Abstract
Purpose
A major challenge in platinum-based cancer therapy is the clinical management of chemoresistant tumors, which have a largely unknown pathogenesis at the level of epigenetic regulation.
Experimental Design
We evaluated the potential of using global loss of 5-hydroxymethylcytosine (5-hmC) levels as a novel diagnostic and prognostic epigenetic marker to better assess platinum-based chemotherapy response and clinical outcome in high-grade serous tumors (HGSOC), the most common and deadliest subtype of ovarian cancer. Furthermore, we identified a targetable pathway to reverse these epigenetic changes, both genetically and pharmacologically.
Results
This study shows that decreased 5-hmC levels are an epigenetic hallmark for malignancy and tumor progression in HGSOC. In addition, global 5-hmC loss is associated with a decreased response to platinum-based chemotherapy, shorter time to relapse, and poor overall survival in patients newly diagnosed with HGSOC. Interestingly, the rescue of 5-hmC loss restores sensitivity to platinum chemotherapy in vitro and in vivo, decreases the percentage of tumor cells with cancer stem cell markers, and increases overall survival in an aggressive animal model of platinum-resistant disease.
Conclusions
Consequently, a global analysis of patient 5-hmC levels should be included in future clinical trials, which use pretreatment with epigenetic adjuvants to elevate 5-hmC levels and improve the efficacy of current chemotherapies. Identifying prognostic epigenetic markers and altering chemotherapeutic regimens to incorporate DNMTi pretreatment in tumors with low 5-hmC levels could have important clinical implications for newly diagnosed HGSOC disease.
Introduction
High-grade serous ovarian cancer (HGSOC) has a high mortality rate despite an initially robust clinical response to platinum-based chemotherapy, and patients often relapse within 2 years following diagnosis (1). Unfortunately, there are no good therapeutic options for patients with HGSOC with intrinsic platinum resistance or patients who acquire resistance as a result of treatment (1). Moreover, newly diagnosed patients are not screened for or stratified on the basis of their initial sensitivity to platinum compounds. Consequently, a major challenge in platinum-based cancer therapy is the clinical management of chemoresistant tumors and the design of more effective treatments for patient cohorts with poor prognosis. It has become increasingly clear that no single genetic event or mutation is responsible for the development of a malignant or chemoresistant tumor phenotype. Moreover, recent advances suggest that distinct aberrant genetic and epigenetic events, including abnormal histone modification and DNA methylation, are all important cancer hallmarks. The discovery of the ten-eleven translocase (TET) enzyme family, which converts 5-mC to 5-hmC, has added an additional layer of complexity to the epigenetic regulation of DNA methylation (2–7). Multiple studies are currently focusing on investigating the effect of epigenetic changes during tumor development and progression (2–7). Research on DNA methylation has been of particular interest recently, mostly targeting specific genetic loci and their promoters (8). For instance, promoter methylation of the BRCA tumor suppressor genes has been implicated in both ovarian and breast cancer malignancies (9–13). Global methylation patterns in solid neoplasms have been difficult to assess in patients until recently (3). New studies suggest that global 5-hmC levels, rather than individual genes and promoter methylation, show a better correlation with clinical outcomes in tumors, such as melanoma (3). Since the dynamic regulation of 5-hmC levels play key roles in the development and pluripotency of somatic stem cells (14–16), it is possible that global 5-hmC patterns may also contribute to the maintenance and regulation of cancer stem cells (CSC), which have been implicated in chemoresistance and poor clinical outcome (17, 18).
A genome-wide epigenetic mapping of melanoma cases recently demonstrated that global loss of 5-hmC is an important hallmark of aggressive tumors with both diagnostic and prognostic implications (3). Moreover, these global epigenetic changes are reversible and the growth of aggressive tumors can be blocked by restoring 5-hmC levels through IDH2 or TET2 overexpression in animal models of disease (3). Additional studies have further validated the clinical utility of 5-hmC loss as a diagnostic tool, particularly with respect to differentiating melanoma micrometastases in sentinel lymph nodes from benign nevi nests and in evaluating the degree of melanocytic dysplasia (19). The correlation between global 5-hmC patterns and clinical outcome has also been shown in glioblastomas as decreased levels of 5-hmC were found to be associated with poor patient survival (20). Here we report that global loss of 5-hmC in high-grade serous ovarian tumors (HGSOC), the most aggressive ovarian cancer subtype, is significantly correlated with poor clinical outcome. Furthermore, an analysis of global 5-hmC patterns could potentially aid in better evaluating tumor response and relapse following platinum-based chemotherapy and overall clinical outcome in HGSOC. In addition, we have found targetable pathways and identified epigenetic adjuvants, which can reverse these epigenetic changes, increase global 5-hmC levels, and subsequently restore sensitivity to platinum chemotherapy. Thus, overexpression of the TET2 enzyme in platinum-resistant ovarian cancer cells is capable of reversing the 5-hmC–deficient epigenetic landscape. Interestingly, this rescue not only reduces the number of tumor cells expressing characteristic CSC markers but also restores platinum sensitivity. Likewise, pretreatment with a DNA methyl transferase inhibitor (DNMTi), 5-azacytidine (5-aza), increases 5-hmC levels via enhanced levels of TET family enzymes, most notably TET2, and subsequently restores chemosensitivity both in vitro and in vivo in an animal model of aggressive platinum-resistant disease. Consequently, newly diagnosed patients with 5-hmC deficiency and intrinsic platinum resistance may benefit from pretreatment with DNMTis to enhance their chemosensitivity and achieve a more effective therapeutic response to standard chemotherapy.
Materials and Methods
5-azacytidine pretreatment therapy
A total of 1 × 105 cells were plated on 10-cm plates and allowed to attach overnight. Tumor cells were then treated with 5-aza at 5, 10, or 20 μmol/L for 72 hours. Tissue culture medium containing 5-aza was replaced every 16 hours. Following 5-aza pretreatment, cells were trypsinized and plated on 96-well plates at 1 × 104 cells per well and allowed to attach overnight. Cells were then treated with CDDP, and cell viability was determined using previously described methods (21). For tumor cells in which the maintenance of 5-hmC levels was observed for 1 week, the medium was replaced with normal RPMI supplemented with 10% FBS (vol/vol) as described post-5-aza treatment.
Cell viability studies
Cells were counted using Cellometer Auto 1000 (Nexcelom Bioscience) and plated on 96-well plates at 10,000 cells per well. They were allowed to attach overnight and then treated with cisplatin (CDDP) at multiple concentrations for 48 hours. Cell viability at each specific CDDP concentration (0, 2.5, 5, 10, 20, 40, 80 μmol/L) was determined using WST-1 (Roche). Cell viability was quantified using a Perkin Elmer 1420 Multi-label Counter at 450 nm. CDDP IC50 values for all tumor lines were calculated using the dose–effect analysis CalcuSyn software (Biosoft).
Dot immunoblot assays
Genomic DNA was extracted using Qiagen Puregene Core Kit A following manufacturer’s instructions and then sonicated. The concentration and quality of DNA were analyzed by using a Nanodrop 1000 (Thermo Fisher Scientific Inc.). DNA was placed on nitrocellulose membranes using a Dot Blot apparatus (Bio-Rad). Blots were incubated with a primary 5-hmC antibody (Active Motif) 1:1,000 and a secondary anti-rabbit (Vector Laboratories Inc.) 1:2,000 antibody. Blots were imaged and quantified using a ChemiDoc XRS+ Imaging System (Bio-Rad); 5-hmC levels were further normalized to the amount of DNA loaded on the blot using Methylene blue staining.
Statistical analysis
Statistical analyses were performed using the GraphPad Prism software. Results were deemed statistically significant if P values were less than 0.05 (P < 0.05). All data were reported as mean ± SE. CDDP IC50 values were calculated using the dose–effect analysis CalcuSyn software (Biosoft). Significance between independent datasets was determined using univariate two-tailed t tests. Kaplan–Meier survival curves were generated using the GraphPad Prism software and the significance when comparing two curves was determined using the log-rank (Mantel–Cox) test. All results were compared with a no-treatment control in which the cells were given the drug vehicle alone. Cox-proportional hazards model was used to calculate the HRs and 95% confidence intervals for the association between 5-hmC levels and survival. Logistic regression was used to compare the 5-hmC levels of patients assumed to have had platinum resistance to patients with long progression-free survival. Both analyses were conducted using SAS version 9.4 (SAS Institute).
Additional Materials and Methods are detailed in Supplementary Data.
Results
Decreased 5-hmC levels are an epigenetic hallmark for malignancy and tumor progression in HGSOC
We evaluated the diagnostic and prognostic potential of 5-hmC as a novel epigenetic marker in the diagnosis of HGSOC, as well at its ability to indicate long-term clinical outcome. Global 5-hmC tumor levels were detected in formalin-fixed, paraffin-embedded tumor tissue via IHC using a specific anti-5-hmC antibody. IHC has been validated as a reliable method for the detection of 5-hmC levels, which can be easily translated into routine clinical care (3). We investigated global patterns of 5-hmC levels in a large biorepository of HGSOC cases by using a patient tumor microarray (TMA) from the Dana-Farber Cancer Institute (DFCI, Boston, MA; ref. 22) and a commercially available TMA (Folio Biosciences). We observed strong 5-hmC staining in normal stroma (Fig. 1A), which was used as an internal staining control. Samples that were deemed to not contain epithelium were excluded from the analysis. We used a composite (multiplicative/product) score, which evaluated both the intensity and percentage of positively stained tumor cells (Supplementary Fig. S1A and S1B), as described previously (3). All normal ovarian surface (OSE) and fallopian tubal epithelial (FTE) samples showed high levels of 5-hmC staining (Fig. 1A, B and D). The OSE and FTE patient control tissues were processed and analyzed separately according to the same protocol. Interestingly, primary HGSOC tumors (n = 203) from the DFCI (n = 136) and Folio (n = 67) patient cohorts were found to have significantly reduced 5-hmC levels when compared with normal OSE (n = 5) and FTE (n = 5), respectively (Fig. 1D). This was true whether the TMAs were analyzed together (Fig. 1D, **, P < 0.01; ***, P < 0.001) or separately (Supplementary Fig. S1C, **, P < 0.01; ***, P < 0.001). In addition, we evaluated 5-hmC levels at different stages of disease progression (Fig. 1E–G) as a subset of the DFCI TMA cases had paired primary and metastatic tumor samples. Interestingly, patients with low primary tumor 5-hmC scores (score < 6, n = 90) maintained low scores in metastatic disease, whereas patients with higher 5-hmC (≥6, n = 22) scores had significantly reduced 5-hmC levels in metastatic tumors (Fig. 1F; **, P < 0.01). This trend of higher 5-hmC primary tumors decreasing with disease progression remained true if a different cutoff 5-hmC staining score of ≥2 (n = 41) was used (Fig. 1G; **, P < 0.01). Patients with low primary tumor 5-hmC scores (n = 71) tended to maintain low scores in metastatic disease, whereas patients with higher 5-hmC (n = 41) scores had significantly reduced 5-hmC levels in metastatic tumors (Fig. 1G; **, P < 0.01). Moreover, the difference between metastatic tumor scores was highly significant between the two patient cohorts (Fig. 1F and G; ***, P < 0.001), indicating intrinsic differences in the epigenetic map of HGSOC tumor subgroups. Furthermore, these results highlight the need for both primary and metastatic lesions to be analyzed for 5-hmC levels to gain a full understanding of the epigenetic landscape and design effective personalized therapeutic regimens.
Figure 1.
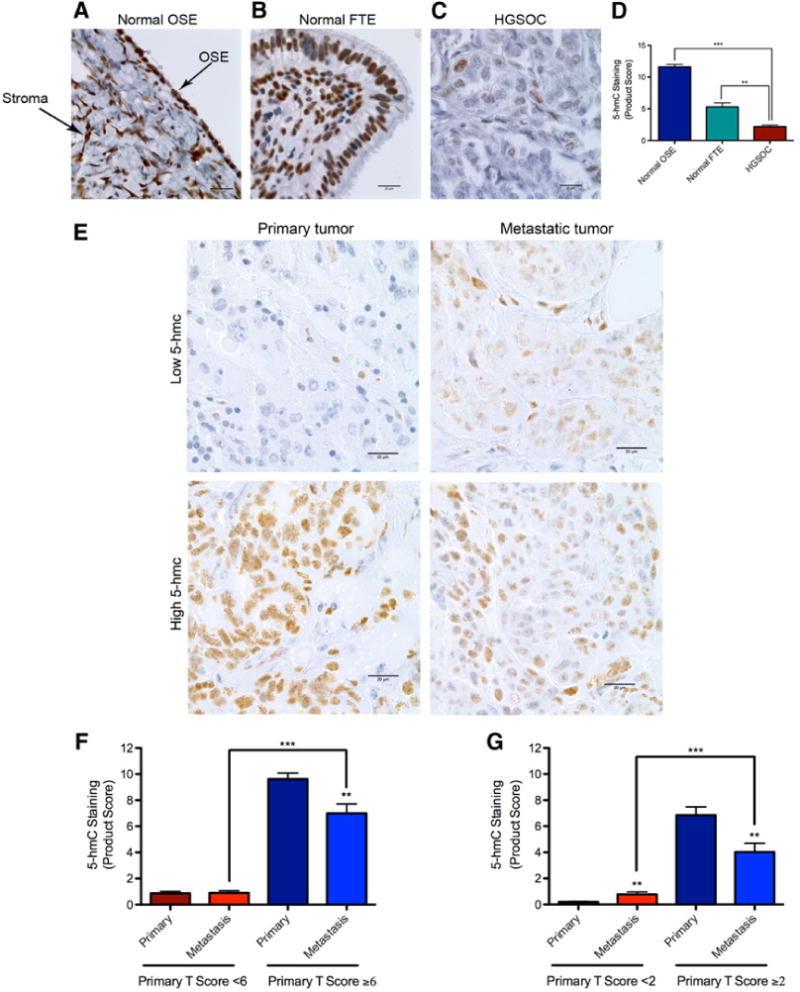
Decreased 5-hmC levels are an epigenetic hallmark for malignancy and tumor progression in HGSOC. A–C, Comparative evaluation of 5-hmC staining in normal ovarian surface epithelium (OSE), normal fallopian tubal epithelium (FTE), and HGSOC patient tumors, respectively, as assessed by IHC studies. The bold arrows indicate normal OSE and ovarian stroma; positive stromal staining was used as an internal staining control (20 μm scale bar, 100 objective lens magnification). D, Quantification of 5-hmC staining scores for normal OSE (n = 5), normal FTE (n = 5), and HGSOC cases (n = 203). Interestingly, HGSOC tumors show significantly lower 5-hmC levels when compared with normal OSE and FTE, respectively (***, P < 0.001; **, P < 0.01). E, Representative 5-hmC levels in primary tumors and corresponding metastatic disease. Shown are examples of a primary tumor with low 5-hmC staining in both the primary tumor and metastatic lesion as well as a primary tumor with higher 5-hmC levels, which decrease upon metastasis (20 μm scale bar, 100× objective lens magnification). F, Quantification of 5-hmC staining scores in primary tumors and corresponding metastatic lesions when patient cohorts are separated by a primary tumor 5-hmC score of 6. Interestingly, patients with low primary tumor 5-hmC scores (n = 90) maintain low scores in metastatic disease, whereas patients with higher 5-hmC (n = 22) scores have significantly reduced 5-hmC levels in metastatic disease (**, P < 0.01; ***, P < 0.001). G, Same conclusions are reached if patient cohorts are separated by a primary tumor 5-hmC staining score of 2. Patients with low primary tumor 5-hmC scores (n = 71) tend to maintain low scores in metastatic disease, whereas patients with higher 5-hmC (n = 41) scores have significantly reduced 5-hmC levels in metastatic disease (**, P < 0.01; ***, P < 0.001).
Loss of 5-hmC is correlated with a shorter time to relapse following platinum-based chemotherapy and poor prognosis in patients newly diagnosed with HGSOC
5-hmC levels were further correlated with clinical outcome and response to platinum-based chemotherapy in a separate Brigham and Women’s Hospital (BWH) TMA (Supplementary Table S1), which included newly diagnosed HGSOCs with long-term clinical follow-up data. The BWH TMA consisted of 1-mm primary tumor cores (up to 3 per case) collected from 107 patients newly diagnosed with HGSOC. Patients were diagnosed between 1998 and 2008 and subsequently enrolled in the New England Case Control Study, a population-based case–control study (23, 24). We first analyzed global 5-hmC levels in a subset of patients with HGSOC (Supplementary Table S2, n = 59) for which platinum treatment relapse data were available. Interestingly, decreased 5-hmC levels (staining score < 2, n = 30) correlate with significantly shorter time to first relapse following platinum-based chemotherapy [HR = 2.6 (1.4–4.7)] when compared with patients with higher (≥2) 5-hmC levels (n = 29; Fig. 2A; **, P < 0.01). In addition, this observation remains significant after adjusting for age, stage, grade, and debulking status [HR = 2.2 (1.1–4.1)]. This further suggests that loss of 5-hmC in newly diagnosed tumors could potentially be used as a prognostic marker for disease relapse following chemotherapy and clinical outcome. Similarly, in the entire BWH HGSOC case set (n = 107), cases with low 5-hmC levels (staining score < 2, n = 53) had a significantly shorter overall survival [HR = 2.5 (1.6–4.1; Fig. 2B; ***, P < 0.001)] when compared with patients with a higher (≥2) 5-hmC score (n = 54) and remains significant after adjusting for age, stage, grade, and debulking status [HR = 1.9 (1.1–3.1)]. Furthermore, when we compared long-term survivors (>3 years) to those assumed to have had platinum resistance (progression-free survival < 12 months), patients with high 5-hmC levels had 12-fold increased odds of having longer progression-free survival than those with low 5-hmC levels [age-adjusted OR = 12 (1.9–79), **, P < 0.01] and this association remained significant after adjusting for age, stage, grade, and debulking status. These results suggest 5-hmC is potentially associated with platinum responsiveness.
Figure 2.
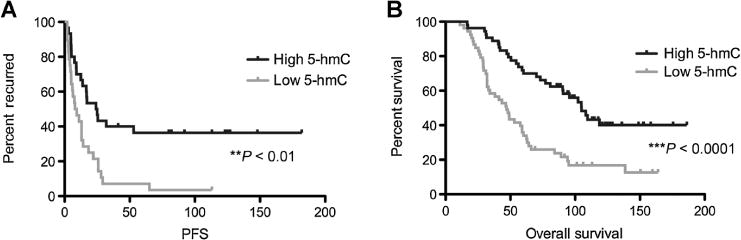
Loss of 5-hmC is correlated with a shorter time to relapse following platinum-based chemotherapy and poor prognosis in patients newly diagnosed with HGSOC. A, Progression-free survival of patients with platinum response data (n = 59) shows a significant decrease in average time to disease relapse for patients with low 5-hmC (<2, n = 30) when compared to patients with high 5-hmC (≥2, n = 29). Patients with high 5-hmC levels experience a significantly longer time to initial disease relapse following platinum-based chemotherapy relative to low 5-hmC patients (**, P < 0.01). B, Kaplan–Meier survival curve of all patients with HGSOC included in the BWH TMA (n = 107). Patients with higher 5-hmC levels (primary tumor score ≥2, n = 54) have a significantly increased overall survival when compared with patients with low 5-hmC levels (primary tumor score <2, n = 53; ***, P < 0.0001).
In addition, we analyzed patient ascites samples collected from newly diagnosed patients with ovarian cancer in a small DFCI/BWH case set for which ascites samples with high tumor content were available. Samples included in the analysis were first confirmed by immunofluorescence (IF) to contain at least 50% tumor cells positive for HGSOC markers PAX8 (25, 26) and EpCAM (refs. 27, 28; Supplementary Fig. S2A). Quantification of 5-hmC levels in patient ascites using a dot immunoblot assay indicated that mean 5-hmC levels of the intrinsic platinum chemoresistant group tended to show a decrease when compared with patients who responded to initial platinum therapy, although the difference did not reach statistical significance possibly due to the small number of samples analyzed (Supplementary Fig. S2B). We further used a HPLC/MS-MS method, which may yield a more accurate 5-hmC quantification, to directly measure relative 5-hmC levels for several platinum-sensitive and intrinsic platinum-resistant samples collected from ovarian tumor ascites and included in the Supplementary Fig. S2A and S2B. As detailed above, the patient samples used for mass spectrometry quantification were first confirmed by IF to contain at least 50% tumor cells positive for HGSOC markers PAX8 and EpCAM (Supplementary Fig. S2A). HPLC-MS/MS analysis was performed using the MassHunter System (Agilent) as described previously (29). Briefly, extracted genomic DNA was digested overnight using DNA Degradase (Zymo Research) for the subsequent HPLC/MS-MS analysis. The purified nucleotides (in 100 μL diluted sample volume) were then subjected to HPLC/MS-MS analysis using manufacturer’s protocol and a C18 column was used for the HPLC. The mass spectrometer was optimized and set up in selected reaction monitoring (SRM) scan mode for monitoring the [M+H+] of dC (m/z 228.1 → 112.1), 4/5mC (m/z 242.1 → 126.1), 5hmC (258.1 → 142.1), and dideoxycytidine (212.1 → 112.1). The Analyst Software recommended by Agilent was used for quantification of the 5-hmC amount in genomic DNA collected from various patient samples. Interestingly, quantification of relative 5-hmC levels in the analyzed samples indicates a statistical significant increase in 5-hmC levels (*, P < 0.05) in platinum-sensitive (ASC 96, HA 01.21.15) when compared with intrinsic platinum-resistant patient samples (ASC 117, HA 01.15.14), as shown in Supplementary Fig. S2C. Taken together, these results led us to further investigate epigenetic reprogramming strategies able to increase 5-hmC levels and possibly improve chemosensitivity and increase overall survival, especially in platinum-resistant disease.
Genetic rescue of low 5-hmC levels via TET2 overexpression restores platinum chemosensitivity
To directly evaluate the potential association between platinum response and 5-hmC profiles, we utilized the platinum-sensitive A2780 ovarian cancer cell line (A2780Sen) and its platinum-resistant counterpart (A2780Res) as an in vitro model for chemoresistant disease. Interestingly, dot immunoblot assay results indicated that the A2780Res line had significantly decreased global 5-hmC levels when compared with its platinum-sensitive A2780Sen counterpart (Fig. 3A; **, P < 0.01). To further investigate whether platinum resistance can be reversed by restoring 5-hmC levels, we overexpressed the catalytically active TET2 domain, which converts 5-mC to 5-hmC 3, 14, 30), in the platinum-resistant A2780Res line (A2780Res+pTET2; Supplementary Fig. S3A). Quantification of the dot immunoblot assay results indicates that TET2 overexpression rescues the low 5-hmC phenotype of the A2780Res line (Fig. 3A; **, P < 0.01). Furthermore, 5-hmC levels in A2780Res+pTET2 cells are similar to those observed in the platinum-sensitive A2780Sen line (Fig. 3A). In addition, we validated the rescue of low 5-hmC levels in A2780Res+pTET2 cells via immunofluorescence (IF) using a specific 5-hmC antibody (Fig. 3B). 5-hmC levels were quantified by measuring mean fluorescence/cell using ImageJ image processing software and normalized for cell number by counting cells with DAPI nuclear staining. Quantification of 5-hmC levels detected via IF demonstrated a statistically significant 5-hmC increase in both the A2780Sen and A2780Res+pTET2 lines when compared with A2780Res tumor cells (Supplementary Fig. S3B; *, P < 0.05). To further confirm the correlation between 5-hmC and TET2 levels, we performed qRT-PCR for the three cell lines and observed higher TET2 mRNA expression in A2780Sen cells when compared with the A2780Res tumor line (Fig. 3C; ***, P < 0.001). As expected, the highest increase in TET2 levels was seen in A2780Res+pTET2 cells (Fig. 3C; ***, P < 0.001). After validating 5-hmC changes within tumor cells as a result of TET2 overexpression, we further explored the platinum response of each cell line. Most significantly, the A2780Res+pTET2 cell line responded to cisplatin (CDDP) therapy with a similar efficacy as the A2780Sen line (Fig. 3D). Both lines show a statistically significant increase in platinum sensitivity (Fig. 3D; **, P < 0.01) when compared with the A2780Res cell line (CDDP IC50 of 22.51 μmol/L for A2780Res, 10.70 μmol/L for A2780Sens, and 7.97 μmol/L for A2780Res+pTET2, respectively). Consequently, TET2 overexpression in chemoresistant cells can lead to a global increase in 5-hmC levels and resensitization to platinum therapy.
Figure 3.
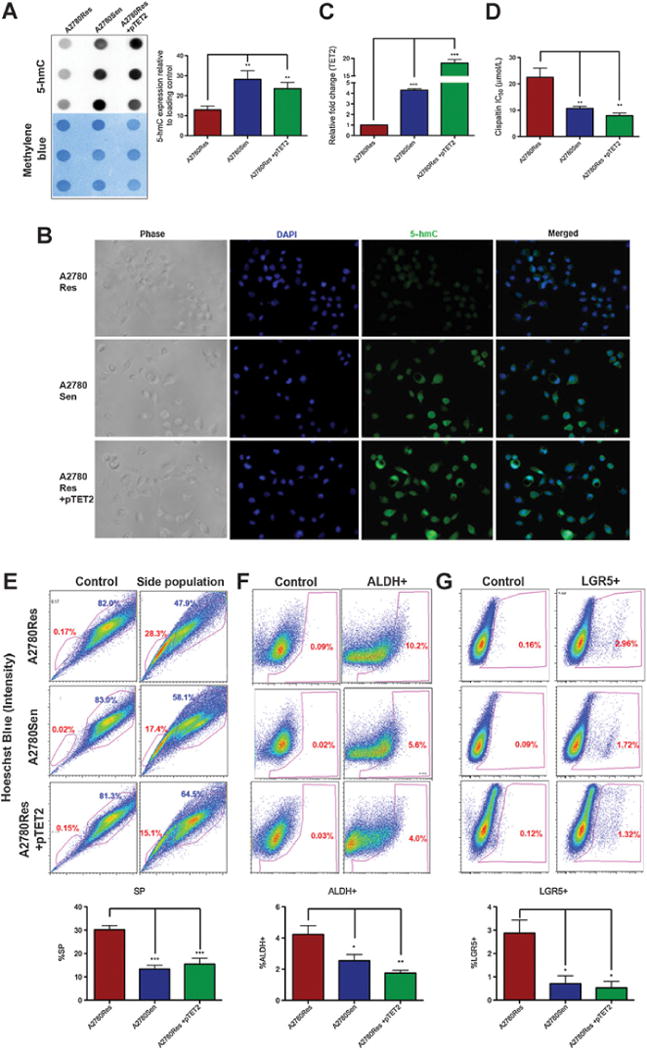
Reversal of low 5-hmC profiles via TET2 overexpression restores platinum sensitivity. A, Representative immunoblot assay results showing global 5-hmC levels in A2780 platinum (CDDP) resistant (A2780Res), sensitive (A2780Sen), and resistant tumor cells with TET2 overexpression (A2780Res+pTET2). Platinum-resistant tumor cells exhibit lower 5-hmC levels when compared with their sensitive counterpart (**, P < 0.01). However, TET2 overexpression in A2780Res cells increases global 5-hmC to statistically higher levels than those seen in the parental A2780Res line (**, P < 0.01) and comparable with those seen in the platinum-sensitive A2780Sen line. B, Immunofluorescence analysis of 5-hmC levels in A2780Res, A2780Sen, and A2780Res+pTET2 tumor lines. Blue color indicates DAPI counterstain of DNA and green color shows 5-hmc levels. C, Relative TET2 mRNA expression in platinum A2780Res, A2780Sen, and resistant tumor cells with TET2 overexpression (A2780Res+pTET2) as assessed by qRT-PCR. Platinum resistant A2780Res tumor cells have significantly lower TET2 levels when compared with their sensitive A2780Sen counterpart (***, P < 0.001) and A2780Res+pTET2 line (***, P < 0.001). D, Comparative chemosensitivity profiles of A2780Res, A2780Sen, and A2780Res+pTET2 tumor lines as assessed by MTT. The CDDP IC50 value of the A2780Res+pTET2 line is similar to the CDDP sensitive counterpart (n.s. P > 0.05) and is significantly lower than the resistant parental line (**, P < 0.01). Thus, TET2 overexpression in the chemoresistant A2780Res line restores the 5-hmC levels and platinum sensitivity of the A2780Sen counterpart. E, A2780Res, A2780Sen, and A2780Res+pTET2 tumor lines were labeled with Hoechst 33342 dye and analyzed by flow cytometry. Control cells were treated with verapamil prior to Hoechst 333422 dye incubation. The A2780Res line has significantly more cancer stem-like cells (SP) when compared with its sensitive counterpart or resistant pTET2 line (***, P < 0.001). F, A2780Res, A2780Sen, and A2780Res+pTET2 tumor lines were labeled with Aldefluor and analyzed by flow cytometry. Similarly, the A2780Res line has significantly more ALDH+ tumor cells when compared with its sensitive A2780Sen counterpart (*, P < 0.05) or resistant pTET2 line (**, P < 0.01), respectively. G, A2780Res, A2780Sen, and A2780Res+pTET2 tumor lines were labeled with the LGR5 antibody and analyzed by flow cytometry. The A2780Res line has significantly more LGR5+ tumor cells as compared with its sensitive A2780Sen counterpart or A2780Res+pTET2 tumor lines (*, P < 0.05).
We further examined changes in tumor subpopulations, specifically those involving cancer stem cell–like markers (CSC). We first analyzed the side population phenotype (SP) whose correlation with platinum chemoresistance has been shown previously (21, 31, 32) for both murine and human cell lines. To this end, murine (4412,4306) and human A2780Res cell lines were sorted by flow cytometry (FACS) into SP and non-SP (NSP) populations. We observed statistically significant decreases in 5-hmC levels for SP when compared with NSP in the tested cell lines (Supplementary Fig. S3C; *, P < 0.05; **, P < 0.01; ***, P < 0.001). We further investigated how TET2 overexpression in platinum-resistant cell lines affected cellular subpopulations with CSC markers, including SP/NSP (21, 31, 32), ALDH (33–35), and LGR5 (36–38), as enrichment in these populations has been linked to chemoresistance (21, 31, 32, 39). We found that the A2780Res+pTET2 line (n = 6, mean 15.6%) had significantly fewer SP cells (Fig. 3E, ***, P < 0.001) than the A2780Res line (n = 6, mean 30.2%), and a similar SP percentage as the platinum-sensitive counterpart A2780Sen (n = 6, mean 15.5%; Fig. 3E). Similarly, TET2 overexpression in A2780Res reduced the numbers of tumor cells with other CSC markers, specifically ALDH (n = 6, mean 4.22% for A2780Res, 2.54% for A2780Sen, and 1.75% for A2780Res+pTET2, respectively) and LGR5 (n = 3, mean 2.87% for A2780Res, 0.76% for A2780Sen, and 0.52%, for A2780Res+pTET2, respectively; Fig. 3F and G; *, P < 0.05; **, P < 0.01). Once we confirmed a TET2-mediated mechanism for restoring global 5-hmC levels and platinum sensitivity in chemoresistant tumor cells, we further sought to identify pharmacologic agents, which could mimic these effects.
Pretreatment with 5-azacytidine increases global 5-hmC levels and platinum chemosensitivity
Thus, we found that pretreatment with 5-azacytidine (5-aza) can restore the 5-hmC profile in A2780Res chemoresistant cells similar to the effect elicited by TET2 overexpression. A2780Res cells were pretreated with 5-aza for 72 hours using a range of concentrations (5, 10, 20 μmol/L) prior to genomic DNA extraction. Quantification of dot immunoblot analysis results revealed a statistically significant increase in 5-hmC levels at all 5-aza doses in the A2780Res line (Fig. 4A; *, P < 0.05; **, P < 0.01). In addition, similar results were obtained using CaOV3, OVCAR4, and OVSAHO tumor cells, which were previously characterized as ovarian HGSOC lines (ref. 40; Fig. 4A, *, P < 0.05; **, P < 0.01; ***, P < 0.001). Interestingly, 5-aza–pretreated A2780Res cells maintained elevated 5-hmC levels while being grown in normal culture media for 1 week after the initial pretreatment, suggesting that 5-aza had a durable effect in temporarily stabilizing increased 5-hmC levels (Supplementary Fig. S4A; ***, P < 0.001). As previously mentioned, we sought to explore how the restoration of 5-hmC levels affected platinum sensitivity. 5-aza–pretreated tumor cells were plated onto 96-well plates and further treated with CDDP. Notably, we observed significant increases in platinum sensitivity following 5-aza pretreatment for 72 hours in A2780Res, CaOV3, OVCAR4, and OVSAHO tumor lines (Fig. 4B; *, P < 0.05; **, P < 0.01; ***, P < 0.001). Additional results confirming increased platinum sensitivity following 5-aza pretreatment in COV318, OVCAR8, and SKOV3 tumor lines are shown in Supplementary Fig. S4B. Interestingly, the CDDP IC50 of the 5-aza (20 μmol/L) pretreated A2780Res line was 8.82 μmol/L, which was close to the IC50 of the sensitive A2780Sen counterpart (10.70 μmol/L) and the IC50 of the genetically altered A2780Res+pTET2 line (7.97 μmol/L). Moreover, A2780Res cells temporarily maintained increased global 5-hmC levels and displayed higher platinum sensitivity after being grown in normal culture medium for 1 week following 5-aza pretreatment with the higher 10 and 20 μmol/L doses (Supplementary Fig. S4A, **, P < 0.01; ***, P < 0.001). Taken together, our results suggest that DNMTis can elicit durable increases in 5-hmC levels and improve chemosensitivity in ovarian cancer cell lines.
Figure 4.
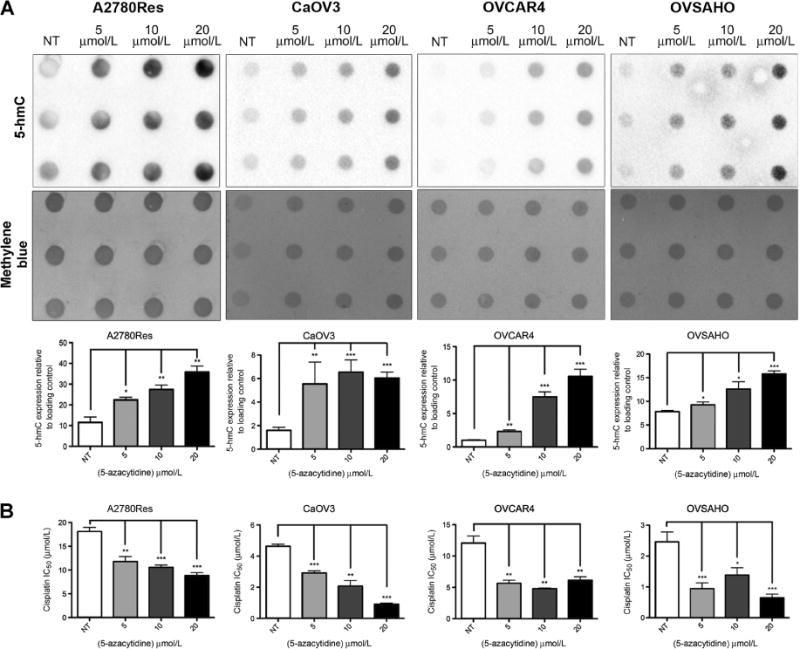
Pretreatment with 5-azacytidine increases global 5-hmC levels and platinum sensitivity. A, 5-hmC immunoblot and quantification of 5-hmC levels in A2780Res, CaOV3, OVCAR4, OVSAHO tumor lines treated for 72 hours with 5-azacytidine (5-aza). Methylene blue staining was used as a DNA loading control. Pretreatment with 5-aza significantly increases 5-hmC levels at all concentrations when compared with untreated (NT) controls in the tested cancer cell lines (*, P < 0.05; **, P < 0.01; ***, P < 0.001). B, The platinum sensitivity of ovarian cancer cell lines increases following pretreatment with 5-aza as shown by MTT analysis. Tumor lines, which were treated for 72 hours with 5, 10, or 20 μmol/L 5-aza followed by CDDP therapy, show statistically significant increases in CDDP IC50 values (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Pretreatment with 5-azacytidine increases TET2 and TET3 expression in ovarian cancer cells
We further investigated whether 5-aza pretreatment induced changes in levels of the TET family of enzymes, specifically TET1, 2, and 3. A2780Res cells were treated for 12, 24, and 48 hours with 5, 10, and 20 μmol/L 5-aza, respectively, and TET mRNA expression was evaluated via qRT-PCR. Interestingly, TET2 and TET3 were significantly upregulated at all treatment timepoints and 5-aza concentrations relative to untreated (NT) cells in the A2780Res line (Fig. 5A; **, P < 0.01; ***, P < 0.001). In addition, we observed significant increases in TET2 and TET3 mRNA expression in the HGSOC cell lines OVCAR4, CaOV3, and OVSAHO (Fig. 5C–E; *, P < 0.05; **, P < 0.01; ***, P < 0.001) following 12 hours of 5-aza (20 μmol/L) pretreatment. We next evaluated TET2 protein levels (TET2 antibody Novus Biologicals, catalog no. NBP2-32104, 1:2000) following 5-aza pretreatment and confirmed that upregulated TET2 mRNA expression correlated with increased protein levels in the A2780Res line as assessed by Western blot analysis (Fig. 5B). In addition, increased TET2 protein expression was seen in A2780Res, OVCAR4, and CaOV3 cell lines following pretreatment with 20 μmol/L 5-aza (Fig. 5F). As we gained a better understanding of the potential mechanism by which 5-aza increases global 5-hmC levels, we continued to investigate the therapeutic potential of 5-aza pretreatment using an in vivo model of aggressive platinum-resistant disease.
Figure 5.
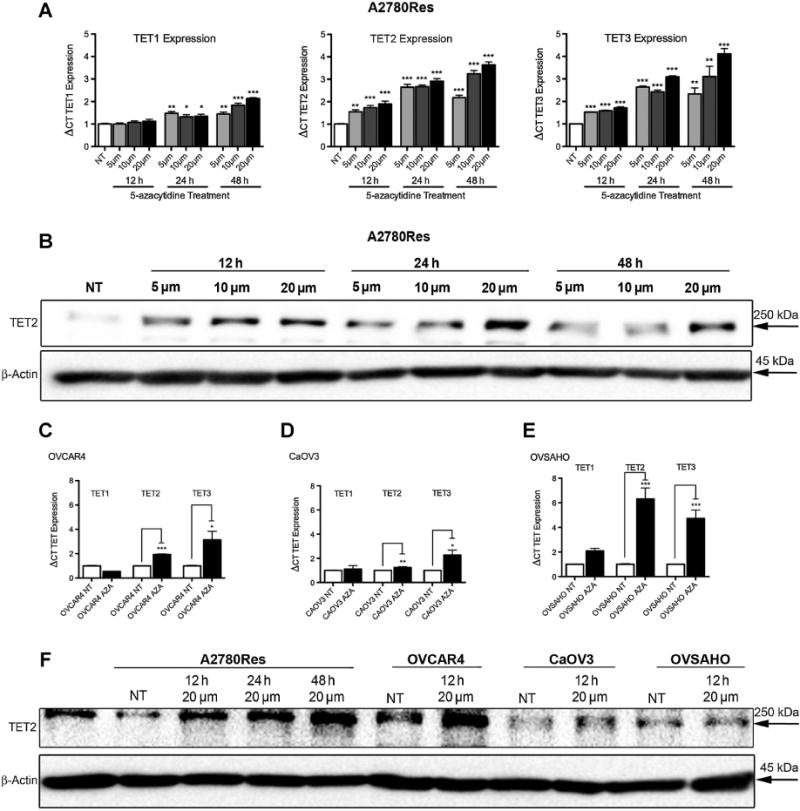
Pretreatment with 5-azacytidine increases TET2 and TET3 expression in ovarian cancer cell lines. A, Comparative mRNA expression levels of TET1, TET2, and TET3 in the A2780Res tumor line following 5-aza treatment (concentrations of 5, 10, and 20 μmol/L, respectively) at various timepoints (treatment time of 12, 24, and 48 hours, respectively) as assessed by qRT-PCR. TET2 and TET3 are significantly upregulated at all concentrations and timepoints relative to untreated cells (NT) (**, P < 0.01; ***, P < 0.001). B, TET2 protein levels in A2780Res cells treated with 5-aza at concentrations of 5, 10, and 20 μmol/L for 12, 24, and 48 hours, respectively, as assessed by Western blot analysis. There is an increase in TET2 expression when compared with untreated (NT) cells. C–E, Comparative mRNA expression levels of TET1, TET2, and TET3 in OVCAR4, CaOV3, and OVSAHO ovarian cancer cell lines, respectively, following 12 hours of 5-aza (20 μmol/L) treatment as assessed by qRT-PCR. TET2 and TET3 are significantly upregulated in all cell lines following 5-aza treatment compared with untreated (NT) cells (*, P < 0.05; **, P < 0.01; ***, P < 0.001). F, TET2 protein levels in ovarian cancer cell lines following treatment with 5-aza as assessed by Western blot analysis.
Pretreatment with 5-azacytidine followed by CDDP therapy increases overall survival in vivo in an aggressive platinum-resistant tumor xenograft model
Following in vitro validation that DNMTis can reverse 5-hmC loss and increase sensitivity to platinum-based therapy, we sought to confirm these findings in vivo using an aggressive platinum tumor xenograft model. A2780Res/luc tumor cells were injected intraperitoneally (i.p.) into immunodeficient NCr Nude mice (Taconic Biosciences) and allowed to propagate within the peritoneal cavity to form tumors. Cohorts were divided into control, 5-aza monotherapy, cisplatin (CDDP) monotherapy, and 5-aza/CDDP combination therapy (n = 5, 5, 8, 8, respectively). Treatments began approximately 1 week following intraperitoneal injection of 1 × 106 A2780Res/luc tumor cells when the bioluminescence signal was easily detectable using the IVIS Lumina II imaging system (PerkinElmer, Inc.). Mice were pretreated twice weekly with 4 mg/kg 5-aza resuspended in PBS and injected intraperitoneally on subsequent days (day 1, 2) followed by 3 mg/kg CDDP therapy on days 3 and 6 of each cycle. Following therapy initiation, mice were observed daily for any visible indicators of tumor development. We did not see a statistical difference in tumor development between the 5-aza–treated cohort and control group as shown in Fig. 6A. Interestingly, we observed a significant delay when visually inspecting for tumor appearance (Fig. 6A; **, P < 0.01) in the combination cohort compared with either monotherapy or control group (mean of 31.8 days for combination therapy, 13.3 days for control, 12 days for 5-aza alone, and 14.4 days for cisplatin monotherapy, respectively). The significant delay in tumor progression is indicative of the ability of the 5-aza pretreatment to increase platinum response when compared with CDDP treatment alone.
Figure 6.
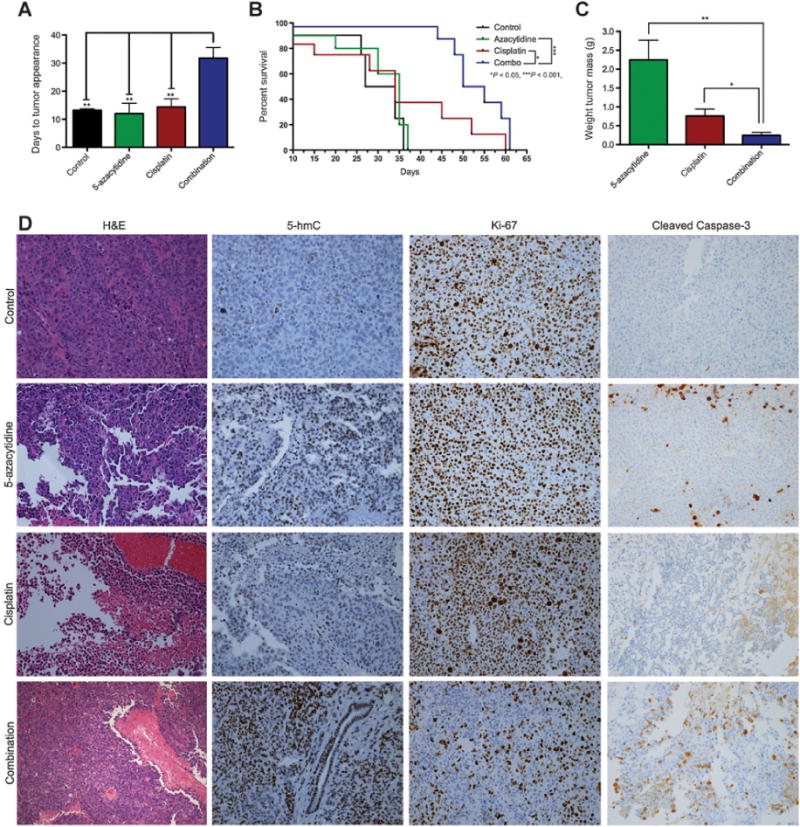
Pretreatment with 5-azacytidine followed by CDDP therapy increases overall survival in vivo in an aggressive platinum A2780Resistant tumor xenograft model. A, Comparative analysis of the tumor development timeline in animal models. A2780Res controls (n = 5) and animals treated with either 5-aza (n = 5) or CDDP monotherapy (n = 8) developed visible tumors in a significantly shorter time than mice treated with combination therapy (n = 8; **, P < 0.01), which received pretreatment with 5-aza followed by CDDP therapy. B, Kaplan–Meier curve showing survival for A2780Res controls (n = 5), 5-aza monotherapy (n = 5), CDDP monotherapy (n = 8), and 5-aza pretreatment + CDDP combination therapy (n = 8) cohorts. The combination group showed a statistically significant survival benefit compared with all the other groups. Of note, the overall survival of mice treated with combination therapy was significantly increased compared with either CDDP alone (*, P < 0.05) or 5-aza monotherapy (***, P < 0.001). C, Comparative analysis in a separate experiment of the average tumor weight in mice treated for 3 treatment cycles with either 5-aza (n = 4) or CDDP monotherapy (n = 6) when compared with combination therapy (n = 6). Most importantly, the analysis shows a significantly reduced tumor burden in A2780Res mice pretreated with 5-aza followed by CDDP therapy when compared with either CDDP alone (*, P < 0.05) or 5-aza alone (**, P < 0.01). D, Comparative IHC analysis of tumor markers in the various treatment cohorts. Tumors in the combination therapy group show increased 5-hmC levels, a lower Ki-67 cellular proliferation index, and higher tumor apoptosis as assessed by cleaved caspase-3 IHC (20× objective lens magnification).
Although decreases in tumor development are important, the ultimate goal in assessing treatment efficacy is to determine whether it increases overall survival. In this survival study, the control group had a mean survival of only 30.5 days, suggesting that this tumor is representative of an aggressive disease model. No statistical differences in overall survival were seen between control and 5-aza groups (Fig. 6B). In addition, the 5-aza and CDDP monotherapy cohorts had similar mean survival times of 35 and 34 days, respectively. The reduced response to CDDP suggests that this is representative of a platinum-resistant disease model. The only group with a clear survival benefit was the 5-aza/CDDP cohort, as the combination treated mice had a mean survival of 52.5 days (Fig. 6B; ***, P < 0.001 compared with 5-aza monotherapy and *, P < 0.05 compared with CDDP monotherapy, respectively). This significant increase in overall survival is consistent with in vitro data (Fig. 4B; Supplementary Fig. S4A and S4B), showing a durable effect of enhanced platinum sensitivity for A2780Res tumor cells pretreated with 5-aza. Since in this survival study, we did not see a statistical difference between the 5-aza–treated cohort and control group, in a separate follow-up study, we focused solely on comparing tumor burden in combination therapy group versus 5-aza and CDDP monotherapies, respectively.
In a follow-up study, we confirmed these results by quantifying the tumor burden of mice injected with A2780Res/luc following 3 weeks of treatment cycles, as previously performed. Each weekly cycle consisted of two pretreatments with 5-aza on days 1 and 2 and two platinum treatments on days 3 and 6 of the cycle. Consistent with Fig. 6A results, the combination therapy cohort (n = 6) had a mean tumor burden of 0.25 g, which was significantly lower when compared with the 5-aza monotherapy cohort (n = 4, 2.25 g; Fig. 6C; **, P < 0.01). In addition, it was also significantly lower when compared with the cisplatin monotherapy cohort (n = 6, 0.76 g; Fig. 6C; *, P < 0.05). The significant reduction in tumor weight confirms increased platinum sensitivity when using 5-aza as a sensitization pretreatment agent as shown by in vitro results. We further analyzed 5-hmC, Ki-67, and cleaved caspase-3 markers in tumors harvested from animals included in the survival study (Fig. 6B and D). Quantification of tumor marker IHC levels in control, 5-aza, cisplatin (CDDP), and combination therapy cohorts is shown in Supplementary Fig. S5A–S5C. Within the control group, tumors showed low 5-hmC, high Ki-67, and low cleaved caspase-3 levels, all indicative of a chemoresistant and aggressive disease, which correlated with a low mean survival of only 30.5 days. In contrast, the 5-aza/CDDP group showed not only increased 5-hmC levels, but also decreased Ki-67 expression and higher tumor apoptosis as assessed by cleaved caspase-3 levels (Fig. 6D; Supplementary Fig. S5). These data suggest that 5-aza pretreatment effectively increases global 5-hmC levels and platinum sensitivity in vivo in the A2780Resistant tumor xenograft model, which further correlate with a significant survival benefit.
Discussion
We report for the first time in a U.S. patient cohort that decreased 5-hmC levels are an epigenetic hallmark for malignancy and tumor progression in HGSOC, the most common and aggressive ovarian cancer subtype. Thus, we observed a significant decrease in 5-hmC levels in metastatic disease within our HGSOC cohort. Interestingly, patients with low primary tumor 5-hmC scores maintain low scores in metastatic disease, whereas patients with higher 5-hmC scores have significantly reduced 5-hmC levels in metastatic disease. Our findings are further supported by recent observations in an Asian patient cohort (41), which analyzed ovarian cancer cases independent of subtype and concluded that decreased 5-hmC levels correlated with higher grade, advanced stage, and increased lymph node metastasis. In addition, low 5-hmC, high 5-mC, and low TET2 levels were all found to be independent prognostic factors of poor survival in ovarian cancer in their particular cohort (41). In addition, decreased 5-hmC levels were recently described in aggressive glioblastomas (20). Thus, low 5-hmC levels may be a hallmark of multiple aggressive solid tumor types, making it crucial to understand the mechanisms responsible for these changes. Furthermore, this study shows that global loss of 5-hmC in newly diagnosed HGSOC is associated with decreased platinum response, a shorter time to relapse following platinum-based chemotherapy, and a poor overall survival. Thus, the patient studies outline a potential association between low 5-hmC levels and intrinsic platinum-resistant disease. Additional investigations are needed to determine whether 5-hmC loss is also associated with acquired platinum resistance, which has a significantly more complex mechanism.
Most importantly, we found that the use of an altered treatment regimen to increase global 5-hmC levels in platinum-resistant tumor cells prior to chemotherapy is important for improving chemosensitivity. The IHC study using a specific anti-5-hmC antibody is easily translatable in the clinic to analyze biopsies at first diagnosis and during subsequent relapses. This could aid in evaluating platinum response at first diagnosis and as the disease progresses. It should be noted that for patients with a higher primary tumor 5-hmC score, despite the drop observed in their metastatic tumors, levels still remain higher than those seen in patients with a low primary tumor score. This suggests that a significant subset of newly diagnosed tumors in either the primary or metastatic locations may be genetically predetermined to elicit a poor response to standard chemotherapy. Thus, when evaluating 5-hmC levels in patients, it is critical to monitor and test epigenetic patterns in metastatic tumors at all invasion sites if available at diagnosis and during disease progression to increase treatment efficacy and spare the patients from toxic and ineffective therapies. It is also recommended that multiple tumor locations should be sampled for 5-hmC levels as various sites may respond differently to platinum therapy due to disease heterogeneity. The routine clinical use of 5-hmC as a global epigenetic marker could aid in assessing tumor response and outcome prior to the delivery of cytotoxic chemotherapeutic treatments.
Loss of 5-hmC in intrinsic platinum-resistant tumor cells can result from multiple mechanisms, including downregulation of the TET family of enzymes, especially TET2 and TET3. In this study, we looked specifically at TET2, although previous studies have indicated that the dysregulation of IDH1 and/or IDH2 can also lead to a decrease in 5-hmC levels (3, 6, 42, 43). Conversely, we demonstrate that overexpression of the catalytic domain of TET2 results in the restoration of 5-hmC levels and a decrease in tumor cells with CSC markers, which could further contribute to the transition from a platinum resistant to a more chemosensitive disease. Our study is the first to exploit the pharmacologic reversal in 5-hmC levels to enhance the chemosensitivity of platinum-resistant tumors in vitro and in vivo. The use of DNMTis, such as 5-aza (44, 45), currently used clinically as Vidaza to treat myelodysplastic syndrome (MDS), may provide benefit to HGSOC patients with 5-hmC loss and intrinsic platinum-resistant disease. On the basis of the positive response in our animal experiments, pretreatment with 5-aza could increase platinum treatment efficacy in chemoresistant tumors, especially in patients newly diagnosed with intrinsic platinum resistance and low 5-hmC epigenetic profiles. This study has revealed new epigenetic reprogramming strategies to reverse global loss of 5-hmC, elicit a durable response to DNMTis, and sustain enhanced 5-hmC levels during administration of chemotherapy. Interestingly, recent reports have shown that DNMTis can increase active demethylation and 5-hmC formation in hepatocarcinoma (46) and during osteogenic differentiation of bone marrow–derived mesenchymal stem cells (47). Thus, Sajadian and colleagues performed an extensive analysis looking at the induction of demethylation via the TET pathway following 5-aza treatment (46). Their work provides further evidence that of the TET enzymes TET2 is the dominant driver behind demethylation (46). Similarly, our work has shown increased TET2 and TET3 levels in ovarian cancer cells pretreated with 5-aza.
Treatment strategies using various hypomethylating agents are starting to be tested in ovarian cancer as well as other solid tumor malignancies (48–50). Previous studies have shown varying levels of success when using DNMTis in a clinical setting. However, these trials did not analyze patients according to their global epigenetic profiles and lacked critical biomarkers predictive of response. The novelty of this study lies in the fact that we have identified a potential personalized marker for initial patient analysis of newly diagnosed tumors and therapy response follow-up in epigenetic trials. Additional validation of our results in a larger patient cohort of newly diagnosed HGSOC cases is required. Moreover, further studies investigating the dysregulation of TET2, TET3, IDH1, and/or IDH2-related markers in newly diagnosed HGSOC and in response to platinum therapy is warranted to accurately stratify patients in future clinical trials. Nevertheless, dose scheduling regimens likely need to include repeated pretreatment with epigenetic adjuvants followed by platinum cycles within a short treatment window in order to increase chemosensitivity in newly diagnosed poor prognostic patients with low 5-hmC scores. Interestingly, our work suggests that if 5-hmC levels can be further increased, DNMTi treatment may even enhance the platinum sensitivity of tumor cells that are already relatively chemosensitive, although this sensitization is clinically most significant for intrinsic platinum-resistant tumors. Consequently, altering chemotherapeutic regimens to incorporate DNMTi pretreatment in tumors with low 5-hmC levels could have clinical implications for poor prognostic, newly diagnosed disease.
Supplementary Material
Translational Relevance.
There are currently no good therapeutic options for patients newly diagnosed with high-grade serous ovarian cancer (HGSOC), the most common and aggressive subtype of ovarian cancer, who present with intrinsic platinum-resistant disease or relapsed patients with acquired platinum resistance. This report shows that global loss of 5-hmC levels is associated with a decreased response to platinum-based chemotherapy, shorter time to relapse, and poor overall survival in patients newly diagnosed with HGSOC. Most importantly, we identify a targetable pathway to rescue 5-hmC loss both genetically and pharmacologically, improve platinum chemosensitivity, and increase survival in poor prognostic platinum-resistant disease. This study could open new avenues for epigenetic reprogramming strategies in newly diagnosed patients with intrinsic platinum-resistant/refractory disease who exhibit decreased 5-hmC levels and may lead to a more durable tumor remission and overall survival.
Acknowledgments
This work is supported by grants awarded to D.M. Dinulescu by the DOD OCRP (W81XWH-10-1-0263, W81XWH-14-1-0092, W81XWH-14-1-0205, W81XWH-15-1-0089, W81XWH-16-1-0687), American Cancer Society (RSG-13-083-01-TBG), NIH (1R03CA189462-01, 2R01CA142746-06A1), Ovarian Cancer Research Fund Liz Tilberis award 330438, Canary Foundation, Burroughs-Wellcome Fund Career Award in the Biomedical Sciences 1005320.01, and the Mildred Moorman Ovarian Cancer Research Fund.
Footnotes
Authors’ Contributions
Conception and design: D.W. Tucker, A.W. Ohman, G. Murphy, C.G. Lian, D.M. Dinulescu
Development of methodology: D.W. Tucker, C.R. Getchell, A.W. Ohman, J.Y. Ko, J.J. Lee, C.G. Lian
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): D.W. Tucker, C.R. Getchell, E.T. McCarthy, A.W. Ohman, S. Xu, J.Y. Ko, M. Gupta, J.E. Medina, J.J. Lee, A. Malik, K.T. Hasselblatt, W. Li, H. Zhang, S.J. Kaplan, J. Liu, U.A. Matulonis, K.L. Terry, C.G. Lian
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): D.W. Tucker, C.R. Getchell, E.T. McCarthy, A.W. Ohman, N. Sasamoto, A.L. Shafrir, J.E. Medina, W. Li, S.J. Kaplan, K.L. Terry, C.G. Lian
Writing, review, and/or revision of the manuscript: D.W. Tucker, C.R. Getchell, E.T. McCarthy, A.W. Ohman, N. Sasamoto, M. Gupta, G. Murphy, J. Liu, U.A. Matulonis, K.L. Terry, C.G. Lian, D.M. Dinulescu
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): C.R. Getchell, E.T. McCarthy, A.W. Ohman, N. Sasamoto, L.A. MacDonald, A. Malik, G. Murphy, M.S. Hirsch, U.A. Matulonis, C.G. Lian
Study supervision: A.W. Ohman, G. Murphy, C.G. Lian, D.M. Dinulescu
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole–genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–94. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 2.Shenker N, Flanagan JM. Intragenic DNA methylation: implications of this epigenetic mechanism for cancer research. Br J Cancer. 2012;106:248–53. doi: 10.1038/bjc.2011.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lian CG, Xu Y, Ceol C, Wu F, Larson A, Dresser K, et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell. 2012;150:1135–46. doi: 10.1016/j.cell.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jäwert F, Hasséus B, Kjeller G, Magnusson B, Sand L, Larsson L. Loss of 5-hydroxymethylcytosine and TET2 in oral squamous cell carcinoma. Anticancer Res. 2013;33:4325–8. [PubMed] [Google Scholar]
- 5.Gambichler T, Sand M, Skrygan M. Loss of 5-hydroxymethylcytosine and ten-eleven translocation 2 protein expression in malignant melanoma. Melanoma Res. 2013;23:218–20. doi: 10.1097/CMR.0b013e32835f9bd4. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Liu Y, Bai F, Zhang JY, Ma SH, Liu J, et al. Tumor development is associated with decrease of TET gene expression and 5-methylcytosine hydroxylation. Oncogene. 2013;32:663–9. doi: 10.1038/onc.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariani CJ, Madzo J, Moen EL, Yesilkanal A, Godley LA. Alterations of 5-hydroxymethylcytosine in human cancers. Cancers. 2013;5:786–814. doi: 10.3390/cancers5030786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heuck CJ, Mehta J, Bhagat T, Gundabolu K, Yu Y, Khan S, et al. Myeloma is characterized by stage-specific alterations in DNA methylation that occur early during myelomagenesis. J Immunol. 2013;190:2966–75. doi: 10.4049/jimmunol.1202493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruscito I, Dimitrova D, Vasconcelos I, Gellhaus K, Schwachula T, Bellati F, et al. BRCA1 gene promoter methylation status in high-grade serous ovarian cancer patients - A study of the tumour Bank ovarian cancer (TOC) and ovarian cancer diagnosis consortium (OVCAD) Eur J Cancer. 2014;50:2090–8. doi: 10.1016/j.ejca.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Rice JC, Massey-Brown KS, Futscher BW. Aberrant methylation of the BRCA1 CpG island promoter is associated with decreased BRCA1 mRNA in sporadic breast cancer cells. Oncogene. 1998;17:1807–12. doi: 10.1038/sj.onc.1202086. [DOI] [PubMed] [Google Scholar]
- 11.Zhu X, Shan L, Wang F, Wang J, Wang F, Shen G, et al. Hypermethylation of BRCA1 gene: implication for prognostic biomarker and therapeutic target in sporadic primary triple-negative breast cancer. Breast Cancer Res Treat. 2015;150:479–86. doi: 10.1007/s10549-015-3338-y. [DOI] [PubMed] [Google Scholar]
- 12.Esteller M, Silva JM, Dominguez G, Bonilla F, Matias-Guiu X, Lerma E, et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst. 2000;92:564–9. doi: 10.1093/jnci/92.7.564. [DOI] [PubMed] [Google Scholar]
- 13.Wang YQ, Yan Q, Zhang JR, Li SD, Yang YX, Wan XP. Epigenetic inactivation of BRCA1 through promoter hypermethylation in ovarian cancer progression. J Obstet Gynaecol Res. 2013;39:549–54. doi: 10.1111/j.1447-0756.2012.01979.x. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Chavez L, Chang X, Wang X, Pastor WA, Kang J, et al. Distinct roles of the methylcytosine oxidases Tet1 and Tet2 in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 2014;111:1361–6. doi: 10.1073/pnas.1322921111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–33. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ficz G, Branco MR, Seisenberger S, Santos F, Krueger F, Hore TA, et al. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature. 2011;473:398–402. doi: 10.1038/nature10008. [DOI] [PubMed] [Google Scholar]
- 17.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 18.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 19.Lian CG, Murphy GF. The genetic evolution of melanoma. N Engl J Med. 2016;374:994–5. doi: 10.1056/NEJMc1515834. [DOI] [PubMed] [Google Scholar]
- 20.Johnson KC, Houseman EA, King JE, von Herrmann KM, Fadul CE, Christensen BC. 5-Hydroxymethylcytosine localizes to enhancer elements and is associated with survival in glioblastoma patients. Nat Commun. 2016;7:13177. doi: 10.1038/ncomms13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAuliffe SM, Morgan SL, Wyant GA, Tran LT, Muto KW, Chen YS, et al. Targeting Notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proc Natl Acad Sci U S A. 2012;109:E2939–48. doi: 10.1073/pnas.1206400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu JF, Hirsch MS, Lee H, Matulonis UA. Prognosis and hormone receptor status in older and younger patients with advanced-stage papillary serous ovarian carcinoma. Gynecol Oncol. 2009;115:401–6. doi: 10.1016/j.ygyno.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Vitonis AF, Titus-Ernstoff L, Cramer DW. Assessing ovarian cancer risk when considering elective oophorectomy at the time of hysterectomy. Obstet Gynecol. 2011;117:1042–50. doi: 10.1097/AOG.0b013e318212fcb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta M, Babic A, Beck AH, Terry K. TNF-α expression, risk factors, and inflammatory exposures in ovarian cancer: evidence for an inflammatory pathway of ovarian carcinogenesis? Hum Pathol. 2016;54:82–91. doi: 10.1016/j.humpath.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ince TA, Sousa AD, Jones MA, Harrell JC, Agoston ES, Krohn M, et al. Characterization of twenty-five ovarian tumour cell lines that phenocopy primary tumours. Nat Commun. 2015;6:7419. doi: 10.1038/ncomms8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karst AM, Drapkin R. Primary culture and immortalization of human fallopian tube secretory epithelial cells. Nat Protoc. 2012;7:1755–64. doi: 10.1038/nprot.2012.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latifi A, Luwor RB, Bilandzic M, Nazaretian S, Stenvers K, Pyman J, et al. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: molecular phenotype of chemoresistant ovarian tumors. PLoS One. 2012;7:e46858. doi: 10.1371/journal.pone.0046858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson VM, Castro CM, Chung J, Miller NC, Ullal AV, Castano MD, et al. Ascites analysis by a micro fluidic chip allows tumor-cell profiling. Proc Natl Acad Sci U S A. 2013;110:E4978–86. doi: 10.1073/pnas.1315370110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Globisch D, Münzel M, Müller M, Michalakis S, Wagner M, Koch S, et al. Tissue distribution of 5-hydroxymethylcytosine and search for active demethylation intermediates. PLoS One. 2010;5:e15367. doi: 10.1371/journal.pone.0015367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–5. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, et al. Ovarian cancer side population defines cells with stem cell- like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006;103:11154–9. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan SL, Medina JE, Taylor MM, Dinulescu DM. Targeting platinum resistant disease in ovarian cancer. Curr Med Chem. 2014;21:3009–20. doi: 10.2174/0929867321666140414102701. [DOI] [PubMed] [Google Scholar]
- 33.Croker AK, Allan AL. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44+ human breast cancer cells. Breast Cancer Res Treat. 2012;133:75–87. doi: 10.1007/s10549-011-1692-y. [DOI] [PubMed] [Google Scholar]
- 34.Liu S, Liu C, Min X, Ji Y, Wang N, Liu D, et al. Prognostic value of cancer stem cell marker aldehyde dehydrogenase in ovarian cancer: a meta-analysis. PLoS One. 2013;8:e81050. doi: 10.1371/journal.pone.0081050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landen CN, Jr, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9:3186–99. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu HC, Liu YS, Tseng KC, Hsu CL, Liang Y, Yang TS, et al. Overexpression of Lgr5 correlates with resistance to 5-FU-based chemotherapy in colorectal cancer. Int J Colorectal Dis. 2013;28:1535–46. doi: 10.1007/s00384-013-1721-x. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi S, Yamada-Okabe H, Suzuki M, Natori O, Kato A, Matsubara K, et al. LGR5-positive colon cancer stem cells interconvert with drug-resistant LGR5-negative cells and are capable of tumor reconstitution. Stem Cells. 2012;30:2631–44. doi: 10.1002/stem.1257. [DOI] [PubMed] [Google Scholar]
- 38.Chen Q, Zhang X, Li WM, Ji YQ, Cao HZ, Zheng P. Prognostic value of LGR5 in colorectal cancer: a meta-analysis. PLoS One. 2014;9:e107013. doi: 10.1371/journal.pone.0107013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang LY, Li PL, Wang TZ, Zhang XC. Prognostic values of 5-hmC, 5-mC and TET2 in epithelial ovarian cancer. Arch Gynecol Obstet. 2015;292:891–7. doi: 10.1007/s00404-015-3704-3. [DOI] [PubMed] [Google Scholar]
- 42.Kroeze LI, Aslanyan MG, van Rooij A, Koorenhof-Scheele TN, Massop M, Carell T, et al. Characterization of acute myeloid leukemia based on levels of global hydroxymethylation. Blood. 2014;124:1110–8. doi: 10.1182/blood-2013-08-518514. [DOI] [PubMed] [Google Scholar]
- 43.Matsuda I, Imai Y, Hirota S. Distinct global DNA methylation status in B-cell lymphomas: immunohistochemical study of 5-methylcytosine and 5-hydroxymethylcytosine. J Clin Exp Hematop. 2014;54:67–73. doi: 10.3960/jslrt.54.67. [DOI] [PubMed] [Google Scholar]
- 44.Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs. conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126:291–9. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sajadian SO, Ehnert S, Vakilian H, Koutsouraki E, Damm G, Seehofer D, et al. Induction of active demethylation and 5hmC formation by 5-aza-cytidine is TET2 dependent and suggests new treatment strategies against hepatocellular carcinoma. Clin Epigenetics. 2015;7:98. doi: 10.1186/s13148-015-0133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan X, Ehnert S, Culmes M, Bachmann A, Seeliger C, Schyschka L, et al. 5-azacytidine improves the osteogenic differentiation potential of aged human adipose-derived mesenchymal stem cells by DNA demethylation. PLoS One. 2014;9:e90846. doi: 10.1371/journal.pone.0090846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu S, Hu W, Iyer R, Kavanagh JJ, Coleman RL, Levenback CF, et al. Phase 1b-2a study to reverse platinum resistance through use of a hypomethylating agent, azacitidine, in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Cancer. 2011;117:1661–9. doi: 10.1002/cncr.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Falchook GS, Fu S, Naing A, Hong DS, Hu W, Moulder S, et al. Methylation and histone deacetylase inhibition in combination with platinum treatment in patients with advanced malignancies. Invest New Drugs. 2013;31:1192–200. doi: 10.1007/s10637-013-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matei D, Fang F, Shen C, Schilder J, Arnold A, Zeng Y, et al. Epigenetic resensitization to platinum in ovarian cancer. Cancer Res. 2012;72:2197–205. doi: 10.1158/0008-5472.CAN-11-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


