Abstract
Regulator of G protein signaling 14 (RGS14) is a multifunctional signaling protein primarily expressed in mouse pyramidal neurons of hippocampal area CA2 where it regulates synaptic plasticity important for learning and memory. However, very little is known about RGS14 protein expression in the primate brain. Here, we validate the specificity of a new polyclonal RGS14 antibody that recognizes not only full length RGS14 protein in primate, but also lower molecular weight forms of RGS14 protein matching previously predicted human splice variants. These putative RGS14 variants along with full-length RGS14 are expressed in the primate striatum. By contrast, only full length RGS14 is expressed in hippocampus, and shorter variants are completely absent in rodent brain. We report that RGS14 protein immunoreactivity is found both pre- and postsynaptically in multiple neuron populations throughout hippocampal area CA1 and CA2, caudate nucleus, putamen, globus pallidus, substantia nigra, and amygdala in adult rhesus monkeys. A similar cellular expression pattern of RGS14 in the monkey striatum and hippocampus was further confirmed in humans. Our electron microscopy data show for the first time that RGS14 immunostaining localizes within nuclei of striatal neurons in monkeys. Taken together, these findings suggest new pre- and postsynaptic regulatory functions of RGS14 and RGS14 variants, specific to the primate brain, and provide evidence for unconventional roles of RGS14 in the nuclei of striatal neurons potentially important for human neurophysiology and disease.
Keywords: RGS14, human, hippocampus, basal ganglia, amygdala, nucleus
INTRODUCTION
Much of neurotransmission is mediated through receptor and heterotrimeric G protein (Gαβγ) signaling near synapses, a process that is tightly regulated by a family of regulators of G protein signaling (RGS) (Gerber et al. 2016) that facilitate the termination of Gαβγ signaling (Brown et al. 2016; Hollinger and Hepler 2002; Ross and Wilkie 2000). RGS proteins comprise a diverse family of signaling molecules that contain various modular domains, but are defined by a conserved RGS domain that serves as a GTPase activating protein (GAP) to catalyze the conversion of active Gα-GTP into inactive Gα-GDP (Hollinger and Hepler 2002; Willars 2006) and limit neurotransmitter signaling. One such RGS family member, RGS14, regulates long-term potentiation (LTP) and synaptic plasticity in mouse pyramidal neurons of hippocampal area CA2 (Lee et al. 2010). RGS14 specifically binds and regulates Gαi/o proteins (Hollinger et al. 2001; Traver et al. 2000) and contains additional signaling domains, including tandem Ras/Rap binding domains and a G protein regulatory (GPR; also known as GoLoco, or GL) motif. The first Ras/Rap binding domain (R1) binds active H-Ras-GTP (Lee et al. 2010; Shu et al. 2010; Vellano et al. 2013; Willard et al. 2009) and active Rap2A-GTP (Mittal and Linder 2006; Traver et al. 2000), which have opposing roles in the expression of LTP in the hippocampus (Fu et al. 2007; Qin et al. 2005; Ryu et al. 2008; Zhu et al. 2002; Zhu et al. 2005). Though at present, it is currently unknown how RGS14 regulates the balance between these two pathways. While the RGS domain binds active Gαi/o-GTP to catalyze the conversion to Gαi/o-GDP, the GPR motif binds inactive Gαi1/3-GDP (Mittal and Linder 2004). Although the GPR motif was previously reported as a guanine nucleotide dissociation inhibitor (Kimple et al. 2001; Kimple et al. 2004), more advanced techniques have revealed that it does not prolong Gαi1-GDP lifetime (Brown et al. 2016), but more likely regulates RGS14 membrane localization (Shu et al. 2007). Of note, RGS14 is capable of interacting with multiple partners simultaneously (Brown et al. 2015; Mittal and Linder 2006), suggesting that its primary cellular function may be beyond that of a GAP.
Our earlier work reported that RGS14 protein is expressed in hippocampal area CA2 of mouse brain (Evans et al. 2014; Lee et al. 2010), a small, enigmatic substructure of the hippocampus located between areas CA1 and CA3. While CA3 Schaffer collateral inputs into CA1 can undergo LTP in response to high frequency stimulation (Florian and Roullet 2004), LTP cannot be induced in CA3 Schaffer collateral projections onto CA2 under the same conditions (Simons et al. 2012; Zhao et al. 2007). However, full expression of LTP at CA3-CA2 Schaffer collateral synapses can be induced by high frequency stimulation upon deletion of RGS14 in mouse. Furthermore, RGS14 knockout mice perform better in a spatial learning task than their wild type counterparts, indicating that RGS14 is a natural suppressor of hippocampal-based learning and memory through blockade of LTP at Schaffer collateral synapses in the mouse CA2 region (Lee et al. 2010). The restoration of LTP in CA2 by RGS14 ablation was blocked by a selective MEK inhibitor, indicating that RGS14 may suppress LTP by regulating signaling through Ras. Consistent with this idea, RGS14 has been shown to inhibit H-Ras/ERK signaling (Shu et al. 2010). Although RGS14’s role in hippocampal area CA2 is well-established, our understanding of its role and expression outside of the hippocampus remains largely unexplored.
Within the mouse brain, RGS14 protein and mRNA expression is mostly confined to CA2, and behavioral tasks in RGS14 knockout animals confirm its role in hippocampal-mediated functions. We recently characterized a new monoclonal antibody against rodent RGS14, and discovered that hippocampal RGS14 in mice is absent at P0, first detected at P7, and reaches its full expression by early adulthood (Evans et al. 2014). However, we also noted modest expression of RGS14 in areas outside of CA2, including the piriform cortex, layers II, III, and V of the neocortex, and areas associated with olfaction. Additionally, various reports have suggested RGS14 may be expressed more broadly in the monkey and human brain (Grafstein-Dunn et al. 2001; Larminie et al. 2004; Lopez-Aranda et al. 2006). Although the Allen Mouse Brain Atlas (http://mouse.brain-map.org) confirms that RGS14 mRNA expression is strongest in the hippocampal CA2 region, mRNA expression atlases for human and non-human primate brains (http://human.brain-map.org and http://www.blueprintnhpatlas.org) report RGS14 transcript not only in CA2, but also in the caudate nucleus and putamen. If RGS14 protein is indeed expressed in regions other than CA2, the functions of RGS14 likely extend beyond the regulation of LTP and hippocampal learning in the primate brain as well. In light of reports of other RGS proteins (RGS2, 4, 7, and 9) known to regulate a broad spectrum of synaptic signaling that may be affected in various psychiatric diseases (Amstadter et al. 2009a; Amstadter et al. 2009b; Gerber et al. 2016; Gold et al. 1997; Han et al. 2006; Hohoff et al. 2015; Ingi and Aoki 2002; Koenen et al. 2009; Labouebe et al. 2007; Lifschytz et al. 2012; Okimoto et al. 2012; Rahman et al. 1999; Seeman et al. 2007), an in-depth knowledge of the pattern of RGS14 protein expression and subcellular localization in the primate brain is needed.
With this goal in mind, the present study characterizes a new specific RGS14 polyclonal antibody that recognizes primate variants of RGS14 and uses this antibody in combination with light and electron microscopy to define in detail the cellular and subcellular localization of RGS14 protein in the human and monkey brain. Our findings demonstrate that RGS14 protein immunoreactivity displays a much broader distribution in the primate brain than in the mouse brain. In addition to being strongly expressed in CA2 and CA1 regions of the hippocampus, robust pre- and postsynaptic labeling was found in basal ganglia nuclei including the caudate nucleus, putamen, globus pallidus, and substantia nigra pars reticulata. Our data also provide evidence for the existence of RGS14 splice variants and their expression in the nucleus of striatal neurons. Altogether, our findings suggest potentially new and diverse functions of RGS14 and RGS14 variants in the primate brain that extend beyond hippocampal learning and memory.
MATERIALS and METHODS
RGS14 Constructs and Materials
RGS14 purification
Full-length human RGS14 (Uniprot O43566) was cloned into a pLic-GST vector (pLic-GST-RGS14) and expressed in BL21 (DE3) Escherichia coli. Bacterial lysate was passed through a glutathione affinity column, washed with phosphate buffered saline, and bead-bound RGS14 purity was verified by coomassie-stained polyacrylamide gel. Rat RGS14 (Uniprot O08773) was purified as described previously (Brown et al. 2015). Briefly, full-length RGS14 was cloned into a pLic-MBP vector with a hexahistidine (H6) tag and a tobacco etch virus (TEV) cleavage site (H6-MBP-TEV-RGS14). Lysate was passed through a Ni2+ affinity column, cleaved overnight at 4°C with 1:200 TEV protease:RGS14, and finally purified on S75/S200 tandem sizing columns.
RGS14 polyclonal antibody
The RGS14 polyclonal antibody was purchased from Proteintech (Rosemont, Il) and stored at −20°C until use, and then 4°C after. The immunogen used to generate this antibody is described by Proteintech as KSLPLGVEELGQLPPVEGPGGRPLRKSFRRELGGTANAALRRESQGSLNSSASLDLGFLAFVSSKSESHRKSLGSTEGESESRPGKYCCVYLPDGTASLALARPGLTIRDMLAGICEKRGLSLPDIKVYLVGNEQALVLDQDCTVLADQEVRLENRITFELELTALERVVRISAKPTKRLQEALQPILEKHGLSPLEVVLHRPGEKQPLDLGKLVSSVAAQRLVLDTLPGVKISKARDKSPCRSQGCPPRTQDKATHPPPASPSSLVKVPSSATGKRQTCDIEGLVELLNRVQSSGAHDQRGLLRKEDLVLPEFLQLPAQGPSSEETPPQTKSAAQPIGGSLNSTTDSAL, which is amino acid 217-566 of human RGS14.
Immunoblotting
Antibody characterization
HEK293 cells were maintained in Dulbecco’s modified eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin. Truncation mutants in rat FLAG-RGS14 were generated as described previously (Evans et al. 2014; Shu et al. 2007). Cells were transfected in 5% FBS DMEM for 24 hours, then lysed and run on an 11% acrylamide gel at 150V for 2 hours, and transferred overnight onto nitrocellulose membranes. RGS14 antibody was incubated at 1:500 in 5% milk for 2 hours at room temperature, then washed for 30 minutes in 0.1% Tween-TBS. Anti-rabbit secondary antibody conjugated to HRP was incubated for 1 hour at room temperature, and washed for another 30 minutes in 0.1% Tween-TBS. Finally, membranes were incubated with ECL and exposed to X-ray film.
Pre-adsorption immunoblotting
Tissue punches containing RGS14 were taken from hippocampus and striatum of one adult wild-type mouse, and RGS14 expression was verified in this tissue as well as three additional mice by light microscopy (data not shown). Similarly, RGS14-containing tissue punches of hippocampus and striatum were taken from one adult rhesus macaque, and RGS14 expression verified in this tissue as well as three additional monkeys by light microscopy as described below. Tissue samples were lysed with 50mM Tris, 150mM NaCl, 1mM EDTA, 2mM DTT, 5mM MgCl2, 1% Triton X-100, and protease inhibitors (Roche, Cat# 04693159001). 50 μg of homogenate was loaded into an 11% acrylamide gel and run at 120V for 2 hours, and transferred overnight onto nitrocellulose membranes. 3.3 μg of RGS14 antibody (Proteintech, Cat# 16258-1-AP) was incubated with 33 μg of purified rat RGS14 (Uniprot O08773) and 33 μg of bead-bound purified human RGS14 (Uniprot O43566) overnight in 250 μL TBS. Membranes were incubated in 1:1000 RGS14 antibody, or 1:1000 pre-adsorbed RGS14 antibody in 5% milk for 2 hours. Membranes were washed for 30 minutes in 0.1% Tween-TBS. Secondary antibody (goat anti-rabbit, HRP-conjugated) at 1:25,000 in 0.1% Tween-TBS was incubated for 1 hour at room temperature. Membranes were washed for another 30 minutes in 0.1% Tween-TBS, then incubated with ECL and exposed to X-ray film.
Animals
All animal housings and procedures were approved by the Emory University Institutional Animal Care and Use Committee (IACUC) and all procedures were approved by IACUC protocol. C57BL/6J mouse tissue used in this study was collected following deep anesthesia with isofluorane and rapid decapitation. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
The non-human primate tissue was collected from three adult (5–7 years old) rhesus monkeys (2 females, 1 male) from the Yerkes National Primate Center colony. After deep anesthesia with pentobarbital (100 mg/kg), the monkeys were transcardially perfused with an oxygenated Ringer solution followed by 2 liters of fixative (4% paraformaldehyde/0.1% glutaraldehyde in phosphate buffer 0.1M, pH 7.4). After perfusion, the brains were cut in 10 mm-thick blocks in the stereotaxic plane and taken out from the skull. They were then post-fixed for 24 hours in 4% paraformaldehyde at 4°C. Following postfixation, the brains were cut in 60 μm-thick sections with a vibrating microtome. Sections were serially collected and stored in an anti-freeze solution (1.4% NaH2PO4-H20, 2.6% Na2HPO4-7H2O, 30% ethylene glycol, 30% glycerol dissolved in distilled water) at −20°C until further use.
Light Microscopy
Monkey tissue
Series of sections (1/24) through the whole brain of three rhesus monkeys were processed to localize RGS14 immunostaining at the light microscopic level as follows: Before any antibody incubations, all sections were treated with a 1% sodium borohydride/PBS solution for 20 minutes and washed in PBS. This was followed by a pre-incubation for 1 hour in a solution containing 1% normal goat serum, 0.3% Triton-X-100, and 1% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO) in PBS. Sections were then incubated for 24 hours at room temperature in a solution containing the rabbit anti-RGS14 antibody (1:4000 dilution) in 1% normal goat serum, 0.3% Triton-X-100, and 1% BSA in PBS. On the following day and after PBS rinses, sections were incubated in a PBS solution containing (secondary) biotinylated horse anti-rabbit IgGs (1:200 dilution; Vector Laboratories, Burlingame, CA) combined with 1% normal goat serum, 0.3% Triton-X-100, and 1% BSA for 90 minutes at room temperature. Sections were exposed to an avidin-biotin-peroxidase complex (ABC; 1:100 dilution, Vector Laboratories) for 90 minutes followed by rinses in PBS and Tris buffer (50mM; pH 7.6). Sections were then incubated with a solution containing 0.025% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich), 10 mM imidazole (Fisher Scientific, Pittsburgh, PA), and 0.006% hydrogen peroxide in Tris buffer for 10 minutes at room temperature. The sections were mounted on slides, air-dried, and dehydrated with increasing dilutions of ethanol followed by xylene, before being being coverslipped with Permount. A ScanScope light microscope (Aperio Technologies; Vista, CA) was used to image the RGS14-immunostained sections.
Human tissue
Post-mortem brain tissue from three individuals (source Emory Alzheimer’s Disease Research Center) with no known brain diseases was collected (average age = 81 ± 9.2 years, 2 females, 1 male). Tissue was collected between 5–7 hours post-mortem, fixed with 8% paraformaldehyde for 1–2 weeks, paraffin-embedded, and cut into 8 μm-thick sections on a microtome. Sections were immunolabeled with RGS14 antibody as follows. Endogenous peroxidase in tissue sections was blocked with 3% H2O2 in methanol for 5 minutes at 40°C. Sections were microwaved for 5 minutes in Citrate buffer (pH 6.0) and allowed to cool to room temperature for 30 minutes. For immunodetection, nonspecific reagent binding was blocked with normal goat serum in 0.1 M TRIS buffer for 15 minutes at 40°C. Sections were incubated in 1:200 RGS14 antibody (rabbit, Proteintech, Cat# 16258-1-AP) overnight at 4°C. After rinsing, sections were incubated for 30 minutes at 38°C in biotinylated secondary antibody (ABC Elite Kit, Vector Labs, Burlingame, CA), rinsed, incubated for 1 hour at 38°C in avidin-biotin complex (Vector Labs), and then developed with diaminobenzidine (DAB) (Vector Labs). Negative controls consist of sections incubated without primary antibody.
Electron Microscopy
Immunoperoxidase
Brain tissue sections from three rhesus macaque monkeys that included the striatum, globus pallidus, substantia nigra or hippocampus were prepared for immunoperoxidase localization of RGS14 at the electron microscopic (EM) level. The staining protocol was similar to that used for light microscopy, except that Triton-X-100 was omitted from incubation solutions. In brief, sections were first put in a cryoprotectant solution, followed by pre-incubation in a solution containing 1% normal goat serum and 1% BSA in PBS for 1 hour at room temperature, and then, a 48 hours incubation in the primary antibody solution containing rabbit anti-RGS14 antibody (1:4000 dilution). On the following day, sections were incubated for 90 minutes at room temperature in biotinylated goat anti-rabbit IgGs (1:200; Vector Laboratories), followed by a 90 minute incubation with the ABC complex (1:100, Vector Laboratories), and DAB (Sigma-Aldrich) processing for 10 minutes. After Phosphate Buffer (0.1 M, pH 7.4) rinses, the tissue underwent treatment with 1% osmium for 20 minutes and 1% uranyl acetate in 70% ethanol for 35 minutes, followed by dehydration with decreasing ethanol concentrations. Sections were placed in propylene oxide, embedded in epoxy resin (Durcupan ACM, Fluka, Buchs, Switzerland) for at least 12 hours, and baked in a 60°C oven for 48 hours. As controls, sections were incubated in a solution containing the pre-adsorbed RGS14 antibody prepared as described above. The rest of the immunostaining protocol remained the same as above.
Samples of regions of interest from the resin-embedded sections were cut and glued onto resin blocks, cut into 60 nm ultrathin sections (Leica Ultracut T2), and stained for 5 minutes with lead citrate for examination under the electron microscope (EM; model 1011, Jeol, Peabody, MA). Immunoreactive elements were digitally collected at 40,000x and 60,000x with a Gatan CCD camera (Model 785; Warrendale, PA) controlled by Digital Micrograph software (version 3.11.1). Preliminary electron microscopic observations were made from the striatum in the three monkeys to determine the animal with the best ultrastructural preservation. Representative electron microscopic data from the striatum, hippocampus and amygdala were then collected from this animal and shown in the present study.
Pre-embedding immunogold
Series of 3–5 sections at the level of the striatum or CA1 hippocampal region were processed for the pre-embedding immunogold procedure to further characterize the subcellular localization of RGS14 in these brain regions. After being processed with the cryoprotectant protocol (see above), these sections were pre-incubated in a PBS solution containing 5% milk for 30 min, followed by an overnight incubation at RT in the primary RGS14 antibody solution consisting of RGS14 antibody (1:4000 dilution) and 1% dry milk in TBS-gelatin buffer (0.02 M, 0.1% gelatin, pH 7.6). On the next day, sections were first incubated for 90 min with secondary goat anti-rabbit Fab’ fragments conjugated to 1.4-nm gold particles (1:100; Nanoprobes, Yaphank, NY) and 1% dry milk in TBS-gelatin to limit cross-reactivity of the secondary antibody. Sections underwent incubation for approximately 10 min in the dark with a HQ Silver Kit (Nanoprobes) to increase gold particle sizes to 30–50-nm through silver intensification, in order to optimize RGS14 visualization. The remaining of the electron microscopy procedure was the same as described above for the immunoperoxidase reaction and described in detail in our previous studies (Gonzales et al. 2013; Kuwajima et al. 2007; Mitrano et al. 2010).
RESULTS
Characterization of the antibody
We recently characterized a monoclonal antibody that recognizes the full-length rodent RGS14 protein to study its postnatal developmental expression in the mouse brain (Evans et al. 2014). We found that, while the mouse hippocampal area CA2 expression of RGS14 protein increases throughout postnatal development and peaks in adulthood, RGS14 expression can also be found in other brain structures, including the piriform cortex, olfactory regions, and in neocortical layers II, III, and V. Other reports have suggested RGS14 may be expressed both in and out of the hippocampus of monkeys and humans (Larminie et al. 2004; Lopez-Aranda et al. 2006). Until recently, technical barriers, including lack of an antibody that recognizes the full-length primate RGS14, have prevented a detailed analysis of RGS14 protein expression in brains of higher mammals. Here, we characterized and assessed the specificity of an RGS14 antibody commercially available from Proteintech (Rosemont, IL). This polyclonal antibody was generated from an immunogen containing the human sequence for the R1 domain through the C-terminus, and therefore was predicted to recognize both primate and rodent RGS14, which share an approximately 90% conserved sequence.
To determine the location of the epitope(s), we generated truncation constructs of rat FLAG-RGS14 (Fig. 1A). We expressed these mutants in HEK293 cells for 24 hours and then immunoblotted with the RGS14 polyclonal antibody, or a FLAG antibody. We found that constructs containing the R1, R2, and GPR domains, but not those containing only the RGS domain, were recognized by the RGS14 antibody, while the FLAG antibody detected even expression across constructs (Fig. 1B). Thus, the RGS14 antibody recognizes the part of the protein that matches the immunogen used to generate it (Fig. 1C). RGS domains have relatively high sequence conservation, however this immunogen lacks the RGS domain and was therefore predicted to be highly specific to RGS14 over all other RGS proteins. To validate this, we expressed RGS14, RGS10, RGS12 (trans-spliced), RGS2, RGS4, and RGS16 in HEK293 cells, and separated lysates alongside mouse hippocampal lysate by gel electrophoresis (Fig. 2). While expression of each construct was verified by blotting for each respective tag (Fig. 2, bottom), the RGS14 antibody recognized only native RGS14 from mouse brain (Fig. 2, lane 1) and recombinant RGS14 (Fig. 2, lane 2)—validating the specificity of the antibody. Though the RGS14 sequence is approximately 90% conserved between rat, mouse, and human, the monoclonal antibody we previously characterized (Evans et al. 2014) does not recognize human RGS14. Therefore, as the synthetic immunogen used to generate the RGS14 antibody was based on the human peptide sequence, we next wanted to verify that the antibody recognized not only rodent RGS14, but also monkey RGS14.
Figure 1. Polyclonal RGS14 antibody recognizes epitopes within the carboxy-terminal half of the protein.
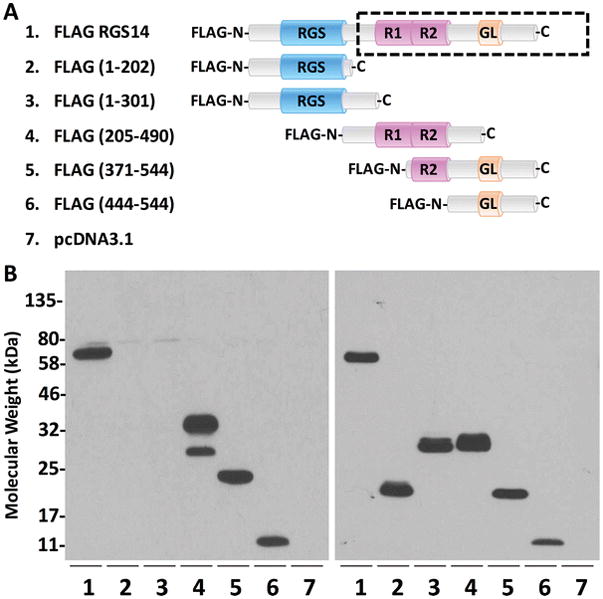
(A) Full-length and truncated forms of FLAG-RGS14 are listed, and their corresponding protein sequences are depicted by cartoon. The human immunogen used to generate this polyclonal antibody is outlined by the dashed box. (B) Constructs in A were cloned into pcDNA3.1, expressed in HEK 293 cells and immunoblotted for RGS14 (left) or FLAG (right). Full-length RGS14 is recognized by the antibody (lane 1). While the front half of the protein (lanes 2–3) is not recognized by the antibody, the back half of RGS14 (lanes 4–6) is, indicating that the epitope(s) are located within the C-terminal end of the protein (from R1 domain to the GPR motif). Expression of each construct is verified by immunoblotting for the FLAG tag, which is universal to all the constructs (right). The epitopes recognized by the polyclonal antibody match the immunogen depicted by the dashed box in (A).
Figure 2. RGS14 antibody specifically recognizes RGS14, but not other RGS proteins.
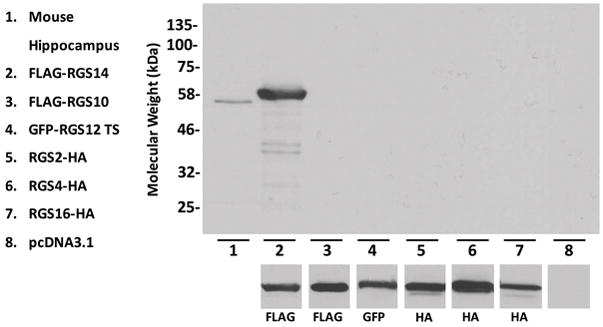
Lysates from hippocampus or HEK 293 cells transfected with RGS2, 4, 10, 12, 14, or 16 were immunoblotted for recognition by the RGS14 antibody or their respective tag. Lane 1: The RGS14 antibody recognizes a band corresponding to full-length RGS14 in the mouse hippocampus. Lanes 2–7: FLAG-RGS14, FLAG-RGS10, GFP-RGS12 trans-spliced (TS), RGS2-HA, RGS4-HA, and RGS16-HA were immunoblotted for RGS14 (top), or their respective tags (bottom). The RGS14 antibody recognized full-length, recombinant FLAG-RGS14 (lane 2), but not the other RGS (lanes 3–7), including RGS10 and RGS12, which are in the same R12 family as RGS14 and thus have a closely related sequence. Immunoblotting for each respective tag confirmed expression of each construct.
Immunoblot analysis of RGS14 expression
To explore species specificity, as well as regional expression of RGS14 in brain tissue, we used flash-frozen (stored at −80°C) fresh hippocampal and striatal punches from an adult wild-type mouse and a rhesus monkey. We immunoblotted 50 μg of total protein from each sample with anti-RGS14. We found that this antibody labeled full-length RGS14 in mouse hippocampal lysate, as we’ve previously shown (Evans et al. 2014; Hollinger et al. 2001; Lee et al. 2010) (Fig. 3A). Reports of mRNA in mouse brain indicate RGS14 is nearly entirely confined to the hippocampus (http://mouse.brain-map.org). Surprisingly, full-length RGS14 was also detected in mouse striatal lysate (Fig. 3A) and in fixed mouse brain tissue (data not shown), indicating a discrepancy between mRNA and RGS14 protein expression in the mouse striatum, which should be explored in future studies.
Figure 3. RGS14 antibody recognizes endogenous RGS14 in the rodent and primate brain.
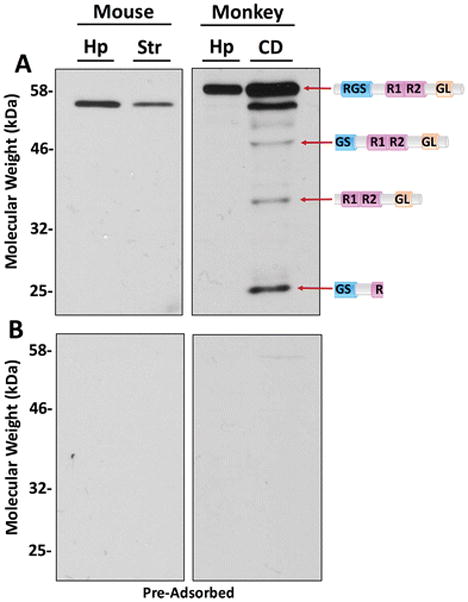
Hippocampal (Hp) and Striatal (Str) punches taken from an adult wild type mouse and a rhesus monkey were lysed and processed through the Bradford assay to quantify protein concentration. The striatal punches in the monkey were taken from the caudate nucleus (CD). Fifty micrograms of protein was loaded into each lane and separated on an 11% acrylamide gel. (A) RGS14 was detected by immunoblotting with 1:1000 diluted RGS14 antibody. Mouse hippocampal and striatal lysates contain full-length RGS14. Monkey hippocampal lysate contains full-length RGS14, and caudate lysate contains full-length RGS14 and three putative splice variants. The putative splice variants match predicted molecular weights: isoform 1 (canonical/full-length; UniProt O43566-7; GenBank EAW85012.1); isoform 2 (UniProt O43566-4; GenBank AAM12650.1); isoform 3 (UniProt O43566-5; GenBank: AAY26402.1); isoform 4 (UniProt O43566-6; GenBank: BAC85600.1). Their predicted protein sequences (Zhao et al. 2013) are represented by cartoons. (B) RGS14 antibody was pre-adsorbed with purified rat RGS14 and purified human RGS14 proteins (10 μg protein: 1 μg antibody). Membranes were subjected to the same antibody dilution (1:1000) and the same immunoblot protocol. Stained RGS14 bands are absent following pre-adsorption, indicating that the antibody is specific and that all detected bands are RGS14.
In the monkey hippocampus and caudate nucleus, we observed full-length RGS14 (Fig. 3A), as expected based on the reported mRNA expression in the non-human primate brain atlas (http://www.blueprintnhpatlas.org). Consistent with predicted full-length protein sequences for mouse (547 amino acids; ~60 kDa) and monkey (566 amino acids; ~62 kDa), monkey RGS14 migrated as a slightly higher molecular weight protein than that observed for mouse. Notably, the monkey caudate immunoblots revealed multiple bands of lower molecular weight compared to full-length RGS14 (Fig. 3A). We hypothesize that these bands may represent splice variants of RGS14 (Fig. 3A), the existence of which have been suggested, but not demonstrated or characterized (Cho et al. 2005; Martin-McCaffrey et al. 2004; Martin-McCaffrey et al. 2005; Zhao et al. 2013). To test whether these bands were in fact splice variants of RGS14, we pre-adsorbed the antibody with purified RGS14 protein (both human and rat species) at a 10:1 mass stoichiometry overnight in 250 μL TBS and used it for immunoblots. We found that virtually all immunoreactivity for both mouse and monkey lysates disappeared, following the same immunoblot protocol as before, when the pre-adsorbed antibody was used (Fig. 3B). This indicates that the bands detected with the RGS14 antibody are specific and likely represent splice variants of the full-length protein. Based on these findings, we used this highly specific antibody to determine the regional expression of RGS14 in the monkey and human brain.
RGS14 localization in Rhesus monkey brain
In the monkey brain, RGS14 immunoreactivity was confined to the caudate nucleus (CD), putamen (PUT), substantia nigra pars reticulata (SNr), globus pallidus (GP), hippocampus (Hp), and amygdala (Am) (Fig. 4). This pattern of expression was robust and very similar in the three monkey brains used in this study. However, it is strikingly different from the RGS14 labeling pattern described in our previous mouse study (Evans et al. 2014). Table 1 summarizes the relative intensity of RGS14 immunoreactivity in key regions of the monkey and human brains.
Figure 4. RGS14 immunoreactivity is expressed in discrete regions throughout the monkey brain.
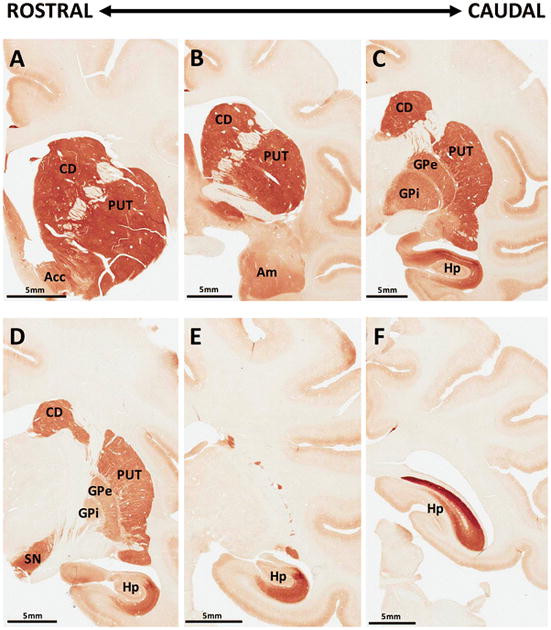
(A–F) Coronal sections through the rostrocaudal extent of the monkey brain show strong RGS14 immunostaining in the caudate nucleus (CD), putamen (PUT), nucleus accumbens (Acc), internal and external globus pallidus (GPi, GPe), substantia nigra (SN), and hippocampus (Hp), while moderate staining was found in the amygdala (Am). Apart from caudal hippocampal labeling, brainstem sections posterior to that displayed in panel F were devoid of RGS14 immunostaining.
Table 1. RGS14 localization within monkey and human brain.
Structures are grouped by functional region, and RGS14 expression is represented by *, where 0 is no expression, * is low expression, and **** is dense expression. NA represents an area that was not examined.
| Functional Region | Structure | RGS14 Expression | |
|---|---|---|---|
| Monkey | Human | ||
| Hippocampus | CA1 | *** | ** |
| CA2 | **** | *** | |
| CA3 | 0 | 0 | |
| Basal Ganglia | Caudate | **** | ** |
| Putamen | **** | ** | |
| Globus Pallidus, internal | *** | *** | |
| Globus Pallidus, external | *** | ** | |
| Substantia Nigra, pars compacta | 0 | NA | |
| Substantia Nigra, pars reticulata | *** | NA | |
| Amygdala | Amygdala, basolateral | ** | NA |
| Amygdala, central lateral | ** | NA | |
| Amygdala, basomedial | ** | NA | |
| Amygdala, lateral | 0 | NA | |
| Amygdalostriatal transition | ** | NA | |
| Amygdalostriatal transition (peri-CeL) | *** | NA | |
To validate the specificity of the polyclonal antibody in these immunohistochemical reactions, we incubated monkey brain tissue with RGS14 antibody pre-adsorbed overnight with purified protein at a 10:1 mass stoichiometry, as we did for the immunoblot experiments, and found that all regions enriched in RGS14 immunolabeling (CD, PUT, SN, GP, hippocampus, and amygdala) were completely devoid of immunoreactivity when exposed to the pre-adsorbed antibody (Fig. 5, control sections from Fig. 4 are next to their pre-adsorbed counterparts for comparison). Note that the molecular layer of the cortex appears labeled in Figure 4 and Figure 5, even in pre-adsorbed sections, indicating that this staining represents non-specific labeling and is likely a common “edge effect” fixation artefact. These findings provide further evidence for the specificity of the RGS14 antibody when applied to the monkey brain. The overall pattern of cellular and subcellular expression of RGS14 in each of the labeled brain regions is described below.
Figure 5. RGS14 labeling in monkey brain is specific.
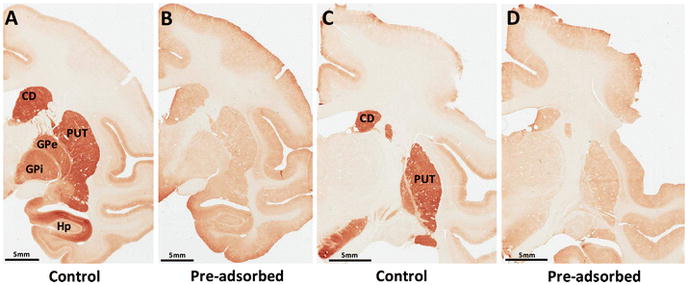
(A–D) RGS14 immunoreactivity was ablated in all regions of the monkey brain after incubation with RGS14 antibody pre-adsorbed with the synthetic immunogenic peptide against which it was made (A versus B; C versus D). Note that brain sections depicted in A and C are also used in Figure 4.
Hippocampus
Overall, the pattern of RGS14 labeling at light and electron microscopic level was similar between the three monkeys used in the present study. The monkey hippocampus was heavily stained when incubated with the RGS14 antibody (Fig. 4C–F; 6A). Robust RGS14 expression was found throughout the CA2-CA1 region, but not in the CA3 area nor the dentate gyrus, of the macaque hippocampus. Consistent with our previous report in mice (Evans et al. 2014; Lee et al. 2010), heavy CA2 labeling of pyramidal cell bodies and dendrites was found throughout the whole rostro-caudal extent of the primate hippocampus. Strong RGS14 expression was also found in CA1, but the labeling in this region was mainly confined to the neuropil, although immunoreactive pyramidal cell bodies and proximal dendritic profiles could also be seen (Fig. 6A). To further determine the cellular make-up of the CA1 neuropil labeling, we used electron microscopy (EM) to characterize the localization of the RGS14 immunoreactivity. Overall, we found that RGS14-positive structures in CA1 comprise both pre- and postsynaptic profiles (Fig. 6B–D). Axon terminals forming asymmetric (i.e. putatively excitatory) axo-dendritic (Fig. 6B) or axo-spinous (Fig. 6C) synapses, dendrites of various sizes, and spines (Fig. 6D) were the main constituents of the CA1 neuropil immunoreactivity. Based on the known projections from CA2 to CA1 (Kohara et al. 2014) and the restricted expression of RGS14 throughout the brain, it is reasonable to suggest that most of the putative glutamatergic RGS14-immunoreactive axon terminals in CA1 originate from RGS14-positive CA2 pyramidal cells.
Figure 6. RGS14 is expressed in CA1 and CA2 pyramidal cell bodies and neuropil in the monkey hippocampus.
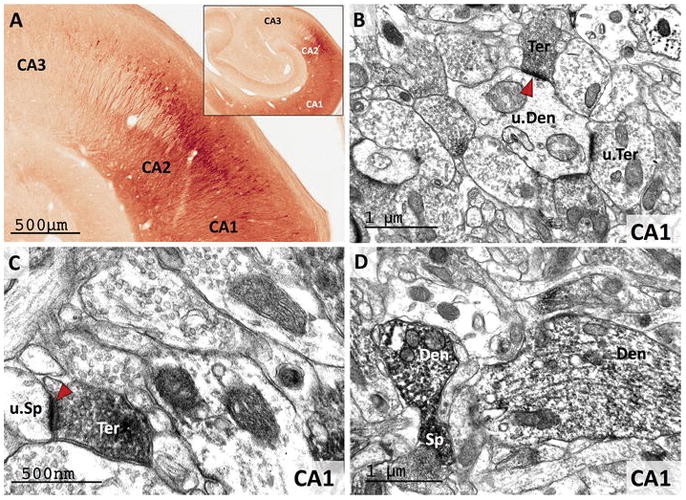
(A) Light microscopy reveals dense RGS14 labeling of pyramidal cell bodies in CA2. By contrast, there is virtually no labeling in CA3. CA1 displays a lighter RGS14 labeling than CA2, which is preferentially localized in the neuropil. (B and C) Electron micrographs of RGS14-positive terminals (Ter) forming asymmetric axo-dendritic (B) or axo-spinous (C) synapses in CA1. In each micrograph, RGS14-immunoreactive axon terminals (Ter), spines (Sp) and dendrites (Den) are indicated, while u.Ter, u.Sp, and u.Den mark unlabeled corresponding elements. The red arrowheads point at asymmetric synapses that involve RGS14-containing terminals, which likely originate from CA2-CA1 axonal projections. (D) Post-synaptic RGS14 labeling in CA1 dendrites and spines.
Striatum
In line with our immunoblot data (Fig. 3A), both the caudate nucleus and putamen displayed strong cellular and neuropil RGS14 immunoreactivity (Fig 7). The labeling was found throughout the rostro-caudal extent of the caudate nucleus and putamen, and in the nucleus accumbens, albeit to a lower intensity than in the dorsal striatum (Fig. 4A–E; 7A). The morphology of labeled striatal cell bodies was consistent with that of previously reported striatal projection neurons, i.e. small- to medium-sized soma with large nuclei surrounded by a thin rim of cytoplasm (Graveland and DiFiglia 1985). At the electron microscopic level, striatal RGS14 immunoreactivity was largely expressed postsynaptically in dendrites and spines frequently contacted by unlabeled putative glutamatergic terminals (Fig 7B–C). Occasionally, RGS14-immunoreactive terminals forming symmetric axo-dendritic synapses were encountered (Fig. 7D). Although there was no clear evidence for immunostaining associated with striatal interneurons, such as fast-spiking parvalbumin-positive neurons, this issue remains to be addressed.
Figure 7. RGS14 is localized in dendrites and spines of GABAergic projection neurons in the monkey striatum.
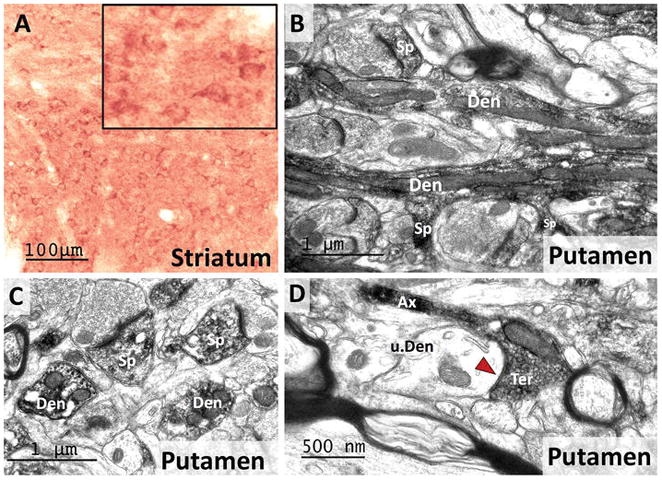
A) A light micrograph shows RGS14-immunoreactive cell bodies alongside a dense neuropil labeling in the monkey striatum. The inset shows a high power view of RGS-positive striatal cell bodies that morphologically resemble those of striatal projection neurons. (B–C) Electron micrographs of RGS14-labeled dendrites (Den) and spines (Sp) in the monkey putamen. Note in the lower part of B, the spines coming off a dendritic process, further confirming the RGS14 expression in spiny projection neurons. (D) Electron micrograph of an RGS14-positive axon terminal that forms a symmetric axo-dendritic synapse (red arrow) with an unlabeled dendrite (u.Den) in the putamen. Although the source of these terminals was not characterized, their ultrastructural features suggest that they may originate from recurrent collaterals of GABAergic striatofugal axons (Wilson and Groves 1980).
Figure 3 demonstrates the existence of putative lower molecular weight RGS14 variants in monkey caudate, which match previously predicted splice variants (Cho et al. 2005; Martin-McCaffrey et al. 2004; Martin-McCaffrey et al. 2005; Zhao et al. 2013). Recombinant RGS14 is a nuclear shuttling protein that contains both a nuclear localization signal (NLS) and a nuclear export signal (NES) (Cho et al. 2005; Shu et al. 2007), and the smallest of these variants retains an NLS but lacks an NES. Therefore, we hypothesized that the caudate may contain a nuclear subset of a short-form of RGS14. To further characterize the subcellular localization of RGS14 in striatal elements, we processed striatal sections with the pre-embedding immunogold method (Fig. 8), which provides a higher level of spatial resolution than the immunoperoxidase technique. Overall, the pattern of cellular distribution of gold labeling was similar to that described above using the immunoperoxidase approach, such that most labeling was found in postsynaptic structures, including dendrites, spines, and neuronal cell bodies (Fig. 8). In general, the bulk of gold labeling was located in the cytosol of immunoreactive elements, often closely associated with vesicular and tubular endoplasmic reticulum-like structures (red arrowheads in Fig. 8). In addition, a significant number of gold particles was also found in the nucleus of labeled cell bodies, suggesting nuclear expression of RGS14 (yellow arrows in Fig. 8C–D). Only a small subset of gold labeling was associated with the plasma membrane of all immunoreactive structures (Fig. 8).
Figure 8. RGS14 localizes in the cytosol and the nucleus of striatal projection neurons.
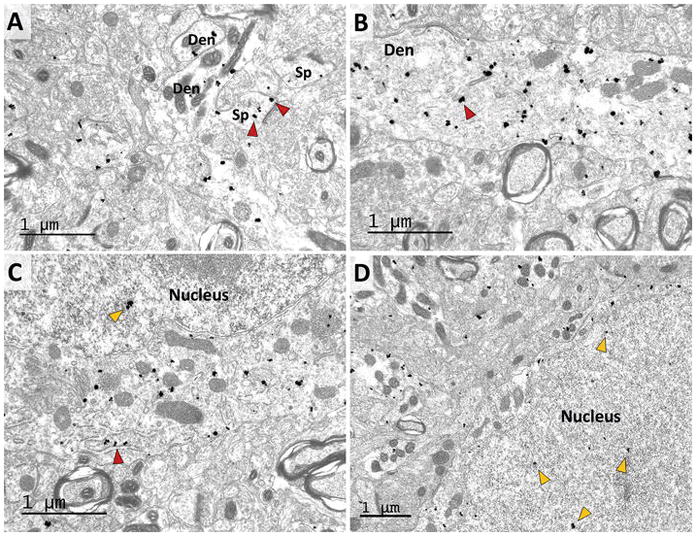
Electron micrographs of RGS14-immunoreactive dendrites (Den) and spines (Sp) (A–B), and neuronal cell bodies (C–D) in the monkey striatum as revealed with the pre-embedding immunogold method. Note that most gold particles are largely located within the cytosol of immunoreactive structures, with rare instances of plasma membrane labeling. In (A), the red arrowheads indicate examples of RGS14 labeling in spines. In (B), the red arrowhead indicates an example of RGS14 labeling in dendrites. In (C) and (D), the staining is associated with the endoplasmic reticulum (red arrowhead) and vesicular organelles in the cytoplasm of immunoreactive cell bodies, while some gold particles are also found in the nucleus of these labeled neurons (yellow arrowheads).
Globus pallidus
Both the external and internal segments of the globus pallidus displayed robust RGS14 immunoreactivity (Fig. 4C–D; 9A). At the light microscopic level, the pallidal labeling was exclusively associated with terminal- and axon-like processes that wrapped around non-immunoreactive dendrites of pallidal neurons (Fig. 9A), a pattern previously described as “woolly fibers” (Haber and Nauta 1983). In light of the previous literature showing that “woolly fibers” originate from striatopallidal GABAergic axons (Haber and Nauta 1983), we used electron microscopy to determine if RGS14 neuropil immunoreactivity in both pallidal segments was, indeed, expressed in GABAergic striatal-like terminals. As shown in Fig. 9, RGS14 immunoreactivity was exclusively expressed presynaptically in small unmyelinated axons and axon terminals that formed symmetric synapses with unlabeled dendrites of pallidal cells (blue arrowheads in Fig. 9B–D). The ultrastructural features and the symmetric membrane specialization of the synapses formed by these terminals was similar to that described for striatopallidal GABAergic terminals in previous studies (Difiglia et al. 1982; Shink and Smith 1995; Smith et al. 1998), thereby indicating that these RGS14-immunoreactive terminals likely originate from striatal projection neurons. The dendrites contacted by the immunoreactive axon terminals also received asymmetric synaptic inputs from unlabeled terminals (red arrowheads in Fig. 9B).
Figure 9. RGS14 is localized presynaptically in striatopallidal GABAergic terminals.
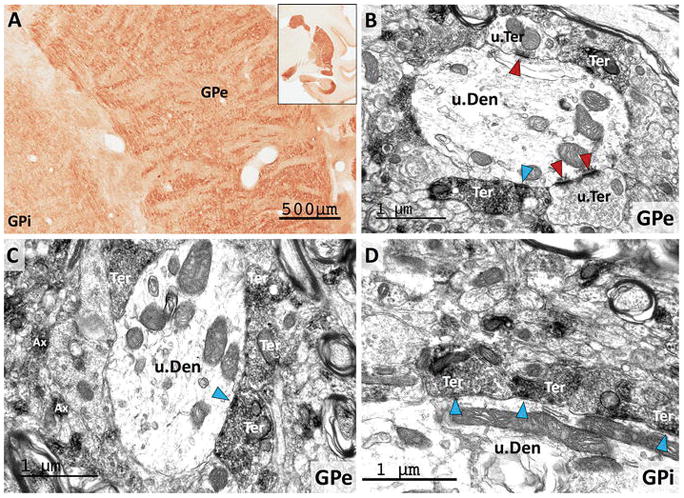
(A) Light micrograph of RGS14-immunoreactive neuropil in both the internal and external globus pallidus (GPi, GPe) displays the “woolly fibers” pattern of labeling previously used to describe the massive striatal GABAergic innervation wrapped around pallidal dendrites (Haber and Nauta 1983). (B–D) Electron micrographs of RGS14 labeling in GPe and GPi confirm that most of the immunoreactivity is found in axon terminals (Ter) that display the ultrastructural features of striatal GABAergic terminals and form symmetric synapses (blue arrowheads) with unlabeled dendrites (u.Den) of pallidal neurons. Note that red arrowheads depict asymmetric synapses formed by unlabeled terminals (u.Ter). Labeled unmyelinated axons (Ax) are also found throughout the neuropil in both pallidal segments (C).
Substantia nigra
Similar to the pallidum, strong RGS14-positive labeling of “woolly fibers” was found in the SNr (Fig. 10A). In contrast, the pars compacta (SNc) was completely devoid of RGS14 labeling (Fig. 10A). At the electron microscopic level, the distribution of labeling was the same as in the globus pallidus, i.e. found mostly in putative striatal-like GABAergic terminals that formed symmetric synapses with unlabeled dendrites (Fig. 10B–D; blue arrowhead in 10C). Consistent with the pattern of “woolly fibers”, some SNr dendrites were completely ensheathed by striatal-like RGS14-labeled boutons (Fig. 10B). In addition to the large amount of putative striatal terminals, another population of RGS14-containing boutons forming asymmetric axo-dendritic synapses (i.e. putatively excitatory) were found in the SNr (red arrowhead in Fig. 10D). Although the exact source(s) of these terminals remains to be established, the central nucleus of the amygdala is one of the RGS14-enriched brain regions (described below) that may contribute to this innervation (Lee et al. 2005; Vankova et al. 1992).
Figure 10. RGS14 is localized pre-synaptically in the monkey substantia nigra pars reticulata.
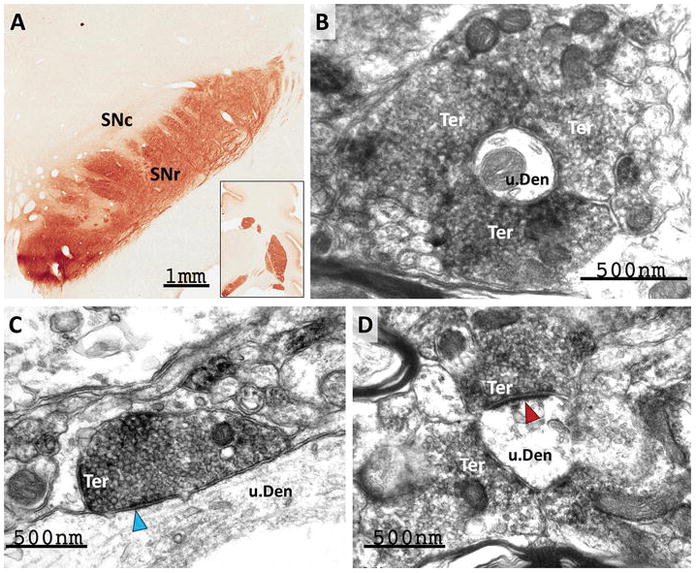
(A) Light micrograph showing dense RGS14 neuropil labeling in the pars reticulata (SNr) of the monkey substantia nigra, while the pars compacta (SNc) is completely devoid of immunoreactivity. As in GPe and GPi, the SNr labeling is made up of rich plexuses of varicosities that display a woolly fibers-like pattern of innervation of dendritic processes. (B–C) At the electron microscope level, the immunostaining is mainly confined to pre-synaptic terminals (Ter) that display the ultrastructural features of striatonigral GABAergic boutons and form symmetric axo-dendritic synapses (blue arrowhead in C). (D) illustrates a subset of RGS14-immunoreactive terminals that forms asymmetric axo-dendritic synapses (red arrowheads). The amygdala is a potential source of these putative excitatory terminals.
Amygdala
Another RGS14-enriched region of the monkey brain was the amygdala (Fig. 11). The basomedial (BM), basolateral (BL) and centrolateral (CeL) nuclei were the most strongly immunoreactive amygdala sub-regions (Fig. 11). In contrast to these regions, the lateral amygdala displayed a far less intense level of RGS14 immunoreactivity (Fig. 11A). In the BM, BL, and CeL, numerous immunoreactive neuronal cell bodies and proximal dendrites laid within a diffuse lightly immunostained neuropil (Fig. 11A; 11D). At higher magnification, some of the positive neurons displayed the morphological features of projection cells (Fig. 11B–C), though double labeling experiments are needed to further characterize their exact chemical phenotype. A dense band of strongly RGS14-immunoreactive cells was also found in the amygdalostriatal transition area (AStr) along the lateral edge of the CeL (Fig. 11D–E; see arrows in E). In adjacent sections immunostained for the ubiquitous neuronal marker, NeuN, this band of labeling corresponds to a sub-region of the AStr that contains a larger neuronal density than neighboring regions (Fig. 11F; see arrows). To further extend our analysis of RGS14 expression in primates, we explored the localization of RGS14 immunoreactivity within the human brain.
Figure 11. RGS14 is expressed throughout the monkey amygdala.
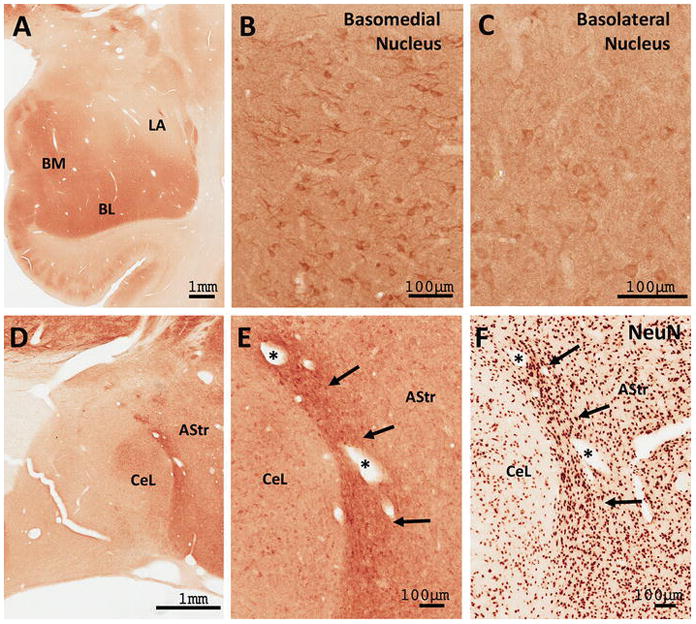
(A) Low power view of the distribution of RGS14 immunoreactivity in the monkey amygdala. (B–C) Light microscope images show discrete RGS14 staining of cell bodies and neuropil in the basomedial (BM) and basolateral (BL) nuclei, but not the lateral (LA) nuclei. (D–E) RGS14 labeling in the central lateral (CeL) and amygdalostriatal region (AStr). The dense band of labeling adjacent to the CeL corresponds to a sub-region of the AStr (arrows in E and F) that contains a larger neuronal density than neighboring AStr regions as revealed by NeuN immunostaining (F).
RGS14 localization in the Human brain
Post-mortem human brain tissue used in this series of experiments was collected from neurologically healthy individuals, immersion-fixed and sectioned (see Methods for details). As shown in the mouse and monkey brains, the CA2 and CA1 hippocampal regions contained a large number of RGS14-immunoreactive pyramidal cell bodies, while the CA3 region was devoid of staining in humans (Fig. 12). The neuropil staining in CA1 and CA2 was considerably lighter in humans than monkeys, likely due to protein degradation during post-mortem delay and immersion (instead of trans-cardiac) fixation of the human tissue. Despite this lower level of immunoreactivity, the general pattern of distribution of RGS14-positive neurons in the human hippocampus was found to be largely consistent with that seen in the monkey and rodent brain.
Figure 12. RGS14 expression in human hippocampus is comparable to that of monkey and mouse.
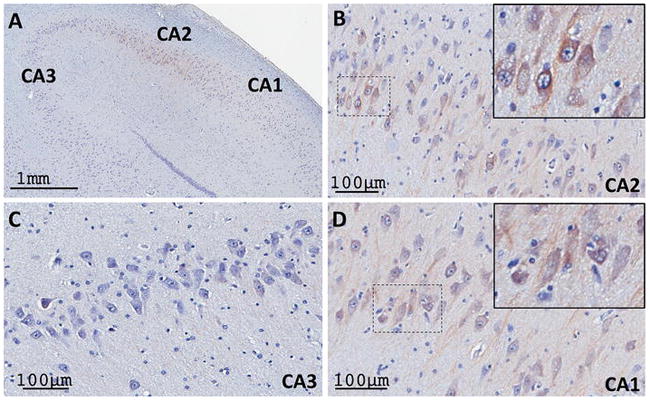
Human hippocampi were double stained with Nissl reagent and RGS14 immunolabeling. (A) Low power view of RGS14 labeling in CA2 and CA1 subfields, but not in CA3, of human hippocampus. (B, D) Higher magnification of RGS14-immunoreactive pyramidal cell bodies in the CA2 (B) and CA1 (D) regions, as shown in the monkey and mouse hippocampus. The insets in the upper right corner of each panel show examples of cell body labeling. There is minimal neuropil labeling in both regions. (C) Micrograph showing thionin-stained CA3 neurons devoid of RGS14 immunoreactivity.
To confirm that the strong RGS14 expression in the monkey basal ganglia could also be found in humans, we examined the cellular localization of RGS14 in the human striatum and globus pallidus. Reminiscent of the monkey data, a moderate neuropil and cellular expression of RGS14 immunoreactivity in the caudate nucleus (Fig. 13A) and putamen (Fig. 13B), combined with a strong woolly fiber-like pattern of axonal labeling in both pallidal segments (Fig. 13C, D), was also observed in humans.
Figure 13. RGS14 expression in the human basal ganglia is consistent with labeling in the monkey basal ganglia.
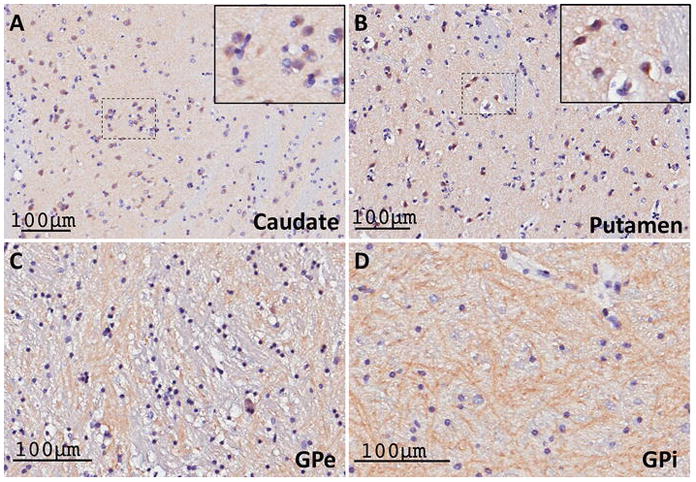
(A–B) Light micrographs of RGS14-positive neuronal cell bodies, double stained with Nissl reagent, within a lightly labeled neuropil in the human caudate nucleus (A) and putamen (B). The insets in the upper right corner of each panel show examples of cell body labeling. (C–D) Dense RGS14-immunoreactive woolly fibers-like neuropil in the human GPe and GPi. The pattern of RGS14 labeling in both the striatum and the globus pallidus in humans is consistent with that described in monkeys.
DISCUSSION
We and others have previously examined RGS14 protein localization and expression throughout the postnatal development of the mouse brain using different antibodies capable of recognizing only rodent RGS14 (Anderson et al. 2009; Evans et al. 2014). Here, we demonstrate the specificity of a new polyclonal antiserum that recognizes the primate RGS14, and used it to map the cellular and subcellular expression of RGS14 and putative RGS14 splice variants in the monkey and human brain (Table 1). We show that RGS14 immunoreactivity is selectively found in areas CA1 and CA2 of the hippocampus, striatum, globus pallidus, substantia nigra pars reticulata, and amygdala in rhesus monkeys. Furthermore, we confirm RGS14 expression in the human hippocampus and dorsal striatum, further validating the study of RGS14 in rodent and monkey models as relevant to human health and disease. Figure 14 summarizes our findings of RGS14 expression in primate brain.
Figure 14. Circuitry diagram of RGS14 protein expression in the primate brain.
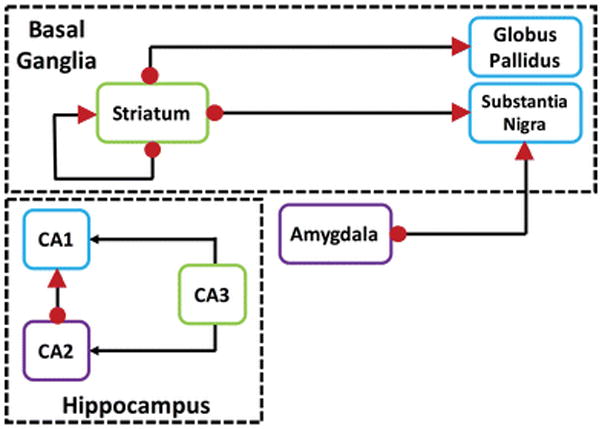
Circuit diagram of RGS14-positive neurons and their connections within the basal ganglia and hippocampus networks. Red arrowheads indicate pre-synaptic RGS14 labeling in terminals. Red dots depict post-synaptic RGS14 labeling in cell bodies, dendrites, and spines. Axonal projections are designated by black lines.
Validation of antibody specificity
To confirm our conclusions regarding RGS14 localization in the human and monkey brain, we determined that the polyclonal RGS14 antibody used in our study was specific and only recognized RGS14 (Figs. 1–3). A previous report described the putative localization of RGS14 in the monkey brain (Lopez-Aranda et al. 2006). Based on our present findings and others (Evans et al. 2014; Hollinger et al. 2001; Lee et al. 2010; Traver et al. 2000; Zhao et al. 2013), it appears that the antibody used in that study was not specific to RGS14. The predicted size of full-length RGS14 is 60 kDa, which matches the size of the band our antibody detects via immunoblot of lysates from both mouse and monkey brain. However, the previously reported antibody recognized a protein that appeared as a single band at 44 kDa in monkey brain membranes, but not supernatant, thereby suggesting that the labeled protein was not RGS14. In their study, the antibody also detected RGS14 expression in astrocytes, while only neuronal labeling was observed in our study, consistent with our previous findings in mice using a different rodent-specific monoclonal antibody (Evans et al. 2014). Noticeable differences in labeling between the two studies were also found in the CA1 and CA2 regions of the hippocampus. Although our findings in primates and those from our previous rodent studies provide evidence for strong RGS14 labeling in CA2 pyramidal neurons (Evans et al. 2014; Haussler et al. 2016; Kohara et al. 2014; Lee et al. 2010), the previous study reported low RGS14 expression in CA2 neurons compared with the CA1 region (Lopez-Aranda et al. 2006). Most importantly, the protein immunostaining patterns reported here match exactly the reported mRNA expression patterns for RGS14 in human and non-human primates (http://human.brain-map.org; http://www.blueprintnhpatlas.org). Based on these observations, we are confident that the immunostaining reported here is truly representative of the cellular and subcellular expression RGS14 protein in the monkey and human brains.
RGS14 in the monkey and human hippocampus
RGS14 has previously been shown to suppress synaptic plasticity as well as hippocampal-dependent learning and memory specifically within the CA2 region of the hippocampus in mice (Lee et al. 2010). Here, we show that RGS14 is also strongly expressed in pyramidal neurons of the CA2 region in both monkey and human hippocampi, but strong cellular and neuropil immunoreactivity could also be found in the primate CA1 region. Our ultrastructural analysis revealed that the bulk of CA1 labeling comprises putative glutamatergic terminals that likely originate from CA2 axonal projections and cell bodies/dendrites/spines of CA1 pyramidal neurons. While there is strong evidence that postsynaptic RGS14 in CA2 pyramidal neurons acts as a natural suppressor of LTP at CA3-CA2 glutamatergic synapses (Lee et al. 2010), the presynaptic function(s) of RGS14 in the CA1 region remains entirely unexplored. Various other RGS proteins have been shown to enhance presynaptic neurotransmitter release by blocking Gβγ-mediated inhibition of Cav2.2 (N-type) calcium channels downstream of GPCRs (Ding et al. 2006; Gerber et al. 2016; Han et al. 2006). It is possible that RGS14 acts in a similar fashion as a dedicated GTPase activating protein, or GAP (Brown et al. 2016), though its presynaptic functions are likely to be more nuanced and complex due to the fact that it contains multiple G protein-binding domains and a growing list of protein binding partners, each with signaling roles. Understanding the presynaptic roles for RGS14 remains a topic of great interest and a focus of ongoing and future studies.
RGS14 in the Basal Ganglia
While the role of RGS14 in the regulation of synaptic physiology in normal and diseased states has largely been centered on its functions in the hippocampus of adult mice (Vellano et al. 2011), our recent study has shown that RGS14 immunoreactivity is also expressed in non-hippocampal regions during early postnatal development of the mouse brain (Evans et al. 2014). Here, we provide evidence for the first time that RGS14 protein is also expressed in the striatum of adult mice. Furthermore, our light and electron microscopic immunohistochemical findings indicate that RGS14 is heavily expressed postsynaptically in dendrites and spines of striatal GABAergic projection neurons and presynaptically in striatopallidal and striatonigral terminals of primates. Therefore, RGS14 is expressed along both the so-called direct and indirect GABAergic striatofugal pathways of the basal ganglia (Albin et al. 1989; Gerfen et al. 1990; Kreitzer and Malenka 2008). Other RGS proteins, including RGS4, contribute to the dopamine-mediated regulation of long term depression (LTD) of corticostriatal glutamatergic synapses in indirect pathway neurons through modulation of postsynaptic mGluR1/5 and D2 dopamine receptors (Lerner and Kreitzer 2012). RGS9-2 and RGS7, which are also highly expressed in the striatum (Anderson et al. 2009), were similarly found to modulate dopamine signaling (Anderson et al. 2010; Rahman et al. 2003). RGS14 could possibly regulate synaptic signaling and plasticity in a similar manner to these other RGS proteins. However, multiple GPCRs and their associated downstream signals are implicated in synaptic plasticity in the basal ganglia (Kreitzer and Malenka 2008), providing many potential targets at which RGS14 could act in both indirect and direct pathway striatal projection neurons.
RGS14 splice variants in the caudate nucleus
Of great interest is our unexpected observation of multiple specific RGS14 bands in the immunoblots of the monkey caudate nucleus (Fig. 3A). While we cannot rule out the possibility that these bands represent proteolyzed and degraded RGS14, a more likely scenario is that they label previously speculated human splice variants of RGS14 (Cho et al. 2005; Martin-McCaffrey et al. 2004; Martin-McCaffrey et al. 2005; Zhao et al. 2013). Notably, these putative splice variants are absent from the monkey hippocampus (Fig. 3A) and have not been reported in rodents. Of the three reported human variants of RGS14, perhaps the most interesting feature is the lack of a functional RGS domain, suggesting a clear function for these RGS14 variants independent from its canonical role as a GAP for G proteins. Two of these splice variants retain a functional GPR motif, which interacts with inactive Gαi1 and Gαi3, and these shorter forms of RGS14 exhibit enhanced binding to Gα in the absence of the RGS domain (Zhao et al. 2013), providing a context for RGS14 expression without an RGS domain. Remarkably, RGS proteins from the R4 family (e.g. RGS4) interact directly with the RBD region of truncated RGS14 lacking an RGS domain, enhancing the GAP activity of the secondary RGS protein (Zhao et al. 2013). This could be a mechanism by which splice variants of RGS14 regulate the function of other RGS proteins specifically within the striatum, as expression of these variants is not seen in the hippocampus.
Independent of RGS14 regulation of G protein functions, some of these variants may serve specific, but as yet undefined, roles within the nucleus of striatal output neurons. We and others have previously demonstrated that full-length recombinant RGS14 contains both a nuclear localization sequence (NLS) and a nuclear export sequence (NES) used to shuttle in and out of the nucleus (Cho et al. 2005; Shu et al. 2007). Of note, the smallest variant (RGS14-S) retains a NLS, but lacks the NES, which is encoded within the GPR motif. Remarkably, our electron microscopy data revealed native RGS14 expression in the nuclei of projection neurons in the monkey caudate nucleus, where the short splice variant is detected by immunoblot. Although this remains to be confirmed, our data suggest that the short splice variant of RGS14 (RGS14-S) might account for RGS14 immunoreactivity within the nucleus of striatal neurons, where it may mediate unique nuclear functions different from the canonical role of RGS14 as a G protein modifier at the plasma membrane. Thus, our findings provide the first evidence for the expression of multiple splice variants of RGS14 in the primate striatum, and suggest for the first time that one or more of these variants displays nuclear localization within striatal projection neurons. Further investigation into the specific roles and localization of these variants within the striatum is of great interest for ongoing and future studies.
RGS14 in the amygdala
We also observed specific RGS14 immunostaining within the basal and centrolateral nuclei of the monkey amygdala. Although this staining was slightly weaker than the robust basal ganglia and hippocampal immunoreactivity, significant cell body and neuropil labeling was expressed in the basomedial, basolateral, and centrolateral nuclei, while the lateral nucleus was largely devoid of immunoreactivity. The amygdala is a central component of the limbic system which plays key roles in the processing of emotional memory, decision making and emotional responses to fear, anxiety and social aggression in primates and rodents (Bocchio et al. 2016; Knox 2016; Lamprecht 2016; Lee et al. 2016). Because the amygdala is made up of glutamatergic principal neurons and various populations of GABAergic interneurons (Mascagni and McDonald 2003; McDonald and Mascagni 2001; Muller et al. 2006), the exact cellular phenotype of RGS14-containing neurons awaits further double labeling studies. However, based on morphological grounds and relative abundance, it is clear that some of the labeled cells belong to the population of amygdalofugal glutamatergic neurons. Whether RGS14 inhibits LTP linked to spatial and contextual learning and memory in the amygdala in a similar fashion as it does in CA2 hippocampal cells (Lee et al. 2010) is unknown. The possibility that amygdala RGS14 modulates neuronal plasticity and LTP associated with emotional learning and memory linked to fear conditioning (Parker et al. 2012), social anxiety, and PTSD (Minkova et al. 2017; Sheynin and Liberzon 2016) remains a topic of great interest for future studies.
CONCLUSIONS
In this study, we provide conclusive evidence for RGS14 protein expression in the monkey and human hippocampus, amygdala, and basal ganglia (Table 1). At the ultrastructural level, our electron microscopy data show that RGS14 displays both a postsynaptic localization in dendrites and spines and a presynaptic localization in terminals of glutamatergic and GABAergic neuronal populations. Furthermore, we provide strong evidence for the existence of predicted human splice variants of RGS14 specifically within the primate striatum. Additionally, we report for the first time electron microscopy data suggesting endogenous RGS14 localization in the nuclei of striatal neurons. Figure 14 illustrates, based on our findings, a circuity diagram of regions enriched in RGS14 and their proposed axonal projection targets in the primate brain. Future research must be performed to determine the role of cytosolic and nuclear RGS14 in these neurons and their functional networks. In particular, understanding the role of the pre- and postsynaptic RGS14 expression in both direct and indirect striatofugal pathways is highly relevant because of the importance of these networks in the pathophysiology of basal ganglia disorders such as Parkinson’s disease (PD). Further investigations aimed at determining whether RGS14 plays a role in the emergence of disrupted synaptic transmission and plasticity at the striatal, pallidal, and nigral level might set the stage for the development of attractive therapeutic targets that could regulate RGS14 and its downstream signaling events in diseased conditions.
Acknowledgments
We would like to acknowledge and thank Marla Gearing, Deborah Cooper, and Susan Jenkins for their very valuable assistance with processing tissue and their continuous guidance. We would also like to acknowledge the Emory Alzheimer’s Disease Research Center for providing the human tissue (AG025688), and the Neuropathology/Histochemistry Core of the Emory NINDS Neurosciences Core Facility (P30 NS055077) for processing the human tissue. This work was supported, in whole or in part, by the National Institutes of Health grants 5R01NS037112 and 1R21 NS087488 (both awarded to JRH), Yerkes National Primate Research Center NIH base grant P51OD011132, and F31NS098648 (awarded to KJG). Additionally, both KJG and KES were supported by NIH training grant T32 GM008602.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Amstadter AB, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, Gelernter J. Variant in RGS2 moderates posttraumatic stress symptoms following potentially traumatic event exposure. J Anxiety Disord. 2009a;23:369–373. doi: 10.1016/j.janxdis.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, Gelernter J. Variation in RGS2 is associated with suicidal ideation in an epidemiological study of adults exposed to the 2004 Florida hurricanes. Arch Suicide Res. 2009b;13:349–357. doi: 10.1080/13811110903266541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GR, et al. R7BP complexes with RGS9-2 and RGS7 in the striatum differentially control motor learning and locomotor responses to cocaine. Neuropsychopharmacology. 2010;35:1040–1050. doi: 10.1038/npp.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GR, Lujan R, Martemyanov KA. Changes in striatal signaling induce remodeling of RGS complexes containing Gbeta5 and R7BP subunits. Mol Cell Biol. 2009;29:3033–3044. doi: 10.1128/MCB.01449-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio M, McHugh SB, Bannerman DM, Sharp T, Capogna M. Serotonin, Amygdala and Fear: Assembling the Puzzle. Front Neural Circuits. 2016;10:24. doi: 10.3389/fncir.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NE, Goswami D, Branch MR, Ramineni S, Ortlund EA, Griffin PR, Hepler JR. Integration of G Protein alpha (Galpha) Signaling by the Regulator of G Protein Signaling 14 (RGS14) J Biol Chem. 2015;290:9037–9049. doi: 10.1074/jbc.M114.634329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NE, Lambert NA, Hepler JR. RGS14 regulates the lifetime of Galpha-GTP signaling but does not prolong Gbetagamma signaling following receptor activation in live cells. Pharmacol Res Perspect. 2016;4:e00249. doi: 10.1002/prp2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Kim DU, Kehrl JH. RGS14 is a centrosomal and nuclear cytoplasmic shuttling protein that traffics to promyelocytic leukemia nuclear bodies following heat shock. J Biol Chem. 2005;280:805–814. doi: 10.1074/jbc.M408163200. [DOI] [PubMed] [Google Scholar]
- Difiglia M, Pasik P, Pasik T. A Golgi and ultrastructural study of the monkey globus pallidus. J Comp Neurol. 1982;212:53–75. doi: 10.1002/cne.902120105. [DOI] [PubMed] [Google Scholar]
- Ding J, et al. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat Neurosci. 2006;9:832–842. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- Evans PR, Lee SE, Smith Y, Hepler JR. Postnatal developmental expression of regulator of G protein signaling 14 (RGS14) in the mouse brain. J Comp Neurol. 2014;522:186–203. doi: 10.1002/cne.23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian C, Roullet P. Hippocampal CA3-region is crucial for acquisition and memory consolidation in Morris water maze task in mice. Behav Brain Res. 2004;154:365–374. doi: 10.1016/j.bbr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Fu Z, Lee SH, Simonetta A, Hansen J, Sheng M, Pak DT. Differential roles of Rap1 and Rap2 small GTPases in neurite retraction and synapse elimination in hippocampal spiny neurons. J Neurochem. 2007;100:118–131. doi: 10.1111/j.1471-4159.2006.04195.x. [DOI] [PubMed] [Google Scholar]
- Gerber KJ, Squires KE, Hepler JR. Roles for Regulator of G Protein Signaling Proteins in Synaptic Signaling and Plasticity. Mol Pharmacol. 2016;89:273–286. doi: 10.1124/mol.115.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci. 1997;17:8024–8037. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales KK, Pare JF, Wichmann T, Smith Y. GABAergic inputs from direct and indirect striatal projection neurons onto cholinergic interneurons in the primate putamen. J Comp Neurol. 2013;521:2502–2522. doi: 10.1002/cne.23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafstein-Dunn E, Young KH, Cockett MI, Khawaja XZ. Regional distribution of regulators of G-protein signaling (RGS) 1, 2, 13, 14, 16, and GAIP messenger ribonucleic acids by in situ hybridization in rat brain. Brain Res Mol Brain Res. 2001;88:113–123. doi: 10.1016/s0169-328x(01)00038-9. [DOI] [PubMed] [Google Scholar]
- Graveland GA, DiFiglia M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 1985;327:307–311. doi: 10.1016/0006-8993(85)91524-0. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Ragsdale CW, Jr, Yoneoka ES, Elde RP. An immunohistochemical study of enkephalins and other neuropeptides in the striatum of the cat with evidence that the opiate peptides are arranged to form mosaic patterns in register with the striosomal compartments visible by acetylcholinesterase staining. Neuroscience. 1981;6:377–397. doi: 10.1016/0306-4522(81)90131-7. [DOI] [PubMed] [Google Scholar]
- Haber SN, Nauta WJ. Ramifications of the globus pallidus in the rat as indicated by patterns of immunohistochemistry. Neuroscience. 1983;9:245–260. doi: 10.1016/0306-4522(83)90291-9. [DOI] [PubMed] [Google Scholar]
- Han J, Mark MD, Li X, Xie M, Waka S, Rettig J, Herlitze S. RGS2 determines short-term synaptic plasticity in hippocampal neurons by regulating Gi/o-mediated inhibition of presynaptic Ca2+ channels. Neuron. 2006;51:575–586. doi: 10.1016/j.neuron.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Haussler U, Rinas K, Kilias A, Egert U, Haas CA. Mossy fiber sprouting and pyramidal cell dispersion in the hippocampal CA2 region in a mouse model of temporal lobe epilepsy. Hippocampus. 2016;26:577–588. doi: 10.1002/hipo.22543. [DOI] [PubMed] [Google Scholar]
- Hohoff C, et al. RGS2 ggenetic variation: association analysis with panic disorder and dimensional as well as intermediate phenotypes of anxiety. Am J Med Genet B Neuropsychiatr Genet. 2015;168B:211–222. doi: 10.1002/ajmg.b.32299. [DOI] [PubMed] [Google Scholar]
- Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54:527–559. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- Hollinger S, Taylor JB, Goldman EH, Hepler JR. RGS14 is a bifunctional regulator of Galphai/o activity that exists in multiple populations in brain. J Neurochem. 2001;79:941–949. doi: 10.1046/j.1471-4159.2001.00629.x. [DOI] [PubMed] [Google Scholar]
- Ingi T, Aoki Y. Expression of RGS2, RGS4 and RGS7 in the developing postnatal brain. Eur J Neurosci. 2002;15:929–936. doi: 10.1046/j.1460-9568.2002.01925.x. [DOI] [PubMed] [Google Scholar]
- Kimple RJ, De Vries L, Tronchere H, Behe CI, Morris RA, Gist Farquhar M, Siderovski DP. RGS12 and RGS14 GoLoco motifs are G alpha(i) interaction sites with guanine nucleotide dissociation inhibitor Activity. J Biol Chem. 2001;276:29275–29281. doi: 10.1074/jbc.M103208200. [DOI] [PubMed] [Google Scholar]
- Kimple RJ, Willard FS, Siderovski DP. Purification and in vitro functional analyses of RGS12 and RGS14 GoLoco motif peptides. Methods Enzymol. 2004;390:416–436. doi: 10.1016/S0076-6879(04)90026-2. [DOI] [PubMed] [Google Scholar]
- Knox D. The role of basal forebrain cholinergic neurons in fear and extinction memory. Neurobiol Learn Mem. 2016;133:39–52. doi: 10.1016/j.nlm.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Amstadter AB, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, Gelernter J. RGS2 and generalized anxiety disorder in an epidemiologic sample of hurricane-exposed adults. Depress Anxiety. 2009;26:309–315. doi: 10.1002/da.20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K, et al. Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat Neurosci. 2014;17:269–279. doi: 10.1038/nn.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima M, Dehoff MH, Furuichi T, Worley PF, Hall RA, Smith Y. Localization and expression of group I metabotropic glutamate receptors in the mouse striatum, globus pallidus, and subthalamic nucleus: regulatory effects of MPTP treatment and constitutive Homer deletion. J Neurosci. 2007;27:6249–6260. doi: 10.1523/JNEUROSCI.3819-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouebe G, et al. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;10:1559–1568. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- Lamprecht R. The Role of Actin Cytoskeleton in Memory Formation in Amygdala. Front Mol Neurosci. 2016;9:23. doi: 10.3389/fnmol.2016.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larminie C, Murdock P, Walhin JP, Duckworth M, Blumer KJ, Scheideler MA, Garnier M. Selective expression of regulators of G-protein signaling (RGS) in the human central nervous system. Brain Res Mol Brain Res. 2004;122:24–34. doi: 10.1016/j.molbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, Holland PC. Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food. J Neurosci. 2005;25:3881–3888. doi: 10.1523/JNEUROSCI.0416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee S, Kim JH. Amygdala Circuits for Fear Memory: A Key Role for Dopamine Regulation. Neuroscientist. 2016 doi: 10.1177/1073858416679936. [DOI] [PubMed] [Google Scholar]
- Lee SE, et al. RGS14 is a natural suppressor of both synaptic plasticity in CA2 neurons and hippocampal-based learning and memory. Proc Natl Acad Sci U S A. 2010;107:16994–16998. doi: 10.1073/pnas.1005362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Kreitzer AC. RGS4 is required for dopaminergic control of striatal LTD and susceptibility to parkinsonian motor deficits. Neuron. 2012;73:347–359. doi: 10.1016/j.neuron.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschytz T, Broner EC, Zozulinsky P, Slonimsky A, Eitan R, Greenbaum L, Lerer B. Relationship between Rgs2 gene expression level and anxiety and depression-like behaviour in a mutant mouse model: serotonergic involvement. Int J Neuropsychopharmacol. 2012;15:1307–1318. doi: 10.1017/S1461145711001453. [DOI] [PubMed] [Google Scholar]
- Lopez-Aranda MF, Acevedo MJ, Carballo FJ, Gutierrez A, Khan ZU. Localization of the GoLoco motif carrier regulator of G-protein signalling 12 and 14 proteins in monkey and rat brain. Eur J Neurosci. 2006;23:2971–2982. doi: 10.1111/j.1460-9568.2006.04838.x. [DOI] [PubMed] [Google Scholar]
- Martin-McCaffrey L, et al. RGS14 is a mitotic spindle protein essential from the first division of the mammalian zygote. Dev Cell. 2004;7:763–769. doi: 10.1016/j.devcel.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Martin-McCaffrey L, Willard FS, Pajak A, Dagnino L, Siderovski DP, D’Souza SJ. RGS14 is a microtubule-associated protein. Cell Cycle. 2005;4:953–960. doi: 10.4161/cc.4.7.1787. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res. 2003;976:171–184. doi: 10.1016/s0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience. 2001;105:681–693. doi: 10.1016/s0306-4522(01)00214-7. [DOI] [PubMed] [Google Scholar]
- Minkova L, et al. Task-dependent modulation of amygdala connectivity in social anxiety disorder. Psychiatry Res. 2017;262:39–46. doi: 10.1016/j.pscychresns.2016.12.016. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Pare JF, Smith Y. Ultrastructural relationships between cortical, thalamic, and amygdala glutamatergic inputs and group I metabotropic glutamate receptors in the rat accumbens. J Comp Neurol. 2010;518:1315–1329. doi: 10.1002/cne.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal V, Linder ME. The RGS14 GoLoco domain discriminates among Galphai isoforms. J Biol Chem. 2004;279:46772–46778. doi: 10.1074/jbc.M407409200. [DOI] [PubMed] [Google Scholar]
- Mittal V, Linder ME. Biochemical characterization of RGS14: RGS14 activity towards G-protein alpha subunits is independent of its binding to Rap2A. Biochem J. 2006;394:309–315. doi: 10.1042/BJ20051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okimoto N, et al. RGS2 mediates the anxiolytic effect of oxytocin. Brain Res. 2012;1453:26–33. doi: 10.1016/j.brainres.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Parker CC, Sokoloff G, Cheng R, Palmer AA. Genome-wide association for fear conditioning in an advanced intercross mouse line. Behav Genet. 2012;42:437–448. doi: 10.1007/s10519-011-9524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, et al. State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev. 2005;19:2000–2015. doi: 10.1101/gad.342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Z, et al. Cloning and characterization of RGS9-2: a striatal-enriched alternatively spliced product of the RGS9 gene. J Neurosci. 1999;19:2016–2026. doi: 10.1523/JNEUROSCI.19-06-02016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Z, et al. RGS9 modulates dopamine signaling in the basal ganglia. Neuron. 2003;38:941–952. doi: 10.1016/s0896-6273(03)00321-0. [DOI] [PubMed] [Google Scholar]
- Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- Ryu J, Futai K, Feliu M, Weinberg R, Sheng M. Constitutively active Rap2 transgenic mice display fewer dendritic spines, reduced extracellular signal-regulated kinase signaling, enhanced long-term depression, and impaired spatial learning and fear extinction. J Neurosci. 2008;28:8178–8188. doi: 10.1523/JNEUROSCI.1944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Ko F, Jack E, Greenstein R, Dean B. Consistent with dopamine supersensitivity, RGS9 expression is diminished in the amphetamine-treated animal model of schizophrenia and in postmortem schizophrenia brain. Synapse. 2007;61:303–309. doi: 10.1002/syn.20368. [DOI] [PubMed] [Google Scholar]
- Sheynin J, Liberzon I. Circuit dysregulation and circuit-based treatments in posttraumatic stress disorder. Neurosci Lett. 2016 doi: 10.1016/j.neulet.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shink E, Smith Y. Differential synaptic innervation of neurons in the internal and external segments of the globus pallidus by the GABA- and glutamate-containing terminals in the squirrel monkey. J Comp Neurol. 1995;358:119–141. doi: 10.1002/cne.903580108. [DOI] [PubMed] [Google Scholar]
- Shu FJ, Ramineni S, Amyot W, Hepler JR. Selective interactions between Gi alpha1 and Gi alpha3 and the GoLoco/GPR domain of RGS14 influence its dynamic subcellular localization. Cell Signal. 2007;19:163–176. doi: 10.1016/j.cellsig.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Shu FJ, Ramineni S, Hepler JR. RGS14 is a multifunctional scaffold that integrates G protein and Ras/Raf MAPkinase signalling pathways. Cell Signal. 2010;22:366–376. doi: 10.1016/j.cellsig.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons SB, Caruana DA, Zhao M, Dudek SM. Caffeine-induced synaptic potentiation in hippocampal CA2 neurons. Nat Neurosci. 2012;15:23–25. doi: 10.1038/nn.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Traver S, et al. RGS14 is a novel Rap effector that preferentially regulates the GTPase activity of galphao. Biochem J. 2000;350(Pt 1):19–29. [PMC free article] [PubMed] [Google Scholar]
- Vankova M, Arluison M, Leviel V, Tramu G. Afferent connections of the rat substantia nigra pars lateralis with special reference to peptide-containing neurons of the amygdalo-nigral pathway. J Chem Neuroanat. 1992;5:39–50. doi: 10.1016/0891-0618(92)90032-l. [DOI] [PubMed] [Google Scholar]
- Vellano CP, Brown NE, Blumer JB, Hepler JR. Assembly and function of the regulator of G protein signaling 14 (RGS14). H-Ras signaling complex in live cells are regulated by Galphai1 and Galphai-linked G protein-coupled receptors. J Biol Chem. 2013;288:3620–3631. doi: 10.1074/jbc.M112.440057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellano CP, Lee SE, Dudek SM, Hepler JR. RGS14 at the interface of hippocampal signaling and synaptic plasticity. Trends Pharmacol Sci. 2011;32:666–674. doi: 10.1016/j.tips.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard FS, et al. Regulator of G-protein signaling 14 (RGS14) is a selective H-Ras effector. PLoS One. 2009;4:e4884. doi: 10.1371/journal.pone.0004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willars GB. Mammalian RGS proteins: multifunctional regulators of cellular signalling. Semin Cell Dev Biol. 2006;17:363–376. doi: 10.1016/j.semcdb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J Comp Neurol. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]
- Zhao M, Choi YS, Obrietan K, Dudek SM. Synaptic plasticity (and the lack thereof) in hippocampal CA2 neurons. J Neurosci. 2007;27:12025–12032. doi: 10.1523/JNEUROSCI.4094-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Nunn C, Ramineni S, Hepler JR, Chidiac P. The Ras-binding domain region of RGS14 regulates its functional interactions with heterotrimeric G proteins. J Cell Biochem. 2013;114:1414–1423. doi: 10.1002/jcb.24483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Zhu Y, et al. Rap2-JNK removes synaptic AMPA receptors during depotentiation. Neuron. 2005;46:905–916. doi: 10.1016/j.neuron.2005.04.037. [DOI] [PubMed] [Google Scholar]


