Abstract
Objectives
(1) To describe repair of complex orbital fractures using computer planning with preoperative virtual reconstruction, mirror image overlay, endoscopy, and surgical navigation. (2) To test the hypothesis that this technique improves outcomes in complex orbital fractures.
Methods
A series of 113 consecutive severe orbital fracture cases was analyzed, 56 of which were performed with mirror image overlay guidance, and 57 of which were repaired without. Data were collected on patient characteristics, fracture severity, diplopia and globe position outcomes, complications, and need for revision surgery.
Results
The mirror image overlay group showed decreased postoperative diplopia in all fracture types (P=.003); the effectiveness was maximal for fractures that involved 3 or 4 walls or the posterior one-third of the orbital floor (P<.001). The need for revision surgery was greatly reduced in this cohort (4% vs 20%; P =.03).
Conclusions
The efficacy of mirror image overlay navigation and orbital endoscopy was studied in one of the largest series of complex orbital fractures in the literature. Based on statistically significant improved outcomes in postoperative diplopia and orbital volume, as well as the decreased need for revision surgery, we accept the hypothesis that mirror image overlay guidance improves outcomes in complex orbital reconstruction and recommend its use for complex orbital fracture repair.
Orbital fracture repair can be among the most challenging of craniofacial reconstructions. The reasons for this are multiple: the surgical approach is often minimized in an attempt to reduce collateral morbidity, exposure of the fracture requires precise dissection to avoid damage to the eyelids and orbital contents, the working space is confined owing to the tight orbital contours and soft tissue that may prolapse to obstruct the surgeon’s view, and for all but isolated single wall fractures, simultaneous viewing of the entire fracture site is typically not possible.
The 3 most common unfavorable outcomes of orbital fracture repair are diplopia, persistent enophthalmos, and infra-orbital nerve hypoesthesia.1 The rates of these complications vary widely in the literature. For diplopia, the incidence ranges from 20% to 42.5% for isolated orbital floor fractures and up to 86% for complex fractures involving multiple walls or the posterior one-third of the orbital floor (also known as the ethmoidal-maxillary strut or “key area”).2–4 For persistent enophthalmos and infraorbital nerve hypoesthesia, complication rates range from 7% to 27.5% and 7% to 59%, respectively.5,6 Such wide ranges for complication rates are due to differences in technique as well as differences in the methods of defining and detecting these conditions.
Another critical outcome parameter is the need for secondary operation, which is most commonly performed for persistent enophthalmos or diplopia. Owing to the large variety of fracture patterns and relative treatment complexities associated with each, it is difficult to determine an accurate rate of secondary operations stratified by fracture severity. Recognizing limitations, a retrospective study of 177 orbital fractures (52% of which were limited to 1 orbital wall) reported a 4% secondary operation rate.7
The single most important factor in minimizing postoperative complications related to globe position is restoration of the orbital bones to their correct anatomic position.8,9 Technological advancements in imaging and surgical computer planning may assist the surgeon in this task. One of the most promising of the emerging technologies is the ability to use computer planning software to create a mirror image overlay (MIO) on a craniofacial computed tomographic (CT) scan. This involves duplicating the contralateral (nontraumatized) orbitozygomatic region, highlighting and reversing (side-to-side) the segment, and superimposing its skeleton onto the fractured, displaced orbit (Figure 1 and Figure 2). When coupled with intraoperative surgical navigation, this technology can provide real-time feedback demonstrating the position and shape of a surgical implant relative to the correct anatomic position of the native bone.
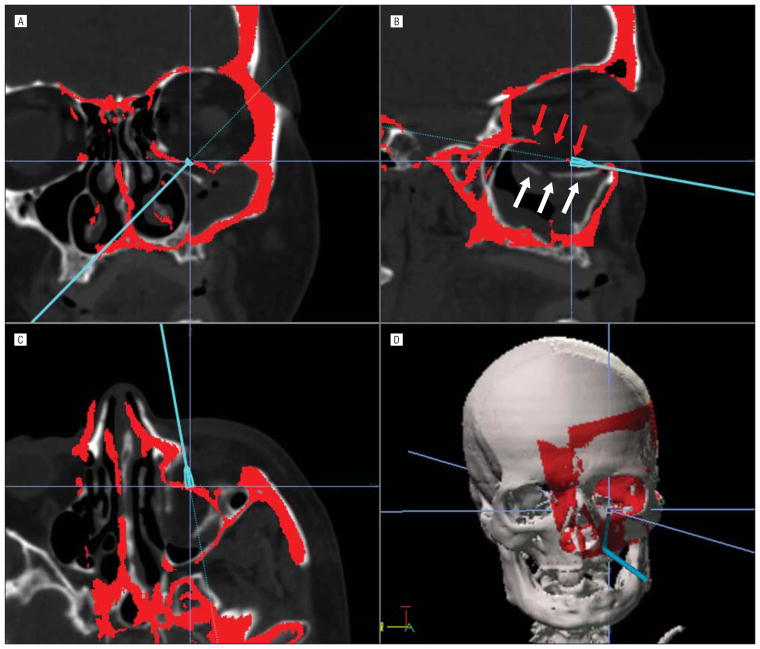
Figure 1.
Mirror image overlay (MIO) from the right unaffected side onto the left fractured side. The cursor is seen confirming the intraoperative placement of the new implant with surgical navigation. A, Coronal image. B, Sagittal image. White arrows point to the displaced orbital floor, and the red arrows point to the MOI orbital floor, or the desired position for the reconstruction. C, Axial image. D, Three-dimensional reconstruction.
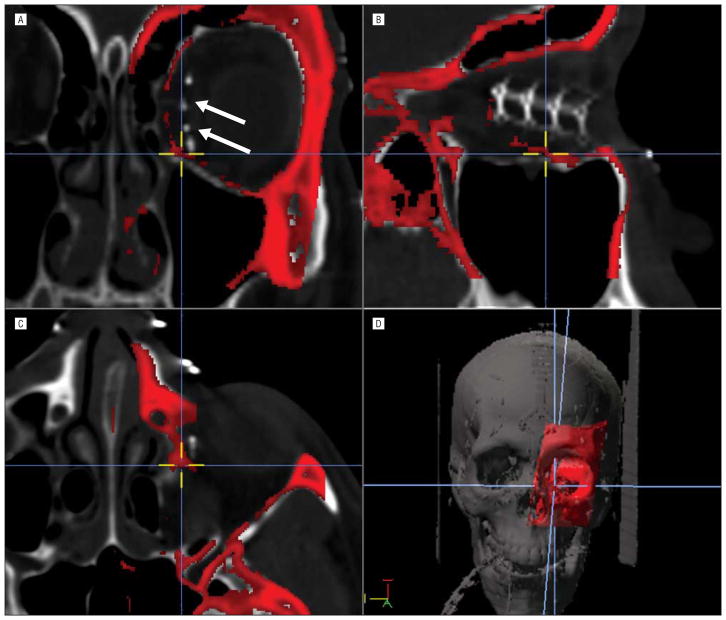
Figure 2.
A second example of the mirror image overlay technique. In this instance it is a revision operation. A, Coronal image. The prior implant (arrows) is seen in a nonfavorable lateralized position. B, Sagittal image. C, Axial image. D, Three-dimensional reconstruction.
Over the past 10 years, multiple studies have examined the feasibility of this technique and reported preliminary outcome data.10–14 A variety of surgical approaches were used, and some of the studies examined stereolitho-graphic models and precontoured implants. The studies suggest that the technique has great potential to aid the surgeon in optimally positioning orbital implants to adequately restore orbital volume and projection. However, all published studies on the MIO technique were limited to case series of less than 25 patients, lacked a comparison group, and were not hypothesis driven. Furthermore, none of these studies used orbital endoscopy.
Orbital endoscopy offers advantages of improved lighting, visualization, and magnification with the ability to use reduced incisions while minimizing globe retraction.15,16 We have found this technique particularly beneficial after completion of the reconstruction to confirm accurate anatomic reconstruction and to ensure that no orbital contents are entrapped by the implant. In 2009, we began using MIO as an additional measure to improve the accuracy of orbital reconstructions.
The objectives of this study were 2-fold: (1) to describe the technique of endoscopic image-guided orbital repair using preoperative virtual reconstruction with MIO, and (2) to test the hypothesis that the MIO technique leads to improved outcomes in the treatment of complex orbital fractures.
METHODS
PATIENT SELECTION
The outcomes of 2 groups of orbital reconstruction cases were compared. The study (MIO) group consisted of a consecutive cohort of all orbital reconstruction cases after the implementation of the MIO technique. Data collection was stopped to allow a minimum of 9 months of follow-up. A historical control group was composed of consecutive operations performed immediately prior to the beginning of MIO use. The following exclusion criteria applied: (1) insufficient documentation (ie, no documented globe position postoperatively), (2) no follow-up beyond postoperative day 7, (3) blindness due to injury, (4) neoplasm, and (5) bilateral orbital fractures. No cases during the initial MIO learning period were eliminated from the study.
SURGICAL TECHNIQUE
The surgical technique was unaltered between the 2 groups except for the use of computer planning and MIO. Orbital endoscopy was used as described previously in all patients, with the exception of 1 patient in the control group who underwent an orbital floor reconstruction.15,16 All cases were performed by, or under the direction of, a single surgeon (K.S.M.).
Transconjunctival (TC) inferior fornix, precaruncular (PC), or lateral retrocanthal (LRC) surgical approaches were used depending on the location of the fracture.17,18 Implants were fabricated intraoperatively by the surgeon.
MIO SETUP AND INTRAOPERATIVE USE
iNtellect Cranial Navigation (Stryker Corp) was used to create the MIO and for surgical navigation. A preoperative CT scan (0.6–1.25 mm cuts) was loaded into the navigation computer, and the MIO was created prior to the patient entering the operating room. Registration using an adhesive mask technique was the only task that needed to be performed with the patient in the operating room. Figure 1 and Figure 2 show 2 examples of the MIO in the computer planning software. Endoscopy was used with navigation to compare the position and conformation of the implant relative to the preoperative MIO surgical plan (Figure 3).
Figure 3.
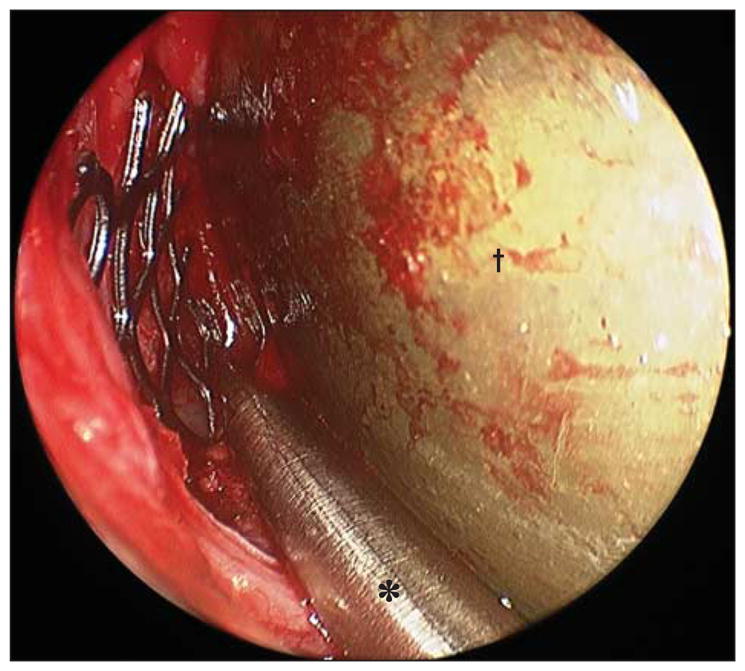
Endoscopic photograph of left orbit confirming correct placement of implant using navigation probe (*). A malleable retractor (†) is seen on the right side of the image retracting the orbital contents.
DATA COLLECTION AND OUTCOME MEASURES
All data were collected from the electronic medical record after approval by the University of Washington institutional review board. All orbital operations performed by the senior author (K.S.M.) within the specified time periods were reviewed. Patient and fracture characteristics as well as outcome measures, including preoperative and postoperative diplopia severity, pre-operative and postoperative globe position (abnormal or normal), and need for secondary operation, were recorded.
Diplopia severity was rated on a 0 to 3 scale (Table 1). This method was derived to emphasize diplopia because it subjectively affected patients in daily life. Grade 2 and grade 3 categories were based on patients either voluntarily reporting diplopia or in response to a question asked to all patients, “Do you have double vision?” Previous literature has suggested that objective diplopia measurements are more sensitive than those reported by patients19; thus, the grade 1 category comprised patients with no subjective double vision reported in daily life but with diplopia detected on examination. Patients were placed in the most severe diplopia category in which they satisfied any 1 of the criteria listed in Table 1.
Table 1.
Diplopia Severity Category Assignment
| Diplopia Severity Grade | Criteria | Assigned Value |
|---|---|---|
| 3 | Diplopia reported as present most of the time or diplopia present in primary gaze. | 3 |
| 2 | Diplopia reported in some aspect of daily life (eg, “only when looking far to my left”), but most of the time. | 2 |
| 1 | Patient reported no diplopia, but on examination was found to have some degree of double vision when asked to track a light source through the 4 cardinal gaze positions: up, down, left, right. | 1 |
| None | No subjective or objective diplopia. | 0 |
Globe position was assessed by the senior author in all patients and was determined to be either normal or abnormal (any discernable amount of globe displacement).
Operative details, including surgical approach, were collected. The number of orbital walls involved in the fracture was noted as well as the status of the posterior one-third of the orbital floor based on radiographic evidence or operative findings. Postoperative complications were recorded and tracked, as was the incidence of revision surgery.
STATISTICAL ANALYSIS
A t test allowing for unequal variances was used to compare means across the 2 groups. Proportions were compared across 2 groups using an unconditional exact χ2 test, which assures proper type I error in small samples sizes.20
RESULTS
A total of 113 consecutive orbital operations performed by the senior author were identified, 56 of which occurred after October 1, 2009, and were performed using the MIO technique, and 57 cases were performed without the MIO technique. Twenty-three patients were excluded from both groups (Table 2). There was 1 case during which the MIO technique and navigation were not functional, and this patient was transferred to the control group for analysis. This yielded a total of 90 operations (45 from the control group and 45 from the MIO group) for data collection and outcomes analysis.
Table 2.
Excluded Patients
| Reason for Exclusion | Groups | |
|---|---|---|
|
| ||
| Control | MIO | |
| Insufficient documentation in medical chart | 1 | 0 |
| Insufficient follow-up | 1 | 1 |
| Blindness due to injury | 8 | 5 |
| Bilateral fractures | 1 | 3 |
| Neoplasm | 1 | 2 |
| Total | 12 | 11 |
Abbreviation: MIO, mirror image overlay.
Patient demographics, mechanism of injury, elapsed time between injury and repair, and patient follow-up are summarized in Table 3. The orbit was previously operated on in 8 cases in the MIO group and 7 in the control group. The period between injury and repair was prolonged owing to a limited number of revision surgical procedures performed approximately 2 years after the initial surgery. With the revision cases excluded, the average number of days until operation was 23 and 29 for the control and MIO groups, respectively.
Table 3.
Summary of Injury Mechanism, Patient Characteristics, Follow-up, Operation Timing, and the Number of Cases in Which the Orbit Was Previously Operated
| Cohort Characteristics | Groups | ||
|---|---|---|---|
|
| |||
| Control | MIO | Both | |
| Injury mechanism, No. | |||
| Assault | 19 | 21 | 40 |
| Motor vehicle crash | 12 | 6 | 18 |
| Fall | 6 | 6 | 12 |
| Sport | 5 | 3 | 8 |
| Othera | 3 | 9 | 12 |
| Patient characteristics | |||
| Age, mean (range), y | 36 (14–82) | 36 (8–62) | 36 (8–82) |
| Female, % | 24 | 29 | 27 |
| Male, % | 76 | 71 | 73 |
| Follow-up, mean (range), d | 303 (9–1209) | 212 (8–748) | 257 (8–1209) |
| Operation timing | |||
| Mean days from injury date until date of operation, No. | 34 | 74 | 54 |
| Mean days from injury date until date of operation (excluding revision operations), No. (range) | 23 (0–293) | 29 (4–862) | 26 (0–862) |
| Cases in which orbit was previously operated, No. | 8 | 7 | 15 |
Abbreviation: MIO, mirror image overlay.
Other injuries occurred while hang gliding, rock climbing, all-terrain vehicle riding, bull riding, and other mishaps involving water balloon launchers and falling trees.
Fracture patterns are reported in Table 4. The most common was 3 or 4 walls, seen in both the control group (42%) and the MIO group (38%). The posterior one-third of the orbital floor, or 2 or more walls, were involved in 80% of cases in the control group and 82% in the MIO group.
Table 4.
Fracture Patterns
| Fracture Pattern | Groups, No. (%) | ||
|---|---|---|---|
|
| |||
| Control | MIO | Both | |
| Isolated orbital floor | 9 (20) | 8 (18) | 17 (19) |
| Isolated medial wall | 2 (4) | 3 (7) | 5 (6) |
| 2 Walls | 15 (33) | 17 (38) | 32 (36) |
| 3 or 4 Walls | 19 (42) | 17 (38) | 36 (40) |
| Posterior one-third of orbital floor involvement of all fracture types | 28 (62) | 30 (67) | 58 (64) |
| Posterior one-third of orbital floor involvement, or ≥2 walls | 36 (80) | 37 (82) | 73 (81) |
Abbreviation: MIO, mirror image overlay.
A combination of TC, PC and LRC surgical approaches were used. In 8 instances, these were extended with a lateral canthotomy. A lateral blepharoplasty incision was used 4 times to access the zygomaticofrontal suture line as well as the lateral orbit wall.
Implants used most frequently were titanium mesh coated with high-density polyethylene (Medpore or Titan by Stryker Corp), followed by bare titanium mesh (Stryker Corp or Synthes Corp). PDS sheet (Ethicon Corp) was rarely used.
Preoperative and postoperative diplopia severity was assigned a score of 0, 1, 2, or 3 as described in Table 1. An improvement factor (possible range of −3 to 3) was calculated by subtracting the postoperative value from the preoperative value for each operation. The control group average improvement was 0.56 (95% CI, 0.27–0.84), whereas the MIO group average improvement was 1.16 (95% CI, 0.88–1.42). Thus, the score for the MIO group was, on average, 0.60 higher than that of the control group (95% CI, 0.21–0.99; P = .003). This represents a greater than 2-fold improvement in the diplopia outcome result compared with the control group. For example, if a patient had grade 2 diplopia preoperatively, there was a 2-fold increased likelihood that the postoperative result would be grade 1 (a result which is not noticeable to the patient in daily life). Table 5 summarizes the diplopia outcomes. There were no cases in which worsening of visual acuity was noted.
Table 5.
Diplopia Results for All Cases
| Diplopia Results | Groups, No. (%) | |
|---|---|---|
| Control | MIO | |
| No. | 45 | 45 |
| Average improvement factora | 0.56 | 1.19 |
| Grade 3 | ||
| Preoperative | 6 (13) | 11 (24) |
| Postoperative | 1 (2) | 1 (2) |
| Grade 2 | ||
| Preoperative | 19 (42) | 24 (53) |
| Postoperative | 10 (22) | 7 (16) |
| Grade 1 | ||
| Preoperative | 8 (18) | 3 (7) |
| Postoperative | 16 (36) | 15 (33) |
| None | ||
| Preoperative | 12 (27) | 7 (16) |
| Postoperative | 18 (40) | 22 (49) |
| Patients with grades 2 and 3 postoperative diplopia | 11 (24) | 8 (19) |
Abbreviation: MIO, mirror image overlay.
Average improvement factor is calculated by assigning a value of diplopia severity and subtracting postoperative scores from preoperative scores.
Values were assigned according to criteria in Table 1: grade 3, grade 2, grade 1, and none. P = .003.
In both the MIO and control groups, 71% of the operations were performed on patients with an abnormal globe position preoperatively (Table 6). In the MIO group, the number of operations that resulted in a change from abnormal (preoperative) to normal (postoperative) was 88%, compared with 69% in the control group (P = .08). Of those with abnormal preoperative globe positions, 31% remained abnormal postoperatively in the control group, whereas only 13% in the MIO group remained abnormal (P = .09). Table 7 summarizes the complications.
Table 6.
Globe Position Results for All Patients
| Globe Position | Groups, No. (%) | P Value | Both Groups, No. (%) | |
|---|---|---|---|---|
|
| ||||
| Control | MIO | |||
| Preoperative | ||||
| Abnormal | 32 (71) | 32 (71) | >.99 | 64 (71) |
| Normal | 13 (29) | 13 (29) | >.99 | 26 (29) |
| Postoperative | ||||
| Abnormal | 10 (22) | 4 (9) | .09 | 14 (16) |
| Normal | 35 (78) | 41 (91) | .09 | 76 (84) |
| Preoperative → postoperative | ||||
| Abnormal → abnormal | 10 (31) | 4 (13) | .09 | 14 (22) |
| Abnormal → normal | 22 (69) | 28 (88) | .08 | 50 (78) |
| Normal → normal | 13 (100) | 13 (100) | >.99 | 26 (100) |
| Normal → abnormal | 0 | 0 | NA | 0 |
Abbreviations: MIO, mirror image overlay; NA, not applicable.
Table 7.
Complications
| Detail | Groups | |
|---|---|---|
|
| ||
| Control | MIO | |
| Extrusion or infection requiring removal of hardwarea | 3 | 2 |
| Entropion | 0 | 2 |
| Ectropion | 0 | 2 |
| Epiphora | 0 | 1 |
| Eyelid abscess | 1 | 0 |
| Retrobulbar hematoma | 1 | 0 |
| Total No. | 5 | 7 |
Abbreviation: MIO, mirror image overlay.
Four of the 5 hardware removals were for plates along the orbital rim, and 1 was for an infected Medpore implant (Stryker Corp).
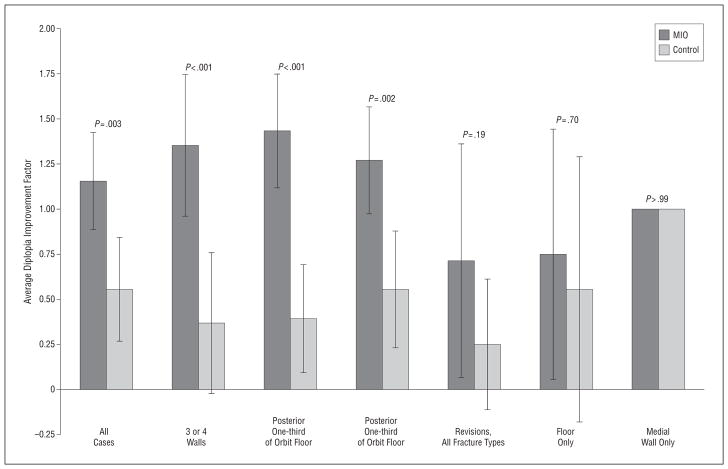
Analyzing outcomes for the revision surgery subset showed an average diplopia improvement factor of 0.71 in the MIO group compared with 0.25 in the control group (Table 8). For globe position outcomes in this subset, 71% of the operations in the MIO group resulted in a normal postoperative globe appearance compared with 50% in the control group (Table 9). This subset included 15 patients, and there was no statistical significance found. Stratifying diplopia based on fracture type and whether it was a revision operation showed that the effect of the MIO technique was greatest in the cases of complex fractures involving 2 or more walls or the posterior one-third of the orbital floor (Figure 4).
Table 8.
Diplopia Outcomes for the Revision Operations
| Diplopia Results | No. % | |
|---|---|---|
|
| ||
| Control Group | MIO Group | |
| No. | 8 | 7 |
| Average improvement factora | 0.25 | 0.71 |
| Grade 3 | ||
| Preoperative | 1 (13) | 0 |
| Postoperative | 0 | 0 |
| Grade 2 | ||
| Preoperative | 5 (63) | 5 (71) |
| Postoperative | 5 (63) | 1 (14) |
| Grade 1 | ||
| Preoperative | 0 | 1 (14) |
| Postoperative | 1 (13) | 4 (57) |
| None | ||
| Preoperative | 2 (25) | 1 (14) |
| Postoperative | 2 (25) | 2 (29) |
| Patients with grades 2 and 3 postoperative diplopia | 5 (63) | 1 (14) |
Abbreviation: MIO, mirror image overlay.
P = .19.
Table 9.
Globe Position Outcomes for the Revision Operations
| Globe Position | Group, No. (%) | ||
|---|---|---|---|
|
| |||
| Control | MIO | Both | |
| Preoperative | |||
| Abnormal | 8 (100) | 7 (100) | 15 (100) |
| Normal | 0 | 0 | 0 |
| Postoperative | |||
| Abnormal | 4 (50) | 2 (29) | 6 (40) |
| Normal | 4 (50) | 5 (71) | 9 (60) |
| Preoperative → postoperative | |||
| Abnormal → abnormal | 4 (50) | 2 (29) | 6 (40) |
| Abnormal → normal | 4 (50) | 5 (71) | 9 (60) |
| Normal → normal | 0 | 0 | 0 |
| Normal → abnormal | 0 | 0 | 0 |
Abbreviation: MIO, mirror image overlay.
Figure 4.
Diplopia outcomes stratified for revisions and fracture types. Error bars indicate 95% confidence intervals. MIO indicates mirror image overlay.
Eleven cases required revision surgery: 2 in the MIO group and 9 in the control group (P = .03). Of the patients requiring a secondary operation, 91% had an abnormal globe position and 73% had grades 1 to 3 diplopia.
COMMENT
This analysis of 90 consecutive complex orbital repairs demonstrated a large, statistically significant improvement in outcomes by the use of preoperative virtual reconstruction with the MIO technique. Specifically, the use of MIO led to a decrease in postoperative diplopia severity (P = .003) and a greater than 4-fold decrease in the need for revision surgery (P = .03) in all cases. The improved effect on diplopia outcomes was more pronounced for the subsets of fractures involving multiple walls or the posterior one-third of the orbital floor (P < .001). A trend toward improved resolution of abnormal globe position was found in the MIO group as well, though this study was not designed to detect subtotal resolution of orbital volume abnormalities.
Furthermore, the data in this study showed markedly improved outcomes in both volume abnormalities and diplopia using the MIO technique in revision cases (diplopia improvement factor of 0.71 for the MIO group compared with 0.25 for the control). This was not statistically significant, partly owing to the small number of revision cases (n = 15). In our practice, however, revision surgery is one of the strongest indications for the use of endoscopic MIO-guided orbital reconstruction. Revision operations are technically more challenging, and reliance on visual analysis for confirmation of anatomic reduction may be less reliable.
The MIO technique seems to be enhanced with the use of orbital endoscopy, which permits simultaneous visualization of the navigation probe coursing along the entire contour of the implant while checking the MIO on the computer to confirm correct placement (Figure 2 and Figure 3). While it is possible to perform this task with standard open techniques, in our experience endoscopy optimizes visualization while minimizing the size of the incision and amount of retraction of the orbital contents, particularly in the posterior orbit.
Grades 2 and 3 subjective diplopia persisted postoperatively in 21.5% of patients in each group. Diplopia of any severity persisted in 56% of patients in both groups, which is comparable with the rates in existing literature despite this study’s predominance of severe fracture patterns (81%). Given the severity of injury in this series, it is quite likely that a significant percentage of patients with persistent diplopia had underlying nerve or muscle damage. This study did not differentiate neuromuscular diplopia etiologies, or others that persist despite correct orbital position.
The average follow-up time was longer in the control group at 10 months compared with 7 months in the MIO group. This must be considered when interpreting the results; however, there is evidence that diplopia may continue to improve up to 12 months postoperatively.21,22 Thus, it is likely that our results underestimate the eventual improvement that some of the MIO population would attain. The minimum time frame for inclusion was 7 days. This short time period was set because many of the patients traveled great distances for treatment—Harborview Medical Center is the level 1 trauma center serving a 5-state area including Alaska. Our practice is to follow local and regional patients for 12 months, but patients from greater distances follow up with a local physician, returning only if a problem develops.
Our preference is to repair fractures 5 to 10 days after injury if there is no urgent indication such as muscle entrapment. However, the number of days elapsed from injury to operation in this series was larger because many of the patients were medically unstable or had sustained severe neurologic injury that mandated a delay in reconstruction.
A potential concern in using MIO and navigation technology is operative time. However, the computer planning and MIO can be performed preoperatively and loaded onto the computer. Surgical navigation set up and registration to a CT scan can be performed in minutes, as is routinely done in endoscopic sinus surgery. We have found that the time spent with setup is reclaimed by shortened operative time owing to increased confidence in appropriate orbital implant placement and a lower rate of secondary surgery. This technique could also be used with preformed implants, although this is not our practice.
Though we found this technique to be reliable, technical challenges may be encountered. The protocol is not useful for bilateral injury because a normal “template” is required for MIO; furthermore, in cases of significant congenital asymmetry, such as hemifacial microsomia, the MIO will not be accurate. To generate a meaningful MIO onto the fractured side, the CT scan should be fine cut per navigation protocol with slices 0.6- to 1.25-mm thick. As seen in Figure 1, the red overlay is not continuous, and some estimation of gaps in information is required. Thus, checking for correct positioning with the surgical navigation takes some practice to become comfortable with the technique. Surgical navigation registration techniques that rely on surface landmarks will have decreased accuracy if the CT scan was performed with edema present that has changed significantly by the day of the operation. This will not affect the accuracy of the actual MIO because that is based on bony anatomy, but if the CT scan is registered inaccurately to the patient, then surgical navigation will be inadequate. This problem occurred in 1 of our cases, and the MIO technique could not be used effectively. After a learning experience of approximately 5 cases, however, the surgical team better understood the potential pitfalls of the technique both preoperatively and intraoperatively. The CT scans were updated as needed, and no major difficulties were encountered after the initial learning curve.
The disposable items used in each case included the facemask for registration and batteries for the navigation probe; these costs totaled approximately $300. We believe this cost is reasonable given the reduced surgical time, decreased need for revision surgery, and decreased need for postoperative CT scans to confirm appropriate implant position.
This study reports one of the largest series of complex orbital fractures in publication and is the only hypothesis-driven retrospective cohort outcome study that we located in a search of the literature. Despite this, there are inherent weaknesses in the study. The evaluation is retrospective, and perimetry was not routinely performed to rigorously analyze the presence or degree of diplopia. The diplopia severity scoring system was created to be pragmatic and highlight diplopia that was functionally significant, recognizing that objective measurements are likely to be more sensitive than subjective patient reporting.19 Future studies of diplopia outcomes may be more robust implementing the Cervical Range of Motion method.23 Regarding orbital volume, Hertel exophthalmometry and postoperative CT scans were performed only when medically indicated—thus, direct volumetric analysis was not possible in this series. There is a possible reporting bias in the determination of globe position; however, assessments of normal or abnormal were by the same surgeon in 90 cases over a 3-year period.
In conclusion, a series of 113 consecutive severe orbital fracture repairs in a 3-year period was analyzed to evaluate the efficacy of the endoscopic MIO technique. Compared with controls, the use of MIO resulted in a significant reduction in postoperative diplopia for complex fractures (P < .001). Furthermore, the incidence of postoperative volume abnormalities was reduced in the MIO cohort. Perhaps even more clinically relevant, the need for revision surgery in severe fractures was reduced from 20% to 4% with the use of this technology (P = .03). Based on experience, another important indication for the use of the MIO is for revision operations. Although not statistically significant, in part due to limited numbers (n = 15), the results in this subset showed improved outcomes in diplopia and globe position.
We therefore accept the hypothesis of the study that MIO improves outcomes in the repair of complex orbital fractures. We conclude that endoscopic MIO reconstruction of the orbit is indicated for the repair of fractures that (1) involve 2 or more walls, (2) include the posterior one-third of the orbital floor, or (3) are revision operations.
Acknowledgments
Funding/Support: This work was supported by the NIH T32 DC00018 training grant.
Footnotes
Conflict of Interest Disclosures: None reported.
Additional Contributions: R. R. Moe provided technical comments and critical evaluation of the manuscript. We also thank the BioRobotics Laboratory at the University of Washington; Blake Hannaford, PhD; and Karthik Balakrishnan, MD.
Author Contributions: Dr Moe had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Bly, Liu, and Moe. Acquisition of data: Bly and Liu Analysis and interpretation of data: Bly, Chang, Cudejkova, Liu, and Moe. Drafting of the manuscript: Bly, Cudejkova, and Moe. Critical revision of the manuscript for important intellectual content: Bly, Chang, Liu, and Moe. Statistical analysis: Bly and Chang. Obtained funding: Bly. Administrative, technical, and material support: Cudejkova and Moe. Study supervision: Chang and Moe.
References
- 1.Brucoli M, Arcuri F, Cavenaghi R, Benech A. Analysis of complications after surgical repair of orbital fractures. J Craniofac Surg. 2011;22(4):1387–1390. doi: 10.1097/SCS.0b013e31821cc317. [DOI] [PubMed] [Google Scholar]
- 2.Greenwald HS, Jr, Keeney AH, Shannon GM. A review of 128 patients with orbital fractures. Am J Ophthalmol. 1974;78(4):655–664. doi: 10.1016/s0002-9394(14)76304-4. [DOI] [PubMed] [Google Scholar]
- 3.Biesman BS, Hornblass A, Lisman R, Kazlas M. Diplopia after surgical repair of orbital floor fractures. Ophthal Plast Reconstr Surg. 1996;12(1):9–16. doi: 10.1097/00002341-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Jaquiéry C, Aeppli C, Cornelius P, Palmowsky A, Kunz C, Hammer B. Reconstruction of orbital wall defects: critical review of 72 patients. Int J Oral Maxillofac Surg. 2007;36(3):193–199. doi: 10.1016/j.ijom.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Hoşal BM, Beatty RL. Diplopia and enophthalmos after surgical repair of blow-out fracture. Orbit. 2002;21(1):27–33. doi: 10.1076/orbi.21.1.27.2598. [DOI] [PubMed] [Google Scholar]
- 6.Vriens JP, van der Glas HW, Moos KF, Koole R. Infraorbital nerve function following treatment of orbitozygomatic complex fractures: a multitest approach. Int J Oral Maxillofac Surg. 1998;27(1):27–32. doi: 10.1016/s0901-5027(98)80091-x. [DOI] [PubMed] [Google Scholar]
- 7.Nowinski D, Messo E, Hedlund A. Treatment of orbital fractures: evaluation of surgical techniques and materials for reconstruction. J Craniofac Surg. 2010;21(4):1033–1037. doi: 10.1097/SCS.0b013e3181e4345d. [DOI] [PubMed] [Google Scholar]
- 8.Charteris DG, Chan CH, Whitehouse RW, Noble JL. Orbital volume measurement in the management of pure blowout fractures of the orbital floor. Br J Ophthalmol. 1993;77(2):100–102. doi: 10.1136/bjo.77.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raskin EM, Millman AL, Lubkin V, della Rocca RC, Lisman RD, Maher EA. Prediction of late enophthalmos by volumetric analysis of orbital fractures. Ophthal Plast Reconstr Surg. 1998;14(1):19–26. doi: 10.1097/00002341-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Gellrich NC, Schramm A, Hammer B, et al. Computer-assisted secondary reconstruction of unilateral posttraumatic orbital deformity. Plast Reconstr Surg. 2002;110(6):1417–1429. doi: 10.1097/01.PRS.0000029807.35391.E5. [DOI] [PubMed] [Google Scholar]
- 11.Schmelzeisen R, Gellrich NC, Schoen R, Gutwald R, Zizelmann C, Schramm A. Navigation-aided reconstruction of medial orbital wall and floor contour in craniomaxillofacial reconstruction. Injury. 2004;35(10):955–962. doi: 10.1016/j.injury.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Bell RB, Markiewicz MR. Computer-assisted planning, stereolithographic modeling, and intraoperative navigation for complex orbital reconstruction: a descriptive study in a preliminary cohort. J Oral Maxillofac Surg. 2009;67(12):2559–2570. doi: 10.1016/j.joms.2009.07.098. [DOI] [PubMed] [Google Scholar]
- 13.He D, Li Z, Shi W, et al. Orbitozygomatic fractures with enophthalmos: analysis of 64 cases treated late. J Oral Maxillofac Surg. 2012;70(3):562–576. doi: 10.1016/j.joms.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 14.Markiewicz MR, Dierks EJ, Potter BE, Bell RB. Reliability of intraoperative navigation in restoring normal orbital dimensions. J Oral Maxillofac Surg. 2011;69(11):2833–2840. doi: 10.1016/j.joms.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 15.Moe KS, Bergeron CM, Ellenbogen RG. Transorbital neuroendoscopic surgery. Neurosurgery. 2010;67(3 suppl operative):ons16–28. doi: 10.1227/01.NEU.0000373431.08464.43. [DOI] [PubMed] [Google Scholar]
- 16.Balakrishnan K, Moe KS. Applications and outcomes of orbital and transorbital endoscopic surgery. Otolaryngol Head Neck Surg. 2011;144(5):815–820. doi: 10.1177/0194599810397285. [DOI] [PubMed] [Google Scholar]
- 17.Moe KS, Jothi S, Stern R, Gassner HG. Lateral retrocanthal orbitotomy: a minimally invasive, canthus-sparing approach. Arch Facial Plast Surg. 2007;9(6):419–426. doi: 10.1001/archfaci.9.6.419. [DOI] [PubMed] [Google Scholar]
- 18.Moe KS. The precaruncular approach to the medial orbit. Arch Facial Plast Surg. 2003;5(6):483–487. doi: 10.1001/archfaci.5.6.483. [DOI] [PubMed] [Google Scholar]
- 19.Fitzsimons R, White J. Functional scoring of the field of binocular single vision. Ophthalmology. 1990;97(1):33–35. doi: 10.1016/s0161-6420(90)32631-3. [DOI] [PubMed] [Google Scholar]
- 20.Barnard GA. A new test for 2 × 2 tables. Nature. 1945;156(3945):177. [Google Scholar]
- 21.Sleep TJ, Evans BT, Webb AA. Resolution of diplopia after repair of the deep orbit. Br J Oral Maxillofac Surg. 2007;45(3):190–196. doi: 10.1016/j.bjoms.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 22.al-Qurainy IA, Stassen LF, Dutton GN, Moos KF, el-Attar A. Diplopia following midfacial fractures. Br J Oral Maxillofac Surg. 1991;29(5):302–307. doi: 10.1016/0266-4356(91)90115-l. [DOI] [PubMed] [Google Scholar]
- 23.Hatt SR, Leske DA, Holmes JM. Comparing methods of quantifying diplopia. Ophthalmology. 2007;114(12):2316–2322. doi: 10.1016/j.ophtha.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]