Abstract
Most IVF-conceived children are healthy, but IVF has also been associated with adverse obstetric and perinatal outcomes as well as congenital anomalies. There is also literature suggesting an association between IVF and neurodevelopmental disorders as well as potentially long-term metabolic outcomes. The main driver for adverse outcomes is the higher risk of multiple gestations in IVF, but as the field moves toward single embryo transfer, the rate of multiple gestations is decreasing. Studies have shown that singleton IVF pregnancies still have a higher incidence of adverse outcomes compared to unassisted singleton pregnancies. Infertility itself may be an independent risk factor. Animal models suggest that epigenetic changes in genes involved in growth and development are altered in IVF during the hormonal stimulation and embryo culture. Further animal research and prospective human data is needed to elucidate the mechanisms by which IVF may contribute to adverse outcomes and to decrease risks.
Keywords: Infertility, IVF, ICSI, perinatal outcomes
Introduction
Infertility affects 7.5 million women in the United States and approximately 1 in 8 couples have trouble conceiving or sustaining a pregnancy.1 Assisted reproductive technologies (ART) are used to treat infertility and include hormonal medications that stimulate the ovulation of one or more oocytes, intrauterine insemination (IUI) in which a processed sample of sperm is instilled into the uterine cavity directly, and in vitro fertilization (IVF). IVF is a particularly successful treatment for infertility. IVF involves ovarian stimulation with gonadotropin hormones, followed by retrieval of oocytes under sedation with subsequent fertilization by sperm in the laboratory, and development of embryos in culture prior to transfer into the uterus (Fig. 1).
Fig 1. The IVF Process.
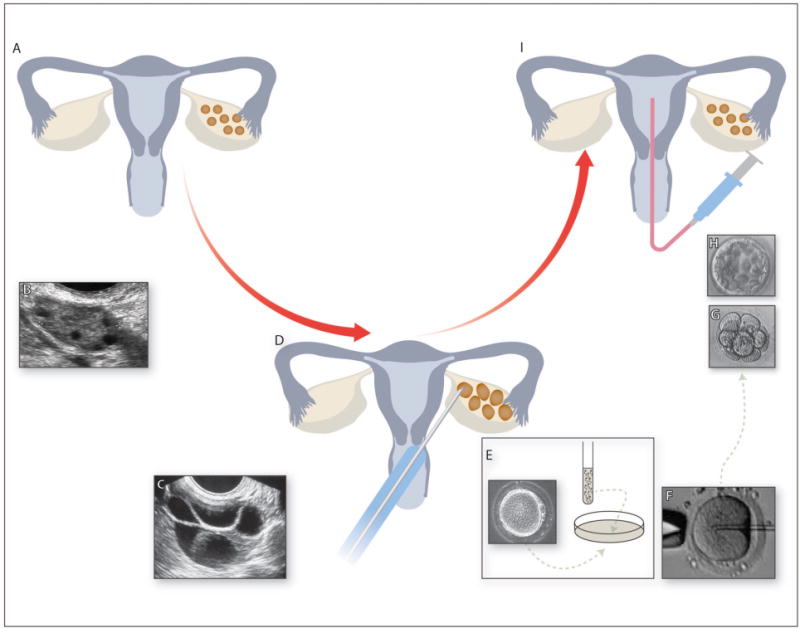
(A) Cartoon showing normal female reproductive system. (B) Transvaginal ultrasound image of unstimulated ovary showing small antral follicles. Ovaries are stimulated by daily gonadotropin injections resulting in the growth of multiple ovarian follicles. (C) Transvaginal ultrasound image of stimulated ovary with multiple growing follicles. (D) When follicles reach a certain size, eggs are retrieved from the follicles under transvaginal ultrasound guidance under sedation. Eggs and sperm are combined in the laboratory either by (E) conventional insemination by combining the sperm and eggs in a dish in the laboratory or by (F) intracytoplasmic sperm injection (ICSI). Embryos are cultured for 3 days (eight-cell embryo - G) to 5 days (blastocyst-stage embryo - H). (I) A selected embryo is then transferred into the uterus and excess good-quality embryos are cryopreserved.
Since the birth of Louise Brown in 1978, over 5 million children have been conceived via IVF2,3 and children conceived after IVF now account for 1.6% of births in the United States.1 IVF may also be combined with intracytoplasmic sperm injection (ICSI), which is a technique for fertilization of the oocyte in the laboratory by directly injecting a single sperm into the cytoplasm of the oocyte (Fig. 1F). Since its development in the early 1990s for the treatment of male infertility, ICSI has gained popularity and, in 2012, ICSI accounted for 93.3% of IVF cycles with male factor infertility and 66.9% of IVF cycles without male factor infertility.4 Embryos produced after IVF or ICSI may be transferred to the uterus during the same cycle of hormonal stimulation (fresh embryo transfer) or they may be cryopreserved and later thawed prior to transfer (frozen/thawed transfer) in a later cycle with more physiologic hormonal levels.
While the majority of IVF-conceived children are healthy, IVF has been associated with an increased risk of adverse obstetric and perinatal outcomes including hypertensive disorders of pregnancy, preterm labor (PTL) and preterm delivery (PTD), and low birth weight (LBW).5,6 IVF pregnancies have also been associated with congenital anomalies, imprinting disorders, and neurodevelopmental disorders.7–9 Furthermore, there is literature that shows that LBW infants are not only at increased risk for adverse neonatal outcomes, but are also at increased risk for adverse metabolic outcomes throughout life including obesity, hypertension, and diabetes.10–16 Many of these outcomes can be attributed to an increased risk of multiple gestations with ART, however, with the increasing use of single embryo transfer, multiple pregnancies have been significantly reduced.17,18 Still, there are conflicting data on whether singleton IVF pregnancies have similar or more adverse outcomes compared to unassisted singleton pregnancies.19–24 This review will examine available data on the association between IVF, with or without ICSI (IVF/ICSI), and adverse perinatal as well as long-term health outcomes and help determine the epidemiological drivers of these outcomes.
Hormonal and Epigenetic Alterations in IVF
In IVF, the oocytes and embryos are exposed to supraphysiological levels of estradiol, which is produced by the ovaries in response to injectable gonadotropins. Vascular endothelial growth factor (VEGF) levels also become elevated in humans and mice after ovarian stimulation and may have a negative impact placentation.25,26 Furthermore, both female and male gametes are manipulated during oocyte retrieval and embryo culture, exposing gametes and embryos to an altered environment at the earliest and, perhaps, most susceptible period. These alterations of the gamete environment may potentially promote changes that lead to adverse perinatal outcomes. The mechanism(s) by which adverse outcomes arise from manipulations of the gamete environment are unknown and likely multifactorial, but epigenetic modification of genes responsible for growth and development may play a role. In fact, animal studies have demonstrated that even in the absence of infertility, procedures and techniques utilized during IVF/ICSI may result in epigenetic changes that lead to long-term changes in neurodevelopment, growth, and metabolism in offspring (Fig. 2).25,27–29
Fig. 2. Summary diagram of adverse maternal and fetal effects associated with IVF.
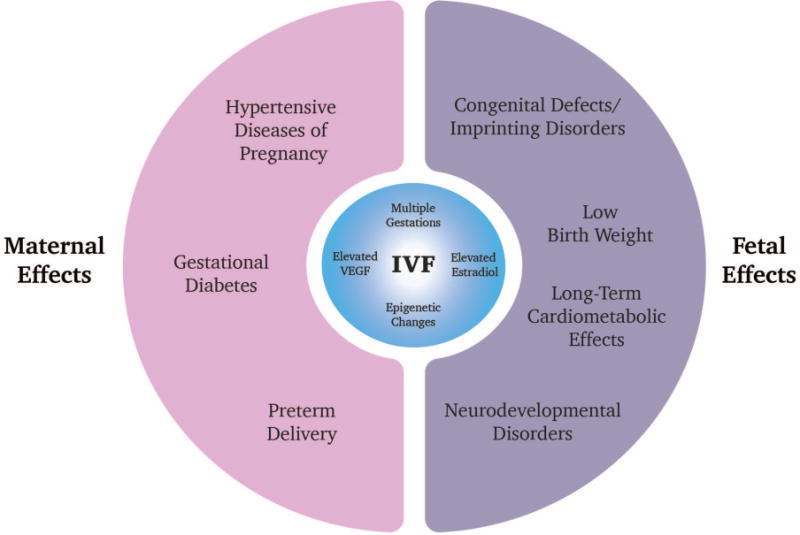
Adverse maternal outcomes associated with IVF include hypertensive diseases of pregnancy, gestational diabetes, and preterm delivery. Fetal effects include low birth weight, congenital defects/imprinting disorders, as well as potentially neurodevelopmental disorders and long-term cardiometabolic effects. The risk of multiple gestations in IVF pregnancies is the main driver for these outcomes, but other mechanisms are actively being investigated. Features of IVF including hormonal stimulation which leads to increased estradiol and VEGF levels may contribute. Epigenetic alterations in imprinted genes after hormonal stimulation and embryo culture may also be a driver for some of these adverse outcomes.
Epigenetics involves molecular mechanisms such as DNA methylation and/or histone modification of chromatin that lead to changes in gene expression and phenotypic characteristics. Imprinting is an epigenetic phenomenon in which genes are expressed by parent-of-origin. Epigenetic adaptations to a stressful intrauterine or embryo culture environment likely lead to long-term effects as shown in several human and animal studies.30,31 Embryo culture conditions have been shown to alter the expression of the imprinted mouse gene H19, which is a regulator of growth.32 Expression of another imprinted gene, Grb10, has been shown to be elevated in the placenta of fetuses derived from the transfer of in vivo derived embryos into a hormonally stimulated recipient mouse.25 Grb10 is a negative growth regulator and an increase in Grb10 was also seen in growth-restricted fetuses.25 Another mouse study demonstrated reduced DNA methylation at the H19/Igf2 imprinting control region (ICR) in the fetal brain and liver of IVF concepti compared to natural controls in one mouse strain, but methylation levels in this region were unchanged in another mouse strain.27 Taken together, the animal data provide convincing evidence that epigenetic changes are occurring during the IVF process and may alter fetal phenotype and long-term health. The fact that these changes are not manifested uniformly is consistent with the theory that only a subset of IVF-conceived children demonstrate adverse outcomes.
Adverse Obstetrical Outcomes
Hypertensive Disorders of Pregnancy
Hypertensive disorders of pregnancy include gestational hypertension, preeclampsia, and eclampsia. A meta-analysis analyzing 15 studies with 12,923 IVF/ICSI pregnancies compared singleton IVF/ICSI pregnancies versus unassisted conceptions and found that the RR of having a hypertensive disorder of pregnancy in IVF/ICSI was 1.49 (95% CI 1.39-1.59).33 A subsequent retrospective cohort study consisting 2641 IVF/ICSI-conceptions and 5282 unassisted conceptions between 2006 and 2014 showed that, among singleton IVF/ICSI conceptions, the odds of having gestational hypertension was 1.99 (95% CI 1.56 – 2.53, p<0.001) compared to singleton unassisted conceptions.34 Singleton IVF/ICSI conceptions also showed a trend toward increased odds of having preeclampsia of 1.71 (95% CI 1.42 – 2.19, p=0.21), but this was not statistically significant. There was no significant difference in incidence of hypertensive disorders of pregnancy between IVF/ICSI twin pregnancies and twins conceived without medical assistance.34 The similarity in the rate of hypertensive disorders in twin IVF/ICSI and unassisted pregnancies is likely because twin pregnancies, regardless of mode of conception, are at higher risk for these disorders. The association between hypertensive disorders of pregnancy in IVF is difficult to ascertain given that women pursuing IVF may be older and may have other comorbidities such as polycystic ovary syndrome (PCOS), which may have prompted infertility treatment. In the studies reviewed, controls were matched by age and eliminated the confounding of maternal age, but comorbidities were not controlled for. Future studies should attempt to control for maternal comorbidities.
Gestational Diabetes
Gestational diabetes (GDM) has also been studied as an outcome in IVF pregnancies because it predisposes to a higher risk of hypertension in pregnancy, fetal macrosomia, operative delivery, and cesarean delivery.35 An increased incidence of GDM in pregnancies conceived via ART has been demonstrated. A cross-sectional study with 215 unassisted pregnancies and 145 singleton ART pregnancies (95 IVF/ICSI pregnancies and 50 IUI pregnancies) excluding patients with conditions that may be confounders including a diagnosis of PCOS, maternal age of 40 years or older, history of diabetes in a first-degree relative, pre-pregnancy diabetes, history of GDM, and prior delivery of a macrosomic infant, determined that the incidence of GDM among IVF/ICSI pregnancies was 43% higher compared to unassisted pregnancies.36 In another retrospective cohort study that did not control for the above confounders, Zhu and colleagues found that GDM was increased in IVF/ICSI singleton pregnancies relative to unassisted singleton pregnancies with odds ratio (OR) in IVF/ICSI singleton pregnancies 2.23 (95% CI 1.85 – 2.69).34 A meta-analysis analyzing six cohort studies and 13,399 IVF/ICSI patients also showed a relative risk of GDM of 1.48 (95% CI 1.33 – 1.66).33 This data suggests an increased incidence of GDM in the ART population in singleton pregnancies. The increased risk may be because women tend to be older when seeking infertility treatment and are therefore at higher risk for GDM due to advancing age. Other studies suggest that the infertility diagnosis itself may confer risk.5 However, even after controlling for confounding factors, an association between ART and GDM remains, which indicates that the ART procedures themselves may contribute to alterations at a molecular level that predispose to the development of GDM.
Preterm Delivery (<37 weeks)
There appears to be an increased risk of preterm delivery in children conceived after IVF. A meta-analysis analyzed 22 studies including 27,819 IVF/ICSI pregnancies and found a higher risk of delivery <37 weeks in IVF/ICSI-conceived children compared to children conceived without medical assistance RR 1.54 (95% CI 1.47 – 1.62).33 Similarly, a prospective cohort study comparing the obstetrical and perinatal outcomes in 1260 women who conceived after IVF, 1899 subfertile women, and 2480 fertile women found that the OR for preterm delivery in IVF was 2.19 (95% CI 1.59 – 3.02).5 An Australian database-linkage study showed that singleton IVF/ICSI pregnancies had a higher odds of preterm delivery: OR 2.20 (95% CI 1.79 – 2.70) for IVF pregnancies with fresh embryo transfer, OR 2.02 (95% CI 1.49 – 2.75) for IVF pregnancies with frozen/thawed embryo transfer, OR 1.63 (95% CI 1.24 – 2.15) for ICSI pregnancies with fresh embryo transfer, but this was not noted in ICSI pregnancies with frozen/thawed embryo transfer OR 1.08 (95% CI 0.60 – 1.94).37 There was no difference in the rate of preterm birth between twin pregnancies conceived with IVF/ICSI or without medical assistance, which is likely due to the higher risk of preterm delivery in twin pregnancies in general, making it difficult to identify a difference if one exists.37 Taken together, the studies indicate that ART procedures may be contributing to the risk of preterm delivery in singleton gestations, but that the risk may be lessened in the setting of frozen/thawed embryo transfers. Some of the risk factors for preterm delivery such as older maternal age and behavioral factors such as smoking may account for some of the risk seen in patients with infertility.38 The frozen/thawed embryo transfer may reduce the risk as it more closely mimics the hormonal levels in an unassisted pregnancy and potential impacts of elevated estradiol and VEGF levels on placentation are avoided. Future studies should attempt to match patients not only by age, but obstetrical and gynecological history as well as other comorbidities to decrease confounding in the data.
Adverse Fetal and Neonatal Outcomes
Low Birth Weight (<2500g)
Abnormalities in birth weight, particularly low birth weight, have been associated with IVF. An increased risk of LBW among children conceived with the assistance of IVF/ICSI compared to those conceived without medical assistance, RR 1.65 (95% CI 1.56-1.75) in a meta-analysis of 19 studies and 28,352 pregnancies.33 The association between IVF/ICSI conception and LBW may be due to the supraphysiological hormonal environment of the IVF cycle. A study by Kalra and others compared the birth weight of singleton infants born after IVF with fresh embryo transfer, versus singletons born after IVF with frozen/thawed embryo transfer.23 The adjusted OR for low birth weight was 1.35 (95% CI 1.20 – 1.51) in fresh embryo transfer IVF cycles compared to birth weight after frozen/thawed embryo transfer.23 Similarly, a retrospective cohort study evaluating births after 95,991 fresh and 16,521 frozen/thawed embryo transfer cycles showed a lower incidence of LBW after frozen/thawed embryo transfer cycles compared to fresh IVF cycles, RR 0.73 (95% CI 0.66 – 0.80), but also a higher incidence of high birth weight after frozen/thawed embryo transfer cycles, RR 1.64 (1.53-1.76).39 However, the effect of the diagnosis of infertility itself is an important consideration as a Dutch population-based sibling study indicated that the birth weight of siblings conceived with IVF was not significantly different from that of siblings conceived without medical assistance and that maternal subfertility itself seemed to contribute to the risk of having a child that is of low birthweight.40
Congenital Defects and Imprinting Disorders
Many studies have highlighted a concern for potentially increased risks of congenital defects and imprinting disorders in IVF pregnancies.9,41–43 However, given the rarity of congenital defects overall, many of these studies have not been adequately powered to detect increased risk if one exists.44 An Australian birth registry study compared the rate of birth defects including cardiovascular, musculoskeletal, and urogenital birth defects in 301 ICSI-conceived infants, 837 IVF-conceived infants, and 4000 control infants conceived without medical assistance.45 The rate of birth defects in IVF and ICSI infants was 8.6% and 9.0%, respectively, compared to 4.2% in control infants.45 However, a subsequent Australian database study with 308,974 births including 6163 assisted conceptions showed that an association between IVF and birth defects disappeared after multivariate analysis accounting for parental factors including maternal age, co-morbid conditions and smoking in pregnancy although an association between ICSI and birth defects remained.46 A history of infertility was also found to be a risk factor for birth defects in this study.46 An infertility diagnosis alone was also shown in a recent study to be associated with an increased risk of birth defects whether conception was assisted or not.47 Thus, there has been conflicting evidence of whether IVF increases the risk of congenital anomalies, or whether the diagnosis of infertility itself is a risk factor for such observed associations.
Imprinting disorders are a class of congenital anomalies affecting growth, development, and metabolism and there has been an association between IVF conceptions and imprinting disorders. Imprinted genes are either maternally or paternally transcribed and their expression is determined by epigenetic modification of parent-specific alleles including DNA methylation, histone modification, and noncoding RNAs. Abnormalities in imprinting are associated with pathological conditions including Beckwith-Wiedemann syndrome (BWS), Angelman syndrome (AS), and Prader-Willi syndromes (PWS).9,48 Imprinting disorders are relatively rare with worldwide estimates of incidence of BWS ranging from 1:287,000 – 1:13,700, incidence of AS ranging from 1: 134,000 to 1:12,000 and PWS estimates ranging from 1:30,000 to 1:10,000.9 BWS is the most widely studied of the imprinting disorders. Studies investigating an association between imprinting disorders and IVF and/or ICSI are conflicting with some studies showing an association up to a nine-fold increase in relative risk (RR) of BWS in IVF/ICSI,49–53 while others show no association or no increased risk.54–56 Many of the studies showing an association between imprinting disorders and ART were questionnaire studies based on registries of patients with BWS and patients with BWS were more likely to be conceived after ART procedures.50–53 One study was a population-based case-control study of patients born in Victoria, Australia between 1983 and 2003 which showed an OR of BWS in IVF of 17.8 with a wide confidence interval (CI 1.8 – 432.9). The studies that showed no increased risk include a large Danish population study that investigated the diagnosis codes for imprinting disorders and clinical symptoms that may be indicative of imprinting disorders in 6052 IVF and 442,349 non-IVF conceived children and found no imprinting disorders in the IVF group,54 a smaller questionnaire study in Ireland and Central England of 2492 ART-conceived children found no imprinting disorders,55 and another Dutch questionnaire study of families of patients with imprinting disorders compared to the larger population which found that after correcting for fertility problems in the parents, there was no increased risk of ART related to imprinting disorders.56 Differences in study methodology and the rarity of imprinting disorders contributes to heterogeneity in these results. However, epigenetic modification of growth genes during hormonal stimulation and embryo culture may put susceptible IVF-concepti at risk for imprinting disorders.27,32
Neurodevelopmental Disorders
The development of the brain may be affected by the manipulation of the hormonal and physical environment of the embryo. Long- and short-term neurodevelopmental outcomes of children conceived after IVF are therefore of interest, but there is conflicting data regarding an association between IVF and neurodevelopmental disorders. A recent study using standardized and validated tools compared the cognitive, motor, and language development of 2-year-old children born after ART to children of the same age conceived without medical assistance found no difference between the groups after adjusting for confounders.57 Other studies have looked at different types of neurodevelopmental disorders with discrepant results.
Cerebral palsy (CP) is an important neurodevelopmental disorder that has been associated with IVF in a large population-based cohort study of children born after IVF (n = 5680) and control children born without medical assistance (n=11,360).7 However, the higher incidence of twin pregnancies, low birth-weight, and prematurity among IVF-conceived children in the study population could potentially account for the higher incidence of CP.7 In another study looking specifically at preterm infants born after IVF, there was a similar incidence of CP between preterm IVF multiples and naturally conceived preterm multiples,58 and a retrospective case-control study showed no increased risk of neurodevelopmental delay in ICSI-conceived children up to 15 months of age.59 An association between IVF or ICSI with CP has been demonstrated in other studies even after adjusting for preterm birth and multiple gestations.60 This conflicting data suggests that more studies and, in particular, long-term prospective data is needed to determine whether an association truly exists and, if so, its magnitude.
Autism and autism spectrum disorders (ASD) have also been the focus of much investigation as the CDC reports that the incidence of ASD has increased 123% from 2002 to 2010 with an incidence of 1 in 68 in 2014.61 While IVF alone has not been shown to be associated with autism in several studies, ICSI has been associated with autism.8,62–64 A Dutch study comparing singleton 5 – 8 year-old children born after IVF (n=92), ICSI (n=87), and without medical assistance (n=85) found a 3.4% incidence of autism in the ICSI group, which is higher than the expected 0.3-0.4% incidence in the population, but this should be cautiously interpreted given the small numbers in the study.62 A subsequent large retrospective cohort study investigating ART-conceived infants born in California between 1997 – 2006 (n = 42,383) with a 5-year follow-up period found an incidence of autism of ~0.8% for singletons and ~1.2% for multiples and the incidence of autism was higher in the ICSI-conceived children compared to IVF-conceived children, even after adjusting for male factor infertility.63 The incidence of autism was lower in singletons whose parents’ diagnosis of infertility was unexplained, and in multiples whose parents were diagnosed with tubal factor infertility, compared to other infertility diagnoses.63 An increased risk of autism and mental retardation was also shown in a prospective cohort study when ICSI was performed for the indication of male factor infertility regardless of whether sperm was ejaculated or surgically extracted.65 Infertility alone may contribute to the development of neurodevelopmental disorders in offspring and alteration of sperm DNA methylation in older fathers may also play a role.66 It is important to note that paternal age alone has also been associated with neuropsychiatric disorders including autism66 and is likely a confounder in these IVF/ICSI studies as older men may be more likely to require ICSI for male factor infertility.
Long-Term Cardiometabolic Outcomes in IVF/ICSI-Conceived Children
As previously mentioned, children born following IVF are more likely to be born with LBW compared to their naturally conceived counterparts. These LBW infants are at increased risk of metabolic abnormalities later in life.12–14,67 However, independent of birthweight, there is evidence that at least some IVF-conceived children may be at increased risk for cardiometabolic disorders including insulin resistance, higher blood pressure, and higher body fat percentage compared to children conceived without medical assistance.68 Most children conceived through IVF are still in their first three decades of life and, thus, limited data exists regarding long-term morbidity and mortality. Surrogate measures of cardiometabolic health, including body mass index (BMI), body fat, blood pressure, serum lipids, inflammatory factors, and glucose tolerance, have been used to predict the risks in IVF-conceived children and young adults.
BMI and Body Fat
Though the effect of IVF on postnatal life has been difficult to elucidate, studies have observed alterations in growth and metabolism in children born following IVF. IVF-conceived children have been shown to have a higher velocity of weight gain in infancy compared to control children conceived without medical assistance,69 though height, weight, head circumference, and serum insulin growth factor levels are similar in children conceived after IVF and those conceived without medical assistance.70,71 This ‘catch-up’ growth has been associated with higher central fat distribution in children in a prospective cohort study in the United Kingdom,72 but was not seen in another study.69 Several studies also report an increase in body fat in IVF-conceived children compared to age-matched controls who were conceived without medical assistance. In the OMEGA study, IVF-conceived children had a significantly higher sum of skinfolds but there was no significant difference in height, weight, or BMI in IVF children compared to controls.73,74 IVF-conceived children had a significantly higher peripheral body fat mass (7.59 ± 4.22 versus 6.69 ± 3.15 kg, p=0.039), a significantly higher percent of peripheral body fat (27.5 ± 9.0 vs. 25.8 ± 8.3%, p=0.030), and a significantly lower percent of lean mass (69.0 ± 8.7 vs 70.5 ± 8.0%, p=0.023).73
Blood Pressure
The data on blood pressure in children conceived after IVF has also been nuanced. Several studies have noted an increase in blood pressure among children conceived via IVF compared to peers conceived without medical assistance. In the OMEGA cohort study, IVF-conceived children were twice as likely as control children to have a higher systolic or diastolic blood pressure even after controlling for potential confounders,74 suggesting that it is actually the interventions utilized during IVF, and not the underlying infertility diagnosis or patient population, that is responsible for this outcome. In a subsequent study by the same group, 106 IVF-conceived children, ages 4-14 years old, were found a higher blood pressure compared to age-matched controls conceived without medical assistance (p <0.001).75 Other studies have examined specific interventions utilized during ART the effect on blood pressure. In several studies of prepubertal children conceived after ovarian stimulation in IVF or after IVF with ICSI, children were found to have higher blood pressure compared to peers conceived without medical assistance or minimal stimulation prior to IVF.76–78 These studies are limited by small sample size and a limited number of blood pressure recordings. However, by the hypothesis of the “developmental origins of adult disease,” the trend toward higher blood pressure in singleton IVF or ICSI children may be consistent with the higher risk of low birthweight leading to catch-up growth and subsequently cardiometabolic disease.79 Therefore, the stimulated environment of the oocyte or embryo, or the manipulation of the embryos in the laboratory may cause epigenetic changes in systems involved in blood pressure regulation. Interestingly, in a similar cohort of singleton adolescent children conceived ICSI, systolic and diastolic baseline blood pressure were similar to age-matched children conceived without medical assistance.80 The sample size in this study was small with blood pressure measurements available for only 217 ICSI-conceived children, and 223 children conceived without medical assistance and continuous blood pressure recordings during a psychological stress test was available for even fewer children, 67 ICSI and 38 unassisted. However, the lack of consistent data from these studies are likely in keeping with the fact that most IVF-conceived children are healthy and only some concepti who are particularly at risk from early epigenetic changes may have adverse cardiometabolic outcomes such as high blood pressure.
Adolescents conceived after IVF or ICSI have also been noted to have impaired systemic and pulmonary vascular dysfunction compared to adolescents conceived from subfertile patients without medical assistance, while adolescents conceived after maternal hormonal stimulation only had normal vascular function.81 This suggests that manipulation of gametes during IVF or ICSI, as opposed to hormonal stimulation, may account for the vascular dysfunction seen. Children conceived without assistance from subfertile parents were used as controls to decrease the effect that subfertility itself may have on cardiometabolic outcomes. The data suggests that, independent of subfertility or infertility, as the number of manipulations to the embryos increases, the risk of adverse effects including long term outcomes may increase. The fact that these effects are only seen in a subset of patients conceived after IVF again suggests that some concepti may be particularly susceptible to environmental perturbations.
Serum lipids
As previously stated, estradiol is produced in response to gonadotropin stimulation during IVF and these levels are supraphysiologic. The supraphysiologic levels of estradiol may contribute to altered lipid profiles in IVF-conceived children as Meng et al. (2015) showed that high estradiol levels achieved during ovarian stimulation were associated with elevated total cholesterol (TC) and low density lipoprotein cholesterol (LDL) levels as well as decreased high density lipoprotein (HDL) in the umbilical cord blood of IVF-conceived children compared to controls who were conceived without medical assistance. There was no significant difference in triglyceride (TG) levels between the IVF and unassisted conception groups.82 In contrast, IVF-conceived children have been shown to have significantly higher TG levels compared to children conceived without medical assistance in another study.75 The differences in TG levels in these studies may relate directly to methodology as umbilical cord blood for term singletons was tested in one study, while blood was collected by venipuncture in children in the other study and multiples were included as well. More study into the effects of IVF on lipids is necessary, but at least one in vitro study has suggested a possible mechanism for lipid derangements. Estradiol was found to stimulate TC and LDL secretion in human hepatoma cells and this effect was blocked by an estrogen receptor antagonist. Estradiol also increased expression of the enzyme 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) in human hepatoma cells, which is necessary for the synthesis of cholesterol.82
Glucose Intolerance
Data on fasting glucose and insulin in children conceived from IVF compared to unassisted pregnancies is limited. Ceelen and others measured fasting glucose and insulin in 131 IVF-conceived children and 131 control children conceived without medical assistance from subfertile parents and used the glucose to insulin ratio and homeostasis assessment model (HOMA) to determine insulin sensitivity.74 There was no significant difference in fasting insulin concentrations, but children conceived after IVF were noted to have higher fasting glucose compared to controls (5.0 ± 0.4 vs. 4.8 ± 0.4 mmol/liter in controls; p = 0.005).74 Conversely, Miles et al. (2007) found that favorable metabolic parameters in children born after IVF, specifically IVF-conceived children had a higher HDL (p = 0.02), lower TG level (p = 0.02), and lower total to HDL-cholesterol ratio (p = 0.01) compared to that of children conceived without medical assistance, but there was no difference in fasting insulin or glucose between the groups.83 These disparate findings indicate a need for further study.
Potential Drivers of Adverse Outcomes
IVF is a safe and highly successful treatment for infertility. Most children born from IVF are healthy, though long-term data is limited given that the oldest IVF-conceived children are in their third decade of life. However, risks of obstetric and perinatal morbidity such as hypertensive disorders of pregnancy, gestational diabetes, preterm delivery, and low birth-weight have been associated with IVF. These adverse outcomes are largely due to an increased risk of multiple gestations in IVF. Single embryo transfer mitigates these risks and there appears to be no significant difference in birth weight and mean gestational age at delivery between children born after single embryo transfer in IVF and children conceived without medical assistance.19 However, single embryo transfer does not eliminate the associations with adverse outcomes altogether, signaling that either the method or a diagnosis of infertility itself may confer some risk.
Several studies looking at the association of IVF with adverse outcomes have highlighted the fact that the diagnosis of infertility alone may be an independent risk factor for adverse perinatal and long-term health outcomes in children. Patients with infertility may be older and more likely to have preexisting comorbid conditions such as hypertension, diabetes, or insulin resistance, or other endocrinopathies such as thyroid derangements or PCOS that may predispose to adverse obstetric and perinatal outcomes. These confounders should be controlled for in future studies.
The potential contribution of IVF to these adverse outcomes must continue to be explored. The administration of gonadotropins during ovarian stimulation leads to the production of supraphysiological estradiol levels from the ovaries. This exposure to supraphysiological estradiol level in the earliest and perhaps most vulnerable time in embryogenesis may alter implantation and placentation thereby predisposing to hypertensive disorders of pregnancy and impaired fetal growth. The elevated estradiol levels may also alter factors involved in metabolism in offspring leading to metabolic derangements. Elevated VEGF levels after ovarian stimulation may also contribute to poor placentation and therefore predispose to hypertensive disorders of pregnancy, and reduced fetal growth.25,26 The mechanisms by which this may occur require further study as data is limited.
Not only are hormonal changes potential drivers for adverse outcomes, but handling of gametes during the various procedures in IVF including oocyte retrieval, conventional insemination or ICSI, embryo culture, and embryo transfer may also contribute. Both human and animal studies suggest that epigenetic alterations due to the manipulation of the hormonal environment and handling of the gametes and embryos at critical stages of development may be responsible.30,31 Further study is needed to elucidate the mechanism(s) responsible for the adverse perinatal outcomes in IVF and to separate out the contribution that a diagnosis of infertility alone may play. Other health outcomes such as neurodevelopmental disorders, congenital anomalies, increased body fat, and insulin resistance, have been associated with IVF in some studies but not in others. IVF-conceived children should be closely monitored long-term to better understand the long-term health risks so that appropriate changes to the practice can be made and early intervention may be employed.
Footnotes
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Sunderam S, Kissin DM, Crawford SB, et al. Assisted Reproductive Technology Surveillance – United States, 2013. MMWR Surveillance Summaries. 2015;64(11):1–25. doi: 10.15585/mmwr.ss6411a1. [DOI] [PubMed] [Google Scholar]
- 2.Adamson GD, Tabangin M, Macaluso M, de Mouzon J. The number of babies born globally after treatment with the assisted reproductive technologies (ART) Fertility and Sterility. 100(3):S42. [Google Scholar]
- 3.Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 4.Boulet SL, Mehta A, Kissin DM, Warner L, Kawwass JF, Jamieson DJ. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA. 2015;313(3):255–263. doi: 10.1001/jama.2014.17985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin J, Sheng X, Wu D, et al. Adverse Obstetric Outcomes Associated With In Vitro Fertilization in Singleton Pregnancies. Reproductive Sciences. 2016;0(0) doi: 10.1177/1933719116667229. 1933719116667229. [DOI] [PubMed] [Google Scholar]
- 6.Qin J-B, Sheng X-Q, Wu D, et al. Worldwide prevalence of adverse pregnancy outcomes among singleton pregnancies after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Archives of Gynecology and Obstetrics. 2016:1–17. doi: 10.1007/s00404-016-4250-3. [DOI] [PubMed] [Google Scholar]
- 7.Strömberg B, Dahlquist G, Ericson A, Finnström O, Köster M, Stjernqvist K. Neurological sequelae in children born after in-vitro fertilisation: a population-based study. The Lancet. 2002;359(9305):461–465. doi: 10.1016/S0140-6736(02)07674-2. [DOI] [PubMed] [Google Scholar]
- 8.Lehti V, Brown AS, Gissler M, Rihko M, Suominen A, Sourander A. Autism spectrum disorders in IVF children: a national case–control study in Finland. Human Reproduction. 2013;28(3):812–818. doi: 10.1093/humrep/des430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermeiden JP, Bernardus RE. Are imprinting disorders more prevalent after human in vitro fertilization or intracytoplasmic sperm injection? Fertil Steril. 2013;99(3):642–651. doi: 10.1016/j.fertnstert.2013.01.125. [DOI] [PubMed] [Google Scholar]
- 10.Jaquet D, Leger J, Levy-Marchal C, Czernichow P. Low birth weight: effect on insulin sensitivity and lipid metabolism. Hormone research. 2003;59(1):1–6. doi: 10.1159/000067940. [DOI] [PubMed] [Google Scholar]
- 11.Jaquet D, Leger J, Czernichow P, Levy-Marchal C. The effect of in-utero undernutrition on the insulin resistance syndrome. Current diabetes reports. 2002;2(1):77–82. doi: 10.1007/s11892-002-0062-x. [DOI] [PubMed] [Google Scholar]
- 12.Jaquet D, Gaboriau A, Czernichow P, Levy-Marchal C. Insulin resistance early in adulthood in subjects born with intrauterine growth retardation. The Journal of clinical endocrinology and metabolism. 2000;85(4):1401–1406. doi: 10.1210/jcem.85.4.6544. [DOI] [PubMed] [Google Scholar]
- 13.Arends NJ, Boonstra VH, Duivenvoorden HJ, Hofman PL, Cutfield WS, Hokken-Koelega AC. Reduced insulin sensitivity and the presence of cardiovascular risk factors in short prepubertal children born small for gestational age (SGA) Clin Endocrinol (Oxf) 2005;62(1):44–50. doi: 10.1111/j.1365-2265.2004.02171.x. [DOI] [PubMed] [Google Scholar]
- 14.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295(7):349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 15.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. The American journal of clinical nutrition. 1999;70(5):811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 16.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. Bmj. 2000;320(7240):967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulkarni AD, Jamieson DJ, Jones HW, Jr, et al. Fertility treatments and multiple births in the United States. N Engl J Med. 2013;369(23):2218–2225. doi: 10.1056/NEJMoa1301467. [DOI] [PubMed] [Google Scholar]
- 18.Criteria for number of embryos to transfer: a committee opinion. Fertil Steril. 2013;99(1):44–46. doi: 10.1016/j.fertnstert.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 19.De Neubourg D, Gerris J, Mangelschots K, et al. The obstetrical and neonatal outcome of babies born after single-embryo transfer in IVF/ICSI compares favourably to spontaneously conceived babies. Hum Reprod. 2006;21(4):1041–1046. doi: 10.1093/humrep/dei424. [DOI] [PubMed] [Google Scholar]
- 20.Fujii M, Matsuoka R, Bergel E, van der Poel S, Okai T. Perinatal risk in singleton pregnancies after in vitro fertilization. Fertility and Sterility. 2010;94(6):2113–2117. doi: 10.1016/j.fertnstert.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Henningsen AK, Pinborg A, Lidegaard O, Vestergaard C, Forman JL, Andersen AN. Perinatal outcome of singleton siblings born after assisted reproductive technology and spontaneous conception: Danish national sibling-cohort study. Fertil Steril. 2011;95(3):959–963. doi: 10.1016/j.fertnstert.2010.07.1075. [DOI] [PubMed] [Google Scholar]
- 22.Sazonova A, Kallen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C. Obstetric outcome after in vitro fertilization with single or double embryo transfer. Hum Reprod. 2011;26(2):442–450. doi: 10.1093/humrep/deq325. [DOI] [PubMed] [Google Scholar]
- 23.Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian Stimulation and Low Birth Weight in Newborns Conceived Through In Vitro Fertilization. Obstetrics & Gynecology. 2011;118(4):863–871. doi: 10.1097/AOG.0b013e31822be65f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schieve LA, Ferre C, Peterson HB, Macaluso M, Reynolds MA, Wright VC. Perinatal outcome among singleton infants conceived through assisted reproductive technology in the United States. Obstet Gynecol. 2004;103(6):1144–1153. doi: 10.1097/01.AOG.0000127037.12652.76. [DOI] [PubMed] [Google Scholar]
- 25.Mainigi MA, Olalere D, Burd I, Sapienza C, Bartolomei M, Coutifaris C. Peri-Implantation Hormonal Milieu: Elucidating Mechanisms of Abnormal Placentation and Fetal Growth. Biology of Reproduction. 2014;90(2):26, 21–29. doi: 10.1095/biolreprod.113.110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Licht P, Neuwinger J, Fischer O, Siebzehnrubl E, Wildt L. VEGF plasma pattern in ovulation induction: evidence for an episodic secretion and lack of immediate effect of hCG. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association. 2002;110(3):130–133. doi: 10.1055/s-2002-29090. [DOI] [PubMed] [Google Scholar]
- 27.de Waal E, Vrooman LA, Fischer E, et al. The cumulative effect of assisted reproduction procedures on placental development and epigenetic perturbations in a mouse model. Hum Mol Genet. 2015;24(24):6975–6985. doi: 10.1093/hmg/ddv400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mainigi M, Rosenzweig JM, Lei J, et al. Peri-Implantation Hormonal Milieu: Elucidating Mechanisms of Adverse Neurodevelopmental Outcomes. Reprod Sci. 2016;23(6):785–794. doi: 10.1177/1933719115618280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song S, Ghosh J, Mainigi M, et al. DNA methylation differences between in vitro- and in vivo-conceived children are associated with ART procedures rather than infertility. Clinical Epigenetics. 2015;7(1):41. doi: 10.1186/s13148-015-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donjacour A, Liu X, Lin W, Simbulan R, Rinaudo PF. In vitro fertilization affects growth and glucose metabolism in a sex-specific manner in an outbred mouse model. Biol Reprod. 2014;90(4):80. doi: 10.1095/biolreprod.113.113134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roseboom TJ, van der Meulen JH, Osmond C, et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944-45. Heart (British Cardiac Society) 2000;84(6):595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doherty AS, Mann MR, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod. 2000;62(6):1526–1535. doi: 10.1095/biolreprod62.6.1526. [DOI] [PubMed] [Google Scholar]
- 33.Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Human Reproduction Update. 2012;18(5):485–503. doi: 10.1093/humupd/dms018. [DOI] [PubMed] [Google Scholar]
- 34.Zhu L, Zhang Y, Liu Y, et al. Maternal and Live-birth Outcomes of Pregnancies following Assisted Reproductive Technology: A Retrospective Cohort Study. Sci Rep. 2016;6:35141. doi: 10.1038/srep35141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol. 2013;122(2 Pt 1):406–416. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 36.Ashrafi M, Gosili R, Hosseini R, Arabipoor A, Ahmadi J, Chehrazi M. Risk of gestational diabetes mellitus in patients undergoing assisted reproductive techniques. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2014;176:149–152. doi: 10.1016/j.ejogrb.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Marino JL, Moore VM, Willson KJ, et al. Perinatal outcomes by mode of assisted conception and sub-fertility in an Australian data linkage cohort. PLoS One. 2014;9(1):e80398. doi: 10.1371/journal.pone.0080398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basso O, Baird DD. Infertility and preterm delivery, birthweight, and Caesarean section: a study within the Danish National Birth Cohort. Human Reproduction. 2003;18(11):2478–2484. doi: 10.1093/humrep/deg444. [DOI] [PubMed] [Google Scholar]
- 39.Maheshwari A, Raja EA, Bhattacharya S. Obstetric and perinatal outcomes after either fresh or thawed frozen embryo transfer: an analysis of 112,432 singleton pregnancies recorded in the Human Fertilisation and Embryology Authority anonymized dataset. Fertility and Sterility. 2016;106(7):1703–1708. doi: 10.1016/j.fertnstert.2016.08.047. [DOI] [PubMed] [Google Scholar]
- 40.Seggers J, Pontesilli M, Ravelli AC, et al. Effects of in vitro fertilization and maternal characteristics on perinatal outcomes: a population-based study using siblings. Fertil Steril. 2016;105(3):590–598.e592. doi: 10.1016/j.fertnstert.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Strawn EY, Bick D, Swanson A. Is it the patient or the IVF? Beckwith-Wiedemann syndrome in both spontaneous and assisted reproductive conceptions. Fertil Steril. 2010;94(2):754.e751–752. doi: 10.1016/j.fertnstert.2010.01.067. [DOI] [PubMed] [Google Scholar]
- 42.Tararbit K, Houyel L, Bonnet D, et al. Risk of congenital heart defects associated with assisted reproductive technologies: a population-based evaluation. European heart journal. 2011;32(4):500–508. doi: 10.1093/eurheartj/ehq440. [DOI] [PubMed] [Google Scholar]
- 43.Tararbit K, Lelong N, Thieulin A-C, et al. The risk for four specific congenital heart defects associated with assisted reproductive techniques: a population-based evaluation. Human Reproduction. 2013;28(2):367–374. doi: 10.1093/humrep/des400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilkins-Haug L. Assisted reproductive technology, congenital malformations, and epigenetic disease. Clin Obstet Gynecol. 2008;51(1):96–105. doi: 10.1097/GRF.0b013e318161d25a. [DOI] [PubMed] [Google Scholar]
- 45.Hansen M, Kurinczuk JJ, Bower C, Webb S. The Risk of Major Birth Defects after Intracytoplasmic Sperm Injection and in Vitro Fertilization. New England Journal of Medicine. 2002;346(10):725–730. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- 46.Davies MJ, Moore VM, Willson KJ, et al. Reproductive Technologies and the Risk of Birth Defects. New England Journal of Medicine. 2012;366(19):1803–1813. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- 47.Levi Setti PE, Moioli M, Smeraldi A, et al. Obstetric outcome and incidence of congenital anomalies in 2351 IVF/ICSI babies. J Assist Reprod Genet. 2016;33(6):711–717. doi: 10.1007/s10815-016-0714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uyar A, Seli E. The impact of assisted reproductive technologies on genomic imprinting and imprinting disorders. Curr Opin Obstet Gynecol. 2014;26(3):210–221. doi: 10.1097/GCO.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halliday J, Oke K, Breheny S, Algar EJ, Amor D. Beckwith-Wiedemann syndrome and IVF: a case-control study. Am J Hum Genet. 2004;75(3):526–528. doi: 10.1086/423902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet. 2003;72(1):156–160. doi: 10.1086/346031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maher ER, Brueton LA, Bowdin SC, et al. Beckwith-Wiedemann syndrome and assisted reproduction technology (ART) J Med Genet. 2003;40(1):62–64. doi: 10.1136/jmg.40.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gicquel C, Gaston V, Mandelbaum J, Siffroi JP, Flahault A, Le Bouc Y. In vitro fertilization may increase the risk of Beckwith-Wiedemann syndrome related to the abnormal imprinting of the KCN1OT gene. Am J Hum Genet. 2003;72(5):1338–1341. doi: 10.1086/374824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sutcliffe AG, Peters CJ, Bowdin S, et al. Assisted reproductive therapies and imprinting disorders–a preliminary British survey. Hum Reprod. 2006;21(4):1009–1011. doi: 10.1093/humrep/dei405. [DOI] [PubMed] [Google Scholar]
- 54.Lidegaard O, Pinborg A, Andersen AN. Imprinting diseases and IVF: Danish National IVF cohort study. Hum Reprod. 2005;20(4):950–954. doi: 10.1093/humrep/deh714. [DOI] [PubMed] [Google Scholar]
- 55.Bowdin S, Allen C, Kirby G, et al. A survey of assisted reproductive technology births and imprinting disorders. Hum Reprod. 2007;22(12):3237–3240. doi: 10.1093/humrep/dem268. [DOI] [PubMed] [Google Scholar]
- 56.Doornbos ME, Maas SM, McDonnell J, Vermeiden JP, Hennekam RC. Infertility, assisted reproduction technologies and imprinting disturbances: a Dutch study. Hum Reprod. 2007;22(9):2476–2480. doi: 10.1093/humrep/dem172. [DOI] [PubMed] [Google Scholar]
- 57.Balayla J, Sheehy O, Fraser WD, et al. Neurodevelopmental Outcomes After Assisted Reproductive Technologies. Obstet Gynecol. 2017;129(2):265–272. doi: 10.1097/AOG.0000000000001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramoğlu M, Kavuncuoğlu S, Aldemir E, Yarar C, Eras Z. Neurodevelopment of preterm infants born after in vitro fertilization and spontaneous multiple pregnancy. Pediatrics International; 2016. pp. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 59.Sutcliffe AG, Saunders K, McLachlan R, et al. A retrospective case-control study of developmental and other outcomes in a cohort of Australian children conceived by intracytoplasmic sperm injection compared with a similar group in the United Kingdom. Fertility and Sterility. 2003;79(3):512–516. doi: 10.1016/s0015-0282(02)04701-5. [DOI] [PubMed] [Google Scholar]
- 60.Zhu JL, Hvidtjørn D, Basso O, et al. Parental infertility and cerebral palsy in children. Human Reproduction. 2010;25(12):3142–3145. doi: 10.1093/humrep/deq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christensen DL, Baio J, Van Naarden Braun K, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years–Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. Morbidity and mortality weekly report Surveillance summaries (Washington, DC: 2002) 2016;65(3):1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knoester M, Helmerhorst FM, van der Westerlaken LAJ, Walther FJ, Veen S. Matched follow-up study of 5–8-year-old ICSI singletons: child behaviour, parenting stress and child (health-related) quality of life. Human Reproduction. 2007;22(12):3098–3107. doi: 10.1093/humrep/dem261. [DOI] [PubMed] [Google Scholar]
- 63.Kissin DM, Zhang Y, Boulet SL, et al. Association of assisted reproductive technology (ART) treatment and parental infertility diagnosis with autism in ART-conceived children. Human Reproduction. 2015;30(2):454–465. doi: 10.1093/humrep/deu338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hvidtjørn D, Grove J, Schendel D, et al. Risk of autism spectrum disorders in children born after assisted conception: a population-based follow-up study. Journal of Epidemiology and Community Health. 2011;65(6):497–502. doi: 10.1136/jech.2009.093823. [DOI] [PubMed] [Google Scholar]
- 65.Sandin S, Nygren K, Iliadou A, Hultman CM, Reichenberg A. Autism and mental retardation among offspring born after in vitro fertilization. JAMA. 2013;310(1):75–84. doi: 10.1001/jama.2013.7222. [DOI] [PubMed] [Google Scholar]
- 66.Jenkins TG, Aston KI, Pflueger C, Cairns BR, Carrell DT. Age-Associated Sperm DNA Methylation Alterations: Possible Implications in Offspring Disease Susceptibility. PLOS Genetics. 2014;10(7):e1004458. doi: 10.1371/journal.pgen.1004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valdez R, Athens MA, Thompson GH, Bradshaw BS, Stern MP. Birthweight and adult health outcomes in a biethnic population in the USA. Diabetologia. 1994;37(6):624–631. doi: 10.1007/BF00403383. [DOI] [PubMed] [Google Scholar]
- 68.Pontesilli M, Painter RC, Grooten IJ, et al. Subfertility and assisted reproduction techniques are associated with poorer cardiometabolic profiles in childhood. Reprod Biomed Online. 2015;30(3):258–267. doi: 10.1016/j.rbmo.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Ceelen M, van Weissenbruch MM, Prein J, et al. Growth during infancy and early childhood in relation to blood pressure and body fat measures at age 8-18 years of IVF children and spontaneously conceived controls born to subfertile parents. Hum Reprod. 2009;24(11):2788–2795. doi: 10.1093/humrep/dep273. [DOI] [PubMed] [Google Scholar]
- 70.Basatemur E, Shevlin M, Sutcliffe A. Growth of children conceived by IVF and ICSI up to 12years of age. Reprod Biomed Online. 2010;20(1):144–149. doi: 10.1016/j.rbmo.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 71.Kai CM, Main KM, Andersen AN, et al. Serum insulin-like growth factor-I (IGF-I) and growth in children born after assisted reproduction. J Clin Endocrinol Metab. 2006;91(11):4352–4360. doi: 10.1210/jc.2006-0701. [DOI] [PubMed] [Google Scholar]
- 72.Ong K, Kratzsch J, Kiess W, Costello M, Scott C, Dunger D. Size at birth and cord blood levels of insulin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-1 (IGFBP-1), IGFBP-3, and the soluble IGF-II/mannose-6-phosphate receptor in term human infants. The ALSPAC Study Team Avon Longitudinal Study of Pregnancy and Childhood. J Clin Endocrinol Metab. 2000;85 doi: 10.1210/jcem.85.11.6998. [DOI] [PubMed] [Google Scholar]
- 73.Ceelen M, van Weissenbruch MM, Roos JC, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Body composition in children and adolescents born after in vitro fertilization or spontaneous conception. J Clin Endocrinol Metab. 2007;92(9):3417–3423. doi: 10.1210/jc.2006-2896. [DOI] [PubMed] [Google Scholar]
- 74.Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab. 2008;93(5):1682–1688. doi: 10.1210/jc.2007-2432. [DOI] [PubMed] [Google Scholar]
- 75.Sakka SD, Loutradis D, Kanaka-Gantenbein C, et al. Absence of insulin resistance and low-grade inflammation despite early metabolic syndrome manifestations in children born after in vitro fertilization. Fertil Steril. 2010;94(5):1693–1699. doi: 10.1016/j.fertnstert.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 76.La Bastide-Van Gemert S, Seggers J, Haadsma ML, et al. Is ovarian hyperstimulation associated with higher blood pressure in 4-year-old IVF offspring? Part II: an explorative causal inference approach. Human Reproduction. 2014;29(3):510–517. doi: 10.1093/humrep/det448. [DOI] [PubMed] [Google Scholar]
- 77.Seggers J, Haadsma ML, La Bastide-Van Gemert S, et al. Is ovarian hyperstimulation associated with higher blood pressure in 4-year-old IVF offspring? Part I: multivariable regression analysis. Human Reproduction. 2014;29(3):502–509. doi: 10.1093/humrep/det396. [DOI] [PubMed] [Google Scholar]
- 78.Belva F, Henriet S, Liebaers I, Van Steirteghem A, Celestin-Westreich S, Bonduelle M. Medical outcome of 8-year-old singleton ICSI children (born >or=32 weeks’ gestation) and a spontaneously conceived comparison group. Hum Reprod. 2007;22(2):506–515. doi: 10.1093/humrep/del372. [DOI] [PubMed] [Google Scholar]
- 79.Barker DJ. The developmental origins of adult disease. Journal of the American College of Nutrition. 2004;23(6 Suppl):588s–595s. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 80.Belva F, Roelants M, De Schepper J, et al. Blood pressure in ICSI-conceived adolescents. Hum Reprod. 2012;27(10):3100–3108. doi: 10.1093/humrep/des259. [DOI] [PubMed] [Google Scholar]
- 81.Scherrer U, Rimoldi SF, Rexhaj E, et al. Systemic and pulmonary vascular dysfunction in children conceived by assisted reproductive technologies. Circulation. 2012;125(15):1890–1896. doi: 10.1161/CIRCULATIONAHA.111.071183. [DOI] [PubMed] [Google Scholar]
- 82.Meng Y, Lv PP, Ding GL, et al. High Maternal Serum Estradiol Levels Induce Dyslipidemia in Human Newborns via a Hepatic HMGCR Estrogen Response Element. Sci Rep. 2015;5:10086. doi: 10.1038/srep10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miles HL, Hofman PL, Peek J, et al. In vitro fertilization improves childhood growth and metabolism. J Clin Endocrinol Metab. 2007;92(9):3441–3445. doi: 10.1210/jc.2006-2465. [DOI] [PubMed] [Google Scholar]


