Abstract
Background
Small airways instability, resulting in premature airway closure, has been recognized as a risk for asthma severity and poor control. Although spirometry has limited sensitivity for detecting small airway dysfunction, a focus on the air-trapping component of obstruction may identify a risk factor for asthma instability.
Objective
To use spirometric measurements to identify patterns of airway obstruction in children, and define obstruction phenotypes that relate to asthma instability.
Methods
Pre- and post-bronchodilation spirometry data were obtained from 560 children in the Asthma Phenotypes in the Inner City study. An air-trapping obstruction phenotype (A Trpg) was defined as forced vital capacity (FVC) Z-score < −1.64, or an increase of FVC ≥ 10% predicted with bronchodilation. The airflow limitation phenotype (A Limit) had forced expiratory volume in 1 s (FEV1)/FVC Z-score < −1.64, but not A Trpg. The None phenotype had neither A Trpg nor A Limit. The 3 obstruction phenotypes were assessed as predictors of number of exacerbations, asthma severity, and airway lability.
Results
The A Trpg phenotype (14% of the cohort) had more exacerbations during the 12-month study, compared with the A Limit (P<0.03) and the None (P<0.001) phenotypes. The A Trpg phenotype also had the highest Composite Asthma Severity Index, the highest asthma treatment step, the greatest variability in FEV1 over time, and the greatest sensitivity to methacholine challenge.
Conclusions
A Trpg and A Limit patterns of obstruction, defined with routine spirometric measurements, can identify obstruction phenotypes that are indicators of risk for asthma severity and instability.
Keywords: Small airways dysfunction, asthma exacerbation, airflow limitation, airway closure
INTRODUCTION
Variable airway obstruction is a defining characteristic of asthma, and in recent years it has become recognized that different patterns of obstruction may have differing associations with asthma severity and instability. Gibbons1 described different patterns of response to bronchial challenge as measured by spirometry, noting that asthma patients having a prominent decrease in the forced vital capacity (FVC) associated with a 20% reduction in the forced expiratory volume in one second (FEV1) were more likely to have a history of frequent exacerbations. They proposed the concept that FEV1 might be partitioned into components of airflow limitation, represented as the fraction of vital capacity exhaled in the first second (FEV1/FVC ratio), and airway closure, represented as a reduction in FVC1. The association of premature airway closure and air-trapping with severe asthma and unstable asthma has been reinforced in multiple studies, using physiological and imaging methods to detect air-trapping during periods of stable asthma2–9. Studies from the Severe Asthma Research Program (SARP) demonstrated that FEV1 can be expressed mathematically as a hyperbolic function of its airflow limitation and air-trapping components5. This approach revealed that the severe asthma group had greater air-trapping relative to the non-severe asthma group, and that a large bronchodilator reversal of FEV1 was mostly due to a reversal of the air-trapping component5. Similarly, the SARP children with severe asthma exhibited air-trapping that was mostly reversed with bronchodilator, while the group with non-severe asthma had normal lung residual volume, and a bronchodilator response that was reflected only in the airflow limitation component6. These studies comparing groups defined by severity suggest that an assessment of airway obstruction patterns also may have value as a prospective marker of asthma instability and severity in individual persons.
The Asthma Phenotypes in the Inner City (APIC) study of the NIAID-sponsored Inner City Asthma Consortium evaluated a large set of variables among urban children to identify asthma clusters, factors related to difficulty in achieving asthma control, and the pathways linked to asthma severity10–12. In these analyses, the level of airway obstruction, defined with FEV1 %predicted and its reversal with bronchodilation, contributed significantly to the models as a risk for severe and difficult-to-control asthma10–12. However, the air-trapping component of obstruction was not assessed in these reports. We hypothesized that a further analysis of the APIC data with regard to obstruction patterns would reveal that individuals with an air-trapping phenotype detected with routine spirometry would exhibit the greatest asthma severity and instability.
METHODS
The APIC study has been described in detail previously11. Children ages 6–17 with physician-diagnosed asthma were recruited from low-income census tracts in 9 urban areas. A requirement for enrollment was a history of at least 2 episodes in the past 12 months that were treated with a short acting beta agonist. After enrollment the participants were followed for 12 months, with visits scheduled every 2 months for clinical assessment and protocol-guided adjustments of treatment based on the Expert Panel Report-3 guidelines13. The protocol was approved by institutional review boards from each participating center, and written informed consent was obtained by the legal guardians of participating children.
Defining obstruction phenotypes
Pre- and post-bronchodilation spirometry data from the final (12-month) visit in the APIC study were used to define the obstruction phenotypes. The 2012 Global Lung Initiative reference equations14 were used to generate predicted values and Z-scores for FEV1, FVC, FEF25-75, and FEV1/FVC ratio. The Global Lung Initiative ethnic groupings 1 (white), 2 (black), and 5 (mixed) were assigned based on the responses to the APIC demographics questionnaires. The 5th centile of the normal population is conventionally used as the lower limit of normal for spirometric variables, which also is a Z-score of −1.64. We used Z < −1.64 for our definitions for consistency with commercial spirometry software reports that include either LLN or Z-score reference points. Airflow limitation was defined as a Z-FEV1/FVC < −1.64. Air-trapping was defined as Z-FVC < −1.64 or a change in the FVC with bronchodilation of ≥10 %predicted (see Fig. E1 in the online repository). These definitions were based on the concept of partitioning FEV1 into components of airflow limitation and air-trapping1,5, and that the air-trapping component in asthma usually improves after bronchodilation5,6. The cut point of 10% predicted change in FVC was selected arbitrarily, lacking published guidelines for a minimally important change for this variable. However, a 10% change in FEV1 with bronchodilation in children has diagnostic specificity for asthma15 and an association with asthma instability16 in children. Because FEV1 changes proportionally with changes in FVC5, our cut point for a change in FVC should be meaningful. Spirometry was performed on standardized equipment by trained technicians, and over-read centrally by certified pulmonary function technologists to ensure that the measurements conformed to ATS guidelines11. Before the spirometry was performed, the participants were questioned to ensure that they had not had a respiratory infection, a cold, or bronchitis in the preceding 4 weeks, and that they had not used short acting bronchodilators within 8 hours, or long acting bronchodilators, anticholinergic agents, leukotriene modifiers, or theophylline within 24 hours. After the baseline spirometry, bronchodilation was achieved with the administration of a total of 4 actuations of an albuterol metered dose inhaler with a valved holding chamber. If the criterion for air-trapping was met, the A Trpg obstruction phenotype was assigned, and if the airflow limitation, but not the air-trapping, criteria were met, the A Limit phenotype was assigned. The None phenotype was assigned if neither air-trapping nor airflow limitation criteria were met. Children with both air-trapping and airflow limitation were included in the A Trpg phenotype to reflect the spectrum of airflow patterns associated with air-trapping, and to ensure that the number of subjects with air-trapping was sufficient for meaningful statistical analyses.
Other APIC variables
Blood samples were obtained at the initial visit of APIC, and processed to obtain counts of blood leukocytes, serum total IgE, and a panel of 20 allergen-specific IgE concentrations11. A panel of 12 common allergens was also applied as skin prick tests. Allergen sensitization was defined as a positive skin prick test or specific IgE > 0.35 kU/L, with 22 total allergens tested11. Allergen sensitizations were further combined into 7 categories: roaches, pets, rodents, pollens, foods, molds, and dust mites11. Serum total IgE was used as a log10 transform in multivariable models.
As an indicator of variability of airway obstruction over time, the standard deviation of the FEV1 %predicted, measured across the multiple APIC visits, was computed for each subject. Methacholine challenge, using the tidal breathing method, was performed in a subgroup of subjects (n=373 in the current analysis), and the provocative concentration associated with a 20% decrease in FEV1 (MeCh PC20) was computed12. The methacholine challenges were done at the 8- or 10-month APIC visit. Plethysmographic lung volumes were measured in a subgroup (n=216) at the clinical pulmonary function labs at 5 of the study sites during the second 6 months of the APIC study.
All the other variables used for the current analyses were obtained at the 12-month APIC visit. The fraction of nitric oxide in exhaled air (FENO) was measured with the NIOX MINO device (Aerocrine, Stockholm, Sweden) as described11. A cut point of >35 ppb was used to define elevated FENO 17. Body mass index (BMI) was computed from height and weight, and expressed as the Z-score11. A Z-BMI > 1.64 (95th centile) was used as the definition of obesity. The Composite Asthma Severity Index (CASI) that has been developed by the Inner City Asthma Consortium18 was employed to quantify asthma severity, using domains of day and night symptoms and albuterol use, FEV1, asthma controller therapy, and exacerbations. A modified CASI score, excluding the FEV1 domain, was used as a dependent variable in multivariable analysis to avoid redundancy with the spirometry- defined independent variables. The total number of exacerbations requiring a burst of systemic corticosteroid therapy during the 12-month study was recorded. The treatment step attained for each subject at the end of the 12-month study was used as another indicator of asthma severity.
Statistical methods
Summary data for normally distributed variables are presented as mean ± SD, and P-values for overall group differences obtained with the general linear model. Non-normally distributed variables are presented as median (IQR), and group differences tested with the Kruskal-Wallis model. Categorical variables are presented as per cents and compared with the Chi Square test.
An exploratory analysis was performed to identify variables that were associated with the individual obstruction phenotypes. A multinomial logistic regression model was constructed with obstruction phenotype as the dependent variable, and the None phenotype as the reference category. Independent variables related to demographics, obesity, allergen sensitization, FENO and blood eosinophils were included in the model, and removed in a stepwise manner, minimizing the Akaike Information Criterion in the final model. This process was repeated for a binary logistic regression model comparing the A Trpg and A Limit phenotypes.
To test the hypothesis that the A Trpg phenotype would have more severe and less stable asthma, we used the categorical obstruction phenotype as an independent variable, and indicators of asthma severity and instability as continuous dependent variables. The CASI score, modified to exclude the FEV1 domain, and the treatment step attained by the end of the APIC study were used as indicators of severity relative to the obstruction phenotype groups. The number of exacerbations during APIC was the primary indicator of asthma instability, tested both as a continuous dependent variable and as a binary categorical variable (<2 vs ≥2) in a logistic regression model. The standard deviation of FEV1 %predicted over multiple APIC visits and the MeCh PC20 were used as indicators of airway lability relative to the obstruction phenotype. The plethysmographic residual lung volumes as a fraction of total lung capacity also were compared among the phenotypes. The Kruskal-Wallis test was used to compare each of these variables among the 3 obstruction phenotypes, and the Conover-Inman test was used for post-hoc pairwise comparisons as indicated. SYSTAT v13.1 (Systat Software, Inc., San Jose, CA) was used for analyses.
RESULTS
Out of the 717 APIC participants, 594 completed the final scheduled visit of the study, and acceptable pre- and post-bronchodilation spirometry measurements were available for 560. After assigning each subject to one of the obstruction phenotype groups there were 300 (54%) in None, 181 (32%) in A Limit, and 79 (14%) in A Trpg. Within the A Trpg group, 49 (62%) also met the criterion for airflow limitation, while 30 (38%) met the criteria only for air-trapping. Table I summarizes the group characteristics. In addition to the defining spirometric criteria, the None group differed from the other obstruction phenotypes, having a larger proportion of Caucasian ethnicity, less obesity, fewer allergen sensitizations, and less inflammation in the univariable comparisons. Predictably, the FEV1 differed among the groups, as it is a global indicator of obstruction that is affected by both airflow limitation and air-trapping. However, the FEV1 was within the normal range for 73% of the A Limit group and 35% of the A Trpg group, indicating that, in this population, an assessment of the FEV1 components may add sensitivity to the detection of obstruction. In the subgroup with plethysmographic lung volume data, the A Trpg phenotype had significantly larger residual lung volume relative to total lung capacity (see Fig. E2 in the online repository), indicating concordance with another measure of air-trapping. Although the plethysmography measurements were obtained over the previous 6 months, the larger residual volumes within the A Trpg phenotype defined at the final visit suggests that the air-trapping was a persistent feature over time.
TABLE I.
Characteristics of the study group and the Obstruction Phenotype subgroups
| All Subjects n=560 | None n=300 | Airflow Limitation n=181 | Air Trapping n=79 | Overall P-value | |
|---|---|---|---|---|---|
|
| |||||
| Age (yr) | 12.4 ± 3.0 | 12.6 ± 3.0 | 12.4 ± 3.0 | 11.9 ± 3.0 | 0.12 |
|
| |||||
| Sex (%M) | 58.4 | 58.0 | 60.2 | 55.7 | 0.78 |
|
| |||||
| Ethnic (%W/B/Mix) | 22.9/67.3/9.8 | 28.0/60.0/12.0 | 16.0/75.1/8.8 | 19.0/77.2/3.8 | 0.002 |
|
| |||||
| BMI | |||||
| Z-score | 1.05 ± 1.17 | 0.89 ± 1.16 | 1.28 ± 1.12 | 1.11 ± 1.24 | 0.002 |
| Z-BMI > 1.64 (%) | 37.7 | 31.0 | 48.1 | 39.2 | <0.001 |
|
| |||||
| Age at Diagnosis* (years) | 2 (1,5) | 3 (1,6) | 2 (1,4) | 2 (<1,3) | <0.001 |
|
| |||||
| Asthma Duration (years) | 9.0 ± 3.7 | 8.7 ± 3.8 | 9.2 ± 3.7 | 9.4 ± 3.6 | 0.14 |
|
| |||||
| Allergen Sensitizations | |||||
| Total Positive of 22* | 8 (3,14) | 7 (2,13) | 9 (4,15) | 12 (5,16) | <0.001 |
| Categories (%Positive): | |||||
| Foods | 49.5 | 44.7 | 55.2 | 54.4 | 0.051 |
| Mites | 51.4 | 45.7 | 54.7 | 65.8 | 0.004 |
| Molds | 45.2 | 36.7 | 50.3 | 65.8 | <0.001 |
| Pets | 56.8 | 52.3 | 58.6 | 69.6 | 0.019 |
| Pollens | 60.4 | 55.3 | 64.1 | 70.9 | 0.02 |
| Roaches | 44.3 | 42.0 | 43.6 | 54.4 | 0.14 |
| Rodents | 30.0 | 27.7 | 30.9 | 36.7 | 0.28 |
|
| |||||
| CASI Score (unmodified) | 4.1 ± 2.8 | 3.2 ± 2.4 | 4.7 ± 2.6 | 6.5 ± 2.9 | <0.001 |
|
| |||||
| FEV1 | |||||
| %Predicted | 93.5 ± 16.9 | 101.3 ± 13.2 | 88.6 ± 13.8 | 75.0 ± 17.4 | <0.001 |
| Z-score | −0.50 ± 1.32 | 0.11 ± 1.05 | −0.88 ± 1.08 | −1.93 ± 1.32 | <0.001 |
|
| |||||
| FVC | |||||
| %Predicted | 105.0 ± 15.0 | 105.8 ± 13.7 | 109.2 ± 13.7 | 92.6 ± 15.9 | |
| Z-score | 0.39 ± 1.21 | 0.46 ± 1.11 | 0.72 ± 1.10 | −0.62 ± 1.27 | |
|
| |||||
| FEV1/FVC | |||||
| ratio | 0.78 ± 0.09 | 0.84 ± 0.05 | 0.71 ± 0.06 | 0.71 ± 0.12 | |
| Z-score | −1.40 ± 1.13 | −0.64 ± 0.73 | −2.33 ± 0.53 | −2.14 ±1.30 | |
|
| |||||
| Δ %Prd FVC | 3.6 ± 6.7 | 1.6 ± 3.9 | 1.9 ± 4.8 | 14.9 ± 7.7 | |
|
| |||||
| Z-FEF25-75 | −1.32 ± 1.19 | −0.57 ± 0.83 | −2.06 ± 0.74 | −2.43 ± 1.25 | <0.001 |
|
| |||||
| FENO | |||||
| ppb* | 24 (13,46) | 19 (12,43) | 28 (13,48) | 29 (16,63) | 0.015 |
| >35 ppb (%) | 35.9 | 31.2 | 40.1 | 44.2 | 0.04 |
|
| |||||
| MeCh PC20 (mg/ml)* | 7.5 (1.9,≥25) | 18.1 (3.1,≥25) | 3.5 (1.3,14.7) | 1.5 (0.6,4.9) | <0.001 |
|
| |||||
| Total IgE (kU/L)* | 249 (82,764) | 193 (57,622) | 252 (103,879) | 506 (152,1398) | <0.001 |
|
| |||||
| Blood EOS (cells/μL)* | 300 (160,448) | 230 (110,400) | 300 (185,485) | 400 (200,600) | 0.001 |
Except as noted, continuous variables are expressed as mean ± SD, with overall P-value obtained from analysis of variance among the 3 obstruction phenotype subgroups. Categorical variables are expressed as percentages, and the P-value determined with a Chi square test.
Expressed as median (interquartile range), and P-value obtained with the Kruskal-Wallis test.
All variables were measured at the 12 month study visit, except blood eosinophils, allergen sensitizations, and serum IgE were measured at the initial visit, and MeCh PC20 was measured at the 8 or 10 month visit.
We employed logistic regression methods to explore demographic characteristics and biomarkers that were associated with the obstruction phenotypes. Multinomial logistic regression comparing A Limit and A Trpg characteristics relative to the None phenotype revealed that a younger age at asthma diagnosis, obesity, black race, and sensitization to molds were risk factors associated with both A Limit and A Trpg (Table II). Additionally, FENO > 35 ppb was associated with the A Limit group, while higher serum total IgE and younger current age were associated with the A Trpg group relative to the None phenotype (Table II). Variables that did not contribute significantly to the model included sex, blood eosinophil count at the initial visit, asthma duration (substituted in the model for age at diagnosis and current age), and the other 6 allergen sensitization categories. Binomial logistic regression modeling of the A Trpg relative to the A Limit phenotype identified younger age at diagnosis and higher serum total IgE as significant predictors of the A Trpg phenotype (Table II).
Table II.
Predictors of the A Limit and A Trpg obstructive phenotypes
| Variable | Odds Ratio | 95% CL (Lower, Upper) | P-value |
|---|---|---|---|
| A Limit vs None | |||
| Age (years) at Diagnosis | 0.91 | 1.85, 0.98 | 0.009 |
| Obese (Z-BMI > 1.64) | 2.17 | 1.46, 3.23 | <0.001 |
| Black Race | 2.29 | 1.45, 3.62 | <0.001 |
| Sensitized to Molds | 1.53 | 1.00, 2.35 | 0.053 |
| FENO > 35 ppb | 1.66 | 1.04, 2.65 | 0.033 |
| A Trpg vs None | |||
| Age (years) at Diagnosis | 0.83 | 0.74, 0.93 | 0.001 |
| Obese (Z-BMI > 1.64) | 1.86 | 1.06, 3.28 | 0.030 |
| Black Race | 1.99 | 1.07, 3.68 | 0.029 |
| Sensitized to Molds | 2.21 | 1.24, 3.96 | 0.007 |
| Log10 Serum IgE | 1.77 | 1.14, 2.76 | 0.012 |
| Current Age (years) | 0.91 | 0.82, 0.999 | 0.049 |
| A Trpg vs A Limit | |||
| Age (years) at Diagnosis | 0.89 | 0.79, 0.99 | 0.028 |
| Log10 Serum IgE | 1.72 | 1.12, 2.62 | 0.012 |
Independent variables contributing to the logistic regression model per minimization of the Akaike Information Criterion.
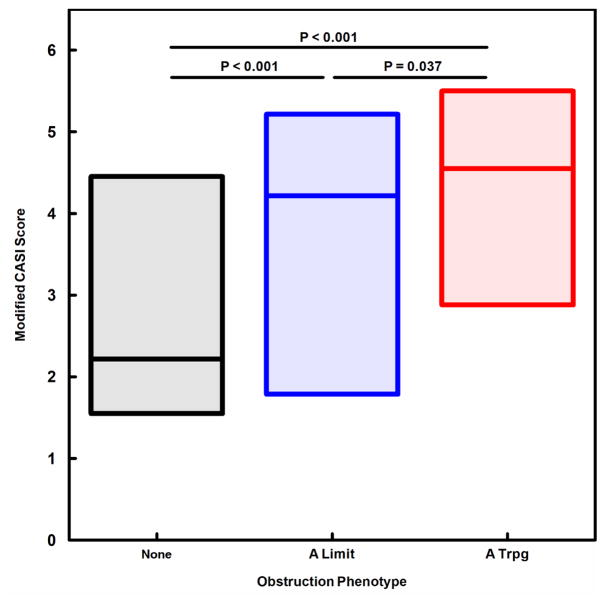
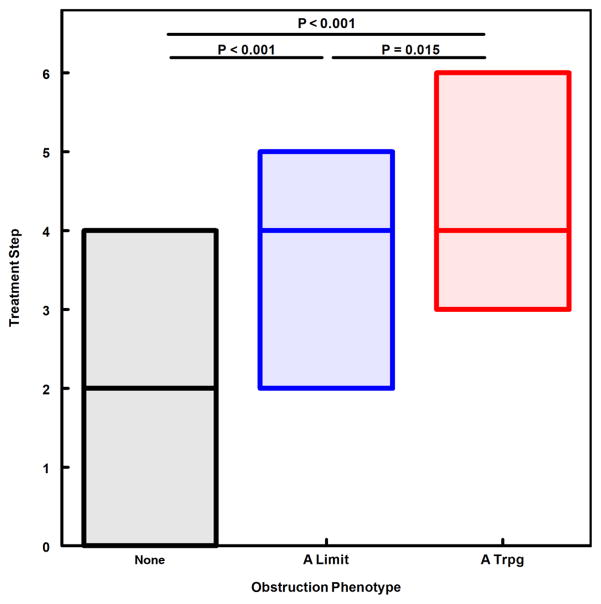
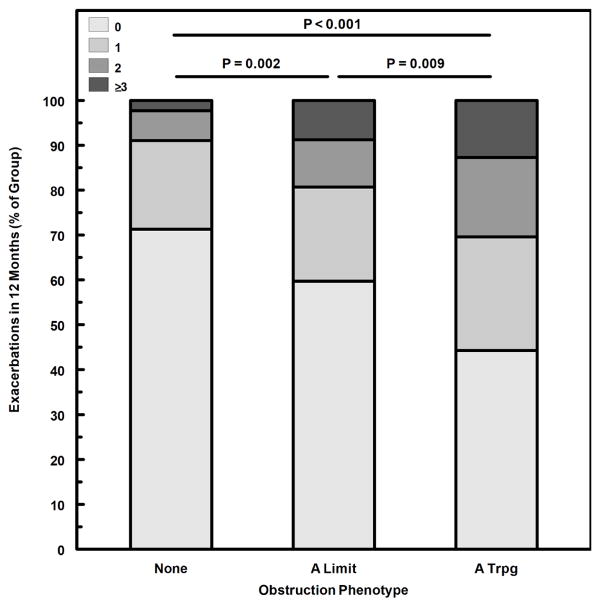
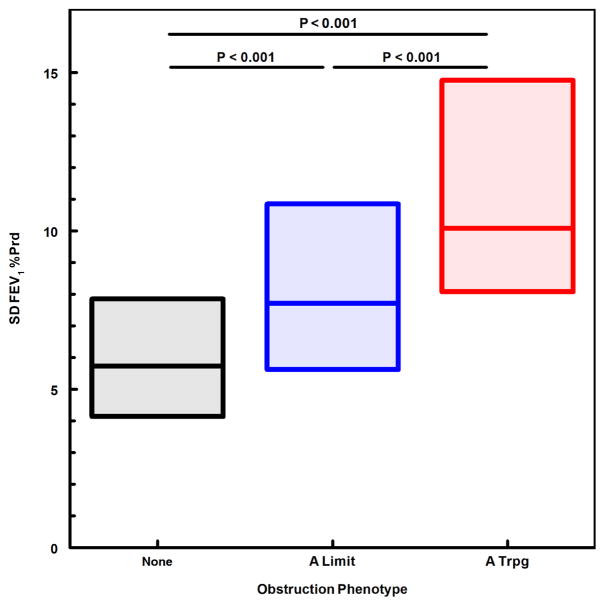
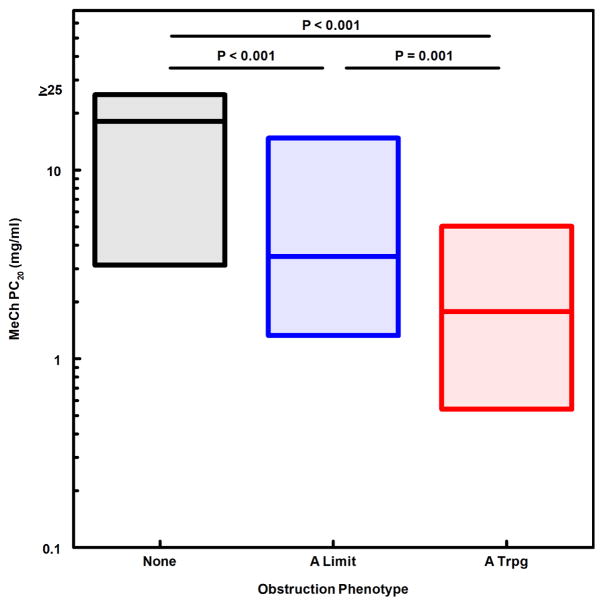
We next evaluated obstruction phenotype as an independent predictor for indicators of asthma severity and instability. A modified CASI score was computed, excluding the FEV1 domain to avoid redundancy of spirometric variables in both dependent and independent variables. The A Limit group had higher modified CASI scores than the None group, and the A Trpg group had scores higher than both the None and A Limit groups (Fig. 1). A similar pattern was found for treatment step at the final visit (fig. 2), indicating that obstruction phenotype was related to the intensity of protocol-guided asthma therapy as well as the current composite severity score. Figure 3 illustrates the number of exacerbations treated with systemic corticosteroids during the 12-month study period for the 3 obstruction phenotypes, the A Trpg group having the greatest exacerbation frequency. In a separate analysis of the risk of having 2 or more exacerbations during the study period, the odds ratios were 4.41 (95%CL 2.37–8.21) for A Trpg phenotype and 2.42 (95%CL 1.41–4.16) for A Limit relative to the None phenotype. We also evaluated indicators of airway lability-- variability of FEV1 over multiple visits and responsiveness to methacholine challenge-- relative to the 3 obstruction phenotypes. Figures 4 and 5 show that the A Trpg phenotype had the greatest variability in FEV1 over time, and was responsive to the lowest concentrations of aerosolized methacholine, compared with the A Limit and None phenotypes. Table E1 in the online repository summarizes these results.
Figure 1.
CASI scores, modified to exclude the FEV1 component, are compared for the 3 obstruction phenotypes, expressed as the median and interquartile range for each phenotype. P-values for paired comparisons obtained with Kruskal-Wallis/Conover-Inman tests.
Figure 2.
Asthma treatment step at the final study visit, expressed as median and interquartile range for each obstruction phenotype group. P-values for paired comparisons obtained with Kruskal-Wallis/Conover-Inman tests.
Figure 3.
Number of asthma exacerbations during the 12-month study period, expressed as the proportion of each group having 0, 1, 2, or ≥3 exacerbations during the study. P-values for comparison of number of exacerbations between groups, obtained with Kruskal-Wallis/Conover-Inman tests.
Figure 4.
Variability of airway obstruction over multiple study visits, expressed as the standard deviation of FEV1 %Predicted measured during the 12-month study period. The median and interquartile range is shown for each obstruction phenotype group. P-values for paired comparisons obtained with Kruskal-Wallis/Conover-Inman tests.
Figure 5.
Methacholine responsiveness, expressed as the PC20 median and interquartile range for each obstruction phenotype group. P-values for paired comparisons obtained with Kruskal-Wallis/Conover-Inman tests.
The forced expiratory flow rate between 75% and 25% of FVC (FEF25-75) may be sensitive to premature airway closure that reduces airflow at lower lung volumes, and therefore we assessed how the FEF25-75 was associated with FEV1/FVC in subjects with (A Trpg group) and without (A Limit and None groups) air-trapping. Using the Z-scores of each airflow variable to compare with like scales, the Z-FEF25-75 was highly and linearly correlated with Z-FEV1/FVC (R2= 0.76, P<0.001; see Fig. E3 in the online repository). A comparison of the air-trapping subgroups reveals similar slopes for the FEF25-75 vs FEV1/FVC, and a small negative shift in the intercept when air-trapping is present (Z-FEF25-75 air-trapping effect = −0.55, P<0.001; Fig. E3). Many of the subjects with air-trapping also had airflow limitation (Z-FEV1/FVC < −1.64; Fig. E3). The FEF25-75 was primarily an indicator of airflow limitation, measuring a value less than the lower limit of normal for only a small proportion of the air-trapping group having FEV1/FVC within the normal range (Fig. E3, lower right quadrant). Thus, the FEF25-75 is slightly more sensitive than FEV1/FVC for the detection of the effects of premature airway closure on airflow limitation, but neither variable functions as a selective indicator of airway closure.
DISCUSSION
The data show that, in a cohort of inner city children with protocol-guided asthma treatment, those meeting spirometry criteria for air-trapping require higher level of treatment, experience more exacerbations and have more airway lability compared with those that have airflow limitation without air-trapping and with those having normal spirometry at the time of evaluation. Collectively, our data strongly support the notion that air-trapping is an indicator of severe asthma in children. Although air-trapping, measured with plethysmography, has been demonstrated in groups of children classified as severe asthma6,8, this is the first study to define an air-trapping phenotype in individual children using routine spirometric measurements and bronchodilation. In a comparison of groups of adults with unstable versus stable asthma history but similar FEV1, premature airway closure, measured with single breath nitrogen washout, was the distinguishing physiological difference between the groups3. Similarly, in the current study the A Trpg and A Limit phenotypes did not differ with regard to FEV1/FVC (Table I), but the presence of detectable air-trapping identified children with increased risk of exacerbations and a need for more intense therapy. Our results are consistent also with studies in children showing that oscillometric indicators of small airway dysfunction are associated with poor asthma control19,20. The association of air-trapping with methacholine responsiveness is also consistent with the studies of Pyrgos, et al, reporting that baseline air-trapping in adults was associated quantitatively with an attenuation of the protective effect of a deep inflation on the response to methacholine21.
Our definition of air-trapping is based on reduced FVC, which is an indicator of elevated lung residual volume, providing that total lung capacity is not reduced substantially. Although total lung capacity may be increased in childhood asthma22, a reduction in lung size is not likely to be associated with asthma. However, in many persons a decrease in FVC may occur without resulting in a measured FVC less than the lower limit of normal, making this criterion for air-trapping relatively insensitive. One feature of asthma is the reversibility of obstruction with bronchodilators, and when airway closure is contributing to the obstruction, there also may be reversal of that component of the obstruction, resulting in an increase in FVC5. Thus, an increase in FVC after bronchodilation implies that air-trapping was present at baseline, and that airway smooth muscle tone was contributing to the closure. This is in contrast to other potential factors causing reduced FVC, such as reduced lung recoil force, restrictive lung disease, mucous plugging, tracheal-bronchial malacia, or paradoxical vocal fold motion, which are not influenced by acute beta-agonist inhalation.
In the current study of children, the subjects with the lowest Z-FEV1/FVC also exhibited air-trapping, although a subgroup of those with air-trapping had normal FEV1/FVC (Fig. E3). The FEV1/FVC ratio is a commonly used indicator of airway obstruction. The FVC is the limiting volume for the FEV1, and thus the ratio is a meaningful conversion of flow units for FEV1 from liters/second to vital capacities/second, given that the FEV1 is a timed expiratory volume. This conversion of units also normalizes FEV1 to the available vital capacity, which reduces intrasubject variability, but there are significant effects of age, sex, race, and height that can be factored into the predictive equations and Z-scores14. When FVC is normal, reduced airflow can be interpreted as reduced airway conductance, or reduced caliber, assuming the lung elastic recoil is normal23. Airway closure will decrease FVC, reducing the denominator of the FEV1/FVC ratio, but it also will interrupt airflow, reducing the numerator of the ratio. The ultimate effect on the ratio will depend on the pattern of airway closure. If airway closure occurs mainly after the initial one second of the forced expiration, the numerator and denominator may be reduced proportionally, resulting in a normal ratio. However, earlier airway closure during forced expiration might have a greater contribution to reducing airflow in the first second, resulting in a lower FEV1/FVC ratio. In adults with severe asthma, there was a parallel decline in the FVC %predicted and the FEV1/FVC %predicted, while in non-severe asthma the FEV1/FVC %predicted was not associated with changes in FVC, indicating different patterns of airway instability5.
Quanjer and colleagues24 analyzed data from a large number of spirometry records from clinical pulmonary function assessments to determine if FEF25-75 was likely to detect airflow abnormalities when FEV1, FVC, and FEV1/FVC were all within the normal range. Using Z-scores to set lower limits of normal at the 5th centile, they reported that Z-FEF25-75 and Z-FEV1/FVC were highly correlated, and that when the FEV1, FVC, and FEV1/FVC were within the normal range, <3% of the FEF25-75 measurements were below normal. They concluded that the FEF25-75 does not offer diagnostic sensitivity or specificity over the other standard spirometric variables. Our data are in agreement with these conclusions, finding that FEF25-75 is primarily a measure of airflow limitation that is highly correlated with FEV1/FVC (Fig E3). There is a small negative shift in the intercept of the Z-FEF25-75 vs Z-FEV1/FVC plot in the subgroup with air-trapping that captured a small number of air-trapping subjects with low FEF25-75 but in the normal range of FEV1/FVC (Fig E3). However, the majority of air-trappers with normal FEV1/FVC also had normal FEF25-75, suggesting that, while it may be affected by premature airway closure, FEF25-75 is not likely to provide additional information about obstruction patterns. One reason for a limited effect of airway closure on the FEF25-75 is that a truncated FVC will shift the volume range at which FEF25- 75 is measured to a higher lung volume relative to total lung capacity, at which maximal flow rates will be higher.
Our exploratory analysis of factors associated with the obstruction phenotypes identified several potential predictors of the A Limit and A Trpg phenotypes relative to the None phenotype (Table II). In multivariable analyses, higher serum IgE levels and lower age of asthma diagnosis were the strongest predictors of the A Trpg phenotype relative to the other obstruction phenotypes. The univariable comparisons (Table I) also were indicative of the strong allergy component in the A Trpg group, with the highest levels of allergen sensitization and blood eosinophils, as well as the highest serum total IgE in that phenotype. In the previous APIC analyses, serum IgE, sensitization to molds, age at asthma diagnosis, and Z-BMI also were identified as important distinguishing characteristics of difficult-to-treat vs easy-to-treat subgroups11.
Premature airway closure resulting in air-trapping is most likely to occur in small membranous airways25, and thus is among the indicators that have been used to assess small airway dysfunction in asthma7. Airway closure is the most extreme case of airway narrowing, and an indicator of airway instability25,26. The current study supports the idea that the presence of air-trapping during a stable period is a marker of airway instability that is likely to result in asthma that is difficult to control. While the underlying causes of small airway instability are poorly understood, the improvement of air-trapping acutely with beta agonist treatment and after a course of anti-inflammatory drug therapy9 suggest that it may be possible to design therapy that can prevent or reverse this insidious form of airway obstruction.
In conclusion, using routine spirometry and bronchodilator response, an air-trapping obstruction pattern can be identified in a subgroup of children with asthma. This obstruction phenotype has characteristics of greater asthma severity and instability and requires more intensive therapy compared with those having normal spirometry or airflow limitation without air-trapping during a stable period. Importantly, this is a method by which a high-risk asthma group may be identified prospectively, using measurements that are safe, inexpensive, easy to perform, and widely available to clinicians. Having additional information regarding risk of asthma instability should be useful to clinicians for making decisions regarding choice of controller therapy, advancing therapy to a higher step, and formulating an action plan for exacerbations. The results support the concept that small airway instability, manifesting as airway closure, is an important component of asthma pathophysiology. Why this phenotype develops in childhood, how it relates to asthma severity and control over time, and how best to treat it are questions that remain to be addressed in future investigations.
Supplementary Material
Clinical Implications.
Small airways dysfunction can be detected as air-trapping with routine spirometric measures, and can identify greater risk for asthma severity and instability in children.
Acknowledgments
Funding: This project has been funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health ((NIH), Department of Health and Human Services (under contract nos. HHSN272200900052C and HHSN272201000052I). Additional support was provided by the National Center for Research Resources (NCRR), and the National Center for Advancing Translational Sciences (NCATS), NIH (under grant nos. NCRR/NIH UL1TR000451, UL1RR025780, UL1TR000075 and NCATS/NIH UL1TR000154, UL1TR001082, UL1TR000077-04, UL1TR000040, UL1TR000150, UL1TR001105 and UM1AI114271). GlaxoSmithKline (GSK) provided Ventolin, Flovent, Advair, and Flonase under a clinical trial agreement with NIH NIAID; GSK did not have a role in the development or approval of the protocol, conduct of the trial, data analysis, manuscript preparation, or the decision to submit the manuscript for publication.
We are grateful to the APIC study participants and their families who gave of themselves to be our investigational partners; our study staff personnel who are dedicated to our inner-city asthma mission and clinical research excellence; and Patrick Heinritz in the Inner-City Asthma Consortium Administrative Center (Madison, Wis) and Samuel Arbes, Michelle Walter, and Herman Mitchell at Rho, Inc, for their leadership, commitment to excellence despite all the challenges, and their legacies in inner-city asthma research.
Abbreviations
- A Limit
Airflow limitation phenotype
- A Trpg
Air-trapping phenotype
- APIC
Asthma Phenotypes in the Inner City study
- BMI
Body mass index
- CASI
Composite Asthma Severity Index
- Δ %Prd FVC
Change in %predicted FVC with bronchodilation
- FENO
Fraction of nitric oxide in expired air
- FEF25-75
Forced expiratory flow rate between 75% and 25% FVC
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- MeCh PC20
Provocative concentration of methacholine HCl associated with 20% decrease in FEV1
- None
Phenotype without airflow limitation or air-trapping criteria
- SARP
Severe Asthma Research Program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gibbons WJ, Sharma A, Lougheed D, Macklem PT. Detection of excessive bronchoconstriction in asthma. Am J Respir Crit Care Med. 1996;153(2):582–9. doi: 10.1164/ajrccm.153.2.8564102. [DOI] [PubMed] [Google Scholar]
- 2.Busacker A, Newell JD, Jr, Keefe T, Hoffman EA, Granroth JC, Castro M, et al. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest. 2009;135(1):48–56. doi: 10.1378/chest.08-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.in ’t Veen CCM, Beekman AJ, Bel EH, Sterk PJ. Recurrent exacerbations in severe asthma are associated with enhanced airway closure during stable episodes. Am J Respir Crit Care Med. 2000;161(6):1902–6. doi: 10.1164/ajrccm.161.6.9906075. [DOI] [PubMed] [Google Scholar]
- 4.Kelly VJ, Sands SA, Harris RS, Venegas JG, Brown NJ, Stuart-Andrews CR, et al. Respiratory system reactance is an independent determinant of asthma control. J Appl Physiol (1985) 2013;115(9):1360–9. doi: 10.1152/japplphysiol.00093.2013. [DOI] [PubMed] [Google Scholar]
- 5.Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, et al. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol (1985) 2008;104(2):394–403. doi: 10.1152/japplphysiol.00329.2007. [DOI] [PubMed] [Google Scholar]
- 6.Sorkness RL, Teague WG, Penugonda M, Fitzpatrick AM National Institutes of Health NHL, Blood Institute’s Severe Asthma Research P. Sex dependence of airflow limitation and air trapping in children with severe asthma. J Allergy Clin Immunol. 2011;127(4):1073–4. doi: 10.1016/j.jaci.2010.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgel PR. The role of small airways in obstructive airway diseases. Eur Respir Rev. 2011;20(119):23–33. doi: 10.1183/09059180.00010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahut B, Peiffer C, Bokov P, Beydon N, Delclaux C. Gas trapping is associated with severe exacerbation in asthmatic children. Respir Med. 2010;104(8):1230–3. doi: 10.1016/j.rmed.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Zeidler MR, Kleerup EC, Goldin JG, Kim HJ, Truong DA, Simmons MD, et al. Montelukast improves regional air-trapping due to small airways obstruction in asthma. Eur Respir J. 2006;27(2):307–15. doi: 10.1183/09031936.06.00005605. [DOI] [PubMed] [Google Scholar]
- 10.Liu AH, Babineau DC, Krouse RZ, Zoratti EM, Pongracic JA, O’Connor GT, et al. Pathways through which asthma risk factors contribute to asthma severity in inner-city children. J Allergy Clin Immunol. 2016;138(4):1042–50. doi: 10.1016/j.jaci.2016.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pongracic JA, Krouse RZ, Babineau DC, Zoratti EM, Cohen RT, Wood RA, et al. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J Allergy Clin Immunol. 2016;138(4):1030–41. doi: 10.1016/j.jaci.2016.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoratti EM, Krouse RZ, Babineau DC, Pongracic JA, O’Connor GT, Wood RA, et al. Asthma phenotypes in inner-city children. J Allergy Clin Immunol. 2016;138(4):1016–29. doi: 10.1016/j.jaci.2016.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tse SM, Gold DR, Sordillo JE, Hoffman EB, Gillman MW, Rifas-Shiman SL, et al. Diagnostic accuracy of the bronchodilator response in children. J Allergy Clin Immunol. 2013;132(3):554–9. e5. doi: 10.1016/j.jaci.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma S, Litonjua AA, Tantisira KG, Fuhlbrigge AL, Szefler SJ, Strunk RC, et al. Clinical predictors and outcomes of consistent bronchodilator response in the childhood asthma management program. J Allergy Clin Immunol. 2008;122(5):921–8. e4. doi: 10.1016/j.jaci.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wildfire JJ, Gergen PJ, Sorkness CA, Mitchell HE, Calatroni A, Kattan M, et al. Development and validation of the Composite Asthma Severity Index--an outcome measure for use in children and adolescents. J Allergy Clin Immunol. 2012;129(3):694–701. doi: 10.1016/j.jaci.2011.12.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y, Aledia AS, Galant SP, George SC. Peripheral airway impairment measured by oscillometry predicts loss of asthma control in children. J Allergy Clin Immunol. 2013;131(3):718–23. doi: 10.1016/j.jaci.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Shi Y, Aledia AS, Tatavoosian AV, Vijayalakshmi S, Galant SP, George SC. Relating small airways to asthma control by using impulse oscillometry in children. J Allergy Clin Immunol. 2012;129(3):671–8. doi: 10.1016/j.jaci.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pyrgos G, Scichilone N, Togias A, Brown RH. Bronchodilation response to deep inspirations in asthma is dependent on airway distensibility and air trapping. J Appl Physiol (1985) 2011;110(2):472–9. doi: 10.1152/japplphysiol.00603.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merkus PJ, van Essen-Zandvliet EE, Kouwenberg JM, Duiverman EJ, Van Houwelingen HC, Kerrebijn KF, et al. Large lungs after childhood asthma. A case-control study. Am Rev Respir Dis. 1993;148(6 Pt 1):1484–9. doi: 10.1164/ajrccm/148.6_Pt_1.1484. [DOI] [PubMed] [Google Scholar]
- 23.Mead J, Turner JM, Macklem PT, Little JB. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol. 1967;22(1):95–108. doi: 10.1152/jappl.1967.22.1.95. [DOI] [PubMed] [Google Scholar]
- 24.Quanjer PH, Weiner DJ, Pretto JJ, Brazzale DJ, Boros PW. Measurement of FEF25-75% and FEF75% does not contribute to clinical decision making. Eur Respir J. 2014;43(4):1051–8. doi: 10.1183/09031936.00128113. [DOI] [PubMed] [Google Scholar]
- 25.Macklem PT. The physiology of small airways. Am J Respir Crit Care Med. 1998;157(5 Pt 2):S181–S3. doi: 10.1164/ajrccm.157.5.rsaa-2. [DOI] [PubMed] [Google Scholar]
- 26.Anafi RC, Beck KC, Wilson TA. Impedance, gas mixing, and bimodal ventilation in constricted lungs. J Appl Physiol (1985) 2003;94(3):1003–11. doi: 10.1152/japplphysiol.00569.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.