bhella PS, Fujimoto N, Shibata S, Palmer MD, Boyd K, Livingston S, Dijk E, Levine BD. The effect of lifelong exercise dose on cardiovascular function during exercise. Journal of Applied Physiology. 2014;116:736–745. doi: 10.1152/japplphysiol.00342.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okazaki K, Iwasaki K-i, Prasad A, Palmer MD, Martini ER, Fu Q, Arbab-Zadeh A, Zhang R, Levine BD. Dose-response relationship of endurance training for autonomic circulatory control in healthy seniors. Journal of Applied Physiology. 2005;99:1041–1049. doi: 10.1152/japplphysiol.00085.2005. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122:1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. Journal of Applied Physiology. 2007;103:867–874. doi: 10.1152/japplphysiol.01106.2006. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Menold E, Dullenkopf A, Reissnecker S, Lormes W, Lehmann M, Steinacker J. Validation of the acetylene rebreathing method for measurement of cardiac output at rest and during high-intensity exercise. Clinical Physiology. 1997;17:171–182. doi: 10.1046/j.1365-2281.1997.02323.x. [DOI] [PubMed] [Google Scholar]
- 16.Goran M, Fields D, Hunter G, Herd S, Weinsier R. Total body fat does not influence maximal aerobic capacity. International journal of obesity. 2000;24:841–848. doi: 10.1038/sj.ijo.0801241. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins C, Bricknell K, Marwick TH. Use of real-time three-dimensional echocardiography to measure left atrial volume: comparison with other echocardiographic techniques. Journal of the American Society of Echocardiography. 2005;18:991–997. doi: 10.1016/j.echo.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Cohen GI, Pietrolungo JF, Thomas JD, Klein AL. A practical guide to assessment of ventricular diastolic function using Doppler echocardiography. Journal of the American College of Cardiology. 1996;27:1753–1760. doi: 10.1016/0735-1097(96)00088-5. [DOI] [PubMed] [Google Scholar]
- 19.Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. The American journal of cardiology. 2007;99:1629–1636. doi: 10.1016/j.amjcard.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. The New England journal of medicine. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 21.Toutouzas K, Trikas A, Pitsavos C, Barbetseas J, Androulakis A, Stefanadis C, Toutouzas P. Echocardiographic features of left atrium in elite male athletes. The American journal of cardiology. 1996;78:1314–1317. doi: 10.1016/s0002-9149(96)00622-4. [DOI] [PubMed] [Google Scholar]
- 22.Dorosz JL, Lezotte DC, Weitzenkamp DA, Allen LA, Salcedo EE. Performance of 3-dimensional echocardiography in measuring left ventricular volumes and ejection fraction: a systematic review and meta-analysis. Journal of the American College of Cardiology. 2012;59:1799–1808. doi: 10.1016/j.jacc.2012.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukunami M, Yamada T, Ohmori M, Kumagai K, Umemoto K, Sakai A, Kondoh N, Minamino T, Hoki N. Detection of patients at risk for paroxysmal atrial fibrillation during sinus rhythm by P wave-triggered signal-averaged electrocardiogram. Circulation. 1991;83:162–169. doi: 10.1161/01.cir.83.1.162. [DOI] [PubMed] [Google Scholar]
- 24.Anderson G, Rhodes E. A review of blood lactate and ventilatory methods of detecting transition thresholds. Sports Medicine. 1989;8:43–55. doi: 10.2165/00007256-198908010-00005. [DOI] [PubMed] [Google Scholar]
- 25.Arbab-Zadeh A, Perhonen M, Howden E, Peshock RM, Zhang R, Adams-Huet B, Haykowsky MJ, Levine BD. Cardiac Remodeling in Response to 1 Year of Intensive Endurance Training. Circulation. 2014;130:2152–61. doi: 10.1161/CIRCULATIONAHA.114.010775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonsen T, Helgesen C, Hjorth N, Bach R, Hoff J. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39:665671. doi: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- 27.Thompson PD, Buchner D, Piña IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 28.Lindsay MM, Dunn FG. Biochemical evidence of myocardial fibrosis in veteran endurance athletes. British journal of sports medicine. 2007;41:447–52. doi: 10.1136/bjsm.2006.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoogsteen J, Schep G, van Hemel NM, van der Wall EE. Paroxysmal atrial fibrillation in male endurance athletes. A 9-year follow up. Europace. 2004;6:222–228. doi: 10.1016/j.eupc.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Grimsmo J, Grundvold I, Maehlum S, Arnesen H. High prevalence of atrial fibrillation in long-term endurance cross-country skiers: echocardiographic findings and possible predictors—a 28–30 years follow-up study. European Journal of Cardiovascular Prevention & Rehabilitation. 2010;17:100–105. doi: 10.1097/HJR.0b013e32833226be. [DOI] [PubMed] [Google Scholar]
- 31.Benito B, Gay-Jordi G, Serrano-Mollar A, Guasch E, Shi Y, Tardif J-C, Brugada J, Nattel S, Mont L. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation. 2011;123:13–22. doi: 10.1161/CIRCULATIONAHA.110.938282. [DOI] [PubMed] [Google Scholar]
- 32.Elosua R, Arquer A, Mont L, Sambola A, Molina L, García-Morán E, Brugada J, Marrugat J. Sport practice and the risk of lone atrial fibrillation: a case–control study. International journal of cardiology. 2006;108:332–337. doi: 10.1016/j.ijcard.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Pelliccia A, Maron BJ, Di Paolo FM, Biffi A, Quattrini FM, Pisicchio C, Roselli A, Caselli S, Culasso F. Prevalence and clinical significance of left atrial remodeling in competitive athletes. Journal of the American College of Cardiology. 2005;46:690–696. doi: 10.1016/j.jacc.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 34.Weiner RB, Baggish AL. Exercise-induced cardiac remodeling. Progress in cardiovascular diseases. 2012;54:380–386. doi: 10.1016/j.pcad.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Baggish AL, Wood MJ. Athlete’s heart and cardiovascular care of the athlete scientific and clinical update. Circulation. 2011;123:2723–2735. doi: 10.1161/CIRCULATIONAHA.110.981571. [DOI] [PubMed] [Google Scholar]
- 36.Eijsvogels TM, Fernandez AB, Thompson PD. Are There Deleterious Cardiac Effects of Acute and Chronic Endurance Exercise? Physiological reviews. 2016;96:99–125. doi: 10.1152/physrev.00029.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bacon AP, Carter RE, Ogle EA, Joyner MJ. VO2max trainability and high intensity interval training in humans: A meta-analysis. PloS one. 2013;8:e73182. doi: 10.1371/journal.pone.0073182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Andrea A, Riegler L, Cocchia R, Scarafile R, Salerno G, Gravino R, Golia E, Vriz O, Citro R, Limongelli G. Left atrial volume index in highly trained athletes. American heart journal. 2010;159:1155–1161. doi: 10.1016/j.ahj.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 39.Brugger N, Krause R, Carlen F, Rimensberger C, Hille R, Steck H, Wilhelm M, Seiler C. Effect of lifetime endurance training on left atrial mechanical function and on the risk of atrial fibrillation. International journal of cardiology. 2014;170:419–425. doi: 10.1016/j.ijcard.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 40.Spence AL, Naylor LH, Carter HH, Buck CL, Dembo L, Murray CP, Watson P, Oxborough D, George KP, Green DJ. A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. The Journal of physiology. 2011;589:5443–5452. doi: 10.1113/jphysiol.2011.217125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiner RB, DeLuca JR, Wang F, Lin J, Wasfy MM, Berkstresser B, Stöhr E, Shave R, Lewis GD, Hutter AM. Exercise-Induced Left Ventricular Remodeling Among Competitive Athletes A Phasic Phenomenon. Circulation: Cardiovascular Imaging. 2015;8:e003651. doi: 10.1161/CIRCIMAGING.115.003651. [DOI] [PubMed] [Google Scholar]
- 42.Anwar AM, Geleijnse ML, Soliman OI, Nemes A, ten Cate FJ. Left atrial Frank–Starling law assessed by real-time, three-dimensional echocardiographic left atrial volume changes. Heart. 2007;93:1393–1397. doi: 10.1136/hrt.2006.099366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.To AC, Flamm SD, Marwick TH, Klein AL. Clinical utility of multimodality LA imaging: assessment of size, function, and structure. JACC: Cardiovascular Imaging. 2011;4:788–798. doi: 10.1016/j.jcmg.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 44.La Gerche A, Claessen G. Increased Flow, Dam Walls, and Upstream Pressure: The Physiological Challenges and Atrial Consequences of Intense Exercise. JACC Cardiovascular imaging. 2016;9:1389–1391. doi: 10.1016/j.jcmg.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 45.D’Ascenzi F, Cameli M, Lisi M, Zacà V, Natali B, Malandrino A, Benincasa S, Catanese S, Causarano A, Mondillo S. Left atrial remodeling in competitive adolescent soccer players. International journal of sports medicine. 2012;33:795. doi: 10.1055/s-0032-1304660. [DOI] [PubMed] [Google Scholar]
- 46.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 47.Levine B, Lane L, Buckey J, Friedman D, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation. 1991;84:1016–1023. doi: 10.1161/01.cir.84.3.1016. [DOI] [PubMed] [Google Scholar]
- 48.Okumura Y, Watanabe I, Ohkubo K, Ashino S, Kofune M, Hashimoto K, Shindo A, Sugimura H, Nakai T, Kasamaki Y. Prediction of the Efficacy of Pulmonary Vein Isolation for the Treatment of Atrial Fibrillation by the Signal-Averaged P-Wave Duration. Pacing and clinical electrophysiology. 2007;30:304–313. doi: 10.1111/j.1540-8159.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- 49.Iwasaki K-i, Zhang R, Zuckerman JH, Levine BD. Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults: how much training for what benefit? Journal of Applied Physiology. 2003;95:1575–1583. doi: 10.1152/japplphysiol.00482.2003. [DOI] [PubMed] [Google Scholar]
- 50.Calvo N, Ramos P, Montserrat S, Guasch E, Coll-Vinent B, Domenech M, Bisbal F, Hevia S, Vidorreta S, Borras R. Emerging risk factors and the dose–response relationship between physical activity and lone atrial fibrillation: a prospective case–control study. Europace. 2015:euv216. doi: 10.1093/europace/euv216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.