Abstract
CONTEXT
The prevalence of pain and its management has been shown to be inversely associated with greater levels of cognitive impairment.
OBJECTIVES
To evaluate whether the documentation and management of pain varies by level of cognitive impairment among nursing home residents with cancer.
METHODS
Using a cross-sectional study, we identified all newly admitted US nursing home residents with a cancer diagnosis in 2011–2012 (n=367,462). Minimum Data Set (MDS) 3.0 admission assessment was used to evaluate pain/pain management in the past five days and cognitive impairment (assessed via the Brief Interview for Mental Status or the Cognitive Performance Scale for 91.6% and 8.4% respectively). Adjusted prevalence ratios (APR) with 95% confidence intervals (CI) were estimated from robust Poisson regression models.
RESULTS
For those with staff-assessed pain, pain prevalence was 55.5% with no/mild cognitive impairment and 50.5% in those severely impaired. Pain was common in those able to self-report (67.9% no/mild, 55.9% moderate, 41.8% severe cognitive impairment). Greater cognitive impairment was associated with reduced prevalence of any pain (APR severe versus no/mild cognitive impairment; self-assessed: 0.77; 95% CI: 0.76–0.78; staff-assessed: 0.96; 95% CI: 0.93–0.99). Pharmacologic pain management was less prevalent in those with severe cognitive impairment (59.4% vs. 74.9% in those with no/mild cognitive impairment).
CONCLUSION
In nursing home residents with cancer, pain was less frequently documented in those with severe cognitive impairment which may lead to less frequent use of treatments for pain. Techniques to improve documentation and treatment of pain in nursing home residents with cognitive impairment are needed.
Keywords: nursing homes, cancer, pain, cognitive impairment
INTRODUCTION
Cancer is the second leading cause of death in the US(1) and the incidence of cancer increases with age.(2,3) Most cancer deaths occur ≥ age 65 years,(4) about 10% of nursing home residents have a cancer diagnosis,(5) and one third of Medicare beneficiaries with cancer receive nursing home care in the last 90 days before death.(6) Nursing home residents with a cancer diagnosis frequently suffer from multiple health conditions which also contribute to pain. Discrete pain management guidelines exist due to common painful conditions associated with cancer and its treatment particularly in later stages and end-of-life care.(7, 8, 9) Given that nursing homes are increasingly important in the care of patients with cancer, understanding the specific issues related to the recognition and management of pain in this vulnerable population is important.
Dementia is common among nursing homes residents affecting about 50% upon admission.(10) The challenges of assessing and managing pain among cognitively impaired residents were noted over 20 years ago in this journal.(11) Patients with cognitive impairment are at high risk for under-treatment of pain(12) and as cognitive functioning decreases, so does the ability to self-report pain(13) due to both intellectual and communication difficulties. These limitations can interfere with diagnosis of other conditions that may contribute to pain.(14) Pain among nursing home residents with dementia is common.(15)
This study sought to evaluate the extent to which the documentation of pain and receipt of pain treatments varied by level of cognitive impairment among newly admitted nursing home residents with a cancer diagnosis. Based on previous research(16), we hypothesized that both the prevalence of pain and its management would be markedly lower in residents with greater levels of cognitive impairment relative to residents with minimal cognitive impairment. The availability of a new national dataset (MDS 3.0) allowed us to provide a more current evaluation of this issue using improved pain measures.
METHODS
This study was approved by the University of Massachusetts Medical School Institutional Review Board.
Data Source
We used the Minimum Data Set (MDS) version 3.0 (17, 18), a comprehensive data source with mandatory assessments for all nursing home residents in Medicaid/Medicare certified facilities. In the US, 96% of nursing homes hold this certification. Full MDS assessments are completed by a health care professional upon admission and annually, with a subset of items collected quarterly or sooner if there is a change in a resident’s clinical status. The MDS 3.0 includes information on resident sociodemographic characteristics, functional status, clinical status, active diagnoses, generalized treatments/procedures and programs. Unlike its predecessor (MDS 2.0), the MDS 3.0 (implemented October 2010) offered residents the opportunity to self-report symptoms such as pain.(19, 20) For certain items, if a resident could not self-report, alternative questions or an authorized proxy are used. The MDS 3.0 is a robust data source that has been widely used and validated.(21)
Sample Selection
We included all newly admitted nursing home residents ≥ 50 years old with an active cancer diagnosis on their admission MDS in 2011 or 2012. Cancer diagnoses reported on MDS assessments have been shown to be adequate for research purposes.(22) Patients who were comatose or who had missing data on covariates were excluded. We identified 367,462 residents meeting criteria of whom most self-reported pain (n=336,733). The remainder had staff-assessed pain (n=30,729). In rare cases where both resident and staff assessed pain, the staff-assessment was used.
Outcome variables
We evaluated two outcomes: 1) pain and 2) pain management. Pain is included in Section J of the MDS 3.0.(23) “Any pain” (yes/no) is recorded if the resident understands pain questions and reports pain occurring within the past five days. If residents are unsure how to answer because pain treatments have resolved their pain, a “No” response is recorded. Thus, an affirmative response to the “any pain” question indicates either pain persisting despite treatments or untreated pain. For those reporting any pain in the past five days, dimensions of pain are assessed including: pain frequency (rarely, occasionally, frequently, almost constantly); pain effect on function (in the past five days, has pain made it hard to sleep? limited day-to-day activities? yes/no); and intensity of pain through either a numeric pain rating scale (1–10 scale of pain intensity) or verbal descriptor scale (mild, moderate, severe, very severe/horrible). For our analysis, we combined pain numeric and pain verbal measures to create one pain severity variable (mild, moderate, severe, very severe). We used the following cutpoints to equate the numeric scale to the verbal descriptor scale: verbal descriptor scale mild≈ numeric rating scale 1 to 4, verbal descriptor scale moderate ≈ numeric rating scale 5 to 7, verbal descriptor scale severe ≈ numeric rating scale 8 to 9, and verbal descriptor scale very severe, horrible ≈ numeric rating scale 10.(24) For residents unable to answer pain questions, staff assess the residents’ pain, documenting pain frequency in the past five days (1–2 days, 3–4 days, daily) based on medical records and/or observation.(23)
The second outcome was receipt of pain treatment. MDS Section J documents receipt of any type of pain management/treatment in the five days prior to the assessment (or since admission if admitted within < 5 days). Pain management regimens include “pharmacological pain management agents prescribed to relieve or prevent pain or its recurrence” without regard to frequency or route of administration.(23) Medications that target treatment of the underlying condition (e.g., chemotherapy, steroids) were excluded. Three types of pain regimens were included: 1) any scheduled pain medication regimen, 2) any as-needed pain medication (pro re nata, PRN), and 3) any non-pharmacological intervention for pain. For pharmacologic pain management, we categorized residents as receiving “any pharmacologic treatment” and whether they received scheduled or PRN treatments. Scheduled regimens define specific time intervals for medication administration, whereas PRN orders include both medication administration as-needed as well as at a time interval (e.g., every four hours as needed for pain). Non-pharmacological interventions for pain include radiotherapy, acupuncture, massage, physical therapy, and biofeedback. Herbal remedies are explicitly excluded.
Cognitive Impairment Classification
We measured cognitive impairment via two MDS 3.0 measures: 1) the Brief Interview for Mental Status (BIMS) and for those unable to complete the BIMS; 2) the Cognitive Performance Scale (CPS). BIMS items include the repetition of words (1 item), temporal orientation (3 items), and recall (3 items) with a score from 0 to 15 correct responses. The BIMS is a validated measure (25,26, 27) with high sensitivity (81%) and specificity (75%) given a <13 score threshold for any cognitive impairment when compared to the Modified Mini-Mental State Examination (score range: 0–100 with a <78 threshold).(28) The CPS (score 0–6) is calculated based on the assessment of short-term memory, daily decision-making skills, ability to be understood by others, independence with eating, and comatose status. We categorized residents into three levels of cognitive impairment based on the Centers for Medicare and Medicaid Services Nursing Home Compendium definition: none to mild (BIMS: 13–15; CPS: 0–2), moderate (BIMS: 8–12; CPS: 3–4), and severe (BIMS: 0–7, CPS: 5–6).(29)
Covariates
Covariates included sociodemographic characteristics, rejects care (yes/no), Activities of Daily Living (ADL) limitations (30) (1–3: minimal, moderate, severe compromise), skilled nursing facility admission (yes/no), hospice use in past 14 days within the nursing home (yes/no), ability to be understood by others (yes/no), understands others (yes/no), painful comorbid conditions, and mental health conditions. The understand/understood variables were derived from four-level MDS items and categorized as always (yes) vs. usually, sometimes, rarely/never (no). Sociodemographic characteristics included: gender (men/women), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic/Latino, other), married (yes/no). Painful comorbid conditions are listed in Table 2. Mental health conditions included: dementia/Alzheimer’s disease, anxiety disorder, and depression.
Table 2.
Potentially painful health conditions of nursing home residents with cancer at admission by cognitive status (n=367,462)
| Level of Cognitive Impairment | ||||
|---|---|---|---|---|
| None/Mild (n=219,147) | Moderate (n=90,633) | Severe (n=57,682) | Overall (n=367,462) | |
| Painful Comorbid conditions | Percentage | |||
| Heart Failure | 17.0 | 18.5 | 16.3 | 17.2 |
| Coronary Artery Diseasea | 24.6 | 26.3 | 24.9 | 25.0 |
| Venous Thromboembolismb | 5.8 | 5.0 | 4.3 | 5.4 |
| Peripheral Vascular/Arterial Disease | 6.8 | 7.0 | 6.3 | 6.8 |
| Inflammatory Bowel Disease/Ulcerative Colitis | 1.5 | 1.2 | 1.0 | 1.3 |
| Skin problemsc | 49.0 | 40.6 | 39.7 | 45.2 |
| Arthritis | 24.3 | 22.2 | 21.0 | 23.3 |
| Osteoporosis | 10.1 | 9.9 | 10.5 | 10.1 |
| Fracture (hip and other) | 13.0 | 11.7 | 10.9 | 12.4 |
| Urinary Tract Infection (last 30 days) | 14.4 | 17.4 | 19.2 | 15.9 |
Includes angina, myocardial infraction, atherosclerotic heart disease etc.
Includes deep vein thrombosis, pulmonary embolism, pulmonary thrombosis embolism
Skin problems include surgical wounds, wound infection, 2nd and 3rd degree burns, open lesions, pressure ulcers, foot problems (infection of the foot, diabetic foot ulcer, other open lesion on the foot)
Analysis
We described sociodemographic and clinical conditions by level of cognitive impairment. Because with large sample sizes even trivial differences are statistically significant, we considered absolute differences in percentages of ≥ 5% noteworthy. We stratified the sample by whether or not the resident responded to pain questions or if they were staff-assessed. We estimated the percent of documented pain (and its management) by level of cognitive impairment for self-reported pain and staff-reported pain separately. To estimate the extent to which differences in pain varied over levels of cognitive impairment, we used robust Poisson models taking into consideration the clustering of residents within facilities.(31) From these models, we estimated crude and adjusted prevalence ratios (APR) and 95% confidence intervals (CI), with intact/mild cognitive impairment used as the reference group.
RESULTS
Sociodemographic and clinical conditions
Half were women and 37.5% were married (Table 1). Those ≥85 years of age represented 24% of those with no/mild cognitive impairments and 43% of those with severe cognitive impairment. Overall, 69.2% of residents were admitted to nursing homes from skilled nursing facilities but fewer (61%) with severe cognitive impairment. About one in five residents with no or mild cognitive impairment had severe ADL compromise compared to 42.1% of those with severe cognitive impairment. Nearly all residents with mild or moderate cognitive impairment could understand others and make themselves understood compared to 40.5% (understand) and 47.2% (understood) of those with severe cognitive impairment. Most conditions were similar across levels of cognitive impairment with few exceptions.
Table 1.
Characteristics of nursing home residents with cancer at admission by cognitive status (n=367,462)
| Level of Cognitive Impairment | ||||
|---|---|---|---|---|
| None/Mild (n=219,147) | Moderate (n=90,633) | Severe (n=57,682) | Overall (n=367,462) | |
| Age, years | Percentage | |||
| 50–64 | 14.5 | 9.2 | 7.4 | 12.1 |
| 65–74 | 25.4 | 18.1 | 14.6 | 21.9 |
| 75–84 | 36.0 | 36.5 | 35.0 | 36.0 |
| 85+ | 24.0 | 36.3 | 43.0 | 30.0 |
| Women | 55.3 | 47.6 | 48.7 | 52.3 |
| Race/Ethnicity | ||||
| Non-Hispanic White | 85.6 | 82.1 | 79.1 | 83.7 |
| Non-Hispanic Black | 9.6 | 11.8 | 13.6 | 10.8 |
| Hispanic or Latino | 3.0 | 3.8 | 4.6 | 3.5 |
| Other | 1.7 | 2.3 | 2.7 | 2.0 |
| Married | 37.0 | 37.6 | 39.3 | 37.5 |
| Skilled nursing facility admission | 71.8 | 68.0 | 61.0 | 69.2 |
| Rejects care | 4.2 | 9.5 | 14.4 | 7.1 |
| Limitations in ADLsa | ||||
| Minimal compromise | 20.4 | 13.1 | 8.2 | 16.7 |
| Moderate compromise | 57.8 | 56.2 | 49.7 | 56.1 |
| Severe compromise | 21.8 | 30.7 | 42.1 | 27.2 |
| Hospice | 4.5 | 9.2 | 14.0 | 7.1 |
| Makes self understoodb | 94.9 | 73.3 | 47.2 | 82.1 |
| Understands others | 92.9 | 68.0 | 40.5 | 78.6 |
| Mental Health conditions | ||||
| Dementia/Alzheimer’s | 6.2 | 25.8 | 49.7 | 17.8 |
| Anxiety disorder | 18.3 | 18.9 | 20.0 | 18.8 |
| Depression | 27.5 | 30.3 | 29.5 | 28.5 |
Activities of daily living
NOTE: This is also an item within the Cognitive Performance Scale (CPS) used to measure cognitive impairment
Pain among residents able to self-report
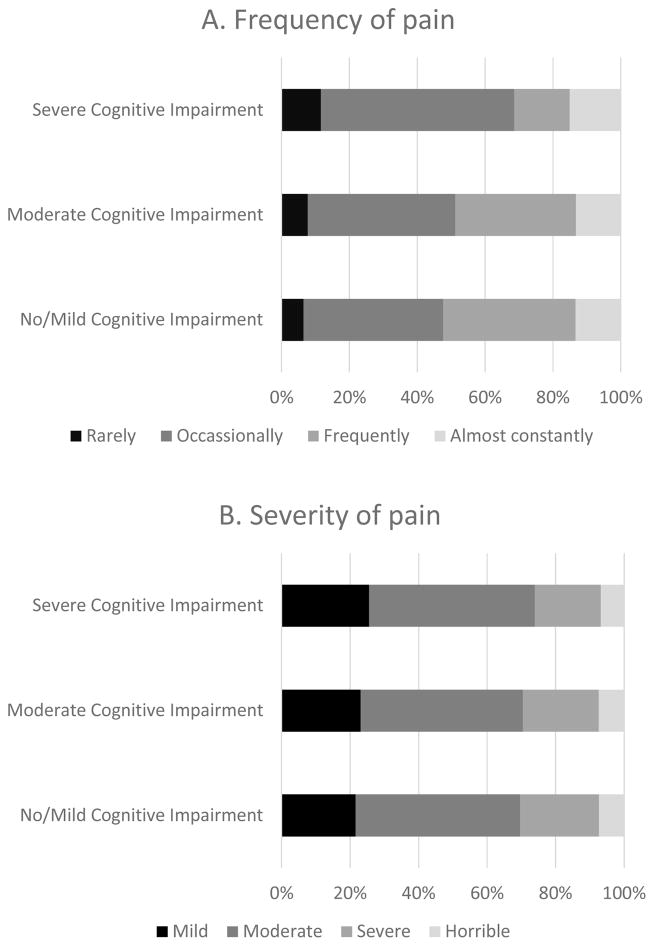
Among those able to self-report (Table 3), 67.9% of those with no/mild cognitive impairment , 55.9% of those with moderate cognitive impairment, and 41.8% of those with severe cognitive impairment had any pain documented. After adjusting for sociodemographic, clinical, and painful conditions, greater cognitive impairment was associated with reduced prevalence of any documented pain (APR moderate versus no/mild cognitive impairment: 0.92; 95% CI: 0.92–0.93; APR severe versus no/mild cognitive impairment: 0.77; 95% CI: 0.76–0.78). The extent to which pain was moderate (48.0%, 47.4%, 48.3%, for no/mild, moderate, severe cognitive impairment, respectively) or severe, very severe or horrible (30.4%, 29.6%, 28.1%, for no/mild, moderate, severe cognitive impairment, respectively) did not vary extensively by level of cognitive impairment (Figure 1). Frequency of pain did not vary by level of cognitive impairment (13.4%, 13.2%, 12.1%, for no/mild, moderate, severe cognitive impairment, respectively). Variations by cognitive impairment were not observed for pain causing sleep difficulty (29.4%, 29.0%, 26.4%, for no/mild, moderate, severe cognitive impairment, respectively) or limiting day to day activities (40.8%, 40.8%, 37.6%, for no/mild, moderate, severe cognitive impairment, respectively).
Table 3.
Association between level of cognitive impairment and any pain among nursing home residents with cancer, prevalence ratios (95% confidence intervals (CI))
| Level of Cognitive Impairment | |||
|---|---|---|---|
| None/Mild | Moderate | Severe | |
| Pain self-assessment and BIMS (n=336,733) | n=210,732 | n=81,911 | n=44,090 |
| % any pain | 67.9 | 55.9 | 41.8 |
| Crude prevalence ratios (95% CI) | 1.0 | 0.83 (0.82 – 0.83) | 0.62 (0.62 – 0.63) |
| Partially adjusted prevalence ratios (95% CI)a | 1.0 | 0.88 (0.87 – 0.89) | 0.71 (0.70 – 0.71) |
| Fully adjusted prevalence ratios (95% CI)b | 1.0 | 0.92 (0.92 –0.93) | 0.77 (0.76 – 0.78) |
| Staff pain assessment and CPS (n=30,729) | n=8,415 | n=8,722 | n=13,592 |
| % any pain | 55.5 | 55.0 | 50.5 |
| Crude prevalence ratios (95% CI) | 1.0 | 1.00 (0.98 – 1.03) | 0.94 (0.91 – 0.96) |
| Partially adjusted prevalence ratios (95% CI)a | 1.0 | 0.98 (0.95 – 1.01) | 0.91 (0.88 – 0.94) |
| Fully adjusted prevalence ratios (95% CI)b | 1.0 | 1.02 (0.99 – 1.05) | 0.96 (0.93 – 0.99) |
Figure 1.
Pain frequency and pain severity by severity of cognitive impairment in residents with self-assessed pain (n=207,364)
Pain among residents unable to self-report (staff-assessed)
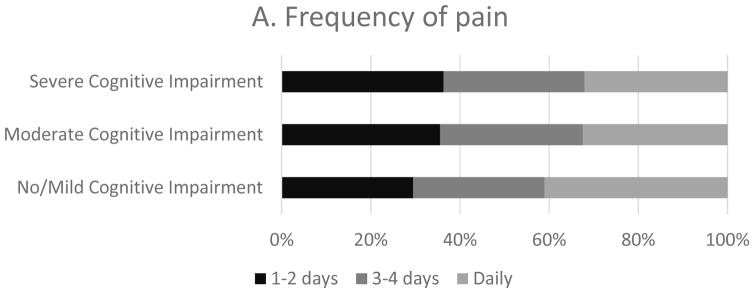
Among residents with staff-assessed pain, more (41.1%) of those with no/mild cognitive impairment had daily pain than those with moderate and severe cognitive impairment (32.4% and 32.1% respectively) (Figure 2). The prevalence of pain (Table 3) was similar between residents with moderate cognitive impairment and those with no/mild cognitive impairment, but lower in those with severe cognitive impairment (APR: 0.96; 95% CI: 0.93–0.99). Pain was noted on 3–4 days for approximately a third of residents regardless of cognitive impairment (no/mild: 29.5%, moderate: 32.0%, and severe: 31.6%) (Figure 2).
Figure 2.
Pain frequency by severity of cognitive impairment in residents with staff assessed pain (n=16,311)
Pain management
Among residents who self-assessed their pain (Table 4), 75.1% of those with no/mild cognitive impairment received pharmacologic pain management relative to 66.7% of those with moderate and 57.9% of those with severe cognitive impairment. This general trend persisted with all pain management subtypes with the exception of scheduled pain regimen only and non-pharmacologic pain management. Among those with staff-assessed pain, frequency of any pharmacologic pain management was similar across levels of cognitive impairment although it was provided least frequently to those with severe cognitive impairment. Overall those with severe cognitive impairment had less documented pain (43.8% vs 67.4% with no/mild cognitive impairment) and received less pharmacologic treatment for pain (59.4% “any pharmacologic treatment” vs 74.9% with no/mild cognitive impairment) (Table 5).
Table 4.
Pain and pain management among nursing home residents with cancer at admission by level of cognitive impairment for all residents and by self- and staff-assessment
| Level of Cognitive Impairment | |||
|---|---|---|---|
| No/Mild | Moderate | Severe | |
| Pain self-assessment and BIMS (n=336,733) | n=210,732 | n=81,911 | n=44,090 |
| Any pain | 67.9 | 55.9 | 41.8 |
| Any pharmacologic pain management | 75.1 | 66.7 | 57.9 |
| Scheduled pain regimen only | 7.1 | 8.7 | 10.1 |
| PRN medication only | 40.1 | 35.1 | 29.5 |
| PRN + Scheduled pain regimen | 27.9 | 22.9 | 18.3 |
| Non-pharmacologic pain management | 35.8 | 30.7 | 26.4 |
| Staff pain assessment and CPS (n=30,729) | n=8,415 | n=8,722 | n=13,592 |
| Any pain | 55.5 | 55.0 | 50.5 |
| Any pharmacologic pain management | 69.4 | 67.2 | 64.3 |
| Scheduled pain regimen only | 7.3 | 9.7 | 11.8 |
| PRN medication only | 34.3 | 32.2 | 29.6 |
| PRN + Scheduled pain regimen | 27.8 | 25.3 | 23.0 |
| Non-pharmacologic pain management | 29.5 | 29.7 | 28.8 |
Table 5.
Summary Table - Pain and pain management among nursing home residents with cancer at admission by level of cognitive impairment for all residents and by self- and staff-assessment
| Level of Cognitive Impairment | ||||
|---|---|---|---|---|
| No/Mild | Moderate | Severe | Overall | |
| % Any Pain | ||||
| Self-Assessed (n= 336,733) | 67.9 | 55.9 | 41.8 | 61.6 |
| Staff-Assessed (n=30,729) | 55.5 | 55.0 | 50.5 | 53.2 |
| Combined (n=367,462) | 67.4 | 55.9 | 43.8 | 60.9 |
| % Any Pharmacologic Treatment)a | ||||
| Self-Assessed (n= 336,733) | 75.1 | 66.7 | 57.9 | 70.8 |
| Staff-Assessed (n=30,729) | 69.4 | 67.2 | 64.3 | 66.5 |
| Combined (n=367,462) | 74.9 | 66.8 | 59.4 | 70.5 |
Includes residents whose pain is controlled
Pain Self-Assessment vs Staff Assessment
For those with no/mild cognitive impairment, self-assessed pain (any pain) was documented for 67.9% but only 55.5% when staff-assessment was employed. Among severely cognitively impaired residents, 41.8% had pain documented using self-assessment while more (50.5%) had pain documented by staff (Table 5). In the self-assessment group, over 75% of residents with no/mild cognitive impairment received “any pharmacologic treatment” for pain compared to 57.9% of severely cognitively impaired. Staff pain assessment was associated with 69.4% of “any pharmacologic treatment” among the least cognitively impaired, and 64.3% of the most severe cognitively impaired residents.
DISCUSSION
In a nationwide study of newly admitted nursing home residents with cancer, we evaluated the extent to which pain reports and receipt of pain management treatment varied by level of cognitive impairment. We found that both the prevalence of documented pain and pain treatment were lower in residents who had greater levels of cognitive impairment, and that this difference was most profound among severely cognitively impaired residents who self-reported their pain. Fewer residents with severe cognitive impairment had any pain documented. When pain was documented, it was similar with respect to severity, frequency, and impact on sleep and activities of daily living regardless of cognitive impairment. This study is innovative given its use of new MDS 3.0 data which incorporated a pain assessment tool that calls for self-report of painful symptoms when able to communicate. We also included in our analysis residents unable to communicate for whom an alternative procedure was used by staff to assess pain.
We hypothesized that both the prevalence of documented pain and its management would be lower among residents with more severe levels of cognitive impairment. Although we detected a clear pattern of greater cognitive impairment associated with lower documentation of pain both overall and among residents self-reporting, we found a different pattern among residents whose pain was staff-assessed. With staff-assessment, mild and moderate cognitive impairment had similar pain prevalence while severe cognitive impairment had less documented pain. The least documented pain was among severely cognitively impaired who self-assessed. Possible explanations include: 1) staff may have misclassified seriously cognitively impaired individuals as sufficiently able to communicate when they were not fully able to do so; and/or 2) the 5-day look-back period employed makes it particularly difficult for residents with severe memory problems to accurately assess pain that is hours or days in the past. Procedures for assessing and documenting pain among severely cognitively impaired residents need to be reevaluated and the role of staff-assessment reconsidered for severely impaired individuals.
Our study, as others (5, 16, 32), showed overall lower use of pharmacologic and nonpharmacologic pain management among those with severe cognitive impairment. This pattern held for residents who self-assessed their pain, but when pain is staff-assessed the proportion receiving treatment across levels of cognitive impairment was more similar. For mild cognitive impairment, pain self-assessment was associated with more pharmacologic treatment while those with staff assessment received less. Severely cognitively impaired residents were the opposite – receiving more treatment when staff assessed than when self-assessed. This seems to indicate that pain self-assessment may be the best option for those who are cognitively intact and less effective with severe cognitive impairment.
We found a surprising number of severely cognitively impaired residents were coded as “always understood” or “always understanding others” (47.2% and 40.5% respectively). Yet despite communication difficulties in over half of severely cognitively impaired residents, all but 8% were administered self-assessments for pain. In other studies over a quarter of moderately to severely impaired residents (MMSE <15) were unable to use words to describe their pain and 14% were unable to locate pain on their own body.(33) Even when patients with cognitive impairment can describe pain, memory may be limited to only immediate recall.(34) The MDS uses a 5-day look-back for pain assessment and impaired short-term memory may make accurate self-assessment impossible. We believe that self-assessment among the severely cognitively impaired may be used too liberally and that the severely impaired may be better served with a staff assessment of their pain experience. Indeed, The European Society for Medical Oncology cancer pain assessment guidelines recommend that “observation of pain-related behaviors and discomfort is indicated in patients with cognitive impairment to assess the presence of pain” (7); the NCCN advises that although “pain intensity must be quantified by the patient whenever possible” self-report depends on the patient’s ability to communicate (8); and the American Society of Anesthesiologists concedes that although self-reported pain is preferred, for some “external observation may be preferable.” (9) Best practices for assessing pain in older adults with dementia have been developed.(35) Although no standardized tool based on nonverbal behavioral pain indicators is accepted as the “gold standard” (36), the American Medical Directors Association recommends the PAINAD scale (Pain Assessment in Advanced Dementia)(37) that rates breathing, negative vocalization, facial expression, body language, and consolability yielding a pain scale from 0 (no pain) to 10 (severe pain).
Pain management in nursing home residents is challenging in the best of circumstances. Effective treatment will reduce the experience of pain, result in improved pain, and reduce deleterious side effects. A trial and reassessment approach to pain medication management, gauging both adverse effects and pain relief is advised (14, 35). Yet, both adverse effects and pain relief may be difficult to measure among the cognitively impaired who may have difficulty evaluating treatment side effects as they also struggle to express their own pain. Intellectual limitations in those with cognitive impairment may render ineffective many non-pharmacologic pain management approaches, like physical therapy, cognitive behavioral therapy, mindfulness, and yoga.(14,38)
The ideal number of people reporting pain who should be treated with pain relieving drugs remains unclear. Some may tolerate pain and may be able to cope with minor/brief episodic pain. The World Health Organization cancer pain ladder recommends that “If pain occurs, there should be prompt oral administration of drugs… until the patient is free from pain”(8, 39) We found >75% of residents with cancer who had the best cognitive functioning and sufficient communication skills to express their pain received the most pain treatment. For those with severe cognitive impairment deemed able to communicate and self-assess their pain, pharmacologic pain treatment was provided to the fewest (57.9%). It would be reasonable to argue that, at minimum, all residents with cancer, regardless of impairment, should have pain detected and treated with analgesics at similar levels. Improved strategies for more effective pain assessment and pain treatment for impaired residents are needed.
This study has several strengths and limitations. It employed a large, standardized national dataset used for comprehensive assessment of residents in nearly all US nursing homes. MDS 3.0 pain measures are an improvement over MDS 2.0 where only two overall pain assessment items were included.(21) MDS 3.0 uses direct resident questioning about subjective states including pain(20) and assesses the frequency and intensity of pain, the effects of pain on functioning, and pain treatment in the past 5 days.(40) Although the MDS 3.0 pain assessment represents an improvement, it is imperfect. With chronic conditions, pain may flare up or recede based on a wide variety of factors including changes in cognitive status.(40) The MDS is administered quarterly or when there is a change of status and is restricted to a 5-day look-back period. Additional limitations include: 1) no information on specific cancer type and staging; 2) lack of information on specific pain medications; 3) no detail on non-pharmacologic pain management strategies that may have been employed. We also know that among various forms of dementia, pain may differ due to a variety of neurobiological factors that may either heighten pain perception or dampen the perception of pain.(41) Evaluating differences by underlying form of dementia was beyond the scope of the current study.
CONCLUSION
Although there has been progress in the recognition and treatment of pain (42), there remains room for continued improvement especially for those with more severe cognitive impairment. Pain self-assessment among the severely cognitively impaired may be used too liberally in a population that, due to cognitive limitations and impaired short-term memory, may be better served with staff assessment. Once pain is recognized by staff, it appears to be treated more frequently when residents are cognitively intact and less frequently when residents are severely impaired. The difference is most profound when residents self-assess their pain. Procedures for assessing and documenting pain among severely cognitively impaired residents need to be reevaluated and the role of staff pain assessment needs to be reconsidered particularly for severely impaired individuals. Thus we conclude that the MDS 3.0 has not entirely solved the challenge of recognizing and treating pain particularly with cognitively impaired residents. Nursing home residents represent some of the most vulnerable members of society – none more exquisitely vulnerable than those suffering from both cancer and cognitive impairment. There is an ethical imperative to provide effective, compassionate care to these patients and to control pain as effectively as possible.(43) Even in light of enhancements to the MDS 3.0, continued improvement in our ability to effectively detect and treat pain among nursing home residents with cancer and dementia is needed.
Acknowledgments
This work was supported by the National Institutes of Health grants 1R21CA198172 and 1TL1TR001454 awarded to Dr. Kate Lapane and supporting the effort of Ms. Mack; and 1F31AG056078 awarded to Mr. Hunnicutt. The funding source had no involvement in study design, collection, analysis or interpretation of data, writing this report, or the decision to submit this article for publication.
Footnotes
DISCLOSURES
The authors have no competing interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2014 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2017. [Accessed December 3, 2017]. Available from: www.cdc.gov/uscs. [Google Scholar]
- 2.Yancik R. Population aging and cancer: a cross-national concern. Cancer J. 2005;11(6):437–41. doi: 10.1097/00130404-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Probability of developing invasive cancer during selected age intervals by sex, US, 2011–2013. [Accessed August 16, 2017];American Cancer Society Cancer Facts & Figures. 2017 Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.htmlf.
- 4.Berger NA, Savvides P, Koroukian SM, et al. Cancer in the Elderly. Trans Am Clin Climatol Assoc. 2006;117:147–156. [PMC free article] [PubMed] [Google Scholar]
- 5.Pimentel CB, Briesacher BA, Gurwitz JH, et al. Pain management in nursing home residents with cancer. J American Geriatr Soc. 2015;63(4):633–641. doi: 10.1111/jgs.13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teno JM, Gozalo PL, Bynum JPW, et al. Change in end-of-life care for Medicare beneficiaries: Site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477. doi: 10.1001/jama.2012.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ripamonti CI, Bandieri E, Roila F. Management of cancer pain: ESMO guidelines. Annals of Oncology. 2011;22(suppl 6):vi69–vi77. doi: 10.1093/annonc/mdr390. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) [Accessed January 22, 2018];Adult Cancer Pain v. 2. 2016 Available from: https://oralcancerfoundation.org/wp-content/uploads/2016/09/pain.pdf.
- 9.American Society of Anesthesiologists Task Force on Pain Management, Cancer Pain Section. Practice guidelines for cancer pain management. A report by the American Society of Anesthesiologists Task Force on Pain Management, Cancer Pain Section. Anesthesiology. 1996;84:1243–1257. [PubMed] [Google Scholar]
- 10.Magaziner J, German P, Zimmerman SI, et al. The prevalence of dementia in a statewide sample of new nursing home admissions aged 65 and older: diagnosis by expert panel. Epidemiology of Dementia in Nursing Homes Research Group. Gerontologist. 2000;40(6):663–72. doi: 10.1093/geront/40.6.663. [DOI] [PubMed] [Google Scholar]
- 11.Ferrell BA, Ferrell BR, Rivera L. Pain in cognitively impaired nursing home patients. J Pain Symptom Manage. 1995;10(8):591–8. doi: 10.1016/0885-3924(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 12.Scherder EJA, Sergeant JA, Swaab DF. Pain processing in dementia and its relation to neuropathology. Lancet Neurol. 2003;2:677–86. doi: 10.1016/s1474-4422(03)00556-8. [DOI] [PubMed] [Google Scholar]
- 13.Kunz M, Mylius V, Scharmann S, Schepelman K, Lautenbacher S. Influence of dementia on multiple components of pain. Eur J Pain. 2009;13:317–325. doi: 10.1016/j.ejpain.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Buffum MD, Hutt E, Chang VT, Craine MH, Snow AL. Cognitive impairment in pain management: review of issues and challenges. J Rehabil Res Devel. 2007;44(2):315–330. doi: 10.1682/jrrd.2006.06.0064. [DOI] [PubMed] [Google Scholar]
- 15.Zwakhalen SMG, Koopmans RTCM, Geels PJEM, Berger MPF, Hamers JPH. The prevalence of pain in nursing home residents with dementia measured using an observational pain scale. Eur J Pain. 2009;13:89–93. doi: 10.1016/j.ejpain.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Bernabei R, Gambassi G, Lapane KL, et al. Management of pain in elderly patients with cancer. JAMA. 1998;279(23):1877–82. doi: 10.1001/jama.279.23.1877. [DOI] [PubMed] [Google Scholar]
- 17.[dataset] Centers for Medicare and Medicaid Services. [Accessed August 15, 2017];Long Term Care Minimum Data Set (MDS) 3.0. 2011 Available from: https://www.resdac.org/cms-data/request/cms-data-request-center.
- 18.Centers for Medicare and Medicaid Services. [Accessed August 16, 2017];Long-Term Care Facility Resident Assessment Instrument 3.0 User’s Manual, Version 1.14. 2016 Oct; Available from: https://downloads.cms.gov/files/MDS-30-RAI-Manual-V114-October-2016.pdf.
- 19.Saliba D, Jones M, Streim J, et al. Overview of significant changes in the Minimum Data Set for nursing homes version 3. 0. J Am Med Dir Assoc. 2012;13:595–601. doi: 10.1016/j.jamda.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Thomas KS, Wysocki A, Intrator O, Mor V. Finding Gertrude: The Resident’s Voice in MDS 3. 0. J Am Med Dir Assoc. 2014;15(11):802–806. doi: 10.1016/j.jamda.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saliba D, Buchanan J. Making the investment count: revision of the Minimum Data Set for nursing homes, MDS 3. 0. J Am Med Dir Assoc. 2012;13(7):602–610. doi: 10.1016/j.jamda.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Gambassi G, Landi F, Peng L, et al. Validity of diagnostic and drug data in standardized nursing home resident assessments: potential for geriatric pharmacoepidemiology. SAGE Study Group. Systematic Assessment of Geriatric drug use via Epidemiology. Med Care. 1998;36(2):167–79. doi: 10.1097/00005650-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Medicare and Medicare Services. RAI Version 3.0 Manual. [Accessed August 16, 2017];Section J: Health Conditions, J0100: Pain Management. 2010 May; Available from: https://www.ahcancal.org/facility_operations/Documents/RAI_3.0/MDS%203.0%20Chapter%203%20-%20Section%20J%20V1.02%20June%2010,%202010.pdf.
- 24.Edelen MO, Saliba D. Correspondence of Verbal Descriptor and Numeric Rating Scales for Pain Intensity: An Item Response Theory Calibration. The Journals of Gerontology Series a, Biological Sciences and Medical Sciences. 2010;65A(7):778–785. doi: 10.1093/gerona/glp215. [DOI] [PubMed] [Google Scholar]
- 25.Saliba D, Buchanan J. [Accessed August 16, 2017];Development & Validation of a Revised Nursing Home Assessment Tool: MDS 3.0. 2008 Apr; Available from: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/downloads/MDS30FinalReport.pdf.
- 26.Saliba D, Buchanan J, Edelen MO, et al. MDS 3. 0: Brief Interview for Mental Status. J Am Med Dir Assoc. 2012;13(7):611–617. doi: 10.1016/j.jamda.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3. 0 Cognitive Function Scale. Med Care. 2017;55(9):e72. doi: 10.1097/MLR.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chodosh J, Edelen MO, Buchanan JL, Yosef JA, Ouslander JG, Berlowitz DR, Streim JE, Saliba D. Nursing home assessment of cognitive impairment: development and testing of a brief instrument of mental status. J Am Geriatr Soc. 2008;56(11):2069–2075. doi: 10.1111/j.1532-5415.2008.01944.x. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Medicare & Meicaid Services. [Accessed December 6, 2017];Nursing Home data Compendium 2015 Edition. Available from: https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/Downloads/nursinghomedatacompendium_508-2015.pdf.
- 30.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54:M546–53. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 31.Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22(6):661–70. doi: 10.1177/0962280211427759. [DOI] [PubMed] [Google Scholar]
- 32.Drageset J, Corbett A, Selbaek G, et al. Cancer-related pain and symptoms among nursing home residents: a systematic review. J Pain Symptom Manage. 2014;48:699–710. doi: 10.1016/j.jpainsymman.2013.12.238. [DOI] [PubMed] [Google Scholar]
- 33.Wynne CF, Ling SM, Remsburg R. Comparison of pain assessment instruments in cognitively intact and cognitively impaired nursing home residents. Geriatr Nurs. 2000;21(1):20–3. doi: 10.1067/mgn.2000.105793. [DOI] [PubMed] [Google Scholar]
- 34.Zwakhalen SMG, Hamers JPH, Abu-Saad HH, Berger MPF. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools. BMC Geriatrics. 2006;6:3. doi: 10.1186/1471-2318-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horgas AL. Assessing pain in older adults with dementia. [Accessed November 14, 2017];Try This: Best Practices in Nursing Care to Older Adults with Dementia (Dementia Series) 2012 :D2. Available from: https://consultgeri.org/try-this/dementia/issue-d2.pdf.
- 36.Warden V, Hurley AC, Volicer L. Development and psychometric evaluation of the pain assessment in advanced dementia (PAINAD) Scale. J Am Med Dir Assoc. 2003;4(1):9–15. doi: 10.1097/01.JAM.0000043422.31640.F7. [DOI] [PubMed] [Google Scholar]
- 37.Herr K, Bjoro K, Decker S. Tools for assessment of pain in nonverbal older adults with dementia: a state-of-the-science review. J Pain Symptom Manage. 2006;31(2):170–92. doi: 10.1016/j.jpainsymman.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Becker WC, Dorflinger L, Edmond SN, et al. Barriers and facilitators to use of non-pharmacological treatments in chronic pain. BMC Fam Pract. 2017;18:41. doi: 10.1186/s12875-017-0608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. [Accessed November 30, 2017];WHO's Cancer Pain Ladder for Adults. Available from: http://www.who.int/cancer/palliative/painladder/en/
- 40.Thielke SM, Sale J, Reid C. Identifying, tracking and managing pain in LTC. Ann Longterm Care. 2010;18(9):42–47. [Google Scholar]
- 41.Scherder E, Herr K, Pickering G, et al. Pain in Dementia. Pain. 2009;145:276–278. doi: 10.1016/j.pain.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Hunnicutt JN, Ulbricht CM, Tjia J, Lapane KL. Pain and pharmacologic pain management in long-stay nursing home residents. Pain. 2017;158(6):1091–1099. doi: 10.1097/j.pain.0000000000000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson VM, Teno JM, Bourbonniere M, Mor V. Palliative care needs of cancer patients in US nursing homes. J Palliat Med. 2005;8(2):273–279. doi: 10.1089/jpm.2005.8.273. [DOI] [PubMed] [Google Scholar]