Abstract
Objective An epidural blood patch (EBP) is the mainstay of treatment for refractory spontaneous intracranial hypotension (SIH). We evaluated the treatment efficacy of targeted EBP in refractory SIH.
Methods All patients underwent brain magnetic resonance imaging (MRI) with contrast and heavily T2-weighted spine MRI. Whole spine computed tomography (CT) myelography with non-ionic contrast was performed in 46 patients, and whole spine MR myelography with intrathecal gadolinium was performed in 119 patients. Targeted EBPs were placed in the prone position one or two vertebral levels below the cerebrospinal fluid (CSF) leaks. Repeat EBPs were offered at 1-week intervals to patients with persistent symptoms, continued CSF leakage, or with multiple leakage sites.
Results Brain MRIs showed pachymeningeal enhancement in 127 patients and subdural hematomas in 32 patients. One hundred fifty-two patients had CSF leakages on heavily T2-weighted spine MRIs. CSF leaks were also detected on CT and MR myelography in 43 and 111 patients, respectively. Good recovery was achieved in all patients after targeted EBP. No serious complications occurred in patients treated with targeted EBP during the 1 to 7 years of follow-up.
Conclusions Targeted and repeat EBPs are rational choices for treatment of refractory SIH caused by CSF leakage.
Keywords: SIH, CSF leakage, targeted EBP, treatment
Introduction
Spontaneous intracranial hypotension (SIH) was initially described in 1938 by Schaltenbrand. 1 SIH is a syndrome characterized by orthostatic headaches, low cerebrospinal fluid (CSF) pressure, and diffuse pachymeningeal enhancement on brain magnetic resonance imaging (MRI) with contrast. 2 3 4 SIH is generally due to CSF leaks from the spinal meningeal diverticula or dural rents, frequently at the cervicothoracic junction, although the cause of leakage is not fully understood. 4 5 Treatment of SIH includes conservative approaches, such as bed rest, adequate hydration, caffeine intake, and abdominal binders and invasive procedures, such as autologous epidural blood patches (EBPs), fibrin injection, or open surgical repair. An EBP is the preferred treatment for patients who have failed a conservative approach. 6
Because of the rarity of SIH, evidence for the efficacy of EBP is insufficient, and a uniform protocol on how to perform an EBP has not been established worldwide; 7 8 9 10 11 Currently, the standard of care for SIH patients is predominantly comprised of conservative treatment in most Chinese medical communities, including patients with refractory SIH. Therefore, in the present study, we assessed the treatment efficacy of targeted EBPs (patches delivered directly to the site of CSF leakage) for refractory SIH patients after identification of the CSF leakage via an imageologic examination in our institution.
Methods
The ethics committee of Center for Intracranial Hypotension Management, Sir Run Run Shaw Hospital, School of Medicine approved this study. Between October 2007 and January 2014, a total of 179 patients (60 males and 119 females) who were diagnosed with SIH due to spontaneous or idiopathic low CSF pressure according to the International Classification of Headache Disorders criteria for headaches attributed to spontaneous (or idiopathic) low CSF pressure. 12 One hundred sixty-five patients with CSF leakages who failed conservative treatments (bed rest and overhydration) over a 2-week period were retrospectively reviewed. The patients who were suspected to have SIH, but did not exhibit a CSF leakage on radiologic imaging, were not be enrolled in this retrospective study.
All 165 SIH patients with refractory CSF leaks were identified by whole spine heavily T2-weighted spine MRI coupled with CT myelography and non-ionic contrast or MR myelography with intrathecal gadolinium. A targeted EBP was performed based on the results of CT myelography or MR myelography first, followed by the results of heavily T2-weighted spine MRI without showing the CSF leakage on CT myelography or MR myelography.
After giving full informed consent, all 165 patients were treated with an EBP targeted to the CSF leakage. The EBP was performed under strict aseptic conditions. Patients were in a prone position and awake during the entire procedure. An 18G needle was inserted in the epidural space using a midline approach at the appropriate level (one or two vertebrae levels below the CT myelography, MR myelography, or heavily T2-weighted spine MRI demonstrating CSF leakage) using the saline loss-of-resistance technique. We slowly injected sterile autologous peripheral unclotted venous blood into the epidural space. We stopped the injection when the patient complained of radicular pain, numbness, or headache. For EBPs at the cervical, cervical-thoracic, lower thoracic, and lumbar levels, the volume of injected blood was ≤10, ≤15, ≤15, and ≤20 mL, respectively. The patients were maintained in the prone position for 30 minute after the procedure and then were placed in the supine position with strict bed rest for the next 48 hours after the EBP had been placed. In patients who had no response to EBP, relapse after EBP, or multiple-level CSF leakages, we considered a repeat EBP at a minimum of a 1-week interval.
Outcomes were defined as follows: good recovery (complete or near-complete resolution of symptoms and returning back to work or school [relief ≥ 75%]); moderate recovery (partial symptomatic resolution (relief ≥ 50%); or no change (relief < 50%).
Results
A total of 165 patients (49 males and116 females; age range, 24–73; mean age, and 37.5 years; Table 1 ) were considered to have SIH refractory to conservative treatment in the current retrospective study.
Table 1. Headache Classification Subcommittee of the International Headache Society: The International Classification of Headache Disorders (2nd ed.).
| A. Diffuse and/or dull headache that worsens within 15 minutes of sitting or standing, at least one of the following, and fulfilling criteria D 1 Neck stiffness 2 Tinnitus 3 Hypacusia 4 Photophobia 5.nausea |
| B. At least one of the following 1. Evidence of low CSF pressure on MRI 2. Evidence of CSF leakage on conventional myelography, CT myelography, or cisternography 3. CSF opening pressure 60 mmH 2 O |
| C. No history of dural puncture or other cause of CSF fistula |
| D. Headache resolved within 72 hours after epidural blood patching |
Abbreviations: CSF, cerebrospinal fluid; CT, computed tomography; MRI, magnetic resonance imaging.
One hundred sixty-one patients had orthostatic headaches, and four patients had orthostatic neck pain as the presenting and core symptom. Other clinical manifestations included nausea, vomiting, dizziness, neck stiffness, shoulder pain, intrascapular pain, diplopia, photophobia, and tinnitus ( Table 2 ). Lumbar punctures were performed in all 165 patients. One hundred thirty-nine patients had low CSF opening pressures ( Table 2 ).
Table 2. Patient demographics and imaging studies.
| Refractory SIH ( n = 165) |
|
|---|---|
| Age (years) | 37.5 (24.1–73.8) |
| Male/female | 49/116 |
| Core symptom | |
| Orthostatic headache | 161 |
| Orthostatic neck pain | 4 |
| Other symptoms | |
| Neck stiffness | 113 |
| Dizziness | 92 |
| Nausea or vomiting | 65 |
| Tinnitus | 31 |
| Shoulder and intrascapular pain | 16 |
| Diplopia | 8 |
| Photophobia | 2 |
| CSF Opening pressure | |
| ≤ 60 mmH 2 O | 139 |
| > 60 mmH 2 O | 26 |
| Diagnostic studies | |
| Brain MRI | 165 |
| Pachymeningeal enhancement | 103 (62.4%) |
| Subdural hematoma | 31(18.8%) |
| Heavily T2-weighted spine MRI | 165 |
| CSF leakage | 152 (92.1%) |
| CT myelography | 48 |
| CSF leakage | 43 (89.6%) |
| MR myelography | 117 |
| CSF leakage | 111 (94.9%) |
| CSF leakage level | |
| Single | 16 (9.7%) |
| Multiple | 149 (90.3%) |
| Cervical | 41 (20%) |
| Cervical-thoracic | 131(63.9%) |
| Mid-lower thoracic | 25(12.2%) |
| Lumbar | 8 (4.8%) |
Abbreviations: CSF, cerebrospinal fluid; CT, computed tomography; MR, magnetic resonance; SIH, spontaneous intracranial hypotension.
Pachymeningeal enhancement was the most common finding on brain MRI with contrast ( Fig. 1 ) and existed in 103 patients ( Table 2 ). Thirty-one patients had subdural hematomas on brain MRI with contrast ( Fig. 2 and Table 2 ). Heavily T2-weighted spinal MRI showed the CSF leakage level ( Fig. 3 ) in 152 patients ( Table 2 ). Of the 48 patients who underwent CT myelography, 43 (89.6%) had CSF leaks ( Fig. 4 and Table 2 ). Of the 117 patients who underwent MR myelography, 111 (94.9%) had CSF leaks ( Fig. 5 and Table 2 ). CSF leakage sites were as follows: cervical level, 41 patients; cervical-thoracic level, 131 patients; mid-lower thoracic level, 25 patients; and lumbar level, 8 patients ( Table 2 ). More than one spinal segment was affected in 149 (90.3%) patients. Eighty-four percent of the leakage sites were located in the cervical spine and cervicothoracic junction. The leakage site of the CSF leak was not determined in 5 patients by CT myelography and in 6 patients by MR myelography; the CSF leakage level was shown on heavily T2-weighed spine MRI in all 11 patients ( Fig. 4 ). All 165 patients were treated with targeted EBP (range, 6–20 mL; mean, 12.24 mL) with CT myelography guidance (43 patients), MR myelography (111 patients), and heavily T2-weighted spine MRI (11 patients) after failure to recover from conservative treatment (bed rest and overhydration).
Fig. 1.
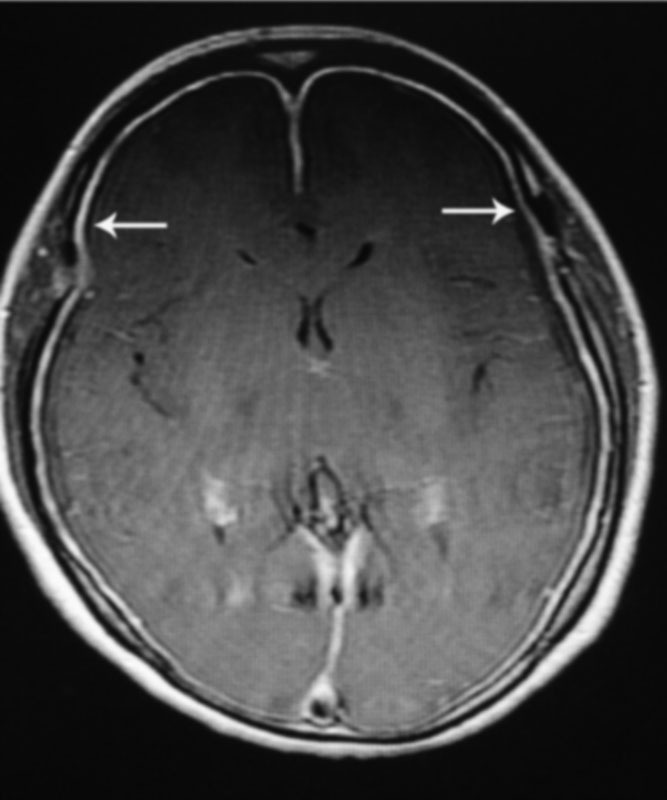
Axial brain MRI with contrast demonstrating diffuse pachymeningeal enhancement (arrow). MRI, magnetic resonance imaging.
Fig. 2.
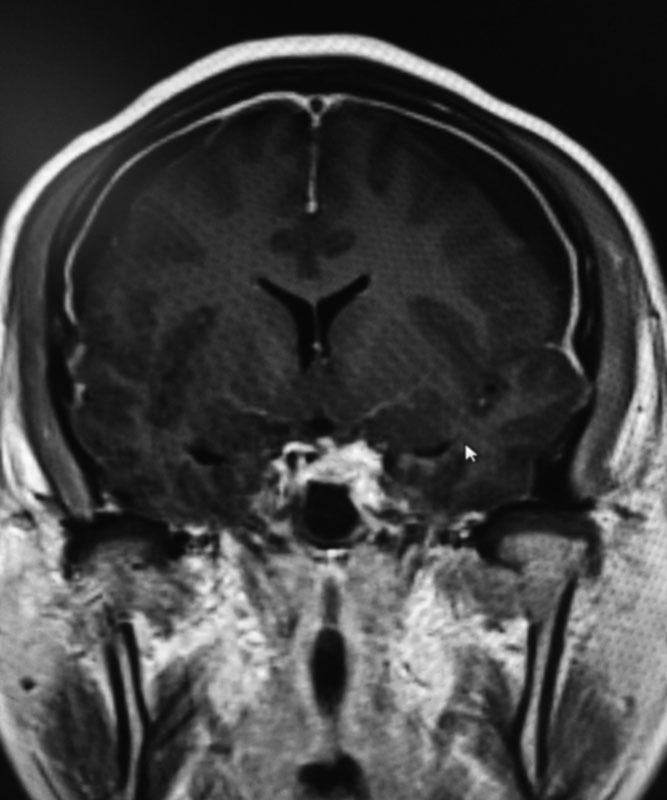
Coronal brain MRI with contrast demonstrating diffuse pachymeningeal enhancement and bilateral subdural hematoma (arrow). MRI, magnetic resonance imaging.
Fig. 3.
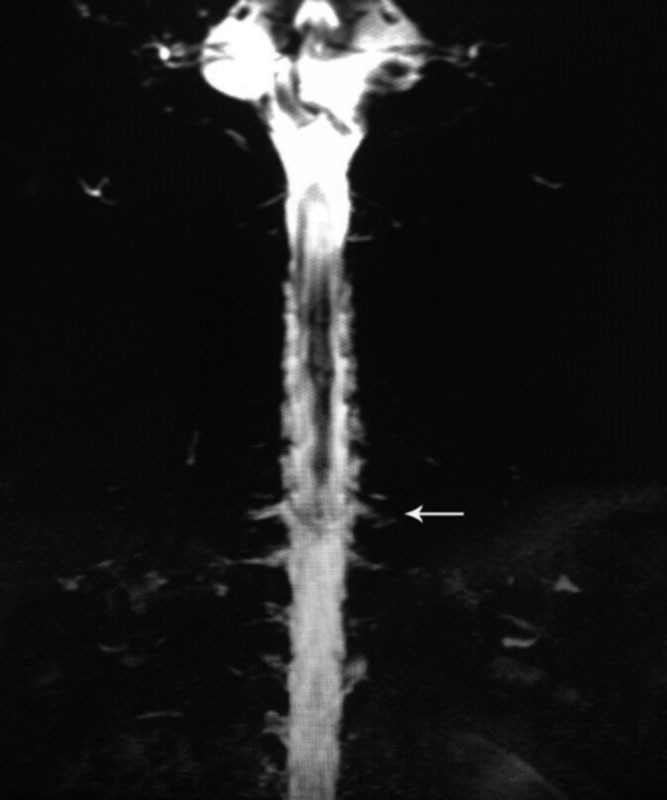
Heavily T2-weighted spine MRI demonstrating multiple meningeal diverticula and dilated nerve root sleeves in cervicothoracic junction region (arrow). MRI, magnetic resonance imaging.
Fig. 4.
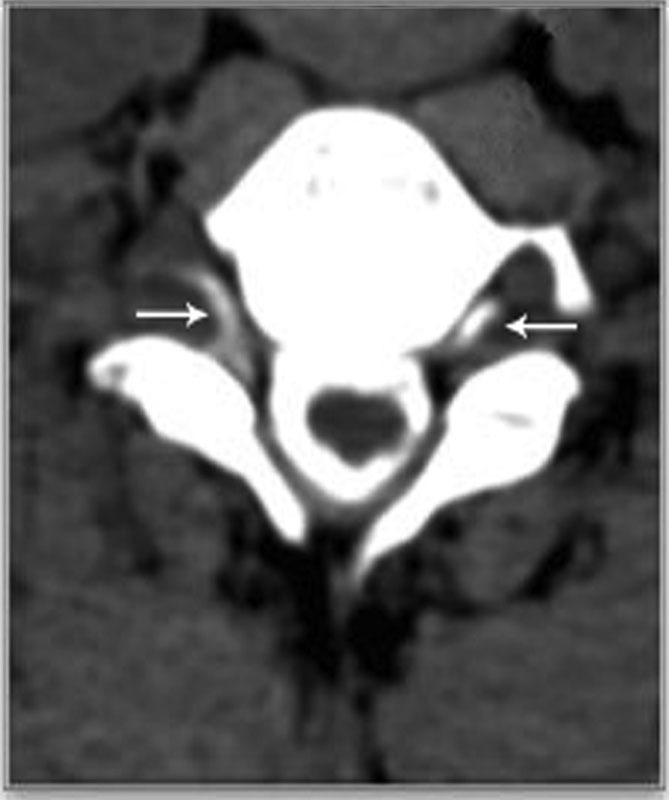
Axial CTM with contrast demonstrating the presence of contrast in the epidural space at the cervicothoracic level (arrows). CTM, CT myelography.
Fig. 5.
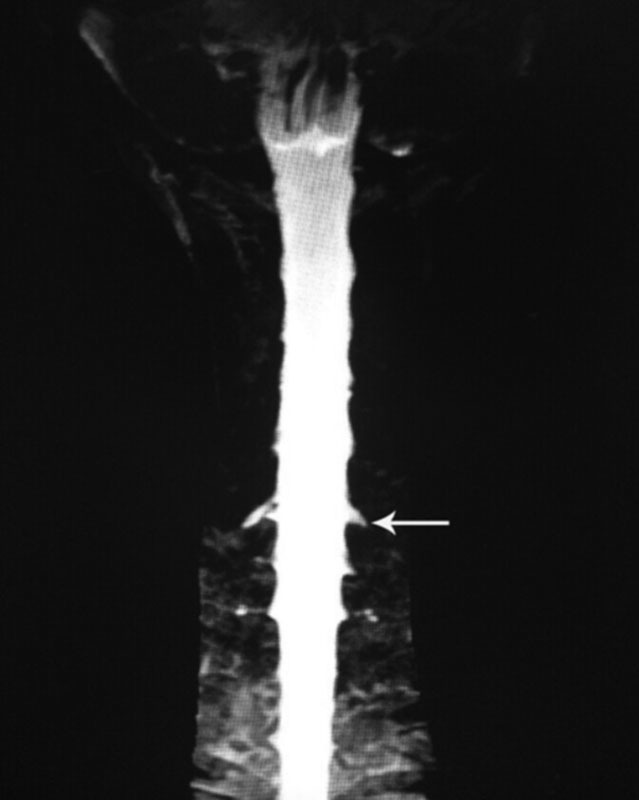
MR myelography with contrast demonstrating CSF leakage located at the C6-T1 levels (straight arrow). MR, magnetic resonance; CSF, cerebrospinal fluid.
During the EBP procedure, 72 patients complained of pain at the puncture site, 65 experienced pain radiating to the upper limb, 29 felt numbness in the upper limb, 27 experienced pain radiating to the lower limb, 12 felt numbness in the lower limb, 21 had neck stiffness, 19 had headaches, and 16 were dizzy; all of these symptoms resolved immediately after injection or within hours after the procedure. Transient bradycardia occurred in 12 patients during epidural blood injection, and 3 patients were treated with atropine intravenous injection. No serious procedure-associated mortalities were observed ( Table 3 ).
Table 3. Side effects/complications in 165 patients during EBP placement.
| Refractory SIH n = 165 |
|
|---|---|
| Puncture site pain | 134 (81.2) |
| Radicular pain to upper limbs | 65(39.3) |
| Numbness in upper limbs | 29(17.6) |
| Radicular pain to lower limbs | 27(16.4) |
| Numbness in lower limbs | 12(7.3) |
| Neck stiffness | 21(12.7) |
| Headache | 19(11.5) |
| Dizziness | 16(9.7) |
| Bradycardia | 12(7.3) |
Abbreviations: EBP, epidural blood patch; SIH, spontaneous intracranial hypotension.
Good recovery was obtained in all 165 patients after one ( n = 145), two ( n = 12), three ( n = 7), or four ( n = 1) targeted EBPs during the initial hospitalization. Additionally, eight patients underwent burr hole drainage because of the mass effect of subdural hematomas before performing targeted EBP. Among the 5 patients, 2 had good recovery after 2 EBPs and the remaining 3 patients recovered after 1 EBP. Amongst the 165 patients, 5 had recurrent CSF leaks after discharge. Among the five patients, three had CSF leak recurrences at the previous leakage site, and two had CSF leak recurrences at a different site. All five patients underwent one EBP during the initial hospitalization and experienced sustained symptomatic relief after a repeat targeted EBP. There were no serious complications during EBP placement and after EBP throughout the follow-up period (range, 3.3 years; mean, 1–7 years).
Discussion
SIH is caused by a CSF leak from a tear in the dura mater. The incidence of SIH is 5 per 100,000/year and is more prevalent in middle-aged women, with a female-to-male ratio of 2:1. 13 14 15 16 17 18 Our results are similar to previous reports. Mokri 19 reported that the clinical manifestations of low CSF pressure include orthostatic headache, nausea, vomiting, dizziness, diplopia, blurred visual, photophobia, neck or intrascapular pain, stupor, and coma. Orthostatic headache is the most common clinical manifestation of SIH, occurring in the upright position and relieved when recumbent. 3 19 In the current study, 161 patients had orthostatic headaches as the presenting and core symptom, which is similar to previous reports.
Treatment for SIH usually begins with a conservative approach, such as bed rest, oral hydration, caffeine intake, and abdominal binders. 19 20 The latency of the efficacy of conservative measures is variable. Outcomes have been poorly analyzed, and no management options have been studied in properly controlled randomized trials. 21 There is no consensus regarding the conservative measures for SIH. Indeed, it is expected that some conservative measures will not yield a durable effect. If the symptoms of SIH persist or worsen, a more aggressive approach may be required, such as surgical CSF leakage repair, placement of fibrin glue, and EBP. 22 23
EBP is considered the current mainstay of treatment for SIH patients who have failed conservative measures. 5 14 24 In our institution, we recommend at least 2 weeks of conservative measures for SIH patients before placing an EBP. An EBP may be targeted to the CSF leakage site or “blinded” distant from the CSF leakage site. Some clinicians perform a “blinded” EBP with a uniform injection site in the lumbar spine. 22 25 26 It is our opinion that EBP performed in the lumbar region, though technically easier, offers a lower likelihood of controlling a large leak in the cervical region. Furthermore, the volume of blood injected is much larger if the blood patch is performed at the precise site of the CSF leak. It has been shown in some studies that targeted EBP is more effective than blind EBP for the treatment of SIH. 14 27 28 29 Therefore, we suggest that EBP procedures should be performed as close to the CSF leakage site as possible to increase the efficacy and success rates.
The key to perform targeted EBP is accurately determining the site of CSF leakage.
There are various imaging techniques for confirming the diagnosis of SIH, searching for the site of CSF leakage, and helping to guide targeted EBP, including heavily T2-weighted spine MRI, CT myelography, and MR myelography. 14 30 Heavily T2-weighted spinal MRI is a non-invasive radiologic procedure that usually shows meningeal diverticula, meningeal enhancement, and extrathecal fluid collections, which often extend along several spinal levels in patients with CSF leaks. 31 Mokri 19 reported that it is not uncommon to identify the level of the leak, but it is uncommon to detect the actual site of the leak by spine MRI. CT and MR myelography are the preferred spinal imaging techniques because the site of CSF leak can effectively and safely be identified, and more accurate localization of the tear can be provided than can be with spine MRI. 14 28
In the current study, all patients underwent detection of CSF leakages by heavily T2-weighted spine MRI coupled with CT or MR myelography. The exact CSF leakage site was demonstrated in 152 of 165 patients. Exact CSF leakage sites were also detected on CT myelography in 43 patients and on MR myelography in 111 patients. Of particular note in this series is that there were 11 patients in whom no exact CSF leakage site was demonstrated on CT or MR myelography. We believe that this failure may be due to slow-flow CSF leakage, which remains problematic and a lingering challenge. 32 In the 11 patients, the CSF leakage level was shown on heavily T2-weighted spine MRI. In the current study, 84% of the detected CSF leakage sites were located in the cervical and cervicothoracic regions, which are the common CSF leakage sites in SIH patients. 4
In our institution, heavily T2-weighted spinal MRI along with CT and MR myelography are used as first-line diagnostic tools for the detection of CSF leaks. Given the less invasive nature, we recommend placing an EBP via the results of spine heavily T2-weighted MRI only when the exact CSF leak is not identified via CT or MR myelography. In this series of patients, 11 (6.7%) patients benefited from CSF leak localization on heavily T2-weighted spine MRI. Radioisotope cisternography is another technique to assess CSF leaks, but not sensitive in localizing the exact leakage sites. 33
The success rate of EBPs for CSF leak has not been thoroughly studied and is less impressive than EBPs for post-dural puncture headaches. 34 Many SIH patients may require more than one EBP. In our series of patients, complete recovery was obtained in all patients after one ( n = 145), two ( n = 12), three ( n = 7), or four ( n = 1) targeted EBPs during the initial hospitalization. The success rate after the first targeted EBP was 87.9%. Five SIH patients had recurrent CSF leaks and achieved sustained symptomatic relief after repeat targeted EBP. We suggest that this good outcome may be due to the detection of CSF leakage sites by iconography and performing targeted EBP for the CSF leak. Strict bed rest for 48 hours after EBP may also play an indispensable role in satisfactory recovery. We also believe that a minimum of a 1-week interval between two EBPs is important so that the EBP can form a scar to seal the previous CSF leak.
It is well known that SIH is sometimes associated with subdural hematomas. In the current study, 31 (18.8%) patients were shown to have subdural hematomas on brain MRI. Whether or not burr hole drainage should be performed as the initial procedure remains controversial. 35 36 Among the 31 patients, 5 (16.1%) had neurosurgical consultations and underwent burr hole drainage because of the mass effect of subdural hematomas before performing targeted EBP. All five patients experienced good recovery after two targeted EBPs (two patients) and one targeted EBP (three patients). For most SIH patients with subdural hematomas, EBP should be considered as the initial treatment prior to irrigation of the hematoma. In some patients in whom the subdural hematoma becomes symptomatic, irrigation of the hematoma should be considered.
Attentions should be paid to physical discomfort during the targeted EBP procedure, as well as for performing blind EBPs. 37 In this study, the cervical and cervicothoracic levels were the frequent sites, and targeted EBP may thus be associated with a higher risks. The adverse effects of targeted EBP include neck stiffness, numbness, radicular pain, dizziness, headache, fever, and puncture site pain. The adverse effects were partly related to the volume and speed of injecting blood. There was no consensus among previous reports regarding the amount of blood volume to be injected while performing EBP. We recommend reducing the speed or stopping the injection of blood when patients complain of pain and numbness or other serious symptoms. In our hands, the volume of injected blood was ≤10, ≤15, ≤15, and ≤20 mL for targeted EBP at the cervical, cervical-thoracic, lower thoracic, and lumbar levels, respectively. In the current study, we slowly injected sterile autologous peripheral unclotted venous blood (range, 6–20 mL; mean, 12.24 mL) into the epidural space. The most common side effects were puncture site pain while injecting blood. Most of these symptoms related to EBP resolved after the procedure. There were no serious complications observed during or after EBP. The puncture site pain and pain or numbness in the limbs result from the traction of adhesion due to spinal fluid in the epidural space. The neck stiffness may be due to the effect of blood in the cervical region. The headache during or after blood injection is usually different from the orthostatic headache related to SIH and may be due to the transient increase in CSF pressure or rebound intracranial hypertension. 19 38
When an EBP fails, epidural injection of fibrin glue or fibrin glue followed by blood may help to close the CSF leak. 39 The effectiveness of fibrin glue has not been established due to limited data. Surgical repair is a treatment of last resort and can be tried for patients who remain refractory to EBP or epidural fibril glue injection. None of the patients in the current series needed epidural fibril glue injection or surgical repair of CSF leakage.
In the current series of patients, five (3%) had recurrent CSF leaks after the initial EBP. Literature pertaining to the recurrence of SIH is lacking, and the incidence has not been previously established. Spontaneous CSF leaks can occur with variable frequency and intervals from the previous leak or from a different site ranging from weeks to years. 32 Among the five patients with recurrent CSF leaks, three had recurrences at the previous leakage site, and two had recurrences from a different site. Repeat EBPs were performed after relocalization of CSF leaks, and symptom relief was obtained. Among the 165 patients, 20 underwent repeat EBPs during the initial hospitalization, and 5 underwent repeat EBPs after the recurrences. In the 25 patients who required more than one EBP, 23 had multiple levels of CSF leakage, which usually represents a significant therapeutic challenge in our institution. Repeat EBPs can increase the success rate of EBP for SIH patients, and a previous EBP failure should not be taken as a signal that a subsequent EBP will fail.
In the present series, targeted EBPs were performed in refractory cases with headaches that did not regress within 2 weeks and along with CSF leakage on imaging techniques despite strict conservative treatment. This is the largest patient study on targeted EBP in the treatment of refractory SIH patients in China. We acknowledge that this study was limited by the retrospective design. Our data confirmed that with accurate localization of the CSF leakage site, targeted and repeat EBPs are safe and highly effective for refractory SIH.
Acknowledgments
This study did not receive any financial support. The data of the study was partly presented at the 15th Annual Meeting of the Japan CSF Hypovolemia Society in Tokyo in March 2016.
Footnotes
Conflicts of Interests The authors declare that they have no conflicts of interests.
References
- 1.Schaltenbrand G. Neuere anschauungen zur pathophysiologie der liquorzirkulation. Zentralbl Neurochir. 1938;3:290–300. [Google Scholar]
- 2.Schievink W I. Spontaneous spinal cerebrospinal fluid leaks. Cephalalgia. 2008;28(12):1345–1356. doi: 10.1111/j.1468-2982.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 3.Mokri B. Spontaneous cerebrospinal fluid leaks: from intracranial hypotension to cerebrospinal fluid hypovolemia--evolution of a concept. Mayo Clin Proc. 1999;74(11):1113–1123. doi: 10.4065/74.11.1113. [DOI] [PubMed] [Google Scholar]
- 4.Mokri B.Low cerebrospinal fluid pressure syndromes Neurol Clin 2004220155–74., vi [DOI] [PubMed] [Google Scholar]
- 5.Rahman M, Bidari S S, Quisling R G, Friedman W A.Spontaneous intracranial hypotension: dilemmas in diagnosis Neurosurgery 201169014–14., discussion 14 [DOI] [PubMed] [Google Scholar]
- 6.Kim S Y, Hong J H. Epidural blood patches in a patient with multi-level cerebrospinal fluid leakage that was induced by spontaneous intracranial hypotension. Korean J Pain. 2010;23(01):46–50. doi: 10.3344/kjp.2010.23.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benzon H T, Nemickas R, Molloy R E, Ahmad S, Melen O, Cohen B. Lumbar and thoracic epidural blood injections to treat spontaneous intracranial hypotension. Anesthesiology. 1996;85(04):920–922. doi: 10.1097/00000542-199610000-00029. [DOI] [PubMed] [Google Scholar]
- 8.Peng P W, Farb R. Spontaneous C1-2 CSF leak treated with high cervical epidural blood patch. Can J Neurol Sci. 2008;35(01):102–105. doi: 10.1017/s0317167100007654. [DOI] [PubMed] [Google Scholar]
- 9.Rai A, Rosen C, Carpenter J, Miele V. Epidural blood patch at C2: diagnosis and treatment of spontaneous intracranial hypotension. AJNR Am J Neuroradiol. 2005;26(10):2663–2666. [PMC free article] [PubMed] [Google Scholar]
- 10.Hannerz J, Dahlgren G, Irestedt L, Meyerson B, Ericson K. Treatment of idiopathic intracranial hypotension: cervicothoracic and lumbar blood patch and peroral steroid treatment. Headache. 2006;46(03):508–511. doi: 10.1111/j.1526-4610.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 11.Sencakova D, Mokri B, McClelland R L. The efficacy of epidural blood patch in spontaneous CSF leaks. Neurology. 2001;57(10):1921–1923. doi: 10.1212/wnl.57.10.1921. [DOI] [PubMed] [Google Scholar]
- 12.Headache Classification Subcommittee of the International Headache Society.The International Classification of Headache Disorders Cephalalgia 2004240179–80. [Google Scholar]
- 13.Schievink W I. Misdiagnosis of spontaneous intracranial hypotension. Arch Neurol. 2003;60(12):1713–1718. doi: 10.1001/archneur.60.12.1713. [DOI] [PubMed] [Google Scholar]
- 14.Schievink W I. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295(19):2286–2296. doi: 10.1001/jama.295.19.2286. [DOI] [PubMed] [Google Scholar]
- 15.Schievink W I, Schwartz M S, Maya M M, Moser F G, Rozen T D. Lack of causal association between spontaneous intracranial hypotension and cranial cerebrospinal fluid leaks. J Neurosurg. 2012;116(04):749–754. doi: 10.3171/2011.12.JNS111474. [DOI] [PubMed] [Google Scholar]
- 16.Shima K, Ishihara S, Tomura S. Pathophysiology and diagnosis of spontaneous intracranial hypotension. Acta Neurochir Suppl (Wien) 2008;102:153–156. doi: 10.1007/978-3-211-85578-2_31. [DOI] [PubMed] [Google Scholar]
- 17.Schievink W I, Maya M M, Moser F, Tourje J, Torbati S. Frequency of spontaneous intracranial hypotension in the emergency department. J Headache Pain. 2007;8(06):325–328. doi: 10.1007/s10194-007-0421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mokri B.Spontaneous intracranial hypotension Continuum (Minneap Minn) 201521(4 headache):1086–1108. [DOI] [PubMed] [Google Scholar]
- 19.Mokri B. Headaches caused by decreased intracranial pressure: diagnosis and management. Curr Opin Neurol. 2003;16(03):319–326. doi: 10.1097/01.wco.0000073933.19076.c0. [DOI] [PubMed] [Google Scholar]
- 20.Paldino M, Mogilner A Y, Tenner M S. Intracranial hypotension syndrome: a comprehensive review. Neurosurg Focus. 2003;15(06):ECP2. [PubMed] [Google Scholar]
- 21.Mokri B. Spontaneous intracranial hypotension. Curr Pain Headache Rep. 2001;5(03):284–291. doi: 10.1007/s11916-001-0045-7. [DOI] [PubMed] [Google Scholar]
- 22.Berroir S, Loisel B, Ducros A et al. Early epidural blood patch in spontaneous intracranial hypotension. Neurology. 2004;63(10):1950–1951. doi: 10.1212/01.wnl.0000144339.34733.e9. [DOI] [PubMed] [Google Scholar]
- 23.Schievink W I, Maya M M, Moser F M. Treatment of spontaneous intracranial hypotension with percutaneous placement of a fibrin sealant. Report of four cases. J Neurosurg. 2004;100(06):1098–1100. doi: 10.3171/jns.2004.100.6.1098. [DOI] [PubMed] [Google Scholar]
- 24.Franzini A, Messina G, Nazzi V et al. Spontaneous intracranial hypotension syndrome: a novel speculative physiopathological hypothesis and a novel patch method in a series of 28 consecutive patients. J Neurosurg. 2010;112(02):300–306. doi: 10.3171/2009.6.JNS09415. [DOI] [PubMed] [Google Scholar]
- 25.Ferrante E, Arpino I, Citterio A, Wetzl R, Savino A. Epidural blood patch in Trendelenburg position pre-medicated with acetazolamide to treat spontaneous intracranial hypotension. Eur J Neurol. 2010;17(05):715–719. doi: 10.1111/j.1468-1331.2009.02913.x. [DOI] [PubMed] [Google Scholar]
- 26.Madsen S A, Fomsgaard J S, Jensen R; Soren Aalb.Epidural blood patch for refractory low CSF pressure headache: a pilot study J Headache Pain 20111204453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mokri B, Maher C O, Sencakova D. Spontaneous CSF leaks: underlying disorder of connective tissue. Neurology. 2002;58(05):814–816. doi: 10.1212/wnl.58.5.814. [DOI] [PubMed] [Google Scholar]
- 28.Schievink W I, Maya M M, Louy C, Moser F G, Tourje J. Diagnostic criteria for spontaneous spinal CSF leaks and intracranial hypotension. AJNR Am J Neuroradiol. 2008;29(05):853–856. doi: 10.3174/ajnr.A0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho K I, Moon H S, Jeon H J, Park K, Kong D S. Spontaneous intracranial hypotension: efficacy of radiologic targeting vs blind blood patch. Neurology. 2011;76(13):1139–1144. doi: 10.1212/WNL.0b013e318212ab43. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y F, Lirng J F, Fuh J L, Hseu S S, Wang S J. Heavily T2-weighted MR myelography vs CT myelography in spontaneous intracranial hypotension. Neurology. 2009;73(22):1892–1898. doi: 10.1212/WNL.0b013e3181c3fd99. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe A, Horikoshi T, Uchida M, Koizumi H, Yagishita T, Kinouchi H. Diagnostic value of spinal MR imaging in spontaneous intracranial hypotension syndrome. AJNR Am J Neuroradiol. 2009;30(01):147–151. doi: 10.3174/ajnr.A1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mokri B. Spontaneous low pressure, low CSF volume headaches: spontaneous CSF leaks. Headache. 2013;53(07):1034–1053. doi: 10.1111/head.12149. [DOI] [PubMed] [Google Scholar]
- 33.Mokri B. Radioisotope cisternography in spontaneous CSF leaks: interpretations and misinterpretations. Headache. 2014;54(08):1358–1368. doi: 10.1111/head.12421. [DOI] [PubMed] [Google Scholar]
- 34.Mokri B. Spontaneous CSF leaks: low CSF volume syndromes. Neurol Clin. 2014;32(02):397–422. doi: 10.1016/j.ncl.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Schievink W I, Maya M M, Moser F G, Tourje J. Spectrum of subdural fluid collections in spontaneous intracranial hypotension. J Neurosurg. 2005;103(04):608–613. doi: 10.3171/jns.2005.103.4.0608. [DOI] [PubMed] [Google Scholar]
- 36.Schievink W I, Maya M M, Pikul B K, Louy C. Spontaneous spinal cerebrospinal fluid leaks as the cause of subdural hematomas in elderly patients on anticoagulation. J Neurosurg. 2010;112(02):295–299. doi: 10.3171/2008.10.JNS08428. [DOI] [PubMed] [Google Scholar]
- 37.Allegri M, Lombardi F, Custodi V M et al. Spontaneous cervical (C1-C2) cerebrospinal fluid leakage repaired with computed tomography-guided cervical epidural blood patch. J Pain Symptom Manage. 2010;40(03):e9–e12. doi: 10.1016/j.jpainsymman.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Kranz P G, Amrhein T J, Gray L. Rebound intracranial hypertension: a complication of epidural blood patching for intracranial hypotension. AJNR Am J Neuroradiol. 2014;35(06):1237–1240. doi: 10.3174/ajnr.A3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gladstone J P, Nelson K, Patel N, Dodick D W. Spontaneous CSF leak treated with percutaneous CT-guided fibrin glue. Neurology. 2005;64(10):1818–1819. doi: 10.1212/01.WNL.0000162029.96759.D2. [DOI] [PubMed] [Google Scholar]


