Abstract
Proactive control refers to the active representation of contextual information to bias cognitive processing and facilitate goal-directed behavior. Despite research suggesting that proactive control may be impaired in autism spectrum disorder (ASD), the associations between proactive control and clinical symptoms of ASD remain underspecified. Here, we combined a children’s version of the AX continuous performance task (AX-CPT) with gold standard clinical assessments in children with ASD (N=35) or typical development (TYP; N=45). After controlling for full-scale IQ, measures of proactive control were similar between ASD and TYP. However, specifically within ASD we observed paradoxical relationships between proactive control and clinical symptoms. Increased reliance on proactive control was associated with reduced attention problems and increased restricted and repetitive behaviors in ASD. Therefore, proactive control appears to represent a double-edged sword in ASD: improved attentional control at the cost of heightened behavioral inflexibility. This represents a compelling and new characterization of the specific association between cognitive control processes isolated in computerized laboratory tasks and the multidimensional cognitive symptoms characteristic of ASD.
Keywords: Autism spectrum disorder, attention, cognitive control, proactive control, restricted and repetitive behaviors
Autism spectrum disorder (ASD) is a common neurodevelopmental disorder comprised of core symptoms including difficulties with communication and social interaction, and the presentation of restricted and repetitive patterns of behavior, interests, and activities [RRBs; (American Psychiatric Association, 2013)]. Attention problems are also common in ASD, with estimates suggesting that the symptom levels of over 50% of individuals are at or near diagnostic criteria for comorbid attention deficit/hyperactivity disorder (ADHD; Leyfer et al., 2006; Simonoff et al., 2008). Studies examining the nature of attention problems in ASD have linked the disorder to impairments with the selection, maintenance, and shifting of attention, as well as aberrant connectivity within attention-related brain networks (Allen & Courchesne, 2001; Elton, Di Martino, Hazlett, & Gao, 2016). Whereas research has often focused on the social dimensions of ASD, RRBs and attention problems represent prominent symptoms in ASD whose underlying mechanisms remain elusive.
A viable mechanism underlying the ASD cognitive phenotype is executive dysfunction. ‘Executive functions’ (EFs) are cognitive abilities primarily sub-served by prefrontal regions including working memory, flexibility, and inhibition. Accumulating neuropsychological evidence suggests that impaired EF–or executive dysfunction–represents a common and replicable symptom of ASD (Hill, 2004; Pennington & Ozonoff, 1996). These findings suggest impairments across dimensions of EF [e.g. working memory (Williams, Goldstein, Carpenter, & Minshew, 2005), flexibility (Ozonoff et al., 2004), and inhibition (Christ, Kester, Bodner, & Miles, 2011)]. Despite the intuitive appeal of a link between executive dysfunction and clinical symptoms in ASD, the data is less clear. Perhaps due to difficulties associated with EF measurement, reliable associations between EF and ASD symptoms have remained elusive (Geurts, Corbett, & Solomon, 2009; Lopez, Lincoln, Ozonoff, & Lai, 2005; Mosconi et al., 2009). In other words, although there is agreement that EF problems exist in ASD, the relationships between specific cognitive processes and clinical symptoms remain underspecified (Geurts, et al., 2009).
To address this gap, cognitive neuroscience work has examined the ‘cognitive control’ processes underlying ASD (e.g. Solomon, Ozonoff, Cummings, & Carter, 2008; Yerys et al., 2015). The cognitive control construct largely overlaps with EF, and refers to the ability to maintain and regulate ongoing cognitive and sensorimotor processing to support goal-directed behavior. The constituent processes and underlying neural circuitry of cognitive control can be carefully parcellated using neuroimaging and noninvasive brain stimulation (Badre, 2008; Nee & D'Esposito, 2016). This level of specificity produces measures with improved sensitivity to clinically-relevant variance in cognitive performance relative to traditional neuropsychological tests (cf., MacDonald & Carter, 2002), and may aid in the development of targeted clinical interventions (Geurts, et al., 2009).
Recent fMRI studies have provided insight into the cognitive control processes impacted in ASD. On the ‘preparing to overcome prepotency’ (POP) task [where participants hold a cue in mind and then inhibit a prepotent response tendency (Barber & Carter, 2005)], participants with typical development (TYP) recruit frontoparietal brain networks anchored in dorsolateral prefrontal cortex (DLPFC) more strongly than individuals with ASD (Solomon et al., 2009; Solomon et al., 2014). A likely function of DLPFC in the POP task is related to the representation of stimulus context, which can facilitate an individual’s ability to select goal-directed responses (Chiew & Braver, 2017). The ability to use context to guide early selection of task-relevant stimuli is often referred to as ‘proactive control’ (Braver, 2012). DLPFC is thought to implement proactive control by actively representing stimulus context through sustained neural activity (Braver, 2012). Therefore, decreased DLPFC recruitment in ASD may be indicative of difficulty recruiting proactive control (Solomon, et al., 2014).
The current study tested the hypothesis that proactive control is impaired in ASD and that this impairment has deleterious clinical effects. Given that ADHD symptoms are associated with impaired context maintenance (Yerys et al., 2009), we hypothesized that reduced proactive control would be associated with increased attention problems in ASD. RRBs in ASD are heterogeneous and comprise both the perseveration of context-inappropriate goals (compulsivity), and repetitive behaviors that lack a clear goal (stereotypy). The relationship between cognitive functioning and RRBs is further complicated by the fact that intelligence is negatively associated with stereotypy (Bishop, Richler, & Lord, 2006). Therefore, we hypothesized that RRBs would be associated with proactive control in ASD, but that the direction and magnitude of these associations may vary across RRB categories. Elucidating the relationship between proactive control and clinical symptoms could help to shape precision medicine approaches for improving cognitive functioning in ASD.
Methods
Participants
Seventy-nine participants were included in the study (NASD=34; NTYP=45). Inclusion criteria were: performance above chance on the task, completion of all clinical assessments, living with at least one biological parent, no motor/vision/hearing problems. ASD participants had to meet both ADOS-2 (ASD or autism) and ADI-R (on either subscale, and within two points on other subscale) clinical cutoffs. For TYP, the criteria included an absence of any learning, developmental, or behavioral disorders or close family members with autism. See Table 1 for detailed characteristics of the sample. Informed written consent was provided by parents and participants assented to the study, which was approved by the UC Davis Institutional Review Board (IRB#: 686644).
Table 1. Summary of current sample.
Yuen’s robust t-tests used for all groupwise comparisons except gender where the Odds Ratio (OR) was used.
| Variables | ASD | TYP | Groupwise Comparison |
|---|---|---|---|
| N | 34 | 45 | N/A |
| Demographic Variables [mean±standard deviation] | |||
| Age (years) | 10.44±1.66 | 10.93±1.34 | tyuen=1.73, p=0.084 |
| Gender (M,F) | (30,4) | (37,8) | OR=0.62, p=0.540 |
| ADOS (calibrated severity score) | 6.94±1.82 | N/A | N/A |
| DAS–Nonverbal IQT | 99.79±18.32 | 107.80±13.29 | tyuen=1.89, p=0.054 |
| DAS–Verbal IQ** | 96.59±22.53 | 110.93±10.70 | tyuen=3.12, p=0.004 |
| DAS–Full-Scale IQ** | 100.74±18.98 | 112.22±9.97 | tyuen=2.84, p=0.007 |
| Clinical Variables [mean±standard deviation] | |||
| Attention Problems*** | 60.85±9.54 | 53.00±4.80 | tyuen=4.21, p<.001 |
| RBS-R Total*** | 24.56±17.55 | 1.60±2.45 | tyuen=5.45, p<0.001 |
| RBS-R Self-Injurious** | 1.85±2.00 | 0.22±0.93 | tyuen=3.13, p=0.006 |
| RBS-R Stereotyped** | 1.82±2.00 | 0.22±0.93 | tyuen=4.04, p=.001 |
| RBS-R Compulsive** | 3.65±3.69 | 0.35±0.80 | tyuen=3.73, p=.005 |
| RBS-R Ritualistic/Sameness | 11.35±9.55 | 0.53±0.84 | tyuen=9.38, p<.001 |
| RBS-R Restricted | 3.50±2.39 | 0.22±0.60 | tyuen=5.95, p=.005 |
| Proactive Control Task Variables [mean±standard deviation] | |||
| AX (RT) | 780±263 | 817±220 | tyuen=0.59, p=0.522 |
| AX (ER) | 0.098±0.061 | 0.082±0.049 | tyuen=1.04, p=0.281 |
| AY (RT) | 1094±318 | 1041±279 | tyuen=0.90, p=0.382 |
| AY (ER)* | 0.407±0.205 | 0.324±0.145 | tyuen=2.12, p=0.027 |
| BX (RT) | 784±362 | 711±220 | tyuen=0.09, p=0.933 |
| BX (ER)T | 0.235±0.180 | 0.144±0.125 | tyuen=1.95, p=0.055 |
| BY (RT) | 745±288 | 697±235 | tyuen=0.09, p=0.925 |
| BY (ER) | 0.151±0.130 | 0.110±0.078 | tyuen=0.77, p=0.428 |
| A-Cue Bias | 0.054±0.592 | −0.041±0.438 | tyuen=0.59, p=0.550 |
| d’-context* | −0.49±1.99 | 0.37±1.51 | tyuen=−2.21, p=0.025 |
p<0.1,
p<0.05,
p<0.01,
p<0.001.
Additional acronyms: Autism Diagnostic Observation Schedule (ADOS), Differential Ability Scale (DAS), Repetitive Behavior Scale-Revised (RBS-R).
Procedure
Attention problems
The parent-report Child Behavior Checklist (CBCL) form of the Achenbach System of Empirically Based Assessment was administered (Achenbach & Rescorla, 2001). We analyzed age- and gender-corrected T-scores on the attention problems subscale of the CBCL. This subscale collapses across symptoms related to inattention (e.g. “can’t concentrate, can’t pay attention for long”), impulsivity/hyperactivity (e.g. “impulsive or acts without thinking”), and some that are not specific to ADHD (e.g. “confused or seems to be in a fog”).
Restricted and repetitive behaviors
The parent-report Repetitive Behavior Scale-Revised (RBS-R) was used to measure RRBs (Bodfish, Symons, & Lewis, 1999). We used a 5-factor breakdown of the RBS-R that was validated for the current study’s age range (Lam & Aman, 2007). This breakdown included self-injurious behaviors (SIB; potential to cause bodily injury; e.g. skin picking), stereotyped behaviors (STB; lacking a clear goal; e.g. hand-flapping), compulsive behaviors (CB; performed according to a rule; e.g. arranging items in a particular pattern), restricted behaviors (REB; limited range of focus; e.g. attachment to particular objects), and ritualistic and sameness behaviors clustered into a single factor (RIB; performed in a consistent fashion and resistant to change; e.g. activities performed in a particular order and difficulty with transitions). We analyzed summed scores for each of the 5-factors.
AX Continuous Performance Test (AX-CPT)
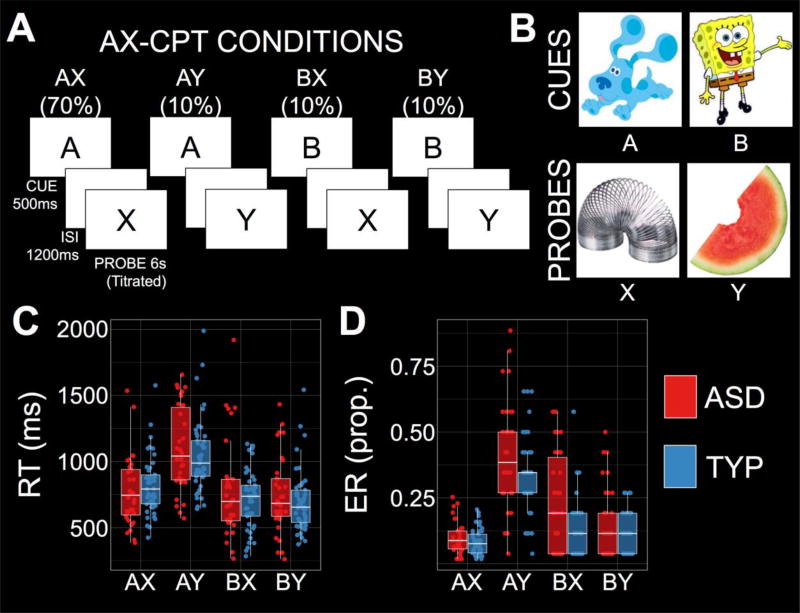
In the AX-CPT, participants see cue-probe sequences and make ‘target’ or ‘nontarget’ responses (Figure 1A). On 70% of trials, an AX sequence is presented and ‘target’ is the correct response. On a minority of trials, an AY (10%), BX (10%), or BY (10%) sequence is presented, and ‘nontarget’ is the correct response. Participants completed 12 practice followed by 120 experimental trials. Cartoon characters were used in lieu of the traditional letter stimuli to make the task amenable to children (Figure 1B; Chatham, Frank, & Munakata, 2009). Our analysis focused on correct trial reaction times (RTs) and error rates (ERs) in each condition. Participants relying on proactive control should demonstrate slower RTs and higher ERs on AY trials relative to all other trial types. Additionally, we computed two measures to index proactive control by combining information from multiple conditions (Gonthier, Macnamara, Chow, Conway, & Braver, 2016; Richmond, Redick, & Braver, 2015). First, A-Cue Bias combines hits on AX trials and false alarms (FA) on AY trials, and is computed as: A-Cue Bias=1/2*(ZAXHITS+ZAYFA) (Gonthier, et al., 2016; Richmond, et al., 2015). Second, d’-context combines hits on AX trials and FA on BX trials, and is computed as: d’-context=ZAXHITS–ZBXFA (Barch et al., 2001; Gonthier, et al., 2016). Both measures merge AX-hits with FA on critical nontarget conditions, reflecting either the tendency to plan a target response after an A-cue leading to more AY-FA (increasing A-Cue Bias), or the tendency to retrieve the context cue to minimize BX-FA (increasing d’-context).
Figure 1.
(A–B) Schematic of the AX-Continuous Performance Task (AX-CPT) and the cartoons used to replace the conventional letter stimuli. (C) Reaction times (RTs) were significantly slower on AY trials in both autism spectrum disorder (ASD) and typical development (TYP). (D) Error rates (ERs) were generally higher in ASD relative to TYP, but the pattern across conditions was consistent: Both groups demonstrated higher ER on AY trials relative to all other conditions.
Results
The data violated assumptions of normality and homoscedasticity and robust inferential tests were used throughout (Field & Wilcox, 2017). Due to unmatched FSIQ scores between ASD and TYP (Table 1), control models were fit for contrasts containing a group difference, and an association between FSIQ and the dependent measure after controlling for group. This approach enabled us to examine whether group differences were extraneously influenced by FSIQ, and was in line with a key assumption of ANCOVA: that the covariate is significantly related to the dependent variable across groups (Dennis et al., 2009). These conditions were met for two measures: BX ER and d’-context.
AX-CPT
Robust four (AX, AY, BX, and BY) by two (ASD, TYP) ANOVAs were conducted on the RT and ER data using ‘bwtrim’ in R (Field & Wilcox, 2017). For RT, there was a significant main effect of condition (Q=69.44, p<0.001, η2=0.614), but neither the effect of group nor the interaction reached significance (group: Q=0.20, p=0.661; interaction: Q=2.32, p=0.097). Planned comparisons were conducted using robust mean comparisons ('yuend'; Field & Wilcox, 2017). The main effect of condition was driven by an RT interference effect on AY trials (MDIFF=245ms, 95%-CI=201 to 288, tyuen=11.32, p<0.001, ξ=0.63; Figure 1C), and facilitative effects on BX and BY trials relative to AX (BX: MDIFF=−69ms, 95%-CI=−113 to −24, tyuen=−3.08, p=0.003, ξ=0.22; BY: MDIFF=−101ms, 95%-CI=−141 to −60, tyuen=−5.03, p<0.001, ξ=0.31; Figure 1C). These RT data were compatible with proactive control in the AX-CPT: A-cues lead participants to anticipate a ‘target’ response that takes time to cancel on AY trials, whereas B-cue trials are faster because the context invariably leads to a nontarget response.
For ER, there were significant effects of condition (Q=87.56, p<0.001, η2=0.605) and group (Q=4.97, p=0.033, η2=0.081), but no interaction (Q=2.11, p=0.123). The main effect of condition was driven by higher ER on AY (MDIFF=0.266, 95%-CI=0.234 to 0.299, tyuen=16.57, p<0.001, η=0.82), BX (MDIFF=0.064, 95%-CI=0.034 to 0.093, tyuen=4.33, p<0.001, ξ=0.47), and BY (MDIFF=0.023, 95%-CI=0.004 to 0.042, tyuen=2.37, p=0.022, ξ=0.20) trials relative to AX (Table 1; Figure 1D). Importantly, ER was higher on AY trials relative to both BX (MDIFF=0.203, 95%-CI=0.163 to 0.243, tyuen=10.23, p<0.001, ξ=0.75) and BY (MDIFF=0.244, 95%-CI=0.211 to 0.278, tyuen=15.17, p<0.001, ξ=0.91) trials. Again, ER data were consistent with a proactive control strategy, participants holding the A-cue in mind are likely to demonstrate a high AY ER.
Planned comparisons between ASD and TYP were conducted using ‘yuenbt’ in R (Field & Wilcox, 2017). AY ERs were higher in ASD relative to TYP (MDIFF=0.084, 95%-CI=0.009 to 0.159, tyuen=2.12, p=0.030, d=0.48). There was a trend towards increased BX ER in ASD relative to TYP (MDIFF=0.092, 95%-CI=−0.003 to 0.188, tyuen=1.95, p=0.056, d=0.60), but this effect was not significant after covarying for FSIQ (tyuen=1.37, p=0.162). Importantly, a failure to recruit proactive control in ASD would be expected to result in decreased AY errors relative to BX. More AY errors than BX errors were observed in both groups (ASD: MDIFF=0.186, 95%-CI=0.102 to 0.269, tyuen=4.61, p<0.001, d=0.55; TYP: MDIFF=0.194, 95%-CI=0.149 to 0.239, tyuen=8.82, p<0.001, d=0.91), and the difference between AY and BX errors was matched between groups (MDIFF=0.004, 95%-CI=−0.068 to 0.077, tyuen=0.13, p=0.906). The current results indicate that both groups employed proactive control during the task.
Lastly, we examined A-Cue Bias and d’-context. Firstly, A-Cue Bias did not differ between groups (MDIFF=−0.073, 95%-CI=−0.165 to 0.310, tyuen=0.59, p=0.550). Second, d’-context was significantly higher in the TYP group relative to ASD (MDIFF=−0.911, 95%-CI=−1.711 to −0.111, tyuen=−2.21, p=0.026, d=0.50), but this effect was not significant after covarying for FSIQ (tyuen=0.99, p=0.282). Therefore, group contrasts suggested that proactive control was broadly matched between ASD and TYP, particularly after controlling for individual differences in general cognitive ability.
Attention Problems and RRBs
Attention problems were higher in ASD (MDIFF=8.48, 95%-CI=4.71 to 12.25, tyuen=4.21, p<0.001, d=1.09). Similarly, each of the five RBS-R factors were higher in ASD: SIB (MDIFF=1.59, 95%-CI=0.55 to 2.63, tyuen=3.13, p=0.006, d=1.10), STB (MDIFF=3.45, 95%-CI=1.61 to 5.30, tyuen=4.04, p=0.002, d=1.41), CB (MDIFF=2.96, 95%-CI=1.32 to 4.61, tyuen=3.73, p=0.003, d=1.32), RIB (MDIFF=9.38, 95%-CI=6.09 to 12.66, tyuen=5.65, p<0.001, d=1.72), and REB (MDIFF=3.41, 95%-CI=2.25 to 4.57, tyuen=5.95, p<0.001, d=2.01).
Associations Between AX-CPT and Clinical Measures
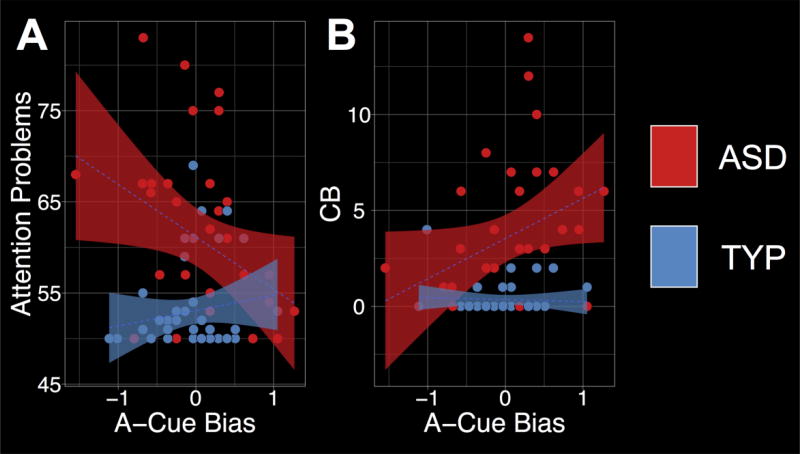
Correlation analyses with robust Bayesian parameter estimation were used to quantify the associations between clinical symptoms and proactive control (Baath, 2014). Both A-Cue Bias and d’-context were correlated with clinical symptoms (attention problems and RBS-R) in each participant group, and then the posterior rho distributions were contrasted to quantify the evidence that the associations differed between groups. First, there was a significant negative association between A-Cue Bias and attention problems in ASD (rhoASD=−0.33, 95%-HDI=−0.63 to −0.02; Figure 2A), which was significantly lower than the association in TYP (rhoTYP=0.08, 95%-HDI=−0.25 to 0.39; rho difference=−0.41, 95%-HDI=−0.78 to −0.02, p=0.041; Figure 2A). Second, there was a significant positive association between A-Cue Bias and CB in ASD (rhoASD=0.34, 95%-HDI=0.009 to 0.61; Figure 2B), which was more positive than the association in TYP at the trend-level (rhoTYP=−0.002, 95%-HDI=−0.30 to 0.30; rho difference=0.33, 95%-HDI=−0.04 to 0.68, p=0.070; Figure 2B). There was no evidence for a dimensional relationship between A-Cue Bias and the other RBS-R subscales (−0.005≤rhos≤0.26, 95%-HDIs contain 0), and d’-context was not associated with any of the clinical measures of interest (−0.25≤rhos≤0.11, 95%-HDIs contain 0).
Figure 2.
(A) Significant negative association between attention problems and A-Cue Bias during the AX-Continuous Performance Task (AX-CPT) in ASD. (B) Significant positive association between A-Cue Bias and compulsive behaviors (CB) in ASD.
Exploratory Post-Hoc Analyses
To evaluate the independence of the associations between A-Cue Bias and clinical symptoms, the two variables that demonstrated significant associations with A-Cue Bias in ASD–i.e. attention problems and CB–were included as covariates in a robust Bayesian multiple linear regression model (Kruschke, Aguinis, & Joo, 2012). The associations were robust to the inclusion of both regressors in the model (attention problems: β=−0.41, 95%-HDI=−0.73 to −0.09; CB: β=0.39, 95%-HDI=0.072 to 0.71; R2=0.28, 95%-HDI=0.11 to 0.44). Furthermore, there was no association between attention problems and CB (rho=0.09, 95%-HDI=−0.25 to 0.44). Therefore, attention problems and CB appear to explain unique variance in A-Cue Bias.
Discussion
Here, we investigated proactive control in ASD. Specifically, we tested two related hypotheses: 1) that proactive control is impaired in ASD, and 2) that this impairment promotes symptoms related to the ASD phenotype (namely, attention problems and RRBs). The current study did not find strong evidence for a proactive control deficit in ASD in late childhood. Importantly, when conducting dimensional analyses to evaluate hypothesis 2, we found that proactive control is associated with both a clinical benefit–reduced attention problems–as well as a clinical cost–heightened RRBs–in ASD.
Previous studies observed impaired cognitive control on the POP task in ASD, thought to reflect diminished proactive control in this population (Solomon, et al., 2008; Solomon, et al., 2009; Solomon, et al., 2014). The current study diverges from this conclusion, and a careful consideration of the similarities between the POP and AX-CPT tasks may reveal what specific controlled processes are disrupted in ASD. The POP task examines interference created by overcoming a prepotent response tendency. This is similar to high conflict trials on the AX-CPT, where a proactive response plan must be modified in light of new information (AY), or inhibited upon presentation of a conflicting probe (BX). In the present study, ASD was associated with significant (AY) or trend-level (BX) increases in error rates in these conditions, suggesting some continuity between the current results and past work. However, the insight provided by the current study is that this finding does not appear to be driven by impaired proactive control per se. Individuals with ASD used the context cues to inform their responses to the probe stimuli, evidenced by greater AY relative to BX error rates, and similar A-Cue Bias in ASD and TYP. Instead, individuals with ASD appear to have an intact ability to deploy proactive control, but difficulty modifying prepared responses under conflict. This pattern is dissociable from the pattern observed in schizophrenia (more BX than AY errors; Barch, et al., 2001), and future studies examining the AX-CPT across patient groups are needed to determine what controlled processes demonstrate convergence across disorders, and what aspects are specific to ASD.
It should be noted that prior studies have suggested that proactive control on the AX-CPT is less mature in early relative to late childhood (Chatham, et al., 2009). Therefore, proactive control difficulties may be greatest during early childhood in ASD, and smaller in magnitude during late childhood. In light of this, the current study might not have had a sufficiently large sample size to detect a relatively small magnitude group difference in proactive control in ASD. To show this empirically, a post-hoc power calculation revealed that the difference observed in d’-context in the current study had an effect size of d=0.49, which would require 91 participants per group to reliably detect differences between ASD and TYP (one-tailed). Therefore, a high-powered longitudinal study of proactive control in children with ASD is needed to provide more conclusive insight into the magnitude and trajectory of proactive control development in ASD.
Regardless of the presence or absence of group differences in proactive control, the present data provide compelling evidence for dissociable dimensional relationships between proactive control and clinical symptoms in ASD. Past studies have provided preliminary evidence linking laboratory-based cognitive tasks and parent-reported clinical symptoms in ASD (Sinzig, Morsch, Bruning, Schmidt, & Lehmkuhl, 2008; Solomon, et al., 2008). The current data provide additional support for these findings using a leading-edge measure of proactive control from the cognitive neuroscience literature. Specifically, the current data suggests that a derived measure (A-Cue Bias) that indexes the combined benefits (AX-hits) and costs (AY-FA) of proactive control appears to be particularly sensitive to individual differences in clinical symptoms–attention problems and RRBs, respectively–in ASD.
Given the significant prevalence of comorbid ADHD and obsessive-compulsive disorder (OCD) diagnoses in the ASD population (Simonoff, et al., 2008), it is unclear whether the observed relationships are specific to ASD. Future transdiagnostic studies are needed to examine the degree to which these dimensional relationships are specific to the ASD cognitive phenotype, or would also be observed in individuals with ADHD (reduced A-Cue Bias) or OCD (increased A-Cue Bias). Future work would also benefit from more refined measures of ADHD that can separate inattention from hyperactivity/impulsivity, which are conflated in the attention problems measure in the CBCL. Finally, it should be noted that it is not possible to determine whether increased proactive control causes, or is caused by, reduced attention problems or increased RRBs in the current study.
The current study supports a recent argument that cognitive control represents a double-edged sword: while it typically confers performance benefits (e.g. keeping attention problems in check), it can disrupt performance when more ‘reactive’ or ‘model-free’ control systems might be more appropriate (e.g. getting stuck on inappropriate goals in RRBs; Amer, Campbell, & Hasher, 2016). The current results suggest that interventions designed to facilitate context maintenance may be useful for reducing attention problems in individuals with ASD and impaired proactive control. Conversely, for individuals with normative proactive control and heightened symptoms related to cognitive rigidity–restricted and repetitive behaviors, or rigid social or linguistic functioning–treatments designed to facilitate cognitive flexibility would represent an appropriate treatment option. More generally, the current work suggests that proactive control can help to shape the design of personalized medicine approaches to cognitive remediation in ASD.
Acknowledgments
Supported by the NIMH (R01MH10651802 & R01MH10328403 held by MS), UC Davis MIND Institute, and Autism Phenome Project (PI: Dr. David G Amaral). The authors thank Elyse Adler, Dr. Brianna Heath, Sarah Mahdavi, Garrett Gower, Kiele Argente, and Ashley Tay for their essential work supporting the current study. We also thank Drs. Christine Wu Nordahl and Corentin Gonthier for insightful comments during preparation of this manuscript.
General Scientific Summary: This study identified a meaningful behavioral marker of proactive control in autism spectrum disorder (ASD). This marker is paradoxically associated with both a clinical benefit (reduced attention problems) and a clinical cost (increased compulsivity) in ASD.
Footnotes
This work was presented at both the Cognitive Neuroscience Society and the American College of Neuropsychopharmacology annual meetings in 2017.
References
- Achenbach TM, Rescorla L. Manual for the ASEBA school-age forms and profiles: An integrated system of multi-informant assessment. Burlington, VT: Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- Allen G, Courchesne E. Attention function and dysfunction in autism. Frontiers in Bioscience: a Journal and Virtual Library. 2001;6:D105–119. doi: 10.2741/allen. [DOI] [PubMed] [Google Scholar]
- Amer T, Campbell KL, Hasher L. Cognitive Control As a Double-Edged Sword. Trends Cogn Sci. 2016;20(12):905–915. doi: 10.1016/j.tics.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and statistical manual of mental disorders (5th ed.) 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Baath R. Bayesian First Aid: a package that imple- ments Bayesian alternatives to the classical *. test functions in R; Paper presented at the Proceedings of UseR! 2014 – the International R User Conference.2014. [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends in Cognitive Sciences. 2008;12(5):193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Barber AD, Carter CS. Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cereb Cortex. 2005;15(7):899–912. doi: 10.1093/cercor/bhh189. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald AW, III, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Richler J, Lord C. Association between restricted and repetitive behaviors and nonverbal IQ in children with autism spectrum disorders. Child Neuropsychol. 2006;12(4–5):247–267. doi: 10.1080/09297040600630288. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Lewis MH. The repetitive behavior scale. Western Carolina Center Research Reports; 1999. [Google Scholar]
- Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci. 2012;16(2):106–113. doi: 10.1016/j.tics.2011.12.010. doi: S1364-6613(11)00261-0 [pii] 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, Frank MJ, Munakata Y. Pupillometric and behavioral markers of a developmental shift in the temporal dynamics of cognitive control. Proc Natl Acad Sci U S A. 2009;106(14):5529–5533. doi: 10.1073/pnas.0810002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew KS, Braver TS. Context processing and cognitive control: From gating models to dual mechanisms. In: Egner T, editor. The Wiley Handbook of Cognitive Control. Chichester, West Sussex, UK: John Wley & Sons; 2017. [Google Scholar]
- Christ SE, Kester LE, Bodner KE, Miles JH. Evidence for selective inhibitory impairment in individuals with autism spectrum disorder. Neuropsychology. 2011;25(6):690–701. doi: 10.1037/a0024256. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc. 2009;15(3):331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Di Martino A, Hazlett HC, Gao W. Neural Connectivity Evidence for a Categorical-Dimensional Hybrid Model of Autism Spectrum Disorder. Biological Psychiatry. 2016;80(2):120–128. doi: 10.1016/j.biopsych.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AP, Wilcox RR. Robust statistical methods: A primer for clinical psychology and experimental psychopathology researchers. Behav Res Ther. 2017 doi: 10.1016/j.brat.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Corbett B, Solomon M. The paradox of cognitive flexibility in autism. Trends Cogn Sci. 2009;13(2):74–82. doi: 10.1016/j.tics.2008.11.006. doi: S1364-6613(08)00260-X [pii] 10.1016/j.tics.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonthier C, Macnamara BN, Chow M, Conway AR, Braver TS. Inducing Proactive Control Shifts in the AX-CPT. Front Psychol. 2016;7:1822. doi: 10.3389/fpsyg.2016.01822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004;8(1):26–32. doi: 10.1016/j.tics.2003.11.003. doi: S1364661303003152 [pii] [DOI] [PubMed] [Google Scholar]
- Kruschke JK, Aguinis H, Joo H. The Time Has Come. Organizational Research Methods. 2012;15(4):722–752. doi: 10.1177/1094428112457829. [DOI] [Google Scholar]
- Lam KS, Aman MG. The Repetitive Behavior Scale-Revised: independent validation in individuals with autism spectrum disorders. J Autism Dev Disord. 2007;37(5):855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, Lainhart JE. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord. 2006;36(7):849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. J Autism Dev Disord. 2005;35(4):445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Carter CS. Cognitive experimental approaches to investigating impaired cognition in schizophrenia: a paradigm shift. J Clin Exp Neuropsychol. 2002;24(7):873–882. doi: 10.1076/jcen.24.7.873.8386. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Kay M, D'Cruz AM, Seidenfeld A, Guter S, Stanford LD, Sweeney JA. Impaired inhibitory control is associated with higher-order repetitive behaviors in autism spectrum disorders. Psychol Med. 2009;39(9):1559–1566. doi: 10.1017/S0033291708004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, D'Esposito M. The hierarchical organization of the lateral prefrontal cortex. Elife. 2016;5 doi: 10.7554/eLife.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Cook I, Coon H, Dawson G, Joseph RM, Klin A, Wrathall D. Performance on Cambridge Neuropsychological Test Automated Battery subtests sensitive to frontal lobe function in people with autistic disorder: evidence from the Collaborative Programs of Excellence in Autism network. J Autism Dev Disord. 2004;34(2):139–150. doi: 10.1023/b:jadd.0000022605.81989.cc. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Richmond LL, Redick TS, Braver TS. Remembering to prepare: The benefits (and costs) of high working memory capacity. J Exp Psychol Learn Mem Cogn. 2015;41(6):1764–1777. doi: 10.1037/xlm0000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Morsch D, Bruning N, Schmidt MH, Lehmkuhl G. Inhibition, flexibility, working memory and planning in autism spectrum disorders with and without comorbid ADHD-symptoms. Child Adolesc Psychiatry Ment Health. 2008;2(1):4. doi: 10.1186/1753-2000-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Cummings N, Carter CS. Cognitive control in autism spectrum disorders. International Journal of Developmental Neuroscience. 2008;26(2):239–247. doi: 10.1016/j.ijdevneu.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, Ly S, Carter CS. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47(12):2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Yoon JH, Ragland JD, Niendam TA, Lesh TA, Fairbrother W, Carter CS. The development of the neural substrates of cognitive control in adolescents with autism spectrum disorders. Biological Psychiatry. 2014;76(5):412–421. doi: 10.1016/j.biopsych.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Carpenter PA, Minshew NJ. Verbal and spatial working memory in autism. J Autism Dev Disord. 2005;35(6):747–756. doi: 10.1007/s10803-005-0021-x. [DOI] [PubMed] [Google Scholar]
- Yerys BE, Antezana L, Weinblatt R, Jankowski KF, Strang J, Vaidya CJ, Kenworthy L. Neural Correlates of Set-Shifting in Children With Autism. Autism Res. 2015;8(4):386–397. doi: 10.1002/aur.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Wallace GL, Sokoloff JL, Shook DA, James JD, Kenworthy L. Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Res. 2009;2(6):322–333. doi: 10.1002/aur.103. [DOI] [PMC free article] [PubMed] [Google Scholar]