Abstract
Background
Respiratory viral infection in early childhood, including that from respiratory syncytial virus (RSV), has been previously associated with the development of asthma.
Objective
We aimed to determine whether ex vivo RSV infection of bronchial epithelial cells (BECs) from children with asthma would induce specific gene expression patterns and whether such patterns were associated with lung function among BEC donors.
Methods
Primary BECs from carefully characterized children with asthma (n = 18) and matched healthy children without asthma (n = 8) were differentiated at an air-liquid interface for 21 days. Air-liquid interface cultures were infected with RSV for 96 hours and RNA was subsequently isolated from BECs. In each case, we analyzed gene expression using RNA sequencing and assessed differences between conditions by linear modeling of the data. BEC donors completed spirometry to measure lung function.
Results
RSV infection of BECs from subjects with asthma, compared with uninfected BECs from subjects with asthma, led to a significant increase in expression of 6199 genes. There was significantly greater expression of 195 genes in BECs from children with asthma and airway obstruction (FEV1/forced vital capacity < 0.85 and FEV1 < 100% predicted) than in BECs from children with asthma without obstruction, or in BECs from healthy children. These specific genes were found to be highly enriched for viral response genes induced in parallel with types I and III interferons.
Conclusions
BECs from children with asthma and with obstructive physiology exhibit greater expression of types I and III interferons and interferon-stimulated genes than do cells from children with normal lung function, and expression of interferon-associated genes correlates with the degree of airway obstruction. These findings suggest that an exaggerated interferon response to viral infection by airway epithelial cells may be a mechanism leading to lung function decline in a subset of children with asthma. (J Allergy Clin Immunol 2017;)
Keywords: Asthma, epithelial cells, sequence analysis, RNA, respiratory syncytial virus, type I interferon
Viral respiratory infections trigger the majority of asthma exacerbations, leading not only to significant morbidity and mortality, but also to substantial direct and indirect societal costs.1,2 Although human rhinovirus (HRV) is the trigger of most acute asthma exacerbations, early respiratory syncytial virus (RSV) infection, especially when severe, has been associated with an increased prevalence of asthma and allergy in childhood and young adulthood.3 Although a causal link between RSV and asthma is controversial, early life severe RSV infection has been associated with significant postinfection respiratory morbidity, asthma, and with lower lung function in children and adults.3–6 Epidemiologic studies have revealed that children with asthma, compared with children without asthma, have lower lung function during childhood that persists into adulthood, and bronchial biopsies from children with asthma demonstrate that features of airway remodeling are already present in early childhood.7–13 These data suggest that structural changes leading to lung function deficits in the airways of patients with asthma occur early in the course of asthma. The role that epithelial innate immune responses to early childhood viral infections such as RSV play in subsequent lung function deficits and/or airway remodeling is unclear.
Because of the strong associations between viral infections and the development and exacerbation of asthma, prior research has considered whether altered antiviral responses in patients with asthma might explain these associations. Models of disease have demonstrated that infection of bronchial epithelial cells (BECs) by RSV leads to both innate and adaptive immune responses via the generation of inflammatory cytokines.14 As part of this response to viral infection, interferons and interferon-stimulated genes (ISGs) are upregulated, resulting in a wide range of effects including interference with viral replication, apoptosis of infected cells, and induction of antiviral measures in neighboring cells.15
On exposure to RSV, the initial viral replication process occurs in the nasopharyngeal epithelium, later invading the bronchial epithelium either by direct cellular propagation or by mucus secretions.16 After entering BECs, the initial epithelial immune response is triggered by the recognition of viral RNA by pattern recognition receptors such as Toll-like receptors, nucleotidebinding oligomerization domain-like receptors, the viral sensor MDA5 (encoded by IFIH1), and the cytosolic receptor retinoic acid-inducible gene 1 (RIG-I).17,18 Subsequently, RIG-I activates the transcription factors nuclear factor κB and interferon regulatory factor 3, leading to expression of the antiviral response genes (see Fig E1 in this article’s Online Repository at www.jacionline.org).19 Among those, the production of types I and III interferons are thought to be an important part of the innate immune response.20
Given the importance of the innate antiviral response, several studies have evaluated the in vitro interferon response of BECs from patients with asthma. Wark et al21 first demonstrated that BECs from patients with asthma that are infected with HRV had increased viral RNA synthesis, significantly reduced IFN-β release, and inhibited apoptosis of infected cells. A type I interferon deficiency in response to HRV was also observed in BECs from children with severe asthma and atopic children without asthma, as well as in other tissues from patients with asthma.22–24 However, other studies25,26 of BECs from subjects with asthma have failed to replicate the differences in interferon synthesis after rhinovirus infection of BECs that was first reported by Wark et al. At present, only 1 study has investigated the interferon response by BECs from children with and without asthma during RSV infection and failed to show any difference in viral replication, types I or III interferon synthesis, or ISG expression between RSV-infected BECs from patients with asthma and healthy control subjects.25
The aim of this study was to assess the ex vivo response to RSV by differentiated primary BECs from children with asthma using unbiased whole transcriptome RNA sequencing and to determine gene expression patterns that associate with lung function among BEC donors. Given the reported associations between early RSV infection and later development of asthma and lung function deficits, together with past reports of altered innate immune responses to respiratory viruses by BECs with patients with asthma, we hypothesized that RSV infection of BECs from children with asthma would elicit unique expression patterns of innate immunity genes that would relate to lung function measures among BEC donors.
METHODS
Subjects
Children ages 6 to 18 years who were undergoing an elective surgical procedure requiring endotracheal intubation and general anesthesia were recruited for this study. A detailed medical history was obtained at enrollment. Inclusion and exclusion criteria for children with atopic asthma included a 1-year history of physician-diagnosed asthma, use of a short-acting β-agonist ≥2 times a month or daily use of an inhaled corticosteroid or leukotriene receptor antagonist, birth at ≥36 weeks gestation, and ≥1 of the following atopic features: history of positive skin prick test or positive serum-specific IgE for a common aeroallergen, elevated serum total IgE, history of physician-diagnosed allergic rhinitis, or history of physician-diagnosed atopic dermatitis. Inclusion and exclusion criteria for healthy children included birth at ≥36 weeks’ gestation, lack of atopy and asthma by the above definitions, lack of any other clinical diagnosis of lung disease, and lack of a family history of asthma.
From each subject, a blood sample was drawn at the time that BECs were obtained and used to measure total serum IgE and allergen-specific IgE to dust mites (Dermatophagoides farinae and Dermatophagoides pteronyssinus), cat epithelium, dog epithelium, Alternaria alternata, Aspergillus fumigatus, and timothy grass. A clinical follow-up visit was completed within 2 months of obtaining BECs from subjects, at which time, lung function measurements were performed. Subjects with asthma were at their clinical baseline during clinical follow-up visits. The fraction of exhaled nitric oxide was measured according to American Thoracic Society/European Respiratory Society guidelines using a NIOX MINO nitric oxide analyzer (Aerocrine, Solna, Sweden).27 Forced vital capacity, FEV1, and forced expiratory flow between 25% and 75% of forced vital capacity were measured according to American Thoracic Society guidelines using a VMAX series 2130 spirometer (VIASYS Healthcare, Hong Kong), and the percentages of predicted parameters were assessed using reference equations by Hankinson et al.28 Among children with asthma, spirometry measurements were obtained when subjects were at their clinical baseline and were repeated 15 minutes following administration of 4 puffs of albuterol from a metered dose inhaler via a spacer to determine whether a bronchodilator response was present.
Written consent was obtained from parents of subjects and assent was obtained for children ≥7 years of age. The Seattle Children’s Hospital Institutional Review Board approved this study.
BEC isolation, proliferation, differentiation, and infection
Immediately after the endotracheal tube was secured, 3 bronchial BEC samples were obtained from subjects while under general anesthesia using 4-mm Harrell unsheathed bronchoscope cytology brushes (CONMED Corporation, Utica, NY). As described by Lane et al,29 the unprotected brush was inserted through an endotracheal tube, advanced until resistance was felt, and rubbed against the airway surface for 2 seconds. Cells were seeded onto T-25 cell culture flasks precoated with type I collagen and proliferated under submerged culture conditions. Using passage 2 or 3 cells, epithelial cells were differentiated at an air-liquid interface for 3 weeks using methods previously described by our lab.30 BEC air-liquid interface cultures were exposed for 2 hours on the apical surface with RSV strain A2 or RSV line 19 at a multiplicity of infection of 0.5, or virus-free control supernatant.
RNA sequencing
Ninety-six hours following RSV infection, or exposure to virus-free control supernatant, RNA was isolated from BECs using the RNAqueous kit for total RNA purification from Ambion-Applied Biosystems (Austin, Tex). RNA concentration and integrity were determined using the Agilent 2100 Bioanalyzer system and Agilent RNA 6000 Nano Chips (Agilent Technologies, Foster City, Calif). Using 1 μg total RNA with an RNA integrity number ≥8, polyadenylated RNA (primarily mRNA) was selected and purified using oligo-dT conjugated magnetic beads. cDNA libraries were then constructed for each sample using the TruSeq Stranded mRNA Sample Prep Kit (#RS-122-2103; Illumina, San Diego, Calif). Final cDNA libraries were analyzed for size distribution using an Agilent Bioanalyzer (DNA 1000 kit, Agilent #5067-1504), quantitated by quantitative PCR (KAPA Library Quant Kit, #KK4824; KAPA Biosystems, Woburn, Mass), then normalized to 2 nmol/L in preparation for sequencing. See Supplemental Methods for further details in this article’s Online Repository at www.jacionline.org.
RSV infection time course experiment
A time course experiment was conducted to determine the peak in gene expression of a panel of innate viral immunity response genes (IFIH1, IFNB1, IRF7, IFIT2, CXCL10, CXCL11, TLR3, and CD40) following RSV infection of human primary BEC air-liquid interface cultures. BEC donors included 3 children with asthma and 3 healthy children. RNA was isolated 6, 24, 48, and 96 hours following infection with RSV strain A2. Real-time PCR was performed using TaqMan probes (Applied Biosystems, subsidiary of Thermo Fisher Scientific, Foster City, Calif). Gene expression peaked at 96 hours or between 48 and 96 hours for each of these genes (see Fig E2 in this article’s Online Repository at www.jacionline.org), supporting our study design to isolate RNA for sequencing at 96 hours following RSV infection.
Protein analysis
Protein levels were measured in BEC supernatant samples harvested 96 hours after RSV infection or exposure to virus-free control supernatant, using a R&D Systems Luminex assay (Minneapolis, Minn) with magnetic beads run on a Luminex 200 series multi-analyte profiling analyzer (Thermo Fisher Scientific, Waltham, Mass).
LDH assay
Lactate dehydrogenase (LDH) activity levels were measured in BEC supernatant samples harvested 96 hours after RSV infection or exposure to virus-free control supernatant, using a Sigma-Aldrich (St Louis, Mo) LDH activity assay.
Statistics
See Supplemental Methods for statistical analyses of RNA sequencing data in this article’s Online Repository.
Clinical, demographic, and protein data was analyzed using GraphPad Prism 7.01 (San Diego, Calif). For comparisons of clinical and demographic data among groups, P values are calculated as appropriate for data: t-test for continuous data and chi-squared for categorical data. Correlation between gene expression and FEV1 percentage of predicted values was assessed by Pearson correlation.
For protein data, 1-way ANOVA or the Kruskal-Wallis test if data in ≥1 groups was nonnormally distributed were used to compare the distributions of protein levels between groups. Post-hoc comparisons between pairs of groups were made using Dunn multiple comparisons test, with a significance level set at P < .05.
RT quantitative PCR data was standardized using glyceraldehyde 3-phospate dehydrogenase as a nonregulated reference gene. Analyses of RT quantitative PCR results were performed using GenEx version 5.0.1 (MultiD Analyses AB, Goteborg, Sweden). Statistical significance was set at P < .05.
RESULTS
RSV A2 infection of BECs from children with asthma leads to altered gene expression
The characteristics of the study population are presented in Table I. All subjects with asthma had mild or moderate persistent asthma severity defined using National Institutes of Health/National Heart, Lung, and Blood Institute Guidelines for the Diagnosis and Management of Asthma (Expert Panel Report 3) criteria.31 Fifteen of the 18 subjects with asthma had evidence of aeroallergen sensitization with ≥1 positive serum-specific IgE and 12 of 18 subjects were using inhaled corticosteroids (ICSs) at the time of sample collection. Healthy subjects were of the same sex and similar in age to subjects with asthma.
TABLE I.
Characteristics of the study population
| Characteristics | Healthy subjects | All asthma subjects | Asthma obstruction | Asthma no obstruction | P value (asthma obstruction vs asthma no obstruction) |
|---|---|---|---|---|---|
| Age (y) | 10.3 ± 4.0 | 11.7 ± 3.5 | 10.8 ± 2.0 | 12.7 ± 4.5 | .27 |
| Sex, n (male/female) | 3/5 | 12/6 | 7/2 | 5/4 | .62 |
| Total IgE (IU/mL) | 37 ± 22 | 522 ± 1204 | 773 ± 1683 | 270 ± 330 | .38 |
| Sensitizations | 0 | 2.8 ± 2.0 | 2.9 ± 2.4 | 2.7 ± 1.8 | .9 |
| ICS use, n (yes/no) | N/A | 12/6 | 7/2 | 5/4 | .62 |
| FENO (ppb) | 10.0 ± 2.6 | 24.0 ± 30.1 | 27.6 ± 44.0 | 20.8 ± 13.4 | .66 |
| FEV1/FVC ratio | 0.93 ± 0.05 | 0.83 ± 0.06 | 0.80 ± 0.05 | 0.85 ± 0.06 | .06 |
| FEV1/FVC % predicted | 107.1 ± 10.4 | 95.7 ± 6.4 | 92.7 ± 5.1 | 98.6 ± 6.5 | <.05 |
| FEV1 % predicted | 104.4 ± 7.7 | 98.7 ± 14.3 | 86.7 ± 8.4 | 110.8 ± 5.9 | <.001 |
| FVC % predicted | 10.0 ± 2.6 | 104.8 ± 13.4 | 94.6 ± 13.4 | 115.1 ± 10.1 | <.001 |
| Bronchodilator response, n (yes) | N/A | 5 | 5 | 0 | <.05 |
| Asthma severity, n (moderate/mild) | N/A | 5/18 | 5/4 | 0/9 | <.001 |
Values are mean ± SD unless otherwise indicated.
FVC, Forced vital capacity; N/A, not applicable; ppb, parts per billion.
Multidimensional scaling of global gene expression showed clustering of samples by individual and virus stimulation, which accounted for the primary sources of variability among samples; age also demonstrated a significant impact on gene expression (see Fig E3 in this article’s Online Repository at www.jacionline.org). Using linear modeling with false discovery rate (FDR) adjustment for multiple testing correction, including a fixed effect for age and a random effect for individual, 6199 genes were differentially expressed in the RSV A2-infected BECs relative to the uninfected cells (FDR < 0.05) (see Fig E4 in this article’s Online Repository at www.jacionline.org). Significantly upregulated genes with a fold change >1.5 were most highly enriched for the gene ontology (GO) biological processes term “immune response” (FDR = 1.3E-22, 77 genes) and related parent and child terms while significantly downregulated genes with a fold change <0.67 were most highly enriched for the GO biological processes term “cell adhesion” (FDR = 4.0E-33) and related terms. Adjusting for use of ICS did not meaningfully impact the differential expression results, and there were no gene expression differences comparing individuals with asthma using an ICS versus not, demonstrating that any expression differences related to steroid effect on the epithelium do not persist after culture.
BECs from children with asthma and airway obstruction show increased expression of a viral response and interferon/ISG gene set with RSV A2 infection
To investigate gene expression patterns by BECs from children with asthma in response to RSV infection that associate with lung function among BEC donors, children were stratified by airway obstruction on spirometry defined as FEV1/forced vital capacity <0.85 and FEV1 percentage predicted <100%. Each group contained 9 individuals and groups were similar in age, sex, atopic status, ICS use, and fraction of exhaled nitric oxide. FEV1 values in the obstruction group ranged from 73% to 98%, predicted with an average of 86.7% (Table I).
Comparison of children with asthma stratified by airway obstruction showed 195 differentially expressed genes at an FDR <0.05 in a group comparison of the RSV A2-infected BECs (Fig 1, A; see Table E1 in this article’s Online Repository at www.jacionline.org). These gene expression differences were present at both the group and individual levels (Fig 1, C). In contrast, there were no differences detected between these groups in the uninfected BECs, demonstrating findings specific to the RSV infection. Of these 195 genes, 184 showed increased expression in the obstruction group relative to the nonobstruction group and 11 showed decreased expression. The genes increased in the obstruction group were highly enriched for the general GO biologic process “response to virus” (FDR = 1.3E-21), which included 24 of 184 genes (13%). Clustering of enriched GO pathways demonstrated several distinct immune responses including positive regulation of I-κB kinase/nuclear factor-κB cascade, ribonucleotide binding, antigen processing and presentation, Toll-like receptor signaling, and activation of caspase activity (Table E2). This gene set includes the type I interferon IFNB1 and the type III interferons, IFNL1 and 1FNL2; furthermore 173 of 184 of the upregulated genes (94%) have previously been shown to be inducible by type I interferons (see Fig E5, A in this article’s Online Repository at www.jacionline.org).32 This viral response and interferon/ISG gene set also includes IFIH1 (encoding the RIG-I-like receptor family member and viral sensor MDA5), several key transcription factors downstream of type I interferon signaling (eg, STAT1, STAT2, and IRF7), as well as multiple effector molecules including MX1 and OAS1 (Fig 1, B). Additionally, critical chemoattractants including CXCL10 and CXCL11, involved in recruiting T cells and other inflammatory leukocytes are upregulated. These genes form a densely interconnected network of known protein-protein interactions that represents the interconnection of the multiple functional pathways (Fig E5, B). Furthermore this viral response and interferon/ISG gene set demonstrates a significant inverse correlation with the degree of airway obstruction measured by FEV1 percentage predicted (r = −0.77, P <.001) among BEC donors (Fig 2). Of note, genes coding for the epithelial-derived cytokines IL-33 and thymic stromal lymphopoietin were not differentially expressed between children with asthma and airway obstruction and children with asthma and normal lung function.
FIG 1.
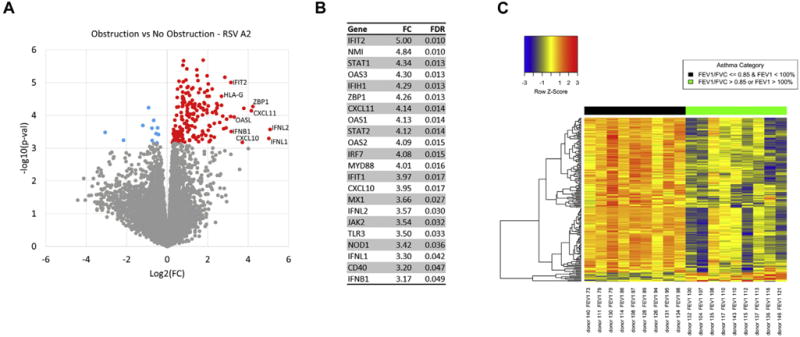
Differential gene expression between children with airway obstruction and children without airway obstruction. A, A volcano plot showing group fold-change differences comparing the RSV A2-infected BECs between the group of children with obstruction and the group without obstruction. Red indicates genes with significantly higher expression in the obstruction group and blue indicates genes with significantly lower expression in the obstruction group (FDR < 0.05). B, List of differentially expressed genes including pattern recognition receptors upstream of type I interferon signaling, ISGs, type III interferons, key transcription factors downstream of type I interferon signaling, and interferon-stimulated chemoattractants regulating T cells and inflammatory leukocytes. C, A heat map of the differentially expressed genes. Gene expression levels are shown as row normalized Z scores with red reflecting higher expression and blue representing lower expression.
FIG 2.
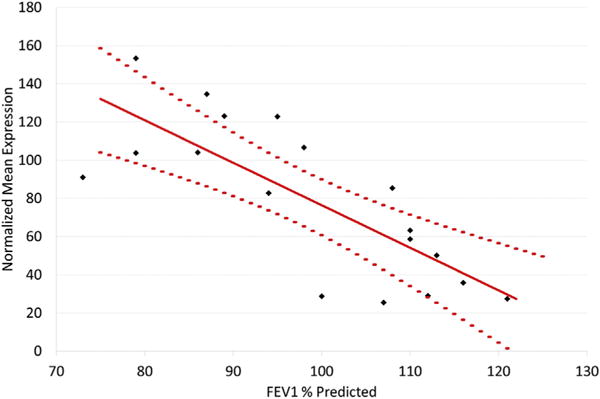
Correlation between expression of the viral response and interferon/ISG gene set and FEV1 percentage predicted. Among children with asthma, the geometric means of the expression values of the 173 viral response and interferon/ISG genes by BECs show significant inverse correlation with degree of airway obstruction as defined by FEV1 percentage predicted. The solid red line represents least squares regression and dotted lines represent the 95% confidence interval.
BECs from children with asthma and airway obstruction also show increased interferon response to RSV strain line 19 infection
We sought to determine the specificity of our findings to BECs from children with asthma and airway obstruction, test the reproducibility of our observations, and to determine whether the findings were RSV strain specific. BECs from 8 age-matched control subjects without asthma, allergic sensitization, or other lung diseases were recruited and infected with RSV strain A2, and BECs from all subjects were also infected with a distinct RSV strain, RSV line 19. Overall gene expression changes elicited by RSV line 19 and RSV A2 were well correlated (r = 0.77, P < .001), particularly among the genes that we previously observed to be differentially expressed following A2 infection (r = 0.94, P < .001) (Fig 3, A). Furthermore, we found that infection of BECs isolated from children with asthma with airway obstruction with either RSV strain led to increased expression of the viral response and interferon/ISG gene set relative to that seen in BECs isolated from children with asthma without obstruction and from healthy children (Fig 3, B), thus confirming our initial findings.
FIG 3.
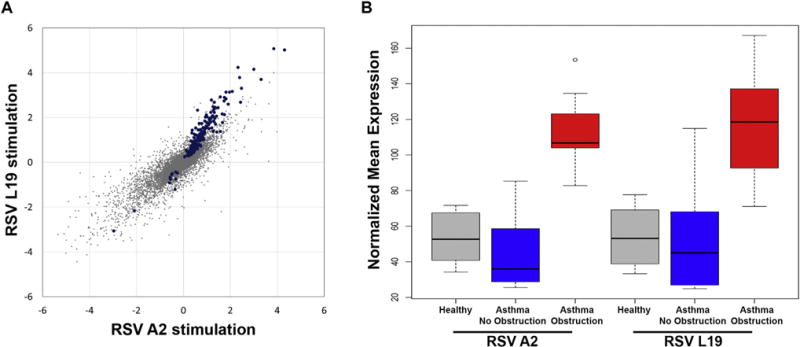
Reproducibility of findings with RSV line 19 stimulation and specificity to airway obstruction. A, Comparison of gene expression differences between asthma obstruction and asthma no obstruction groups were assessed separately in the RSV line 19-infected BECs and RSV A2-infected BECs. The fold-change values for each comparison are plotted demonstrating a high degree of correlation between the 2 datasets. Identical differences between the 2 comparisons would be along the 45° diagonal. Blue dots indicate the 195 statistically significant genes. B, A box plot of the geometric means of the upregulated genes in the asthma no obstruction (blue), asthma obstruction (red), and healthy children (gray) groups after RSV infection shows very similar responses to RSV A2 and RSV line 19 strains, and increased expression of this gene set specific to the asthma obstruction group. Airway obstruction was defined as an absolute FEV1/FVC ratio <0.85 and FEV1 percentage predicted <100%.
Finally, we assessed whether there was a relationship between BEC donor bronchodilator response (BDR) and expression of the viral response and interferon/ISG gene set elicited by RSV A2 infection in ex vivo BEC cultures. Five of the 18 children with asthma were BDR-positive. There was a trend toward increased RSV A2-induced mean expression of the viral response and interferon/ISG gene set by BECs from children with asthma who were BDR-positive (n = 5) compared with those who were BDR-negative (n = 13) (P = .17; data not shown); however, this was likely driven by the fact that all 5 of the children with asthma with BDR met our criteria for obstruction. A subgroup analysis within those children with asthma and airway obstruction (BDR-positive, n = 5; BDR-negative, n = 4) demonstrated a nonsignificant trend toward greater geometric mean expression of the of the viral response and interferon/ISG gene set by BECs from children who were BDR-negative (P = .12; data not shown).
Levels of protein secretion of types I and III interferons and ISGs by BECs are consistent with gene expression patterns
To determine whether the gene expression changes we observed led to increases in protein expression, we used ELISA to quantify several candidate types I and III interferon response proteins. Consistent with gene expression results, we observed greater protein concentrations of IFNβ1, IFNλ1 (IL-29), CXCL10, CXCL11, and CD40 in supernatant of BECs from children with asthma and airway obstruction compared to BECs from children with asthma and normal lung function, as well as compared to BECs from healthy children (Fig 4). There was not a significant difference in IFNλ1 (IL-28A) concentrations in supernatant by BECs among the 3 subject groups.
FIG 4.
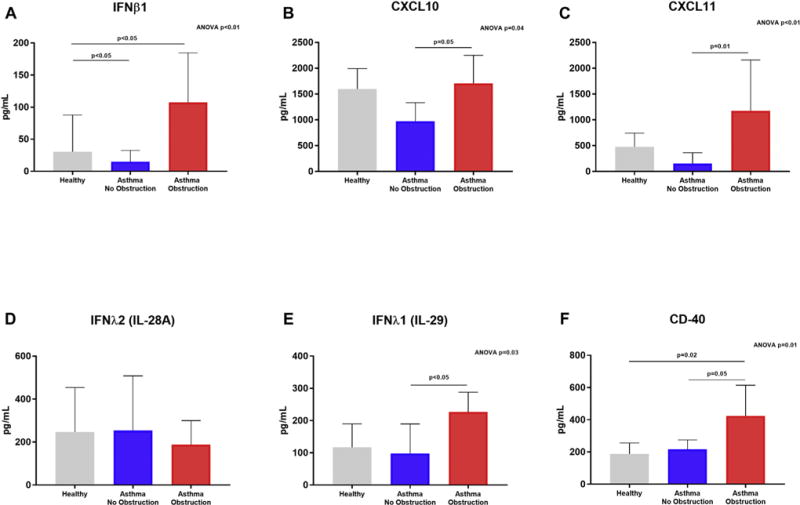
Protein concentrations were measured in BEC supernatant samples harvested 96 hours after RSV infection. IFNβ1 (A), CXCL10 (B), CXCL11 (C), IFNλ1/IL-29 (E), and CD40 (F) protein concentrations were significantly different among supernatants of BECs from children with asthma and airway obstruction, children with asthma and normal lung function, and healthy children (ANOVA, P< .05), and concentrations of these proteins were significantly greater in supernatants from BECs of children with asthma and airway obstruction as compared to supernatants of BECs from children with asthma and normal lung function (Dunn test, P < .05). There was not a significant difference in IFNλ2/IL-28A concentrations in supernatant by BECs between the 3 subject groups.
Viral replication and cell viability in ex vivo BEC cultures
RSV copy number in BECs assayed by PCR 96 hours following infection was similar between BECs from children with asthma and airway obstruction, BECs from children with asthma and normal lung function, and BECs from healthy children (see Fig E6 in this article’s Online Repository at www.jacionline.org). LDH activity as a proxy for cell necrosis/death was also similar among BECs from the 3 groups (see Fig E7 in this article’s Online Repository at www.jacionline.org).
DISCUSSION
Numerous previous studies have demonstrated long-term consequences of early childhood respiratory infections. RSV infection is a risk factor in the development of asthma, and children who develop RSV bronchiolitis have been shown to have persistent airway obstruction as well as airway hyperresponsiveness, even 30 years after infection.5,33 In this present study, we observed significantly increased expression of a set of type I (IFNB1) and III IFNs (IFNL1, IFNL2) and interferon-inducible genes in ex vivo RSV-infected BECs from children with asthma and airway obstruction compared with children with asthma and normal lung function, as well as with healthy children. Analysis of protein in BEC supernatant confirmed that the differences observed in types I and III interferons and ISGs were also present at the protein level. Furthermore, we showed that this interferon response is consistent with complex interconnected biologic functions including likely types I and III interferon amplification, inhibition of viral replication, antigen presentation, upregulation of Toll-like receptor pathways, and chemoattraction of leukocytes. We also show the results were reproducible with 2 different RSV strains.
The magnitude of the interferon response showed significant correlation with the degree of airway obstruction, suggesting a possible mechanistic connection between altered BEC types I and III interferon responses and airway remodeling. However, airflow obstruction does not constitute direct evidence of airway remodeling, and direct assessment of airway remodeling by histopathology in children is limited by human subject research regulations. Although we did not observe differential expression of matrix metalloproteinases (MMPs) by BECs alone (data not shown), a plausible mechanism by which exaggerated types I and III interferon responses to RSV infection could lead to lower lung function would be that interferons and ISGs stimulate airway leukocytes and/or interstitial cells to increase their expression of remodeling-associated proteases such as MMPs 8, 9, and 10.34–36 An imbalance between proteases and antiproteases for example would bias toward a profibrotic airway milieu. MMPs 2, 3, 8, and 9 are proteases implicated in both inflammation and airway remodeling in asthma.37–40 MMP-8 in BAL samples has been inversely correlated with FEV1 in adults with asthma, and MMP-9 levels have been reported to be higher in the sputum of patients with asthma than in healthy subjects, associated with asthma severity, and associated with a fall in FEV1 following allergen challenge.38,39,41,42 Future studies using coculture of BECs with relevant leukocytes and interstitial stromal cells will be critical to test such hypotheses.
An ISG with potential mechanistic significance for which we observed increased RNA expression and protein secretion in RSV-infected BECs from donors with lower lung function is CXCL10. CXCL10 is a chemokine that acts as a strong chemoattractant for multiple leukocytes including macrophages, T cells, and natural killer cells, and has been shown to play a role in mediating leukocyte recruitment and airway inflammation in the setting of viral infections, including HRV.43,44 Furthermore, CXCL10 has also been shown to be secreted by rhinovirus-infected BECs and to act as a chemotactic for airway fibroblasts, suggesting that viral triggered BEC secretion of CXCL10 may contribute to airway remodeling in asthma through recruitment and activation of fibroblasts.45 A potentially more general mechanism linking BEC types I and III interferon responses to lower lung function could simply be that an exaggerated BEC interferon response leads to an overzealous cellular immune response with resultant “collateral damage” to the airway, which given the intrinsic wound-healing defect of the epithelium in asthma leads to airway remodeling with reticular basement membrane thickening secondary to deposition of extracellular matrix.46,47
There has been heterogeneity with regard to the results of prior studies evaluating the innate immune response of BECs from patients with asthma to viral infection. Early research described an impaired type I (IFNB1) and type III interferon (IFNL1, IFNL2) responses to HRV infection by airway epithelial cells from patients with asthma.21,48 Wark et al21 posited that the reduced production of IFN-β resulted in less caspase activity, thereby causing increased viral replication due to a lack of epithelial cell apoptosis. A subsequent study by the same group using cells from adults with well-controlled atopic asthma and healthy adults evaluated the epithelial response to HRV and did not find any significant differences in interferon or ISG expression between BECs from patients with asthma and healthy subjects.49 With regard to RSV specifically, a study by Patel et al25 did not find any significant differences in viral replication or IFNB1, IFNL1, IFNL2/3, OAS1, or MX1 mRNA expression; however, this comparison only compared adults with asthma to healthy control subjects and did not look at subgroups of patients with asthma. In the present study, we have specifically compared BECs from children with asthma and airway obstruction to children with asthma who have normal lung function and have leveraged an unbiased whole transcriptome analysis. Through this approach we have demonstrated significantly increased expression of coordinated types I and III interferon immune responses that specifically correlate with severity of airway obstruction among children with asthma. Together with the findings from the Wark et al21 and Patel et al25 studies, our observations suggest that the interferon response to virus by BECs from children with asthma is more nuanced than simply over- or underexpression and likely depends on virus type and asthma phenotype. Furthermore, our data show that the interferon responses represent a multifaceted immune response activating multiple mechanistic pathways that could contribute to disease.
Despite differences in the expression of types I and III interferons and ISGs observed between BECs from children with asthma and airway obstruction and BECs from children with asthma and normal lung function, we did not observe any differences in RSV copy number between the groups. Changes in viral copy number may require leukocyte effector cells activated by increased interferon and ISG activity, which was not modeled in our experiments. Alternatively, it is possible the interferon and ISG responses to RSV by the BECs from healthy children and children with asthma and normal lung function represent a “normal” viral innate immune response and that the increased and potentially “excessive” response by BECs from children with asthma and airway obstruction does not further decrease RSV replication.
Although the findings from this study are compelling given the reproducible results found when BECs were infected with a different strain of RSV (line 19), there are some limitations of our study design. First, as with all in vitro or ex vivo studies of isolated BECs, it is unclear whether the findings are generalizable to in vivo conditions. However, a resultant strength of our model system is that the epithelial response to viral infection was evaluated in isolation from other cells, without the inflammatory milieu and cellular interactions from lymphocytes and other components of the innate immune system, allowing for identification of pure epithelial cell responses to RSV. Second, our population of children with asthma exhibited mild airflow obstruction, consistent with milder phenotypes of childhood asthma. Finally, we did not infect our cultures with other respiratory viruses (eg, HRV, influenza, parainfluenza). Although beyond the scope of this investigation, given the importance of HRV as a trigger of acute asthma exacerbations, it will be important in future investigations to assess the innate immune response of BECs from patients with asthma to HRV in the context of BEC donor lung function measures.
In summary, using primary cells differentiated ex vivo from children who have been carefully characterized, we have shown that RSV infection of BECs from children with asthma and baseline airway obstructive physiology demonstrate greater expression of types I and III interferons and ISGs compared with cells from children with asthma without airway obstruction. Furthermore, we observed that the degree of airway obstruction was inversely correlated with the magnitude of BEC interferon-related gene expression. These observations suggest that an exaggerated interferon response to viral infection by airway epithelial cells may be a mechanism leading to lung function decline in a subset of children with asthma. Additional studies are needed to determine whether a similar, exaggerated interferon response is elicited by HRV in BECs from children with asthma and airway obstruction and to investigate potential genetic risk variants upstream of types I and III interferons that may explain the excessive or deficient interferon responses of BECs from some children with asthma.
Supplementary Material
Key messages.
RSV infection of BECs from children with asthma and with obstructive physiology, compared with cells from children with asthma and normal lung function as well as to cells from healthy children, demonstrate greater expression of types I and III interferon and interferon-associated genes.
The degree of airway obstruction is inversely correlated with the magnitude of epithelial cell interferon-related gene expression in this model.
Exaggerated interferon response to viral infection by airway epithelial cells may be a mechanism leading to lung function decline in a subset of children with asthma.
Acknowledgments
Supported by grants from the National Institutes of Health (R01HL128361,1U19AI125378) and Amgen,Inc.
We would like to thank Mike Comeau for his valuable assistance with the initial coordination of this project.
Abbreviations
- BEC
Bronchial epithelial cell
- BDR
Bronchodilator responsive
- FDR
False discovery rate
- GO
Gene ontology
- HRV
Human rhinovirus
- ICS
Inhaled corticosteroid
- ISG
Interferon-stimulated gene
- LDH
Lactate dehydrogenase
- MMP
matrix metalloproteinase
- RIG-I
Receptor retinoic acid-inducible gene I
- RSV
Respiratory syncytial virus
Footnotes
Disclosure of potential conflict of interest: K. M. Misura is an employee and owns stock in Amgen, Inc. R. G. James received a grant from the National Institutes of Health. S. F. Ziegler received a grant from the National Institutes of Health. J. S. Debley received a grant from the National Institutes of Health and Amgen. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett SBL, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127:145–52. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–52. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 4.Korppi M, Piippo-Savolainen E, Korhonen K, Remes S. Respiratory morbidity 20 years after RSV infection in infancy. Pediatr Pulmonol. 2004;38:155–60. doi: 10.1002/ppul.20058. [DOI] [PubMed] [Google Scholar]
- 5.Backman K, Piippo-Savolainen E, Ollikainen H, Koskela H, Korppi M. Adults face increased asthma risk after infant RSV bronchiolitis and reduced respiratory health-related quality of life after RSV pneumonia. Acta Paediatr. 2014;103:850–5. doi: 10.1111/apa.12662. [DOI] [PubMed] [Google Scholar]
- 6.Zomer-Kooijker K, van der Ent CK, Ermers MJ, Uiterwaal CS, Rovers MM, Bont LJ, et al. Increased risk of wheeze and decreased lung function after respiratory syncytial virus infection. PLoS One. 2014;9:e87162. doi: 10.1371/journal.pone.0087162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 8.Phelan PD, Robertson CF, Olinsky A. The Melbourne Asthma Study: 1964-1999. J Allergy Clin Immunol. 2002;109:189–94. doi: 10.1067/mai.2002.120951. [DOI] [PubMed] [Google Scholar]
- 9.Tai A, Tran H, Roberts M, Clarke N, Gibson A-M, Vidmar S, et al. Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol. 2014;133:1572–8e3. doi: 10.1016/j.jaci.2013.12.1033. [DOI] [PubMed] [Google Scholar]
- 10.Barbato A, Turato G, Baraldo S, Bazzan E, Calabrese F, Panizzolo C, et al. Epithelial damage and angiogenesis in the airways of children with asthma. Am J Respir Crit Care Med. 2006;174:975–81. doi: 10.1164/rccm.200602-189OC. [DOI] [PubMed] [Google Scholar]
- 11.Malmström K, Pelkonen AS, Malmberg LP, Sarna S, Lindahl H, Kajosaari M, et al. Lung function, airway remodelling and inflammation in symptomatic infants: outcome at 3 years. Thorax. 2011;66:157–62. doi: 10.1136/thx.2010.139246. [DOI] [PubMed] [Google Scholar]
- 12.Payne DNR, Rogers AV, Adelroth E, Bandi V, Guntupalli KK, Bush A, et al. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med. 2003;167:78–82. doi: 10.1164/rccm.200205-414OC. [DOI] [PubMed] [Google Scholar]
- 13.Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007;176:858–64. doi: 10.1164/rccm.200702-212OC. [DOI] [PubMed] [Google Scholar]
- 14.Hallstrand TS, Hackett TL, Altemeier WA, Matute-Bello G, Hansbro PM, Knight DA. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clin Immunol. 2014;151:1–15. doi: 10.1016/j.clim.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Levy DE, Marié IJ, Durbin JE. Induction and function of type I and III interferon in response to viral infection. Curr Opin Virol. 2011;1:476–86. doi: 10.1016/j.coviro.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman SJ, Laham FR, Polack FP. Mechanisms of illness during respiratory syncytial virus infection: the lungs, the virus and the immune response. Microbes Infect. 2004;6:767–72. doi: 10.1016/j.micinf.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Loo Y-M, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82:335–45. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukherjee S, Lukacs NW. Innate immune responses to respiratory syncytial virus infection. Curr Top Microbiol Immunol. 2013;372:139–54. doi: 10.1007/978-3-642-38919-1_7. [DOI] [PubMed] [Google Scholar]
- 19.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol. 2007;81:1401–11. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villenave R, Thavagnanam S, Sarlang S, Parker J, Douglas I, Skibinski G, et al. In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo. Proc Natl Acad Sci U S A. 2012;109:5040–5. doi: 10.1073/pnas.1110203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–47. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S-H, Lim K-H, Park H-K, Lee S-Y, Kim S-H, Kang H-R, et al. Reduced IRF7 response to rhinovirus unrelated with DNA methylation in peripheral mononuclear cells of adult asthmatics. Asia Pac Allergy. 2015;5:114–22. doi: 10.5415/apallergy.2015.5.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards MR, Regamey N, Vareille M, Kieninger E, Gupta A, Shoemark A, et al. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 2013;6:797–806. doi: 10.1038/mi.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baraldo S, Contoli M, Bazzan E, Turato G, Padovani A, Marku B, et al. Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. J Allergy Clin Immunol. 2012;130:1307–14. doi: 10.1016/j.jaci.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Patel DA, You Y, Huang G, Byers DE, Kim HJ, Agapov E, et al. Interferon response and respiratory virus control are preserved in bronchial epithelial cells in asthma. J Allergy Clin Immunol. 2014;134:1402–12e7. doi: 10.1016/j.jaci.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sykes A, Macintyre J, Edwards MR, Del Rosario A, Haas J, Gielen V, et al. Rhino-virus-induced interferon production is not deficient in well controlled asthma. Thorax. 2014;69:240–6. doi: 10.1136/thoraxjnl-2012-202909. [DOI] [PubMed] [Google Scholar]
- 27.American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 28.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 29.Lane C, Burgess S, Kicic A, Knight D, Stick S. The use of non-bronchoscopic brushings to study the paediatric airway. Respir Res. 2005;6:53. doi: 10.1186/1465-9921-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Guisa JM, Powers C, File D, Cochrane E, Jimenez N, Debley JS. Airway epithelial cells from asthmatic children differentially express proremodeling factors. J Allergy Clin Immunol. 2012;129:990–7e6. doi: 10.1016/j.jaci.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(suppl 5):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 32.Rusinova I, Forster S, Yu S, Kannan A, Masse M, Cumming H, et al. Interferome v20: an updated database of annotated interferon-regulated genes. Nucleic Acids Res. 2013;41:D1040–6. doi: 10.1093/nar/gks1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–41. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 34.Foronjy RF, Taggart CC, Dabo AJ, Weldon S, Cummins N, Geraghty P. Type-I interferons induce lung protease responses following respiratory syncytial virus infection via RIG-I-like receptors. Mucosal Immunol. 2015;8:161–75. doi: 10.1038/mi.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dabo AJ, Cummins N, Eden E, Geraghty P. Matrix metalloproteinase 9 exerts antiviral activity against respiratory syncytial virus. PLoS One. 2015;10:e0135970. doi: 10.1371/journal.pone.0135970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kong MYF, Whitley RJ, Peng N, Oster R, Schoeb TR, Sullender W, et al. Matrix metalloproteinase-9 mediates RSV infection in vitro and in vivo. Viruses. 2015;7:4230–53. doi: 10.3390/v7082817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemjabbar H, Gosset P, Lamblin C, Tillie I, Hartmann D, Wallaert B, et al. Contribution of 92 kDa gelatinase/type IV collagenase in bronchial inflammation during status asthmaticus. Am J Respir Crit Care Med. 1999;159:1298–307. doi: 10.1164/ajrccm.159.4.9708080. [DOI] [PubMed] [Google Scholar]
- 38.Prikk K, Maisi P, Pirilä E, Reintam M-A, Salo T, Sorsa T, et al. Airway obstruction correlates with collagenase-2 (MMP-8) expression and activation in bronchial asthma. Lab Invest. 2002;82:1535–45. doi: 10.1097/01.lab.0000035023.53893.b6. [DOI] [PubMed] [Google Scholar]
- 39.Vignola AM, Riccobono L, Mirabella A, Profita M, Chanez P, Bellia V, et al. Sputum metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio correlates with airflow obstruction in asthma and chronic bronchitis. Am J Respir Crit Care Med. 1998;158:1945–50. doi: 10.1164/ajrccm.158.6.9803014. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki R, Kato T, Miyazaki Y, Iwata M, Noda Y, Takagi K, et al. Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in sputum from patients with bronchial asthma. J Asthma. 2001;38:477–84. doi: 10.1081/jas-100105868. [DOI] [PubMed] [Google Scholar]
- 41.Wenzel SE, Balzar S, Cundall M, Chu HW. Subepithelial basement membrane immunoreactivity for matrix metalloproteinase 9: association with asthma severity, neutrophilic inflammation, and wound repair. J Allergy Clin Immunol. 2003;111:1345–52. doi: 10.1067/mai.2003.1464. [DOI] [PubMed] [Google Scholar]
- 42.Cataldo DD, Bettiol J, Noël A, Bartsch P, Foidart J-M, Louis R. Matrix metalloproteinase-9, but not tissue inhibitor of matrix metalloproteinase-1, increases in the sputum from allergic asthmatic patients after allergen challenge. Chest. 2002;122:1553–9. doi: 10.1378/chest.122.5.1553. [DOI] [PubMed] [Google Scholar]
- 43.Romagnani P, Crescioli C. CXCL10: a candidate biomarker in transplantation. Clin Chim Acta. 2012;413:1364–73. doi: 10.1016/j.cca.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Leigh R, Proud D. Virus-induced modulation of lower airway diseases: pathogenesis and pharmacologic approaches to treatment. Pharmacol Ther. 2015;148:185–98. doi: 10.1016/j.pharmthera.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shelfoon C, Shariff S, Traves SL, Kooi C, Leigh R, Proud D. Chemokine release from human rhinovirus-infected airway epithelial cells promotes fibroblast migration. J Allergy Clin Immunol. 2016;138:114–22e4. doi: 10.1016/j.jaci.2015.12.1308. [DOI] [PubMed] [Google Scholar]
- 46.Stevens PT, Kicic A, Sutanto EN, Knight DA, Stick SM. Dysregulated repair in asthmatic paediatric airway epithelial cells: the role of plasminogen activator inhibitor-1. Clin Exp Allergy. 2008;38:1901–10. doi: 10.1111/j.1365-2222.2008.03093.x. [DOI] [PubMed] [Google Scholar]
- 47.Holgate ST, Holloway J, Wilson S, Bucchieri F, Puddicombe S, Davies DE. Epithelial-mesenchymal communication in the pathogenesis of chronic asthma. Proc Am Thorac Soc. 2004;1:93–8. doi: 10.1513/pats.2306034. [DOI] [PubMed] [Google Scholar]
- 48.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PAB, Bartlett NW, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–6. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 49.Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Jarjour NN, Gern JE. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


