Abstract
Purpose
The aims were to 1) determine feasibility of measuring physical function in our ICU Recovery Clinic (RC), 2) determine if physical function was associated with 6-month re-hospitalization and 1-year mortality and 3) compare ICU survivors’ physical function to other comorbid populations.
Materials and Methods
We established the Wake Forest ICU RC. Patients were seen in clinic 1 month following hospital discharge. Testing included the Short Form-36 questionnaire and Short Physical Performance Battery (SPPB). We related these measures to 6 month re-hospitalizations and 1 year mortality, and compared patients’ functional performance with other comorbid populations.
Results
Thirty-six patients were seen in clinic from July 2014 to June 2015; the median SPPB score was 5 (IQR 5). The median SF-36 physical component summary score was 21.8 (IQR 28.8). Mortality was 14% at 1 year. Of those who did not die by 1 year, 35% were readmitted to our hospital within 6 months of hospital discharge. SPPB scores demonstrated a non-significant trend with both mortality (p=0.06) and readmissions (p=0.09). ICU survivors’ SPPB scores were significantly lower than those of other chronically ill populations (p<0.001).
Conclusions
Physical function measurement in a recovery clinic is feasible and may inform subsequent morbidity and mortality.
Keywords: critical illness, clinic, recovery, physical function, healthcare utilization, intensive care unit
Introduction
More than 4 million Americans are hospitalized with critical illness annually [1–3]. Recent advances in critical care have led to an increasing number of survivors of critical illness [3–5]. Despite this success, emerging data demonstrate significant long-term physical, cognitive, and neuropsychiatric morbidity in survivors of sepsis and the Acute Respiratory Distress Syndrome (ARDS), two of the most common causes of critical illness [6–13]. Physical dysfunction affects approximately two-thirds of patients surviving critical illness [12–16]. Common impairments include loss of muscle mass and function, joint immobility, exercise limitation, fatigue, and decreased quality of life.
Despite an increase in reports of significant impairments following critical illness, there are no formal, evidence-based guidelines by which to deliver post-discharge care. The National Institute for Health and Care Excellence issued recommendations for follow up care in critically ill patients in England and Whales in 2009 [17]. However, these recommendations were based on a paucity of data [18, 19]. More recently, a survey of post-discharge clinics in the United Kingdom (UK) demonstrated that only 30% of Intensive Care Units (ICUs) in the UK had a follow-up clinic, and that of those clinics 55% were nurse-led [20]. There was no consensus on timing of follow up visits, appropriate interventions, or which tool(s) should be used in these clinics to assess physical and psychological recovery. Barriers to broader implementation of ICU follow-up clinics included a lack of funding and a lack of supporting evidence for efficacy [20]. Published data regarding ICU follow-up clinics in the United States are minimal [21, 22]. Although there have not been any efficacy studies to date, there is growing interest in ICU Recovery Clinics, on the part of patients, families, clinicians, and researchers [23–25].
Current evidence suggests that objective measures of physical function are highly correlated with both hospitalizations [26] and mortality [27–29] regardless of the underlying disease process [30]. Given these data, many clinicians have advocated for the incorporation of physical function measures into routine care [31, 32]. Physical function has been measured in post-ICU populations, but to date, these have either been in the context of research studies or only evaluated via a subjective measure. One purpose of the Wake Forest ICU Recovery Clinic was to assess the trajectory of patients in terms of their healthcare burden and recovery. Therefore, we sought to use physical function measures as a novel way to assess patients in an ICU Recovery Clinic as a part of routine clinical care. The aims of this study were to 1) determine our ability to administer objective and subjective measures of physical function in an ICU recovery clinic, 2) to determine if physical function at 1 month post-ICU discharge was associated with 6-month re-hospitalization and 1-year mortality and 3) to compare an objective measure of ICU survivors’ physical function to that of other populations with comorbidities.
Materials and Methods
The Wake Forest ICU Recovery Clinic took place at a tertiary care academic medical center with a 33 bed Medical ICU (MICU). Participants were recruited between July 2014 and June 2015. Patients 18 years of age or older admitted to the MICU with septic shock and/or acute hypoxic or mixed respiratory failure requiring mechanical ventilation for more than 24 hours were screened for inclusion in the ICU Recovery Clinic. Patient who were bed- or wheelchair-bound prior to admission or who had active metastatic cancer were excluded. Baseline physical function was not assessed during hospitalization. Convenience sampling was used and an iterative process was utilized to try to optimize attendance at the Recovery Clinic. Selected patients were identified and scheduled for a clinic appointment approximately one month following hospital discharge. Structured clinic visits included evaluations by a clinical pharmacist and a clinical assessment by a pulmonary and critical care- trained physician.
ICU Recovery Clinic visits began with medication reconciliation, evaluation, and counselling by the clinical pharmacist. The patients then underwent hand-grip strength testing, completed a Short Physical Performance Battery (SPPB), and then were administered a Short Form-36 questionnaire (SF-36). The hand grip strength testing was done using an isometric hydraulic hand dynamometer (Jamar, Bolingbrook, IL) and has been reported in other studies [33]. Testing takes approximately 3 minutes. The test is performed by asking a seated patient to hold his/her arm at 90 degrees; they were then instructed to squeeze the dynamometer to the best of their ability. A measurement in handgrip strength (kg of force) is obtained. After each attempt the indicator needle was reset to zero and the maneuver repeated for a total of three times in each hand. An average for each hand is taken and reported [34]. The SPPB is an integrated measure of physical function and consists of 3 components (balance testing, 4 meter gait speed, and repeated chair stands). Each component is graded on a scale of zero to four points, with a total sum score maximum of 12 [35–37]. Administration of the SPPB typically requires less than 10 minutes. To meet the layout of the clinic, we typically performed balance testing first, then repeated chair stands followed by gait speed. The SF-36 is a quality of life questionnaire that measures the following domains: physical function, role physical, bodily pain, mental health, role emotional, social function, general health, and vitality [38]. SF-36 testing typically requires 8-12 minutes. Physician evaluations were then completed and patients were informed of the recommended treatments, studies, and referrals. In select cases of significant impairment and/or need for coordination, patients were invited back to the Wake Forest ICU Recovery Clinic for a follow-up visit and repeat assessment.
A retrospective cohort study of Wake Forest ICU Recovery Clinic patients was approved by the Wake Forest Institutional Review Board (Wake Forest IRB 00031295). Preliminary data has been presented in abstract form [39]. For the purposes of this study, patient demographics and characteristics were obtained through chart review. The Charlson Comorbidity Index [40] and APACHE II [41] scores were calculated from retrospective chart review. The APACHE II scores were calculated using the worst physiologic values recorded at the time of admission to the ICU or within the first 24 hours of ICU stay. The Glasgow Coma Score (GCS) is not routinely measured at the time of ICU admission and only one patient had a GCS recorded. All missing GCS’s were imputed at 15 in order to calculate a best-case APACHE II score. Healthcare utilization was assessed through a review of the electronic medical record (EMR) in order to note readmissions to Wake Forest within 6 months of ICU discharge. Additionally, mortality information was abstracted from the EMR as well as the North Carolina State Center for Health Statistics. For all analyses, descriptive statistics were utilized with mean (standard deviation (SD)) and median (interquartile range (IQR)) being reported. Data were analyzed using the Student’s t-test, Wilcoxon rank sum test, Pearson Chi-squared test, Fisher’s exact test, and logistic regression when appropriate. All logistic regression analyses for 6 month readmissions and 1 year mortality were bivariate; sample size precluded multivariate logistic regression. All statistical analyses were performed with Excel 2010 (Microsoft, Redmond, WA) and STATA SE 13.1 (StataCorp, College Station, TX).
Comparison groups for SPPB were older study participants with defined comorbidities who were enrolled in other studies supported by the Wake Forest Claude D. Pepper Older Americans Independence Center. Baseline SPPB assessments in all comparison groups were performed before any study interventions. The comparison study populations comprised the following: 1) adults with renal failure who were candidates for transplantation[42], 2) adults with congestive heart failure with preserved ejection fraction[43], 3) adults with chronic obstructive pulmonary disease[44], 4) adults who were at high risk for cardiovascular disease[45], and 5) healthy older adults [46, 47].
Results
Recovery Clinic Screening and Enrollment
Between July 25th, 2014 and June 26th, 2015 MICU patients were screened Monday through Friday. We evaluated 36 patients in the clinic—7 with septic shock, 20 with acute hypoxic and/or mixed respiratory failure (ARF), and 9 with both. Patients were seen an average of 31 days following hospital discharge.
Patient Characteristics
The median age of patients upon admission to the ICU was 64.5 (IQR 27.5) (Table 1). Fifty-three percent of patients were male and the overall median BMI of 27.4 (IQR 11.7). The median hospital length of stay overall was 13.0 days (10.3). The median APACHE II score at admission was 28.5 (IQR 8.0) and the median Charlson Comorbidity Index was 4.0 (IQR 2.3). Notably, 47% received steroids and 14% received continuous neuromuscular blockade. Forty-four percent required vasopressors and/or inotropes and twenty-five percent were diagnosed with ARDS.
Table 1.
Baseline Characteristics
| Patient Characteristic | (n=36) |
|---|---|
| Age, median (IQR) | 64.5 (27.5) |
| Sex, Male (%) | 19 (53%) |
| BMI, median (IQR) | 27.4 (11.7) |
| APACHE II (at admission)*, median (IQR) | 28.5 (8.0) |
| Charlson Comorbidity Index, median (IQR) | 4.0 (3.3) |
| Diagnosed with ARDS, n (%) | 9 (25%) |
| Required RRT, n (%) | 5 (14%) |
| In-hospital cardiac arrest, n (%) | 0 (0%) |
| Received steroids, n (%) | 17 (47%) |
| Received neuromuscular blockade†, n (%) | 5 (14%) |
| Received vasopressors‡, n (%) | 16 (44%) |
| Days on ventilator, median (IQR) | 3.0 (4.3) |
| Hospital LOS, median (IQR) | 13.0 (10.3) |
Baseline Characteristics of Wake Forest ICU Recovery Clinic Patients.
Abbreviations: IQR=interquartile range; BMI= Body Mass Index in kg/m2; APACHE= Acute Physiologic and Chronic Health Evaluation; ARDS = Acute Respiratory Distress Syndrome; RRT= Renal Replacement Therapy; LOS= length of stay.
Values used were taken retrospectively through chart review during the first 24 hours of admission to the ICU, GCS of 15 imputed if missing data.
Exclusive of neuromuscular blockade for intubation.
Vasopressors include norepinephrine, epinephrine, dopamine and phenylephrine.
Feasibility of Physical Function and Health-Related Quality of Life Testing
A total of 35 patients underwent hand grip strength testing with a median recording of 19.5 Kg force (IQR 16.1) for the right hand (Table 2). Thirty-five patients completed the SPPB with a median score of 5 (IQR 5) on a scale of 0–12. The median for each sub-score (4 possible points each) was: 3 (IQR 3) for balance, 1 (IQR 1) for gait speed, and 1 (IQR 2) for the chair stands. Thirty of the 36 patients completed SF-36 testing. Patients had a median PCS score of 21.8 (IQR 28.8) and MCS score of 78.7 (IQR 61.7). The median SF-36 physical function score was 35 (IQR 30).
Table 2.
Objective and Subjective Physical Function Measures at the Time of ICU Recovery Clinic Visit Related to Readmissions and Mortality
| Patient Characteristic | ALL PATIENTS n=36 | 6 MONTH READMISSION | 1 YEAR MORTALITY OR | ||||
|---|---|---|---|---|---|---|---|
| YES | NO | OR (95%CI), p-value | YES | NO | (95%CI), p-value | ||
|
| |||||||
| Hand grip strength*, Kg force, median (IQR) | |||||||
| Left | 20.7 (14.0) | 14 (21) | 21.67 (13.2) | OR 0.98 (0.91-1.04), p 0.47 | 13.67 (8.3) | 21.7 (14) | OR 0.96 (0.88-1.05), p 0.42 |
| Right | 19.5 (16.1) | 14 (10.7) | 21 (16.5) | OR 0.95 (0.88-1.02), p 0.16 | 13.3 (3) | 19.8 (16.7) | OR 0.96 (0.88-1.06), p 0.42 |
|
| |||||||
| SPPB*, median (IQR) | |||||||
| Balance Subscore | 3 (3) | 2 (4) | 3 (2) | OR 0.74 (0.47-1.15), p 0.18 | 0 (2) | 3 (2) | OR 0.52 (0.27-0.99), p 0.03 |
| Gait Subscore | 1 (1) | 0 (2) | 1.5 (1.5) | OR 0.53 (0.25-1.11), p 0.07 | 1 (1) | 1 (2) | OR 0.65 (0.25-1.71), p 0.36 |
| Chair Subscore | 1 (2) | 1 (1) | 1 (2) | OR 0.70 (0.35-1.41), p 0.29 | 0 (1) | 1 (2) | OR 0.37 (0.09-1.54), p 0.09 |
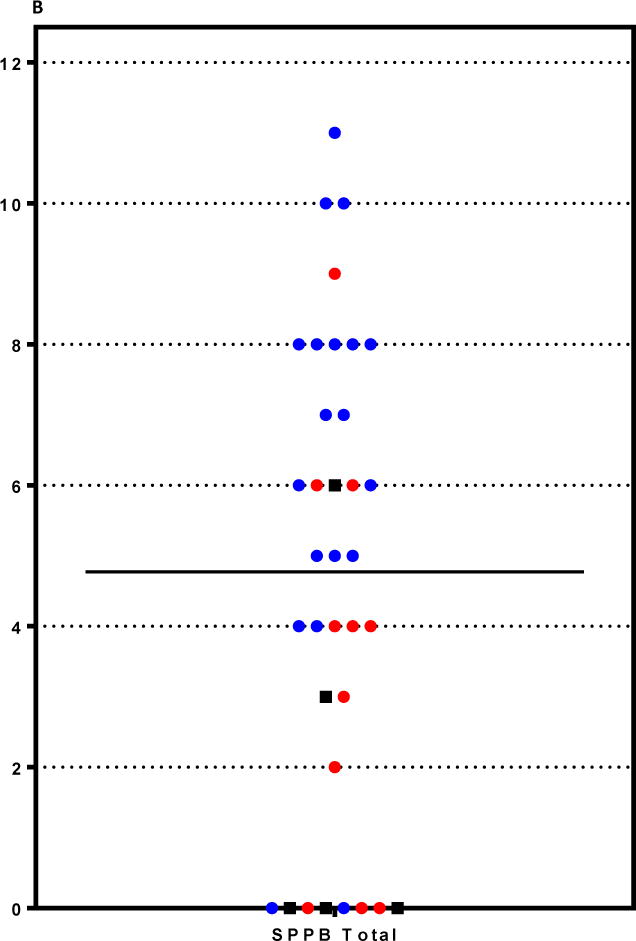
| TOTAL Score | 5 (5) | 4 (6) | 6 (4.5) | OR 0.83 (0.66-1.05), p 0.11 | 2 (3) | 5.5 (4) | OR 0.73 (0.52-1.04), p 0.053 |
|
| |||||||
| Short Form-36†, median(IQR) | |||||||
| Physical Functioning | 35 (30) | 20 (27.5) | 40 (30) | OR 0.97 (0.93-1.01), p 0.18 | 15 (22.5) | 40 (30) | OR 0.94 (0.88-1.01), p 0.11 |
| Physical Role | 0 (25) | 0 (12.5) | 0 (25) | OR 1.0 (0.97-1.03), p 0.95 | 0 (0) | 0 (25) | — |
| Emotional Role | 66.7 (100) | 66.7 (100) | 66.7 (100) | OR 1.0 (0.98-1.02), p 0.91 | 50 (66.7) | 66.7 (100) | OR 1.0 (0.97-1.02), p 0.78 |
| Vitality | 30 (30) | 30 (25) | 30 (35) | OR 0.99 (0.96-1.03), p 0.66 | 22.5 (15) | 35 (30) | OR 0.98 (0.93-1.03), p 0.35 |
| Mental Health | 68 (44) | 60 (42) | 68 (36) | OR 1.0 (0.97-1.03), p 0.92 | 70 (22) | 64 (48) | OR 1.0 (0.96-1.04), p 0.99 |
| Social Functioning | 50 (50) | 75 (43.75) | 50 (37.5) | OR 1.02 (0.99-1.05), p 0.14 | 56.3 (56.3) | 50 (62.5) | OR 1.0 (0.96-1.03), p 0.78 |
| Bodily Pain | 42.5 (57.5) | 30 (60) | 47.5 (57.5) | OR 0.99 (0.97-1.01), p 0.43 | 51.3 (78.8) | 42.5 (57.5) | OR 1.0 (0.97-1.02), p 0.95 |
| General Health | 40 (30) | 42.5 (15) | 40 (35) | OR 1.01 (0.97-1.06), p 0.58 | 25 (17.5) | 45 (25) | OR 0.92 (0.84-1.00), p 0.051 |
| Physical Summary Score | 21.8 (28.8) | 14.7(22.1) | 26.9 (27.4) | OR 0.98 (0.94-1.02), p 0.36 | 5.6 (41.2) | 25.9 (27.4) | OR 0.95 (0.89-1.01), p 0.12 |
| Mental Summary Score | 78.7 (61.7) | 69.4 (58.0) | 78.7 (60.1) | OR 1.01 (0.98-1.03), p 0.61 | 58.6 (59.0) | 78.7 (63.0) | OR 1.00 (0.97-1.03), p 0.99 |
Objective and subjective physical function testing completed at the time of the ICU Recovery Clinic visit.
Abbreviations: Kg= Kilograms; IQR= Interquartile Range; SPPB= Short Physical Performance Battery; OR= Odds Ratio; CI= Confidence Interval.
Missing data for 1 patient.
Missing data for 6 patients.
Readmissions comprised readmissions to Wake Forest only.
Healthcare Utilization and Mortality
Fourteen percent (5/36) of all patients seen in the ICU Recovery Clinic were deceased at 1 year following their initial hospital stay. Excluding those who died in the year following their ICU stay, 11 of 31 (35%) patients were readmitted to Wake Forest Baptist Medical Center within the 6 months following their critical illness. Handgrip was not significantly associated with readmission or 1 year mortality (right handgrip p= 0.16, Odds Ratio (OR) 0.95 (95% Confidence Interval (CI) 0.88-1.02) and p= 0.42, OR 0.96 (95% CI 0.88-1.06), respectively, Table 2). The SPPB balance score, but not gait speed or chair stands, was significantly associated with 1 year mortality (p= 0.03, OR 0.52 (95% CI 0.27-0.99), Figure 1). The SPPB sum score approached significance for an association with 1 year mortality (p= 0.053, OR 0.73 (95% CI 0.52-1.04)) and for 6 month readmissions (p=0.11, OR 0.83 (95% CI 0.66-1.05)). The SF-36 component scores were not significantly associated with readmissions and 1 year mortality with the following exception: the SF-36 general health component was nearly significantly associated with 1 year mortality (p= 0.051, OR 0.92 (95% CI 0.84-1.00)). The SF-36 physical functioning component demonstrated a possible trend for association with both readmissions and mortality (p=0.18, OR 0.97 (95% CI 0.93-1.01) and p=0.11 (OR 0.94 (0.88-1.01) respectively).
Figure 1. SPPB Scores in Relation to Six Month Readmissions and 1 Year Mortality.
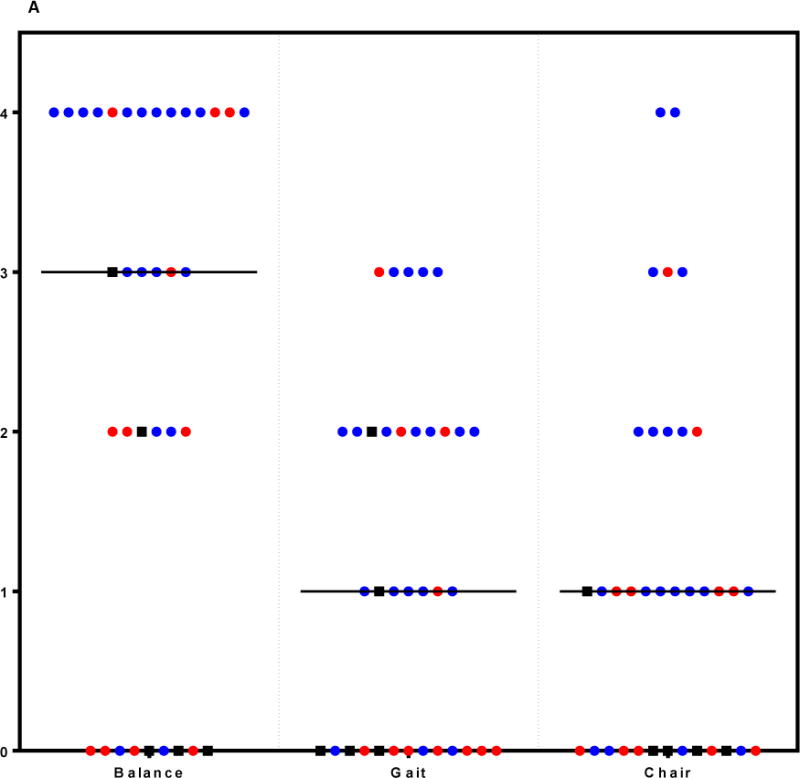
A: SPPB Component scores in ICU Recovery Clinic Patients. Each patient seen in the ICU Recovery Clinic is represented by a circle or square for each component of the SPPB (balance testing, gait speed, and repeated chair stands; range for each 0-4). Black boxes demonstrate deaths by 1 year after ICU discharge. Red circles denote readmissions to Wake Forest within the six months after ICU discharge. Blue circles denote patients who returned to the ICU Recovery Clinic and are not known to be dead at 1 year or have been readmitted to Wake Forest within six months of ICU discharge.
B: Total SPPB Scores in ICU Recovery Clinic Patients. Each patient seen in the ICU Recovery Clinic is represented by a circle or square for their total SPPB score (range 0-12). Black boxes demonstrate deaths by 1 year after ICU discharge. Red circles denote readmissions to Wake Forest within the six months after ICU discharge. Blue circles denote patients who returned to the ICU Recovery Clinic and are not known to be dead at 1 year or have been readmitted to Wake Forest within six months of ICU discharge.
Abbreviations: SPPB= Short Physical Performance Battery; Gait= Gait Speed; Chair= Repeated Chair Stands.
Comparison of SPPB in ICU survivors compared to other populations
We compared SPPB data from the ICU survivors seen in clinic with existing data from other studies of older patients with particular comorbidities (Table 3). The SPPB component scores and overall score for ICU survivors were significantly lower than those from each comparator group, with p-values for each <0.01 (Figure 2).
Table 3.
Other Co-morbid Cohorts used for comparison
| Study Population | Number of Subjects | Mean Age (SD) | Overall SPPB Mean (SD) | p-value for comparison with ICU Recovery Cohort |
|---|---|---|---|---|
| End Stage Renal Failure Patients Awaiting Organ Transplantation | n= 26 | 67 (4.9) | 8.4 (2.8) | <0.001 |
| Patients with Congestive Heart Failure with a Preserved Ejection Fraction | n= 52 | 70 (7.5) | 9.7(1.6) | <0.001 |
| Patients with COPD | n= 153 | 67 (9.8) | 10.4 (1.5) | <0.001 |
| Patients at High Risk of Cardiovascular Disease | n= 279 | 66 (7.5) | 10.3 (1.5) | <0.001 |
| Healthy Older Adults | n= 54 | 70 (7.7) | 11.3 (0.8) | <0.001 |
Number of subjects, age, and SPPB scores for other co-morbid cohorts used for comparison to the ICU Recovery Cohort.
Abbreviations: n= number; SD= Standard Deviation; SPPB= Short Physical Performance Battery.
Figure 2. SPPB scores at ICU Recovery Clinic compared to other older, co-morbid populations.
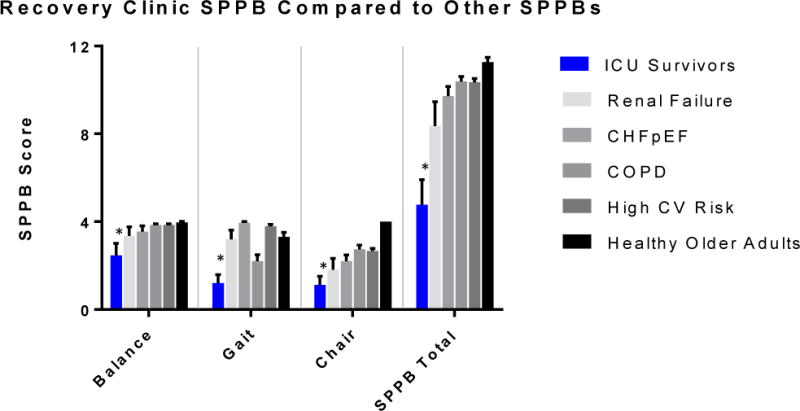
SPPB of ICU Recovery Clinic Patients compared to those of other co-morbid conditions. Data are graphed as mean with 95% confidence interval. Each comparison between SPPB scores of ICU Recovery Clinic patients and cohorts with other conditions was statistically significant with p<0.01 (denoted by *).
Discussion
To our knowledge, this is the first report of a post-ICU clinic with both objective and subjective measures of physical function outside the context of a research study. We were able to perform SPPB, handgrip, and SF-36 within the context of the ICU Recovery Clinic visit, demonstrating feasibility. Additionally, we found that SPPB had a nearly significant association with 6-month readmissions and 1-year mortality. Finally, we demonstrated that SPPB measures in the Wake Forest ICU Recovery Clinic population were significantly lower than other older, comorbid populations. It is important to note as well, the high rates of 6 month readmissions and 1 year mortality (44% combined overall).
In our ICU Recovery Clinic, the SPPB, handgrip, and SF-36—were all feasible as demonstrated by at least an 83% completion rate for each test [48]. The SPPB generally takes less than 10 minutes to complete; handgrip testing requires less than 3 minutes; and the Short Form-36 less than 10 minutes. While these did add a significant amount of time to each clinic visit, we had intentionally scheduled 1 hour clinic visits for our patients. Additionally, in the future all three of these tests may not be necessary; rather the SPPB might be a higher-yield test to complete. We propose that the SPPB be considered for part of standard measures in an ICU Recovery Clinic given 1) its ease of administration, 2) its clinical relevance based on its face validity, and 3) its potential ability to predict 6 month re-hospitalizations and 1 year mortality in this population.
Face validity of the SPPB is simply based on the fact that it is easy for a clinician to understand how the inability to rise from a chair or the inability to walk at a reasonable gait speed might affect a patient. There appears to be more face validity to the components of the SPPB than for example, handgrip or even the SF-36. In fact, the SPPB was first described and validated in 1995 when Guralnik, et al. showed that in 70 year old, nondisabled, community dwellers lower-extremity function measured using the SPPB was highly predictive of subsequent disability [49]. In their cohort, those that scored 4, 5 or 6 on the SPPB were 4.2 to 4.9 times more likely to have disability in activities of daily living or mobility-related disability at four years when compared to those with scores of 10, 11 or 12 [35]. Subsequent studies have demonstrated that the prognostic value of the SPPB applies to patients following hospital discharge and in those with COPD [37, 50, 51].
Compared to patients with other chronic illnesses, our patients scored significantly lower on the SPPB. This demonstrates the profound disability that many of our ICU survivors have 1 month after discharge from the hospital. It is important to note, however, that our patients are not clinically stable—rather their physical function is in flux and has been shown to improve on the average over the next 5 months although there is significant heterogeneity in the clinical course [13, 14, 52, 53]. Despite the differing trajectories of our patients’ physical function, our small cohort demonstrated a possible trend toward a significant association between SPPB score and 6-month readmissions as well as 1-year mortality. This highlights that the SPPB may be highly clinically relevant, particularly in a recovery clinic, when a clinician might not routinely have access to other data (eg. APACHE or Charlson) that might help predict outcomes.
Finally, comparison of our ICU survivors’ SPPB scores with those of other patient populations can provide insight into the degree of physical impairments that these patients have. Notably, SPPB scores for each of the components of balance, gait, and chair-stands were significantly lower than each of the comparator comorbid groups; the SPPB overall score was also significantly lower for ICU survivors than for each of the comparator groups. This was inclusive of groups with chronic diseases including renal failure, congestive heart failure, and chronic obstructive pulmonary disease. These data highlight a significant need for methods to mitigate the development of physical impairments and/or improve the physical function of these patients.
There are limitations to this study. First, it is important to note the nature of our intervention and our study design as the retrospective nature of the data collection limits data availability. Also, our patient sample was a convenience sample of patients with a high severity of illness while in the ICU who were both willing and able to return to a Recovery Clinic appointment. These patients may not be representative of the larger post-ICU population; this may limit our ability to make generalizations to the broader population of ICU survivors. Third, the APACHE II scores were calculated using the patient’s admission data and the GCS is not routinely recorded upon admission to the ICU. However, the best-case GCS was used, so our reported APACHE data likely underestimate severity of illness. Fourth, the elements of the SPPB were performed out of the typical ordering of components for the SPPB, which may impact the results. Additionally, there was some missing data for handgrip, SPPB, and SF-36. Finally, the comparison of our patients’ physical function as measured by SPPB to that of other chronically ill groups has limitations in that the patient groups are not matched in terms of key variables such as age, sex, or comorbidities.
Despite these limitations, this cohort study does have external validity. First, even though our sample size was small and concerns about the generalizability of our sample may be valid, it should be noted that the SF-36 scores in our cohort were remarkably similar to those of Herridge et al at 3 months of follow-up of survivors of ARDS [54]. Also, our SPPB scores at 1 month post-ICU discharge were similar to those from a recent trial of standardized rehabilitation therapy [36]. Given these observations, the population we followed should be reasonably representative of the larger population.
Conclusions
In sum, this study is the first to report both subjective and objective measures of physical function in an ICU recovery clinic. We also demonstrated the novel use of the SPPB in the post-ICU clinic. The SPPB is well-validated in other patient cohorts and is a strong predictor of future disability, hospital readmission and mortality in older adults. In fact, it may be a measure that has enduring utility across a variety of patient populations. If the trends in our data are true, it could be a powerful motivator to demonstrate to a clinician or even a patient or family member the predicted reduction in odds of readmission and/or mortality per point increase in the SPPB score. Future, larger studies of ICU Recovery Clinics should consider use of the SPPB to measure physical function and help prognosticate risk for readmission and long term mortality.
Highlights.
-
–
It is feasible to measure physical function as part of an ICU recovery clinic.
-
–
Physical function measured by the SPPB may predict readmissions and mortality.
-
–
SPPB scores in ICU survivors were significantly lower than other comorbid cohorts.
Acknowledgments
We would like to thank Lori Flores, Carolann Young, Shannon Shields, Daniel Lipford, Lina Purcell, and Alexandra Bolick for their assistance with this project. We would like to also thank our ICU Recovery Clinic Patients and their caregivers for their strength and resilience.
Sources of Support: This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. We would like to acknowledge the assistance of the Wake Forest Clinical and Translational Science Institute (WF CTSI), which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001420. Drs. Bakhru and Files received support from the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332).
Primary Source of Funding: None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: All authors made substantial contributions to the conception or design of the study; the acquisition, analysis or interpretation of data; and drafting or revising the manuscript.
Data Supplement: None
Conflict of interest statement: All authors have no conflict of interest to declare.
Abbreviations: None
References
- 1.Carson SS, Cox CE, Holmes GM, Howard A, Carey TS. The changing epidemiology of mechanical ventilation: a population-based study. J Intensive Care Med. 2006;21(3):173–82. doi: 10.1177/0885066605282784. [DOI] [PubMed] [Google Scholar]
- 2.Needham DM, Bronskill SE, Calinawan JR, Sibbald WJ, Pronovost PJ, Laupacis A. Projected incidence of mechanical ventilation in Ontario to 2026: Preparing for the aging baby boomers. Critical care medicine. 2005;33(3):574–9. doi: 10.1097/01.ccm.0000155992.21174.31. [DOI] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis*. Critical care medicine. 2014;42(3):625–31. doi: 10.1097/CCM.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD, Network NNA Recent trends in acute lung injury mortality: 1996–2005. Critical care medicine. 2009;37(5):1574–9. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171(4):340–7. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 7.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson JC, Hart RP, Gordon SM, Hopkins RO, Girard TD, Ely EW. Post-traumatic stress disorder and post-traumatic stress symptoms following critical illness in medical intensive care unit patients: assessing the magnitude of the problem. Crit Care. 2007;11(1):R27. doi: 10.1186/cc5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–16. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson LV. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(1):50–6. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 11.Jackson JC, Pandharipande PP, Girard TD, Brummel NE, Thompson JL, Hughes CG, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. The Lancet Respiratory medicine. 2014;2(5):369–79. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–93. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 13.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 364(14):1293–304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 14.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Critical care medicine. 2014;42(4):849–59. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matte A, Barr A, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174(5):538–44. doi: 10.1164/rccm.200505-693OC. [DOI] [PubMed] [Google Scholar]
- 16.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Shanholtz C, Husain N, et al. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med. 2012;185(5):517–24. doi: 10.1164/rccm.201103-0503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan T, Brett SJ, Stokes T, Guideline Development G Rehabilitation after critical illness: summary of NICE guidance. BMJ. 2009;338:b822. doi: 10.1136/bmj.b822. [DOI] [PubMed] [Google Scholar]
- 18.Cuthbertson BH, Rattray J, Campbell MK, Gager M, Roughton S, Smith A, et al. The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ. 2009;339:b3723. doi: 10.1136/bmj.b3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones C, Skirrow P, Griffiths RD, Humphris GH, Ingleby S, Eddleston J, et al. Rehabilitation after critical illness: a randomized, controlled trial. Critical care medicine. 2003;31(10):2456–61. doi: 10.1097/01.CCM.0000089938.56725.33. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths JA, Barber VS, Cuthbertson BH, Young JD. A national survey of intensive care follow-up clinics. Anaesthesia. 2006;61(10):950–5. doi: 10.1111/j.1365-2044.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- 21.Khan BA, Lasiter S, Boustani MA. CE: critical care recovery center: an innovative collaborative care model for ICU survivors. The American journal of nursing. 2015;115(3):24–31. doi: 10.1097/01.NAJ.0000461807.42226.3e. quiz 4, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modrykamien AM. The ICU follow-up clinic: a new paradigm for intensivists. Respiratory care. 2012;57(5):764–72. doi: 10.4187/respcare.01461. [DOI] [PubMed] [Google Scholar]
- 23.Prinjha S, Field K, Rowan K. What patients think about ICU follow-up services: a qualitative study. Crit Care. 2009;13(2):R46. doi: 10.1186/cc7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huggins EL, Bloom SL, Stollings JL, Camp M, Sevin CM, Jackson JC. A Clinic Model: Post-Intensive Care Syndrome and Post-Intensive Care Syndrome-Family. AACN Adv Crit Care. 2016;27(2):204–11. doi: 10.4037/aacnacc2016611. [DOI] [PubMed] [Google Scholar]
- 25.Society of Critical Care Medicine THRIVE. 2017 http://www.sccm.org/Research/Quality/thrive/Pages/default.aspx.
- 26.Peel NM, Navanathan S, Hubbard RE. Gait speed as a predictor of outcomes in post-acute transitional care for older people. Geriatr Gerontol Int. 2014;14(4):906–10. doi: 10.1111/ggi.12191. [DOI] [PubMed] [Google Scholar]
- 27.Pavasini R, Guralnik J, Brown JC, di Bari M, Cesari M, Landi F, et al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016;14(1):215. doi: 10.1186/s12916-016-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legrand D, Vaes B, Mathei C, Adriaensen W, Van Pottelbergh G, Degryse JM. Muscle strength and physical performance as predictors of mortality, hospitalization, and disability in the oldest old. Journal of the American Geriatrics Society. 2014;62(6):1030–8. doi: 10.1111/jgs.12840. [DOI] [PubMed] [Google Scholar]
- 29.Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–93. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 30.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bierman AS. Functional status: the six vital sign. Journal of general internal medicine. 2001;16(11):785–6. doi: 10.1111/j.1525-1497.2001.10918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson J, Letts L, Chan D, Officer A, Wojkowski S, Oliver D, et al. Monitoring physical functioning as the sixth vital sign: evaluating patient and practice engagement in chronic illness care in a primary care setting–a quasi-experimental design. BMC family practice. 2012;13:29. doi: 10.1186/1471-2296-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali NA, O’Brien JM, Jr, Hoffmann SP, Phillips G, Garland A, Finley JC, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178(3):261–8. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 34.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age and ageing. 2011;40(4):423–9. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 35.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of gerontology. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 36.Morris PE, Berry MJ, Files DC, Thompson JC, Hauser J, Flores L, et al. Standardized Rehabilitation and Hospital Length of Stay Among Patients With Acute Respiratory Failure: A Randomized Clinical Trial. JAMA. 2016;315(24):2694–702. doi: 10.1001/jama.2016.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volpato S, Cavalieri M, Sioulis F, Guerra G, Maraldi C, Zuliani G, et al. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66(1):89–96. doi: 10.1093/gerona/glq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–4. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davison J, Files DC, Bakhru RN, Griffin K, Morris PE. American Thoracic Society. Colorado: 2015. The Design and Implementation of a MICU Survivors’ Clinic: A Fellow’s Journey Starting from Square One; p. A4499. [Google Scholar]
- 40.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 41.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical care medicine. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 42.Hartmann EL, Kitzman D, Rocco M, Leng X, Klepin H, Gordon M, et al. Physical function in older candidates for renal transplantation: an impaired population. Clin J Am Soc Nephrol. 2009;4(3):588–94. doi: 10.2215/CJN.03860808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitzman DW, Hundley WG, Brubaker PH, Morgan TM, Moore JB, Stewart KP, et al. A randomized double-blind trial of enalapril in older patients with heart failure and preserved ejection fraction: effects on exercise tolerance and arterial distensibility. Circ Heart Fail. 2010;3(4):477–85. doi: 10.1161/CIRCHEARTFAILURE.109.898916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foy CG, Wickley KL, Adair N, Lang W, Miller ME, Rejeski WJ, et al. The Reconditioning Exercise and Chronic Obstructive Pulmonary Disease Trial II (REACT II): rationale and study design for a clinical trial of physical activity among individuals with chronic obstructive pulmonary disease. Contemp Clin Trials. 2006;27(2):135–46. doi: 10.1016/j.cct.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, et al. Sarcopenia, obesity, and inflammation–results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005;82(2):428–34. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- 46.Stehle JR, Jr, Leng X, Kitzman DW, Nicklas BJ, Kritchevsky SB, High KP. Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67(11):1212–8. doi: 10.1093/gerona/gls178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reeves GR, Whellan DJ, Patel MJ, O’Connor CM, Duncan P, Eggebeen JD, et al. Comparison of Frequency of Frailty and Severely Impaired Physical Function in Patients >/=60 Years Hospitalized With Acute Decompensated Heart Failure Versus Chronic Stable Heart Failure With Reduced and Preserved Left Ventricular Ejection Fraction. The American journal of cardiology. 2016;117(12):1953–8. doi: 10.1016/j.amjcard.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, et al. Defining Feasibility and Pilot Studies in Preparation for Randomised Controlled Trials: Development of a Conceptual Framework. PloS one. 2016;11(3):e0150205. doi: 10.1371/journal.pone.0150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bernabeu-Mora R, Medina-Mirapeix F, Llamazares-Herran E, Garcia-Guillamon G, Gimenez-Gimenez LM, Sanchez-Nieto JM. The Short Physical Performance Battery is a discriminative tool for identifying patients with COPD at risk of disability. Int J Chron Obstruct Pulmon Dis. 2015;10:2619–26. doi: 10.2147/COPD.S94377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher S, Ottenbacher KJ, Goodwin JS, Graham JE, Ostir GV. Short Physical Performance Battery in hospitalized older adults. Aging clinical and experimental research. 2009;21(6):445–52. doi: 10.1007/bf03327444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prescott HC, Langa KM, Liu V, Escobar GJ, Iwashyna TJ. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;190(1):62–9. doi: 10.1164/rccm.201403-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Needham DM, Dinglas VD, Morris PE, Jackson JC, Hough CL, Mendez-Tellez PA, et al. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up. Am J Respir Crit Care Med. 2013;188(5):567–76. doi: 10.1164/rccm.201304-0651OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–93. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]


