Abstract
Background
Patients with low-density lipoprotein cholesterol (LDL-C) ≥190mg/dL are at high-risk of atherosclerotic cardiovascular disease (ASCVD) events. Treatment guidelines recommend intensive treatment in these patients. Variation in the use of lipid-lowering therapies (LLT) in these patients in a national sample of cardiology practices is not known.
Methods and Results
Using data from the American College of Cardiology’s NCDR® PINNACLE® registry, we assessed the proportion of patients with LDL-C ≥190mg/dL (n=49,447) receiving statin, high-intensity statin, LLT associated with ≥50% LDL-C lowering, ezetimibe or a PCSK9 inhibitor between January 2013 and December 2016. We assessed practice-level rates and variation in LLT use using median rate ratio (MRR) adjusted for patient and practice characteristics. MRR represent the likelihood that 2 random practices would differ in treatment of “identical” patients with LDL-C ≥190mg/dL. The proportion of patients receiving a statin, high-intensity statin, LLT associated with ≥50% LDL-C reduction, ezetimibe, or PCSK9 inhibitor were 58.5%, 31.9%, 34.6%, 8.5%, and 1.5%, respectively. Median practice-level rates and adjusted MRR for statin (56% [IQR= 47.3%–64.8%]; MRR 1.20 [95% CI 1.17–1.23]), high-intensity statin (30.2% [IQR= 12.1%–41.1%]; MRR 2.31, [95% CI = 2.12–2.51]), LLT with ≥50% LDL-C lowering (31.8% [IQR= 15.3%–45.5%]; MRR 2.12 (95% CI = 1.95–2.28)]; ezetimibe (5.8% [IQR= 2.8%–9.8%]; MRR 2.42 [2.21–2.63]), and PCSK9 inhibitors (0.16% [0–1.9%], MRR 2.38 [2.04–2.72]) indicated significant gaps and >200 percent variation in receipt of several of these medications for patients across practices. Among those without concomitant ASCVD, even larger treatment gaps were noted (proportion of patients on a statin, high-intensity statin, LLT with ≥50% LDL-C reduction, ezetimibe or PCSK9 inhibitor were 50.8%, 25.25%, 26.8%, 4.9%, and 0.74%, respectively).
Conclusion
Evidence-based LLT use remains low among patients with elevated LDL-C with significant variation in care. System-level interventions are needed to address these gaps and reduce variation in care of these high-risk patients.
Keywords: elevated LDL-C, statin, high-intensity statin, quality of care, variation in care
Journal Subject Terms: Lipids and Cholesterol, Cardiovascular Disease, Quality and Outcomes
Introduction
The Adult Treatment Panel (ATP III) cholesterol guideline from 20011 and the 2013 American College of Cardiology (ACC)\ American Heart Association (AHA) guideline2 recommend intensive treatment of patients with primary elevation of low-density lipoprotein cholesterol (LDL-C). This is based on the finding that patients with LDL-C levels ≥ 190 mg/dL have a very-high lifetime risk of atherosclerotic cardiovascular disease (ASCVD) events.3,4 Recent studies have shown that although the prevalence of genetic mutations associated with familial hypercholesterolemia (FH) is low among patients with LDL-C ≥190 mg/dL, the risk of future ASCVD events remains high in these patients even in the absence of FH defining mutations.4 Studies have shown that the risk of coronary heart disease (CHD) is accelerated by 10 to 20 years in men and 20 to 30 years in women with LDL-C levels ≥190 mg/dL.3 This increased risk can be mitigated by early identification and treatment of these patients. Statin therapy has been shown to significantly lower future risk of ASCVD events in these patients.5–7 The 2013 ACC/AHA cholesterol treatment guideline therefore identified LDL-C ≥190 mg/dL as one of the 4 statin benefit groups.2 In these patients, the guidelines recommend consideration for early initiation of high-intensity statin therapy.2,8 Non-statin lipid lowering therapies including ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have also been shown to lower LDL-C levels in these patients.9,10
Recent studies have shown large treatment gaps in the care of patients with LDL-C ≥ 190 mg/dL.11–13 These studies were performed either outside the United States12 or included patients seeking care in specialty lipid clinics within the United States.11 In addition, the use of non-statin lipid lowering medications (e.g. ezetimibe or PCSK9 inhibitors) in these patients has not been formally evaluated. Another aspect of quality in the care of patients with LDL-C ≥ 190 mg/dL is to ensure that the prescription of evidence-based lipid lowering medications does not vary based on where a patient is receiving care (i.e. care for this high-risk group should be uniform). To our knowledge, no study has evaluated the use of evidence-based lipid lowering therapies among these patients seeking care in a nationally representative sample of cardiology practices in the United States. Similarly, practice-level variation in the care of these patients is not known. Using data from the National Cardiovascular Data Registry (NCDR®) Practice Innovation and Clinical Excellence (PINNACLE) Registry®, we assessed the frequency and practice-level variation in the use of evidence-based lipid lowering therapies among patients with LDL-C ≥ 190 mg/dL seeking care in cardiology practices across the United States.
Methods
Cohort Development
The data, methods used in the analysis, and materials used to conduct the research will not be made available to any researcher for purposes of reproducing the results or replicating the procedure. We utilized data from the NCDR® PINNACLE® registry. PINNACLE® is the first and the largest prospective U.S. based outpatient cardiac quality improvement registry with voluntary participation from both academic and non-academic practices (predominantly cardiology practices) throughout the United States.14,15 Data in the registry are directly extracted using algorithms mapped to data in the electronic health records. Quality control is ensured using a standard data collection tool with written definitions, uniform data entry and transmission requirements, and data quality checks.15,16
Study Population
For the current analyses, we first identified patients seeking outpatient cardiology care in the PINNACLE® registry from January 1, 2013 to December 31, 2016 with available LDL-C levels (n= 2,463,535). Among these patients, we identified 49,447 patients with any LDL-C ≥ 190 mg/dL, which represents the 98th percentile among all available LDL-C levels in the PINNACLE registry. These 49,447 patients were seeking care in 476 practices across the United States.
Because we wanted to assess both no-treatment and under-treatment of these patients, we evaluated the use of statin therapy, high-intensity statin therapy, lipid lowering therapy associated with ≥ 50% LDL-C reduction, ezetimibe therapy, or PCSK9 inhibitors (alirocumab or evolocumab) among patients with LDL-C ≥ 190 mg/dL. High-intensity statin therapy was defined per the 2013 ACC/AHA cholesterol guideline2 as the use of statin therapy which would on average lower LDL-C levels by ≥ 50%. Given that prior studies show that the use of LDL-C lowering ≥ 50% serves as a good indicator of future ASCVD outcomes among treated patients17 and can be used as an indicator of quality of care in patients with elevated LDL-C12, we also evaluated any combination of lipid lowering therapy which would be associated with at least a 50% LDL-C reduction as described in a previous study.12 These included the use of a high-intensity statin (with or without ezetimibe or a PCSK9 inhibitor), use of a moderate-intensity statin plus ezetimibe, or the use of a PCSK9 inhibitor with or without a statin or ezetimibe. Since patients could have multiple outpatient cardiology visits during the study interval, documentation of the use of a lipid lowering therapy (statin, ezetimibe or PCSK9 inhibitor) at any encounter was taken as evidence of the use of these medications. To further account for dose-dependent statin intolerance, we used the highest documented intensity of statin therapy at any encounter during the study interval to calculate the intensity of statin therapy.
Study Outcomes and Analyses
We first assessed the proportion of patients with LDL-C ≥ 190 mg/dL on statin therapy and on high-intensity statin therapy. We also assessed the proportion of these patients receiving lipid lowering therapy associated with ≥ 50% LDL-C reduction, ezetimibe or a PCSK9 inhibitor.
We compared several baseline characteristics among patients receiving statin therapy and those not receiving statin therapy using the Chi-square statistic or Student’s t-test for categorical and continuous variables, respectively. Similar analyses were performed to compare patients receiving high-intensity statin versus those not receiving high-intensity statin therapy. Baseline characteristics included patient’s age, sex, race, insurance type, history of hypertension, diabetes, current smoking, coronary artery disease (CAD), myocardial infarction (MI), percutaneous coronary intervention (PCI), peripheral artery disease (PAD), stroke, or transient ischemic attack (TIA).
We then calculated median practice-level rates and interquartile range (IQR) for the use of statin therapy, high-intensity statin therapy, lipid lowering therapy associated with ≥ 50% LDL-C reduction, ezetimibe, or a PCSK9 inhibitor. To calculate this, the number of patients on each of these therapies in a practice was used as the numerator and the total number of patients with LDL-C ≥ 190 mg/dL in a practice was used as the denominator. We first examined these rates across practice using descriptive plots. To further examine and quantify the extent of practice-level variation in statin use, we constructed multivariable hierarchical regression models to determine the median rate ratio (MRR), which is a well-described measure to assess variation in care.14,18–20 These were 2-level hierarchical models to adjust for clustering of patients within practices, with the individual practices modeled as a random effect and patient characteristics as filter effects within each practice.21 This approach allowed us to control for measured confounding between practices, as the use of hierarchical models ensured that patients with similar baseline characteristics were compared with each other. We first calculated unadjusted MRR. We then adjusted for patient characteristics (age, sex, insurance status [private vs. public vs. others], history of hypertension, diabetes, current smoking, history of CAD, stroke or PAD) followed by adjustment for practice-level variables (geographical location of the practice where the patient is seeking care and the number of years that the practice has been participating in the PINNACLE® registry) to assess if there was any attenuation in MRRs following these adjustments. The calculated MRR can be interpreted as the likelihood that 2 random practices would differ in treatment of “identical” patients. For example, a MRR of 1 suggests no practice-level variation and similar treatment at two random practices for identical patients with LDL-C ≥ 190mg/dL, while a MRR of 1.50 suggests 50% probability of differing treatment for identical patients with LDL-C ≥ 190mg/dL between two random practices. Based on prior studies, MRRs of >1.20 are generally considered to indicate significant practice-level variation.14,18–20 Similar analyses were performed for high-intensity statin use, use of lipid lowering therapy associated with ≥ 50% LDL-C reduction, ezetimibe use, and PCSK9 inhibitor use.
We also performed several sensitivity analyses. These included evaluating the proportion of patients aged <50 years with LDL-C ≥ 190mg/dL who were on a statin, high-intensity statin, lipid lowering therapy associated with ≥50% LDL-C reduction, ezetimibe and PCSK9 inhibitor. We also evaluated these results after excluding patients with LDL-C ≥ 190 mg/dL with concomitant ASCVD (history of CAD, PAD, stroke or TIA). Analyses were performed using SAS version 9.3 (SAS Institute, Cary, North Carolina). All p values described are two-sided. A p value <0.05 was considered significant. The protocol was approved by the Institutional Review Boards at Baylor College of Medicine and the Michael E. DeBakey VA Medical Center.
Results
Our final study cohort included 49,447 patients with LDL-C levels ≥ 190 mg/dL. The distribution of LDL-C levels in these patients was right skewed (Figure 1) with mean (SD) and median (IQR) LDL-C levels of 214 (24.5) and 205 (196–222) mg/dL, respectively. The number (%) of patients with LDL-C levels between 190–219 mg/dL, 220–249 mg/dL, 250–279 mg/dL, 280–309 mg/dL, and ≥310 mg/dL were 35,699 (72.2%), 9243 (18.7%), 2689 (5.4%), 1053 (2.1%), and 763 (1.5%), respectively. 11.8% of the patients were receiving care in practices located in the West region, 20.8% in practices located in the Midwest, 16.75% in practices located in the Northeast, and 50.65% in practices located in the South.
Figure 1.
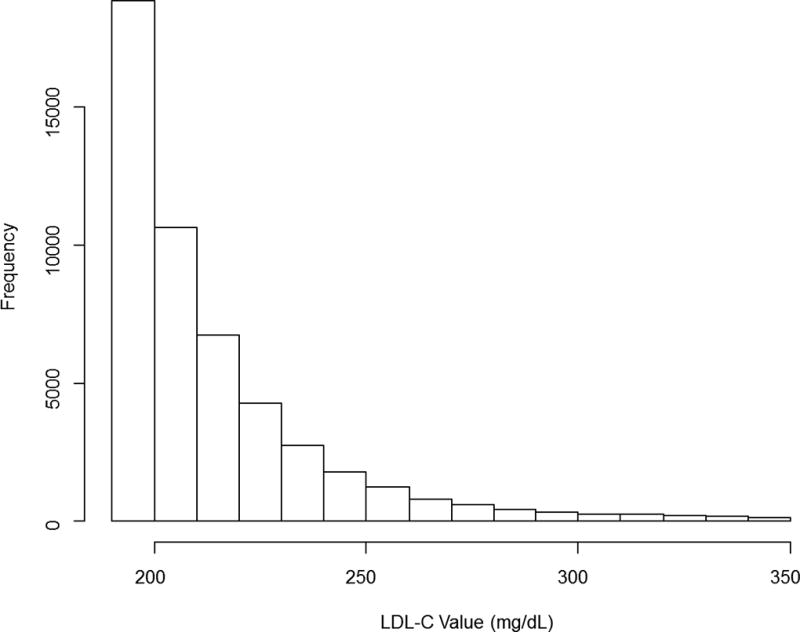
Distribution of LDL-C values among those with LDL-C ≥ 190mg/dL
In this cohort of 49,447 patients, the number of patients (percentage) receiving statin therapy, high-intensity statin therapy, lipid lowering therapy associated with ≥ 50% LDL-C reduction, ezetimibe, or a PCSK9 inhibitor were 28,950 (58.5%), 15,791 (31.9%), 17,094 (34.6%), 4194 (8.5%), and 732 (1.5%), respectively. Patients receiving statin therapy compared to those not receiving statins (Table 1) were slightly younger, more likely to be males, current smokers, more likely to be receiving care in practices in the West region and less likely to be Caucasian or receiving care in practices in South region. Patients receiving statins also had a higher prevalence of CAD, diabetes mellitus, hypertension, chronic kidney disease, MI, PCI, stroke, or TIA compared to those not receiving statin therapy. Patients receiving statins had minimally higher LDL-C levels (215 mg/dL) compared to those not receiving statin therapy (212 mg/dL) and were also more likely to receive ezetimibe or either alirocumab or evolocumab. Qualitatively similar results were seen when comparing patients receiving high-intensity statin therapy versus those not receiving high-intensity statin therapy (Table 2).
Table 1.
Baseline characteristics of patients with LDL-C ≥ 190mg/dL receiving statin therapy compared to those not receiving statin therapy
| Patients receiving statin therapy (n= 28,950) |
Patients not receiving statin therapy (n=20,497) |
p-value | |
|---|---|---|---|
| Age, y, mean/SD | 62.2/12.6 | 63.4/14.0 | <.0001 |
| Male sex, n (%) | 11,271 (39.0%) | 6,415 (31.3%) | <.0001 |
| Race, n (%) | <.0001 | ||
| White | 16,962 (58.6%) | 13,523 (66.0%) | |
| Black | 2,397 (8.3%) | 1,300 (6.4%) | |
| Other | 9,575 (33.1%) | 5,665 (27.6%) | |
| Practice Region | <0.001 | ||
| Midwest region | 6,106 (21.1%) | 4,150 (20.26%) | |
| Northeast region | 4,777 (16.51%) | 3,500 (17.08%) | |
| South region | 14,285 (49.37%) | 10,750 (52.47%) | |
| West region | 3,766 (13.02%) | 2,088 (10.19%) | |
| Coronary artery disease, n (%) | 13,164 (45.5%) | 6,709 (32.7%) | <.0001 |
| Diabetes mellitus, n (%) | 6,700 (23.1%) | 3,666 (17.9%) | <.0001 |
| Hypertension, n (%) | 20,158 (69.6%) | 13,124 (64.0%) | <.0001 |
| Current smoking, n (%) | 6,293 (22.4%) | 3,829 (19.5%) | <.001 |
| Peripheral artery disease, n (%) | 2,539 (8.8%) | 1,270 (6.2%) | <.0001 |
| Chronic kidney disease, n (%) | 1,276 (4.4%) | 728 (3.6%) | <.0001 |
| Myocardial infarction, n (%) | 3,865 (13.4%) | 1,736 (8.5%) | <.0001 |
| PCI, n (%) | 5,163 (17.8%) | 2,451 (12.0%) | <.0001 |
| Stroke, n (%) | 3,052 (10.5%) | 1,625 (7.9%) | <.0001 |
| Low density lipoprotein (LDL) cholesterol (mg/dL), mean/SD | 215.2/27.4 | 212.4/25.0 | <.0001 |
| Patients on alirocumab, n (%) | 144 (0.5%) | 140 (0.7%) | 0.003 |
| Patients on evolocumab, n (%) | 218 (0.8%) | 230 (1.1%) | <.0001 |
| Patients on ezetimibe, n (%) | 3,070 (10.6%) | 1,124 (5.5%) | <.0001 |
PCI = percutaneous coronary intervention, SD = standard deviation
Table 2.
Baseline characteristics of patients with LDL-C ≥ 190 mg/dL receiving high-intensity statin therapy compared to those not receiving high-intensity statin therapy
| Patients receiving high-intensity statin therapy (n= 15,791) |
Patients not receiving high-intensity statin therapy (n=33,656) |
p-value | |
|---|---|---|---|
| Age, y, mean/SD | 62.0/12.5 | 63.0/13.5 | <.0001 |
| Male sex, n (%) | 6,441 (40.8%) | 11,245 (33.4%) | <.0001 |
| Race, n (%) | <.0001 | ||
| White | 9,079 (57.5%) | 21,406 (63.6%) | |
| Black | 1,293 (8.2%) | 2404 (7.2%) | |
| Other | 5,410 (34.3%) | 9,830 (29.2%) | |
| Practice Region | <0.001 | ||
| Midwest region | 3,022 (19.15%) | 7,234 (21.5%) | |
| Northeast region | 3,003 (19.03%) | 5,274 (15.68%) | |
| South region | 7,599 (48.15%) | 17,436 (51.83%) | |
| West region | 2,158 (13.67%) | 3,696 (10.99%) | |
| Insurance type, n (%) | <0.001 | ||
| Private | 8,827 (55.9%) | 17,015 (50.55%) | |
| Public | 2,279 (14.45%) | 5,248 (15.6%) | |
| None | 82 (0.5%) | 352 (1.05%) | |
| Others/unknown | 4,603 (29.15%) | 11,040 (32.8%) | |
| Coronary artery disease, n (%) | 7,521 (47.6%) | 12,352 (36.7%) | <.0001 |
| Diabetes mellitus, n (%) | 4030 (25.5%) | 6,336 (18.8%) | <.0001 |
| Hypertension, n (%) | 11,034 (69.9%) | 22,248 (66.1%) | <.0001 |
| Current smoking, n (%) | 3,521 (22.8%) | 6,601 (20.5%) | <.001 |
| Peripheral artery disease, n (%) | 1619 (10.3%) | 2,190 (6.5%) | <.0001 |
| Chronic kidney disease, n (%) | 843 (5.3%) | 1161 (3.5%) | <.0001 |
| Myocardial infarction, n (%) | 2,137 (13.5%) | 3,464 (10.3%) | <.0001 |
| PCI, n (%) | 3,669 (23.2%) | 3,945 (11.7%) | <.0001 |
| Stroke, n (%) | 2,113 (13.4%) | 2,564 (7.6%) | <.0001 |
| Low density lipoprotein (LDL) cholesterol (mg/dL), mean/SD | 215.4/27.4 | 213.3/26.0 | <.0001 |
| Patients on alirocumab, n (%) | 115 (0.7%) | 169 (0.5%) | 0.004 |
| Patients on evolocumab, n (%) | 167 (1.1%) | 281 (0.8%) | 0.031 |
| Patients on ezetimibe, n (%) | 2,193 (13.9%) | 2001 (6.0%) | <.0001 |
PCI = percutaneous coronary intervention, SD = standard deviation
Practice-level variation in statin and high-intensity statin therapy use
Median practice-level rates (IQR) for statin therapy (Table 3, Figure 2) were 56% (IQR=47.3%–64.8%). As noted in Figure 2, there was significant practice-level variation in the proportion of patients with LDL-C ≥ 190 mg/dL receiving statin therapy varying from just above 10% of patients in some practices to >90% of patients in others. Unadjusted MRR for statin therapy use was 1.24, which was minimally attenuated in a model adjusting for patient and practice-level variables (adjusted MRR 1.20, 95% CI 1.17–1.23).
Table 3.
Practice-level rates of lipid-lowering therapy use and its variation among patients with LDL-C ≥ 190 mg/dL
| Medication Class |
Practice-level rates of Use of a medication class Median (IQR) |
MRR (95% CI) (unadjusted) |
MRR (95% CI) (adjusted for patient characteristics)* |
MRR (95% CI) (adjusted for patient and practice characteristics)† |
|---|---|---|---|---|
| Any statin | 56% (47.3%–64.8%) | 1.24 (1.21–1.27) | 1.22 (1.19–1.24) | 1.20 (1.17–1.23) |
| High-intensity statin therapy | 30.2% (12.1%–41.1%) | 2.51 (2.29–2.73) | 2.34 (2.15–2.54) | 2.31 (2.12–2.51) |
| Lipid lowering therapy associated with ≥50% LDL-C reduction | 31.8% (15.3%–45.5%) | 2.29 (2.11–2.47) | 2.21 (2.04–2.38) | 2.12 (1.95–2.28) |
| Ezetimibe therapy | 5.8% (2.8%–9.8%) | 2.56 (2.34–2.78) | 2.42 (2.22–2.62) | 2.42 (2.21–2.63) |
| PCSK9 inhibitors | 0.16% (0–1.9%) | 2.76 (2.37–3.14) | 2.56 (2.12–2.79) | 2.38 (2.04–2.72) |
IQR = interquartile range, MRR= median rate ratio, CI = confidence interval, PCSK9 = proprotein convertase subtilisin/kexin
Adjusted for patient’s age, sex, insurance status [private vs. public, vs. others], history of hypertension, diabetes, current smoking, history of coronary artery disease, stroke, or peripheral artery disease
Adjusted for patient characteristics plus adjusted for practice-level characteristics (geographical location of the practice where patient is seeking care and the number of years that the practice is participating in the PINNACLE® registry)
Figure 2.
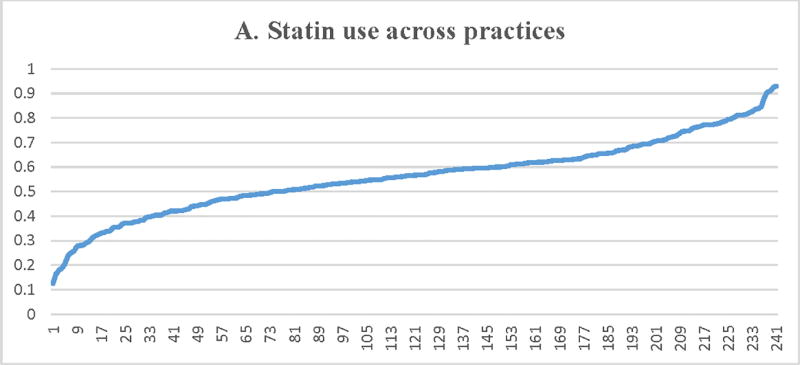
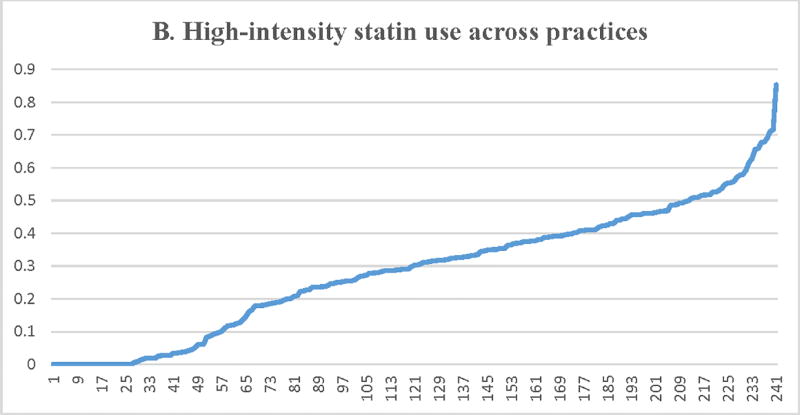
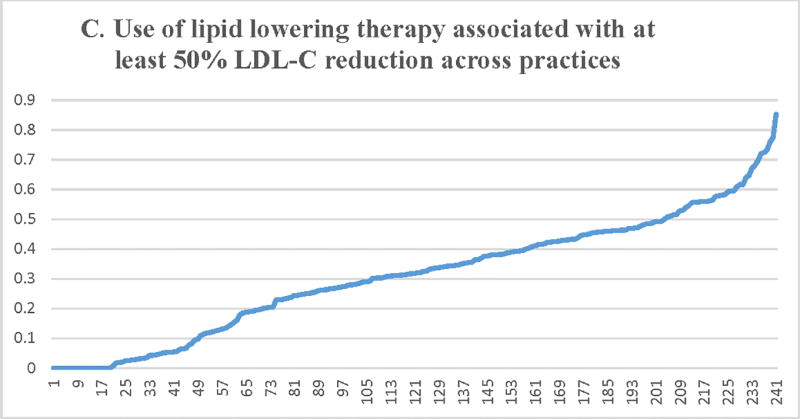
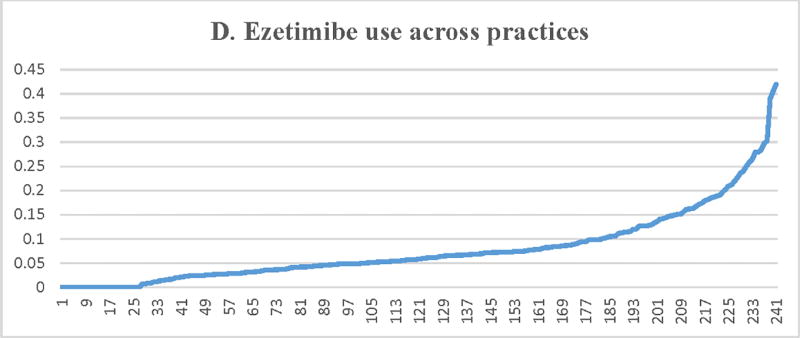
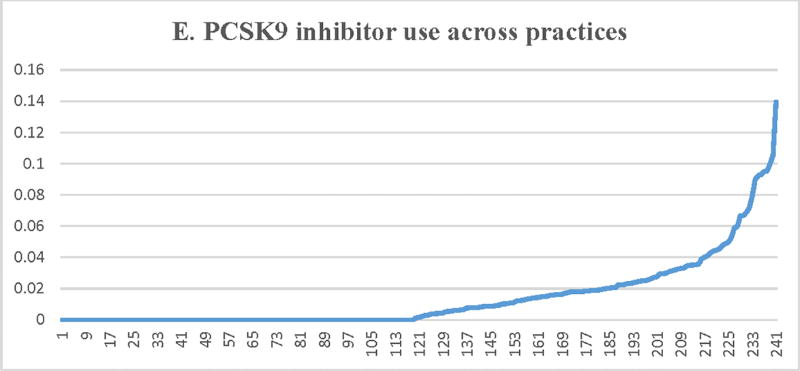
Proportion of patients with LDL-C ≥ 190 mg/dL receiving a statin (A) or a high-intensity statin (B), lipid lowering therapy associated with ≥50% LDL-C reduction (C), ezetimibe (D), or a PCSK9 inhibitor (E) across participating practices*. X axis = random practice ID, Y axis = proportion of patients with LDL-C ≥190 mg/dL in a practice receiving statin therapy (A), high-intensity statin therapy (B), lipid lowering therapy associated with ≥50% LDL-C reduction (C), ezetimibe (D), or a PCSK9 inhibitor (E). *Includes practices with at least 30 patients with LDL-C ≥ 190 mg/dL
Median practice-level rates (IQR) for high-intensity statin use were 30.2% (IQR= 12.1%–41.1%) (Table 3 and Figure 2). There was substantial practice-level variation in high-intensity statin therapy use with several practices having less than 10% of patients on high-intensity statin therapy and a few practices having more than 50% of patients on high-intensity statin therapy. There was substantial practice-level variation in the use of high-intensity statin therapy (unadjusted MRR = 2.51, 95% CI 2.29–2.73), which was modestly attenuated after adjusting for patient and practice-level characteristics (adjusted MRR = 2.31, 95% CI = 2.12–2.51). These results indicate large practice-level variation in high-intensity statin therapy use between two similar patients with LDL-C ≥ 190 mg/dL treated at 2 random practices with one patient 131% more likely to receive a high-intensity statin compared to another patient with similar characteristics.
Practice-level variation in the use of lipid lowering therapy associated with ≥ 50% LDL-C reduction
Median practice-level rates for the use of lipid lowering therapy associated with ≥ 50% LDL-C reduction (Table 3, Figure 2) were 31.8% (IQR= 15.3%–45.5%). There was substantial practice-level variation. The fully adjusted MRR across practices was 2.12 (95% CI = 1.95–2.28). History of hypertension, diabetes, current smoking, CAD, ischemic stroke, or PAD were associated with a higher likelihood of receiving lipid lowering therapy associated with ≥ 50% LDL-C reduction. Increasing age of the patient, female sex, and location of the practice in the Midwest or South (compared to the West region) were associated with a lower likelihood of receiving lipid lowering therapy associated with ≥ 50% LDL-C reduction (Supplemental Table 1). Patient’s type of insurance or the number of years of participation by the practice in PINNACLE® registry were not significantly associated with receipt of lipid lowering therapy regimen associated with ≥ 50% LDL-C reduction.
Practice-level variation in the use non-statin therapies
Median (IQR) practice-level rates for ezetimibe and PCSK9 inhibitor use was 5.8% (2.8%–9.8%) and 0.16% (0–1.9%), respectively. As noted (Table 3, Figure 2), there was significant practice-level variation in the use of ezetimibe and PCSK9 inhibitors among these patients, with minimal impact of patient and practice-level characteristics (adjusted MRR [95% CI] = 2.42 [2.21–2.63] for ezetimibe therapy use and 2.38 [2.04–2.72] for PCSK9 inhibitor use).
Sensitivity analyses
Among patients <50 years of age with LDL-C ≥ 190 mg/dL (n=7,456), the proportion on a statin, high-intensity statin, lipid lowering therapy regimen associated with ≥50% LDL-C reduction, ezetimibe and PCSK9 inhibitor were 58.1%, 31.6%, 33.4%, 4.96%, and 1.08%, respectively.
Among patients (n=21,091) without any history of CAD, PAD, stroke or TIA, the use of lipid lowering therapies was even lower (proportion of patients on a statin, high-intensity statin, lipid lowering regimen associated with ≥50% LDL-C reduction, ezetimibe or PCSK9 inhibitor were 50.8%, 25.25%, 26.8%, 4.9%, and 0.74%, respectively).
We also evaluated quarterly time trends in the use of ezetimibe or PCSK9 inhibitors in our study cohort (Supplemental Figures 1,2). As seen, the rate for the use of ezetimibe in our study cohort gradually increased throughout the study interval but have plateaued around 10–11% since the last quarter of 2015. For PCSK9 inhibitors, there was a steady increase in their use from the third quarter of 2015 (0.34%) to their highest level in the second quarter of 2016 (3.39% of the patients) but have since stabilized.
Discussion
In these analyses from a national registry of outpatients receiving care in cardiology practices across the United States, we found that roughly 4 in 10 patients with LDL-C ≥ 190 mg/dL were not on statin therapy and less than one-third were on high-intensity statin therapy. Our results also show a 20% and 131% variation in the use of statin and high-intensity statin therapy among two similar patients with LDL-C ≥ 190 mg/dl receiving care at two random practices. Despite their high LDL-C levels, the use of non-statin therapies (ezetimibe or PCSK9 inhibitors) was low. Although treatment guidelines recommend at least a 50% reduction in LDL-C levels as the first step in these high-risk patients,2 only 35% percent of the patients were on a lipid lowering regimen which would lead to at least a 50% LDL-C reduction. These results indicate that a lack of treatment, under treatment, and substantial variation in care independent of patient characteristics are common amongst these patients.
Our results are broadly consistent with results shown in a registry of 1295 adults with heterozygous FH from 11 lipid clinics in the United States.11 In that study, roughly 25% were not receiving statin therapy and 42% were receiving high-intensity statin therapy. Only 41% of the patients achieved ≥ 50% LDL-C reduction. The lower number of patients on statin or high-intensity statin therapy in our study compared to this study can be explained by differences in practices enrolled in the study (specialized lipid clinics versus general cardiology practices). To our knowledge, this is the first study evaluating the quality of cholesterol care in this high-risk patient population among a national sample of cardiology practices. Similar results have also been described in a registry of 4132 patients in Spain.12
Studies have shown that patients with LDL-C ≥ 190 mg/dL (even in the absence of FH defining mutations) have a very-high lifetime risk of ASCVD.3,4 In the Cardiovascular Lifetime Risk Pooling Project, individuals with LDL-C ≥ 190 mg/dL had up to 5 times higher risk of CHD at 30 years of follow-up compared to those with LDL-C <130 mg/dL.3 It is important to note that although relative risk was higher with higher LDL-C levels in younger individuals, the absolute risk still remains low due to low event rates when compared to those with established ASCVD.22 Conversely, CHD risk was accelerated by 10 to 20 years in men and 20 to 30 years in women with LDL-C ≥190 mg/dL and therefore, elevated LDL-C levels in this population represent a modifiable risk factor. For example, the event rate of 2.6 events (CHD death or nonfatal MI) per 1000-person years of follow-up among 20–29 year-old men was comparable to an event rate of 1.5 per 1000 person-years of follow-up among men 30–39 years of age with LDL-C <130 mg/dL and 4.0 events per 1000-person years of follow-up among 40–49 year old men with LDL-C <130 mg/dL. Similarly, while the risk of CHD has been described as 22-fold higher among patients with FH defining mutations (likely due to high lifetime exposure to elevated LDL-C levels), the future risk of CHD remains 6-fold higher among those with LDL-C ≥ 190 mg/dL and no FH related mutations.4 Recent studies have also shown that as opposed to prevalence of heterozygous FH of 1 in 250, prevalence of LDL-C ≥ 190 mg/dL may be as high as 5–7% in the general population and therefore, the population attributable risk associated with this phenotype (and its undertreatment) is very high.3,13
Although the exact reasons for the undertreatment of these high-risk patients (treatment-risk paradox) are not well known, our prior national surveys might explain some reasons for clinical inertia23,24 in the treatment of these patients.25,26 These national surveys, which included both cardiologists and internal medicine providers, showed that 52% of the providers would not perform a risk discussion regarding possible initiation of statin therapy in patients with LDL-C ≥190 mg/dL but would rather continue with lifestyle modification alone, perform a 10 year ASCVD risk estimation, perform a coronary calcium score or carotid intima media thickness, or reassure the patient and repeat cholesterol levels in 5 years. In the same survey, only 29% of the providers knew the definition of low, moderate and high-intensity statin therapy.25 These results indicate that providers may not think of patients with LDL-C ≥ 190 mg/dL as high-risk. In addition to explaining why such a large proportion of these patients are not on statin therapy, it may also explain why the intensity of lipid lowering therapy is low in these patients. These gaps at the level of individual providers may also explain why there is a significant residual variation in care even after adjustments are made for a large number of patient and practice-level variables. It is important to note that patients referred to cardiology practices are inherently high-risk due to referral bias, as these patients are already deemed to have signs and symptoms suggestive of ASCVD. This is evident from the fact that roughly 40% of the patients in our cohort already had concomitant CAD, 8% had PAD, 10–15% had history of stroke or TIA and 21% had diabetes, which would make use of statin and moderate to high-intensity statin therapy a Class I indication even without elevated LDL-C levels. In the absence of these comorbid conditions, treatment gaps are even worse, as shown in our sensitivity analyses. A much larger treatment gap and a more significant magnitude of practice-level variation for the use of high-intensity statin therapy, LLT with ≥ 50% LDL-C reduction and non-statins compared with statin use indicates that although clinicians may agree on the need for lipid lowering therapy in these patients, the intensity of therapy that is employed is not uniform. Lastly, some patients with LDL-C ≥190mg/dL could also not perceive themselves as high-risk. These patient perceptions may not be very well accounted for by variables used in our adjustment model.
Our results also show significant practice-level variation in the use of statin, high-intensity statin therapy, lipid lowering therapy associated with ≥ 50% LDL-C reduction, and non-statin therapy use among these patients. These results indicate that there is heterogeneity among practices in the treatment of patients with LDL-C ≥190 mg/dL with some practices more successful in treating these patients with evidence-based medications. Differences in patient-related factors (or tolerance to statin therapy) across practices could also account for some of these differences. Attenuation of some practice-level variation after adjustment for patient characteristics but persistence of significant practice-level variation indicates that efforts to address these gaps should target both clinicians and patients.
What can be done to address these gaps and reduce variation in the care of this high-risk group? An Institute of Medicine (IOM) report noted that it takes 17 years for knowledge to be implemented in routine clinical practice.27 This time-lag between generation of knowledge and its implementation in routine clinical practice is unacceptable. Prior studies have shown that passive diffusion of knowledge via guideline publication may not suffice. These efforts will need to be supplemented with active interventions like audit and feedback to the providers on quality of care that is delivered to these high-risk patients28 and patient-centered interventions such as mutual goal setting. These tools should provide cognitive support to providers at the point-of-care to first identify these high-risk patients, prompt them to perform high quality risk discussion to help patients understand the importance of treating such high LDL-C levels, tools to initiate evidence-based lipid lowering therapies, and tools to encourage providers to perform lipid testing on other first-degree relatives of the identified patients. This must be done with careful review of existing workflows, garnering participation and buy-in by the clinical users, and using human factors best practices, as clinicians are notably over-burdened with information, and studies have shown clinical reminder burn-out that results in ignoring the decision support.29,30 Several recent initiatives by the FH foundation (creation of CASCADE registry,11 deployment of advanced informatics to identify FH patients, provider and patient resources) and the ACC NCDR® PINNACLE registry (dashboards for practices on their performance in the use of evidence-based lipid lowering therapies in patients with LDL-C ≥ 190 mg/dL) should further assist in improving the care of these high-risk patients.
Our study has limitations. These results represent whether a statin, high-intensity statin or a non-statin therapy was prescribed to the patient. Given the limitations of data captured in the PINNACLE® registry, we are not able to comment on whether patients were adherent to their prescribed medications. We also do not have data on family history of premature ASCVD or family history of elevated LDL-C levels, which would allow us to determine how many of these patients fulfill clinical criteria for FH. Also, given that the patient population in PINNACLE® mostly reflects a referred population, baseline pre-treatment LDL-C are generally not available for the cohort of patients studied. To overcome this issue, we assessed any value of LDL-C during the study interval as well as the highest intensity of statin therapy noted on any encounter during that time. Of course, this approach will exclude some patients as a function of the intensity of their treatment but will tend to underestimate the degree of undertreatment we report. For non-statin therapies, especially PCSK9 inhibitors, formulary restrictions and difficulties obtaining prior approval could have contributed to the low number of patients on this therapy. It is possible that the low number of patients prescribed statin therapy could represent patient refusal to accept statin therapy after risk discussion or patient intolerance to statin therapy. Our post-hoc analyses show that among those not on a statin, only 152 (0.7%) patients refused a statin as documented by the providers. Although partial or complete statin intolerance could explain some of our results, a large prior registry of 7924 patients showed the prevalence of muscular symptoms to be close to 10.5% in statin treated patients.31 Recent studies have also shown that many of these “statin intolerant” patients can tolerate some form of statin therapy.32,33 Our results (Table 1 and 2) also show that on average, the use of non-statin therapies was higher among patients receiving statin or high-intensity statin therapy. If statin intolerance were to be responsible for this treatment gap, then the proportion of patients receiving non-statin therapies (especially ezetimibe due to its easy availability) should have been higher among patients not receiving a statin or a high-intensity statin. Some patients with high LDL-C in our cohort could have a secondary cause of hyperlipidemia that we could not account for such as severe hypothyroidism, nephrotic syndrome or cholestasis. Although this is possible, prevalence of these disorders in a population referred to outpatient cardiology practices is likely low with at least some patients with these secondary lipid disorders still requiring lipid lowering therapy. Finally, our results represent quality of care among practices participating in the PINNACLE® registry. By participating in a quality improvement registry, these practices are likely different compared to other cardiology practices and therefore, treatment gaps could be worse in average cardiology practices across the United States.
Conclusion
Significant treatment gaps persist in evidence-based lipid lowering therapy use among patients with elevated LDL-C levels in a national sample of cardiology practices. Similarly, there is a significant practice-level variation in the use of evidence-based lipid lowering therapies in these patients. System-level interventions are needed to address these gaps and to reduce variation in care for this high-risk group.
Supplementary Material
What is Known
Patients with low-density lipoprotein cholesterol (LDL-C) ≥190 mg/dL are at a high long-term risk of atherosclerotic cardiovascular disease (ASCVD) events. Treatment guidelines recommend consideration for early initiation of high-intensity statin therapy in these patients.
What the Study Adds
This study shows that the use of evidence-based therapies remains very low among patients with elevated LDL-C, with only 58.5% of these patients on a statin and 31.9% on high-intensity statin therapy.
This study also shows that there is a significant practice-level variation in the proportion of patients with LDL-C ≥ 190 mg/dL receiving statin and high-intensity statin therapy, with a >200% variation in the receipt of high-intensity statin therapy among two identical patients receiving care at two random practices participating in the PINNACLE® registry.
Practice-level rates for use of non-statin therapies were also low, with significant practice-level variation in the use of ezetimibe and PCSK9 inhibitors among these patients.
These results indicate that system-level interventions are needed to address these treatment gaps and to reduce variation in care of these patients
Acknowledgments
Sources of funding: This research was supported by the American College of Cardiology Foundation’s (ACCF) National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the ACCF. Learn more about the ACC NCDR at https://cvquality.acc.org/NCDR-Home. Dr. Virani is supported by research funding from the Department of Veterans Affairs Health Services Research and Development Service, American Diabetes Association, and the American Heart Association.
Disclosures:
Dr. Virani reports honoraria from American College of Cardiology (Associate Editor Innovations, ACC.org, significant), and National Lipid Association (modest).
Dr. Morris reports advisory board or consultant work for Amgen, Sanofi, Regeneron, and is on steering committees for Esperion, Amgen (all modest).
Dr. Masoudi serves as the Chief Science Officer for the National Cardiovascular Data Registry (significant) and has a contract with the American College of Cardiology (significant)
Dr. Bittner reports grant/research support to her institution from Sanofi/Regeneron (significant), Astra Zeneca (modest), Esperion (modest), Dalcor (modest), Amgen (modest); and clinical trial site PI support (paid to the institution, not individual) from Astra Zeneca (significant) and Bayer Healthcare (significant).
Dr. Ballantyne reports grant/research support to his institution from Abbott Diagnostic (significant), Amarin (significant), Amgen (significant), Esperion (significant), Ionis (significant), Novartis (significant), Pfizer (significant), Regeneron (significant), Roche Diagnostic (significant), SanofiSynthelabo (significant), NIH (significant), AHA (significant), and ADA (significant). He reports consultant work for Abbott Diagnostics (modest), Amarin (modest), Amgen (modest), Astra Zeneca (significant), Boehringer Ingelheim (significant), Eli Lilly (modest), Esperion (modest), Ionis (modest), Matinas BioPharma Inc (modest), Merck (significant), Novartis (modest), Pfizer (significant), Regeneron (modest), Roche Diagnostic (modest), and Sanofi-Synthelabo (significant).
Footnotes
The views expressed in this manuscript do not represent those of the Department of Veterans Affairs or the American College of Cardiology.
References
- 1.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Perak AM, Ning H, de Ferranti SD, Gooding HC, Wilkins JT, Lloyd-Jones DM. Long-Term Risk of Atherosclerotic Cardiovascular Disease in US Adults With the Familial Hypercholesterolemia Phenotype. Circulation. 2016;134:9–19. doi: 10.1161/CIRCULATIONAHA.116.022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khera AV, Won HH, Peloso GM, Lawson KS, Bartz TM, Deng X, van Leeuwen EM, Natarajan P, Emdin CA, Bick AG, Morrison AC, Brody JA, Gupta N, Nomura A, Kessler T, Duga S, Bis JC, van Duijn CM, Cupples LA, Psaty B, Rader DJ, Danesh J, Schunkert H, McPherson R, Farrall M, Watkins H, Lander E, Wilson JG, Correa A, Boerwinkle E, Merlini PA, Ardissino D, Saleheen D, Gabriel S, Kathiresan S. Diagnostic Yield and Clinical Utility of Sequencing Familial Hypercholesterolemia Genes in Patients With Severe Hypercholesterolemia. J Am Coll Cardiol. 2016;67:2578–89. doi: 10.1016/j.jacc.2016.03.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallejo-Vaz AJ, Robertson M, Catapano AL, Watts GF, Kastelein JJ, Packard CJ, Ford I, Ray KK. LDL-Cholesterol Lowering for the Primary Prevention of Cardiovascular Disease Among Men with Primary Elevations of LDL-Cholesterol Levels of 190 mg/dL or Above: Analyses from the WOSCOPS 5-year Randomised Trial and 20-year Observational Follow-Up. Circulation. 2017;136:1878–1891. doi: 10.1161/CIRCULATIONAHA.117.027966. [DOI] [PubMed] [Google Scholar]
- 6.Neil A, Cooper J, Betteridge J, Capps N, McDowell I, Durrington P, Seed M, Humphries SE. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J. 2008;29:2625–33. doi: 10.1093/eurheartj/ehn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Versmissen J, Oosterveer DM, Yazdanpanah M, Defesche JC, Basart DC, Liem AH, Heeringa J, Witteman JC, Lansberg PJ, Kastelein JJ, Sijbrands EJ. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ. 2008;337:a2423. doi: 10.1136/bmj.a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orringer CE, Jacobson TA, Saseen JJ, Brown AS, Gotto AM, Ross JL, Underberg JA. Update on the use of PCSK9 inhibitors in adults: Recommendations from an Expert Panel of the National Lipid Association. J Clin Lipidol. 2017;11:880–890. doi: 10.1016/j.jacl.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Kawashiri MA, Nohara A, Noguchi T, Tada H, Nakanishi C, Mori M, Konno T, Hayashi K, Fujino N, Inazu A, Kobayashi J, Mabuchi H, Yamagishi M. Efficacy and safety of coadministration of rosuvastatin, ezetimibe, and colestimide in heterozygous familial hypercholesterolemia. Am J Cardiol. 2012;109:364–9. doi: 10.1016/j.amjcard.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Ginsberg HN, Rader DJ, Raal FJ, Guyton JR, Baccara-Dinet MT, Lorenzato C, Pordy R, Stroes E. Efficacy and Safety of Alirocumab in Patients with Heterozygous Familial Hypercholesterolemia and LDL-C of 160 mg/dl or Higher. Cardiovasc Drugs Ther. 2016;30:473–483. doi: 10.1007/s10557-016-6685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.deGoma EM, Ahmad ZS, O'Brien EC, Kindt I, Shrader P, Newman CB, Pokharel Y, Baum SJ, Hemphill LC, Hudgins LC, Ahmed CD, Gidding SS, Duffy D, Neal W, Wilemon K, Roe MT, Rader DJ, Ballantyne CM, Linton MF, Duell PB, Shapiro MD, Moriarty PM, Knowles JW. Treatment Gaps in Adults With Heterozygous Familial Hypercholesterolemia in the United States: Data From the CASCADE-FH Registry. Circ Cardiovasc Genet. 2016;9:240–9. doi: 10.1161/CIRCGENETICS.116.001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez de Isla L, Alonso R, Watts GF, Mata N, Saltijeral Cerezo A, Muñiz O, Fuentes F, Diaz-Diaz JL, de Andrés R, Zambón D, Rubio-Marin P, Barba-Romero MA, Saenz P, Sanchez Muñoz-Torrero JF, Martinez-Faedo C, Miramontes-Gonzalez JP, Badimón L, Mata P, SAFEHEART Investigators Attainment of LDL-Cholesterol Treatment Goals in Patients With Familial Hypercholesterolemia: 5-Year SAFEHEART Registry Follow-Up. J Am Coll Cardiol. 2016;67:1278–85. doi: 10.1016/j.jacc.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Abul-Husn NS, Manickam K, Jones LK, Wright EA, Hartzel DN, Gonzaga-Jauregui C, O'Dushlaine C, Leader JB, Lester Kirchner H, Lindbuchler DM, Barr ML, Giovanni MA, Ritchie MD, Overton JD, Reid JG, Metpally RP, Wardeh AH, Borecki IB, Yancopoulos GD, Baras A, Shuldiner AR, Gottesman O, Ledbetter DH, Carey DJ, Dewey FE, Murray MF. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science. 2016;354:aaf7000. doi: 10.1126/science.aaf7000. [DOI] [PubMed] [Google Scholar]
- 14.Hira RS, Kennedy K, Nambi V, Jneid H, Alam M, Basra SS, Ho PM, Deswal A, Ballantyne CM, Petersen LA, Virani SS. Frequency and Practice-Level Variation in Inappropriate Aspirin Use for the Primary Prevention of Cardiovascular Disease: Insights From the National Cardiovascular Disease Registry's Practice Innovation and Clinical Excellence Registry. J Am Coll Cardiol. 2015;65:111–21. doi: 10.1016/j.jacc.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 15.Chan PS, Oetgen WJ, Buchanan D, Mitchell K, Fiocchi FF, Tang F, Jones PG, Breeding T, Thrutchley D, Rumsfeld JS, Spertus JA. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry's PINNACLE (Practice Innovation And Clinical Excellence) program. J Am Coll Cardiol. 2010;56:8–14. doi: 10.1016/j.jacc.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messenger JC, Ho KK, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ, Reynolds MR, Rokos IC, Spertus JA, Wang TY, Winston SA, Rumsfeld JS, Masoudi FA, NCDR Science and Quality Oversight Committee Data Quality Workgroup The National Cardiovascular Data Registry (NCDR) data quality brief: the NCDR data quality program in 2012. J Am Coll Cardiol. 2012;60:1484–8. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Bangalore S, Fayyad R, Kastelein JJ, Laskey R, Amarenco P, DeMicco DA, Waters DD. 2013 Cholesterol Guidelines Revisited: Percent LDL Cholesterol Reduction or Attained LDL Cholesterol Level or Both for Prognosis? Am J Med. 2016;129:384–91. doi: 10.1016/j.amjmed.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Hira RS, Kennedy K, Jneid H, Alam M, Basra SS, Petersen LA, Ballantyne CM, Nambi V, Chan PS, Virani SS. Frequency and practice-level variation in inappropriate and nonrecommended prasugrel prescribing: insights from the NCDR PINNACLE registry. J Am Coll Cardiol. 2014;63:2876–7. doi: 10.1016/j.jacc.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Pokharel Y, Akeroyd JM, Ramsey DJ, Hira RS, Nambi V, Shah T, Woodard LD, Winchester DE, Ballantyne CM, Petersen LA, Virani SS. Statin Use and Its Facility-Level Variation in Patients With Diabetes: Insight From the Veterans Affairs National Database. Clin Cardiol. 2016;39:185–91. doi: 10.1002/clc.22503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pokharel Y, Gosch K, Nambi V, Chan PS, Kosiborod M, Oetgen WJ, Spertus JA, Ballantyne CM, Petersen LA, Virani SS. Practice-Level Variation in Statin Use Among Patients With Diabetes: Insights From the PINNACLE Registry. J Am Coll Cardiol. 2016;68:1368–9. doi: 10.1016/j.jacc.2016.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein H. Multilevel Statistical Models. 4. Wiley; London, UK: 2010. [Google Scholar]
- 22.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, CANTOS Trial Group Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor PJ. Commentary--improving diabetes care by combating clinical inertia. Health Serv Res. 2005;40:1854–61. doi: 10.1111/j.1475-6773.2005.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virani SS, Woodard LD, Chitwood SS, Landrum CR, Urech TH, Wang D, Murawsky J, Ballantyne CM, Petersen LA. Frequency and correlates of treatment intensification for elevated cholesterol levels in patients with cardiovascular disease. Am Heart J. 2011;162:725–732. e1. doi: 10.1016/j.ahj.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Virani SS, Pokharel Y, Steinberg L, Chan W, Akeroyd JM, Gowani SA, Kalra A, Polsani V, Miedema MD, Jones PH, Nambi V, Petersen LA, Ballantyne CM. Provider understanding of the 2013 ACC/AHA cholesterol guideline. J Clin Lipidol. 2016;10:497–504. e4. doi: 10.1016/j.jacl.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Pokharel Y, Steinberg L, Chan W, Akeroyd JM, Jones PH, Nambi V, Nasir K, Petersen L, Ballantyne CM, Virani SS. Case-based educational intervention to assess change in providers' knowledge and attitudes towards the 2013 American College of Cardiology/American Heart Association Cholesterol Management Guideline. Atherosclerosis. 2016;246:115–20. doi: 10.1016/j.atherosclerosis.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001. [PubMed] [Google Scholar]
- 28.Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane Effective Practice and Organization of Care Review Group. BMJ. 1998;317:465–8. doi: 10.1136/bmj.317.7156.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh H, Thomas EJ, Mani S, Sittig D, Arora H, Espadas D, Khan MM, Petersen LA. Timely follow-up of abnormal diagnostic imaging test results in an outpatient setting: are electronic medical records achieving their potential? Arch Intern Med. 2009;169:1578–86. doi: 10.1001/archinternmed.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hysong SJ, Sawhney MK, Wilson L, Sittig DF, Esquivel A, Singh S, Singh H. Understanding the management of electronic test result notifications in the outpatient Setting. BMC Med Inform Decis Mak. 2011;11:22. doi: 10.1186/1472-6947-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–14. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Plutzky J, Shubina M, Turchin A. Continued Statin Prescriptions After Adverse Reactions and Patient Outcomes: A Cohort Study. Ann Intern Med. 2017;167:221–227. doi: 10.7326/M16-0838. [DOI] [PubMed] [Google Scholar]
- 33.Mampuya WM1, Frid D, Rocco M, Huang J, Brennan DM, Hazen SL, Cho L. Treatment strategies in patients with statin intolerance: the Cleveland Clinic experience. Am Heart J. 2013;166:597–603. doi: 10.1016/j.ahj.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


