Abstract
Objective Nasal and paranasal malignant tumors invading the skull base are rare and poorly studied. We evaluated postoperative complications in patients undergoing salvage surgery for such tumors.
Design Retrospective study.
Setting Kobe University Hospital.
Participants Among 48 patients who underwent surgery for tumors involving the skull base between 1993 and 2015, 21 patients had squamous cell carcinoma, 13 had olfactory neuroblastoma, 5 had adenocarcinoma, 2 had sarcoma, 2 had adenoid cystic carcinoma, and 1 each had malignant melanoma, poorly differentiated carcinoma, undifferentiated carcinoma, myoepithelial carcinoma, and malignant peripheral nerve sheath tumor. Prior to skull base surgery, radiotherapy, chemoradiotherapy (CRT), particle radiotherapy, chemotherapy, or surgery were applied in 3, 15, 4, 5, and 3 patients, respectively.
Main Outcome Measures Main outcome measures were postoperative complications in patients who underwent skull base surgery after concomitant CRT and/or particle therapy.
Results Major postoperative complications were observed in 14 surgical procedures (29%; 2 patients with cerebral herniation, 3 with cerebrospinal fluid leakages, 3 with meningitis, 1 with hydrocephalus, 6 with epidural abscesses, 2 with local infections, and 2 with partial flap necrosis). Four patients developed ≥2 complications. One patient died of postoperative lung infarction. Three (16.7%) of 18 patients without prior treatment and 9 (50%) of 18 patients who underwent preoperative radiotherapy/CRT had severe postoperative complications. Two (50%) of four patients treated with particle radiotherapy had postoperative complications.
Conclusions CRT or particle radiotherapy were significantly associated with a high risk of severe postoperative complications after skull base surgery. Meticulous care should be taken in patients treated with radiotherapy/particle therapy prior to skull base surgery.
Keywords: chemoradiotherapy, complications, malignant nasal and paranasal tumors, particle radiotherapy, salvage surgery, skull base surgery
Introduction
Nasal and paranasal malignant tumors invading the skull base are relatively rare, accounting for ∼3 to 5% of all malignant head and neck tumors. 1 In view of the rarity of cancer developing at this anatomical site and due to the multiplicity of histological types, no randomized clinical trials have been completed to generate treatment recommendations. Operative mortality rates are reported to be <5%, but the overall complication rate for craniofacial resection (CFR) has been reported to range from 25 to 65%. 2 3 4 5 6 Recently, chemoradiotherapy (CRT) and particle radiotherapy have been employed as alternative treatment options for these cancers in patients who decline CFR.
Skull base surgery can be employed as a rescue treatment for recurrent cancers. Yet, in our previous study, we reported that postoperative complications were significantly higher in patients who first underwent radiotherapy with a dose of more than 60 Gy. Similarly, severe complications are also expected in patients who undergo particle radiotherapy, but to date, no report has focused on the postoperative complications of skull base surgery after concomitant CRT or particle therapy.
Methods
A retrospective analysis was conducted in patients with nasal or paranasal sinus tumors invading the skull base, who had been surgically treated at Kobe University Hospital between 1993 and 2015. A total of 26 men and 22 women were included in the analysis, with an average age of 59 years (range, 13−73 years). Forty-seven patients underwent 48 skull base surgeries for tumors involving the skull base during this period. Thirty patients had the following treatments prior to skull base surgery: CRT ( n = 15), radiotherapy ( n = 3), particle radiotherapy ( n = 4), chemotherapy ( n = 5), and surgery ( n = 3). All patients had malignant tumors, and squamous cell carcinoma was the most common pathology (squamous cell carcinoma [ n = 21], olfactory neuroblastoma [ n = 13], adenocarcinoma [ n = 5], and others [ n = 9]). Follow-up periods after treatment ranged from 1 to 215 months (median, 36 months). Overall survival rates, disease-free survival rates, and local control rates were calculated using the Kaplan − Meier method. For statistical analysis, Fisher's exact probability test was used, and p < 0.05 was considered statistically significant.
Results
Surgical Management
The surgical approach was classified based on the extent of resection required. Fig. 1 illustrates pattern of dissection. Fig. 1A included en bloc resection of the tumor, involving the ethmoid and/or sphenoid sinuses. A bifrontal craniotomy was performed to access the anterior skull base, incorporating the most medial aspect of the tumor involving the anterior skull base. Orbital contents were preserved in this procedure. Defects in the anterior skull base were reconstructed with a pericranial flap. Fig. 1B used an infratemporal-subtemporal approach via a frontolateral craniotomy and total maxillectomy with infratemporal dissection, through a classic or modified Weber–Fergusson incision. Any defect in the anterolateral skull base was reconstructed using a free flap of rectus abdominal muscle. Procedures for Fig. 1A and B were performed in 21 and 23 patients, respectively. Atypical approaches were performed on the remaining patients.
Fig. 1.
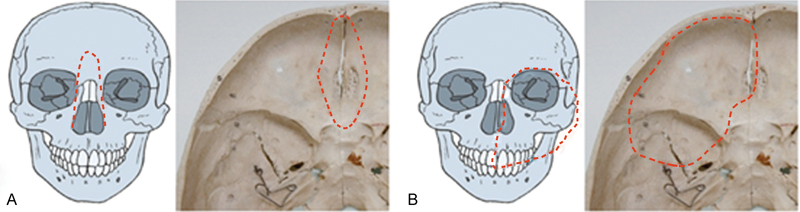
( A ) Dissection pattern includes en bloc resection of the tumor involving ethmoid and/or sphenoid sinuses. Bifrontal craniotomy is performed to access the anterior skull base. Dotted lines denote resection of cribriform plate and nasal bone. ( B ) Dissection pattern entails infratemporal-subtemporal approach through anterolateral craniotomy and total maxillectomy with infratemporal dissection, through a classic or modified Weber–Fergusson incision. Dotted lines denote resection of anterolateral skull base and orbital contents, maxilla.
Oncological Outcome, Postoperative Mortality, and Major Complications
Kaplan–Meier curves for local control rate, disease-free survival rate, and overall survival rate are given in Fig. 2 . The 5-year local control rate, disease-free survival rate, and overall survival rate were 76%, 55.6%, and 66.7%, respectively.
Fig. 2.
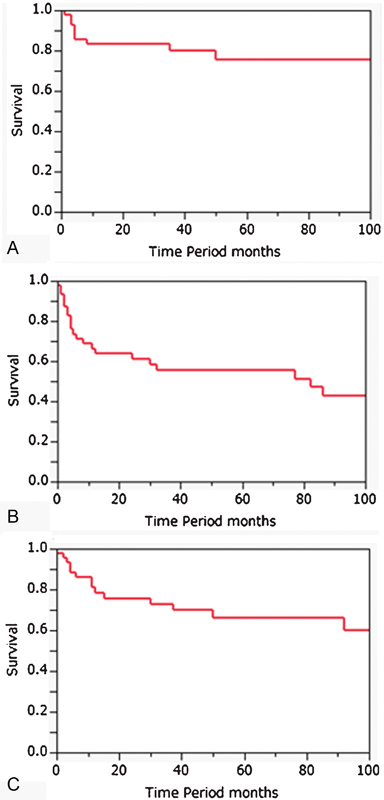
Kaplan–Meier survival curves of actuarial 5-year local control ( A ), disease-free survival ( B ), and overall survival ( C ) for the treatment of nasal and paranasal malignant tumors involving the skull base during the allocated period.
In our series of 47 patients undergoing surgery for nasal and paranasal malignant tumors involving the skull base, one (2.1%) patient died perioperatively due to a lung infarction. Major postoperative complications were observed after 14 (29.7%) surgical procedures. Two or more complications were observed in four patients. Postoperative complications are reported in Table 1 . Complications involving the central nervous system occurred in 14 (29.2%) patients, systemic complications occurred in one (2.4%) patient, and wound complications were observed in 10 (21.2%) patients. Postoperative complications according to patients' variables predictive for mortality are shown in Table 2 . Patients >60 years of age and previous treatments were not significant predictors of postoperative complications. However, previous radiotherapy or CRT was a significant predictor of postoperative complications ( p < 0.03).
Table 1. Postoperative complications.
| Complications | No. of patients |
|---|---|
| Central nervous system complications | |
| Cerebrospinal fluid leakage | 3 (6.3%) |
| Meningitis | 3 (6.3%) |
| Cerebral herniation | 2 (4.2%) |
| Encephalitis | 1 (2.1%) |
| Hydrocephalus | 1 (2.1%) |
| Wound complications | |
| Epidural abscess | 6 (12.5%) |
| Partial flap necrosis | 2 (4.2%) |
| Local infections | 2 (4.2%) |
| Systemic complication | |
| Death | 1 (2.4%) |
Table 2. Postoperative complications according to patients' factors.
| Factor | No. of patients | Major Local Complications | p -Value |
|---|---|---|---|
| Age (year) | NS | ||
| <60 | 23 | 6 | |
| ≧60 | 25 | 8 | |
| Previous treatment | NS | ||
| No | 18 | 2 | |
| Yes | 30 | 12 | |
| Radiotherapy | p = 0.03 | ||
| None | 26 | 3 | |
| RT, CRT or, particle radiotherapy | 22 | 11 | – |
Abbreviations: CRT, chemoradiotherapy; RT, radiotherapy.
Major Postoperative Complications According to Extent of Surgery
Postoperative complications according to surgical extent, as predictors of mortality, are shown in Table 3 . There were no significant differences in complications associated with resection of the orbit, frontal lobe, or dura mater. However, Fig. 1B (anterolateral skull base) resection had a significantly higher rate of complications compared with a Fig. 1A (anterior skull base) resection.
Table 3. Postoperative major complications according to extent of surgery.
| Surgical extent | No. of patients | Major local complications | p -Value |
|---|---|---|---|
| Orbital contents | p = 0.057 | ||
| Preserved | 24 | 3 | |
| Resected | 24 | 11 | |
| Cranial base resection | p < 0.05 | ||
| Anterior ( Fig. 1A ) | 21 | 2 | |
| Anterolateral ( Fig. 1B ) | 23 | 11 | |
| Others | 4 | 1 | |
| Frontal lobe | NS | ||
| Preserved | 43 | 11 | |
| Resected | 5 | 3 | − |
| Dura mater | NS | ||
| Preserved | 37 | 9 | |
| Resected | 11 | 5 |
Abbreviation: NS, non-significant.
Major Postoperative Local Complications According to RT, CRT, or Particle Therapy
Table 4 summarizes the major postoperative complications according to prior RT, CRT, or particle radiotherapy. Nine (50%) of 18 patients treated with prior radiotherapy or CRT had postoperative complications, and 2 (50%) of 4 patients treated with particle radiotherapy also had severe postoperative complications. In particular, patients who received >60 Gy of radiotherapy or particle radiotherapy had more severe complications. For example, one patient had encephalitis, three had CSF leakage, and one had unresectable calcification of the dura.
Table 4. Postoperative major local complications according to RT, CRT, or particle therapy.
| Factor | No. of patients | Major local complications | Details |
|---|---|---|---|
| Radiotherapy | 18 | 9 | |
| <60 Gy | 7 | 4 | Meningitis 1, epidural abscess 2, and partial flap necrosis 2 |
| ≧60 Gy | 11 | 5 | CSF leakage 2,meningitis 1, epidural abscess 2, and unresectable dura for calcification 1 |
| Particle radiotherapy | 4 | 2 | |
| Proton | 3 | 2 | Encephalitis 1, meningitis 1, CSF leakage 1, and epidural abscess 2 |
| Carbon | 1 | 0 |
Abbreviation: CSF, cerebrospinal leakage; RT, radiotherapy; CRT, chemoradiotherapy.
Discussion
In this report, we analyzed oncological outcomes and perioperative complications in patients who underwent skull base surgery at our hospital. We found that prior radiotherapy, CRT, or particle radiotherapy as well as the extent of surgery required were significant predictors of severe postoperative complications.
The introduction of CFR by Ketcham et al in the 1960s 1 was a major advancement in the management of nasal and paranasal malignancies. By including the anterior skull base in the surgery, this procedure has markedly improved local control of tumors invading the skull base. Although this approach was associated with significant morbidity and severe perioperative mortality in the early days, recent advances in skull base reconstruction and the improvement in perioperative management have generalized the use of this surgical technique. In 1994, Kraus et al 3 reviewed 85 consecutive patients who underwent anterior CFR and found that local major complications occurred in 26 (31%) patients, local minor complications occurred in 7 patients (8%), and systemic complications in 5 (6%). 2 These findings are in accordance with the findings of the present study. Postoperative morbidity has been reported previously and ranges from 0 to 4.7%. 2 3 4 5 6 7 Donald 8 also reported a complication rate of 50.5%. 8 At our institution, one patient died due to lung infarction. Following this, we stopped introducing a femoral central intravenous catheter during the perioperative period and gave particular attention to the possibility of deep vein thrombosis. Reported causes of postoperative death include systemic disease, such as acute myocardial infarction, unexpected variceal bleeding due to liver cirrhosis, and cerebrovascular complications, such as infarction of the brain, cerebral edema, and rupture of the internal carotid artery. 7 8
The presence of a medical comorbidity, prior radiation exposure, and both dural and brain involvement were also reported to be associated with severe complications. 3 In the present study, prior irradiation was an important predictive factor of postoperative complications, in accordance with our previous study. 6 We also found that prior particle radiotherapy was a predictive factor for postoperative complications. Patel et al. 9 showed the physical advantage of a rapid dose fall-off beyond the Bragg peak, which allows more conformal treatment with a better targeted dose-coverage of nasal and paranasal tumors. However, as is evident from the present study, particle therapy resulted in poor survivability and frequent postoperative complications.
All patients with prior CRT or particle radiotherapy in our series refused skull base surgery as the initial therapy and received these treatments at other hospitals. However, the previously reported skull base survival rates of patients with squamous cell carcinoma involving skull base invasion, who were treated with particle radiotherapy or CRT, were significantly poorer than those of such patients in the present series. 10 11 Considering these results, we highly recommend skull base surgery as the initial treatment for patients with nasal and paranasal malignant tumors involving the skull base.
In the present study, types of skull base resections had significant correlations with postoperative complications. Anterolateral resection required reconstruction with a free musculocutaneous flap as well as a longer operating time. Consequently, complications associated with the use of free flaps, such as necrosis and epidural abscesses, developed. Taken together, several factors contributed to the occurrence of postoperative complications associated with anterior and anterolateral cranial base surgery. This includes the extent of the surgical approach, whether more than a single surgery was required, prior radiotherapy or CRT, and the experience of the surgical team. As is shown in Table 1 , the most common postoperative complication was epidural abscess. To avoid the occurrence of this complication and to protect the reconstructed dura using an adequate volume of well-vascularized muscle tissue, careful attention needs to be paid to obliterating the skull base defect. In addition, the mucosal remnant of the frontal sinus should be removed in order to prevent sinusitis, and a drainage route from the skull base to the nasal cavity should be secured to prevent epidural fluid accumulation.
Recently, endoscopic endonasal surgery and endoscopic-assisted skull base surgery for nasal and paranasal tumors invading the skull base have been reported, with excellent oncological and functional results. 12 13 14 It, therefore, seems advisable that patients with nasal and paranasal malignancies involving the skull base should be treated with these advanced forms of skull base surgery to avoid unnecessary postoperative complications and to afford good control of tumors.
Conclusion
Patients who underwent definitive CRT or particle radiotherapy were at significantly higher risk of severe postoperative complications after skull base surgery; these patients, therefore, require meticulous care.
Footnotes
Conflict of Interest None.
References
- 1.Ketcham A S, Wilkins R H, Vanburen J M, Smith R R. A combined intracranial facial approach to the paranasal sinuses. Am J Surg. 1963;106:698–703. doi: 10.1016/0002-9610(63)90387-8. [DOI] [PubMed] [Google Scholar]
- 2.Ganly I, Patel S G, Singh B et al. Complications of craniofacial resection for malignant tumors of the skull base: report of an International Collaborative Study. Head Neck. 2005;27(06):445–451. doi: 10.1002/hed.20166. [DOI] [PubMed] [Google Scholar]
- 3.Kraus D H, Shah J P, Arbit E, Galicich J H, Strong E W. Complications of craniofacial resection for tumors involving the anterior skull base. Head Neck. 1994;16(04):307–312. doi: 10.1002/hed.2880160403. [DOI] [PubMed] [Google Scholar]
- 4.Patel S G, Singh B, Polluri A et al. Craniofacial surgery for malignant skull base tumors: report of an international collaborative study. Cancer. 2003;98(06):1179–1187. doi: 10.1002/cncr.11630. [DOI] [PubMed] [Google Scholar]
- 5.Deschler D G, Gutin P H, Mamelak A N, McDermott M W, Kaplan M J. Complications of anterior skull base surgery. Skull Base Surg. 1996;6(02):113–118. doi: 10.1055/s-2008-1058652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nibu K, Sasaki T, Kawahara N, Sugasawa M, Nakatsuka T, Yamada A.Complications of craniofacial surgery for tumors involving the anterior cranial base Neurosurgery 19984203455–461., discussion 461–462 [DOI] [PubMed] [Google Scholar]
- 7.Kim Y S, Moon K S, Kim G W et al. Recent role of craniofacial resection for malignant tumors involving the anterior skull base: surgical experience in a single institution. Brain Tumor Res Treat. 2015;3(02):81–88. doi: 10.14791/btrt.2015.3.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donald P J. Complications in skull base surgery for malignancy. Laryngoscope. 1999;109(12):1959–1966. doi: 10.1097/00005537-199912000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Patel S H, Wang Z, Wong W W et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol. 2014;15(09):1027–1038. doi: 10.1016/S1470-2045(14)70268-2. [DOI] [PubMed] [Google Scholar]
- 10.Morimoto K, Demizu Y, Hashimoto N et al. Particle radiotherapy using protons or carbon ions for unresectable locally advanced head and neck cancers with skull base invasion. Jpn J Clin Oncol. 2014;44(05):428–434. doi: 10.1093/jjco/hyu010. [DOI] [PubMed] [Google Scholar]
- 11.Sakashita T, Hayashi R, Homma A et al. Multi-institutional retrospective study for the evaluation of ocular function-preservation rates in maxillary sinus squamous cell carcinomas with orbital invasion. Head Neck. 2015;37(04):537–542. doi: 10.1002/hed.23639. [DOI] [PubMed] [Google Scholar]
- 12.de Almeida J R, Su S Y, Koutourousiou M et al. Endonasal endoscopic surgery for squamous cell carcinoma of the sinonasal cavities and skull base: oncologic outcomes based on treatment strategy and tumor etiology. Head Neck. 2015;37(08):1163–1169. doi: 10.1002/hed.23731. [DOI] [PubMed] [Google Scholar]
- 13.Hanna E, DeMonte F, Ibrahim S, Roberts D, Levine N, Kupferman M. Endoscopic resection of sinonasal cancers with and without craniotomy: oncologic results. Arch Otolaryngol Head Neck Surg. 2009;135(12):1219–1224. doi: 10.1001/archoto.2009.173. [DOI] [PubMed] [Google Scholar]
- 14.Kassam A B, Thomas A, Carrau R Let al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap Neurosurgery 2008630101ONS44–ONS52., discussion ONS52–ONS53 [DOI] [PubMed] [Google Scholar]


