Abstract
Up to 35% of very preterm infants survive with neurodevelopmental impairments (NDI) such as cognitive deficits, cerebral palsy, and attention deficit disorder. Advanced MRI quantitative tools such as brain morphometry, diffusion MRI, magnetic resonance spectroscopy, and functional MRI at term-equivalent age are ideally suited to improve current efforts to predict later development of disabilities. This would facilitate application of targeted early intervention therapies during the first few years of life when neuroplasticity is optimal. A systematic search and review identified 47 published studies of advanced MRI to predict NDI. Diffusion MRI and morphometry studies were the most commonly studied modalities. Despite several limitations, studies clearly showed that brain structural and metabolite biomarkers are promising independent predictors of NDI. Large representative multicenter studies are needed to validate these studies.
Keywords: Infant, Premature, Magnetic resonance imaging (MRI), Morphometry, Diffusion MRI, Functional MRI, Magnetic resonance spectroscopy, Brain metabolites, Microstructure, Cerebral palsy, Cognitive impairment, Neurodevelopmental impairment
Introduction
Every year in the United States, more than 100,000 babies are born very preterm (at ≤32 weeks gestational age). Up to 35% of these infants develop cognitive, behavioral, and/or psychological abnormalities and 10% develop cerebral palsy (CP), thereby increasing their risk for poor educational, health, and social outcomes.1–3 The continuing high incidence of preterm births in the United States and worldwide—1 out of every 9 births4—coupled with improving survival rates that exceed 90% in developed countries is contributing to an increased prevalence of survivors with such neurodevelopmental impairments (NDI).5,6 The societal economic impact of lifetime care for persons born with CP and cognitive deficits in the United States is estimated to be $15 billion and $64 billion annually, respectively.7
Children with CP typically do not receive a clinical diagnosis until 2 years of age. Cognitive deficits and behavioral/psychological abnormalities cannot be reliably diagnosed until 3–5 years of age.8–10 Yet, in the first 3 years after birth, the brain undergoes dramatic growth, and trillions of synaptic connections are laid down.11 These sensitive early years are critical for neuroplasticity.12 Early diagnosis of developmental disabilities using traditional means is however unlikely because neurologic function is still very immature at birth. Development of imaging prognostic biomarkers at birth could fill this critical need for early diagnosis. Such an advance would facilitate targeted delivery of evidence-based infant stimulation programs or new neuroprotective interventions13–15 after neonatal intensive care unit (NICU) discharge to preserve brain development and/or promote neuroplasticity. Prognostic biomarkers could also be developed into surrogate endpoints of NDI at term-equivalent age (TEA) for more efficient testing of neuroprotective clinical trials during the initial neonatal intensive care stay.
How accurately do existing biomarkers or statistical models in the neonatal period predict NDI in very preterm infants? Several large studies from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network have attempted to answer this question using clinical risk factors at the time of preterm birth, during the NICU hospitalization, and at NICU discharge.16–18 Two of these studies also developed risk prediction calculators to help clinicians counsel families about their infant’s risk of NDI.16,17 While these studies did improve prediction accuracy over existing prognostic models, they are still unable to accurately identify eligible babies for early intervention therapies and neurodevelopmental follow-up. Further, they did not examine the value of conventional structural MRI (sMRI) during the initial NICU hospitalization or at TEA. Hintz et al.19 examined the incremental value of sMRI over clinical factors and early and late cranial ultrasound (US) findings in predicting NDI or death at 18–22 months’ corrected age (CA) in 480 extremely preterm infants. The addition of sMRI had only a small impact on prediction accuracy because most major lesions other than cerebellar hemorrhages were readily visible on cranial US. These results are similar to other large qualitative sMRI studies20 and to a recent meta-analysis of all sMRI studies at TEA in very preterm infants.21 This meta-analysis examined the prognostic value of white matter abnormalities on term MRI to predict individual and combined NDIs. For moderate to severe WMA, the sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio to predict CP at ≥18 months was, 67%, 92%, 8.1, and 0.4, respectively and for mental development was, 38%, 87%, 3.0, and 0.7, respectively. Prognostic test properties for a qualitative diagnosis of diffuse excessive high signal intensity (DEHSI) in predicting NDI were even lower.21
Clearly, a critical gap continues to exist and there is an urgent need for more effective imaging tools and early prognostic biomarkers. In order to improve prediction accuracy, such biomarkers have to be objective, thereby reducing or ideally eliminating measurement error. They also need to be sensitive, so that subtle structural and functional connectivity and metabolic abnormalities can be accurately diagnosed. Advanced MRI techniques such as volumetric MRI (vMRI), diffusion tensor imaging and diffusion MRI (dMRI), magnetic resonance spectroscopy (MRS), and resting-state functional connectivity MRI (fcMRI) appear to be ideally suited to address these needs (see Toa and Neil22 for a technical review). Each of these modalities appears to be more sensitive than sMRI and able to offer complementary brain measurements such as regional volumes, metabolites, microstructural connectivity, and functional network connectivity. The image analysis tools for these novel technologies are increasingly being automated, thereby eliminating subjective human assessments. Their quantitative nature lends far greater study power as compared to categorical measures from cranial US and sMRI. The higher reliability and reduced measurement error also increase study power and improve prediction validity. Overall, these advanced modes of MRI give the best opportunity to identify and develop powerful prognostic biomarkers and surrogate endpoints for clinical trials. The goal of this review is to determine the independent ability of advanced brain MRI biomarkers at TEA to predict NDI in very preterm infants.
Methods
A systematic search strategy was employed to identify and critique all published early advanced MRI studies that predicted one or more neurodevelopmental impairments at 18 months CA or later in very preterm infants. The following inclusion criteria and definitions were used to select eligible studies: (1) very preterm infants, born at or below 32 weeks gestational age (GA) or very low-birth-weight infants (BW < 1500 g); (2) advanced brain MRI: any MRI study that performed dMRI, MRS, fMRI, or quantitative measures of brain macrostructure (morphometry) or lesions; (3) TEA: 37–42 weeks postmenstrual age (PMA); and (4) neurodevelopmental impairment: CP, cognitive or intellectual impairments, social–emotional problems, and/or behavioral/psychological abnormalities diagnosed at a minimum age of 18 months CA or later. Additionally, only longitudinal cohort, nested cohort, and case–control studies were included in the analyses. A few studies that predominantly studied very preterm or very low-birth-weight infants but also included slightly more mature infants (e.g., 33 and 34 weeks’ GA) were permitted for inclusion. Only full-text articles were included in this study.
Medline/PubMed database was searched on 3/15/16 using the following systematic search strategy that included Medical Subject Heading (MeSH) and few non-MeSH search terms: (“infant, premature” OR “infant, low-birth-weight”) AND (“diffusion tensor imaging” OR “diffusion magnetic resonance imaging” OR “connectome” OR “DTI” OR “diffusion tensor tractography” OR “DTT” OR “functional neuroimaging” OR “magnetic resonance imaging” OR “fMRI” OR “fcMRI” OR “functional connectivity” OR “magnetic resonance spectroscopy” OR “brain/metabolism” OR “brain mapping” OR “brain volume”) AND (“neurodevelopmental disorders” OR “developmental disabilities” OR “disability evaluation” OR “cerebral palsy” OR “motor disorders” OR “cognition disorders” OR “cognitive” OR “intellectual disability” OR “intelligence” OR “language development disorders” OR “Bayley” OR “behavior” OR “mental competency” OR “mental disorders” OR “autism spectrum disorder” OR “autistic disorder” OR “attention deficit disorder with hyperactivity” OR “ADHD” OR “child behavior disorders”). The PsycINFO database was also searched for additional relevant articles.
Retrieved articles were screened based on the title and abstract for definite exclusions. For the remainder, full text of each article was accessed and the eligibility criteria applied. Last, the bibliography of all eligible full-text articles was hand-searched for additional eligible articles. Eligible articles were critically appraised using the Critical Appraisal Worksheet for prognostic studies.23 Answers to the following questions were sought: (1) Were infants representative of the underlying population; (2) Was reliability of image processing methods adequately tested; (3) Were outcomes clearly defined and evaluated masked to imaging and other prognostic data; (4) What was the follow-up rate at ≥18 months CA; (5) Were there systematic differences in infants with and without follow-up in relation to prognostic factors; (6) Were advanced MRI biomarkers independently/incrementally predictive over known prognostic factors of NDI; (7) Were prognostic test properties such as sensitivity, specificity, and likelihood ratios reported; (8) How precise were the estimates; and (9) Was the prognostic model internally validated?
Results
The search strategy retrieved 438 articles published between August 2001 and December 2015. After articles were reviewed for definite exclusions and the bibliography of eligible articles was hand-searched, 47 articles met the inclusion criteria. The Table presents a list of each of the studies and summarizes their inclusion criteria; advanced MRI measures tested, identified prognostic biomarkers and associated NDI. Overall, the following three types of advanced MRI methods have been tested as prognostic biomarkers of NDI in very preterm infants: (1) morphometric; (2) MRS; and (3) dMRI. No fMRI studies attempting to predict NDI were found. Nine studies evaluated more than one advanced MRI method. Morphometric and dMRI were the most common type of studies, with each modality being tested and reported in 25 studies each. Although MRS has been around the longest, only five studies investigated brain metabolites in very preterm infants. None of the studies reported the value of multimodal advanced MRI biomarkers in predicting NDI in very preterm infants. Most studies used a 1.5 T MRI scanner but more recent studies reported use of 3.0 T scanners in neonates. While a vast majority of studies reported outcomes at 18–24 months CA, a few, primarily from Australia, predicted outcomes up to 7 years of age. The most common outcomes studied included cognitive, language, and motor development at 18–24 months CA as assessed on the Bayley Scales of Infant and Toddler Development (Second or Third Editions; Bayley-II or Bayley-III) and neuromotor exam. More than 90% of these studies were single-center cohorts from level three academic centers from developed nations around the world. There was great variability in image processing tools, regions of brain studied, and outcomes. Due to such excessive heterogeneity, no attempt was made to pool study results for a meta-analysis of prognostic test properties (Table).
Table.
Summary of studies found via systematic search of early advanced MRI in very preterm infants and prediction of NDI at 18 months CA or later.
| Citation | Subjects | MRI details | Biomarker(s) | Outcome |
|---|---|---|---|---|
| Morphometric analyses | ||||
| Valkama et al.24 | 50 Infants <34 weeks GA and <1800 g BW | 1.0 T magnet; eight 1-dimensional brain stem measurements | Medulla oblongata, pons, and mesencephalon | CP and permanent hearing loss at 18 months CA |
| Peterson et al.25 | 10 “Medically stable” infants (GA and BW not provided) | 1.5 T; total brain WM, GM, ventricular, and parcellated lobar volumes | Right sensorimotor and midtemporal WM volumes | Bayley Scales II MDI scores at 18–20 months CA |
| Woodward et al.26 | 76 Infants ≤32 weeks GA and <1500 g BW | 1.5 T; automated regional and total tissue volumes | Sensorimotor, parietooccipital, and premotor regional volumes | Object working memory task |
| Kapellou et al27 and Rathbone et al.28 | 63 and 82 Infants <30 weeks GA, respectively; TEA as late as 48 weeks PMA | 1 T; cerebrum volume and cortical surface area segmented semi-automatically between 24 and 44 weeks PMA | Cortical surface area and cerebral volume growth | Multiple cognitive and psychological outcomes at 2 and 6 years of age |
| Shah et al.29 | 83 Infants ≤32 weeks GA | 1.5 T; manually segmented cerebellar volume | None | No association with Bayley II MDI or PDI scores |
| Shah et al.30 | 68 Infants ≤33 weeks GA and <1500 g BW | 1.5 T; automated volumes of five cerebral tissue subtypes and eight regions bilaterally | Inferior occipital regional volume | Several measures of oculomotor control |
| Beauchamp et al.31 | 156 Infants <30 weeks GA and <1250 g BW | 1.5 T; manual segmentation of hippocampi and automated regional tissue and lobar volumes | Bilateral hippocampal volumes | Delayed alternation, a measure of working memory |
| Thompson et al.32,33 and Rogers et al.34 | 184 Infants <30 weeks GA and <1250 g BW | 1.5 T; manually segmented hippocampi volume and shape; bifrontal diameter, bilateral frontal heights, and transverse cerebellar diameter | Hippocampal volume | Mental development at 2 years CA; social–emotional difficulties at 5 years; verbal and visual memory at 7 years |
| Boardman et al.35 | 80 Infants ≤34 weeks GA | 1.5 T; regional volumes derived from deformation-based morphometry | None | No association with Griffiths DQ at mean chronological age of 28 months |
| Lind et al.36,37 | 97 and 164 infants, respectively with BW <1501 g and GA <37 weeks | 0.23 T and 1.5 T; total and regional tissue and structural volumes segmented manually | Cerebellum | Motor outcome; no associations with behavioral measures at age 5 years |
| Tich et al.38 | 187 Infants <30 weeks GA and <1250 g BW | 1.5 T; bifrontal, biparietal and transverse cerebellar diameter | Biparietal diameter | Mental and psychomotor development on the Bayley II MDI and PDI at 24 m CA |
| van Kooij et al.39 | 112 Infants <31 weeks GA | 3.0 T; automated cerebellar volumes | Cerebellar volumes | Cognitive scores on Bayley-III (no association with motor scores) |
| Thompson et al.40 | 106 Infants <30 weeks GA and/or <1250 g BW | 1.5 T; corpus callosum area and shape | Circular corpus callosum | Mental development on the Bayley II MDI at 2 years CA |
| He et al.41 | 38 infants ≤1000 g BW | 3 T; volume of objectively defined DEHSI abnormalities using clinical T2-weighted images | DEHSI volume | Cognitive and language development on the Bayley III at 18–22 months CA |
| Bora et al.42 | 110 Infants ≤32 weeks GA | 1.5 T; cortical and subcortical GM, WM, and CSF volumes | Total brain tissue volume and dorsal prefrontal, subgenual, and sensorimotor regional volumes | Inattention/hyperactivity subscale of the strengths and difficulties questionnaire at ages 4, 6, and 9 years |
| Kidokoro et al.43 | 297 Infants from three cohorts with GA <30 weeks GA and MRI at TEA | 1.5 T and 3.0 T; biparietal width and inter-hemispheric distance measured on coronal T2w images | Biparietal width and inter-hemispheric distance | Mental and psychomotor development on the Bayley II MDI and PDI at 2 years CA |
| Park et al.44 | 90 Infants <1000 g BW and 1000— 1499 g BW with clinical indication for MRI (10 were from latter group) | 3.0 T; anterior–posterior length of the CC and transcerebellar diameter on conventional sMRI | Anterior–posterior length of the CC and transcerebellar diameter | Mental and psychomotor development on the Bayley II MDI and PDI; CP; and NDI |
| Paul et al.45 | 27 Infants <30 weeks GA without severe brain injury and scans at TEA | 3.0 T; total cerebral volume and cortical surface area | None | No association with Bayley III cognitive, language, or motor scores |
| Skiold et al.46 | 27 Infants <27 weeks GA at birth with no injury on conventional MRI at TEA | 1.5 T; voxel based morphometry; automated regional and total brain volumes | Cerebellum, white matter, and cortical gray matter volumes | Cognitive and language development on Bayley III at 30 months CA |
| Young et al.47 | 65 Infants <33 weeks GA and MRI at TEA | 1.5 T; automated total and regional brain volumes | Volumes of caudate, putamen, and globus pallidus nuclei | Cognitive, language, and visual motor integration on standardized tests at 4 years |
| Ullman et al.48 | 153 Infants <30 weeks GA and <1250 g BW | 1.5 T; deformation-based morphometry | Insula and putamen volumes | Mathematical ability and working memory at 5 and 7 years |
| Magnetic resonance spectroscopy | ||||
| Augustine et al.49 | 36 Infants ≤32 weeks GA and/or ≤1500 g BW | 1.5 T; multivoxel MRS from deep nuclear GM and superior WM/GM | None | No association with Bayley II MDI or PDI scores |
| van Kooij et al.39 | 58 Infants <31 weeks GA | 3.0 T; single-voxel MRS in cerebellum | NAA/choline in cerebellum | Cognitive scores on Bayley-III (no association with motor scores) at 2 years CA |
| Chau et al.50 | 177 Infants 24–32 weeks GA | 1.5 T; multivoxel chemical shift imaging in the centrum semiovale and basal ganglia | NAA/choline in centrum semiovale WM and basal ganglia | Cognitive, language, and motor outcomes on Bayley III at 18 months CA |
| Bapat et al.51 | 38 Infants ≤1000 g BW | 3.0 T; single-voxel MRS in hippocampus, subventricular zone and cortex | NAA/choline in subventricular zone and cortex; NAA/myoinositol in subventricular zone | Cognitive and language development on Bayley- III at 18–22 months CA |
| Hart et al.52 | 67 Infants <35 weeks GA | 1.5 T; single-voxel MRS in anterior and posterior WM | NAA/choline, NAA/Cr and lactate in posterior WM | Cognitive, language, and fine motor outcomes on Bayley III at 18 months CA |
| Diffusion MRI | ||||
| Arzoumanian et al.53 | 63 Infants <34 weeks GA and <1800 g BW with no gross injury on anatomic MRI | 1.5 T; 6-direction DTI: whole-brain histogram analyses; manual ROI placements in the CC, cerebral WM, anterior and posterior limb of the internal capsule, subcortical GM nuclei | FA of posterior limb of the internal capsule (right) | CP and minor motor abnormalities 18–24 months CA |
| Krishnan et al.54 | 38 Infants ≤34 weeks GA and without severe WM abnormality | 1.5 T; 3-direction DWI; ADC from manually drawn ROIs in centrum semiovale bilaterally | Centrum semiovale ADC | DQ on Griffith Mental Development Scales at 2 years |
| Drobyshevsky et al.55 | 24 Infants <32 weeks GA | MRI strength not stated; 6-direction DTI with manually placed ROIs in 19 locations throughout the brain | Change in FA between early and TEA scan in internal capsule and occipital WM | Motor development on Bayley II PDI at 24 months CA |
| Rose et al.56 | 24 Infants <32 weeks GA, <1500 g BW with and without long-term motor abnormalities | 1.5 T; 6-direction DTI; manual ROIs in bilateral PLIC | FA in PLIC | Gross motor outcomes determined using standardized gait and motor testing |
| Kaukola et al.57 | 30 Infants <30 weeks GA, <1000 g BW | 1.5 T; 3-direction DWI; manual ROIs in the PLIC, pons, centrum semiovale, and corona radiata | ADC in corona radiata | Gross motor function on Griffiths gross motor subscale at 2 years CA |
| Rose et al.58 | 78 Infants <32 weeks GA | 1.5 T; 6-direction DTI; manual ROIs in bilateral ALIC and PLIC and genu and splenium of the CC | FA in PLIC and splenium | Motor development on Bayley II PDI at 18–22 months CA; no correlation with MDI |
| Boardman et al.35 | 80 Infants ≤34 weeks GA | 1.5 T; Manually placed ROIs in multiple regions of interest | ADC values in centrum semiovale | Griffiths DQ at mean chronological age of 28 months |
| Thompson et al.40 | 106 Infants <30 weeks GA and <1250 g BW | 1.5 T; 6-direction DTI and probabilistic tractography of CC, manually segmented | FA for CC bundle and splenium of CC | Bayley II PDI scores at 2 years CA |
| van Kooij et al.59 | 69 Infants <31 weeks GA | 3.0 T; 32-direction DTI and deterministic tractography; manually segmented PLIC and CC fiber bundles | None significant in primary analyses | Several associations with Bayley III scores in secondary analyses |
| Kidokoro et al.60 | 160 Infants <30 weeks GA and/or <1250 g BW | 1.5 T; 6-direction DTI; 6 manually placed ROIs in centrum semiovale | FA in anterior and middle centrum semiovale | Mental and motor scores on Bayley II subscales at 2 years CA |
| Rogers et al.34 | 80 Infants <30 weeks GA and <1250 g BW | 1.5 T; several manual ROIs placed in frontal, temporal, & occipital lobes | MD in right orbitofrontal cortex | Peer problem scores on the strengths and difficulties questionnaire at 5 years |
| van Kooij et al.61 | 63 Infants <31 weeks GA | 3.0 T; 32-direction DTI; whole-brain automated tract-based spatial statistics | FA in multiple WM regions | Cognitive, motor, and fine motor scores on Bayley III at 2 years CA |
| Aeby et al.62 | 41 Infants ≤32 weeks GA and/or ≤1500 g BW without severe brain injury | 1.5 T; 32-direction DTI; voxel based analysis | MD, AD, and RD in superior temporal gyrus | Language scores on Bayley III at 2 years CA; no relation with cognition or motor scores |
| Ball et al.63 | 55 Infants <35 weeks GA without severe brain injury | 3.0 T; 15-direction DTI; multiple ROI analyses in the cortex and whole-brain automated DTI analyses | MD in the cortex | DQ on Griffith Mental Development Scales at 2 years |
| Chau et al.50 | 177 Infants 24–32 weeks GA; infants with large parenchymal venous infracts excluded | 1.5 T; 12-direction DTI; FA measured in various WM regions and deep nuclear GM manually placed ROIs | FA measured in various WM regions and deep nuclear GM | Cognitive, language, and motor outcomes on Bayley III at 18 months CA |
| deBruine et al.64 | 84 Infants <32 weeks GA | 3.0 T; 32-direction DTI and deterministic tractography; manually segmented PLIC, genu and splenium of the CC fiber bundles | PLIC FA and fiber length; ADC and fiber length of splenium | Cerebral palsy and psychomotor score on Bayley III PDI score at 2 years CA |
| Parikh et al.65 | 41 Infants ≤1000 g BW | 3.0 T; diffusivity and volume measures in objectively defined DEHSI regions | DEHSI volume and diffusivity metrics | Cognitive and language scores on Bayley III at 18–22 months CA |
| Brouwer et al.66 | 93 Infants <31 weeks GA | 3.0 T; 3-direction DWI; manual ROI measures in cerebellum and several cerebral WM regions | Cerebellum ADC | Motor outcome on Bayley III motor subscale at 24 months CA |
| Hart et al.52 | 67 Infants <35 weeks GA | 1.5 T; ADC from 22 regions of interest | None | No association with CP or Bayley III scores |
| Pogribna et al.67 | 42 Infants with BW ≤1000 g | 3.0 T; 15-direction DTI; manual ROI analyses throughout the WM | Centrum semiovale MD; subventricular zone FA | Cognitive and language scores on Bayley III at 18–22 months CA |
| Skiold et al.46 | 29 Infants <27 weeks GA at birth with no injury on conventional MRI at TEA | 1.5 T; whole-brain tract-based spatial statistics | None | No correlation seen with cognitive and language scores on Bayley III at 30 months CA |
| Thompson et al.68 | 96 Infants <30 weeks GA and/or <1250 g BW | 1.5 T; 6-direction DTI; automatically selected ROIs in cerebellum and cerebral WM | Inferior occipital and cerebellar MD | Motor and executive function (Movement ABC and Tower of London) at 7 years |
| Duerden et al.69 | 153 Infants between 24 and 32 weeks GA | 1.5 T; 12-direction DTI; whole-brain tract-based spatial statistics | FA and RD in multiple WM regions including CC and corticospinal tract | Motor and cognitive scores on the Bayley III at 18 months CA |
| Rose et al.70 | 66 Infants <1500 g BW | 3.0 T; 25-direction DTI; semi-automated atlas based segmentation into 126 regions | PLIC MD and genu MD and FA | Cognitive and motor scores on Bayley III at 18–22 months CA and gait velocity |
| Ullman et al.48 | 93 Infants <30 weeks GA and <1250 g BW | 1.5 T; whole-brain group-averaged FA map derived using an automated atlas | Whole-brain FA map | Mathematical ability and working memory at 5 years |
AD, axial diffusivity; ADC, apparent diffusion coefficient; BW, birth weight; CA, corrected age; CC, corpus callosum; DQ, developmental quotient; DTI, diffusion tensor imaging; DWI, diffusion weighted imaging; FA, fractional anisotropy; GA, gestational age; GM, gray matter; MD, mean diffusivity; MDI, metal development index; PDI, psychomotor development index; PLIC, posterior limb of the internal capsule; RD, radial diffusivity; ROI, region of interest; T, tesla; TEA, term-equivalent age; WM, white matter.
The majority of morphometric studies measured brain volumes using conventional or 3-dimensional (3D) MRI sequences. Most studies used manual segmentation approaches to measure structural volumes and neonatal brain atlases for automated tissue/regional volumes. Smaller studies examining brain volumes tended to show a stronger correlation with outcomes, while some larger studies showed no association with cognitive, language, or motor outcomes. A few studies measured brain regions or structures on single slices (e.g., 1D or 2D width and/or height) or determined the shape of structures. Several of these morphometric measures were predictive of NDI. In particular, biparietal width and inter-hemispheric distance were both predictive of mental and psychomotor development at 2 years CA.43
Three studies employed voxel/deformation-based morphometry to determine differences in brain volumes between very preterm and healthy term controls to identify prognostic biomarkers.35,46,48 Two of these studies reported an association with long-term developmental outcomes up to 7 years of age.46,48 Two studies reported different automated methods for quantifying DEHSI abnormalities, the most common finding on brain MRI at TEA. Both studies found a strong association between volume of DEHSI and cognitive and language scores on the Bayley-III at 2 years of age.41,65
Of the five studies that evaluated the association between MRS and NDI, four identified several prognostic biomarkers, including NAA/choline and NAA/myoinositol metabolite ratios in a variety of white and gray matter regions that were predictive of cognitive, language, and/or motor scores on the Bayley-III.39,50–52 Three studies utilized single-voxel and two evaluated multi-voxel chemical shift imaging to identify biomarkers. Measurements of NAA/Choline in the central white matter and deep nuclear gray matter were the most predictive of NDI.
Overall, 25 published studies examined regional and/or global microstructural brain development on dMRI and associated these quantitative measurements with one or more NDI outcomes. The vast majority of these studies (23 of 25) reported at least one significant association. While a few studies performed diffusion weighted imaging only (three directions), the rest performed diffusion tensor imaging and none reported use of higher order dMRI models such as diffusion spectrum imaging and high angular resolution diffusion imaging to predict NDI. Diffusion tensor tractography was reported in three studies; all three examined the genu, splenium, and/or whole CC fiber bundle.40,59,64 Two also examined sensorimotor fiber microstructure running through the posterior limb of the internal capsule.59,64 In all, 9 of the 25 studies used semi-automated or automated methods to query regional and whole-brain microstructure.
Several patterns emerged following critical appraisal of all the studies. All but two of the studies were from single-center, academic level III/IV NICUs and hence were not representative of the underlying population. For studies that used manual segmentation or region of interest placements, inter-rater reliability (and intra-rater reliability for some studies) was often not reported. Increasingly however, studies employed automated methods to reduce measurement variability. The accuracy and availability of these methods in neonates has improved greatly over time. All studies defined NDI outcomes, but only half reported that outcome assessors were masked to imaging and/or other prognostic data. The follow-up rate at 18 months CA or older was not reported in a few studies and rates for the other studies ranged from 59% to 100%. Assessment for systematic differences in prognostic factors between infants with and without follow-up was not possible for a majority of studies because this data was not reported. Approximately two-thirds of the studies demonstrated that advanced MRI biomarkers were independently predictive over known prognostic factors (e. g., sMRI) of NDI. Three studies reported prognostic test properties such as sensitivity and specificity and none reported likelihood ratios. Two out of the 47 studies reported that they internally validated their prognostic model.48,67
Discussion
This systematic search yielded 47 unique studies that examined the value of advanced MRI biomarkers in predicting NDI at 18 months CA or older. A majority of these studies identified one or more novel brain metabolite, morphometric, or microstructural prognostic biomarkers that were predictive of NDI (Figure 1). Studies that tested dMRI parameters were more likely to report identification of one or more significant prognostic biomarker(s) as compared to vMRI studies. However, the risk of publication bias cannot be ruled out because negative studies are less likely to be submitted or accepted for publication.71,72 Irrespective of the type of advanced MRI modality utilized, a few brain regions were identified in three or more studies to be predictive of outcomes: corpus callosum, cerebellum, centrum semiovale, sensorimotor, subcortical nuclei, and posterior limb of the internal capsule. Most studies reported outcomes around 2 years CA. Because of our currently limited ability to accurately evaluate cognitive, executive, and behavior function at this early age, additional studies with longer follow-up are needed to fully assess the value of prognostic biomarkers.8
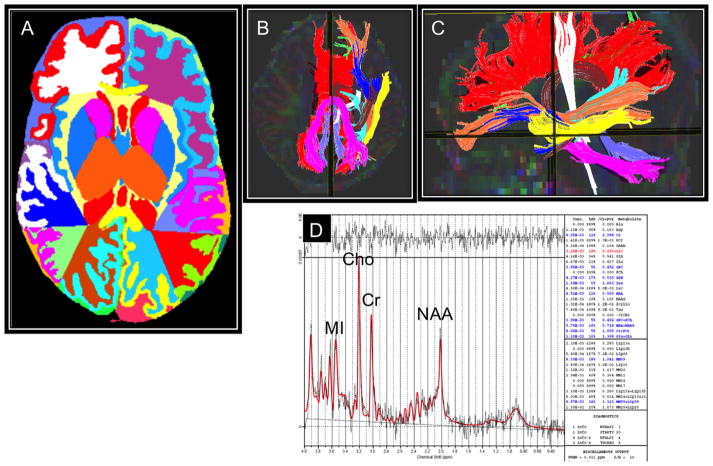
Fig. 1.
Examples of brain advanced MRI measurements including morphometry (A), diffusion tractography (B and C), and magnetic resonance spectroscopy (D). Representative advanced MRI examples at term-equivalent age display an extremely low-birth-weight infant’s brain that was segmented into tissues classes, subcortical structures, and lobes (A), 10 white matter tracts, displayed in axial and sagittal orientations (B and C), and a proton MRS spectrum displaying the four main metabolites, including N-acetylaspartate (NAA), creatine (Cr), choline (Cho), and myoinositol (MI).
Considering the recent development of this area of research, most studies were not representative of the underlying population. Study sample sizes ranged from 10 to 297 very preterm infants with a large majority enrolling less than 100 subjects. About one-third of studies simply compared quantitative MRI measurements between infants with and without NDI and/or did not evaluate the incremental predictive value of significant biomarkers over known prognostic factors of NDI such as injury on sMRI. Analyzed studies rarely reported sensitivity, specificity and likelihood ratios or performed internal validation using cross-validation techniques such as bootstrap.73 The objective of many of these studies was likely to uncover mechanisms rather than to predict outcomes. Nevertheless, reporting prognostic test properties facilitates ready adoption into clinical practice and ideally improves counseling of families. Furthermore, as scientific investigations have increasingly come under scrutiny for lack of reproducibility, internal validation (followed by external validation) is more important than ever.74
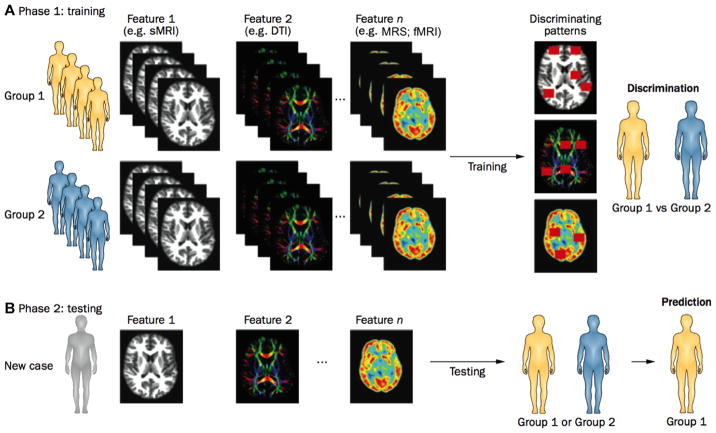
Despite such limitations, these studies show that quantitative brain structural and metabolite measurements are promising as prognostic biomarkers of NDI. However, in order to move closer to clinical translation, studies will need to (1) enroll an inception cohort from a geographically defined region, (2) use more objective measures of brain development/injury, and (3) employ a combination of promising multimodal biomarkers to yield robust prognostic models capable of accurate prediction of individual impairments. In addition to utilizing proven approaches such as multivariable regression techniques, use of newer multivariable pattern classification algorithms (e.g., machine learning) may further enhance the ability to accurately predict long-term NDI.75 These algorithms would ideally discriminate between NDI subgroups of cognitive, behavioral, or motor impairments on the basis of multimodal neuroimaging data (Figure 2). This approach is particularly suited for the development of MRI-based biomarkers that can be used for phenotypic stratification of patients with different types of developmental impairments.
Fig. 2.
Use of multivariable pattern classification to discriminate between multiple subgroups of very preterm infants with cognitive, behavioral, or motor impairments on the basis of multimodal neuroimaging data. (A) Pattern classification models are initially trained (Phase 1) on well-characterized phenotypic data obtained by structural MRI, DTI, MRS, and/or fMRI to identify patterns of potentially discriminative features. (B) These patterns can then be used to determine whether an individual patient in the validation cohort should be assigned to the impaired or control group (Phase 2). Abbreviations: sMRI, structural MRI; DTI, diffusion tensor imaging; MRS, magnetic resonance spectroscopy; fMRI, functional MRI. (Adapted with permission from Ecker and Murphy,75 copyright 2014.)
Of the advanced MRI studies that reported a strength of association with cognitive outcomes, the two studies that objectively quantified DEHSI in the centrum semiovale WM, showed the strongest correlation between DEHSI volume and cognitive and language scores.41,65 Four additional studies that examined the centrum semiovale also found a significant correlation between diffusion metrics or brain NAA/Choline and cognitive and language scores at 18–24 months CA.35,50,54,60 This finding is in distinct contrast to studies that have qualitatively evaluated DEHSI and found no association with outcomes.76–79 One likely explanation for such contrasting outcomes is the inherent subjectivity of qualitative MRI readings. Qualitative assessment of the presence of DEHSI exhibits low intra- and inter-rater reliability.80,81 Even advanced MRI studies are susceptible to such concerns. Several of the advanced MRI studies in this review did not report the reliability of brain measurements and those that did usually did not report more robust measures such as within-subject standard deviation and repeatability.82,83 These data suggest a need and an opportunity for objective measures of brain injury and aberrant development. The recent development of neonatal atlases and automated image processing methods can directly address such concerns and reduce measurement error.
There has been increasing interest in studying functional brain networks with resting-state and task-based fMRI. Perhaps because of the more recent development of this tool and associated infant-friendly image-processing techniques, this systematic search did not identify any studies using fMRI functional measures to predict outcomes. Nevertheless, early studies that have examined development of functional networks in very preterm infants have identified dramatic changes in sensory, motor, and executive functional networks over the first 6 months of life that may prove to be independent predictors of NDI.84–86 For example, new unpublished data from an ongoing longitudinal cohort showed significant connectivity differences in 4 somatosensory and motor networks in 18 very preterm infants without CP as compared to 5 very preterm infants with CP, as diagnosed at 24 months CA (Figure 3). For each of the four regions studied, preterm infants with CP exhibited an overall reduced sensorimotor connectivity as compared to infants without CP.
Fig. 3.
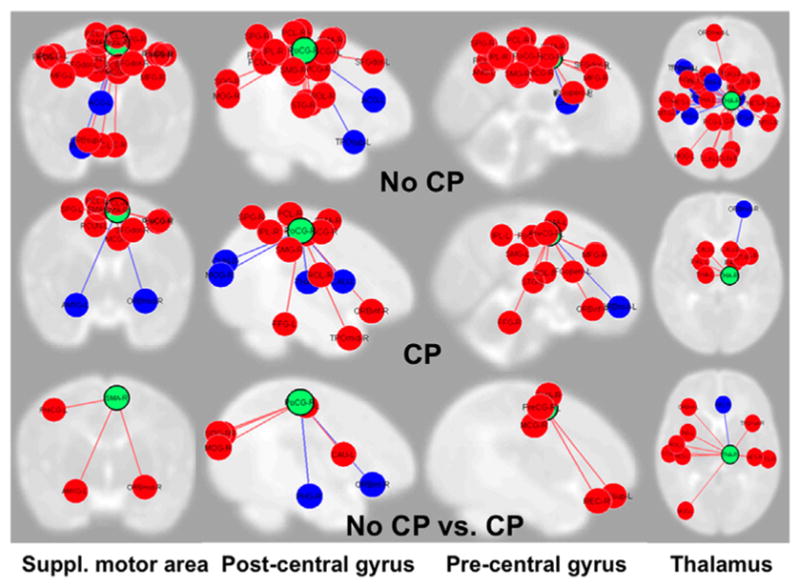
Functional connectivity MRI from 4 somatosensory and motor networks from 5 very preterm infants with cerebral palsy (CP) and 18 without CP. The columns and green circles represent the four sensorimotor regions of interest, including supplementary motor area, post-central gyrus, pre-central gyrus, and thalamus. The red and blue circles represent regions of the brain they are connected with; red signifies a positive correlation while blue represents a negative one. Infants with CP exhibited fewer sensorimotor connections (middle panel) than those without CP (top panel). The last panel displays several networks that were present in infants without CP (red connections) but were absent in infants with CP and a few hubs (blue) where infants with CP (blue) had more connections than those without CP.
This systematic review has several limitations. Only two databases were searched and non-English studies were not evaluated. Additionally, only the study author performed the systematic review and critical appraisal. However, hand searches of reference lists should have reduced such biases. Also, few non-English studies were identified and the likelihood that other databases (e.g., CINAHL and EMBASE) would exclusively index neonatal advanced MRI studies is low. The value of predicting NDI outcomes for clinical care remains a matter of debate and discussion.87,88 Part of this debate arose from inappropriate use of early imaging results to limit intensive care. Unfortunately, the data to support this practice is not available and therefore is prone to errors in prediction and self-fulfilling prophecies. While the evidence is limited,89 most parents are desirous for more accurate long-term prognostic information, especially by TEA, in order to properly plan post-discharge early intervention support services.
Clinical translation aside, there is great enthusiasm for applying prediction biomarkers/models for early risk stratification so that randomized trials can offer targeted early intervention or neuroprotective therapies to the highest risk infants. To date, clinical trials of neuroprotective interventions have shown small to moderate effect sizes. This is not surprising considering there is genotypic and phenotypic variability with NDI, even when you consider impairments individually. With successful early risk stratification, treatments and interventions can be tailored to the patient’s specific predicted motor, cognitive, or behavioral outcome phenotypes. This approach could significantly increase the effect size of interventions in large-scale clinical trials. The development of advanced MRI prognostic biomarkers as surrogate outcomes could also facilitate testing of neuroprotective interventions soon after birth and well before NICU discharge. Currently, it costs approximately $2.6 billion to bring a successful drug to market due to the many drugs that fail in the pipeline and the huge costs of clinical trials.90 Surrogate outcomes could facilitate: (1) shorter pre-clinical development phase for neuro-protective drugs; (2) use of the same outcome measure for experimental and early phase human trials; and (3) reduced need to evaluate NDI for phase I/II trials.
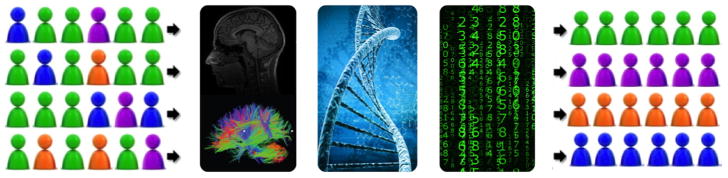
What are some exciting future developments to expect in this fast evolving research area? One likely development will be the use of combined advanced MRI, genomic/epigenetic biomarkers and sophisticated pattern classification techniques to make personalized predictions for high-risk infants a reality (Figure 4). Newer developments in infant magnetoencephalography91 and bedside monitoring tools (e.g., high-density EEG and near infrared spectroscopy)92,93 may also yield novel complementary biomarkers. Such advances will facilitate targeted early intervention therapies and novel neuroprotective interventions to enable improved developmental outcomes. These findings may also be readily translatable to full-term newborns with neonatal encephalopathy, neonatal stroke, and complex congenital heart disease that face an equally high risk of developing disabilities.
Fig. 4.
The combined use of advanced MRI, genomic/epigenetic biomarkers and sophisticated pattern classification algorithms will make personalized predictions for high-risk infants a reality. A combination of biomarkers from advanced brain MRI and genomic/epigenetic biologic samples and pattern classification algorithms such as machine learning are ideally suited to classify the current heterogeneous mix of very preterm infants into different risk groups. This will facilitate the delivery of more effective personalized treatments for very preterm infants.
Acknowledgments
Supported in part by the National Institute of Neurological Disorders and Stroke, United States of NIH Grant R01NS094200-01A1. The author thanks Lili He, PhD, for her thoughtful comments on a previous version of this article.
References
- 1.Woodward LJ, Clark CA, Bora S, Inder TE. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PloS One. 2012;7(12):e51879. doi: 10.1371/journal.pone.0051879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson S. Cognitive and behavioural outcomes following very preterm birth. Semin Fetal Neonatal Med. 2007;12(5):363–373. doi: 10.1016/j.siny.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res. 2011;69(5 Pt 2):11R–18R. doi: 10.1203/PDR.0b013e318212faa0. [DOI] [PubMed] [Google Scholar]
- 4.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 5.Behrman RE, Butler AS, editors. Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention. Washington DC: National Academies Press (US); 2007. pp. 354–373. [PubMed] [Google Scholar]
- 6.Lorenz JM, Wooliever DE, Jetton JR, Paneth N. A quantitative review of mortality and developmental disability in extremely premature newborns. Arch Pediatr Adolesc Med. 1998;152(5):425–435. doi: 10.1001/archpedi.152.5.425. [DOI] [PubMed] [Google Scholar]
- 7.Honeycutt AA. Economic Costs of Mental Retardation, Cerebral Palsy, Hearing Loss, and Vision Impairment. London, England: Elsevier Science Ltd; 2003. pp. 207–228. [Google Scholar]
- 8.Hack M, Taylor HG, Drotar D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116(2):333–341. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 9.Ment LR, Vohr B, Allan W, et al. Change in cognitive function over time in very low-birth-weight infants. J Am Med Assoc. 2003;289(6):705–711. doi: 10.1001/jama.289.6.705. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt B, Anderson PJ, Doyle LW, et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. J Am Med Assoc. 2012;307(3):275–282. doi: 10.1001/jama.2011.2024. [DOI] [PubMed] [Google Scholar]
- 11.Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48(10):757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston MV. Plasticity in the developing brain: implications for rehabilitation. Dev Disabil Res Rev. 2009;15(2):94–101. doi: 10.1002/ddrr.64. [DOI] [PubMed] [Google Scholar]
- 13.Nordhov SM, Ronning JA, Dahl LB, Ulvund SE, Tunby J, Kaaresen PI. Early intervention improves cognitive outcomes for preterm infants: randomized controlled trial. Pediatrics. 2010;126(5):e1088–e1094. doi: 10.1542/peds.2010-0778. [DOI] [PubMed] [Google Scholar]
- 14.Achenbach TM, Howell CT, Aoki MF, Rauh VA. Nine-year outcome of the Vermont intervention program for low birth weight infants. Pediatrics. 1993;91(1):45–55. [PubMed] [Google Scholar]
- 15.Spittle A, Orton J, Anderson PJ, Boyd R, Doyle LW. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev. 2015;11:CD005495. doi: 10.1002/14651858.CD005495.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD for the National Institute of Child Health and Human Development Neonatal Research Network. Intensive care for extreme prematurity—moving beyond gestational age. N Engl J Med. 2008;358(16):1672–1681. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ambalavanan N, Carlo WA, Tyson JE, et al. Outcome trajectories in extremely preterm infants. Pediatrics. 2012;130(1):e115–e125. doi: 10.1542/peds.2011-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broitman E, Ambalavanan N, Higgins RD, et al. Clinical data predict neurodevelopmental outcome better than head ultrasound in extremely low birth weight infants. J Pediatr. 2007;151(5):500–505. 505.e1–2. doi: 10.1016/j.jpeds.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hintz SR, Barnes PD, Bulas D, et al. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics. 2015;135(1):e32–e42. doi: 10.1542/peds.2014-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355(7):685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 21.Van’t Hooft J, van der Lee JH, Opmeer BC, et al. Predicting developmental outcomes in premature infants by term equivalent MRI: systematic review and meta-analysis. Syst Rev. 2015;4:71. doi: 10.1186/s13643-015-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao JD, Neil JJ. Advanced magnetic resonance imaging techniques in the preterm brain: methods and applications. Curr Pediatr Rev. 2014;10(1):56–64. doi: 10.2174/157339631001140408121106. [DOI] [PubMed] [Google Scholar]
- 23.Centre for Evidence-Based Medicine UoO. 2010 http://www.cebm.net/wp-content/uploads/2014/04/cebm-prognosis-worksheet.pdf. Retreived 02.05.16.
- 24.Valkama AM, Tolonen EU, Kerttul LI, Paakko EL, Vainionpaa LK, Koivist ME. Brainstem size and function at term age in relation to later neurosensory disability in high-risk, preterm infants. Acta Paediatr. 2001;90(8):909–915. [PubMed] [Google Scholar]
- 25.Peterson BS, Anderson AW, Ehrenkranz R, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111(5 Pt 1):939–948. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- 26.Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128(Pt 11):2578–2587. doi: 10.1093/brain/awh618. [DOI] [PubMed] [Google Scholar]
- 27.Kapellou O, Counsell SJ, Kennea N, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3(8):e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rathbone R, Counsell SJ, Kapellou O, et al. Perinatal cortical growth and childhood neurocognitive abilities. Neurology. 2011;77(16):1510–1517. doi: 10.1212/WNL.0b013e318233b215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah DK, Anderson PJ, Carlin JB, et al. Reduction in cerebellar volumes in preterm infants: relationship to white matter injury and neurodevelopment at two years of age. Pediatr Res. 2006;60(1):97–102. doi: 10.1203/01.pdr.0000220324.27597.f0. [DOI] [PubMed] [Google Scholar]
- 30.Shah DK, Guinane C, August P, et al. Reduced occipital regional volumes at term predict impaired visual function in early childhood in very low birth weight infants. Invest Ophthalmol Vis Sci. 2006;47(8):3366–3373. doi: 10.1167/iovs.05-0811. [DOI] [PubMed] [Google Scholar]
- 31.Beauchamp MH, Thompson DK, Howard K, et al. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain. 2008;131(Pt 11):2986–2994. doi: 10.1093/brain/awn227. [DOI] [PubMed] [Google Scholar]
- 32.Thompson DK, Wood SJ, Doyle LW, et al. Neonate hippocampal volumes: prematurity, perinatal predictors, and 2-year outcome. Ann Neurol. 2008;63(5):642–651. doi: 10.1002/ana.21367. [DOI] [PubMed] [Google Scholar]
- 33.Thompson DK, Adamson C, Roberts G, et al. Hippocampal shape variations at term equivalent age in very preterm infants compared with term controls: perinatal predictors and functional significance at age 7. NeuroImage. 2013;70:278–287. doi: 10.1016/j.neuroimage.2012.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers CE, Anderson PJ, Thompson DK, et al. Regional cerebral development at term relates to school-age social–emotional development in very preterm children. J Am Acad Child Adolesc Psychiatry. 2012;51(2):181–191. doi: 10.1016/j.jaac.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boardman JP, Craven C, Valappil S, et al. A common neonatal image phenotype predicts adverse neurodevelopmental outcome in children born preterm. NeuroImage. 2010;52(2):409–414. doi: 10.1016/j.neuroimage.2010.04.261. [DOI] [PubMed] [Google Scholar]
- 36.Lind A, Haataja L, Rautava L, et al. Relations between brain volumes, neuropsychological assessment and parental questionnaire in prematurely born children. Eur Child Adolesc Psychiatry. 2010;19(5):407–417. doi: 10.1007/s00787-009-0070-3. [DOI] [PubMed] [Google Scholar]
- 37.Lind A, Parkkola R, Lehtonen L, et al. Associations between regional brain volumes at term-equivalent age and development at 2 years of age in preterm children. Pediatr Radiol. 2011;41(8):953–961. doi: 10.1007/s00247-011-2071-x. [DOI] [PubMed] [Google Scholar]
- 38.Tich SN, Anderson PJ, Hunt RW, Lee KJ, Doyle LW, Inder TE. Neurodevelopmental and perinatal correlates of simple brain metrics in very preterm infants. Arch Pediatr Adolesc Med. 2011;165(3):216–222. doi: 10.1001/archpediatrics.2011.9. [DOI] [PubMed] [Google Scholar]
- 39.Van Kooij BJ, Benders MJ, Anbeek P, Van Haastert IC, De Vries LS, Groenendaal F. Cerebellar volume and proton magnetic resonance spectroscopy at term, and neurodevelopment at 2 years of age in preterm infants. Dev Med Child Neurol. 2012;54(3):260–266. doi: 10.1111/j.1469-8749.2011.04168.x. [DOI] [PubMed] [Google Scholar]
- 40.Thompson DK, Inder TE, Faggian N, et al. Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. NeuroImage. 2012;59(4):3571–3581. doi: 10.1016/j.neuroimage.2011.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He L, Parikh NA. Atlas-guided quantification of white matter signal abnormalities on term-equivalent age MRI in very preterm infants: findings predict language and cognitive development at two years of age. PloS One. 2013;8(12):e85475. doi: 10.1371/journal.pone.0085475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bora S, Pritchard VE, Chen Z, Inder TE, Woodward LJ. Neonatal cerebral morphometry and later risk of persistent inattention/hyperactivity in children born very preterm. J Child Psychol Psychiatry. 2014;55(7):828–838. doi: 10.1111/jcpp.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kidokoro H, Anderson PJ, Doyle LW, Woodward LJ, Neil JJ, Inder TE. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics. 2014;134(2):e444–e453. doi: 10.1542/peds.2013-2336. [DOI] [PubMed] [Google Scholar]
- 44.Park HW, Yoon HK, Han SB, et al. Brain MRI measurements at a term-equivalent age and their relationship to neurodevelopmental outcomes. AJNR Am J Neuroradiol. 2014;35(3):599–603. doi: 10.3174/ajnr.A3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paul RA, Smyser CD, Rogers CE, et al. An allometric scaling relationship in the brain of preterm infants. Ann Clin Transl Neurol. 2014;1(11):933–937. doi: 10.1002/acn3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skiold B, Alexandrou G, Padilla N, Blennow M, Vollmer B, Aden U. Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. J Pediatr. 2014;164(5):1012–1018. doi: 10.1016/j.jpeds.2013.12.051. [DOI] [PubMed] [Google Scholar]
- 47.Young JM, Powell TL, Morgan BR, et al. Deep grey matter growth predicts neurodevelopmental outcomes in very pre-term children. NeuroImage. 2015;111:360–368. doi: 10.1016/j.neuroimage.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 48.Ullman H, Spencer-Smith M, Thompson DK, et al. Neonatal MRI is associated with future cognition and academic achievement in preterm children. Brain. 2015;138(Pt 11):3251–3262. doi: 10.1093/brain/awv244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Augustine EM, Spielman DM, Barnes PD, et al. Can magnetic resonance spectroscopy predict neurodevelopmental outcome in very low birth weight preterm infants? J Perinatology. 2008;28(9):611–618. doi: 10.1038/jp.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chau V, Synnes A, Grunau RE, Poskitt KJ, Brant R, Miller SP. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology. 2013;81(24):2082–2089. doi: 10.1212/01.wnl.0000437298.43688.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bapat R, Narayana PA, Zhou Y, Parikh NA. Magnetic resonance spectroscopy at term-equivalent age in extremely preterm infants: association with cognitive and language development. Pediatr Neurol. 2014;51(1):53–59. doi: 10.1016/j.pediatrneurol.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hart AR, Smith MF, Whitby EH, et al. Diffusion-weighted imaging and magnetic resonance proton spectroscopy following preterm birth. Clin Radiol. 2014;69(8):870–879. doi: 10.1016/j.crad.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Arzoumanian Y, Mirmiran M, Barnes PD, et al. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR Am J Neuroradiol. 2003;24(8):1646–1653. [PMC free article] [PubMed] [Google Scholar]
- 54.Krishnan ML, Dyet LE, Boardman JP, et al. Relationship between white matter apparent diffusion coefficients in preterm infants at term-equivalent age and developmental outcome at 2 years. Pediatrics. 2007;120(3):e604–e609. doi: 10.1542/peds.2006-3054. [DOI] [PubMed] [Google Scholar]
- 55.Drobyshevsky A, Bregman J, Storey P, et al. Serial diffusion tensor imaging detects white matter changes that correlate with motor outcome in premature infants. Dev Neurosci. 2007;29(4–5):289–301. doi: 10.1159/000105470. [DOI] [PubMed] [Google Scholar]
- 56.Rose J, Mirmiran M, Butler EE, et al. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev Med Child Neurol. 2007;49(10):745–750. doi: 10.1111/j.1469-8749.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- 57.Kaukola T, Perhomaa M, Vainionpaa L, et al. Apparent diffusion coefficient on magnetic resonance imaging in pons and in corona radiata and relation with the neurophysiologic measurement and the outcome in very preterm infants. Neonatology. 2010;97(1):15–21. doi: 10.1159/000226603. [DOI] [PubMed] [Google Scholar]
- 58.Rose J, Butler EE, Lamont LE, Barnes PD, Atlas SW, Stevenson DK. Neonatal brain structure on MRI and diffusion tensor imaging, sex, and neurodevelopment in very-low-birthweight preterm children. Dev Med Child Neurol. 2009;51(7):526–535. doi: 10.1111/j.1469-8749.2008.03231.x. [DOI] [PubMed] [Google Scholar]
- 59.van Kooij BJ, van Pul C, Benders MJ, van Haastert IC, de Vries LS, Groenendaal F. Fiber tracking at term displays gender differences regarding cognitive and motor outcome at 2 years of age in preterm infants. Pediatr Res. 2011;70(6):626–632. doi: 10.1203/PDR.0b013e318232a963. [DOI] [PubMed] [Google Scholar]
- 60.Kidokoro H, Anderson PJ, Doyle LW, Neil JJ, Inder TE. High signal intensity on T2-weighted MR imaging at term-equivalent age in preterm infants does not predict 2-year neurodevelopmental outcomes. AJNR Am J Neuroradiol. 2011;32(11):2005–2010. doi: 10.3174/ajnr.A2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Kooij BJ, de Vries LS, Ball G, et al. Neonatal tract-based spatial statistics findings and outcome in preterm infants. AJNR Am J Neuroradiol. 2012;33(1):188–194. doi: 10.3174/ajnr.A2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aeby A, De Tiege X, Creuzil M, et al. Language development at 2 years is correlated to brain microstructure in the left superior temporal gyrus at term equivalent age: a diffusion tensor imaging study. NeuroImage. 2013;78:145–151. doi: 10.1016/j.neuroimage.2013.03.076. [DOI] [PubMed] [Google Scholar]
- 63.Ball G, Srinivasan L, Aljabar P, et al. Development of cortical microstructure in the preterm human brain. Proc Natl Acad Sci U S A. 2013;110(23):9541–9546. doi: 10.1073/pnas.1301652110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Bruine FT, Van Wezel-Meijler G, Leijser LM, et al. Tractography of white-matter tracts in very preterm infants: a 2-year follow-up study. Dev Med Child Neurol. 2013;55(5):427–433. doi: 10.1111/dmcn.12099. [DOI] [PubMed] [Google Scholar]
- 65.Parikh NA, He L, Bonfante-Mejia E, et al. Automatically quantified diffuse excessive high signal intensity on MRI predicts cognitive development in preterm infants. Pediatr Neurol. 2013;49(6):424–430. doi: 10.1016/j.pediatrneurol.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brouwer MJ, van Kooij BJ, van Haastert IC, et al. Sequential cranial ultrasound and cerebellar diffusion weighted imaging contribute to the early prognosis of neurodevelopmental outcome in preterm infants. PloS One. 2014;9(10):e109556. doi: 10.1371/journal.pone.0109556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pogribna U, Burson K, Lasky RE, Narayana PA, Evans PW, Parikh NA. Role of diffusion tensor imaging as an independent predictor of cognitive and language development in extremely low-birth-weight infants. AJNR Am J Neuroradiol. 2014;35(4):790–796. doi: 10.3174/ajnr.A3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson DK, Lee KJ, Egan GF, et al. Regional white matter microstructure in very preterm infants: predictors and 7 year outcomes. Cortex. 2014;52:60–74. doi: 10.1016/j.cortex.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duerden EG, Foong J, Chau V, et al. Tract-based spatial statistics in preterm-born neonates predicts cognitive and motor outcomes at 18 months. AJNR Am J Neuroradiol. 2015;36(8):1565–1571. doi: 10.3174/ajnr.A4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rose J, Cahill-Rowley K, Vassar R, et al. Neonatal brain microstructure correlates of neurodevelopment and gait in preterm children 18–22 mo of age: an MRI and DTI study. Pediatr Res. 2015;78(6):700–708. doi: 10.1038/pr.2015.157. [DOI] [PubMed] [Google Scholar]
- 71.Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and related biases. Health Technol Assess (Rockv) 2000;4(10):1–115. [PubMed] [Google Scholar]
- 72.Dwan K, Altman DG, Arnaiz JA, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PloS One. 2008;3(8):e3081. doi: 10.1371/journal.pone.0003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moons KG, Kengne AP, Woodward M, et al. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart. 2012;98(9):683–690. doi: 10.1136/heartjnl-2011-301246. [DOI] [PubMed] [Google Scholar]
- 74.Begley CG, Ioannidis JP. Reproducibility in science: improving the standard for basic and preclinical research. Circ Res. 2015;116(1):116–126. doi: 10.1161/CIRCRESAHA.114.303819. [DOI] [PubMed] [Google Scholar]
- 75.Ecker C, Murphy D. Neuroimaging in autism—from basic science to translational research. Nat Rev Neurol. 2014;10(2):82–91. doi: 10.1038/nrneurol.2013.276. [DOI] [PubMed] [Google Scholar]
- 76.de Bruine FT, van den Berg-Huysmans AA, Leijser LM, et al. Clinical implications of MR imaging findings in the white matter in very preterm infants: a 2-year follow-up study. Radiology. 2011;261(3):899–906. doi: 10.1148/radiol.11110797. [DOI] [PubMed] [Google Scholar]
- 77.Jeon TY, Kim JH, Yoo SY, et al. Neurodevelopmental outcomes in preterm infants: comparison of infants with and without diffuse excessive high signal intensity on MR images at near-term-equivalent age. Radiology. 2012;263(2):518–526. doi: 10.1148/radiol.12111615. [DOI] [PubMed] [Google Scholar]
- 78.Hart A, Whitby E, Wilkinson S, Alladi S, Paley M, Smith M. Neurodevelopmental outcome at 18 months in premature infants with diffuse excessive high signal intensity on MR imaging of the brain. Pediatr Radiol. 2011;41(10):1284–1292. doi: 10.1007/s00247-011-2155-7. [DOI] [PubMed] [Google Scholar]
- 79.Skiold B, Vollmer B, Bohm B, et al. Neonatal magnetic resonance imaging and outcome at age 30 months in extremely preterm infants. J Pediatr. 2012;160(4):559–566e1. doi: 10.1016/j.jpeds.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 80.Hart AR, Smith MF, Rigby AS, Wallis LI, Whitby EH. Appearances of diffuse excessive high signal intensity (DEHSI) on MR imaging following preterm birth. Pediatr Radiol. 2010;40(8):1390–1396. doi: 10.1007/s00247-010-1633-7. [DOI] [PubMed] [Google Scholar]
- 81.Slaughter LA, Bonfante-Mejia E, Hintz SR, Dvorchik I, Parikh NA. Early conventional MRI for prediction of neurodevelopmental impairment in extremely-low-birth-weight infants. Neonatology. 2016;110(1):47–54. doi: 10.1159/000444179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bland JM, Altman DG. Measurement error. Br Med J. 1996;312(7047):1654. doi: 10.1136/bmj.312.7047.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bland JM, Altman DG. Measurement error and correlation coefficients. Br Med J. 1996;313(7048):41–42. doi: 10.1136/bmj.313.7048.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doria V, Beckmann CF, Arichi T, et al. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci U S A. 2010;107(46):20015–20020. doi: 10.1073/pnas.1007921107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smyser CD, Inder TE, Shimony JS, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He L, Parikh NA. Brain functional network connectivity development in very preterm infants: the first six months. Early Hum Dev. 2016;98:29–35. doi: 10.1016/j.earlhumdev.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 87.Janvier A, Barrington K. Trying to predict the future of expreterm infants: who benefits from a brain MRI at term? Acta Paediatr. 2012;101(10):1016–1017. doi: 10.1111/j.1651-2227.2012.02788.x. [DOI] [PubMed] [Google Scholar]
- 88.Pearce R, Baardsnes J. Term MRI for small preterm babies: do parents really want to know and why has nobody asked them? Acta Paediatr. 2012;101(10):1013–1015. doi: 10.1111/j.1651-2227.2012.02767.x. [DOI] [PubMed] [Google Scholar]
- 89.Harvey ME, Nongena P, Gonzalez-Cinca N, Edwards AD, Redshaw ME ePRT. Parents’ experiences of information and communication in the neonatal unit about brain imaging and neurological prognosis: a qualitative study. Acta Paediatr. 2013;102(4):360–365. doi: 10.1111/apa.12154. [DOI] [PubMed] [Google Scholar]
- 90.DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 91.Roberts TP, Paulson DN, Hirschkoff E, et al. Artemis 123: development of a whole-head infant and young child MEG system. Front Hum Neurosci. 2014;8:99. doi: 10.3389/fnhum.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grieve PG, Isler JR, Izraelit A, et al. EEG functional connectivity in term age extremely low birth weight infants. Clin Neurophysiol. 2008;119(12):2712–2720. doi: 10.1016/j.clinph.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kenosi M, Naulaers G, Ryan CA, Dempsey EM. Current research suggests that the future looks brighter for cerebral oxygenation monitoring in preterm infants. Acta Paediatr. 2015;104(3):225–231. doi: 10.1111/apa.12906. [DOI] [PubMed] [Google Scholar]