Abstract
Asthma in older adults (often classified as those ≥ 65 years of age) is relatively common, underdiagnosed, and sub-optimally treated. It is an important health problem, as the population of the United States continues to age. Unfortunately, asthma morbidity and mortality rates are highest in this age group. Alterations of the innate and adaptive immune responses occur with aging, and contribute to pathophysiologic differences and subsequent treatment challenges. The symptoms of asthma may differ from younger populations, and often include fatigue. There are unique factors that can complicate asthma management among older adults, including comorbidities, menopause, care giver roles, and depression. Pharmacologic therapies are often not as effective as in younger populations, and may have greater side effects. Spirometry, peak flow measurements, and asthma education are typically underutilized, and may contribute to delays in diagnosis as well as worse outcomes. There are specific strategies that health care providers can take to improve the care of older adults with asthma.
Keywords: Asthma, Older Adults, Elderly, Spirometry, Immunosenescence, Depression, Menopause, Education, Asthma COPD Overlap Syndrome (ACOS)
Introduction
To many, there is the belief that asthma occurs in childhood and resolves over time. However, studies show that a significant number of people first develop asthma at a relatively older age, and that it can persist throughout the lifespan. Understanding asthma in older adults is critical to health care providers and researchers, as the number of those above age 65 in the United States is projected to increase to 85 million individuals by the year 2045, a 77% increase since 2015 (Figure 1).1 Additionally, by 2030, the percent of older adults will increase to 20% of the total population, and therefore those who manage adult asthma will likely have many patients above the age of 65. This review covers unique features of asthma in older adults, discusses challenges of diagnostic and management strategies, and highlights areas where further research is needed to address knowledge gaps.
Figure 1.
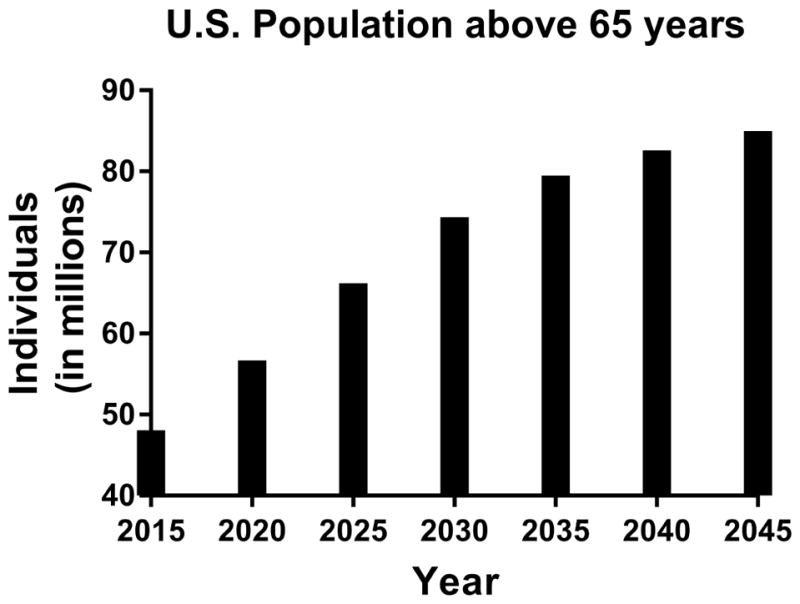
Projected U.S. population ≥ 65 years. Projections based on 2015 analysis of U.S Census data by Population Reference Bureau
Epidemiology
Accurate data on the prevalence of asthma in older adults can be difficult to obtain. In part, this is due to the fact that many asthma trials specifically exclude individuals above the age of 65. Additionally, in the older adult with respiratory symptoms, often times those with a minimal, remote, or even nonexistent smoking history are misdiagnosed as having COPD rather than asthma,2, 3 even though data suggests that approximately 20 pack years are typically required to develop COPD.4 A 2015 Centers for Disease Control and Prevention (CDC) survey found that among adults, the prevalence of asthma is highest in those age 45 – 64 years, with 8.4% having a current diagnosis of asthma.5 This was the same prevalence as the overall rate for children age 0 – 18. Therefore, asthma remains a common chronic disease across all ages.
Results of the CDC Asthma Call Back Survey (~900,000 individuals) did find that the assumption of asthma typically beginning in childhood is often, but not always, true.6 Among all children, asthma incidence was 12.5 per 1000. However, new cases of asthma occurred frequently at any age. In adults over the age of 18 years of age, the greatest incidence rate was in those age 55 – 64 years (4.6 per 1000). In those above the age of 65, the incidence rate was 3.1 per 1000. More recently, Baptist et al. reported in a study in the Journal that found that among 180 persistent asthma patients over the age of 55, 46% were diagnosed after the age of 40.7 Taken together, these data indicate that asthma is common in those above 65, and that many cases begin later in life.
There appear to be clinical differences between those who develop asthma in childhood, adolescence, or young adult-hood (termed “long standing asthma”, or LSA) and those who develop asthma after the age of 40 (termed “late onset asthma”, or LOA). Those with LOA are more likely to have frequent symptoms, less atopy, and a decreased response to standard inhaled corticosteroids and beta agonists,8, 9 though some authors have suggested those with LSA may in fact have worsened outcomes.10 Recent cluster analyses of older adults has been published, and will help to clarify these issues as well as identify specific phenotypes that may respond preferentially to one treatment over another.7, 11
What does seem clear is that older adults have some of the highest asthma morbidity and mortality rates. For example, as shown in Figure 2, the mortality rate from asthma steadily increases with age. Additionally, studies of severe asthma have shown that older adults may have a more difficult-to-control phenotype than younger populations, with decreased responsiveness to standard medications.7, 12, 13 Of those presenting to the emergency department for asthma, adults ≥ 65 years have the highest rate who subsequently require hospitalization (approximately 25% admission rate compared to 7.9% for all ages) and longest length of stay.14 It is important to note that asthma is frequently underdiagnosed, and therefore these numbers may be underestimations.
Figure 2.
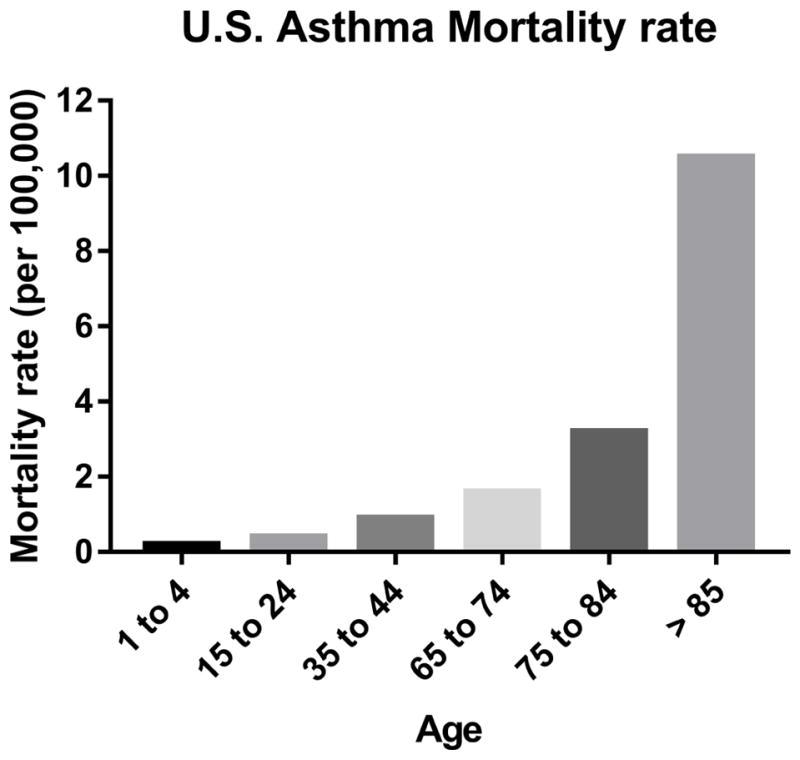
Mortality rates for asthma among different age groups (young children, adolescents, young adults, and older/very old adults). Adapted from: Kochanek KD, Murphy SL, Xu JQ, Tejada-Vera B. Deaths: Final data for 2014. National vital statistics reports; vol 65 no 4. Hyattsville, MD: National Center for Health Statistics. 2016
Immunosenescence
With increasing age, there are alterations in both the innate and adaptive immune responses which likely impact the pathology, and consequently the treatment, of asthma in older adults. Two distinct alterations of aging on the innate and adaptive immune responses have been described and can occur simultaneously. One, “immunosenescence,” is a “blunted” response after a pathogenic threat or tissue injury. However, despite an inability to proliferate, some senescent cells remain alive, functioning at an altered capacity. This results in the second process, “inflammaging,” an increased low-grade basal systemic inflammation (e.g., IL1-β, IL-6 and TNF-α) in the absence of an overt infection.15 The mechanisms of immunosenescence and inflammaging are the consequence of both “random” (e.g., environmental exposures, accumulation of reactive oxygen species [ROS] from metabolic activity, mutagenesis) and “regulated” (e.g., genetic) events.16
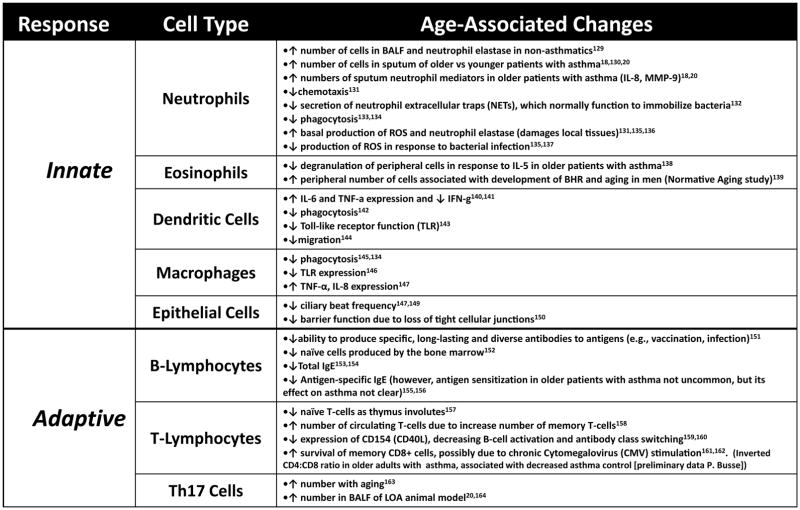
The possible impacts of immunosenescence and inflammaging on asthma are briefly outlined in Figure 3. Collectively, alterations in innate and adaptive cells likely have several clinical implications including decreasing the response to vaccinations and increasing rates of infections, which in turn may exacerbate asthma, or play a role in the inception of LOA.17 Furthermore, older patients with asthma may have altered airway and systemic inflammation (i.e. higher neutrophils with increased IL-6, IL-8, C-reactive protein) resembling changes seen in a phenotype of severe asthma in younger patients which is often less responsive to corticosteroid treatment.18–20
Figure 3.
Impacts of immunosenescence and inflammaging on asthma. A list of corresponding citations is available online.
Symptoms and Diagnosis
Many of the asthma symptoms common in younger patients (e.g., intermittent chest tightness, nocturnal wheezing and dyspnea) are present in older patients. However, there are important considerations. Older adults frequently note fatigue as a principal symptom, even in the absence of typical asthma manifestations.21 Dyspnea is commonly associated with other chronic disorders in older patients (e.g., heart failure, other lung diseases, anemia), therefore asthma as the cause of these symptoms may be overlooked. Furthermore, some older patients may limit their activity to avoid becoming dyspneic, or attribute it to aging itself. Older adults may have a decreased perception of dyspnea even with significant airflow obstruction.22, 23 As smoking can induce symptoms of wheeze, cough and sputum production, asthma may be confused with COPD. Prominent gastroesophageal reflux disease (GERD) may present with a chronic cough, hoarseness and wheezing, and therefore needs to be considered in the differential diagnosis of asthma.24
The same objective measures used to diagnose asthma in younger patients are employed in older patients. However, similar to symptoms of asthma in this group, there are age-related factors which must be considered. First, the FEV1/FVC ratio decreases with aging, and therefore it is essential to use age-adjusted values. 25, 26 Second, with aging, bronchial hyperresponsiveness (BHR) to methacholine increases;27–29 therefore, provocation testing may be less accurate in this age group. Bronchoprovocation challenges may be contraindicated in some older patients with low baseline lung function or cardiac co-morbidities. Third, spirometry involves effort dependent maneuvers. Over 80% of older persons can achieve ATS acceptable results; however, it may be difficult for those who are frail.30–36 Poor coordination and muscle weakness in some patients may produce inaccurate readings of peak expiratory flow.37, 38 Unfortunately, objective measures of lung function such as spirometry and peak flow measurements are generally underutilized in older patients and contribute to the delay in diagnosis.34
Other tests such as the carbon monoxide diffusing capacity of the lung (DLCO) may help distinguish between asthma and COPD. Chest Computed Tomography (CT) may demonstrate increased wall thickness, and increased air-trapping in older asthmatic patients.39 The use of exhaled nitric oxide (eNO) in older patients is not completely established, and aging itself can increase eNO.40, 41 Some studies using eNO as a marker for asthma diagnosis and a measure of control in older patients have shown it to be beneficial, 42–46 whereas others have not.47
Unique factors that can complicate asthma management among older adults – and strategies to address them
Comorbidities
In recent years, there has been a significant increase in the literature demonstrating that comorbidities significantly influence asthma management and control. A 2016 meta-analysis of 11 studies involving 460,000 patients found that asthma patients were more likely to have cardiovascular disease (odds ratio (OR) = 1.9), obesity (OR = 1.51), gut or urinary comorbidities (OR = 1.62), and hypertension (OR = 1.91) compared to those without asthma (p < 0.0001 for all comparisons listed).48 The National Health and Nutrition Examination Survey (NHANES) revealed that adult asthma patients with comorbidities were more likely to have asthma symptoms, activity limitations, and emergency department visits for asthma compared to asthma patients without comorbidities.49 Data specifically looking at adults ≥ 65 years has confirmed that in this age group, comorbidities are directly related to asthma hospitalizations and emergency department visits.50 As people age, the number of medical conditions and medications to manage these conditions increase. Therefore, the negative impact of comorbidities and asthma management has a greater impact on older adults.
In older adults, comorbidities can impact asthma management in a number of ways. There can be interactions between conditions, similar symptom presentation of asthma and comorbidities, decreased adherence with multiple medications, and conflicting recommendations on care from numerous specialists.51 In a qualitative study, older adults with asthma and cardiac disease describe having been instructed by their physician to try medications for both conditions simultaneously and/or sequentially during episodes of dyspnea.21 Additionally, in patients with multiple comorbidities, asthma may take a ‘back seat’ during a primary care office visit, and be sub-optimally addressed.
To improve the care of older adults with asthma, clinicians should identify, acknowledge, and address comorbidities where appropriate. A recent study by Tay et. al in the Journal found that asthma outcomes improved when asthma specialists identified and subsequently treated or referred patients for specific comorbidities (e.g. GERD, sleep apnea, or obesity).52 Additionally, emphasizing to a patient that suboptimal asthma control can make exercise and weight loss problematic may impress upon some the importance of regular asthma care. With improved control, it may be possible for the older adult to reduce the burden of other conditions such as cardiac disease, diabetes, and obesity.
Treatment of co-morbid allergic rhinitis with nasal corticosteroids is a relatively safe approach in older adults, and may be an option to improve asthma control without stepping up controller therapy.53 Specific immunotherapy improves allergic rhinitis in older patients, but risks including anaphylaxis and the use of epinephrine in patients with significant cardiac disease must be considered.54 Sublingual immunotherapy has a lower incidence of serious adverse effects and may be an attractive alternative in older adults, though currently most formulations are approved only to the age 65.
Depression
While depression is an asthma comorbidity, it deserves additional mention due to recent literature showing its negative effects on elderly asthmatic patients. Depression is relatively common in adults above the age of 65, affecting up to 20% of older adults in Western countries.55 A recent article in the Journal demonstrated that older adults with asthma and depression were nearly twice as likely to have poor asthma outcomes across several indicators, including asthma-related ED/urgent care visits, compared with those without depression.56 The mechanisms responsible for depression worsening asthma outcomes are not clearly established, and may relate to inflammation, cerebral anatomic changes, autonomic nervous dysfunction, decreased adherence, or ineffective self-management behaviors.57
Small trials have shown modest benefit of pharmacotherapy and/or behavioral therapy in the treatment of adults with coexisting asthma and depression, though none were exclusively performed in an older population.58–61 Given the prevalence of depression among older adults, its known effects on asthma, and the potential benefit of therapy, asthma providers should consider screening patients for depression (for example, with the Geriatric Depression Scale)62 and treat or refer as appropriate.
Menopause and Hormone Replacement Therapy (HRT)
Several studies have documented the effects of hormones such as estrogen and progesterone on airway caliber and asthma exacerbations. A study of 2322 peri-menopausal women found the risk of new-onset asthma was 2.4–3.4 times higher compared to pre-menopausal women,63 and menopause was associated with an accelerated lung function decline.64 A separate meta-analysis reported that post-menopausal women on HRT had an increased risk of asthma compared to pre-menopausal women.65 Conversely, in women with pre-existing asthma, HRT may improve respiratory symptoms and decrease exacerbations.66
Menopause may also increase the number of asthma exacerbations.67, 68 There is a spike in exacerbations among asthmatic women that occurs at age 50,69 the mean age of menopause in the United States. This may indicate that sex hormones have a protective effect in asthma, or that fluctuations in hormonal levels can be particularly detrimental. Asthma in women that begins after menopause is frequently severe, non-atopic, and often requires oral corticosteroids for control.12, 70 The risks and benefits of HRT must be considered, and the asthma care provider is in a unique position to offer specific information to assist in making decisions about HRT.
Caregiver roles and transportation difficulties
Older adults are ever more frequently taking on greater care giving roles – whether that of a spouse with significant medical problems, a child who is living at home, or a grandchild while the parents work. Being in a stressful caregiver position has been associated with poorer self-reported health, more negative health behaviors, and greater use of health care.71 It may be beneficial to inquire and acknowledge the challenges that providing care to others entails, and to highlight that to be optimally effective in such a role requires management of one’s own health – including asthma.
Transportation can also be problematic for many older adults. Loss of driving privileges can make follow-up care difficult. The asthma provider can help work with senior service agencies, case managers, or social workers to document the need for follow-up care, and may also try to schedule visits on the same day as other medical appointments.72
Complementary and Alternative Medicine
The use of complementary and alternative medicine (CAM) to manage asthma symptoms is between 20 – 30%.73 An analysis of over 7000 adults above the age of 55 from the Asthma Call Back Survey found that this number was even higher at 39%.74 The most frequent modalities included breathing exercises, vitamins, and herbal therapy. Other research has shown that older adults with asthma rarely, if ever, discuss CAM with their physician.21 Therefore, the provider caring for the older adult with asthma should inquire about CAM usage, discuss potential risks/benefits of such therapies as compared to traditional medicine, and discuss how CAM can be used in conjunction with traditional therapies.
Financial concerns
The current rate among adults over the age of 65 living in poverty is approximately 10%, and this number is expected to rise in the upcoming years.75 Poverty has numerous detrimental effects on health, such as the inability to afford insurance copays, increased exposure to pollution and/or environmental triggers, and increased psychological stress which worsens asthma. Patients living in poverty often feel ashamed, and few bring up the inability to pay with their provider.76 Tangible steps that providers can use include empathetic questions on the ability to afford medications while on a fixed income, prescribing medications with the lowest copay, provision of list of financial resources, and referral to social work when appropriate.77
Pharmacologic challenges/strategies
Much of the recommendations for asthma management in older patients is based upon therapeutic studies mostly in patients primarily <60 years of age,78 and a recent ATS workshop report on management of asthma in the elderly specifically noted difficulties in making concrete pharmacologic recommendations.79 There are several important challenges, both in relationship to potential age-related responses to therapies, and decreases in cognitive and physical functioning. For example, older patients with decreased cognitive function had poorer inhaler technique.80 To improve medication delivery and adherence, older patients can be prescribed breath-activated medication devices,81 spacers to attach to metered dose inhalers (MDI) or medications which can be delivered by nebulizer(including corticosteroids for daily controller use, although this is not currently an FDA indication for patients with asthma over 12 years of age), or an ElliptaTM DPI device.82 Furthermore, like treatment of other medical conditions in geriatric medicine, there are issues of polypharmacy, drug-drug interactions and cost of medications which can significantly impact optimal treatment. The following sections will address issues of rescue and controller asthma therapies in the aged.
Rescue medications
Although not recently investigated, the literature suggests that older patients may have a more pronounced bronchodilator response to anti-cholinergic compared to beta2 agonist therapy.83, 84 With aging, there may be a decline in beta2-receptor density, responsiveness and affinity.85, 86 Older patients may be more sensitive to adverse effects of beta2-agonists, particularly those with unstable cardiovascular disorders.87, 88 Research in adults comparing albuterol to levalbuterol has demonstrated a similar rate of side effects (including headache, tremor, hyperglycemia and tachycardia), though studies in an predominantly elderly population are lacking.89, 90 Although more likely with oral agents, cognitive impairment, falls, symptomatic urinary outlet obstruction and closed angle glaucoma are potential risks of high doses of inhaled anti-cholinergics.91
Controller therapies
Inhaled corticosteroids (ICS) are important controller therapies in patients with persistent asthma, yet appear to be underutilized in older patients.92–94 There are possible reasons for their underuse in this age group, including concerns for adverse effects of higher doses and under-diagnosis of asthma in older adults. Older patients, in particular women, receiving higher dose ICS (e.g., >1000mcg/day budesonide equivalent) should be monitored closely for decreased bone mineral density and increased fracture risk.95–98 Older patients should be given an ICS with a lower bioavailability and at the lowest dose to control their disease, with consideration of step-down therapy if he/she is well controlled for at least three months. To decrease the effects of corticosteroids on bone resorption, patients should be encouraged to exercise (if possible), avoid excess alcohol intake, and use daily supplemental calcium with vitamin D. Observational studies in the elderly have suggested that ICS have a small, but significant risk of subcapsular and nuclear cataracts99, 100 and a small risk for developing glaucoma; however, further studies are needed.101
In regard to the effectiveness, data suggests that ICS therapy is less effective in older adults compared to younger age groups. Th-17 mediated asthma, marked by neutrophilic inflammation, has been recognized to be less responsive to ICS therapy, and is likely more common among older asthmatics.102 Additionally, some older patients with asthma may have a component of fixed airway obstruction.103 A large, retrospective review of 10 landmark asthma trials in patients with mild-moderate disease on ICS therapy, found an age-related increase in treatment failures.104 In this analysis, the number of patients receiving inhaled corticosteroids was significantly higher than all other therapies suggesting that it may have been underpowered to detect a similarly increased failures with other controller medications.
Despite these findings, ICS use reduces hospital admissions and mortality in older adults,105 but similar to younger patients with asthma, there are likely specific disease phenotypes which responds better to corticosteroid therapy. The use of ultra-fine particle ICS medications may be beneficial in older asthmatics, who are reported to have more small airway involvement,106 but this has not been formally evaluated. Despite this data, ICS therapy is still currently the preferred medication for older patients with persistent disease.
The safety of long acting beta-agonist (LABA) use in older patients, particular those with underlying cardiovascular disease, has been most studied in COPD. Studies specifically focused on the safety of this class of drugs in the elderly asthma patients are lacking; however, the boxed warning for use of LABAs as monotherapy applies to all patients with asthma. A recent large FDA-mandated study demonstrated the safety of LABAs as add-on therapy in asthma, and included 11% of individuals ≥ 64 years. While no increased evidence of adverse events was found in older adults, the study was not adequately powered for that subpopulation.107 Long acting muscarinic antagonists have been shown to be efficacious as add-on therapy in asthmatics up to age 75 years.108, 109 Their use in older patients with asthma requires further study, particularly if this age group has a preferential bronchodilator response to anti-cholinergics.
The use of oral controller asthma therapies for older patients is attractive in that it would likely increase compliance due to its ease of use. There are a few studies examining the efficacy of leukotriene receptor antagonists (LTRAs) in older patients. Although significant improvement in asthma indices was observed in older patients receiving LTRAs, it was less pronounced than in younger patients 110 and in older patients treated with ICS therapy.111, 112 The use of methylxanthines (e.g. theophylline) in older adults, is limited by its relatively weak bronchodilator properties, along with its many side effects and drug interactions.113
Injectable biologics offer a potentially easier administration and effective medication for older patients with asthma. Most studies of anti-IgE (omalizumab) treatment in older patients with asthma have demonstrated clinical improvement in asthma symptoms and exacerbations, though the response may be less than in younger patients. 114–117 Analysis from data collected from 2 randomized clinical trials investigating the use of reslizumab (an anti-IL-5 monoclonal antibody) demonstrate a reduction in asthma exacerbations and improved lung function in patients with LOA,.118
Nonpharmacologic treatments
A recent review in the Journal discussed the current state of nonpharmacologic treatment for severe asthma. While this review was not focused on older adults, it reported that both pulmonary rehabilitation and breathing techniques may be helpful in a subset of patients without causing harm.119 Among older adults, there are limited studies that appear to support these techniques.120, 121 Additionally, older patients with asthma have been shown to have a poorer understanding of their medication use and self-management.122 Asthma educational strategies appear beneficial in older adults, although these strategies are frequently underutilized in this population.123, 124 For example, a 6-session randomized controlled trial of a self-regulation behavioral intervention demonstrated an improvement in health care utilization, asthma control and quality of life compared to the control group.125
Asthma COPD Overlap (ACO)
A specific entity in the differential diagnosis that deserves mention is Asthma COPD Overlap (ACO), which has also been referred to as Asthma COPD Overlap Syndrome (ACOS). A recent workshop by the NIH and American Thoracic Society described this disorder as a condition in which patients with asthma have features of COPD, or patients with COPD have features of asthma.126 Patients with ACO appear to have more hospitalizations, worse quality of life, and greater symptom burden than those with asthma or COPD alone.127, 128 Although precise definition, diagnosis, testing, and treatment options for ACO are still being established, what is most germane to this review is that a majority of ACO patients will likely be above the age of 50. As the NIH workshop participants note, at this time there is not a single, universal definition of ACO for diagnosis and treatment, but several research themes are emerging. Unquestionably, older adults with asthma will play an important role in future investigations of ACO.126
Conclusion
Asthma among older adults is a common disorder, and as the population of the United States grows older, it will be encountered by anyone who manages adult asthma. As demonstrated in Tables 1 and 2, there are unique features related to diagnosis, medication, and non-pharmaceutical concerns that must be identified and properly managed in order to provide optimal care. As further research is performed in older adults with asthma, important medical discoveries will ensure that management in the golden years is truly golden.
Table 1.
Important non-medication considerations for older 357 adults with asthma
| Item | Possible solution |
|---|---|
| Symptoms of asthma are often similar to other age groups, but differences do exist | Ask about non-traditional asthma symptoms, such as fatigue |
| Older adults may have a decreased perception of dyspnea | Consider use of a peak flow meter to assist in assessment of airflow obstruction |
| Bronchial hyper-responsiveness to methacholine increases with age | Interpret results with caution, and consider a higher threshold for an abnormal test |
| Older adults with decreased cognition have poor inhaler technique | Consider breath-activated devices, spacers, and nebulizers |
| Comorbidities are common among older adults, and can negatively impact asthma | Identify, acknowledge, and address comorbidities where appropriate |
| Depression is especially problematic asthma management for older adults | Consider screening, treatment and/or referral for depression |
| Menopause is often associated with asthma exacerbations | The risks and benefits of hormone replacement therapy should be carefully considered in difficult to control asthma |
| Older adults frequently have caregiver roles for their spouse, children, or grandchildren | Acknowledge the challenges, and stress that to be optimally effective you must take care of your own health |
| Transportation can be problematic | Work with senior service agencies and social workers; schedule multiple appointments on the same day |
| Frequent use of complementary and alternative medicine (CAM) for asthma | Discuss risks and benefits of such therapies with your patient; consider how breathing exercises and asthma education can be incorporated |
| Poverty is a growing problem among older adults, and can adversely affect health | Use empathetic communication to discuss financial issues; prescribe medications with the lowest copay; refer to appropriate financial services |
Table 2.
Medication considerations in older 360 adults with asthma
| Medication | Consideration |
|---|---|
| Short acting beta agonists |
|
| Short acting muscarinic antagonists |
|
| Inhaled corticosteroids |
|
| Long acting beta agonists |
|
| Long acting muscarinic antagonists |
|
| Leukotriene receptor antagonists |
|
| Specific immunotherapy |
|
| Omalizumab |
|
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alan P. Baptist, University of Michigan.
Paula J. Busse, Icahn School of Medicine at Mount Sinai.
References
- 1.Mather M, Jacobsen LA, Pollard KM. Aging in the United States. Population Bulletin. 2015;70(2):1–17. [Google Scholar]
- 2.Bellia V, Battaglia S, Catalano F, Scichilone N, Incalzi RA, Imperiale C, et al. Aging and disability affect misdiagnosis of COPD in elderly asthmatics: the SARA study. Chest. 2003 Apr;123(4):1066–1072. doi: 10.1378/chest.123.4.1066. [DOI] [PubMed] [Google Scholar]
- 3.Tinkelman DG, Price DB, Nordyke RJ, Halbert RJ. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006 Jan-Feb;43(1):75–80. doi: 10.1080/02770900500448738. [DOI] [PubMed] [Google Scholar]
- 4.Straus SE, McAlister FA, Sackett DL, Deeks JJ. The accuracy of patient history, wheezing, and laryngeal measurements in diagnosing obstructive airway disease. CARE-COAD1 Group. Clinical Assessment of the Reliability of the Examination-Chronic Obstructive Airways Disease. JAMA. 2000 Apr 12;283(14):1853–1857. doi: 10.1001/jama.283.14.1853. [DOI] [PubMed] [Google Scholar]
- 5.National Health Interview Survey, 2015. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention; Table A-2. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2015_SHS_Table_A-2.pdf. [Google Scholar]
- 6.Winer RA, Qin X, Harrington T, Moorman J, Zahran H. Asthma incidence among children and adults: findings from the Behavioral Risk Factor Surveillance system asthma call-back survey--United States, 2006–2008. J Asthma. 2012 Feb;49(1):16–22. doi: 10.3109/02770903.2011.637594. [DOI] [PubMed] [Google Scholar]
- 7.Baptist AP, Hao W, Karamched KR, Kaur B, Carpenter L, Song PXK. Distinct Asthma Phenotypes among Older Adults with Asthma. The journal of allergy and clinical immunology. In practice. 2017 Jul 27; doi: 10.1016/j.jaip.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Nijs SB, Venekamp LN, Bel EH. Adult-onset asthma: is it really different? Eur Respir Rev. 2013 Mar 01;22(127):44–52. doi: 10.1183/09059180.00007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baptist AP, Ross JA, Clark NM. Older adults with asthma: does age of asthma onset make a difference? J Asthma. 2013 Oct;50(8):836–841. doi: 10.3109/02770903.2013.816967. [DOI] [PubMed] [Google Scholar]
- 10.Herscher ML, Wisnivesky JP, Busse PJ, Hanania NA, Sheng T, Wolf MS, et al. Characteristics and outcomes of older adults with long-standing versus late-onset asthma. J Asthma. 2017 Apr;54(3):223–229. doi: 10.1080/02770903.2016.1211141. [DOI] [PubMed] [Google Scholar]
- 11.Park HW, Song WJ, Kim SH, Park HK, Kim SH, Kwon YE, et al. Classification and implementation of asthma phenotypes in elderly patients. Ann Allergy Asthma Immunol. 2015 Jan;114(1):18–22. doi: 10.1016/j.anai.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010 Feb 15;181(4):315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw DE, Sousa AR, Fowler SJ, Fleming LJ, Roberts G, Corfield J, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015 Nov;46(5):1308–1321. doi: 10.1183/13993003.00779-2015. [DOI] [PubMed] [Google Scholar]
- 14.Rui P, Kang K. National Hospital Ambulatory Medical Care Survey: 2014 Emergency Department Summary Tables. Available from: http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2014_ed_web_tables.pdf.
- 15.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014 Jun;69( Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013 Jun 06;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer BA, Reed CE, Yunginger JW, Wollan PC, Silverstein MD. Incidence and outcomes of asthma in the elderly. A population-based study in Rochester, Minnesota. Chest. 1997 Feb;111(2):303–310. doi: 10.1378/chest.111.2.303. [DOI] [PubMed] [Google Scholar]
- 18.Nyenhuis SM, Schwantes EA, Evans MD, Mathur SK. Airway neutrophil inflammatory phenotype in older subjects with asthma. J Allergy Clin Immunol. 2010 May;125(5):1163–1165. doi: 10.1016/j.jaci.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood LG, Baines KJ, Fu J, Scott HA, Gibson PG. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest. 2012 Jul;142(1):86–93. doi: 10.1378/chest.11-1838. [DOI] [PubMed] [Google Scholar]
- 20.Busse PJ, Birmingham JM, Calatroni A, Manzi J, Goryachokovsky A, Fontela G, et al. Effect of aging on sputum inflammation and asthma control. J Allergy Clin Immunol. 2017 Jun;139(6):1808–1818. e1806. doi: 10.1016/j.jaci.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baptist AP, Deol BB, Reddy RC, Nelson B, Clark NM. Age-specific factors influencing asthma management by older adults. Qual Health Res. 2010 Jan;20(1):117–124. doi: 10.1177/1049732309355288. [DOI] [PubMed] [Google Scholar]
- 22.Quadrelli SA, Roncoroni A. Features of asthma in the elderly. J Asthma. 2001 Aug;38(5):377–389. doi: 10.1081/jas-100000259. [DOI] [PubMed] [Google Scholar]
- 23.Weiner P, Magadle R, Waizman J, Weiner M, Rabner M, Zamir D. Characteristics of asthma in the elderly. Eur Respir J. 1998 Sep;12(3):564–568. doi: 10.1183/09031936.98.12030564. [DOI] [PubMed] [Google Scholar]
- 24.Raiha I, Impivaara O, Seppala M, Knuts LR, Sourander L. Determinants of symptoms suggestive of gastroesophageal reflux disease in the elderly. Scandinavian journal of gastroenterology. 1993 Nov;28(11):1011–1014. doi: 10.3109/00365529309098301. [DOI] [PubMed] [Google Scholar]
- 25.Vaz Fragoso CA, McAvay G, Van Ness PH, Casaburi R, Jensen RL, MacIntyre N, et al. Phenotype of Normal Spirometry in an Aging Population. Am J Respir Crit Care Med. 2015 Oct 1;192(7):817–825. doi: 10.1164/rccm.201503-0463OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012 Dec;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renwick DS, Connolly MJ. The relationship between age and bronchial responsiveness: evidence from a population survey. Chest. 1999 Mar;115(3):660–665. doi: 10.1378/chest.115.3.660. [DOI] [PubMed] [Google Scholar]
- 28.Cuttitta G, Cibella F, Bellia V, Grassi V, Cossi S, Bucchieri S, et al. Changes in FVC during methacholine-induced bronchoconstriction in elderly patients with asthma: bronchial hyperresponsiveness and aging. Chest. 2001 Jun;119(6):1685–1690. doi: 10.1378/chest.119.6.1685. [DOI] [PubMed] [Google Scholar]
- 29.Scichilone N, Messina M, Battaglia S, Catalano F, Bellia V. Airway hyperresponsiveness in the elderly: prevalence and clinical implications. Eur Respir J. 2005 Feb;25(2):364–375. doi: 10.1183/09031936.05.00080204. [DOI] [PubMed] [Google Scholar]
- 30.Bellia V, Battaglia S, Matera MG, Cazzola M. The use of bronchodilators in the treatment of airway obstruction in elderly patients. Pulmonary pharmacology & therapeutics. 2006;19(5):311–319. doi: 10.1016/j.pupt.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Luoto JA, Elmstahl S, Wollmer P, Pihlsgard M. Incidence of airflow limitation in subjects 65–100 years of age. Eur Respir J. 2016 Feb;47(2):461–472. doi: 10.1183/13993003.00635-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen SC, Ragab S. Ability to learn inhaler technique in relation to cognitive scores and tests of praxis in old age. Postgrad Med J. 2002 Jan;78(915):37–39. doi: 10.1136/pmj.78.915.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen SC, Yeung P. Inability to draw intersecting pentagons as a predictor of unsatisfactory spirometry technique in elderly hospital inpatients. Age Ageing. 2006 May;35(3):304–306. doi: 10.1093/ageing/afj090. [DOI] [PubMed] [Google Scholar]
- 34.Bellia V, Pistelli R, Catalano F, Antonelli-Incalzi R, Grassi V, Melillo G, et al. Quality control of spirometry in the elderly. The SA.R.A. study. SAlute Respiration nell'Anziano = Respiratory Health in the Elderly. Am J Respir Crit Care Med. 2000 Apr;161(4 Pt 1):1094–1100. doi: 10.1164/ajrccm.161.4.9810093. [DOI] [PubMed] [Google Scholar]
- 35.Bellia V, Pistelli R, Catalano F, Antonelli-Incalzi R, Grassi V, Melillo G, et al. Quality Control of Spirometry in the Elderly. American Journal of Respiratory and Critical Care Medicine. 2000 Apr 01;161(4):1094–1100. doi: 10.1164/ajrccm.161.4.9810093. [DOI] [PubMed] [Google Scholar]
- 36.Sherman CB, Kern D, Richardson ER, Hubert M, Fogel BS. Cognitive Function and Spirometry Performance in the Elderly. American Review of Respiratory Disease. 1993 Jul 01;148(1):123–126. doi: 10.1164/ajrccm/148.1.123. [DOI] [PubMed] [Google Scholar]
- 37.Enright PL, McClelland RL, Buist AS, Lebowitz MD. Correlates of peak expiratory flow lability in elderly persons. Chest. 2001 Dec;120(6):1861–1868. doi: 10.1378/chest.120.6.1861. [DOI] [PubMed] [Google Scholar]
- 38.Enright PL, Burchette RJ, Peters JA, Lebowitz MD, McDonnell WF, Abbey DE. Peak flow lability: association with asthma and spirometry in an older cohort. Chest. 1997 Oct;112(4):895–901. doi: 10.1378/chest.112.4.895. [DOI] [PubMed] [Google Scholar]
- 39.Inoue H, Niimi A, Takeda T, Matsumoto H, Ito I, Matsuoka H, et al. Pathophysiological characteristics of asthma in the elderly: a comprehensive study. Ann Allergy Asthma Immunol. 2014 Nov;113(5):527–533. doi: 10.1016/j.anai.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Toren K, Murgia N, Schioler L, Bake B, Olin AC. Reference values of fractional excretion of exhaled nitric oxide among non-smokers and current smokers. BMC pulmonary medicine. 2017 Aug 25;17(1):118. doi: 10.1186/s12890-017-0456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malerba M, Damiani G, Carpagnano GE, Olivini A, Radaeli A, Ragnoli B, et al. Values in Elderly People for Exhaled Nitric Oxide Study. Rejuvenation research. 2016 Jun;19(3):233–238. doi: 10.1089/rej.2015.1706. [DOI] [PubMed] [Google Scholar]
- 42.Bozek A, Filipowski M, Fischer A, Jarzab J. Characteristics of atopic bronchial asthma in seniors over 80 years of age. Biomed Res Int. 2013;2013:689782. doi: 10.1155/2013/689782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porsbjerg CM, Gibson PG, Pretto JJ, Salome CM, Brown NJ, Berend N, et al. Relationship between airway pathophysiology and airway inflammation in older asthmatics. Respirology. 2013 Oct;18(7):1128–1134. doi: 10.1111/resp.12142. [DOI] [PubMed] [Google Scholar]
- 44.Langley SJ, Goldthorpe S, Craven M, Morris J, Woodcock A, Custovic A. Exposure and sensitization to indoor allergens: association with lung function, bronchial reactivity, and exhaled nitric oxide measures in asthma. J Allergy Clin Immunol. 2003 Aug;112(2):362–368. doi: 10.1067/mai.2003.1654. [DOI] [PubMed] [Google Scholar]
- 45.Dweik RA, Sorkness RL, Wenzel S, Hammel J, Curran-Everett D, Comhair SA, et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med. 2010 May 15;181(10):1033–1041. doi: 10.1164/rccm.200905-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Godinho Netto AC, Dos Reis TG, Matheus CF, Aarestrup BJ, Aarestrup FM. Fraction of exhaled nitric oxide measurements in the diagnoses of asthma in elderly patients. Clin Interv Aging. 2016;11:623–629. doi: 10.2147/CIA.S94741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawamatawong T, Siripongpun S, Rerkpattanapipat T. Role of eosinophilic inflammation and atopy in elderly asthmatic patients. Asia Pacific allergy. 2016 Jul;6(3):181–186. doi: 10.5415/apallergy.2016.6.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su X, Ren Y, Li M, Zhao X, Kong L, Kang J. Prevalence of Comorbidities in Asthma and Nonasthma Patients: A Meta-analysis. Medicine (Baltimore) 2016 May;95(22):e3459. doi: 10.1097/MD.0000000000003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel MR, Janevic MR, Heeringa SG, Baptist AP, Clark NM. An examination of adverse asthma outcomes in u.s. Adults with multiple morbidities. Annals of the American Thoracic Society. 2013 Oct;10(5):426–431. doi: 10.1513/AnnalsATS.201302-032OC. [DOI] [PubMed] [Google Scholar]
- 50.Hsu J, Chen J, Mirabelli MC. Asthma Morbidity, Comorbidities, and Modifiable Factors among Older Adults. The journal of allergy and clinical immunology. 2017 Jul 19; doi: 10.1016/j.jaip.2017.06.007. In practice. [DOI] [PMC free article] [PubMed]
- 51.Bayliss EA, Steiner JF, Fernald DH, Crane LA, Main DS. Descriptions of barriers to self-care by persons with comorbid chronic diseases. Annals of family medicine. 2003 May-Jun;1(1):15–21. doi: 10.1370/afm.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tay TR, Lee J, Radhakrishna N, Hore-Lacy F, Stirling R, Hoy R, et al. A Structured Approach to Specialist-referred Difficult Asthma Patients Improves Control of Comorbidities and Enhances Asthma Outcomes. The journal of allergy and clinical immunology. In practice. 2017 Jul-Aug;5(4):956–964. e953. doi: 10.1016/j.jaip.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 53.Oka A, Hirano T, Yamaji Y, Ito K, Oishi K, Edakuni N, et al. Determinants of Incomplete Asthma Control in Patients with Allergic Rhinitis and Asthma. The journal of allergy and clinical immunology. In practice. 2017 Jan-Feb;5(1):160–164. doi: 10.1016/j.jaip.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Asero R. Efficacy of injection immunotherapy with ragweed and birch pollen in elderly patients. Int Arch Allergy Immunol. 2004 Dec;135(4):332–335. doi: 10.1159/000082328. [DOI] [PubMed] [Google Scholar]
- 55.Volkert J, Schulz H, Harter M, Wlodarczyk O, Andreas S. The prevalence of mental disorders in older people in Western countries - a meta-analysis. Ageing Res Rev. 2013 Jan;12(1):339–353. doi: 10.1016/j.arr.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Patel PO, Patel MR, Baptist AP. Depression and Asthma Outcomes in Older Adults: Results from the National Health and Nutrition Examination Survey. The journal of allergy and clinical immunology. In practice. 2017 Jun 17; doi: 10.1016/j.jaip.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 57.Wang L, Wang T, Liu S, Liang Z, Meng Y, Xiong X, et al. Cerebral anatomical changes in female asthma patients with and without depression compared to healthy controls and patients with depression. J Asthma. 2014 Nov;51(9):927–933. doi: 10.3109/02770903.2014.927482. [DOI] [PubMed] [Google Scholar]
- 58.Brown ES, Vornik LA, Khan DA, Rush AJ. Bupropion in the treatment of outpatients with asthma and major depressive disorder. Int J Psychiatry Med. 2007;37(1):23–28. doi: 10.2190/D235-2285-2121-6724. [DOI] [PubMed] [Google Scholar]
- 59.Mancuso CA, Sayles W, Allegrante JP. Randomized trial of self-management education in asthmatic patients and effects of depressive symptoms. Ann Allergy Asthma Immunol. 2010 Jul;105(1):12–19. doi: 10.1016/j.anai.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown ES, Howard C, Khan DA, Carmody TJ. Escitalopram for severe asthma and major depressive disorder: a randomized, double-blind, placebo-controlled proof-of-concept study. Psychosomatics. 2012 Jan-Feb;53(1):75–80. doi: 10.1016/j.psym.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 61.Yorke J, Adair P, Doyle AM, Dubrow-Marshall L, Fleming S, Holmes L, et al. A randomised controlled feasibility trial of Group Cognitive Behavioural Therapy for people with severe asthma. J Asthma. 2017 Jun;54(5):543–554. doi: 10.1080/02770903.2016.1229335. [DOI] [PubMed] [Google Scholar]
- 62.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 63.Triebner K, Johannessen A, Puggini L, Benediktsdottir B, Bertelsen RJ, Bifulco E, et al. Menopause as a predictor of new-onset asthma: A longitudinal Northern European population study. J Allergy Clin Immunol. 2016 Jan;137(1):50–57. e56. doi: 10.1016/j.jaci.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 64.Triebner K, Matulonga B, Johannessen A, Suske S, Benediktsdottir B, Demoly P, et al. Menopause Is Associated with Accelerated Lung Function Decline. Am J Respir Crit Care Med. 2017 Apr 15;195(8):1058–1065. doi: 10.1164/rccm.201605-0968OC. [DOI] [PubMed] [Google Scholar]
- 65.Zemp E, Schikowski T, Dratva J, Schindler C, Probst-Hensch N. Asthma and the menopause: a systematic review and meta-analysis. Maturitas. 2012 Nov;73(3):212–217. doi: 10.1016/j.maturitas.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 66.Kos-Kudla B, Ostrowska Z, Marek B, Ciesielska-Kopacz N, Kajdaniuk D, Kudla M. Effects of hormone replacement therapy on endocrine and spirometric parameters in asthmatic postmenopausal women. Gynecol Endocrinol. 2001 Aug;15(4):304–311. [PubMed] [Google Scholar]
- 67.Balzano G, Fuschillo S, Melillo G, Bonini S. Asthma and sex hormones. Allergy. 2001 Jan;56(1):13–20. doi: 10.1034/j.1398-9995.2001.00128.x. [DOI] [PubMed] [Google Scholar]
- 68.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev. 2012 Feb;33(1):1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skobeloff EM, Spivey WH, St Clair SS, Schoffstall JM. The influence of age and sex on asthma admissions. JAMA. 1992 Dec 23–30;268(24):3437–3440. [PubMed] [Google Scholar]
- 70.Balzano G, Fuschillo S, De Angelis E, Gaudiosi C, Mancini A, Caputi M. Persistent airway inflammation and high exacerbation rate in asthma that starts at menopause. Monaldi Arch Chest Dis. 2007 Sep;67(3):135–141. doi: 10.4081/monaldi.2007.484. [DOI] [PubMed] [Google Scholar]
- 71.Son J, Erno A, Shea DG, Femia EE, Zarit SH, Stephens MA. The caregiver stress process and health outcomes. J Aging Health. 2007 Dec;19(6):871–887. doi: 10.1177/0898264307308568. [DOI] [PubMed] [Google Scholar]
- 72.Dugdale DC, Epstein R, Pantilat SZ. Time and the patient-physician relationship. J Gen Intern Med. 1999 Jan;14( Suppl 1):S34–40. doi: 10.1046/j.1525-1497.1999.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Slader CA, Reddel HK, Jenkins CR, Armour CL, Bosnic-Anticevich SZ. Complementary and alternative medicine use in asthma: who is using what? Respirology. 2006 Jul;11(4):373–387. doi: 10.1111/j.1440-1843.2006.00861.x. [DOI] [PubMed] [Google Scholar]
- 74.Ward CE, Baptist AP. Characteristics of Complementary and Alternative Medicine (CAM) use among older adults with asthma. J Asthma. 2016 Jan 19;:1–7. doi: 10.3109/02770903.2015.1116090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.National Council on Aging. [Accessed Jan 18, 2017];Economic Security for Seniors Facts. https://www.ncoa.org/news/resources-for-reporters/get-the-facts/economic-security-facts/
- 76.Alexander GC, Casalino LP, Meltzer DO. Patient-physician communication about out-of-pocket costs. JAMA. 2003 Aug 20;290(7):953–958. doi: 10.1001/jama.290.7.953. [DOI] [PubMed] [Google Scholar]
- 77.Hardee JT, Platt FW, Kasper IK. Discussing health care costs with patients: an opportunity for empathic communication. J Gen Intern Med. 2005 Jul;20(7):666–669. doi: 10.1111/j.1525-1497.2005.0125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007 Nov;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 79.Skloot GS, Busse PJ, Braman SS, Kovacs EJ, Dixon AE, Vaz Fragoso CA, et al. An Official American Thoracic Society Workshop Report: Evaluation and Management of Asthma in the Elderly. Annals of the American Thoracic Society. 2016 Nov;13(11):2064–2077. doi: 10.1513/AnnalsATS.201608-658ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allen SC, Jain M, Ragab S, Malik N. Acquisition and short-term retention of inhaler techniques require intact executive function in elderly subjects. Age Ageing. 2003 May;32(3):299–302. doi: 10.1093/ageing/32.3.299. [DOI] [PubMed] [Google Scholar]
- 81.Chapman KR, Love L, Brubaker H. A comparison of breath-actuated and conventional metered-dose inhaler inhalation techniques in elderly subjects. Chest. 1993 Nov;104(5):1332–1337. doi: 10.1378/chest.104.5.1332. [DOI] [PubMed] [Google Scholar]
- 82.Ishiura Y, Fujimura M, Shiba Y, Ohkura N, Hara J, Abo M, et al. A Comparison of the Efficacy of Once-Daily Fluticasone Furoate/Vilanterole with Twice-Daily Fluticasone Propionate/Salmeterol in Elderly Asthmatics. Drug research. 2017 Sep 19; doi: 10.1055/s-0043-118536. [DOI] [PubMed] [Google Scholar]
- 83.van Schayck CP, Folgering H, Harbers H, Maas KL, van Weel C. Effects of allergy and age on responses to salbutamol and ipratropium bromide in moderate asthma and chronic bronchitis. Thorax. 1991 May;46(5):355–359. doi: 10.1136/thx.46.5.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barros MJ, Rees PJ. Bronchodilator responses to salbutamol followed by ipratropium bromide in partially reversible airflow obstruction. Respiratory medicine. 1990 Sep;84(5):371–375. doi: 10.1016/s0954-6111(08)80071-6. [DOI] [PubMed] [Google Scholar]
- 85.Connolly MJ. Ageing, late-onset asthma and the beta-adrenoceptor. Pharmacology & therapeutics. 1993 Dec;60(3):389–404. doi: 10.1016/0163-7258(93)90029-d. [DOI] [PubMed] [Google Scholar]
- 86.Connolly MJ, Crowley JJ, Charan NB, Nielson CP, Vestal RE. Impaired bronchodilator response to albuterol in healthy elderly men and women. Chest. 1995 Aug;108(2):401–406. doi: 10.1378/chest.108.2.401. [DOI] [PubMed] [Google Scholar]
- 87.Bellia V, Pedone C, Catalano F, Zito A, Davi E, Palange S, et al. Asthma in the elderly: mortality rate and associated risk factors for mortality. Chest. 2007 Oct;132(4):1175–1182. doi: 10.1378/chest.06-2824. [DOI] [PubMed] [Google Scholar]
- 88.Gupta P, O'Mahony MS. Potential adverse effects of bronchodilators in the treatment of airways obstruction in older people: recommendations for prescribing. Drugs Aging. 2008;25(5):415–443. doi: 10.2165/00002512-200825050-00005. [DOI] [PubMed] [Google Scholar]
- 89.Donohue JF, Hanania NA, Ciubotaru RL, Noe L, Pasta DJ, Schaefer K, et al. Comparison of levalbuterol and racemic albuterol in hospitalized patients with acute asthma or COPD: a 2-week, multicenter, randomized, open-label study. Clin Ther. 2008;30(Spec No):989–1002. doi: 10.1016/j.clinthera.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 90.Nowak R, Emerman C, Hanrahan JP, Parsey MV, Hanania NA, Claus R, et al. A comparison of levalbuterol with racemic albuterol in the treatment of acute severe asthma exacerbations in adults. Am J Emerg Med. 2006 May;24(3):259–267. doi: 10.1016/j.ajem.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 91.Collamati A, Martone AM, Poscia A, Brandi V, Celi M, Marzetti E, et al. Anticholinergic drugs and negative outcomes in the older population: from biological plausibility to clinical evidence. Aging Clin Exp Res. 2015 May 1; doi: 10.1007/s40520-015-0359-7. [DOI] [PubMed] [Google Scholar]
- 92.Enright PL, McClelland RL, Newman AB, Gottlieb DJ, Lebowitz MD. Underdiagnosis and undertreatment of asthma in the elderly. Cardiovascular Health Study Research Group. Chest. 1999 Sep;116(3):603–613. doi: 10.1378/chest.116.3.603. [DOI] [PubMed] [Google Scholar]
- 93.Hartert TV, Togias A, Mellen BG, Mitchel EF, Snowden MS, Griffin MR. Underutilization of controller and rescue medications among older adults with asthma requiring hospital care. J Am Geriatr Soc. 2000 Jun;48(6):651–657. doi: 10.1111/j.1532-5415.2000.tb04723.x. [DOI] [PubMed] [Google Scholar]
- 94.Sin DD, Tu JV. Underuse of inhaled steroid therapy in elderly patients with asthma. Chest. 2001 Mar;119(3):720–725. doi: 10.1378/chest.119.3.720. [DOI] [PubMed] [Google Scholar]
- 95.Etminan M, Sadatsafavi M, Ganjizadeh Zavareh S, Takkouche B, FitzGerald JM. Inhaled corticosteroids and the risk of fractures in older adults: a systematic review and meta-analysis. Drug Saf. 2008;31(5):409–414. doi: 10.2165/00002018-200831050-00005. [DOI] [PubMed] [Google Scholar]
- 96.Battaglia S, Cardillo I, Lavorini F, Spatafora M, Scichilone N. Safety considerations of inhaled corticosteroids in the elderly. Drugs Aging. 2014 Nov;31(11):787–796. doi: 10.1007/s40266-014-0213-1. [DOI] [PubMed] [Google Scholar]
- 97.Suissa S, Baltzan M, Kremer R, Ernst P. Inhaled and nasal corticosteroid use and the risk of fracture. Am J Respir Crit Care Med. 2004 Jan 01;169(1):83–88. doi: 10.1164/rccm.200305-640OC. [DOI] [PubMed] [Google Scholar]
- 98.Mortimer KJ, Harrison TW, Tattersfield AE. Effects of inhaled corticosteroids on bone. Ann Allergy Asthma Immunol. 2005 Jan;94(1):15–21. doi: 10.1016/S1081-1206(10)61280-X. quiz 22–13, 79. [DOI] [PubMed] [Google Scholar]
- 99.Garbe E, Suissa S, LeLorier J. Association of inhaled corticosteroid use with cataract extraction in elderly patients. JAMA. 1998 Aug 12;280(6):539–543. doi: 10.1001/jama.280.6.539. [DOI] [PubMed] [Google Scholar]
- 100.Ernst P, Baltzan M, Deschenes J, Suissa S. Low-dose inhaled and nasal corticosteroid use and the risk of cataracts. Eur Respir J. 2006 Jun;27(6):1168–1174. doi: 10.1183/09031936.06.00043005. [DOI] [PubMed] [Google Scholar]
- 101.Leone FT, Fish JE, Szefler SJ, West SL. Systematic review of the evidence regarding potential complications of inhaled corticosteroid use in asthma: collaboration of American College of Chest Physicians, American Academy of Allergy, Asthma, and Immunology, and American College of Allergy, Asthma, and Immunology. Chest. 2003 Dec;124(6):2329–2340. doi: 10.1378/chest.124.6.2329. [DOI] [PubMed] [Google Scholar]
- 102.Busse PJ, Birmingham JM, Calatroni A, Manzi J, Goryachokovsky A, Fontela G, et al. The effect of aging on sputum inflammation and asthma control. J Allergy Clin Immunol. 2016 Oct 7; doi: 10.1016/j.jaci.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Braman SS, Kaemmerlen JT, Davis SM. Asthma in the elderly. A comparison between patients with recently acquired and long-standing disease. Am Rev Respir Dis. 1991 Feb;143(2):336–340. doi: 10.1164/ajrccm/143.2.336. [DOI] [PubMed] [Google Scholar]
- 104.Dunn RM, Lehman E, Chinchilli VM, Martin RJ, Boushey HA, Israel E, et al. Impact of Age and Gender on Response to Asthma Therapy. Am J Respir Crit Care Med. 2015 Jun 11; doi: 10.1164/rccm.201503-0426OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sin DD, Tu JV. Inhaled corticosteroid therapy reduces the risk of rehospitalization and all-cause mortality in elderly asthmatics. Eur Respir J. 2001 Mar;17(3):380–385. doi: 10.1183/09031936.01.17303800. [DOI] [PubMed] [Google Scholar]
- 106.Inoue H, Niimi A, Takeda T, Matsumoto H, Ito I, Matsuoka H, et al. Pathophysiological characteristics of asthma in the elderly: a comprehensive study. Annals of Allergy, Asthma & Immunology. 2014;113(5):527–533. doi: 10.1016/j.anai.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 107.Stempel DA, Raphiou IH, Kral KM, Yeakey AM, Emmett AH, Prazma CM, et al. Serious Asthma Events with Fluticasone plus Salmeterol versus Fluticasone Alone. N Engl J Med. 2016 May 12;374(19):1822–1830. doi: 10.1056/NEJMoa1511049. [DOI] [PubMed] [Google Scholar]
- 108.Kerstjens HAM, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in Asthma Poorly Controlled with Standard Combination Therapy. New England Journal of Medicine. 2012;367(13):1198–1207. doi: 10.1056/NEJMoa1208606. [DOI] [PubMed] [Google Scholar]
- 109.Kerstjens HAM, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. The Lancet Respiratory Medicine. 3(5):367–376. doi: 10.1016/S2213-2600(15)00031-4. [DOI] [PubMed] [Google Scholar]
- 110.Korenblat PE, Kemp JP, Scherger JE, Minkwitz MC, Mezzanotte W. Effect of age on response to zafirlukast in patients with asthma in the Accolate Clinical Experience and Pharmacoepidemiology Trial (ACCEPT) Ann Allergy Asthma Immunol. 2000 Feb;84(2):217–225. doi: 10.1016/S1081-1206(10)62759-7. [DOI] [PubMed] [Google Scholar]
- 111.Creticos P, Knobil K, Edwards LD, Rickard KA, Dorinsky P. Loss of response to treatment with leukotriene receptor antagonists but not inhaled corticosteroids in patients over 50 years of age. Ann Allergy Asthma Immunol. 2002 Apr;88(4):401–409. doi: 10.1016/S1081-1206(10)62372-1. [DOI] [PubMed] [Google Scholar]
- 112.Bozek A, Warkocka-Szoltysek B, Filipowska-Gronska A, Jarzab J. Montelukast as an add-on therapy to inhaled corticosteroids in the treatment of severe asthma in elderly patients. The Journal of asthma : official journal of the Association for the Care of Asthma. 2012 Jun;49(5):530–534. doi: 10.3109/02770903.2012.680638. [DOI] [PubMed] [Google Scholar]
- 113.Shannon M, Lovejoy FH., Jr The influence of age vs peak serum concentration on life-threatening events after chronic theophylline intoxication. Archives of internal medicine. 1990 Oct;150(10):2045–2048. [PubMed] [Google Scholar]
- 114.Maykut RJ, Kianifard F, Geba GP. Response of older patients with IgE-mediated asthma to omalizumab: a pooled analysis. J Asthma. 2008 Apr;45(3):173–181. doi: 10.1080/02770900701247277. [DOI] [PubMed] [Google Scholar]
- 115.Korn S, Schumann C, Kropf C, Stoiber K, Thielen A, Taube C, et al. Effectiveness of omalizumab in patients 50 years and older with severe persistent allergic asthma. Ann Allergy Asthma Immunol. 2010 Oct;105(4):313–319. doi: 10.1016/j.anai.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 116.Sposato B, Scalese M, Latorre M, Scichilone N, Matucci A, Milanese M, et al. Effects of omalizumab in severe asthmatics across ages: A real life Italian experience. Respiratory medicine. 2016 Oct;119:141–149. doi: 10.1016/j.rmed.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 117.Tat TS, Cilli A. Evaluation of long-term safety and efficacy of omalizumab in elderly patients with uncontrolled allergic asthma. Ann Allergy Asthma Immunol. 2016 Nov;117(5):546–549. doi: 10.1016/j.anai.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 118.Brusselle G, Germinaro M, Weiss S, Zangrilli J. Reslizumab in patients with inadequately controlled late-onset asthma and elevated blood eosinophils. Pulmonary pharmacology & therapeutics. 2017 Apr;43:39–45. doi: 10.1016/j.pupt.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 119.Hall C, Nici L, Sood S, ZuWallack R, Castro M. Nonpharmacologic Therapy for Severe Persistent Asthma. The journal of allergy and clinical immunology. 2017 Jul-Aug;5(4):928–935. doi: 10.1016/j.jaip.2017.04.030. In practice. [DOI] [PubMed] [Google Scholar]
- 120.Gomieiro LT, Nascimento A, Tanno LK, Agondi R, Kalil J, Giavina-Bianchi P. Respiratory exercise program for elderly individuals with asthma. Clinics (Sao Paulo) 2011;66(7):1163–1169. doi: 10.1590/S1807-59322011000700007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Majewski M, Dabrowska G, Pawik M, Rozek K. Evaluation of a Home-Based Pulmonary Rehabilitation Program for Older Females Suffering from Bronchial Asthma. Advances in clinical and experimental medicine : official organ Wroclaw Medical University. 2015 Nov-Dec;24(6):1079–1083. doi: 10.17219/acem/31679. [DOI] [PubMed] [Google Scholar]
- 122.Soones TN, Lin JL, Wolf MS, O'Conor R, Martynenko M, Wisnivesky JP, et al. Pathways linking health literacy, health beliefs, and cognition to medication adherence in older adults with asthma. J Allergy Clin Immunol. 2017 Mar;139(3):804–809. doi: 10.1016/j.jaci.2016.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O'Conor R, Martynenko M, Gagnon M, Hauser D, Young E, Lurio J, et al. A qualitative investigation of the impact of asthma and self-management strategies among older adults. J Asthma. 2017 Jan 02;54(1):39–45. doi: 10.1080/02770903.2016.1193602. [DOI] [PubMed] [Google Scholar]
- 124.Goeman D, Jenkins C, Crane M, Paul E, Douglass J. Educational intervention for older people with asthma: a randomised controlled trial. Patient Educ Couns. 2013 Dec;93(3):586–595. doi: 10.1016/j.pec.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 125.Baptist AP, Ross JA, Yang Y, Song PX, Clark NM. A randomized controlled trial of a self-regulation intervention for older adults with asthma. J Am Geriatr Soc. 2013 May;61(5):747–753. doi: 10.1111/jgs.12218. [DOI] [PubMed] [Google Scholar]
- 126.Woodruff PG, van den Berge M, Boucher RC, Brightling C, Burchard EG, Christenson SA, et al. American Thoracic Society/National Heart, Lung, and Blood Institute Asthma-Chronic Obstructive Pulmonary Disease Overlap Workshop Report. Am J Respir Crit Care Med. 2017 Aug 01;196(3):375–381. doi: 10.1164/rccm.201705-0973WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kauppi P, Kupiainen H, Lindqvist A, Tammilehto L, Kilpelainen M, Kinnula VL, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011 Apr;48(3):279–285. doi: 10.3109/02770903.2011.555576. [DOI] [PubMed] [Google Scholar]
- 128.de Marco R, Pesce G, Marcon A, Accordini S, Antonicelli L, Bugiani M, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PloS one. 2013;8(5):e62985. doi: 10.1371/journal.pone.0062985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meyer KC, Soergel P. Variation of bronchoalveolar lymphocyte phenotypes with age in the physiologically normal human lung. Thorax. 1999 Aug;54(8):697–700. doi: 10.1136/thx.54.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ducharme ME, Prince P, Hassan N, Nair P, Boulet LP. Expiratory flows and airway inflammation in elderly asthmatic patients. Respiratory medicine. 2011 Sep;105(9):1284–1289. doi: 10.1016/j.rmed.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 131.Sapey E, Greenwood H, Walton G, Mann E, Love A, Aaronson N, et al. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 2014 Jan 9;123(2):239–248. doi: 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tseng CW, Kyme PA, Arruda A, Ramanujan VK, Tawackoli W, Liu GY. Innate immune dysfunctions in aged mice facilitate the systemic dissemination of methicillin-resistant S. aureus. PLoS One. 2012;7(7):e41454. doi: 10.1371/journal.pone.0041454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fortin CF, Larbi A, Lesur O, Douziech N, Fulop T., Jr Impairment of SHP-1 down-regulation in the lipid rafts of human neutrophils under GM-CSF stimulation contributes to their age-related, altered functions. J Leukoc Biol. 2006 May;79(5):1061–1072. doi: 10.1189/jlb.0805481. [DOI] [PubMed] [Google Scholar]
- 134.Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol. 2004 Aug;76(2):291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- 135.Verschoor CP, Loukov D, Naidoo A, Puchta A, Johnstone J, Millar J, et al. Circulating TNF and mitochondrial DNA are major determinants of neutrophil phenotype in the advanced-age, frail elderly. Mol Immunol. 2015 May;65(1):148–156. doi: 10.1016/j.molimm.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 136.Baptista G, Dupuy AM, Jaussent A, durant R, Ventura E, Sauguet P, et al. Low-grade chronic inflammation and superoxide anion production by NADPH oxidase are the main determinants of physical frailty in older adults. Free Radic Res. 2012 Sep;46(9):1108–1114. doi: 10.3109/10715762.2012.692784. [DOI] [PubMed] [Google Scholar]
- 137.Ogawa K, Suzuki K, Okutsu M, Yamazaki K, Shinkai S. The association of elevated reactive oxygen species levels from neutrophils with low-grade inflammation in the elderly. Immun Ageing. 2008 Oct 24;5:13. doi: 10.1186/1742-4933-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mathur SK, Schwantes EA, Jarjour NN, Busse WW. Age-related changes in eosinophil function in human subjects. Chest. 2008 Feb;133(2):412–419. doi: 10.1378/chest.07-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Annema JT, Sparrow D, O'Connor GT, Rijcken B, Koëter GH, Postma DS, et al. Chronic respiratory symptoms and airway responsiveness to methacholine are associated with eosinophilia in older men: the Normative Aging Study. Eur Respir J. 1995 Jan;8(1):62–69. doi: 10.1183/09031936.95.08010062. [DOI] [PubMed] [Google Scholar]
- 140.Agrawal A, Tay J, Ton S, Agrawal S, Gupta S. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J Immunol. 2009 Jan 15;182(2):1138–1145. doi: 10.4049/jimmunol.182.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Canaday DH, Amponsah NA, Jones L, Tisch DJ, Hornick TR, Ramachandra L. Influenza-induced production of interferon-alpha is defective in geriatric individuals. Journal of clinical immunology. 2010 May;30(3):373–383. doi: 10.1007/s10875-010-9374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007 Jun 01;178(11):6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- 143.Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010 Mar 01;184(5):2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao J, Legge K, Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. The Journal of clinical investigation. 2011 Dec;121(12):4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging cell. 2004 Aug;3(4):161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 146.van Duin D, Mohanty S, Thomas V, Giner S, Montgomery RR, Fikrig E, et al. Age-associated defect in human TLR-1/2 function. J Immunol. 2007 Jan 15;178(2):970–975. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- 147.Oishi Y, Manabe I. Macrophages in age-related chronic inflammatory diseases. Aging and Mechanisms of Disease. 2016;(2):16018. doi: 10.1038/npjamd.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ho JC, Chan KN, Hu WH, Lam WK, Zheng L, Tipoe GL, et al. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med. 2001 Mar;163(4):983–988. doi: 10.1164/ajrccm.163.4.9909121. [DOI] [PubMed] [Google Scholar]
- 149.Svartengren M, Falk R, Philipson K. Long-term clearance from small airways decreases with age. Eur Respir J. 2005 Oct;26(4):609–615. doi: 10.1183/09031936.05.00002105. [DOI] [PubMed] [Google Scholar]
- 150.Ghadially R, Brown BE, Sequeira-Martin SM, Feingold KR, Elias PM. The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. The Journal of clinical investigation. 1995 May;95(5):2281–2290. doi: 10.1172/JCI117919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005 Oct 1;366(9492):1165–1174. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 152.Kogut I, Scholz JL, Cancro MP, Cambier JC. B cell maintenance and function in aging. Seminars in immunology. 2012 Oct;24(5):342–349. doi: 10.1016/j.smim.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 153.Barbee RA, Halonen M, Lebowitz M, Burrows B. Distribution of IgE in a community population sample: correlations with age, sex, and allergen skin test reactivity. J Allergy Clin Immunol. 1981 Aug;68(2):106–111. doi: 10.1016/0091-6749(81)90167-6. [DOI] [PubMed] [Google Scholar]
- 154.Sapigni T, Biavati P, Simoni M, Viegi G, Baldacci S, Carrozzi L, et al. The Po River Delta Respiratory Epidemiological Survey: an analysis of factors related to level of total serum IgE. Eur Respir J. 1998 Feb;11(2):278–283. doi: 10.1183/09031936.98.11020278. [DOI] [PubMed] [Google Scholar]
- 155.Jarvis D, Luczynska C, Chinn S, Potts J, Sunyer J, Janson C, et al. Change in prevalence of IgE sensitization and mean total IgE with age and cohort. J Allergy Clin Immunol. 2005 Sep;116(3):675–682. doi: 10.1016/j.jaci.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 156.Kerkhof M, Droste JH, de Monchy JG, Schouten JP, Rijcken B. Distribution of total serum IgE and specific IgE to common aeroallergens by sex and age, and their relationship to each other in a random sample of the Dutch general population aged 20–70 years. Dutch ECRHS Group, European Community Respiratory Health Study. Allergy. 1996 Nov;51(11):770–776. doi: 10.1111/j.1398-9995.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 157.Meyer KC. The role of immunity and inflammation in lung senescence and susceptibility to infection in the elderly. Seminars in respiratory and critical care medicine. 2010 Oct;31(5):561–574. doi: 10.1055/s-0030-1265897. [DOI] [PubMed] [Google Scholar]
- 158.Tsukamoto H, Clise-Dwyer K, Huston GE, Duso DK, Buck AL, Johnson LL, et al. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc Natl Acad Sci U S A. 2009 Oct 27;106(43):18333–18338. doi: 10.1073/pnas.0910139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005 Jun 1;174(11):7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 160.Britanova OV, Putintseva EV, Shugay M, Merzlyak EM, Turchaninova MA, Staroverov DB, et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol. 2014 Mar 15;192(6):2689–2698. doi: 10.4049/jimmunol.1302064. [DOI] [PubMed] [Google Scholar]
- 161.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002 Aug 15;169(4):1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 162.Heron M, Grutters JC, ten Dam-Molenkamp KM, et al. Bronchoalveolar lavage cell pattern from healthy human lung. Clinical and Experimental Immunology. 2012;167(3):523–531. doi: 10.1111/j.1365-2249.2011.04529.x. 11/14/accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schmitt V, Rink L, Uciechowski P. The Th17/Treg balance is disturbed during aging. Exp Gerontol. 2013 Dec;48(12):1379–1386. doi: 10.1016/j.exger.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 164.Birmingham JM, Gillespie VL, Srivastava K, Li XM, Busse PJ. Influenza A infection enhances antigen-induced airway inflammation and hyperresponsiveness in young but not aged mice. Clin Exp Allergy. 2014 Sep;44(9):1188–1199. doi: 10.1111/cea.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]