Abstract
In this work, a new composite materials of graphene oxide (GO)-incorporated metal-organic framework (MOF)(UiO-66-NH2/GO) were in-situ synthesized, and were found to exhibit enhanced high performances for CO2 capture. X-ray diffraction (XRD), scanning electron microscope (SEM), N2 physical adsorption, and thermogravimetric analysis (TGA) were applied to investigate the crystalline structure, pore structure, thermal stability, and the exterior morphology of the composite. We aimed to investigate the influence of the introduction of GO on the stability of the crystal skeleton and pore structure. Water, acid, and alkali resistances were tested for physical and chemical properties of the new composites. CO2 adsorption isotherms of UiO-66, UiO-66-NH2, UiO-66/GO, and UiO-66-NH2/GO were measured at 273 K, 298 K, and 318 K. The composite UiO-66-NH2/GO exhibited better optimized CO2 uptake of 6.41 mmol/g at 273 K, which was 5.1% higher than that of UiO-66/GO (6.10 mmol/g). CO2 adsorption heat and CO2/N2 selectivity were then calculated to further evaluate the CO2 adsorption performance. The results indicated that UiO-66-NH2/GO composites have a potential application in CO2 capture technologies to alleviate the increase in temperature of the earth’s atmosphere.
Keywords: graphene oxide (GO), metal-organic framework (MOF), UiO-66-NH2, carbon dioxide adsorption
1. Introduction
Metal-organic frameworks (MOFs) are formed by the coordination between metal clusters and organic linkers. Due to their large capacity for the adsorption of gases and structural and chemical tenability, they have gained much attention to the potential applications of CO2 capture [1,2]. The UiO-66 sample with good thermal stability (>500 °C) and water resistance shows some improved CO2 adsorption performance [3,4,5]. Moreover, when compared with the performance of industrial adsorbent (zeolite 13x, for example), the adsorption capacity of UiO-66 demonstrates an improved value [1,6]. In order to enrich this type MOFs, some functional groups were introduced to the skeleton to synthesize the functional series UiO-66 type of MOFs, which could maintain the topology of the original constant [7,8,9,10]. However, after functionalization, although the series UiO-66 type of MOFs still displayed the same skeletal structure, the thermal stability, chemical stability, etc., exhibited degradation to some extent. Kandiah et al. [11,12] compared the thermal stability and the chemical stability of three kinds of UiO-66 types of MOFs (UiO-66-NH2, UiO-66-NO2, and UiO-66-Br), and the results showed that, for UiO-66-NH2 and UiO-66-NO2, the thermal stability fell at 350–400 °C, which was due to the ligand decomposition. Moreover, UiO-66-NH2, UiO-66-NO2, and UiO-66-Br prepared in water and HCl solution with pH = 1 displayed the stability of the structure, while UiO-66-NH2 and UiO-66-Br that were prepared in the NaOH solution with pH = 14 exhibited the collapsed structure partially or completely at the above experimental condition. Yang et al. [13,14] reported that, when compared to UiO-66, the CO2 adsorption capacity of UiO-66-NH2 increased, which was due to the existence of amino. However, the stability of UiO-66-NH2 was inferior to that of UiO-66. Therefore, it is necessary to find a new kind of UiO-66 type of MOFs, which can display outstanding performance on thermal stability, chemical stability, and gas adsorption.
Simultaneously, as reported in the literature, the introduction of NH2 could improve the CO2/N2 selectivity in the coal-fired flue gas [15,16]. MOFs have plenty of porosities, but the large pore space in MOFs was not in favor of gas molecules storage. GO has a layered structure and its surface has some groups (–OH, C–O–C, –C=O). The incorporation of GO to MOFs could effectively reduce the MOFs pore space [17,18].
Hence, in this paper, UiO-66-NH2/GO composites were in-situ synthesized with the addition of graphene oxide (GO) into UiO-66-NH2. Moreover, the CO2 adsorption performances for the UiO-66-NH2/GO and UiO-66-NH2 samples were investigated in order to discuss the influence factor effects on the structure and chemical properties, which could be beneficial for the development of high stability, high gas adsorption materials.
2. Experimental
2.1. Synthesis of UiO-66-NH2/GO Composites
UiO-66-NH2 was solvothermally synthesized based on the method of Abid et al. [19]. Zirconium chloride (ZrCl4, 1.47 g) and 2-aminoterephthalic acid (H2BDC-NH2, 1.06 g) were dissolved in N,N-dimethylformamide (DMF, 150 mL). Then, the mixture was transferred into a 200 mL Teflon lined stainless-steel autoclave for homogeneous reaction, which was maintained at 393 K for 24 h. The mixture was cooled to ambient temperature and repeatedly washed three times with DMF and ethanol, respectively. Yellow crystal was obtained by drying at 393 K.
The composites of UiO-66-NH2/GO were synthesized as the following procedure. Firstly, graphene oxide (GO) was prepared according to Hummers’ technique [17,18]. A given mass of dry scattered GO was added to 150 mL DMF solvent with ultrasonic treatment for 5 h to obtain a GO suspension. Then, ZrCl4 (1.47 g) and H2BDC-NH2 (1.06 g) were added to the GO suspension to synthesize UiO-66-NH2/GO. The expected amount of GO is 5 wt % in UiO-66-NH2/GO, which is the most appropriate ratio that is found in our previous studies of UiO-66/GO samples [17]. The obtained gray mixture was transferred into a 200 mL Teflon lined stainless-steel autoclave for homogeneous reaction, which was maintained at 393 K for 24 h. The mixture was cooled to ambient temperature and repeatedly washed three times with DMF and ethanol, respectively. Finally, gray crystal was obtained by drying at 393 K.
2.2. Characterizations
For X-ray diffraction (XRD) measurements, the samples were grounded with DMF (MOF series) or ethanol (GO series) in a small agate mortar, then, the mixture was smear-mounted onto a glass slide and was air-dried. The samples were analyzed by using a Philips X’Pert X-ray diffractometer (Cu Kα radiation, with a voltage of 40 kV and current of 40 mA, Philips (China) Investment Co. Ltd, Shanghai, China)
Infrared spectrum (FT-IR) was performed by using a MB154S-FTIR spectrometer (Bomem, Quebec City, QC, Canada) according to the attenuated total reflectance method (ATR).
Thermogravimetric analysis (TGA) was analyzed by using a SDTQ600 thermal analyzer (Perkin-Elmer Pyris Diamond, Waltham, MA, USA). Experiments were carried out under a N2 atmosphere (30 mL/min flow rate) at a heating rate of 10 °C/min from room temperature to 800 °C.
ASAP 2010 analyzer (Micromeritics, Norcross, GA, USA) was applied to evaluate the N2 adsorption isotherms at −196 °C. Before the experiment, the samples were decorated 12 h at 393 K. The surface area SBET, the total pore volume Vpore, the micropore volume Vmic, and the mesopore volume Vmes were obtained from the isotherms.
Scanning electron microscope (SEM) was performed on a S-4800 field emission scanning electron microscope (Hitachi High-Technologies corporation, Tokyo, Japan). Scanning was ran on a powder sample that was previously dried and also should be coated with a thinness of gold to avoid charging in the case of MOFs and the composites.
2.3. CO2 Adsorption Experiment
CO2 adsorption measurements under 0~1.2 bar were performed by an apparatus for physical adsorption (V-Sorbet 2008S, Beijing Jinaipu Science & Technology Co., Ltd., Beijing, China). The experiments were operated at 273 K, 298 K and 318 K. The sample tube was immersed in a heated dewar flask filled with water to control the adsorption temperature. Before the experiment, the samples (0.1–0.2 g) in the analysis chamber were subjected to a vacuum at ambient temperature for 12 h. The reversibility of the CO2 adsorption process on the samples was studied by multiple CO2 adsorption cycles. Regeneration experiments were conducted at 393 K for 12 h in vacuum, and then the corresponding adsorption isotherms were collected.
2.4. Chemical Stability Test
At room temperature, newly synthesized UiO-66-NH2/GO (0.2 g) was exposed to air humidity (70–90%) for 30 days, or 20 mL pH = 1 HCl solution (or pH = 14 in NaOH solution for 2 h. XRD testing was carried out on samples after filtration drying.
3. Results and Discussion
3.1. Structure Characterization
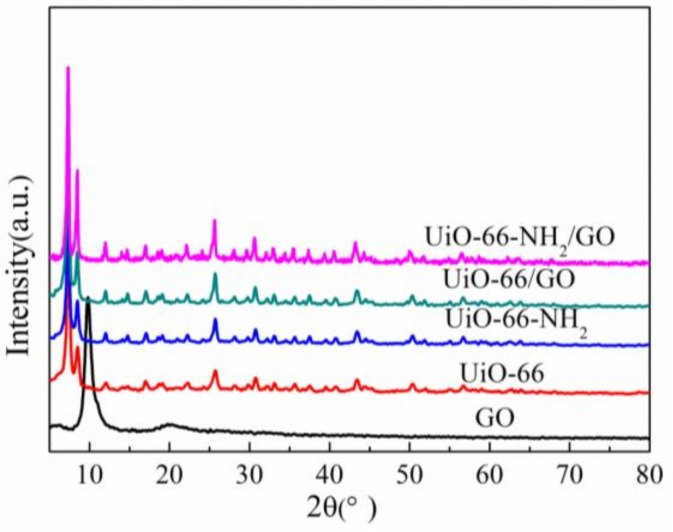
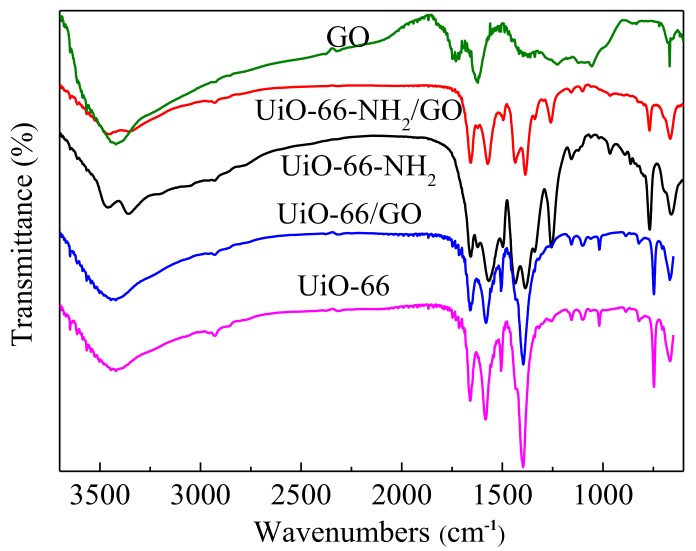
X-ray diffraction (XRD) of different samples is illustrated in Figure 1. As shown in Figure 1, the main diffraction peaks of UiO-66-NH2 are similar to those of UiO-66, revealing that the introduction of –NH2 groups did not change the crystal structure of matrix UiO-66, which is consistent with the results of previous reports [20,21,22]. UiO-66-NH2/GO composites have the same diffraction peak position and a slightly stronger peak intensity when compared with UiO-66-NH2. It proved that UiO-66-NH2 is the main component of UiO-66-NH2/GO composites and UiO-66-NH2/GO composites still maintained a good crystal structure. The main diffraction peak of GO (2θ = 10°) does not appear in UiO-66-NH2/GO composites, which is consistent with the situation of UiO-66/GO. The reason could be concluded that the very low content (5%) of GO in the composites, the high dispersion of GO with polar solvents (DMF), which were known to disperse GO very well, and the interaction of GO with UiO-66-NH2.
Figure 1.
X-ray diffraction patterns of the different samples.
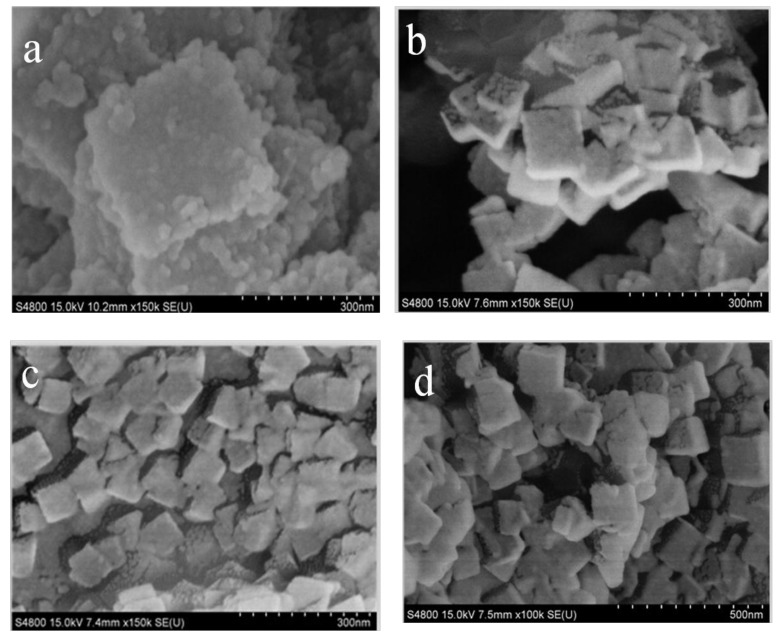
SEM images of different samples were showed in Figure 2. As seen from Figure 2a,b, UiO-66-NH2 has the same cubic structure as UiO-66 crystal. Owing to the influence of synthesis condition, the particle size of UiO-66-NH2 is around 150 nm, which is smaller than that of UiO-66 (300 nm). The result is in accordance with the previous reported in the literature [22]. UiO-66-NH2 and UiO-66/GO composites have similar cubic crystals with a much smaller particle size of approximately 80–100 nm and are of irregular appearance (as shown in Figure 2c,d), which could be attributed to the introduction of GO. It can be explained that the oxygen-containing functional groups in GO combined with Zr4+, inhibiting the crystal growth of UiO-66-NH2.
Figure 2.
Scanning electron microscope (SEM) images of UiO-66 (a) [17]; UiO-66-NH2 (b); UiO-66/GO (c) [17]; and UiO-66-NH2/GO (d).
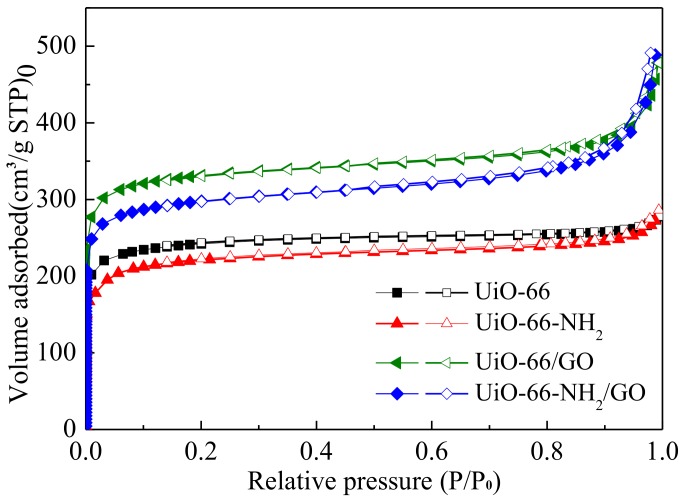
Figure 3 presents the N2 adsorption-desorption isotherm of different materials. N2 adsorption-desorption isothermal of all the synthetic samples reveal a typical type I isotherm. Pore structure parameters and Density Functional Theory (DFT) pore size distribution are presented in Figure 4 and Table 1.
Figure 3.
N2 adsorption-desorption isotherms of the different samples at 77 K.
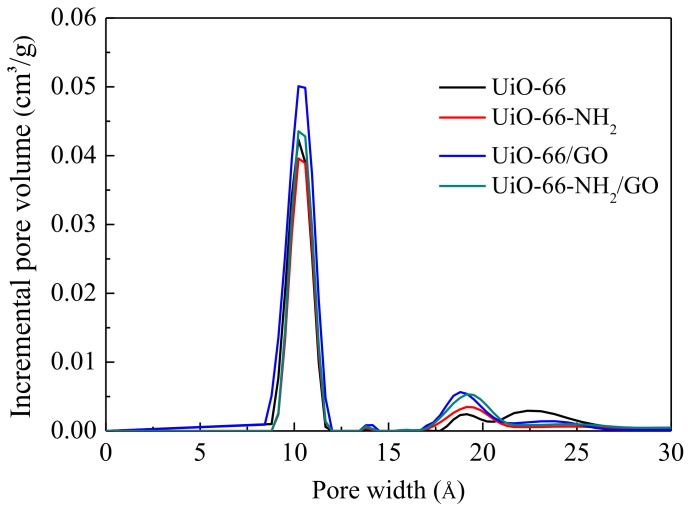
Figure 4.
Pore size distribution of the different samples.
Table 1.
Data of porous structure of the different samples.
| Sample | SBET (m2/g) | Vpore (cm3/g) | Vmic (cm3/g) | Vmic/Vpore (%) |
|---|---|---|---|---|
| UiO-66 | 838 | 0.245 | 0.224 | 91 |
| UiO-66-NH2 | 822 | 0.236 | 0.214 | 90 |
| UiO-66/GO | 1184 | 0.384 | 0.304 | 79 |
| UiO-66-NH2/GO | 1052 | 0.345 | 0.286 | 83 |
SBET is the BET surface area; Vpore is total pore volume; Vmic is micropore volume.
As can be seen from Table 1, the SBET and Vpore of UiO-66-NH2 is 822 m2/g and 0.236 cm3/g respectively, which is similar to that of UiO-66, in conformity with the results that were reported by Nik et al. [22], but slightly lower those that were reported by Garibay et al. [8]. This slight difference may result from the different activation temperatures. GO has no adverse effect on composite materials. Properly adding GO significantly improves the SBET and Vpor of UiO-66/GO. For SBET, Vpore, and Vmic of UiO-66-NH2/GO are 1052 m2/g, 0.345 cm3/g, and 0.286 cm3/g, respectively, which increases by 28%, 46%, and 46% than UiO-66-NH2. The incorporation of GO does not negatively affect the porosity of UiO-66-NH2, it is in accordance with the previous report. The new pores could generate on the interface between the GO layers and the MOF “blocks”, which could improve the surface area.
As shown in Figure 4, the pore size of the composite material is 8~12 Å. In this range, the pore size distribution of UiO-66/GO and UiO-66-NH2/GO is more than the corresponding parent material UiO-66 and UiO-66-NH2, which is good for CO2 adsorption. It can also be seen that the pore sizes of these materials are partly distributed between 16 Å and 22 Å. During this interval, the pore size distribution of UiO-66-NH2 and UiO-66 is similar, and that of UiO-66/GO and UiO-66-NH2/GO is similar. The pore volume of the composite materials is higher than that of the parent material, which is also good for CO2 adsorption.
3.2. Chemical Characteristics
Figure 5 is the FT-IR spectra of different samples. In the spectrum of UiO-66-NH2, new peaks appeared at 3461 cm−1 and 3361 cm−1, respectively, when compared with UiO-66, corresponding to the symmetric and asymmetric vibration peak of –NH2 [23]. The peak at 1621 cm−1 corresponds to the N–H bending vibration. The peaks at 1482 cm−1 and 1382 cm−1 can be attributed to the N–H bending vibration and C–N stretching vibration, respectively [12]. The IR spectrum of UiO-66/GO was almost the same as that of UiO-66, with a slight decrease in strength.
Figure 5.
FT-IR spectra of the different samples.
When comparing to the UiO-66-NH2, the spectrum of UiO-66-NH2/GO composites was relatively unchanged, while the peak strength decreased slightly. The main peaks of GO also do not appear in the composite materials. The oxygen functional groups on the GO layers could bond with the open metal sites of UiO-66-NH2, resulting in the band disappearance of GO over UiO-66-NH2/GO composites in the FTIR spectra. In addition, small changes in the composite materials were observed at 1158 cm−1, 893 cm−1, 961 cm−1, and 698 cm−1. The reason for this is that the introduction of functional groups that are carried by the GO surface and carboxyl coordinated with metal ion slightly changed the chemical environment of the Terephthalic acid ligand.
3.3. Stability Analysis
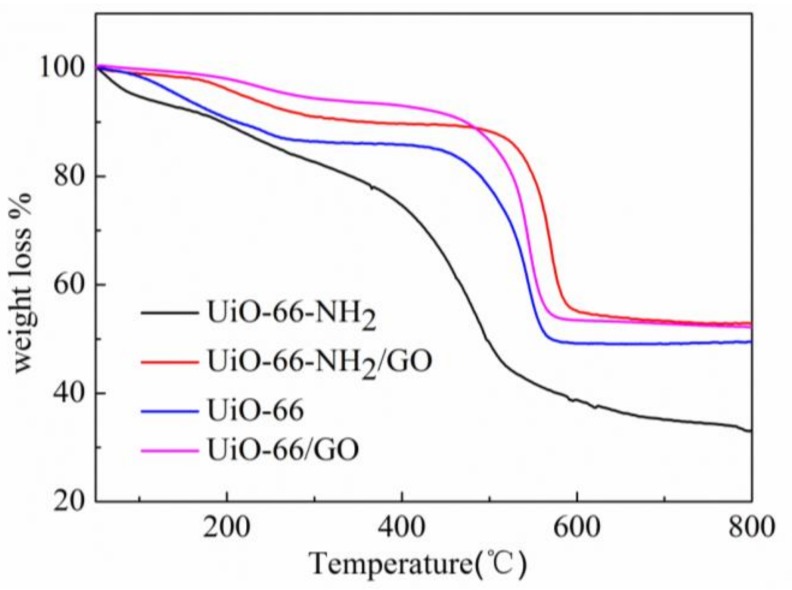
Figure 6 shows the TG curves of the different samples. The thermal stability of UiO-66-NH2 decreased when compared with that of UiO-66. The terephthalic acid ligand in UiO-66 skeleton materials decomposed above 480 °C, but the decomposition of amino terephthalic acid ligand occurred from 380 °C, which is 100 °C lower than that of UiO-66 materials. This is consistent with the UiO-66-NH2 collapse of skeleton temperature, as reported by the literature [8]. The decomposition temperature of other types of amine-MOF is also lower than that of the matrix [24,25]. Finally, when the temperature reaches 650 °C, the UiO-66-NH2 has the largest quality loss of approximately 65 wt %, and the quality loss of the UiO-66 is about 52 wt %. This shows that the thermostability of the latter is better. In addition, the thermostability of UiO-66-NH2/GO composites is better than UiO-66, which is similar to UiO-66/GO composites. Their decomposition temperature is in the range of 500–650 °C, with a similar quality loss of 47 wt %. To sum up, the thermal stability of UiO-66-NH2/GO composites is obviously higher than that of UiO-66-NH2. The addition of the right amount of GO clearly improved the thermostability of UiO-66-NH2.
Figure 6.
Thermo-gravimetric analysis for the different samples.
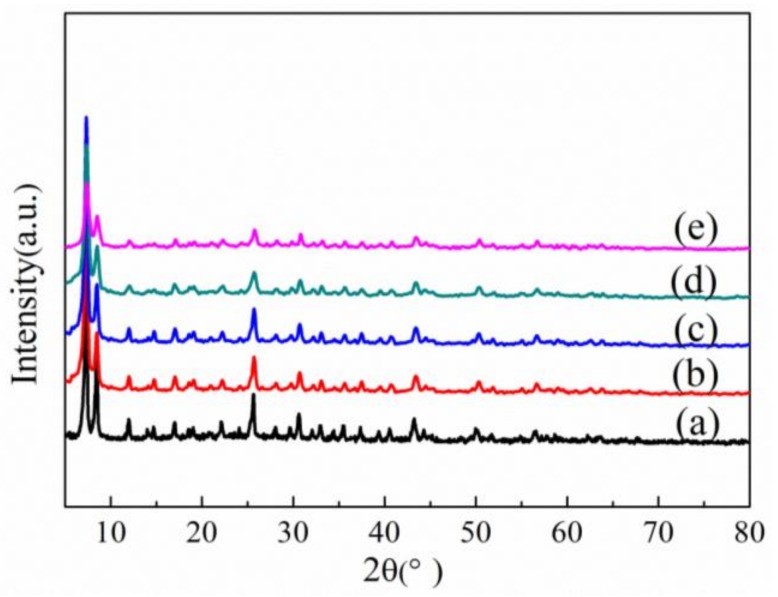
In addition to the above investigation of the thermal stability, we also studied the chemical stability of the UiO-66-NH2/GO. The stability of UiO-66-NH2/GO in the air, water, acid, and alkali qualitative is characterized by XRD. As can be seen from Figure 7b,c, after the UiO-66-NH2/GO exposed in the air for 30 days, or is soaked in water for 10 days at room temperature, the crystal structure did not change, which proved that the UiO-66-NH2/GO has a high water stability that is the same as that of matrix UiO-66 and UiO-66/GO [17]. The introduction of GO means that the UiO-66-NH2 can improve the stability of the network structure under the condition of humidity.
Figure 7.
XRD patterns for UiO-66-NH2/GO: (a) as-prepared; (b) desolvated; (c) in air for 30 days; (d) soaked in water for 10 days; (e) immersed in in HCl solution (pH = 1) for 2 h; and, (f) NaOH solution (pH = 14) for 2 h.
As shown in Figure 7d,e, after the UiO-66-NH2/GO was soaked in HCl solution (pH = 1) and NaOH solution (pH = 14) for 2 h, the strength of the main peak got slightly smaller, but was still able to maintain crystal structure. According to the literature, after soaking in the same condition of the NaOH solution, the skeleton of UiO-66-NH2, UiO-66-NO2, and UiO-66-Br completely collapsed, whereas the UiO-66 structure was damaged, and it was converted into a state of lower crystallinity [11]. A chemical stability test showed that UiO-66-NH2/GO have excellent water resistance, acid resistance, and alkali resistance. Among them, the alkali resistance has a significant increase when compared with the other reported UiO-66 type of MOFs.
3.4. Low Pressure CO2 Adsorption
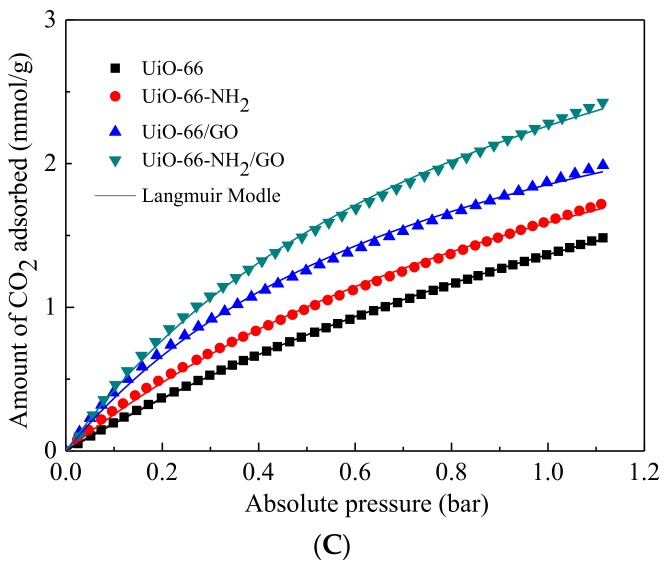
The CO2 adsorption performance of the samples was elevated using a physical adsorption instrument under the condition of low voltage static. Before the gas adsorption test, all of the samples were dried under vacuum for 12 h at 393 K to completely remove the solvent molecules from inorganic metal element Zr [13]. CO2 adsorption isotherms of different samples are shown in Figure 8. The tests were carried out at 273 K, 298 K, and 318 K, respectively, and 0~1.2 bar.
Figure 8.
CO2 adsorption isotherms measured at 273 K (A); 298 K (B); and, 318 K (C).
The CO2 adsorption capacities of all of the samples decrease with the increase of temperature. The CO2 uptakes of all the samples under three temperatures followed the same order: UiO-66-NH2/GO > UiO-66/GO > UiO-66-NH2 > UiO-66. As can be seen from the isotherms, the CO2 adsorption capacity of UiO-66 and UiO-66/GO clearly increased with the decrease of temperature, which proved that the adsorption mechanism can be assigned to physical adsorption. The incorporation of GO to UiO-66 led to the increase of the mesopore volume and micropore volume. The mesopore was in favor of the transmission of CO2 to the adsorption site with high energy and the micropore was in favor of the strong retention of CO2 molecules. As showed in Figure 8, the adsorption capacity of UiO-66-NH2 at 273 K below 1.0 bar is 3.93 mmol/g, which is 7.3% higher than that of UiO-66 (3.66 mmol/g). A similar situation occurs when the adsorption temperature increases to 318 K. The UiO-66-NH2 adsorption capacity is 1.60 mmol/g, which is 16.0% higher than that of UiO-66 1.38 mmol/g. The UiO-66-NH2/GO adsorption capacity at 273 K is 6.41 mmol/g, which is 5.1% higher than that of UiO-66/GO (6.10 mmol/g), while the UiO-66-NH2/GO adsorption capacity at 318 K is 2.26 mmol/g, which is 20.2% higher than that of UiO-66 1.88 mmol/g. As known in Table 1, the SBET of UiO-66-NH2 is a bit smaller than that of UiO-66. Similarly, the specific surface area of UiO-66-NH2/GO is slightly smaller than that of UiO-66/GO. Moreover, in terms of CO2 adsorption capacity, the opposite is true: UiO-66-NH2 is above UiO-66, and UiO-66-NH2/GO is higher than UiO-66/GO. This phenomenon shows that, for UiO-66-NH2 and UiO-66-NH2/GO, in addition to the physical adsorption sites of CO2 adsorption, there are other adsorption sites, and the adsorption reaction between CO2 and amino groups improves the CO2 adsorption capacity. Moreover, the high temperature is advantageous in terms of the reaction on the adsorption sites and being conducive to the physical adsorption at low temperature, high temperature reaction adsorption sites when strength is more obvious. The existence of reaction adsorption sites results in strong interaction and better adsorption selectivity between the adsorbate and adsorbent, which favors the important factors of gas purification.
In Table 2, several traditional adsorbent and synthetic samples for CO2 adsorption capacity under 1.0 bar are listed. As can be seen from the Table 2, when compared with other typical porous materials, such as 13 x molecular sieve, activated carbon adsorption, and some common MOFs, the UiO-66-NH2/GO composite has a higher CO2 adsorption capacity. Therefore, the UiO-66-NH2/GO composite has certain industrial application prospects.
Table 2.
Adsorption capacity of the prepared composite and some other adsorbents for selected CO2 reported from the literature at 1 bar.
| Sample | Chemical Formula | Q (mmol/g) | Temperature (K) | Ref. |
|---|---|---|---|---|
| MOF-5 | Zn4O(BDC)3 | 2.10 | 296 | [26] |
| IRMOF-1 | Zn4O(BDC)3 | 1.92 | 208 | [2] |
| MOF-177 | Zn4O(BTB)2 | 0.8 | 298 | [27] |
| zeolite 13X | - | 1.77 | 293 | [28] |
| Activated carbon | - | 1.5 | 298 | [29] |
| UiO-66 | Zr6O4(OH)(BDC)6 | 1.77 | 298 | [4] |
| UiO-66-NH2 | Zr6O4(OH)(BDC-NH2)6 | 3.05 | 298 | [4] |
| UiO-66 | Zr6O4(OH)(BDC)6 | 2.27 | 298 | present work |
| UiO-66/GO | - | 3.37 | 298 | present work |
| UiO-66-NH2 | Zr6O4(OH)(BDC-NH2)6 | 2.59 | 298 | present work |
| UiO-66-NH2/GO | - | 3.80 | 298 | present work |
Q is CO2 adsorption capacities.
In order to determine the reason for the difference in CO2 adsorption capacity, the calculation of CO2 adsorption heat was carried out. When compared with UiO-66, UiO-66-NH2, and UiO-66/GO, the CO2 adsorption capacity of UiO-66-NH2/GO has an excellent performance. The reason for this may be related to the adsorption heat ΔH.
Langmuir isotherm equation had been proposed to describe the experimental data, according to the following Langmuir equation:
| (1) |
where Qe and Pe are equilibrium adsorption and pressure, respectively. Qm is the monolayer capacity and b is the Langmuir equilibrium constant. Isotherm parameters at all of the temperatures are summarized in Table 3.
Table 3.
Data of Langmuir isotherm for CO2 adsorption on the different samples.
| Sample | 273 K | 298 K | 318 K | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qm | b | R2 | Qm | b | R2 | Qm | b | R2 | |
| UiO-66 | 11.78 | 0.45 | 0.999 | 15.49 | 0.17 | 0.999 | 4.42 | 0.45 | 0.999 |
| UiO-66-NH2 | 12.63 | 0.45 | 0.999 | 8.28 | 0.45 | 0.999 | 3.82 | 0.71 | 0.998 |
| UiO-66/GO | 10.83 | 1.26 | 0.997 | 6.03 | 1.20 | 0.997 | 3.37 | 1.22 | 0.997 |
| UiO-66-NH2/GO | 10.92 | 1.39 | 0.996 | 6.38 | 1.41 | 0.996 | 4.39 | 1.06 | 0.998 |
From the isothermal measurements performed at three different temperatures (Figure 8 and Figure 9), it is possible to calculate the net isosteric heat of adsorption from Clausius-Clapeyron equation [30]:
| lnP = −(ΔH/RT) + C | (2) |
where ΔH is the amount of adsorption heat (kJ/mol), p is the pressure (MPa), T is the temperature (K), R is the gas constant, and C is integration constant. The lnP plot of 1/T is a straight line with slope—(ΔH/R). According to the low voltage (0.01 MPa to 0.1 MPa), under the 273 K, 298 K and 318 K adsorption isotherm, we obtain relevant data generation according to the type, and CO2 isothermal adsorption heat is calculated. The isosteric heat of adsorptions for CO2 were 24.3~22.9 kJ/mol for UiO-66, 24.6~23.2 kJ/mol for UiO-66-NH2, 28.9~27.8 kJ/mol for UiO-66/GO, and 30.5~29.4 kJ/mol for UiO-66-NH2/GO, depending on the degree of CO2 loading. There was no significant difference in Q values and the maximum variation between the highest and lowest values did not exceed 5%. Therefore, an average Q was calculated. The average isosteric heat of adsorption for CO2 on the samples studied. UiO-66, UiO-66-NH2, UiO-66/GO, and UiO-66-NH2/GO CO2 isothermal adsorption heat is, respectively, 23.5 kJ/mol, 24.2 kJ/mol, 28.3 kJ/mol, and 29.9 kJ/mol. Among them, the UiO-66 and UiO-66-NH2 CO2 isothermal adsorption heat is the same as the literature. Hui et al. [31] measured CO2 UiO-66 when the high coverage adsorption heat is 22 kJ/mol. Aprea et al. [32] reported that the 13x molecular sieve CO2 isothermal adsorption heat is 32.5 kJ/mol. Generally, the smaller the adsorption heat of the materials, the lower renewable energy. However, for low adsorption heat, the adsorption selectivity will decrease, leading to the capture of the lower gas purity. The UiO-66-NH2/GO CO2 isothermal adsorption heat is 29.9 kJ/mol, which is higher than the UiO-66-NH2 CO2 isothermal adsorption heat of 24.2 kJ/mol. The UiO-66/GO CO2 isothermal adsorption heat is 28.3 kJ/mol, which is higher than that of UiO-66 isothermal adsorption heat 23.5 kJ/mol. This is mainly due to holes or defects in the interface between the MOF and the GO in the crystal structure, boosting the interaction between the skeleton and CO2, and thus increasing the CO2 adsorption capacity. At the same time, CO2 intermolecular interactions in the channel may also increase △H. The UiO-66-NH2 CO2 isothermal adsorption heat is 24.2 kJ/mol, which is slightly higher than that of the UiO-66 CO2 isothermal adsorption heat of 23.5 kJ/mol. The UiO-66-NH2/GO CO2 isothermal adsorption heat is 29.9 kJ/mol, which is slightly higher than the UiO-66/GO isothermal adsorption heat of 28.3 kJ/mol. This can be attributed to the existence of -NH2 and the reaction adsorption sites between CO2, raising the heat of adsorption. The interaction between CO2 is enhanced, the CO2 adsorption capacity increases, and thus, eventually, there was a corresponding conformity.
Figure 9.
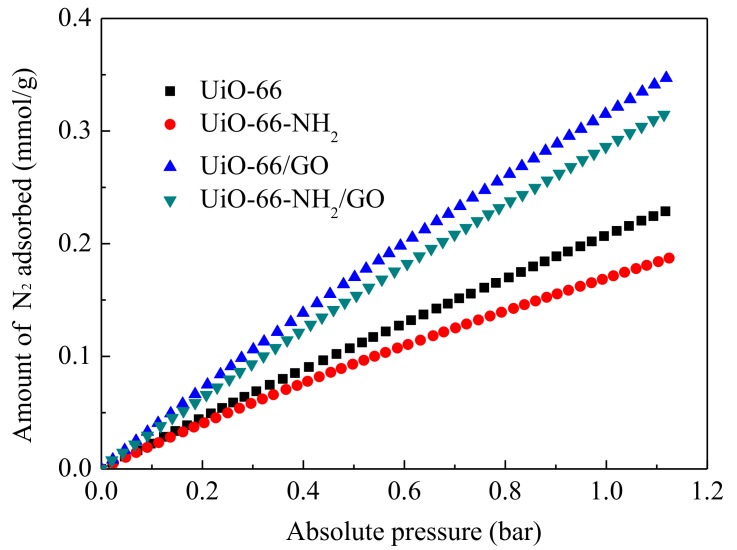
N2 adsorption isotherms measured at 298 K on the sample.
3.5. Adsorption Selectivity of CO2/N2
UiO-66-NH2/GO may be a potential adsorbent for flue gas containing CO2/N2 in view of its high stability and CO2 adsorption capacity. In order to get N2 adsorption isotherms, the sample is tested at 298 K in the pressure range from 0 to 1 bar. As shown in Figure 9, the adsorption capacity order of N2 on the sample is: UiO-66/GO > UiO-66-NH2/GO > UiO-66 > UiO-66-NH2. This is different from that of CO2 on the sample, the latter is: UiO-66-NH2/GO > UiO-66/GO > UiO-66-NH2 > UiO-66. When compared with parent materials, the N2 adsorption capacity of UiO-66-NH2 and UiO-66-NH2/GO composites decreases, which is helpful for improving the CO2/N2 adsorption selectivity of the sample.
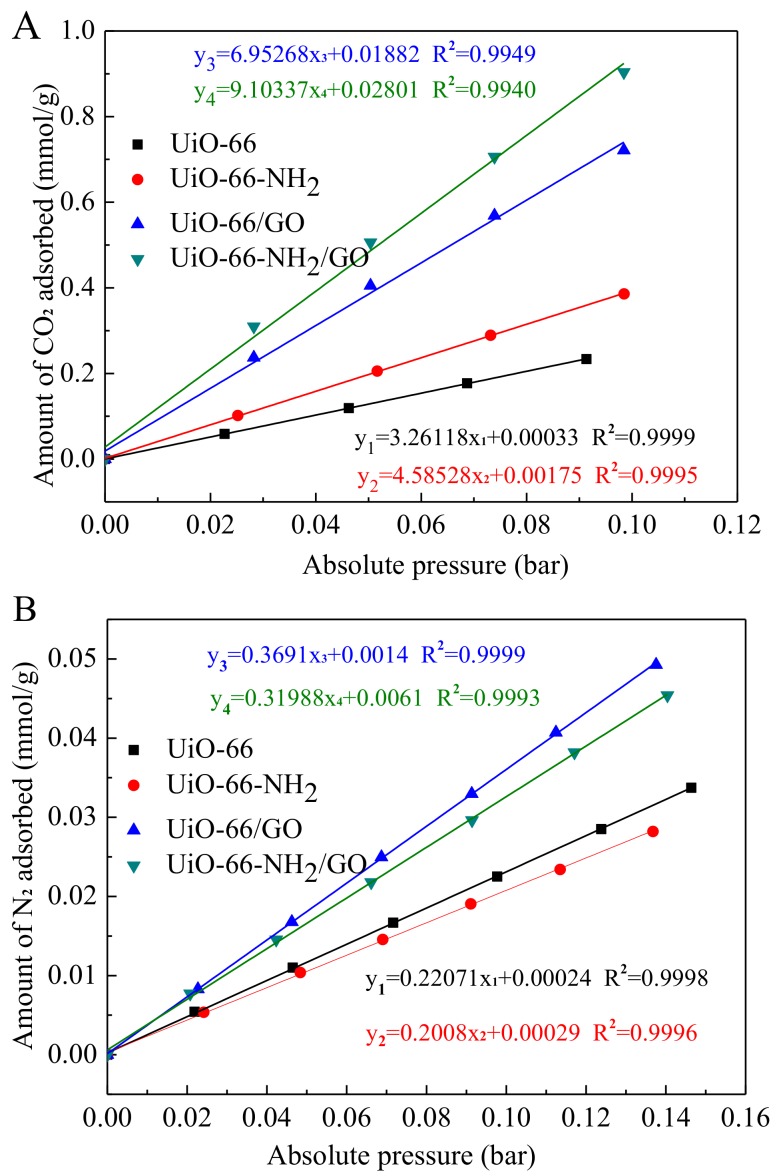
According to the CO2 and N2 adsorption isotherm of the samples in Figure 8B and Figure 9, CO2/N2 selectivity can be calculated. The initial slope (Henry’s constant) of the CO2 and N2 adsorption isotherm at the low pressure section can be used to calculate the selectivity factor and the single-component adsorption isotherm can be used to estimate the selectivity factor. The selectivity factor is defined as the ratio of the initial slope of CO2 and N2 [33]. Figure 10 shows the result according to the initial slope of CO2 and N2 adsorption isotherm.
Figure 10.
Initial slopes calculation for CO2 (A) and N2 (B) collected at 298 K.
The selectivities of UiO-66, UiO-66/GO, UiO-66-NH2, and UiO-66-NH2/GO to CO2/N2 were 14.78, 18.84, 22.83, and 28.45. The selectivity of UiO-66 to CO2/N2 is 14.78. The selectivity of UiO-66-NH2 to CO2/N2 is 22.83, which has increased by 54.5%. The selectivity of UiO-66/GO to CO2/N2 is 18.84 and UiO-66-NH2/GO to CO2/N2 is 28.45. The latter has increased by 51.0%. Similar to the MOFs of the amine ligand, the selectivity of both UiO-66-NH2 and UiO-66-NH2/GO composites clearly improve. This is because the adsorption of UiO-66-NH2 and UiO-66-NH2/GO to CO2 contain chemical reaction adsorption sites, in addition to physical adsorption sites because of -NH2, which makes their adsorption capacity to CO2 increase significantly. Furthermore, the adsorption capacity of UiO-66-NH2 and UiO-66-NH2/GO to N2 decrease. The selectivity order of the sample to CO2/N2 is: UiO-66-NH2/GO > UiO-66-NH2 > UiO-66/GO > UiO-66. Saha et al. [34] reported that the selectivity of MOF-5 and MOF-177 to CO2/N2 is 17.48 and 17.73, respectively, at 298 K and 1 bar. When compared with MOF-5 and MOF-177, the selectivity of UiO-66-NH2 and UiO-66-NH2/GO synthesized to CO2/N2 is much higher. To sum up, UiO-66-NH2/GO and UiO-66-NH2 have good prospects for industrial applications.
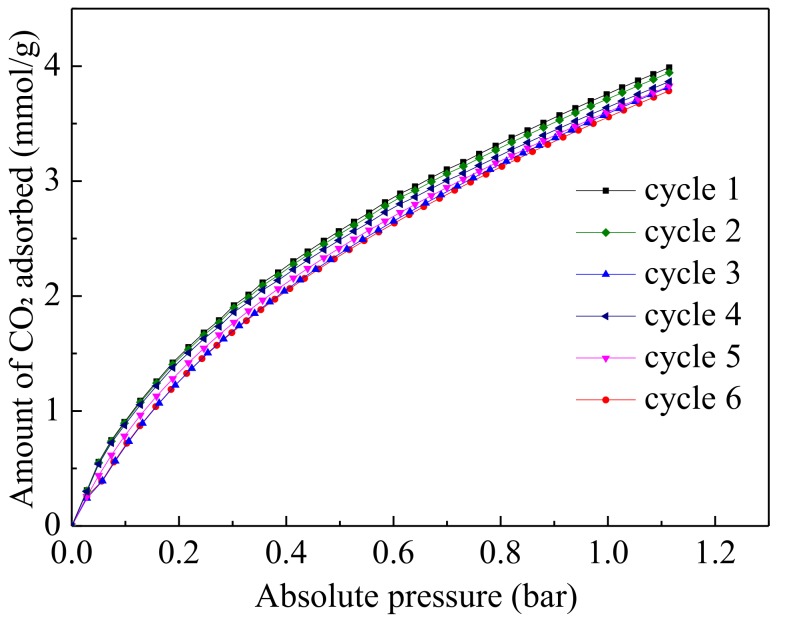
3.6. Multiple Cycles of CO2 Adsorption–Desorption
Figure 11 depicts the reversibility of the CO2 adsorption process on UiO-66-NH2/GO at 298 K from 0 bar to 1.2 bar. The isotherms in the diagram have been obtained after 12 h heating at 393 K in the vacuum condition. The adsorption capacity of the UiO-66-NH2/GO was lowered by only 4% after six cycles, from 3.80 to 3.64 mmol/g at 298 K and 1.0 bar. The six isotherms overlapped partly. The cyclical data reveals that the new UiO-66-NH2/GO material that is synthesized in this work is a more stable adsorbent after six adsorption/desorption cycles under mild regeneration conditions.
Figure 11.
Six cycles of CO2 adsorption isotherms on UiO-66-NH2/GO at 298 K.
4. Conclusions
In this work, new composite materials of graphene oxide (GO)-incorporated metal-organic framework (MOF)(UiO-66-NH2/GO) were in-situ synthesized, and exhibited enhanced high performances for CO2 capture. The sample stabilities of this new sample were also investigated in the presence of water attack or acid treatment or base treatment. In terms of stability, the results were good. UiO-66-NH2/GO have been characterized by kinds of technical indicators and have been tested for CO2 adsorption, with comparison of counterparts UiO-66-NH2, UiO-66/GO, and UiO-66 samples. SBET, Vpore, and Vmic of UiO-66-NH2/GO are, respectively, 1052 m2/g, 0.345 cm3/g, and 0.286 cm3/g larger than UiO-66-NH2 but slightly smaller than UiO-66/GO. The CO2 absorbance of UiO-66-NH2/GO achieves 6.41 mmol/g at 273 K, which is 5.1% higher than that of UiO-66/GO (6.10 mmol/g). In addition to physical adsorption sites, chemical adsorption sites (amino groups) also improved the CO2 adsorption capacity. CO2 adsorption heat and CO2/N2 selectivity was 29.9 kJ/mol and 28.45, which is higher than the selectivity of UiO-66-NH2 and UiO-66/GO. UiO-66-NH2/GO exhibited better optimized CO2 adsorption and CO2/N2 selectivity. So, UiO-66-NH2/GO composites has a potential application in CO2 capture technologies to alleviate the increase in temperature of the earth’s atmosphere.
Acknowledgments
This work was supported by the funding of Ecological Building Materials and Environmental Protection Equipment Collaborative Innovation Center Project of Jiang Su Province (1142658).
Author Contributions
Yan Cao provided the experimental methods, analyzed the experimental data and wrote this manuscript. Fujiao Song, Hongmei Zhang, Tao Huang and Jiayu Ji participated in experimental work. Qin Zhong gave some advice of experimental methods. Wei Chu and Qi Xu made some guides to the use of instruments, reviewed the manuscript.
Conflicts of Interest
There is no conflicts of interest.
References
- 1.Sumida K., Rogow D.L., Mason J.A., McDonald T.M., Bloch E.D., Herm Z.R., Bae T.H., Long J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012;112:724–781. doi: 10.1021/cr2003272. [DOI] [PubMed] [Google Scholar]
- 2.Millward A.R., Yaghi O.M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005;127:17998–17999. doi: 10.1021/ja0570032. [DOI] [PubMed] [Google Scholar]
- 3.Cavka J.H., Jakobsen S., Olsbye U., Guillou N., Lamberti C., Bordiga S., Lillerud K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008;130:13850–13851. doi: 10.1021/ja8057953. [DOI] [PubMed] [Google Scholar]
- 4.Huang Q.H., Ding J., Huang X., Wei X.L., Wang W.L. Experimental and Computational Investigation of CO2 Capture on Mix-ligand Metal-organic Framework UiO-66. Energy Procedia. 2017;105:4395–4401. doi: 10.1016/j.egypro.2017.03.933. [DOI] [Google Scholar]
- 5.Chavan S., Vitillo J.G., Gianolio D., Zavorotynska O., Civalleri B., Jakobsen S., Nilsen M.H., Valenzano L., Lamberti C., Lillerud K.P., et al. H2 storage in isostructural UiO-67 and UiO-66 MOFs. Phys. Chem. Chem. Phys. 2012;14:1614–1626. doi: 10.1039/C1CP23434J. [DOI] [PubMed] [Google Scholar]
- 6.Yang Q., Jobic H., Salles F., Kolokolov D., Guillerm V., Serre C., Maurin G. Probing the dynamics of CO2 and CH4 within the porous zirconium terephthalate UiO-66(Zr): A synergic combination of neutron scattering measurements and molecular simulations. Chemistry. 2011;17:8882–8889. doi: 10.1002/chem.201003596. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y., Qin W., Li Z., Li Y. Enhanced stability and CO2 affinity of a UiO-66 type metal-organic framework decorated with dimethyl groups. Dalton Trans. 2012;41:9283–9285. doi: 10.1039/c2dt30950e. [DOI] [PubMed] [Google Scholar]
- 8.Garibay S.J., Cohen S.M. Isoreticular synthesis and modification of frameworks with the UiO-66 topology. Chem. Commun. 2010;46:7700–7702. doi: 10.1039/c0cc02990d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cmarik G.E., Kim M., Cohen S.M., Walton K.S. Tuning the adsorption properties of UiO-66 via ligand functionalization. Langmuir. 2012;28:15606–15613. doi: 10.1021/la3035352. [DOI] [PubMed] [Google Scholar]
- 10.Ethiraj J., Albanese E., Civalleri B., Vitillo J.G., Bonino F., Chavan S., Shearer G.C., Lillerud K.P., Bordiga S. Carbon dioxide adsorption in amine-functionalized mixed-ligand metal-organic frameworks of UiO-66 topology. ChemSusChem. 2014;7:3382–3388. doi: 10.1002/cssc.201402694. [DOI] [PubMed] [Google Scholar]
- 11.Kandiah M., Nilsen M.H., Usseglio S., Jakobsen S., Olsbye U., Tilset M., Larabi C., Quadrelli E.A., Bonino F., Lillerud K.P. Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem. Mater. 2010;2:6632–6640. doi: 10.1021/cm102601v. [DOI] [Google Scholar]
- 12.Kandiah M., Usseglio S., Svelle S., Olsbye U., Lillerud K.P., Tilset M. Post-synthetic modification of the metal-organic framework compound UiO-66. J. Mater. Chem. 2010;20:9848–9851. doi: 10.1039/c0jm02416c. [DOI] [Google Scholar]
- 13.Yang Q., Wiersum A.D., Llewellyn P.L., Guillerm V., Serre C., Maurin G. Functionalizing porous zirconium terephthalate UiO-66(Zr) for natural gas upgrading: A computational exploration. Chem. Commun. 2011;47:9603–9605. doi: 10.1039/c1cc13543k. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q., Wiersum A.D., Jobic H., Guillerm V., Serre C., Llewellyn P.L., Maurin G. Understanding the thermodynamic and kinetic behavior of the CO2/CH4 gas mixture within the porous zirconium terephthalate UiO-66(Zr): A joint experimental and modeling approach. J. Phys. Chem. C. 2011;115:13768–13774. doi: 10.1021/jp202633t. [DOI] [Google Scholar]
- 15.Stavitski E., Pidko E.A., Couck S., Remy T., Hensen E.J., Weckhuysen B.M., Denayer J., Gascon J., Kapteijn F. Complexity behind CO2 capture on NH2-MIL-53(Al) Langmuir. 2011;27:3970–3976. doi: 10.1021/la1045207. [DOI] [PubMed] [Google Scholar]
- 16.Vaidhyanathan R., Iremonger S.S., Dawson K.W., Shimizu G.K.H. An amine-functionalized metal organic framework for preferential CO2 adsorption at low pressures. Chem. Commun. 2009;35:5230–5232. doi: 10.1039/b911481e. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y., Zhao Y.X., Lv Z.J., Song F.J., Zhong Q. Preparation and enhanced CO2 adsorption capacity of UiO-66/graphene oxide composites. J. Ind. Eng. Chem. 2015;27:102–107. doi: 10.1016/j.jiec.2014.12.021. [DOI] [Google Scholar]
- 18.Zhao Y.X., Ding H.L., Zhong Q. Synthesis and characterization of MOF-aminated graphite oxide composites for CO2 capture. Appl. Surf. Sci. 2013;284:138–144. doi: 10.1016/j.apsusc.2013.07.068. [DOI] [Google Scholar]
- 19.Abid H.R., Shang J., Ang H., Wang S. Amino-functionalized Zr-MOF nanoparticles for adsorption of CO2 and CH4. Int. J. Smart Nano Mater. 2013;4:72–82. doi: 10.1080/19475411.2012.688773. [DOI] [Google Scholar]
- 20.Gascon J., Aktay U., Hernandezalonso M., Vanklink G., Kapteijn F. Amino-based metal-organic frameworks as stable, highly active basic catalysts. J. Catal. 2009;261:75–87. doi: 10.1016/j.jcat.2008.11.010. [DOI] [Google Scholar]
- 21.Boutin A., Couck S., Coudert F., Serra-Crespo P., Gascon J., Kapteijn F., Fuchs A.H., Denayer J.F.M. Thermodynamic analysis of the breathing of amino-functionalized MIL-53(Al) upon CO2 adsorption. Microporous Mesoporous Mater. 2011;140:108–113. doi: 10.1016/j.micromeso.2010.07.009. [DOI] [Google Scholar]
- 22.Nik O.G., Chen X.Y., Kaliaguine S. Functionalized metal organic framework-polyimide mixed matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2012;413–414:48–61. doi: 10.1016/j.memsci.2012.04.003. [DOI] [Google Scholar]
- 23.Vermoortele F., Ameloot R., Vimont A., Serre C., Vos D.D. An amino-modified Zr-terephthalate metal-organic framework as an acid-base catalyst for crossaldol condensation. Chem. Commun. 2011;47:1521–1523. doi: 10.1039/C0CC03038D. [DOI] [PubMed] [Google Scholar]
- 24.Chen X.Y., Vinh-Thang H., Rodrigue D., Kaliaguine S. Amine-functionalized MIL-53 metal-organic framework in polyimide mixed matrix membranes for CO2/CH4 separation. Ind. Eng. Chem. Res. 2012;51:6895–6906. doi: 10.1021/ie3004336. [DOI] [Google Scholar]
- 25.Marx S., Lleist W., Huang H., Maciejewski M., Baiker A. Tuning functional sites and thermal stability of mixed-linker MOFs based on MIL-53(Al) Dalton Trans. 2010;39:3795–3798. doi: 10.1039/c002483j. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Z., Li Z., Lin Y.S. Adsorption and diffusion of carbon dioxide on metal-organic framework (MOF-5) Ind. Eng. Chem. Res. 2009;48:10015–10020. doi: 10.1021/ie900665f. [DOI] [Google Scholar]
- 27.Walton K.S., Millward A.R., Dubbeldam D., Frost H., Low J., Yaghi O.M., Sunrr R.Q. Understanding inflections and steps in carbon dioxide adsorption isotherms in metal-organic frameworks. J. Am. Chem. Soc. 2017;130:406–407. doi: 10.1021/ja076595g. [DOI] [PubMed] [Google Scholar]
- 28.Liang Z., Marshall M., Chaffee A.L. CO2 Adsorption-based separation by metal organic framework (Cu-BTC) versus Zeolite (13X) Energy Fuels. 2009;23:2785–2789. doi: 10.1021/ef800938e. [DOI] [Google Scholar]
- 29.Skoulidas A.I., Sholl D.S. Self-diffusion and transport diffusion of light gases in metal-organic framework materials assessed using molecular dynamics simulations. J. Phys. Chem. B. 2015;109:15760–15768. doi: 10.1021/jp051771y. [DOI] [PubMed] [Google Scholar]
- 30.Kondo S., Tatsuo I. AbeI, Adsorption Science. Chemistry Industry Press; Beijing, China: 2005. [Google Scholar]
- 31.Hui W., Shen Y.C., Vaiva K., Madhusudan T., Ping C., Taner Y., Wei Z. Unusual and highly tunable missing-linker defects in zirconium metal-organic framework UiO-66 and their important effects on gas adsorption. J. Am. Chem. Soc. 2013;135:10525–10532. doi: 10.1021/ja404514r. [DOI] [PubMed] [Google Scholar]
- 32.Aprea P., Caputo D., Gargiulo N., Iucolano F., Pepe F. Modeling carbon dioxide adsorption on microporous substrates: Comparison between Cu-BTC metal-organic framework and 13X zeolitic molecular sieve. J. Chem. Eng. Data. 2010;55:3655–3661. doi: 10.1021/je1002225. [DOI] [Google Scholar]
- 33.An J., Geib S.J., Rosi N.L. High and selective CO2 uptake in a cobalt adeninate metal-organic framework exhibiting pyrimidine and amino-decorated pores. J. Am. Chem. Soc. 2010;132:38–39. doi: 10.1021/ja909169x. [DOI] [PubMed] [Google Scholar]
- 34.Saha D., Bao Z., Jia F., Deng S.G. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A. Environ. Sci. Technol. 2010;44:1820–1826. doi: 10.1021/es9032309. [DOI] [PubMed] [Google Scholar]