Abstract
Background
Apparent diffusion coefficient (ADC) imaging is a biomarker of cytotoxic injury that predicts edema formation and outcome after ischemic stroke. It therefore has the potential to serve as a “tissue clock” to describe the extent of ischemic injury and potentially predict response to therapy. The goal of this study was to determine the relationship between baseline ADC signal intensity, revascularization and edema formation.
Methods
We examined the ADC signal intensity ratio (ADCr) of the stroke lesion (defined as the baseline DWI hyperintense region) compared to the contralateral normal hemisphere in 65 subjects from the Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) trial. The associations between ADCr, neurologic outcome and cerebral edema were examined. Finally, we explored the interaction between baseline ADCr and vessel recanalization at day 7 on post-stroke edema.
Results
We found that lower initial ADCr was associated with worse outcome on the modified Rankin scale at 90 days (52.2% of those with ADCr < 64% were mRS 5–6 vs 19.1% with ADCr ≥ 64%, p = 0.006). Those subjects with reconstitution of flow distal to the initial vessel occlusion showed greater normalization of ADCr on follow up scan (increase in ADCr of 16.4±2.07% vs. 1.99±4.33%, p = 0.0039). In those patients with low baseline ADCr, successful revascularization was associated with reduced edema (median swelling volume 164ml [IQR 53.3–190ml] vs 20.7ml [IQR 3.20–55.1ml], p = 0.024).
Conclusions
This study reaffirms the association of ADCr with outcome after stroke, supports the idea that reperfusion may attenuate rather than enhance post-stroke edema, and indicates that the degree of edema with and without revascularization may be predicted by ADCr.
Keywords: Acute ischemic stroke, Acute stroke imaging, Cerebral edema, Diffusion-weighted imaging, MRI of acute stroke
Introduction
Current guideline-endorsed treatment strategies for acute ischemic stroke are focused on rapid reperfusion – either the first 4.5 hours for intravenous tissue plasminogen activator[1–5], or within 6 hours for endovascular clot retrieval[6–11]. While current guidelines rely on this time clock to determine treatment eligibility, it is likely that time merely serves as an inexact surrogate for progression of underlying pathology at a variable pace across patients[12,13]. Implementing a “tissue clock” may improve the selection of patients, enhancing safety and extending treatment to those who might not otherwise be eligible.
Apparent diffusion coefficient (ADC) imaging is a biomarker of cytotoxic injury that has the potential to serve as a “tissue clock” for ischemic damage. ADC signal intensity correlates with degree of lactate production and histologic evidence of tissue injury in animal models[14]. ADC signal similarly correlates with cerebral metabolic rate of oxygen (CMRO2) in animal models[15,16], where ADC values above 80% of baseline were associated with normalization following reperfusion. Case series in humans have found the relationship between ADC and oxygen metabolism to be less direct, except at very low levels of ADC[17,18]. Relative ADC signal intensity during MCAO in rats can be used to predict final infarct volume following reperfusion[19]. We have previously shown that the ratio of ADC signal in the region of stroke to the normal hemisphere (ADCr) on baseline MRI is predictive of edema formation[20] and outcome after ischemic stroke[21]. What is unknown, however, is the effect of revascularization on the evolution of ADC and the ability of ADC to predict edema and outcome in the setting of vessel recanalization. The goal of this study is to understand the interaction between initial cytotoxic injury, revascularization and post-stroke edema formation.
Methods
Patients
The Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) trial was a phase 2b randomized, controlled, open-label/blinded outcome trial conducted at 22 centers in North America between 2004 and 2011[22]. Patients were enrolled who were between 18 and 85 years-old and presented within 8 hours of last seen well with a large vessel, anterior circulation stroke and National Institutes of Health Stroke Scale (NIHSS) score of 6 to 29. Patients underwent pretreatment imaging with multimodal CT or MRI to identify the presence of a penumbral pattern with a small infarct core and large area of tissue at risk. Those patients with a penumbral pattern were randomized to receive either standard care or mechanical thrombectomy. Patients who received IV tPA were eligible for inclusion if CT or MR angiography demonstrated a persistent large vessel occlusion.
In total, 127 patients were randomized, of which 82 had acute and 7-day follow up MRI available for analysis in the current study (41 in each treatment arm). Baseline demographic data and past medical history were documented for all patients. Outcome data was available in the form of 90-day modified Rankin Scale score (mRS). All patients had revascularization assessed based on 7-day magnetic resonance angiography (MRA) or computed tomography angiography (CTA). As part of the initial trial analysis, revascularization on day 7 MRA/CTA was scored in both the thrombectomy and non-thrombectom treatment arms according to the arterial occlusive lesion (AOL) score[23]. Scoring was defined as follows: 0=no recanalization of the primary lesion, 1=incomplete or partial recanalization of the primary lesion with no distal flow, 2=incomplete or partial recanalization of the primary lesion with distal flow, and 3=complete recanalization of the primary lesion with distal flow.
ADC ratio calculation
Investigators (MBB, TWKB and ACO) blinded to clinical and laboratory data performed all image analysis. Patients were excluded who had bilateral infarcts or who had poor image quality (motion degradation) that limited accurate identification of the region of stroke. Region of interest analysis was performed using Analyze 11.0 or Analyze Pro (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN) using our previously described methods[20]. Briefly, the hyper intense acute stroke lesion and the entire contralateral hemisphere were outlined on DWI using a semi-automated method. These image maps were then transferred to ADC images. CSF signal was identified and subtracted from the image maps. Volume and signal intensity was calculated for the stroke and contralateral hemisphere. ADCr was calculated by taking the ratio of signal intensity of the stroke over that of the entire contralateral hemisphere. Normalization to the contralateral hemisphere allows us to adjust for variation among individuals and technical variation between different scanner settings[24], including different B values used in the generation of diffusion sequences at different institutions. ADCr was calculated on both initial and follow up MRI, with delta ADCr = (follow up ADCr) − (baseline ADCr).
Swelling volume calculation
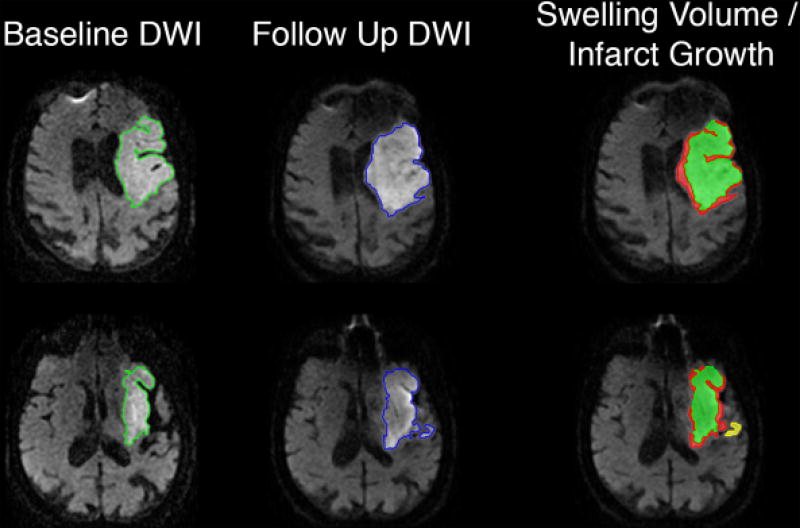
Swelling volume was determined using our previously described methods[20] as outlined in Figure 1. The hyper-intense lesion volume was determined on baseline and follow-up DWI, and the total stroke lesion expansion (ΔDWI) was defined as the change in DWI lesion between initial and follow up MRI. Baseline and follow-up B0 sequences were co-registered using the SPM module in Matlab with manual verification, with the same transformation then applied to the DWI and ADC sequences. Co-registered images were then compared side-by-side simultaneously in the axial, sagittal, and coronal planes using the Analyze 11.0 3D Voxel Registration module. Areas of new regional diffusion abnormality were outlined by an expert rater on follow-up MRI and categorized as definite new infarction. Any areas of hemorrhagic conversion were excluded. The swelling volume was calculated based on the relationship: Swelling volume = Total stroke lesion expansion volume − Definite new infarct volume. Any negative value for calculated swelling volume was assumed to be due to resolution of early edema between baseline and follow up imaging. All negative swelling volumes were assigned a value of zero for analysis.
Figure 1.
Swelling volume was calculated using our previously described methods. Briefly, the baseline and follow up B0 sequences were co-registered using SPM, with the same transformation then applied to DWI sequences. The region of DWI hyperintensity was outlined on both baseline (green) and follow up (blue) MRIs, using a semi-automated seed-based technique. The follow up image was compared to the baseline in axial, coronal and sagittal planes, and any diffusion abnormality in a distinct anatomic region was defined as infarct growth (yellow). Finally, the baseline DWI lesion volume was subtracted from the resulting map of follow up DWI lesion and infarct growth, with any residual volume classified as swelling (red).
Statistical analysis
Descriptive statistics of baseline variables were performed, reported as mean ± standard deviation (for normally distributed continuous data), median with interquartile range (for non-normal or ordinal data), and proportions (for binary data). The association between dichotomized ADCr and outcome was assessed using Fisher’s Exact Test. Comparison between recanalization status and change in ADCr was performed using ANOVA with Dunnett’s test for multiple comparisons using persistent occlusion as the control. The effect of dichotomized recanalization score on delta ADCr and follow up ADCr was assessed using a Student’s t-test. Comparisons between dichotomized ADCr, dichotomized AOL score, and swelling volume were performed using Wilcoxon rank sum tests. All statistical analyses were performed using JMP Pro 12.0 (SAS Institute, Cary, NC, USA).
Results
Study Cohort
Of the 82 subjects in MR RESCUE with baseline MRI available, 9 were excluded from the current study due to motion degradation or other artifact. A further 4 were excluded due to missing diffusion sequences. Four (4) subjects were excluded due to baseline ADC stroke volume of less than 5mL to limit any effects of volume averaging on signal intensity analysis. In total, 65 subjects were used for baseline ADCr analysis in this study. Of these, 3 were excluded from follow up analysis due to the development of bilateral strokes and one subject was missing follow up ADC images. In total, 61 subjects had follow up MRI available for analysis. Clinical data for the 65 subjects included in the current study are summarized in Table 1. The two treatment arms were combined for analysis given the low rate of successful endovascular intervention in the original study with only 8 (24%) of the 33 endovascular subjects in our study cohort achieving TICI 2b-3 recanalization at the time of angiography. Importantly, there was no significant difference in the rate of AOL 2–3 recanalization on the day 7 MRA or CTA between treatment arms (90% in medical arm vs. 73% in endovascular arm, p = 0.08).
Table 1.
Characteristics of the study cohort
| n=65 | |
|---|---|
| Age (years), mean ± SD | 64.7 ± 15.4 |
| Sex, male, N (%) | 35 (53.8%) |
| Comorbidities, N (%) | |
| Diabetes | 16 (24.6%) |
| Hypertension | 48 (73.8%) |
| Hyperlipidemia | 35 (53.8%) |
| Atrial fibrillation | 20 (30.7%) |
| Prior stroke | 11 (16.9%) |
| Treatment, N (%) | |
| IV tPA | 26 (40.0%) |
| Randomized to endovascular intervention | 33 (50.8%) |
| Laterality (left), N (%) | 34 (52.3%) |
| Admission NIHSS, median [IQR] | 17 [13–21] |
| 90-day mRS, median [IQR] | 4 [3, 5] |
| Time from LSW to MRI (hrs), mean ± SD | 4.6 ± 1.4 |
| Time from baseline to follow up MRI (days), median [IQR] | 7 [6,7] |
| Admission ADC volume (mL), median [IQR] | 39.7 [17.1–89.7] |
| Follow up ADC volume (mL), median [IQR]* | 97.6 [43.6–191] |
| Swelling volume (mL), median [IQR] | 23 [3.7–38] |
| Admission ADC ratio, mean ± SD | 67.4 ± 7.63% |
| Follow up ADC ratio, mean ± SD* | 81.1 ± 13.5% |
| AOL 2–3 on day 7 MRA/CTA, n (%) | 51 (81.0%) |
Follow up scans were analyzed in 61 patients; 3 subjects were excluded due to development of bilateral stroke, 1 subject had missing follow up diffusion images
ADCr is associated with outcome
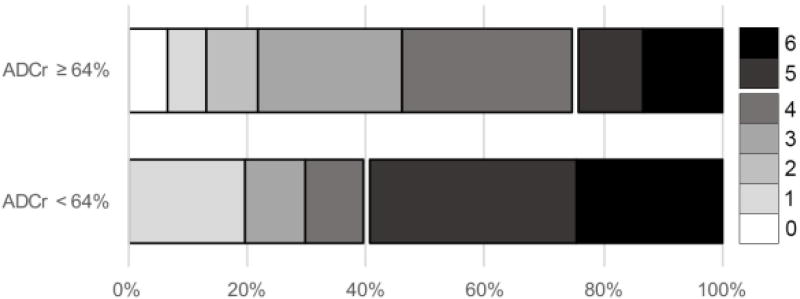
Subjects were dichotomized based on an ADCr cutoff of 64%, which we previously found to be predictive of good (mRS 0–2) 90-day outcome with a sensitivity of 0.81 and specificity of 0.53[21]. This cutoff represents the lowest tertile of ADCr measured in the current cohort. Those patients with baseline ADCr < 64% had worse outcome as demonstrated by a shift in mRS at 90 days (Figure 2, p = 0.028). Dichotomized ADCr was not predictive of good (mRS 0–2) vs poor (mRS 3–6) outcome in this cohort with relatively severe stroke (median 90 day mRS 4 [IQR 3, 5]). However, those with baseline ADCr < 64% were more likely to be severely disabled or dead (mRS 5–6, 52.2% with ADCr < 64% vs 19.1% with ADCr ≥ 64%, p = 0.006). As a sensitivity analysis, we performed similar comparisons of outcome at different cut points for ADCr (Supplemental Figure 1). Worse outcome across the entire mRS scale was associated with ADCr below the 50th and 66th percentiles, in addition to the 33rd percentile as described above. There was a greater likelihood of mRS 5–6 in those patients with ADCr below the 25th (67% vs 25% with ADCr above the 25th percentile, p = 0.003), 33rd (52% vs 19%, p = 0.006) and 50th (48% vs 22%, p = 0.036) percentiles. Based on these results, and given that the 33rd percentile ADCr of 64% corresponded with the level identified in our prior work[21] as predictive of outcome, all further comparisons were carried out with an ADCr cut point of 64%.
Figure 2.
Baseline ADCr of ≥ 64% is associated with improved 90-day outcome. Higher baseline ADCr was associated with a shift in overall mRS (p = 0.028). Additionally, a greater proportion of patients with baseline ADCr < 64% were severely disabled or dead at 90 days (mRS 5–6, 52.2% with ADCr < 64% vs 19.1% with ADCr ≥ 64%, p = 0.006).
Revascularization is associated with normalization of ADCr
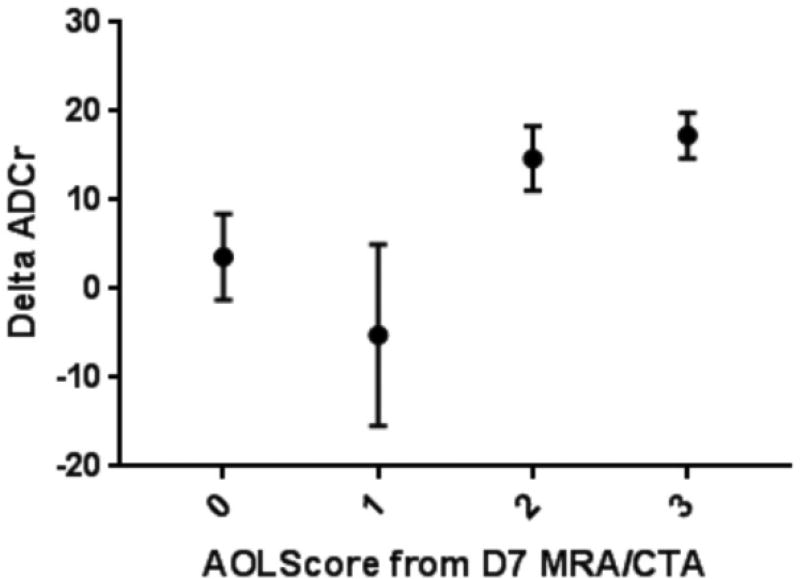
There was a significant association between recanalization score and follow up ADCr (Figure 3, p = 0.008). Pairwise comparison using persistent occlusion as the control found that those patients with complete recanalization (AOL 3) had significantly greater delta ADCr (p = 0.0385). If AOL scores are dichotomized, those with restoration of distal flow (AOL 2–3) have significantly greater delta ADCr (16.4±2.07% vs. 1.99±4.33% for AOL 0–1, p = 0.0039). There was no difference in baseline ADCr between those subjects that did and did not have recanalization at day 7 (67.8±2.3% for AOL 0–1 vs 67.4±1.1% for AOL 2–3, p = 0.87). However, absolute follow up ADCr was higher in those with AOL 2–3 (84.0±1.79% vs. 70.2±3.75% for AOL 0–1, p = 0.0015).
Figure 3.
Association between recanalization and ADCr evolution. Change in ADCr between baseline and follow up MRI (delta ADCr) is associated with vessel recanalization (ANOVA, p = 0.0248). Multiple comparison analysis found that delta ADCr was significantly greater in patients with AOL 3 compared to those with persistent occlusion (AOL 0; Dunnett’s test, p = 0.0385). If AOL scores are dichotomized, those with restoration of distal flow (AOL 2–3) have significantly greater mean delta ADCr (16.4±2.07% vs. 1.99±4.33% for AOL 0–1, p = 0.0039).
Revascularization limits swelling in patients with low baseline ADCr
There was a trend towards greater swelling volume in those patients with baseline ADCr < 64%, who had a median swelling volume of 25ml (IQR 5.3–73ml) versus a median of 21 (IQR 2.8–32ml) in those with ADCr ≥ 64%. This difference did not reach significance (p = 0.19). Similarly, there was a trend towards greater swelling volume in those patients with AOL 0–1 (median 27ml [IQR, 13–124ml]) versus those with AOL 2–3 (median 21ml [IQR, 3.2–35]). This also did not reach significance (p = 0.13). We assessed the relationship between swelling volume and other known predictors of cerebral edema, including age, baseline NIHSS, DWI lesion volume and baseline glucose level (Supplementary Table 1). Of these, NIHSS (r2 = 0.14, p = 0.0023) and DWI volume (r2 = 0.17, p = 0.0005) were significantly associated with swelling volume. In multivariable linear regression, only DWI volume remained significantly associated with swelling (adjusted r2 = 0.22, p = 0.013).
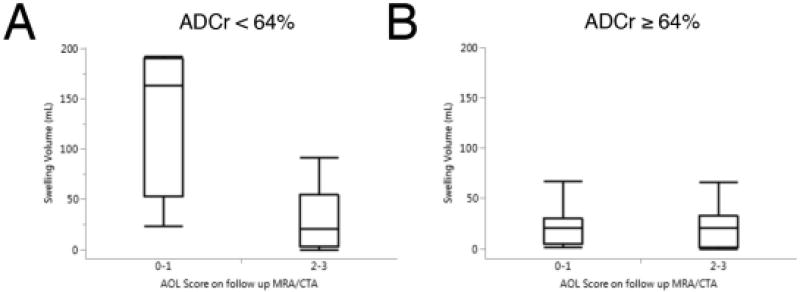
To evaluate the interplay between baseline ADCr and revascularization, we separately analyzed the effect of vessel recanalization in those patients with baseline ADCr above and below the 64% threshold. Those patients with baseline ADCr < 64% who did not achieve restoration of distal flow (AOL 0–1) had significantly greater swelling than those who did (Figure 4a; median 164ml [IQR 53.3–190ml] vs 20.7ml [IQR 3.20–55.1ml] in AOL 2–3, p = 0.024). There was no effect of recanalization on swelling in those patients with baseline ADCr ≥ 64% (Figure 4b, p = 0.827). Linear regression analysis of the stratified data found that both DWI volume (r2 = 0.33, p = 0.0078) and dichotomized AOL score (r2 = 0.33, p = 0.0099) were significantly associated with swelling volume in only the low ADCr group. Multivariable linear regression shows that both AOL score and DWI volume remain independent predictors of swelling volume (adjusted r2 = 0.47, p = 0.020 for DWI volume, p = 0.020 for AOL 0–1 vs 2–3).
Figure 4.
Baseline ADCr predicts the effect of recanalization on swelling. In those patients with a low baseline ADCr (< 64%), lack of distal reconstitution was associated with greater swelling (median 164ml [IQR 53.3–190ml] vs 20.7ml [IQR 3.20–55.1ml] for AOL 2–3, p = 0.024). There was no significant effect of recanalization on swelling volume in patients with baseline ADCr ≥ 64% (median 21.0ml [IQR 4.56–30.5ml] for AOL 0–1 vs. 20.7ml [IQR 1.8–32.6ml] for AOL 2–3).
Discussion
The ratio of ADC signal intensity between ischemic and normal tissue can be used as a marker of cytotoxicity that is predictive of edema[20] and outcome[21] following ischemic stroke. In the current study, we demonstrate the ability of ADCr to predict outcome in a second cohort, further supporting its utility as an imaging biomarker. We find that reopening of the initial vascular occlusion is associated with normalization of ADC on follow up imaging, emphasizing the fact that diffusion imaging is dynamic and does not necessarily represent tissue fate when examined at a single time point. Perhaps most importantly, we demonstrate that the vessel recanalization is associated with less edema, but only in those patients with low baseline ADCr, representative of more severe cytotoxic injury. Those patients with high ADCr at baseline did not appear to be at as much risk for edema in this cohort, such that recanalization did not reduce the already low swelling volume.
Our finding that vessel recanalization was associated with reduced edema was somewhat surprising in the context of animal data that suggests a substantial role for reperfusion edema in models of focal ischemia[25–28]. In humans, there is a suggestion that thrombolysis may reduce the incidence of edema[29]. Furthermore, the finding that persistent vessel occlusion was a predictor of swelling in large MCA infarct[30] is consistent with our observations in the current study. While there are likely many differences between the animal models and human disease to explain the discrepancy[31], one highlight is the difference in timing. Most animal studies looked at edema shortly after reperfusion, while the MR RESCUE cohort only has follow up imaging available a median of 7 days after presentation.
The findings in the current study are complementary to our recent analysis of perfusion imaging in the same cohort[32]. We found that the degree of change in perfusion-weighted imaging between baseline and follow up MRI was associated with swelling volume, with greater degree of reperfusion associated with less swelling. These two studies highlight the importance of multimodal imaging in fully understanding the complex relationships between initial cytotoxic injury, vessel reopening, tissue reperfusion and development of edema. The advantage of ADC is its utility in aiding in the prediction of edema even when only measured at an acute time point, however it is likely that multiple imaging modalities are needed for patient selection and outcome prediction in acute stroke.
This study has several limitations. The MR RESCUE cohort includes only those patients who presented with a penumbral pattern of ischemia, so the findings cannot be extrapolated to other patterns. The trial itself was unsuccessful, owing largely to an inability of the endovascular intervention to achieve rates of recanalization better than those in the medical arm[22]. We address that here by dividing our subjects based on actual observed vessel recanalization rather than treatment arm. However, this introduces a second limitation in that we do not know the timing of revascularization, as we only have vessel imaging in all subjects at 7 days post-stroke. Vessel opening could have occurred at any point between subject enrollment and the follow up CTA/MRA. We therefore cannot extrapolate these results to understand the effect of early thrombectomy or tPA administration on cerebral edema. However, if vessel opening did occur late, it would seem that this should bias against finding a significant effect of recanalization on change in ADCr, given our prior finding ADCr declines to a nadir at 24 hours after stroke onset[20]. In addition, while the benefit of vessel recanalization was only demonstrated at low baseline ADCr, this is based on analysis of swelling volume rather than clinical outcome. It would be useful to try to replicate our results in larger trials of MR imaging-based selection of patients for endovascular intervention[33,34].
The single, 7-day follow up time point may also limit our ability to accurately estimate the degree of edema. While case series of patients with large hemispheric infarction have found that most edema and related neurologic deterioration occurs within 48 hours[35], it can also be observed out to 10 days post-stroke[36]. The infarct volumes in the current cohort were generally smaller than in these series and none of the patients examined here underwent decompressive craniectomy, but it is likely that the timing of edema is similar. It is possible that the swelling volumes measured at this late time point may partially reflect the use of hyperosmolar therapy, leading us to underestimate the effects of persistent vessel occlusion and lower ADCr on swelling volume. Data on rates of hyperosmolar use in this cohort are not available.
Overall, this study provides further evidence for the utility of ADC as an imaging biomarker of edema and outcome after stroke. Further analysis of ADC as part of a multimodal imaging strategy is warranted in an effort to enrich patient selection for acute stroke therapy.
Supplementary Material
Acknowledgments
Funding
The funding for the MR RESCUE trial was provided by NIH P50NS044378 (C.S.K). Analysis was funded in part by NIH K23NS076597, NIH R01NS099209 and the Andrew David Heitman Neurovascular Research Foundation (W.T.K). Dr. Kimberly has also received funding from Remedy Pharmaceuticals and AHA 14GRNT19060044.
Footnotes
Conflicts of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship or publication of this article.
Literature Cited
- 1.Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, Pereira VM, et al. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 4.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. N Engl J Med. 2015;372:1–11. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 5.Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 6.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 7.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 8.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with Alteplase 3 to 4.5 Hours after Acute Ischemic Stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 9.Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CBLM, Dippel DW, et al. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA. 2016;316:1279. doi: 10.1001/jama.2016.13647. [DOI] [PubMed] [Google Scholar]
- 10.Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJB, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 11.Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 AHA/ASA Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment. 2015 doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler HM, Mlynash M, Inoue M, Tipirnini A, Liggins J, Bammer R, et al. The growth rate of early DWI lesions is highly variable and associated with penumbral salvage and clinical outcomes following endovascular reperfusion. Int J Stroke. 2015;10:723–729. doi: 10.1111/ijs.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidwell CS, Wintermark M, De Silva DA, Schaewe TJ, Jahan R, Starkman S, et al. Multiparametric MRI and CT models of infarct Core and favorable penumbral imaging patterns in acute ischemic stroke. Stroke. 2013;44:73–79. doi: 10.1161/STROKEAHA.112.670034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neumann-Haefelin T, Moseley ME, Albers GW. New magnetic resonance imaging methods for cerebrovascular disease: emerging clinical applications. Ann Neurol. 2000;47:559–570. [PubMed] [Google Scholar]
- 15.Sakoh M, Ostergaard L, Gjedde a, Røhl L, Vestergaard-Poulsen P, Smith DF, et al. Prediction of tissue survival after middle cerebral artery occlusion based on changes in the apparent diffusion of water. J Neurosurg. 2001;95:450–8. doi: 10.3171/jns.2001.95.3.0450. [DOI] [PubMed] [Google Scholar]
- 16.Sakoh M, Ohnishi T, Ostergaard L, Gjedde A. Prediction of tissue survival after stroke based on changes in the apparent diffusion of water (cytotoxic edema) Acta NeurochirSuppl. 2003;86:137–140. doi: 10.1007/978-3-7091-0651-8_29. [DOI] [PubMed] [Google Scholar]
- 17.Guadagno JV, Warburton EA, Aigbirhio FI, Smielewski P, Fryer TD, Harding S, et al. Does the acute diffusion-weighted imaging lesion represent penumbra as well as core? A combined quantitative PET/MRI voxel-based study. J Cereb Blood Flow Metab. 2004;24:1249–1254. doi: 10.1097/01.WCB.0000141557.32867.6B. [DOI] [PubMed] [Google Scholar]
- 18.Guadagno JV, Jones PS, Fryer TD, Barret O, Aigbirhio FI, Carpenter TA, et al. Local relationships between restricted water diffusion and oxygen consumption in the ischemic human brain. Stroke. 2006;37:1741–1748. doi: 10.1161/01.STR.0000232437.00621.86. [DOI] [PubMed] [Google Scholar]
- 19.Tudela R, Soria G, Pérez-De-Puig I, Ros D, Pavía J, Planas AM. Infarct volume prediction using apparent diffusion coefficient maps during middle cerebral artery occlusion and soon after reperfusion in the rat. Brain Res. 2014;1583:169–178. doi: 10.1016/j.brainres.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Battey TW, Karki M, Singhal AB, Wu O, Sadaghiani S, Campbell BC, et al. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke. 2014;45:3643–3648. doi: 10.1161/STROKEAHA.114.006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bevers MB, Vaishnav NH, Pham L, Battey TWK, Kimberly WT. Hyperglycemia is associated with more severe cytotoxic injury after stroke. J Cereb Blood Flow Metab. 2017;37 doi: 10.1177/0271678X16671730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–23. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T. Revascularization end points in stroke interventional trials: Recanalization versus reperfusion in IMS-I. Stroke. 2005;36:2400–2403. doi: 10.1161/01.STR.0000185698.45720.58. [DOI] [PubMed] [Google Scholar]
- 24.Tsujita N, Kai N, Fujita Y, Hiai Y, Hirai T, Kitajima M, et al. Interimager variability in ADC measurement of the human brain. [cited 2016 May 17];Magn Reson Med Sci. 2014 13:81–7. doi: 10.2463/mrms.2012-0098. [DOI] [PubMed] [Google Scholar]
- 25.Pillai DR, Dittmar MS, Baldaranov D, Heidemann RM, Henning EC, Schuierer G, et al. Cerebral ischemia-reperfusion injury in rats--a 3 T MRI study on biphasic blood-brain barrier opening and the dynamics of edema formation. J Cereb Blood Flow Metab. 2009;29:1846–55. doi: 10.1038/jcbfm.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell BA, Symon L, Branston NM. CBF and time thresholds for the formation of ischemic cerebral edema, and effect of reperfusion in baboons. J Neurosurg. 1985;62:31–41. doi: 10.3171/jns.1985.62.1.0031. [DOI] [PubMed] [Google Scholar]
- 27.Gartshore G, Patterson J, Macrae IM. Influence of ischemia and reperfusion on the course of brain tissue swelling and blood-brain barrier permeability in a rodent model of transient focal cerebral ischemia. Exp Neurol. 1997;147:353–360. doi: 10.1006/exnr.1997.6635. [DOI] [PubMed] [Google Scholar]
- 28.Nour M, Scalzo F, Liebeskind DS. Ischemia-reperfusion injury in stroke. Interv Neurol. 2013;1:185–99. doi: 10.1159/000353125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudolf J, Grond M, Stenzel C, Neveling M, Heiss WD. Incidence of space-occupying brain edema following systemic thrombolysis of acute supratentorial ischemia. Cerebrovasc Dis. 1998;8:166–171. doi: 10.1159/000015843. [DOI] [PubMed] [Google Scholar]
- 30.Thomalla G, Hartmann F, Juettler E, Singer OC, Lehnhardt FG, Köhrmann M, et al. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: A prospective multicenter observational study. Ann Neurol. 2010;68:435–445. doi: 10.1002/ana.22125. [DOI] [PubMed] [Google Scholar]
- 31.Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irvine HJ, Ostwaldt A-C, Bevers MB, Dixon S, Battey TW, Campbell BC, et al. Reperfusion after ischemic stroke is associated with reduced brain edema. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17720559. 0271678X1772055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wouters A, Lemmens R, Christensen S, Wilms G, Dupont P, Mlynash M, et al. Magnetic resonance imaging-based endovascular versus medical stroke treatment for symptom onset up to 12 h. Int J Stroke. 2016;11:127–33. doi: 10.1177/1747493015607503. [DOI] [PubMed] [Google Scholar]
- 34.Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 1995;0:1581–1587. doi: 10.1016/S1474-4422(16)30177-6. [DOI] [PubMed] [Google Scholar]
- 35.Qureshi AI, Suarez JI, Yahia AM, Mohammad Y, Uzun G, Suri MFK, et al. Timing of neurologic deterioration in massive middle cerebral artery infarction: A multicenter review. Crit Care Med. 2003;31:272–277. doi: 10.1097/00003246-200301000-00043. [DOI] [PubMed] [Google Scholar]
- 36.Robertson SC, Lennarson P, Hasan DM, Traynelis VC. Clinical course and surgical management of massive cerebral infarction. Neurosurgery. 2004;55:55–61. doi: 10.1227/01.neu.0000126875.02630.36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.