Introduction
The idiopathic nature of Urological Chronic Pelvic Pain Syndrome (UCPPS), which encompasses interstitial cystitis/bladder pain syndrome (IC/BPS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), has prompted an intense search for clinical biomarkers. Identification of clinically relevant, validated biologic markers of UCPPS has great potential to inform our understanding of UCPPS pathophysiology, development, and progression, and ultimately clinical management. To date, the cause of this syndrome is unknown and there is no ability to determine who is at risk of progression or who will respond to specific therapy. Accurate noninvasive tests, based on objective, specific, and definable levels of validated biomarkers, are essential to improve and standardized clinical guidelines and for more targeted management strategies based on patient profiles.
The MAPP Research Network was established by the NIDDK of the NIH and represents a comprehensive approach to the study of UCPPS 1, 2. A major goal of this initiative is to better understand pathophysiology and identify potential therapeutic targets. In this MAPP Network study, we have utilized a biologically-driven, candidate biomarker discovery strategy, grounded in the basic biochemistry and physiology of this syndrome, to evaluate candidate non-invasive biomarkers of UCPPS.
Reviews of the literature have revealed a number of candidate protein biomarker targets. Increased VEGF levels have been detected in bladder biopsy samples from patients with IC compared to controls, with levels correlating with pain severity 3, 4. Prior studies also suggest an important connection between neovascularization and IC, in that new blood vessels in biopsy samples of IC patients showed significantly lower levels of pericyte coverage of the nascent endothelium, evidence consistent with the known association between high levels of VEGF and immature vessel formation 3. Moreover, VEGF originally discovered as VPF (Vascular Permeability Factor), 5, 6 is the most potent endogenous vascular permeability factor and may mediate the dysregulated vascular permeability postulated to be important in UCPPS 7–9. Given these associations, we chose to include VEGF and its receptor VEGFR1, as biomarker candidates.
Inflammation has been suggested to be an underlying pathophysiologic mechanism in UCPPS 10 and increased concentrations of pro-inflammatory cytokines (IL-1β, TNF-α, IL-8) have been reported in patients with UCPPS as compared with controls 11, 12. In this respect, MMPs are of particular interest. Originally thought to be associated exclusively with tissue remodeling and destruction, it is now widely appreciated that MMPs play a role in a variety of biologic processes including cytokine and growth factor release, tumor growth and progression, angiogenesis and a number of inflammatory conditions 13–17. During the inflammatory response, MMPs and their complexes are released from connective tissue cells in response to pro-inflammatory cytokines. Upregulation of MMP activity results in the recruitment of pro-inflammatory cells to the site of tissue injury. MMPs have also been implicated in the regulation of the immune response because of their ability to cleave inflammatory mediators and stimulate the clearance of inflammatory cells 18–21. Given that MMPs may play an important role in UCPPS as a function of the inflammatory phenotype, abnormal vasculature and the dysregulation of ECM (extracellular matrix) turnover present in this and other potentially related diseases we included these proteins in our study.
NGAL, also known as Lipocalin-2 (Lcn2), is upregulated in numerous chronic and acute inflammatory conditions, including chronic kidney disease, ulcerative colitis and myocardial infarction 22, 23. There exists an intricate interplay between NGAL and other inflammatory cytokines as well, such that NGAL upregulation can be triggered by multiple inflammatory cytokines, including TNF-α, IL-1β and IL-17 22, 23. Moreover, NGAL itself is a modulator of the levels of other inflammatory cytokines, as well as of the behavior of inflammatory leukocytes 24–26. In our laboratory, we have demonstrated that NGAL is a significant stimulator of VEGF levels in breast tumor cells and can also independently induce the epithelial to mesenchymal transition in breast cancer, as well as being a noninvasive biomarker of this disease 27–29. NGAL can also form a complex with MMP-9, representing a distinct protein complex referred to as MMP-9/NGAL complex. We have previously shown that when NGAL complexes with MMP-9, it protects MMP-9 from degradation preserving its enzyme activity 16, 30. The MMP-9/NGAL complex has also been detected in urine samples from patients with various diseases. Based on these findings, we chose to measure NGAL as well as MMP-9/NGAL complex in the urine of individuals with UCPPS and correlate them with clinical symptoms.
Here, we report sex-specific comparisons of candidate biomarkers among UCPPS and control cohorts to gain new insights into underlying pathophysiology. In addition, associations of biomarkers with symptom severity within the UCPPS cohort are examined. We anticipate that the identified biological markers may serve as potential candidates for further clinical evaluation, including in the development of new treatment strategies directed toward novel targets, as objective measures for patient classification schemes, and evaluation of clinical outcomes, as well as further characterization of underlying mechanism.
Methods and Materials
Urine collection, storage, shipping
Biologic specimens collected in the Trans-MAPP Epidemiology/Phenotyping Study (EPS) 2 included urine collected at baseline visits. Clean-catch midstream urine was collected using alcohol-free Triad Medical-Benzalkonium chloride antiseptic towelettes (Allegro Medical) 2. After collection, the urine was immediately frozen at −80°C and shipped to the MAPP Network Tissue Analysis and Technology Core for centralized processing, where it was then divided into 3 mL aliquots and stored at −80°C until further analysis. All specimens were de-identified to allow for blinded testing 2. Documentation of sample origin, acquisition, transportation, processing, and storage was tracked and recorded for each sample for quality assurance and quality control purposes.
Protein assays
Urine samples were thawed, aliquoted, and assayed as below. Remaining samples at the time of aliquoting were then stored at −80°C. Total protein concentration was determined using the Bradford method with bovine serum albumin as the standard. Samples were read on a DU 640 spectrophotometer (Beckman Coulter).
Enzyme-linked immunosorbent assay (ELISA)
Monospecific ELISAs (Quantikine, R&D Systems, Inc.) for human MMP-2, MMP-9, MMP-9/NGAL complex, NGAL, VEGF and VEGFR1 were performed according to manufacturer’s instructions. All analyses were conducted in duplicate in a double-blinded manner as previously reported by us 27, 29, 31–33.
Study Population
The Trans-MAPP EPS enrolled male and female participants in three cohorts: 1) UCPPS participants, 2) positive controls (PC) and 3) healthy controls (HC) 1, 2. UCPPS participants were defined by a nonzero response on a pelvic pain scale. PC were defined by the presence of a non-urological associated syndrome (NUAS) (fibromyalgia, chronic fatigue syndrome, and/or irritable bowel syndrome), and healthy controls were defined by the absence of both UCPPS and other chronic pain conditions. Detailed inclusion and exclusion criteria are described 2. Stratified random sampling was used to select participants for this substudy. UCPPS participants with the most severe urologic pain and urinary symptoms and HCs and PCs with the least severe symptoms were more likely to be selected to maximize the likelihood of detecting differences between UCPPS and control cohorts. Sampling was conducted within sex, and severity was determined by the sum of the Genitourinary Pain Index (GUPI) 34 pain and urinary subscores, which was categorized as mild (less than 13), moderate (greater than or equal to 13 and less than 23), or severe (greater than or equal to 23 for a maximum of 33) for selection. Demographic data were collected, and additional clinical characteristics were assessed by the RAND Interstitial Cystitis Epidemiology Study criteria 35, Brief Pain Inventory (BPI) short form 36, and Interstitial Cystitis Symptom Index (ICSI) 37. For analyses of associations of symptom severity with candidate proteins, GUPI Pain and Urinary subscores and items of the ICSI symptom index are reflected in two primary symptom constructs, pain severity and urinary severity 38.
Statistical analyses
Duplicate values of candidate proteins were evaluated for quality prior to analysis. Reliability analyses were conducted using Bland-Altman plots, Coefficients of Variation (CVs) and Intraclass Correlation Coefficients (ICCs). Undetectable concentration values reported as zero were imputed with half the limit of detection (LOD), and values above the maximum detectable limit (MDL) were removed (n=11). Unlike values below the LOD, which we know lie between 0 and the LOD, it cannot be assumed that values above the MDL are indeed that large. Values below the LOD but greater than 0 remained as measured. Results of reliability analyses, LODs and MDLs are provided in Tables S1 and S2.
Imputed concentration values were divided by total urinary protein per sample to normalize concentration to total urinary protein to correct for various degrees of hydration and protein excretion. Concentrations (“classic concentration”, ng/mL or pg/mL) and concentration normalized to total protein (“normalized concentration”, pg/µg) were then log-transformed. Candidate biomarker levels were compared within sex using Student’s t-test. We compare UCPPS and HC to assess markers of illness and pain and UCPPS and PC to identify markers unique to UCPPS. Markers that differed significantly between UCPPS and respective controls in univariable analysis after accounting for multiple testing were entered into a multivariable logistic regression with UCPPS versus HC or UCPPS versus PC as the outcome. Corresponding ROC curves were generated. Models did not include any variables beyond the indicated proteins. We accounted for multiple testing by controlling the False Discovery Rate 39 (FDR), a more powerful alternative to the standard Bonferroni correction. The FDR is a generalization of type I error under a single hypothesis test to multiple hypothesis tests. This method of adjusting for multiple testing considers variable thresholds for the significance of ranked p-values that result from a set of hypothesis tests instead of a single, stringent threshold applied to all tests. The FDR was set to 0.05, and the reference thresholds for p-values for each test were determined by a formula based on the number of tests 39. FDR adjustment for six tests of classic concentration was applied separately for tests of UCPPS versus HC and UCPPS versus PC, and similarly to seven tests of normalized concentration for each marker and additionally total protein. Locally weighted scatterplot smoothing was performed to visualize associations of classic and normalized concentration with pain and urinary severity 38 in all three cohorts. Among UCPPS patients only, linear regression was used to quantify the association of pain and urinary severity with classic and normalized concentrations of candidate biomarkers, and associated markers in univariable analyses were entered into separate multivariable models for each symptom. Multivariable models again included markers significant after adjustment for multiple comparisons and did not adjust for other factors. Pearson correlation coefficients among markers were generated to evaluate the potential for multicollinearity in regression models.
Results
Table 1 summarizes selected demographic data, prevalence of non-urological associated syndromes (NUAS), and the primary urologic pain and urinary severity levels of the Trans-MAPP EPS participants included and not included in these analyses (see Materials and Methods). The evaluated subset included 259 UCPPS (139 female, 120 male) participants, 125 healthy controls (75 female, 50 male), and 107 positive controls (75 female, 32 male). UCPPS participants selected for this biomarker study reported longer duration of symptoms (mean (SD) 9.7 (11.6) vs. 6.6 (8.6), p=0.002) and were more likely to have a NUAS than those not in the study (42.9% vs. 30.9%, p=0.014), and selected healthy and positive controls had less severe urinary (p<0.001 HC, p<0.001 PC) and pain (p=0.003 HC, p<0.001 PC) symptoms than those not included. These results were expected given the sampling strategy. Further, males were overrepresented among PC relative to the prevalence of males among all MAPP PC participants, also by design.
Table 1.
Characteristics of Sample and Study Groups
| Variable | Category | UCPPS | Healthy Controls (HC) | Positive Controls (PC) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Included1 | Not Included |
Total | P value |
Included1 | Not Included |
Total | P value |
Included1 | Not Included |
Total | P value |
||
| N | 259 | 165 | 424 | 125 | 290 | 415 | 107 | 92 | 199 | ||||
| Age | Mean (SD) | 43.3 (14.7) | 43.5 (15.8) | 43.4 (15.1) | 0.918 | 42.5 (13.3) | 39.7 (14.3) | 40.5 (14.1) | 0.062 | 41.6 (13.1) | 41.3 (14.4) | 41.5 (13.7) | 0.899 |
| Duration of UCPPS Symptoms* | Mean (SD) | 9.7 (11.6) | 6.6 (8.6) | 8.5 (10.6) | 0.002 | – | – | – | – | – | – | – | – |
| Sex* | Female | 139 (53.7%) | 94 (57.0%) | 233 (55.0%) | 0.505 | 75 (60.0%) | 158 (54.5%) | 233 (56.1%) | 0.299 | 75 (70.1%) | 82 (89.1%) | 157 (78.9%) | 0.001 |
| Male | 120 (46.3%) | 71 (43.0%) | 191 (45.0%) | 50 (40.0%) | 132 (45.5%) | 182 (43.9%) | 32 (29.9%) | 10 (10.9%) | 42 (21.1%) | ||||
| Race | White | 229 (88.4%) | 145 (87.9%) | 374 (88.2%) | 0.8592 | 90 (72.0%) | 226 (77.9%) | 316 (76.1%) | 0.810 | 76 (71.0%) | 73 (79.3%) | 149 (74.9%) | 0.513 |
| Black | 10 (3.9%) | 6 (3.6%) | 16 (3.8%) | 16 (12.8%) | 32 (11.0%) | 48 (11.6%) | 12 (11.2%) | 10 (10.9%) | 22 (11.1%) | ||||
| Ethnicity | Hispanic | 19 (7.4%) | 9 (5.5%) | 28 (6.6%) | 0.441 | 8 (6.4%) | 27 (9.3%) | 35 (8.4%) | 0.328 | 10 (9.4%) | 3 (3.3%) | 13 (6.6%) | 0.080 |
| Non-Hispanic | 239 (92.6%) | 156 (94.5%) | 395 (93.4%) | 117 (93.6%) | 263 (90.7%) | 380 (91.6%) | 96 (90.6%) | 89 (96.7%) | 185 (93.4%) | ||||
| NUAS3* | Any | 111 (42.9%) | 51 (30.9%) | 162 (38.2%) | 0.014 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 107 (100.0%) | 92 (100.0%) | 199 (100.0%) | ||
| None | 148 (57.1%) | 114 (69.1%) | 262 (61.8%) | 125 (100.0%) | 290 (100.0%) | 415 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||||
| Pain* Severity | Mean (SD) | 15.2 (6.1) | 14.7 (4.7) | 15.0 (5.6) | 0.353 | 0.1 (0.6) | 0.4 (1.2) | 0.3 (1.0) | 0.003 | 1.8 (3.7) | 5.8 (5.5) | 3.6 (5.0) | 0.000 |
| Urinary* Severity | Mean (SD) | 12.7 (6.6) | 12.5 (5.5) | 12.6 (6.2) | 0.767 | 1.6 (2.0) | 3.5 (3.1) | 2.9 (2.9) | 0.000 | 4.4 (4.4) | 8.8 (6.0) | 6.4 (5.6) | 0.000 |
| BPS4 Type | BPS: No | 48 (18.5%) | 26 (15.8%) | 74 (17.5%) | 0.463 | 117 (93.6%) | 268 (92.4%) | 385 (92.8%) | 0.669 | 83 (77.6%) | 58 (63.0%) | 141 (70.9%) | 0.025 |
| BPS: Yes | 211 (81.5%) | 139 (84.2%) | 350 (82.5%) | 8 (6.4%) | 22 (7.6%) | 30 (7.2%) | 24 (22.4%) | 34 (37.0%) | 58 (29.1%) | ||||
| Pain Region5 | Pelvic Pain & Beyond | 197 (76.1%) | 119 (72.1%) | 316 (74.5%) | 0.364 | 48 (38.4%) | 127 (43.8%) | 175 (42.2%) | 0.307 | 85 (79.4%) | 77 (83.7%) | 162 (81.4%) | 0.442 |
| Pelvic Pain only | 62 (23.9%) | 46 (27.9%) | 108 (25.5%) | 77 (61.6%) | 163 (56.2%) | 240 (57.8%) | 22 (20.6%) | 15 (16.3%) | 37 (18.6%) | ||||
denotes variables for which there was a significant difference between participants that were selected for the substudy compared to those who were not selected for the substudy in at least one participant cohort (UCPPS, HC, or PC)
Participants were selected for this substudy by stratified random sampling within sex and cohort. UCPPS participants with the most severe urologic pain and urinary symptoms and HC and PC with the least severe symptoms were more likely to be selected to maximize the likelihood of detecting differences between UCPPS and control cohorts.
The comparison of race between included and not included subjects was based on a 7-level race variable within the categories of white, black, Asian, multi-race, native Hawaiian, other, and unknown. White and black are shown for brevity.
NUAS denotes the presence of a nonurological associated sydrome, defined as Chronic Fatigue Syndrome, Irritable Bowel Sydrome, or Fibromyalgia
BPS Type indicates Bladder Pain Syndrome type. Patients classified as ‘yes’ reported the presence of painful filling or painful urgency on the RAND Interstitial Cystitis Epidemiology Study criteria38
Pain region indicates the widespreadness of pain. ‘Pelvic Pain only’ indicates the presence of pain in the pelvic region only, and ‘Pelvic Pain and Beyond’ indicates pain outside of the pelvic region. All participants were classified as pelvic pain only by default and designated as pelvic pain and beyond by indicating pain outside of the pelvic region on a modified version of the Brief Pain Inventory short form36.
Quality control analyses utilizing the duplicate values for each of these 6 biomarkers and total protein, resulted in coefficients of variation (CVs) of 118.2% (MMP-2), 25.9% (MMP-9), 74.4% (MMP-9/NGAL complex), 26.2%(NGAL), 23.5%(VEGF), 57.3% (VEGF-R1), and 7.2% (total protein). ICCs resulting from these duplicate values were 0.837 (MMP-2), 0.990 (MMP-9), 0.972 (MMP-9/NGAL complex), 0.971 (NGAL), 0.980 (VEGF), 0.793 (VEGF-R1), and 0.997 (total protein).
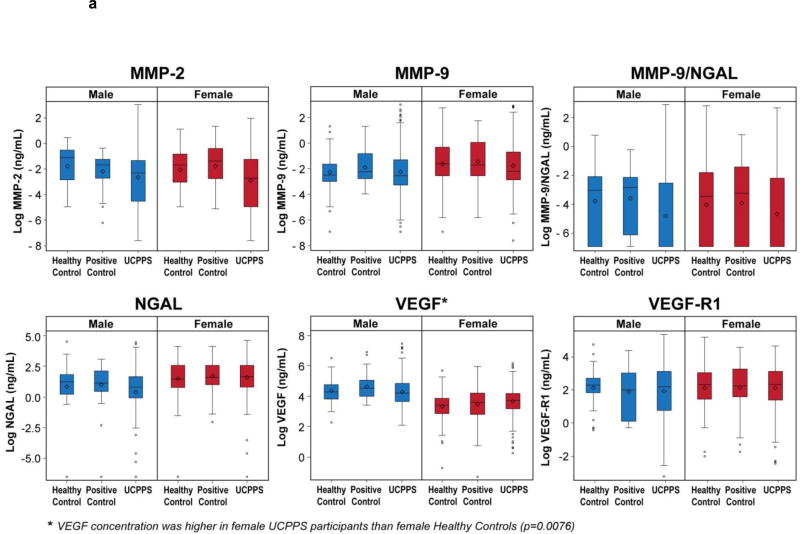
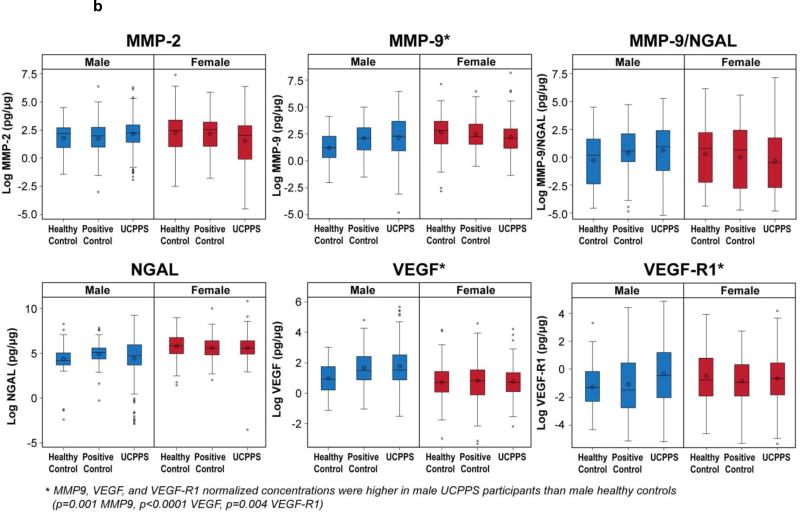
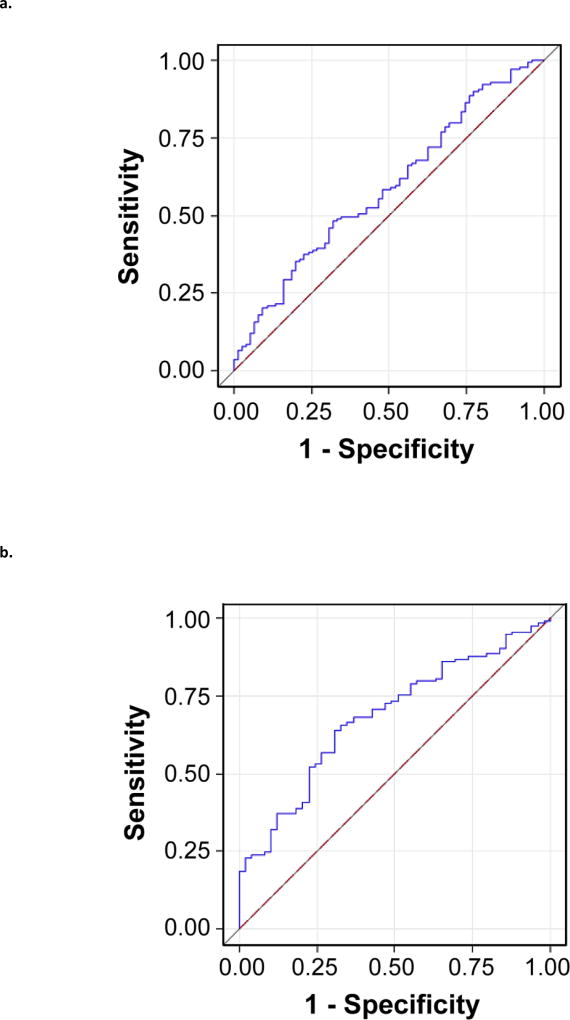
Figure 1a and Figure 1b display biomarker concentration (ng/mL or pg/mL) and normalized concentrations (pg/µg), respectively, by cohort and sex. Although boxplots suggest differences in median levels for some proteins among cohorts, large variability in measurements largely prevented such differences from reaching statistical significance. Nonetheless, urinary VEGF classic concentration was significantly higher in female UCPPS than HC (mean UCPPS=3.72, mean HC=3.46; p=0.0076). ROC analysis suggested, however, that VEGF did not distinguish UCPPS participants from HC (AUC=0.58, Figure 2a). Males with UCPPS had significantly higher levels of normalized VEGF (mean UCPPS = 1.78, mean HC =0.98, p<0.0001), VEGF-R1 (mean UCPPS=-0.33, mean HC=-1.25, p=0.004), and MMP-9 (mean UCPPS=2.16, mean HC=1.22, p=0.001) than male HC. In multivariable analysis these three markers fairly distinguished UCPPS from HC (AUC=0.68, Figure 2b).
Figure 1.
Log biomarker concentrations (ng/mL or pg/mL, subfigure 1A) or normalized concentrations (pg/µg) are presented for male (blue) and female (red) participants by patient cohort (Healthy Control, Positive Control, or UCPPS). Candidate proteins that differed significantly by cohort within sex are indicated by ‘*’.
a Biomarker Concentration (ng/mL or pg/mL) by Cohort and Sex
b Biomarker Concentration Normalized to Total Protein (pg/µg) by Cohort and Sex
Figure 2.
Receiver Operating Characteristic (ROC) curves are presented for markers that differed significantly between cohorts within sex to demonstrate the capacity for candidate proteins to distinguish Urological Chronic Pelvic Pain Syndrome (UCPPS) participants from Healthy Controls (HC). Satisfactory performance is indicated by an area under the ROC curve (AUC) of 0.70. Subfigure 2A shows the potential of candidate protein VEGF concentration to distinguish between female UCPPS and HC. Results are based on a logistic regression model with UCPPS vs HC as the outcome and VEGF concentration as the predictor, fit among female participants only. Subfigure 2B shows the potential of normalized concentrations of MMP9, VEGF, and VEGF-R1 to distribution between male UCPPS and male HC. Results are based on a multivariable logistic regression model with UCPPS vs. HC as the outcome and normalized MMP9, VEGF, and VEGFR1 as predictors, fit among male participants only.
a. ROC Analysis of VEGF (ng/mL) for Discriminating UCPPS versus Healthy Control Females, AUC=0.58
b. ROC Analysis of MMP-9, VEGF, and VEGF-R1 (pg/µg) for Discriminating UCPPS versus Healthy Control Males, AUC=0.68
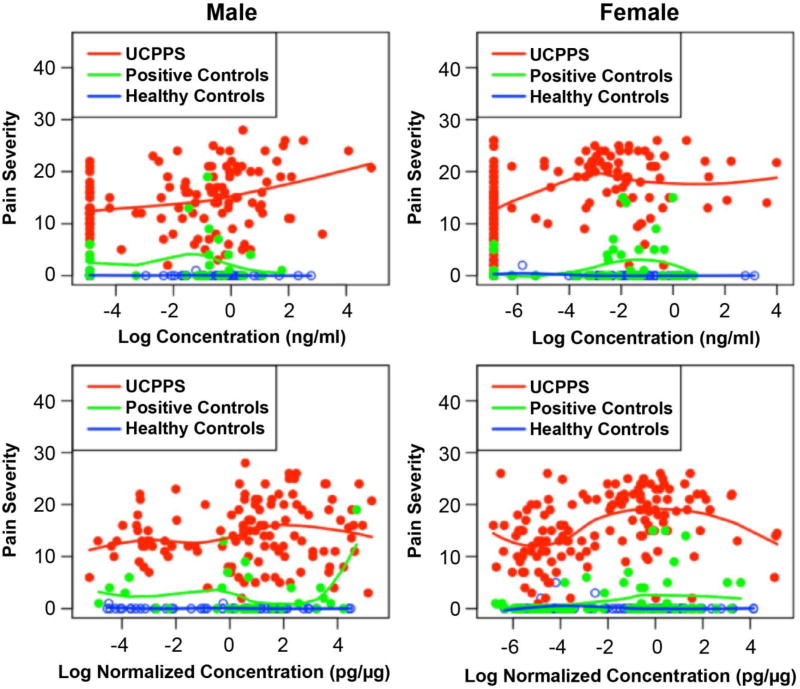
Scatterplots suggested a positive association of concentration and normalized concentration with pain severity and urinary severity for several candidate proteins (eg. MMP9/NGAL and pain severity (Figure 3); urinary severity and other candidate proteins not shown). Tables 2a and 2b show the complete results of univariable and multivariable linear regression, respectively, of the two primary measures of UCPPS symptom severity 38 with respect to concentrations of each of the six candidate biomarkers within the UCPPS patient cohort, stratified by sex. In UCPPS females, pain severity was significantly positively associated with concentrations of all biomarkers except NGAL (MMP-2 p=0.0113, MMP-9 p=0.0094, MMP-9/NGAL complex p<.0001, VEGF p <.0001, VEGF-R1 p=0.0012), and urinary severity was significantly positively associated with concentrations of all biomarkers except VEGF-R1 in univariable analysis (MMP-2 p=0.0004, MMP-9 p=0.0012, MMP9/NGAL p <.0001, NGAL p=0.0149, VEGF p <.0001). After adjustment for other markers in multivariable analysis, concentrations of MMP-9/NGAL complex, VEGF, and VEGF-R1 remained significantly positively associated with pain severity (MMP-9/NGAL complex p=0.0013, VEGF p=0.0169, VEGF-R1 p=0.0092), and only MMP-9/NGAL complex was significantly positively associated with urinary severity in females (p=0.0211). In males pain severity was significantly positively associated with the classic concentration of MMP-9 (p=0.0006) and MMP-9/NGAL complex (p=0.0007), and urinary severity was significantly positively associated with the classic concentrations of MMP-9 (p=0.0001), MMP-9/NGAL complex (p=0.0001), and VEGF-R1 (p=0.0107) (Table 2a). In multivariable analysis none of these markers were associated with pain or urinary severity in males, likely due in part to the apparent collinearity among markers (Table 2b, ρ=0.68 MMP9 and MMP9/NGAL complex; ρ=0.16 MMP9 and VEGF-R1; ρ=0.22 MMP9/NGAL complex and VEGF-R1 among male UCPPS).
Figure 3.
Pain severity is plotted against MMP9/NGAL log concentration (ng/ml, top) or log normalized concentration (pg/µg, bottom) separately for male (left) and female (right) Urological Chronic Pelvic Pain Syndrome participants (UCPPS, red), Positive Controls (PC, green), and Healthy Controls (HC, blue). Trend lines were determined by locally weighted scatterplot smoothing.
Pain Severity and MMP9/NGAL by Cohort and Sex
Table 2.
| a. Univariable Regression Analyses for Symptoms and Biomarker Concentration by Gender within UCPPS | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Biomarker | FEMALE | MALE | ||||||
| ß | 95% CI | P value | R-Square | ß | 95% CI | P value | R-Square | ||
| PAIN SEVERITY | MMP-2 (ng/mL) | 0.684 | (0.16, 1.21) | 0.0113 | 0.0463 | 0.214 | (−0.48, 0.90) | 0.5406 | 0.0032 |
| MMP-9 (ng/mL) | 0.988 | (0.25, 1.73) | 0.0094 | 0.0496 | 0.979 | (0.43, 1.53) | 0.0006 | 0.0977 | |
| MMP-9/NGAL (ng/mL) | 0.919 | (0.56, 1.28) | <.0001 | 0.1604 | 0.725 | (0.31, 1.14) | 0.0007 | 0.0955 | |
| NGAL (ng/mL) | 0.774 | (−0.10, 1.65) | 0.0818 | 0.0235 | 0.157 | (−0.20, 0.52) | 0.3874 | 0.0064 | |
| VEGF (pg/mL) | 2.392 | (1.36, 3.42) | <.0001 | 0.1334 | 0.633 | (−0.39, 1.65) | 0.2211 | 0.0129 | |
| VEGF-R1 (pg/mL) | 1.452 | (0.58, 2.32) | 0.0012 | 0.0741 | 0.447 | (−0.28, 1.18) | 0.2280 | 0.0123 | |
| URINARY SEVERITY | MMP-2 (ng/mL) | 0.995 | (0.46, 1.53) | 0.0004 | 0.0910 | 0.641 | (−0.15, 1.43) | 0.1099 | 0.0217 |
| MMP-9 (ng/mL) | 1.275 | (0.51, 2.04) | 0.0012 | 0.0763 | 1.281 | (0.65, 1.91) | 0.0001 | 0.1248 | |
| MMP-9/NGAL (ng/mL) | 0.917 | (0.53, 1.30) | <.0001 | 0.1449 | 0.938 | (0.48, 1.40) | 0.0001 | 0.1233 | |
| NGAL (ng/mL) | 1.143 | (0.23, 2.06) | 0.0149 | 0.0462 | 0.301 | (−0.11, 0.71) | 0.1515 | 0.0176 | |
| VEGF (pg/mL) | 2.251 | (1.15, 3.35) | <.0001 | 0.1086 | −0.217 | (−1.41, 0.97) | 0.7179 | 0.0011 | |
| VEGF-R1 (pg/mL) | 0.910 | (−0.02, 1.84) | 0.0563 | 0.0269 | 1.080 | (0.26, 1.90) | 0.0107 | 0.0544 | |
| b. Multivariable Regression Analyses for Symptoms and Biomarker Concentration by Gender within UCPPS | |||||||
|---|---|---|---|---|---|---|---|
| Outcome | Biomarker | FEMALE (Models 5 and 7) | MALE (Models 1 and 3) | ||||
| ß | 95% CI | P value | ß | P value | R-Square | ||
| PAIN SEVERITY | MMP-2 (ng/mL) | −0.13 | (−.69, 0.42) | 0.6376 | - | - | - |
| MMP-9 (ng/mL) | −0.41 | (−1.3, 0.53) | 0.3909 | 0.71 | (−.05, 1.47) | 0.0660 | |
| MMP-9/NGAL (ng/mL) | 0.87 | (0.35, 1.39) | 0.0013 | 0.33 | (−.24, 0.91) | 0.2488 | |
| NGAL (ng/mL) | - | - | - | - | - | - | |
| VEGF (pg/mL) | 1.49 | (0.27, 2.70) | 0.0169 | - | - | - | |
| VEGF-R1 (pg/mL) | 1.15 | (0.29, 2.02) | 0.0092 | - | - | - | |
| URINARY SEVERITY | MMP-2 (ng/mL) | 0.43 | (−.16, 1.02) | 0.1506 | - | - | - |
| MMP-9(ng/mL) | −0.10 | (−1.2, 0.99) | 0.8562 | 0.69 | (−.16, 1.54) | 0.1119 | |
| MMP-9/NGAL (ng/mL) | 0.70 | (0.11, 1.30) | 0.0211 | 0.52 | (−.13, 1.17) | 0.1160 | |
| NGAL (ng/mL) | 0.26 | (−.68, 1.21) | 0.5804 | - | - | - | |
| VEGF (pg/mL) | 1.10 | (−.22, 2.41) | 0.1018 | - | - | - | |
| VEGF-R1 (pg/mL) | - | - | - | 0.69 | (−.12, 1.51) | 0.0957 | |
ß denotes the change in mean symptom severity associated with a 1-unit increase in the log-transformed classic concentration
FDR thresholds out of 6 tests are 0.05, 0.042, 0.033, 0.025, 0.016, 0.0083. Significant values are bolded for emphasis.
ß denotes the change in mean symptom severity associated with a 1-unit increase in the log-transformed concentration, holding other markers constant. Significant values are bolded for emphasis.
Tables 3a and 3b show analogous regression analyses for normalized concentrations (pg/µg). In females, pain and urinary symptom severity at baseline were each significantly positively associated with MMP-9/NGAL complex (p<0.0001). Further, increased MMP-2 and VEGF were significantly associated with greater urinary (p=0.0074) and pain severity (p=0.0127), respectively. In multivariable analysis MMP-9/NGAL complex remained significantly positively associated with pain severity in females (p=0.0001). Similarly, in the multivariable model for urinary severity in females, MMP-9/NGAL complex was significantly associated with symptom severity (p=0.0004). In male UCPPS participants, no significant correlates of pain and urinary severity were found among normalized concentration levels of candidate biomarkers in univariable analysis, however total urinary protein was positively associated with pain severity (p=0.0042).
Table 3.
| a. Univariable Regression Analyses for Symptoms and Biomarker Normalized Concentration by Gender within UCPPS | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Biomarker | FEMALE | MALE | ||||||
| ß | 95% CI | P value | R-Square | ß | 95% CI | P value | R-Square | ||
| PAIN SEVERITY | TOTAL PROTEIN | 0.572 | (−0.30, 1.45) | 0.1992 | 0.0120 | 0.982 | (0.32, 1.65) | 0.0042 | 0.0674 |
| MMP-2 (pg/µg) | 0.440 | (−0.07, 0.95) | 0.0906 | 0.0209 | −0.644 | (−1.26, −0.02) | 0.0422 | 0.0345 | |
| MMP-9 (pg/µg) | 0.508 | (−0.17, 1.18) | 0.1390 | 0.0164 | 0.216 | (−0.30, 0.73) | 0.4056 | 0.0060 | |
| MMP-9/NGAL (pg/µg) | 0.845 | (0.48, 1.21) | <.0001 | 0.1349 | 0.313 | (−0.10, 0.72) | 0.1335 | 0.0193 | |
| NGAL (pg/µg) | 0.386 | (−0.37, 1.15) | 0.3174 | 0.0078 | −0.130 | (−0.54, 0.28) | 0.5318 | 0.0034 | |
| VEGF (pg/µg) | 1.280 | (0.28, 2.28) | 0.0127 | 0.0445 | −0.692 | (−1.42, 0.04) | 0.0627 | 0.0295 | |
| VEGF-R1 (pg/µg) | 0.366 | (−0.22, 0.95) | 0.2198 | 0.0111 | −0.257 | (−0.71, 0.20) | 0.2638 | 0.0106 | |
| URINARY SEVERITY | TOTAL PROTEIN | 0.593 | (−0.32, 1.51) | 0.2033 | 0.0120 | 0.859 | (0.08, 1.64) | 0.0308 | 0.0392 |
| MMP-2 (pg/µg) | 0.723 | (0.20, 1.25) | 0.0074 | 0.0523 | −0.183 | (−0.91, 0.54) | 0.6179 | 0.0021 | |
| MMP-9 (pg/µg) | 0.714 | (0.01, 1.41) | 0.0455 | 0.0300 | 0.521 | (−0.07, 1.11) | 0.0826 | 0.0262 | |
| MMP-9/NGAL (pg/µg) | 0.837 | (0.45, 1.23) | <.0001 | 0.1200 | 0.545 | (0.08, 1.01) | 0.0211 | 0.0454 | |
| NGAL (pg/µg) | 0.614 | (−0.19, 1.42) | 0.1325 | 0.0179 | 0.091 | (−0.38, 0.57) | 0.7040 | 0.0012 | |
| VEGF (pg/µg) | 1.125 | (0.06, 2.19) | 0.0378 | 0.0316 | −1.070 | (−1.91, −0.23) | 0.0126 | 0.0529 | |
| VEGF-R1 (pg/µg) | 0.117 | (−0.50, 0.74) | 0.7092 | 0.0010 | 0.038 | (−0.49, 0.56) | 0.8851 | 0.0002 | |
| b. Multivariable Regression Analyses for Symptoms and Biomarker Normalized Concentration by Gender within UCPPS | ||||
|---|---|---|---|---|
| Outcome | Independent Variable |
FEMALE (Models 13 and 16) | ||
| ß | 95% CI | P value | ||
| PAIN SEVERITY | MMP-2 (ng/mL) | - | - | - |
| MMP-9 (ng/mL) | - | - | - | |
| MMP-9/NGAL (ng/mL) | 0.77 | (0.38, 1.16) | 0.0001 | |
| NGAL (ng/mL) | - | - | - | |
| VEGF (pg/mL) | 0.59 | (−.42, 1.61) | 0.2502 | |
| VEGF-R1 (pg/mL) | - | - | - | |
| URINARY SEVERITY | MMP-2 (ng/mL) | 0.35 | (−.18, 0.89) | 0.1922 |
| MMP-9(ng/mL) | - | - | - | |
| MMP-9/NGAL (ng/mL) | 0.75 | (0.34, 1.17) | 0.0004 | |
| NGAL (ng/mL) | - | - | - | |
| VEGF (pg/mL) | - | - | - | |
| VEGF-R1 (pg/mL) | - | - | - | |
ß denotes the change in mean symptom severity associated with a 1-unit increase in the log-transformed normalized concentration FDR thresholds out of 7 tests: 0.0500, 0.0429, 0.0357, 0.0286, 0.0214, 0.0143, 0.007. Significant values are bolded for emphasis.
ß denotes the change in mean symptom severity associated with a 1-unit increase in the log-transformed normalized concentration, holding other markers constant. Significant values are bolded for emphasis.
Conclusion
There is a lack of understanding of the etiology and risk factors of UCPPS and diagnosis still relies primarily on patient reported symptoms.1 It was previously believed that the symptoms of UCPPS were primarily seen in women. However, emerging data from the MAPP Network has shown that a large percentage of men with pelvic pain also have similar bladder symptoms 40. Unfortunately, to date, patient therapies are generally ineffective 1.
To identify non-invasive protein biomarkers for UCPPS that may inform underlying pathophysiology and provide targets for further clinical evaluation and hypothesis testing, the extensive resources and expertise of the MAPP Network have been leveraged to perform the largest UCPPS non-invasive biomarker analysis to date involving biological samples from over 491 individuals. Ultimately, identification and subsequent validation of such non-invasive, unbiased and accurate biomarkers may aid in identifying the presence of this syndrome, reveal clinically relevant patient sub-groups, monitor disease progression and therapeutic efficacy; predict flares, and objectively assess hallmarks of this syndrome, such as pain severity, urinary severity and other clinical manifestations. The results of this MAPP Network biomarker study provide important insights for addressing such long term goals through demonstrating that males with UCPPS had significantly higher normalized protein concentration of VEGF, VEGF-R1, and MMP-9 than healthy male controls. Furthermore, males with UCPPS had significantly higher concentrations of VEGF than female healthy controls. Additionally, pain and urinary symptoms were found to be positively associated with the MMP9/NGAL complex in women.
While the underlying mechanisms of UCPPS are not understood, inflammation, abnormal vascular activation, fibrosis and extracellular matrix remodeling have all been suggested to be contributing factors. Increased levels of urinary VEGF, as well as MMP-9, detected in UCPPS may reflect the upregulation of these pathways in patients. For instance, VEGF is known to regulate endothelial activation via upregulation of MMP-2 and MMP-9 in several cell types. MMP-2, in turn, may regulate VEGF expression on a transcriptional level via an integrin/PI3-kinase-dependent pathway 41. Increased VEGF has been reported in IC/BPS and VEGF levels were reduced after onabotulinumtoxinA injections and hydrodistention suggesting that VEGF may contribute to the pathogenesis 42. During angiogenesis, endothelial cells are guided into a vascular area by macromolecules such as VEGF. VEGF binds and activates 2 tyrosine kinase receptors (VEGF-R1 and VEGF-R2) and plays a role in migration of inflammatory cells and inflammation-induced lymphangiogenesis and angiogenesis 43. Of importance in UCPPS may be the relationship of VEGF to hypoxia and ischemia, a stimulus for angiogenesis 44, 45. A relative ischemia in the bladder of IC/BPS patients during filling compared to controls has been reported 46 and the density of blood vessels in the bladder subepithelium of IC/BPS patients is decreased 47. In parallel to UCPPS in humans, animal models of chronic bladder ischemia from partial bladder outlet obstruction results in increased VEGF expression 48. Bladder epithelial cells taken from patients with overactive bladder symptoms (urinary frequency and urgency), as opposed to asymptomatic patients, respond to stretch stimuli by a 1.5 fold increase in hypoxia-inducible factors 1α and 2α and a greater than 3-fold increase in VEGF 49. Similarly, expression of HIF-1a and VEGF are increased in the umbrella cells of IC/BPS patients. Finally, macrophages stimulated in low oxygen environment also secrete soluble VEGR1 50. Identifying the factors that contribute to hypoxia/ischemia in UCPPS could reveal potential therapeutic targets.
In a murine stress model, in addition to VEGF release, bladder vascular permeability was also increased 51. This relationship may explain the association of worsening symptoms by stress in patients with IC/BPS 52, 53. Corticotropin releasing hormone stimulates mast cells to release newly synthesized VEGF which causes vasodilation 54. The effect is mediated through CRH receptor 2 (CRH-R2) 51 and elevated VEGF levels may explain this association. In men, receptors for VEGF are present not only on endothelial cells, but also in prostate cancer and glandular and basal cells in BPH tissue specimens 53–56. In prostate cancer cell lines, inflammation results in increased levels of MMP-9 57. VEGF is also an immunosuppressive cytokine 58, 59.
In addition to roles in inflammation and angiogenesis, VEGFR1 and MMP-9 are implicated in pain regulation. VEGFR1 contributes to modulating the response to VEGFR2 58 and has recently been described to influence pain. In mouse models, injection of VEGFA produces no spontaneous pain response, but animals develop significant hypersensitivity to graded von Frey filaments and noxious heat stimuli. This response was blocked by neutralizing antibodies to VEGFR1 but not by antibodies against VEGFR2. Recently, it has been reported that ligands of the VEGF family can augment pain sensitivity through selective activation of VEGFR1 expressed in sensory neurons in human cancer and mouse models 60. In these studies, systemic blockade of VEGFR1 prevented tumor-induced nerve remodeling and reduced cancer pain, suggesting that the VEGF/VEGFR1 targeting by antiangiogenic therapies could provide a palliative effect in these models 60.
MMP-9 plays a role in regulating the acute phase of neuropathic pain. MMP2 is constitutively expressed in many tissues whereas MMP-9 is inducible 61. After sciatic nerve ligation, MMP-9 is induced in less than one day and inhibition of MMP-9 attenuated neuropathic pain at day 1 62. The mechanism of MMP-9 sensitization is through activation of IL-1β and microglia 61. Increased MMP-9 levels could reflect ongoing acute neurogenic inflammation.
Another possible contributing factor is the presence of microorganisms. The NIH sponsored CPCRN found that significant bacteria were present in men with CP/CPPS but also in asymptomatic controls 63. Although prior history of urinary tract infection is more common in women with IC/PBS from childhood 64, bacteria are not usually found in the bladder 65. In the MAPP study, in women with IC/PBS, it was shown that gram negative bacteria bind the TLR-4 receptor and the response to TLR-4 stimulation correlated with pain 66. Exposure of prostate epithelial cells to LPS, on the outer membrane of gram negative bacteria, resulted in TLR-4-mediated release of both TGFβ1 and VEGF through activation of NF-κB 67. Taken together, specific bacterial stimulation in the proper setting could contribute to increased levels of VEGF regardless of gender.
In the present study, females had increased urinary MMP-9/NGAL complex in correlation with pain and urinary symptoms. The finding of elevated MMP-9/NGAL complex suggests a possible link to bacterial infection. NGAL is rapidly and locally produced in the urinary tract by TLR receptors in response to bacterial exposure 68. NGAL is involved in protection from bacterial infection by competing for iron with the bacteria and selecting for bacteria with alternative chemistry of siderophores used for iron acquisition 69. A recurring theme in patients with IC/BPS is a history of urinary tract infection that was treated, but had persistence of symptoms including pain. Elevated MMP-9/NGAL may signal bacterial involvement in pain and urinary symptoms development. NGAL may also be a marker for ongoing inflammation and perhaps apoptosis.
Interestingly, our analyses demonstrated variability in the utility of particular protein markers depending on whether concentration values or concentration normalized to total protein values were used. Both concentration and normalized concentration values were analyzed because particular proteins values may be independent of overall total protein concentration and, therefore, when normalized, their ability to discriminate is lost. A limitation of our study is the variation of duplicate measurements. CVs of ≤20% are desirable for duplicate laboratory values, and for MMP-2, MMP-9/NGAL complex, and VEGF-R1 CVs exceeded 50% while MMP-9, NGAL, and VEGF had CVs of 25.9%, 26.2%, and 23.5%, respectively. Normalized concentrations of MMP-9, VEGF and VEGF-R1 distinguished UCPPS men from healthy controls, but only total protein in the univariate analysis of normalized concentrations and symptom severity was associated with pain severity in men with UCPPS (Table 3a). Elevated levels of total urinary protein may be a reflection of more concentrated urine samples possibly due to decreased fluid intake to limit painful and frequent voiding. Specifically in men, total urinary protein may also reflect additional prostatic secretions that contribute to overall urinary protein. What remains unclear in most urinary biomarker studies, is whether a particular marker is a reflection of the local (bladder or prostate) versus overall systemic changes, or a combination. This lack of specificity may contribute to the variability associated with total urinary protein normalization as total urinary protein can also change based on local injury or inflammation or hydration status. While not all UCPPS patients demonstrate inflammation, these results support the convergence of inflammation, neuropathic pain and infection in UCPPS. Indeed, prior findings from the MAPP Network on the role of the TLR4 receptor response support these relationships 70.
The non-invasive nature of these biomarkers would allow for frequent and economical testing in a variety of settings and research studies and ultimately in a clinical setting if warranted. In addition to their potential to provide insights into underlying disease mechanisms, these biomarkers may potentially prove useful as targets for development of novel interventions, pending future evaluation. Indeed, FDA-approved drugs that target some of these proteins, such as VEGF, are currently in clinical use 58. Such markers, once sufficiently validated, and may provide objective correlates to patient-reported symptoms and enhance strategies for multiplexing diverse phenotypic information to predict symptom progression and response to therapy for UCPPS patient sub-groups. Ongoing characterizations of these and other biomarkers are focused on validation and further assessment of relationships with clinical symptoms in a longitudinal study design.
Supplementary Material
Acknowledgments
Funding for the MAPP Research Network was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) (DK82370, DK82342, DK82315, DK82344, DK82325, DK82345, DK82333, and DK82316). Funding for the present study was obtained as part of this cooperative agreement from the NIDDK, NIH (DK103227).
Footnotes
ClinicalTrials.gov Identifier: NCT01098279
Conflicts of Interest
The authors have nothing to declare.
References
- 1.Clemens JQ, Mullins C, Kusek JW, et al. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol. 2014;14:57. doi: 10.1186/1471-2490-14-57. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landis JR, Williams DA, Lucia MS, et al. The MAPP research network: design, patient characterization and operations. BMC Urol. 2014;14:58–75. doi: 10.1186/1471-2490-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiuchi H, Tsujimura A, Takao T, et al. Increased vascular endothelial growth factor expression in patients with bladder pain syndrome/interstitial cystitis: its association with pain severity and glomerulations. BJU Int. 2009;104:826–831. doi: 10.1111/j.1464-410X.2009.08467.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee JD, Lee MH. Increased expression of hypoxia-inducible factor-1alpha and vascular endothelial growth factor associated with glomerulation formation in patients with interstitial cystitis. Urology. 2011;78:971, e11–e15. doi: 10.1016/j.urology.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 5.Moses MA, Harper J. The Regulation of Tumor Angiogenesis: From the Angiogenic Switch Through Tumor Progression. In: Bignold LP, editor. Cancer: Cell Structures, Carcinogens and Tumor Pathogenesis. EXS Basel; Birkhauser: 2006. pp. 223–268. [Google Scholar]
- 6.Senger DR, Perruzzi CA, Feder J, et al. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. [PubMed] [Google Scholar]
- 7.Nishijima S, Sugaya K, Kadekawa K, et al. High-dose tranilast administration to rats creates interstitial cystitis-like symptoms with increased vascular permeability. Life Sci. 2013;93:897–903. doi: 10.1016/j.lfs.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Rooney P, Srivastava A, Watson L, et al. Hyaluronic acid decreases IL-6 and IL-8 secretion and permeability in an inflammatory model of interstitial cystitis. Acta Biomater. 2015;19:66–75. doi: 10.1016/j.actbio.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 9.Rudick CN, Chen MC, Mongiu AK, et al. Organ cross talk modulates pelvic pain. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1191–R1198. doi: 10.1152/ajpregu.00411.2007. [DOI] [PubMed] [Google Scholar]
- 10.Homma Y, Nomiya A, Tagaya M, Oyama T, Takagaki K, Nishimatsu H, Igawa Y. Increased mRNA expression of genes involved in pronociceptive inflammatory reactions in bladder tissue of interstitial cystitis. J Urol. 2012;190(5):1925–1931. doi: 10.1016/j.juro.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 11.Corcoran AT, Yoshimura N, Tyagi V, Jacobs B, Leng W, Tyagi P. Mapping the cytokine profile of painful bladder syndrome/interstitial cystitis in human bladder and urine specimens. World J Urol. 2013;31(1):241–246. doi: 10.1007/s00345-012-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy SF, Schaeffer AJ, Thumbikat P. Immune mediators of chronic pelvic pain syndrome. Nat Rev Urol. 2014;11(5):259–69. doi: 10.1038/nrurol.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dufour A. Degradomics of matrix metalloproteinases in inflammatory diseases. Front Biosci (Schol Ed) 2015;7:150–167. doi: 10.2741/S430. [DOI] [PubMed] [Google Scholar]
- 14.Jia D, Roy R, Moses MA. MMPs in Biology and Medicine. In: Sagi I, Gaffney J, editors. Matrix Metalloproteinase Biology, Chapt 10. New York: John Wiley and Sons, Inc; 2014. pp. 183–213. 2014. [Google Scholar]
- 15.Roy R, Zhang B, Moses MA. Making the cut: protease-mediated regulation of angiogenesis. Exp Cell Res. 2006;312:608–622. doi: 10.1016/j.yexcr.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Yan L, Borregaard N, Kjeldsen L, et al. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP9 activity by NGAL. J Biol Chem. 2001;276:37258–37265. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B, Yan L, Tsang PC, et al. Matrix metalloproteinase-2 (MMP-2) expression and regulation by tumor necrosis factor alpha (TNFalpha) in the bovine corpus luteum. Mol Reprod Dev. 2005;70:122–132. doi: 10.1002/mrd.20196. [DOI] [PubMed] [Google Scholar]
- 18.Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007;(6):1375–1381. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]
- 19.Hall CJ, Boyle RH, Sun X, Wicker SM, Misa JP, Krissansen GW, Print CG, Crosier KE, Crosier PS. Epidermal cells help coordinate leukocyte migration during inflammation through fatty acid-fuelled matrix metalloproteinase production. Nat Commun. 2014;5:3880. doi: 10.1038/ncomms4880. [DOI] [PubMed] [Google Scholar]
- 20.Song J, Wu C, Zhang X, Sorokin LM. In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1β-induced peritonitis. J Immunol. 2013;190(1):401–10. doi: 10.4049/jimmunol.1202286. [DOI] [PubMed] [Google Scholar]
- 21.Moore BA, Manthey CL, Johnson DL, Bauer AJ. Matrix metalloproteinase-9 inhibition reduces inflammation and improves motility in murine models of postoperative ileus. Gastroenterology. 2011;141(4):1283–1292. doi: 10.1053/j.gastro.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakraborty S, Kaur S, Guha S, et al. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826:129–169. doi: 10.1016/j.bbcan.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Chan YR. Lipocalin 2 regulation and its complex role in inflammation and cancer. Cytokine. 2011;56:435–441. doi: 10.1016/j.cyto.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Sickinger S, Maier H, Konig S, et al. Lipocalin-2 as mediator of chemokine expression and granulocyte infiltration during ischemia and reperfusion. Transpl Int. 2013;26:761–769. doi: 10.1111/tri.12116. [DOI] [PubMed] [Google Scholar]
- 25.Warszawska JM, Gawish R, Sharif O, et al. Lipocalin 2 deactivates macrophages and worsens pneumococcal pneumonia outcomes. J Clin Invest. 2013;123:3363–3372. doi: 10.1172/JCI67911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Wu Y, Zhang Y, et al. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol Endocrinol. 2008;22:1416–1426. doi: 10.1210/me.2007-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Bielenberg DR, Rodig SJ, et al. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci U S A. 2009;106:3913–3918. doi: 10.1073/pnas.0810617106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, McNeish B, Butterfield C, et al. Lipocalin 2 is a novel regulator of angiogenesis in human breast cancer. FASEB J. 2013;27:45–50. doi: 10.1096/fj.12-211730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coticchia CM, Curatolo AS, Zurakowski D, et al. Urinary MMP-2 and MMP9 predict the presence of ovarian cancer in women with normal CA125 levels. Gynecol Oncol. 2011;123:295–300. doi: 10.1016/j.ygyno.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez CA, Yan L, Louis G, et al. The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin Cancer Res. 2005;11:5390–5395. doi: 10.1158/1078-0432.CCR-04-2391. [DOI] [PubMed] [Google Scholar]
- 31.Smith ER, Zurakowski D, Saad A, et al. Urinary biomarkers predict brain tumor presence and response to therapy. Clin Cancer Res. 2008;14:2378–2381. doi: 10.1158/1078-0432.CCR-07-1253. [DOI] [PubMed] [Google Scholar]
- 32.Roy R, Moses MA. ADAM12 induces estrogen-independence in breast cancer cells. Breast Cancer Res Treat. 2012;131:731–741. doi: 10.1007/s10549-011-1431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith ER, Manfredi M, Scott RM, et al. A recurrent craniopharyngioma illustrates the potential usefulness of urinary matrix metalloproteinases as noninvasive biomarkers: case report. Neurosurgery. 2007;60:E1148–E1149. doi: 10.1227/01.NEU.0000255464.37634.3C. [DOI] [PubMed] [Google Scholar]
- 34.Clemens JQ, Calhoun EA, Litwin MS, McNaughton-Collins M, Kusek JW, Crowley EM. Landis JR and Urologic Pelvic Pain Collaborative Research Network Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology. 2009;74:983–987. doi: 10.1016/j.urology.2009.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry SH, Bogart LM, Pham C, Liu K, Nyberg L, Stoto M, Suttorp M, Clemens JQ. Development, validation and testing of an epidemiological case definition of interstitial cystitis/painful bladder syndrome. J Urol. 2010;183:1848–1852. doi: 10.1016/j.juro.2009.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cleeland C. The brief pain inventory user guide. 2009. Charles S Cleeland; Houston, TX Google Scholar: 2014. [Google Scholar]
- 37.O'Leary MP, Sant GR, Fowler FJ, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49:58–63. doi: 10.1016/s0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- 38.Griffith JW, Stephens-Shields AJ, Hou X, et al. Pain and urinary symptoms should not be combined into one score: Psychometric findings from the Multi-disciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. J Urol. 2015;195:949–954. doi: 10.1016/j.juro.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 40.Clemens JQ, Clauw DJ, Kreder K, et al. Comparison of baseline urological symptoms in men and women in the MAPP research cohort. J Urol. 2015;193:1554–1558. doi: 10.1016/j.juro.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desch A, Strozyk EA, Bauer AT, et al. Highly invasive melanoma cells activate the vascular endothelium via an MMP-2/integrin alphavbeta5-induced secretion of VEGF-A. Am J Pathol. 2012;181:693–705. doi: 10.1016/j.ajpath.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Peng CH, Jhang JF, Shie JH, et al. Down regulation of vascular endothelial growth factor is associated with decreased inflammation after intravesical OnabotulinumtoxinA injections combined with hydrodistention for patients with interstitial cystitis--clinical results and immunohistochemistry analysis. Urology. 2013;82:1452, e1–6. doi: 10.1016/j.urology.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Saban R, Saban MR, Maier J, et al. Urothelial expression of neuropilins and VEGF receptors in control and interstitial cystitis patients. Am J Physiol Renal Physiol. 2008;295:F1613–F1623. doi: 10.1152/ajprenal.90344.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harper J, Yan L, Loureiro RM, et al. Repression of vascular endothelial growth factor expression by the zinc finger transcription factor ZNF24. Cancer Res. 2007;67:8736–8741. doi: 10.1158/0008-5472.CAN-07-1617. [DOI] [PubMed] [Google Scholar]
- 45.Marti HH, Risau W. Angiogenesis in ischemic disease. Thromb Haemost. 1999;82:44–52. [PubMed] [Google Scholar]
- 46.Pontari MA, Hanno PM, Ruggieri MR. Comparison of bladder blood flow in patients with and without interstitial cystitis. J Urol. 1999;162:330–334. [PubMed] [Google Scholar]
- 47.Rosamilia A, Cann L, Dwyer P, et al. Bladder microvasculature in women with interstitial cystitis. J Urol. 1999;161:1865–1870. [PubMed] [Google Scholar]
- 48.Ghafar MA, Anastasiadis AG, Olsson LE, et al. Hypoxia and an angiogenic response in the partially obstructed rat bladder. Lab Invest. 2002;82:903–909. doi: 10.1097/01.lab.0000021135.87203.92. [DOI] [PubMed] [Google Scholar]
- 49.Christiaansen CE, Sun Y, Hsu YC, et al. Alterations in expression of HIF-1alpha, HIF-2alpha, and VEGF by idiopathic overactive bladder urothelial cells during stretch suggest role for hypoxia. Urology. 2011;77:1266 e7–1266 e11. doi: 10.1016/j.urology.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roda JM, Sumner LA, Evans R, et al. Hypoxia-inducible factor-2alpha regulates GM-CSF-derived soluble vascular endothelial growth factor receptor 1 production from macrophages and inhibits tumor growth and angiogenesis. J Immunol. 2011;187:1970–1976. doi: 10.4049/jimmunol.1100841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boucher W, Kempuraj D, Michaelian M, et al. Corticotropin-releasing hormone-receptor 2 is required for acute stress-induced bladder vascular permeability and release of vascular endothelial growth factor. BJU Int. 2010;106:1394–1399. doi: 10.1111/j.1464-410X.2010.09237.x. [DOI] [PubMed] [Google Scholar]
- 52.Lutgendorf SK, Kreder KJ, Rothrock NE, et al. Stress and symptomatology in patients with interstitial cystitis: a laboratory stress model. J Urol. 2000;164:1265-–1269. [PubMed] [Google Scholar]
- 53.Ullrich PM, Turner JA, Ciol M, et al. Stress is associated with subsequent pain and disability among men with nonbacterial prostatitis/pelvic pain. Ann Behav Med. 2005;30:112–118. doi: 10.1207/s15324796abm3002_3. [DOI] [PubMed] [Google Scholar]
- 54.Cao J, Papadopoulou N, Kempuraj D, et al. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 55.Ferrer FA, Miller LJ, Lindquist R, et al. Expression of vascular endothelial growth factor receptors in human prostate cancer. Urology. 1999;54:567–572. doi: 10.1016/s0090-4295(99)00156-9. [DOI] [PubMed] [Google Scholar]
- 56.Hahn D, Simak R, Steiner GE, et al. Expression of the VEGF-receptor Flt-1 in benign, premalignant and malignant prostate tissues. J Urol. 2000;164:506–510. [PubMed] [Google Scholar]
- 57.Debelec-Butuner B, Alapinar C, Varisli L, et al. Inflammation-mediated abrogation of androgen signaling: an in vitro model of prostate cell inflammation. Mol Carcinog. 2014;53:85–97. doi: 10.1002/mc.21948. [DOI] [PubMed] [Google Scholar]
- 58.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 59.Gottfried E, Kreutz M, Mackensen A. Tumor-induced modulation of dendritic cell function. Cytokine Growth Factor Rev. 2008;19:65–77. doi: 10.1016/j.cytogfr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 60.Selvaraj D, Gangadharan V, Michalski CW, et al. A Functional Role for VEGFR1 Expressed in Peripheral Sensory Neurons in Cancer Pain. Cancer Cell. 2015;27:780–796. doi: 10.1016/j.ccell.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji RR, Xu ZZ, Wang X, et al. Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol Sci. 2009;30:336–340. doi: 10.1016/j.tips.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawasaki Y, Xu ZZ, Wang X, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14 doi: 10.1038/nm1723. 331- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nickel JC, Alexander RB, Schaeffer AJ, et al. Leukocytes and bacteria in men with chronic prostatitis/chronic pelvic pain syndrome compared to asymptomatic controls. J Urol. 2003;170:818–822. doi: 10.1097/01.ju.0000082252.49374.e9. [DOI] [PubMed] [Google Scholar]
- 64.Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73:258–262. doi: 10.1016/j.urology.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 65.Al-Hadithi HN, Williams H, Hart CA, Frazer M, Adams EJ, Richmond DH, Tincello DG. Absence of bacterial and viral DNA in bladder biopsies from patients with interstitial cystitis/chronic pelvic pain syndrome. J Urol. 2005;174:151–154. doi: 10.1097/01.ju.0000161605.14804.a9. [DOI] [PubMed] [Google Scholar]
- 66.Schrepf A, O'Donnell M, Luo Y, et al. Inflammation and inflammatory control in interstitial cystitis/bladder pain syndrome: Associations with painful symptoms. Pain. 2014;155:1755–1761. doi: 10.1016/j.pain.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pei Z, Li H, Guo Y, et al. Sodium selenite inhibits the expression of VEGF, TGFbeta(1) and IL-6 induced by LPS in human PC3 cells via TLR4-NF-(K)B signaling blockage. Int Immunopharmacol. 2010;10:50–56. doi: 10.1016/j.intimp.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 68.Steigedal M, Marstad A, Haug M, et al. Lipocalin 2 imparts selective pressure on bacterial growth in the bladder and is elevated in women with urinary tract infection. J Immunol. 2014;193:6081–6089. doi: 10.4049/jimmunol.1401528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 70.Schrepf A, Bradley CS, O’Donnell M, et al. Toll-like receptor 4 and comorbid pain in Interstitial Cystitis/Bladder Pain Syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Brain Behav Immun. 2015;49:66–74. doi: 10.1016/j.bbi.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.