Abstract
Objective
To conduct a pilot randomized trial of Interpersonal and Social Rhythm Therapy plus Data-Informed Referral (IPSRT+DIR) versus DIR-alone for adolescents at-risk for bipolar disorder (BP).
Method
Eligible participants included youth (12–18) with a BP parent; youth with BP were excluded. Participants (n=42) were randomized to receive IPSRT+DIR to treat any psychiatric disorders present at baseline, or DIR-alone. A blind evaluator assessed outcomes at baseline, 3- and 6-months. Participants wore an actigraph to measure sleep/wake patterns for 7 days at baseline and 6-months. Primary outcomes included mood and non-mood symptoms and sleep disturbance.
Results
Youth randomized to IPSRT+DIR attended approximately half of scheduled IPSRT sessions. Although 33% of DIR-alone youth were referred for mental health services at intake (another 33% were already engaged in services), none initiated new services over follow-up. No youth developed new-onset mood disorder over follow-up. Self- and parent-reported mood and non-mood psychiatric symptoms did not distinguish the groups, although youth in DIR-alone tended to have higher baseline scores on most measures. Per clinician ratings, 1 youth receiving IPSRT+DIR displayed subthreshold hypo/manic symptoms, versus 2 receiving DIR-alone (OR=14.7, p=0.03), possibly signaling less subthreshold hypo/manic symptoms, and for fewer weeks (χ2=11.06, p=0.0009), over 6-months with IPSRT+DIR. We found a small effect for youth in the IPSRT+DIR group to evidence more WASO at pre-treatment, but less at follow-up (cohen’s d=0.28).
Limitations
Small sample size limits statistical power, and we are unable to definitively attribute group differences to IPSRT versus greater clinical contact. Ability to examine distal/rare (i.e., BP onset) outcomes was limited.
Conclusions
Adolescents at-risk for BP present challenges to psychosocial treatment engagement and retention. IPSRT merits further study as an acceptable intervention for at-risk youth, though necessary frequency and intensity to affect outcomes should be examined. The potential to delay or prevent subthreshold hypo/manic symptoms via enhanced sleep continuity is an area for further examination. Future studies with larger samples and extended follow-up can help determine whether IPSRT may delay or prevent syndromal hypo/mania in youth at-risk.
Keywords: Bipolar disorder, offspring, risk, early intervention
Introduction
Bipolar disorder (BP) is an episodic mood disorder that affects 3–5% of individuals. The illness is of substantial public health import given its elevated risk for negative outcomes including substance misuse, disability, and suicide (Goodwin & Jamison, 2007).
A positive family history of BP is the most potent risk factor for developing BP (Mortensen, Pedersen, Melbye, Mors, & Ewald, 2003), with first-degree relatives at greatest risk. While not all individuals who develop BP have a family history, those with a family history demonstrate earlier illness onset (Singh et al., 2007). Furthermore, those with early illness onset exhibit the most severe illness course (Post et al., 2015). Studies indicate between 5–19% of offspring of parents with BP (OPB) develop BP themselves by young adulthood (Axelson et al., 2015; DelBello & Geller, 2001). OPB are also at greater risk for other psychiatric conditions including depression, anxiety, and behavioral disorders. Although estimates vary between studies, likely due to differing methodology (Duffy et al., 2011), up to 75% exhibit at least one axis I disorder during childhood (DelBello & Geller, 2001). In 20% of cases, other psychiatric disorders precede development of BP in OPB by young adulthood, whereas 55% display other psychiatric conditions but do not develop BP by young adulthood (Axelson et al., 2015). Thus, OPB represent a readily identifiable population at ultra-high risk for early onset of psychiatric disorder, and specifically BP.
Intervention for OPB has the potential to alleviate early psychiatric symptoms, delay illness onset, minimize illness severity, and possibly even prevent illness onset altogether, yet little is known about effective interventions for this population. “Clinical staging models” of chronic medical disease management are increasingly being applied in the field of psychiatry, whereby interventions are matched with individuals based on the individual’s status along an illness continuum (i.e., high-risk, asymptomatic=0; family history and non-specific symptoms/subthreshold manic symptoms=1; full hypo/manic episode=2; recurrent/chronic illness with functional impairment=3–4). This approach asserts treatments with lower risk-benefit ratio and higher acceptability are warranted during earlier stages of illness, at which time short-term symptoms and prevention of disease progression are targeted (McGorry, Hickie, Yung, Pantelis, & Jackson, 2006; Scott et al., 2013).
With regard to early intervention (i.e., stages 0–1), results from psychopharmacological studies are mixed (DelBello, Adler, Whitsel, Stanford, & Strakowski, 2007) and concerns regarding use of medications with potentially concerning side effects in minimally symptomatic youth exist. Psychosocial treatment may mitigate the effects of biopsychosocial factors that contribute to risk (e.g., sleep, stress), and may be more acceptable to youth and families in early illness stages (McHugh, Whitton, Peckham, Welge, & Otto, 2013; Scott, Hickie, & McGorry, 2012; Vallarino et al., 2015). Yet, the only psychosocial intervention specifically evaluated for adolescent OPB to date is Family Focused Therapy for High-Risk Children (FFT-HR). FFT-HR targets OPB age 9–17 with active mood symptoms, and a diagnosis of BP Not Otherwise Specified (NOS), cyclothymia, or depressive disorder. A small randomized trial showed more rapid recovery from mood symptoms, more weeks in remission, and less mania symptoms over follow-up among OPB who received FFT-HR versus an educational control (Miklowitz et al., 2013). Nadkarni and Fristad (2010) also reported lower rates of conversion to BP among depressed youth who received their multi-family psychoeducational intervention, although this sample was not expressly selected for biological risk. These studies included youth who had already developed mood disorders. Ongoing trials of other treatment approaches for youth at-risk for BP, including group CBT, individual CBT, and Mindfulness-based CBT, are pending (Vallarino et al., 2015). Thus, there are currently no other available data on psychosocial intervention for high-risk youth who do not already meet criteria for a BP spectrum or mood disorder (i.e., stage 0 or 1). Experts identify this as a high priority area, particularly amidst interest in clinical staging models for classifying and treating BP (Benarous, Consoli, Milhiet, & Cohen, 2016; Vallarino et al., 2015).
In the experimental therapeutics approach to treating and preventing mental disorders (Report of the National Advisory Mental Health Council’s Workgroup, 2010), a measurable target, or “mechanism of action” hypothesized to underlie the cause of a disorder and/or a treatment’s efficacy is identified and engaged. In BP, neurobiological risk markers (i.e., endophenotypes) for course and outcome among OPB are being explored (e.g., genes, brain function) (Chang, Howe, Gallelli, & Miklowitz, 2006), yet no evidence to date supports their ability to predict illness trajectories. Another promising endophenotype for BP involves abnormalities of the sleep and circadian systems. The instability model of BP (Goodwin & Jamison, 2007) posits that aberrant circadian genes and their modulation underlie the pathophysiology of the illness. Indeed, dysregulated sleep is a core symptom of manic and depressive episodes in BP, and sleep frequently remains disrupted between episodes (Geoffrey et al., 2014). Sleep disturbance is also a pathway to recurrence in biologically vulnerable individuals (Kasper & Wehr, 1992).
Sleep studies in OPB indicate that sleep and circadian dysregulation are risk markers for BP. As compared with controls, adolescent OPB report more variable sleep duration and timing (Stoleru, Nottelmann, Belmont, & Ronsaville, 1997), more severe and persistent sleep problems (Giles, DelBello, Stanford, & Strakowski, 2007), later bedtimes (Levenson et al., 2015), greater sleep fragmentation and efficiency via actigraphy (Ankers & Jones, 2009), and more REM sleep disturbances via EEG (Friess, Modell, Brunner, Tagaya, & Lauer, 2008). In one sample of OPB, the most common non-affective disorder preceding a mood disorder was a sleep disorder (Duffy, Alda, Crawford, Milin, & Grof, 2007); in another, baseline sleep variables, including frequent nighttime awakenings and inadequate sleep, predicted development of BP over follow-up (Levenson et al., 2015). Critically, sleep and circadian disturbances are modifiable risk factors, rendering them promising targets for early intervention among OPB.
Furthermore, recent studies document epic rates of sleep deprivation and circadian dysregulation among adolescents generally, attributable to an interaction between biological and psychosocial factors unique to this developmental stage (Keyes, Maslowsky, Hamilton, & Schulenberg, 2015). Such sleep disturbances during adolescence are linked to risk for a host of negative outcomes including substance use, automobile accidents, and obesity (Carskadon, Acebo, & Jenni, 2004). Perhaps most alarming is the association between sleep disturbance and suicide in adolescents (Goldstein, Bridge, & Brent, 2008). Sleep is therefore a critical domain for intervention that may underlie multiple negative health-related outcomes.
Sleep and social rhythms may become disrupted via stressful life events. Among OPB, the family environment, characterized by high levels of conflict, can serve as a stressor (Miklowitz & Chang, 2008); family conflict positively correlates with severity of offspring psychopathology (Grgoroiu-Serbanescu, Totoescu, Jipescu, Marinewscu, & Ardelean, 1989). Households with a parent with BP also exhibit less cohesion and organization than normative households (Grgoroiu-Serbanescu et al., 1989), possibly contributing further to offsprings’ irregular social rhythms. In the face of such environmental stress, social support serves a protective function (Heponiemi, Elovainio, Kivimaki, Pulkki, & Keltingas-Jarvinen, 2006). Yet, OPB report less social support (Pellegrini et al., 1986). Therefore, additional social support, particularly around the stressful circumstance of having a parent with BP, may be protective for OPB.
Interpersonal and Social Rhythm Therapy (IPSRT)(Frank, 2005) is an intervention developed for adults with BP. IPSRT is based on the Social Zeitgeber theory (Ehlers, Kupfer, & Monk, 1993), and builds on interpersonal psychotherapy for depression (IPT) (Klerman, Weissman, Rounsaville, & Chevron, 1984), emphasizing the connection between mood symptoms and stress from interpersonal relationships. The central IPSRT treatment goal is to regularize daily rhythms and facilitate good sleep hygiene to promote mood stability. In adults, IPSRT is associated with more regular social rhythms (Frank, 2005) and depressive remission (Miklowitz et al., 2007). Hlastala and Frank adapted IPSRT for adolescents with BP. An open study indicates mood and functional improvement with treatment (Hlastala, Kotler, McClellan, & McCauley, 2010). Based on these encouraging results, we modified the adolescent IPSRT model for adolescent OPB who are at-risk for BP (but have not yet developed BP themselves) targeting sleep, social rhythm disturbance, psychoeducation and support around parental BP. Results from our open trial (n=13) (Goldstein et al., 2013) indicate challenges to initial engagement, but high satisfaction with the model, and change in select sleep/circadian patterns (i.e., less weekend oversleeping) with treatment.
As a next step, we conducted a pilot randomized trial of IPSRT plus referral for community treatment for any psychiatric conditions identified through intake psychiatric assessment (i.e., Data-Informed Referral, DIR) versus DIR-alone for adolescent OPB. In keeping with our prior work, we included OPB who have not yet developed BP but may be exhibiting symptoms of other psychiatric disorders. We hypothesized that at-risk youth receiving IPSRT+DIR would demonstrate: 1) less severe mood and non-mood psychiatric symptoms; and 2) more regular sleep and social rhythms (via objective and subjective measures).
Methods
Study Design Overview
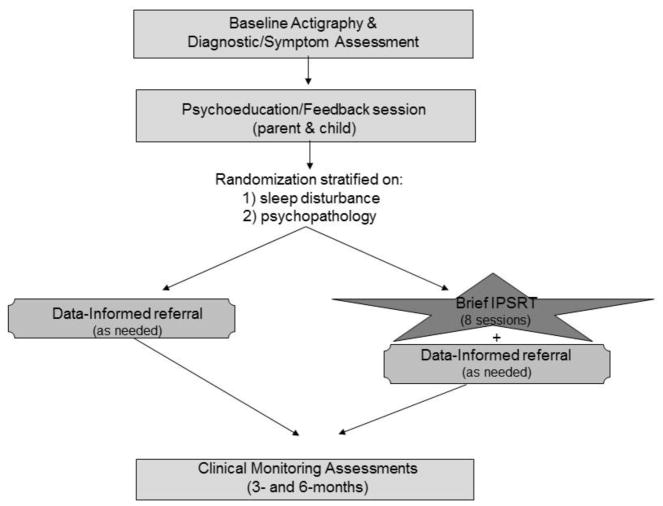
All participants received a thorough assessment of psychiatric and sleep disturbance at intake, followed by a feedback session (Figure 1). All youth were offered DIR as clinically indicated for any psychiatric symptoms/disorders identified at intake. Youth were then randomly allocated (see Stratified Randomization) to also receive either IPSRT or no IPSRT. We carefully considered the selection of a control condition in the trial. Data support great heterogeneity in both type and severity of psychopathology among OPB. As such, we felt no single treatment model or program for the control condition was likely to sufficiently address the breadth of this population’s needs. Furthermore, DIR-alone mirrors community practice and is thus widely generalizable.
Figure 1.
Study design
All participants completed clinical assessments at intake, 3- and 6-months, and wore an actigraph to objectively assess sleep/wake patterns for 7 days at intake and 6-months.
Procedures
The study was approved by the University of Pittsburgh Institutional Review Board and is in accordance with the Helsinki Declaration of 1975. Prior to initiation of any study procedures, study staff explained all procedures to participants and parents, and obtained written informed consent/assent. The trial was registered with clinical trials.gov (NCT03203707).
Inclusion Criteria
Participants included youth age 12 years 0 months to 18 years 11 months with a biological parent diagnosed with BP (see Parental Diagnosis). Youth with a primary BP and/or sleep disorder diagnosis were excluded (see Diagnostic Evaluation), as were youth with intellectual disability, autism spectrum, or organic central nervous system disorder.
Recruitment
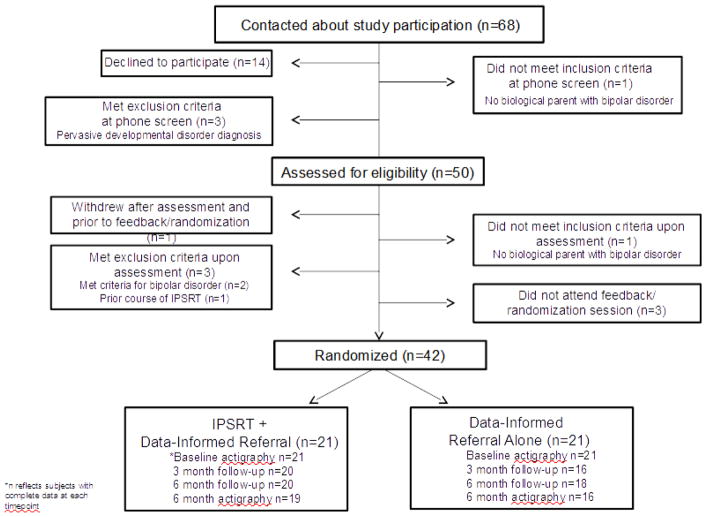
OPB were recruited through multiple venues (outpatient psychiatric services n=17, ongoing research studies n=13, adult BP support groups n=3, and advertisements n=9). We screened 68 families, 50 of whom consented and were assessed for eligibility. Of these, 42 were randomized (see CONSORT diagram, Figure 2).
Figure 2.
Study CONSORT diagram
Diagnostic Evaluation
The independent study evaluator was a master’s level clinician who completed Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (KSADS-PL) (Kaufman et al., 1997), Adolescent Longitudinal Interval Follow-up Evaluation (ALIFE) (Keller et al., 1987), and Structured Clinical Interview for DSM-IV Disorders (SCID) (First, Spitzer, Gibbon, & Williams, 1996) training and achieved an acceptable level of reliability with the first author (kappas>0.8). Inter-rater reliability (IRR) on 5 randomly selected interviews indicated high IRR for KSADS and SCID diagnoses and ALIFE PSR mood ratings (ICC>=0.8).
We evaluated for current and past psychiatric disorders among youth at intake using the KSADS-PL (Kaufman et al., 1997) and the KSADS mania (Axelson et al., 2003) and depression rating scales. The evaluator administered the KSADS first to the parent/guardian and subsequently the adolescent. Summary scores were based on a consensus between informants. All evaluations were staffed with a study-affiliated child psychologist or psychiatrist to confirm diagnoses. Primary sleep disorders were assessed at intake using the Structured Interview for DSM-IV Sleep Disorders (University of, Pittsburgh, 2010).
Confirmation of Parent’s Diagnosis
Parents with records documenting a BP diagnosis from a mental health professional within one year were offered the opportunity to sign release forms for study personnel to obtain their records (n=40). In one case, no records were available. The study evaluator then confirmed the BP diagnosis (i.e., hypo/manic episode) via administration of the SCID mania module with the participating parent. One other participant was adopted at birth; we confirmed this participant’s biological parent’s diagnosis via records provided by the adoptive family.
Stratified Randomization
To account for the expected diagnostic heterogeneity among OPB (Duffy et al., 2007) that would render differential rates of DIR in the study design, and the finding that OPB with sleep disturbance at intake are more likely to develop BP over follow-up (Levenson et al., 2015), we stratified randomization on intake psychopathology and sleep disturbance collected during the requisite intake assessment. Psychopathology was operationalized as presence/absence of any lifetime KSADS Axis I diagnosis. Based on prior findings (T.R. Goldstein et al., 2013), we defined sleep disturbance as: 1) Objective sleep disturbance evidenced by actigraphy data on ≥2 days indicating: sleep efficiency<75%; nighttime awakenings>3; total sleep time<6 or>12 hours; bedtime/waketime variability from weekday to weekend>2 hours; and/or sleep latency>30 minutes; and/or 2) Subjective sleep disturbance indicating: Insomnia Severity Index>7 (indicative of subthreshold insomnia) (Bastien, Vallieres, & Morin, 2001); poor subjective sleep quality per School Sleep Habits Survey (Wolfson et al., 2003) items 14 (“big problem with daytime sleepiness,” i.e.,>3), 18 (self-identified poor sleeper) and/or 19 (“I never get enough sleep”); and/or Dep-P sleep item (#15) >3 (indicating moderate difficulty with any of the following: insomnia, circadian reversal, non-restorative sleep, sleeplessness). We used a modification of Efron’s biased coin toss procedure (Efron, 1971) to randomly assigned participants to groups.
Feedback Session/Data-Informed Referral
Within two weeks of completing the intake assessment, adolescents and their parent(s) attended a 45-minute feedback session with a study therapist that included: 1) Review of findings from the intake clinical assessment (i.e., diagnoses and symptoms); 2) Review of findings from the intake sleep assessment, including the computer-generated actigraphy report; 3) Provision of DIR--clinically-indicated referrals for treatment based on data gathered from intake assessment. We developed a referral guide for study staff with community treatment resources for common childhood psychiatric difficulties. Participants were informed of treatment group assignment upon completion of the feedback session.
IPSRT
The IPSRT intervention for OPB was described in our prior publication (T.R. Goldstein et al., 2013). The intervention includes: 1) Psychoeducation about risk for BP; 2) Social rhythm therapy (SRT) aiming to establish and maintain stable routines to protect against onset of mood symptoms in vulnerable individuals; and 3) Interpersonal psychotherapy (IPT) centering on the adolescent’s feelings about being OPB, and linking stressful family events to mood. We delivered the intervention in 8 in-person sessions over 6 months. Parents were involved in psychoeducation sessions, and further involvement was determined as clinically appropriate. Therapists indicated the primary IPSRT component(s) covered in the session on a therapy tracking form.
IPSRT Therapist Training and Supervision
Four experienced therapists (3 Master’s level Licensed Clinical Social Workers, 1 Doctoral level Clinical Psychologist; mean years of clinical experience = 18.5, Range 8–27) conducted the feedback sessions and the IPSRT intervention. All staff attended training with the first and senior authors that included manual review, role plays, and discussion of videotaped pilot IPSRT sessions. Therapists participated in weekly group supervision involving videotape review and discussion of session content.
IPSRT Treatment Fidelity
A doctoral-level rater trained and maintained at an acceptable level of IRR (ICC>=0.8) with the senior author rated a random sample of 10 sessions from each study therapist using a modified version of the 22-item IPSRT Therapy Rating Scale (Wagner, Frank, & Steiner, 1992). Mean total scores indicated high fidelity to the model among all therapists for each of the three treatment phases (1–5 scale where 1=no IPSRT focus and 5=extensive IPSRT focus; mean score initial phase=4.3, intermediate phase=3.9, final phase=3.9).
Measures
Participants met with the study evaluator (blind to treatment condition) at intake, 3- and 6-month timepoints. In addition, participants completed self-/parent-report measures every 6 weeks (intake, 1.5-, 3-, 4.5- and 6-months) via a secure internet portal.
Mood Episodes and Symptom Severity
The evaluator administered the ALIFE (Keller et al., 1987) to assess week-by-week changes in severity of mood symptoms over participation and to establish onset of new affective disorder diagnoses. Participants completed the self-report Mood and Feelings Questionnaire (MFQ) (Daviss et al., 2006), parent-report Child Mania Rating Scale (CMRS) (Pavuluri, Henry, Devineni, Carbray, & Birmaher, 2006), and self-/parent-report Strengths and Difficulties Questionnaire (SDQ) (Goodman & Goodman, 2009).
Subjective Sleep
Adolescents completed the self-report Adolescent School Sleep Habits Survey (ASHS) to assess subjective report of sleep/wake habits (Wolfson et al., 2003) and the Insomnia Severity Index (ISI). Given that adolescents are more reliable reporters of their sleep than are their parents, and that combined reports show no significant improvement over adolescent report alone (Fatima et al., 2016), no parent-report measures of adolescent sleep were included.
Actigraphy
Participants wore an actigraph (Actiwatch Spectrum Plus from Philips Respironics, Murrysville, PA), a wristwatch-sized device that is the gold standard objective ambulatory monitoring method for assessing sleep and circadian rhythm disruptions in an individual’s natural environment (Marino et al., 2013) on the wrist of their non-dominant hand for 7 consecutive days at intake and 6-months.
Psychosocial Service Utilization
Receipt of mental health services (pharmacotherapy and psychosocial) was documented via the Child and Adolescent Services Assessment (CASA) (Ascher, Farmer, Burns, & Angold, 1996) detailed service form.
Data Analysis
First, we explored demographic and clinical differences between the IPST+DIR versus DIR-alone groups using nonparametric Wilcoxon tests for continuous variables and chi-squared or Fisher’s exact tests for categorical variables. After observing an autoregressive correlation structure in estimated autocorrelation and partial-autocorrelation functions, we implemented logistic generalized estimating equations (GEE) using a first-order autoregressive covariance pattern to analyze the weekly LIFE PSR data (mixed effects models failed to converge). We further analyzed participants’ rate of weeks with subthreshold PSR symptoms using offset zero-inflated Poisson regression (i.e., each participant’s count of weeks with offset accounting for total weeks). Analyses examining self-report mood symptom severity scales (SDQ, MFQ, CMRS) and self-report sleep scales (ASHS, ISI) were conducted using mixed effects linear regression (random intercept models). All regressions controlled for participants’ baseline scale measurements.
Actigraphy
We operationalized specific sleep variables of interest from the actigraphy data, as follows: total sleep time (total time spent sleeping per 24 hour period), sleep efficiency (% of time asleep/time in bed), midsleep time (midpoint between bedtime and waketime defined as minutes after midnight), and wake after sleep onset (WASO, time awake after sleep onset). Analyses of actigraphy data employed mixed linear regression models (random intercept models acount for multiple daily measurements within participant during follow-up period) after square root transforming each outcome variable. All models controlled for each participant’s mean baseline assessments, weekday vs. weekend, school day vs. non-school day, and demographics and clinical variables significant at 0.2. Variability models were also fit analyzing standard deviations, coefficients of variation, and absolute differences between weekday and weekend measures for the aforementioned sleep outcomes.
Power to detect significant differences at the 0.05 level was greater than 82% for all models. Regression analyses were performed on complete cases only; if subjects were missing follow-up data, those observations were excluded from corresponding regression analyses. Linear effect sizes were estimated via Cohen’s d as computed with raw group means and standard deviations for intake comparisons and least square t-statistics for longitudinal comparisons. Logistic effect sizes were estimated via odds ratios.
Results
Study Sample
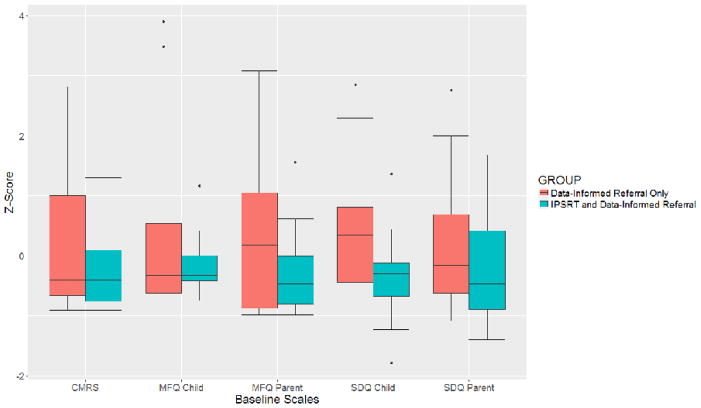
Sample demographic and clinical characteristics can be seen in Table 1. The sample included 42 OPB with a mean age of 14 (50% male). Thirty-two (76%) participants met criteria for at least one lifetime psychiatric disorder, and 27 (64%) had at least one current psychiatric disorder. Although there were no statistically significant between-group differences on any of the intake demographic or clinical variables examined (p>0.1 for all), in general, the DIR-alone group tended to have higher scores on most measures (see Figure 3).
Table 1.
Sample Demographic and Clinical Characteristics
| Variable | IPSRT + DIR (n=21) | DIR Alone (n=21) |
|---|---|---|
|
| ||
| Age (years) | 14.1 (1.7) | 14.2 (1.9) |
|
| ||
| Sex (male) | 9 (43%) | 12 (57%) |
|
| ||
| Race | ||
| White | 14 (67%) | 14 (67%) |
| African-American | 5 (24%) | 6 (29%) |
| More than 1 race | 2 (10%) | 1 (5%) |
|
| ||
| Socioeconomic Status (SES)a | 38.8 (15.3) | 42.7 (17.1) |
|
| ||
| Living Situation (both biological parents) | 6 (29%) | 10 (48%) |
|
| ||
| Parent with BP (Mother) | 15 (71%) | 16 (76%) |
|
| ||
| Parent BP Subtype | ||
| BPI | 12 (57%) | 10 (48%) |
| BPII | 5 (24%) | 4 (19%) |
| BPNOS | 4 (19%) | 7 (33%) |
|
| ||
| Lifetime Axis I Diagnosisb | ||
| No Diagnosis | 6 (29%) | 4 (19%) |
| At least 1 Diagnosis | 15 (71%) | 17 (81%) |
| Mood Disorder | 6 (29%) | 8 (38%) |
| MDDc | 1 (5%) | 3 (14%) |
| Depressive Disorder NOSd | 6 (29%) | 2 (10%) |
| Mood Disorder NOS | 0 (0%) | 4 (19%) |
| Dysthymia | 0 (0%) | 1 (5%) |
| Anxiety Disorder | 7 (33%) | 6 (29%) |
| Behavioral Disordere | 8 (38%) | 14 (66%) |
| Other Disorder | 7 (33%) | 5 (24%) |
| # Lifetime Diagnoses | 1.7 (1.6; range 0–6) | 2.0 (1.5; range 0–5) |
|
| ||
| Current Axis I Diagnosis | ||
| No Diagnosis | 10 (47%) | 5 (24%) |
| At least 1 Diagnosis | 11 (53%) | 16 (76%) |
| Mood Disorder | 2 (10%) | 7 (33%) |
| MDD | 0 (0%) | 2 (10%) |
| Depressive Disorder NOS | 2 (10%) | 1 (5%) |
| Mood Disorder NOS | 0 (0%) | 4 (19%) |
| Anxiety Disorder | 7 (33%) | 5 (24%) |
| Behavioral Disorder | 7 (33%) | 14 (66%) |
| Other Disorder | 0 | 4 (19%) |
| # Current Diagnoses | 0.9 (1.2) | 1.5 (1.3) |
|
| ||
| Any Psychotropic Medications at Intake | 2 (10%) | 5 (24%) |
| Antidepressant | 2 (10%) | 4 (19%) |
| Stimulant | 0 | 3 (14%) |
| Other | 1 (5%) | 1 (5%) |
Hollingshead Redlich criteria
per KSADS-PL(38) and the KSADS mania(41) and depression rating scales
MDD = Major Depressive Disorder
NOS= Not Otherwise Specified
Includes Attention Deficit Hyperactivity Disorder and Oppositional Defiant Disorder
Figure 3.
Group differences in baseline clinical assessment measures at study intake
Treatment Engagement
IPSRT+Data-Informed Referral
On average, youth randomized to receive IPSRT+DIR attended 4.1 (SD=2.9, Range 0–8; mode=6) IPSRT sessions. None of the participants randomized to IPSRT formally withdrew from the treatment. Rather, missed sessions were a result of cancellations, no-shows and/or failure to respond to therapists’ attempts to schedule subsequent sessions. IPSRT therapists indicated 58% of sessions included psychoeducational content, 38% SRT content, and 53% IPT content.
At intake, 4 (19%) of the participants randomized to receive IPSRT+DIR were already engaged in (and continued as DIR) mental health treatment. Two (10%) were referred for DIR as an adjunct to IPSRT following intake; one declined the referral, and the other did not engage in any adjunctive mental health services over follow-up (per CASA data).
Data-Informed Referral Alone
At intake 7 (33%) of the participants randomized to DIR-alone were already engaged in (and continued as DIR) mental health treatment. An additional 7 (33%) were provided a specific referral following intake; of these, three declined the referral. Of the four who accepted, none engaged in mental health services over follow-up.
Treatment Response
Clinician-Rated Mood Episodes (ALIFE PSR Ratings)
No participant in either group developed a new threshold mood episode (i.e., mania, hypomania or depression) over follow-up. Treatment groups did not significantly differ in likelihood to display subthreshold symptoms of depression on the ALIFE PSR over follow-up (IPSRT: n=3, median number of weeks=17; DIR: n=9, median number of weeks=21; OR=4.2, z=1.66, p=0.1). However, participants receiving IPSRT+DIR, as compared with DIR-alone, were significantly less likely to display subthreshold symptoms of hypo/mania over follow-up (IPSRT: n=1, median number of weeks=2; DIR: n=2, median number of weeks=41.5; OR=14.7, p=0.03), and exhibited a significantly lower rate of weeks spent with subthreshold hypo/mania (χ2=11.06, p=0.0009).
Self-/Parent-Reported Mood and Non-Mood Symptom Severity (MFQ, CMRS, SDQ)
There were no significant differences between groups over time on total self- or parent-reported MFQ, CMRS or SDQ scores (ps>0.1; see Table 2). Effect sizes were small, ranging from 0.04–0.11.
Table 2.
Change in Subjective Clinical Measures with Treatment by Group
| Variable | IPSRT + DIR (n=21) | DIR Only (n=19) | Group Contrasts | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intake Mean (SD) | Follow-Up Mean (SE)* | Intake Mean (SD) | Follow-Up Mean (SE)* | Intake | Follow-Up | |||||
| Cohen’s d | t Stat | p-value | Cohen’s d | t Stat | p-value | |||||
| SDQ Child Total Score | 8.0 (3.5) | 8.9 (0.7) | 11.6 (6.6) | 8.6 (0.9) | 0.68 | 2.05 | 0.05 | 0.06 | 0.26 | 0.79 |
| SDQ Parent Total Score | 10.6 (6.3) | 8.2 (0.8) | 12.5 (7.1) | 8.8 (0.8) | 0.28 | 0.82 | 0.42 | 0.11 | 0.48 | 0.63 |
| MFQ Child Total Score | 6.6 (5.6) | 7.5 (1.2) | 11.6 (16.4) | 6.9 (1.4) | 0.41 | 1.26 | 0.22 | 0.07 | 0.33 | 0.74 |
| MFQ Parent Total Score | 9.9 (9.7) | 7.9 (1.6) | 17.3 (16.6) | 7.5 (1.7) | 0.54 | 1.58 | 0.13 | 0.04 | 0.17 | 0.87 |
| CMRS | 6.9 (6.7) | 6.8 (1.2) | 12.1 (12.8) | 6.3 (1.3) | 0.51 | 1.48 | 0.15 | 0.06 | 0.28 | 0.78 |
| ASHS | 2.6 (0.4) | 2.4 (0.1) | 2.4 (0.6) | 2.3 (0.1) | 0.39 | −0.72 | 0.47 | 0.40 | 1.42 | 0.16 |
| ISI Total Score | 4.8 (4.1) | 4.3 (0.7) | 6.7 (6.2) | 4.2 (0.7) | 0.36 | 1.17 | 0.25 | 0.02 | 0.08 | 0.94 |
Follow-up means, standard errors, and test statistics were estimated via mixed effects linear regression controlling for intake scores.
SDQ = Strengths and Difficulties Questionnaire; MFQ = Mood and Feelings Questionnaire; CMRS = Child Mania Rating Scale;ASHS = Adolescent School Sleep Habits Survey
Sleep. (Subjective and Objective)
There were no significant differences between groups over time on either of the subjective measures of sleep (ASHS Cohen’s d=0.40, F=2.01, p=0.2; ISI Cohen’s d=0.02, F=0.01, p=0.9; Table 2). Thirty-five participants (16 DIR-alone, 19 IPSRT+DIR) had both pre- and post-treatment actigraphy data [mean=7 intake days (SD=0.6) and 7 post-treatment days (SD=0.8)]. Via actigraphy, the IPSRT+DIR group evidenced significantly more WASO at pre-treatment, but less at follow-up (Cohen’s d=0.28, F=3.92, p=0.05). The other actigraphy variables did not significantly change from pre-treatment to post-treatment in either group (ps>0.1, Cohen’s ds=0.07–0.19; see Table 3).
Table 3.
Objective Sleep Variables (via Actigraphy) by Treatment Group
| Variable | IPSRT + DIR (n=19) | DIR Only (n=16) | Cohen’s d | F Stat | p-value | ||
|---|---|---|---|---|---|---|---|
| Timepoint (months) | Timepoint (months) | ||||||
| 0 | 6 | 0 | 6 | ||||
| Scored Total Sleep Time (Min) | 447.6 (90.9) | 434.9 (111.4) | 431.3 (102.4) | 438.0 (83.0) | 0.19 | 1.73 | 0.19 |
| Wake after Sleep Onset (Min) | 52.5 (32.8) | 49.2 (27.2) | 42.3 (19.1) | 49.3 (24.0) | 0.28 | 3.92 | 0.05 |
| Sleep Efficiency (%) | 87.3 (6.1) | 87.2 (6.2) | 88.1 (6.2) | 87.5 (5.1) | 0.12 | 0.68 | 0.41 |
| Midsleep Time (Min after Midnight) | 243.5 (104.1) | 238.5 (108.2) | 239.9 (100.0) | 247.5 (104.5) | 0.07 | 0.21 | 0.65 |
| Sleep Maintenance (%) | 89.8 (5.5) | 89.8 (5.1) | 91.1 (3.8) | 90.0 (4.3) | 0.08 | 0.29 | 0.59 |
Discussion
The ability to delay or prevent onset of BP in individuals at high risk represents a critical area for further study. This is the first randomized trial to investigate a psychosocial intervention for youth at genetic risk of BP who are not already symptomatic of the illness. We found that recruitment and engagement of OPB for psychosocial treatment research presented unique challenges. OPB engaged in IPSRT at high rates, rendering the treatment generally acceptable. However, attendance was irregular (50% attendance rate). In contrast, youth referred for DIR for heterogeneous psychopathology who were not already engaged in treatment at intake were highly unlikely to pursue any mental health services despite demonstrated need. None of the subjective measures (self- or parent-report) of mood, non-mood psychiatric symptoms or sleep significantly distinguished the DIR-alone group from the IPSRT+DIR group over follow-up. Although present in very few subjects, we detected a signal for between-group differences in clinician ratings of mood episodes (blind to treatment group) and objective measure of sleep (wrist actigraphy). Specifically, 1 youth in IPSRT+DIR, versus 2 in DIR_alone, exhibited subthreshold hypo/mania over 6-month follow-up via clinician ratings. Additionally, although youth receiving IPSRT demonstrated greater objective actigraphy-derived WASO (an index of sleep continuity) at intake, we did detect a small effect indicative of lower WASO at follow-up.
Primarily, it is important to note the challenges in conducting this work. We approached far more families regarding participation than enrolled. In keeping with our prior work, parents expressed great interest in having their children engage in psychotherapy, whereas offspring were more likely to decline. However, the rate of refusal prior to intake assessment among those contacted about participation in the present study (14/68; 21%) represents a significant improvement over that in our initial open pilot study (67%)(Goldstein et al., 2013). Based on our prior experience in the open study whereby many OPB refused IPSRT on the assertion that there was “nothing wrong” with them (i.e., engaging in treatment implies they are ill), in this subsequent trial, we highlighted to a greater extent the universal IPSRT themes of interpersonal relationships and sleep. It appears these efforts to enhance acceptability and engagement were effective.
Most youth randomized to IPSRT did engage in the treatment, indicating high general acceptability. Yet, similar to our open pilot trial, youth only attended half of the scheduled IPSRT sessions. It remains unclear whether more regular sessions would have differentially impacted outcomes. Our 50% attendance rate among OPB receiving IPSRT is in stark contrast to the 97% rate achieved in an open trial of IPSRT for adolescents diagnosed with BP (Hlastala et al., 2010). It may be that youth with BP experiencing acute symptoms, and their parents (who are often responsible for scheduling and transporting them to treatment), are in greater distress and therefore more motivated to attend treatment regularly. It is also possible that OPB feel their needs are met with fewer IPSRT sessions, as compared with youth who have already developed BP. As such, the optimal “dose” of IPSRT for OPB, as well as additional approaches to engagement for at-risk youth remain important areas for future study.
Our data support the need for intervention for the heterogeneous psychiatric conditions (e.g., ADHD, anxiety) present in OPB that precede BP onset. Addressing these conditions may be another promising path to preventing or delaying BP in OPB. Yet our study indicates low rates of acceptance and follow-through with data-informed treatment referrals. Indeed, a substantial body of research aims to enhance mental health help-seeking in youth (Gulliver, Griffiths, Christensen, & Brewer, 2012). Greater understanding of reasons for not engaging with community-based referrals for the heterogeneous psychopathology common in OPB may represent an important step toward helping at-risk youth engage in early intervention.
Neither self- nor parent-report measures indicated any group differences in mood symptoms. However, we detected a small but possibly meaningful signal for group differences in subthreshold hypo/mania via blinded clinician-ratings. Although only observed in a small subset of participants, the possibility that IPSRT may be associated with decreased risk for subthreshold hypo/mania may offer hope for the intervention’s long-term preventive capacity. Specifically, recent findings from the Pittsburgh Bipolar Offspring Study indicate the single strongest prospective categorical predictor of new-onset BP among OPB is subthreshold hypo/manic symptoms (HR=7.6; Axelson et al., 2015), and when measured dimensionally, subsyndromal hypo/manic symptoms reliably and significantly increase over time up to the point of BP conversion (Hafeman et al., 2016). As such, to the extent that any early intervention delays or prevents subthreshold hypo/manic symptoms among OPB, onset of BP may be thwarted. It is noteworthy that Miklowitz et al. (2013) found an effect on the trajectory of hypomanic symptoms with FFT-HR for symptomatic OPB via clinician ratings, but similarly had limited follow-up to determine long-term effects on development of threshold BP. These findings bring attention to the clinical importance of early subclinical hypo/manic presentations among OPB, and highlight the potential for early psychosocial intervention to modify illness trajectories. Studies with larger samples and extended follow-up periods are needed to determine the long-term effects of these early interventions on syndromal outcomes.
To our knowledge, this is the first psychosocial intervention to date that has carefully assessed and targeted a specific known endophenotype in this population. Given that sleep disturbance is broadly accepted as a critical part of the prodrome of BP among high-risk offspring (Duffy et al., 2014), and that IPSRT has an explicit focus on regularizing sleep and social rhythms, we hypothesized that OPB receiving IPSRT would demonstrate more regular sleep and social rhythms following treatment. We were surprised that we did not find improvements in sleep timing and/or variability via actigraphy with IPSRT. However, our experience aligns with other school-based sleep studies (Wing, Chan, & Man, 2015) indicating minimal impact of intervention on adolescents’ bed- and wake-times. Teens may be more amenable to make changes toward consolidating sleep, as opposed to altering bed- or wake-times per se, given both biological and social influences unique to adolescence (Carskadon, 2002). Along these lines, although the IPSRT+DIR group evidenced significantly more wake after sleep onset (WASO, a measure of sleep continuity) at pre-treatment via actigraphy, they exhibited less WASO than the DIR-alone group at follow-up. Decreasing WASO with treatment may be reflecting a more consistent daily schedule resulting in more consolidated sleep, or possibly improved sleep hygiene practices (e.g., not leaving one’s cell phone beside the bed with the alerts on). It may be that the IPSRT foci on sleep hygiene and/or resolving interpersonal stress may be targeting consolidation of sleep, as reflected by decreased WASO. If replicated, such data would lend further support for IPSRT’s effect on the hypothesized mechanism of action—sleep and social rhythms.
Although we demonstrated improvement in subjective report of weekend oversleep with IPSRT in our initial pilot study with OPB (Goldstein et al., 2013), and Frank et al’s (2005) landmark study showed improvement in subjective social rhythm regularity among adults with BPI receiving IPSRT, in the present study we did not find group differences on subjective measures of sleep and social rhythms. Research indicates a significant discrepancy between subjective perception and objective sleep data among adolescents (Arora, Broglia, Pushpakumar, Lodhi, & Taheri, 2013), and this disparity is correlated with severity of sleepiness and depressive symptoms (Tsuchiyama, Nagayama, Kojima, & Yamada, 2003). Thus, multiple levels of analysis should be considered when assessing these complex constructs moving forward.
Limitations
This study was a pilot randomized trial, and therefore was likely underpowered to detect some meaningful differences between groups. This is particularly true given the sample’s heterogeneity in terms of diagnoses, sleep patterns, and medications at intake, as well as the tendency (despite randomized stratification) for the DIR-alone group to exhibit greater (though largely not statistically significant) psychopathology at study intake. Although we controlled for baseline symptom severity in all models, it is possible that regression to the mean (among the DIR Alone group) further explains similar levels of outcome variables at post-treatment. Yet, the literature indicates that the youth targeted herein are both representative of the population of OPB, and those most important to target in the interests of prevention and early intervention (Birmaher et al., 2009; Miklowitz & Chang, 2008). The youth included in the study, while not expressly treatment-seeking, were amenable to intervention if randomized to IPSRT. It is unclear whether this is representative of the larger population of offspring of parents with BP. Additionally, we cannot definitively state that results can be explained by IPSRT per se, as opposed to more clinical contact in the IPSRT arm. Additionally, we did not assess whether IPSRT-relevant topics were addressed in DIR, which could have created a confound. Yet, receipt of DIR services was very limited in both groups (i.e., IPSRT + DIR n=4, 19%; DIR-alone n=7, 33%), and importantly, pre-dated study participation for all subjects. Furthermore, with no measure of skill practice, generalization of skills is unknown. We also did not examine change in another IPSRT target--family stress/relationships—which will be a critical mediator to examine in future work. Yet, the study was underpowered to expressly examine mediators of IPSRT response. Furthermore, our ability to examine effects of IPSRT on distal outcomes (i.e., onset of BP) was limited. We see the efforts of this work as critical preparation to inform subsequent trials examining outcomes over longer periods.
Conclusions
IPSRT merits further study as an acceptable intervention for at-risk youth; critical questions regarding necessary frequency and intensity of early intervention for this population should be further explored. Furthermore, efforts to understand and target specific barriers to engagement and retention in mental health treatment in this high-risk population are warranted. These initial steps toward “preemption” of BP by employing early detection and intervention as informed by targeting biomarkers represent a promising area for further examination. Specifically, the potential to delay or prevent subthreshold hypo/manic symptoms via enhanced sleep continuity may hold promise. Future studies with larger samples and extended follow-up can help determine whether IPSRT may delay or prevent syndromal hypo/mania in youth at-risk.
Highlights.
Interpersonal and Social Rhythm Therapy (IPSRT) is feasible and acceptable to deliver to youth at high risk for bipolar disorder by virtue of a first-degree family history of the illness.
Few youth referred for community mental health services at intake initiated services over 6 month follow-up, highlighting the need for engagement efforts with this population.
No youth developed new-onset mood disorder over follow-up. Self- and parent-reported mood and non-mood psychiatric symptoms did not distinguish youth receiving IPSRT + Data-Informed Referral (DIR) for any psychiatric disorders present at baseline versus DIR-alone. Per clinician ratings, 1 youth receiving IPSRT+DIR displayed subthreshold hypo/manic symptoms, versus 2 receiving DIR-alone over 6-months, possibly signaling less subthreshold hypo/manic symptoms, and for fewer weeks.
Although no self-reported measure of sleep distinguished the groups, we found a small effect for youth in the IPSRT+DIR group to evidence more wake after sleep onset (WASO; an index of sleep continuity) via actigraphy over follow-up.
Acknowledgments
The authors thank study therapists Nina Hotkowski LCSW, Amy Schlonski LCSW, and Timothy Winbush LCSW; study staff Kelly Monk RN, Reality Price MA, Rachael Fersch-Podrat LCSW, Dawn Rone MA, Sarah Gratzmiller BA, Annette Wood; Joel Sherrill from NIMH; Jamie Feldman; and the staff of the Child and Adolescent Bipolar Spectrum Services (CABS) clinic. Funding: R34 (MH 091177)
Statistical Expert: John Merranko MS
Funding Source
This work was supported by the National Institute of Mental Health (R34 MH091177; Goldstein).
Footnotes
Disclosures
Dr. Goldstein has received grant funding from NIMH, The Brain and Behavior Foundation, the American Foundation for Suicide Prevention, and receives royalties from Guilford Press. Dr. Franzen has received grant funding from NIMH, NIDA and The Brain and Behavior Foundation. Dr. Levenson has received grant funding from NHLBI, the American Sleep Medicine Foundation, and royalties from the American Psychological Association Books. Dr. Axelson has received research support from NIMH, royalties from UpToDate, and serves as a consultant to Janssen Global Services, LLC. Dr. Birmaher receives royalties from American Psychiatric Publishing, Random House, Lippincott Williams & Wilkins, and UpToDate and has served as a consultant to Janssen. Dr. Frank serves as an editorial consultant for American Psychiatric Press, and advisory board member for Servier International, receives royalties from the American Psychological Association Press and Guilford Press, and holds stock in Psychiatric Assessments, Incorporated. The other authors report no financial relationships with commercial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ankers D, Jones S. Objective assessment of circadian activity and sleep patterns in individuals at behavioural risk of hypomania. J Clin Psychol. 2009;65:1–16. doi: 10.1002/jclp.20608. [DOI] [PubMed] [Google Scholar]
- Arora T, Broglia E, Pushpakumar D, Lodhi T, Taheri S. An investigation of the strength of the association and agreement levels between subjective and objective sleep duration in adolescents. PLos One. 2013;8:e72406. doi: 10.1371/journal.pone.0072406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher BH, Farmer EMZ, Burns BJ, Angold A. The child and adolescent services assessment (CASA): Description and psychometrics. J Emot Behav Disord. 1996;4:12–20. [Google Scholar]
- Axelson D, Goldstein B, Goldstein T, Monk K, Yu H, Hickey M, Sakolsky D, Diler R, Hafeman D, Merranko J, Iyengar S, Brent D, Kupfer D, Birmaher B. Diagnostic precursors to bipolar disorder in offspring of parents with bipolar disorder: A longitudinal study. Am J Psychiatry. 2015;172:638–646. doi: 10.1176/appi.ajp.2014.14010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelson DA, Birmaher B, Brent D, Wassick S, Hoover C, Bridge J, Ryan N. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-age Children Mania Rating Scale for Children and Adolescents. J Child Adolesc Psychopharmacol. 2003;13:463–470. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Benarous X, Consoli A, Milhiet V, Cohen D. Early interventions for youths at high risk for bipolar disorder: a developmental apporach. Eur Child Adolesc Psychiatry. 2016;25:217–233. doi: 10.1007/s00787-015-0773-6. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, Obreja M, Ehmann M, Iyengar S, Shamseddeen W, Kupfer D, Brent D. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: The Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66:287–296. doi: 10.1001/archgenpsychiatry.2008.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA. Adolescent sleep patterns: Biological, social and psychological influences. Cambridge University Press; Cambridge: 2002. [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann NY Acad Sci. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Chang K, Howe M, Gallelli KA, Miklowitz D. Prevention of pediatric bipolar disorder: Integration of neurobiological and psychosocial processes. Ann NY Acad Sci. 2006;1094:235–247. doi: 10.1196/annals.1376.026. [DOI] [PubMed] [Google Scholar]
- Daviss WB, Birmaher B, Melhem N, Axelson DA, Michaels SM, Brent DA. Criterion validity of the Mood and Feelings Questionnaire for depressive episodes in clinic and non-clinic subjects. J Child Psychol Psychiatry. 2006;47:927–934. doi: 10.1111/j.1469-7610.2006.01646.x. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Adler CM, Whitsel RM, Stanford KE, Strakowski SM. A 12-week single-blind trial of quetiapine for the treatment of mood symptoms in adolescents at high risk for developing bipolar I disorder. J Clin Psychiatry. 2007;68:789–795. doi: 10.4088/jcp.v68n0520. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Geller B. Review of studies of child and adolescent offspring of bipolar parents. Bipolar Disord. 2001;3:325–334. doi: 10.1034/j.1399-5618.2001.30607.x. [DOI] [PubMed] [Google Scholar]
- Duffy A, Alda M, Crawford L, Milin R, Grof P. The early manifestations of bipolar disorder: a longitudinal prospective study of the offspring of bipolar parents. Bipolar Disord. 2007;9:828–838. doi: 10.1111/j.1399-5618.2007.00421.x. [DOI] [PubMed] [Google Scholar]
- Duffy A, Doucette S, Lewitzka U, LAlda M, Hajek T, Grof P. Findings from bipolar offspring studies: methodology matters. Early Interv Psychiatry. 2011;5:181–191. doi: 10.1111/j.1751-7893.2011.00276.x. [DOI] [PubMed] [Google Scholar]
- Duffy A, Horrocks J, Doucette S, Keown-Stoneman C, McCloskey S, Grof P. The developmental trajectory of bipolar disorder. Br J Psychiatry. 2014;204:122–128. doi: 10.1192/bjp.bp.113.126706. [DOI] [PubMed] [Google Scholar]
- Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58:403–417. [Google Scholar]
- Ehlers CL, Kupfer DJ, Monk TH. Biological rhythms and depression: The role of zeitgeibers and zeitstorers. Depression. 1993;1:285–293. [Google Scholar]
- Fatima Y, Doi S, O’Callaghan M, Williams G, Najman J, Mamun A. Parent and adolescent reports in assessing adolescent sleep problems: results from a large population study. Acta Paediatr. 2016;105:433–439. doi: 10.1111/apa.13404. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (Version 2.0) New York: 1996. [Google Scholar]
- Frank E. Treating bipolar disorder: A clinician’s guide to interpersonal and social rhythm therapy. Guilford Press; New York: 2005. [Google Scholar]
- Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, Fagiolini A. Two year outcomes for Interpersonal and Social Rhythm Therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62:996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- Friess E, Modell S, Brunner H, Tagaya H, Lauer CJ. The Munich vulnerability study on affective disorders: microstructure of sleep in high-risk subjects. Eur Arch Psychiatry Clin Neurosci. 2008;258:285–291. doi: 10.1007/s00406-007-0795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffrey P, Boudebesse C, Bellivier F, Lajnef M, Henry C, Leboyer M, Scott J, Etain B. Sleep in remitted bipolar disorder: a naturalistic case-control study using actigraphy. J Affect Disord. 2014;158:1–7. doi: 10.1016/j.jad.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Giles L, DelBello MP, Stanford K, Strakowski SM. Child Behavior Checklist profiles of children and adolescents with and high risk for developing bipolar disorder. Child Psychiatry Hum Dev. 2007;38:47–55. doi: 10.1007/s10578-006-0041-6. [DOI] [PubMed] [Google Scholar]
- Goldstein TR, Bridge JA, Brent DA. Sleep and suicidal behavior in adolescents. J Consult Clin Psychol. 2008;76:84–91. doi: 10.1037/0022-006X.76.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein TR, Fersch RK, Axelson DA, Gilbert A, Hlastala S, Birmaher B, Frank E. Early Intervention for adolescents at high risk for the development of bipolar disorder: Pilot study of Interpersonal and Social Rhythm Therapy (IPSRT) Psychotherapy. 2014;51(1):180–189. doi: 10.1037/a0034396. 2013. [DOI] [PubMed] [Google Scholar]
- Goodman A, Goodman R. Strengths and difficulties questionnaire as a dimensional measure of child mental health. J Am Acad Child Adolesc Psychiatry. 2009;48:400–403. doi: 10.1097/CHI.0b013e3181985068. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Course and outcome. In: Goodwin FK, Jamison KR, editors. Manic-depressive illness. Oxford University Press; New York: 2007. pp. 119–154. [Google Scholar]
- Grgoroiu-Serbanescu MCD, Totoescu A, Jipescu I, Marinewscu E, Ardelean V. Psychopathology in children aged 10–17 of bipolar parents: Psychopathology rate and correlates of the severity of the psychopathology. J Affect Disord. 1989;16:167–179. doi: 10.1016/0165-0327(89)90071-2. [DOI] [PubMed] [Google Scholar]
- Gulliver A, Griffiths K, Christensen H, Brewer J. A sustematic review of help-seeking interventions for depression, anxiety and general psychological distress. BMC Psychiatry. 2012;12:81. doi: 10.1186/1471-244X-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman D, Merranko J, Axelson D, Goldstein B, Goldstein T, Monk K, Hickey M, Sakolsky D, Diler R, Iyengar S, Brent D, Kupfer D, Birmaher B. Toward the definition of a bipolar prodrome: Dimensional predictors of bipolar spectrum disorders in at-risk youths. Am J Psychiatry. 2016;173:695–704. doi: 10.1176/appi.ajp.2015.15040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heponiemi T, Elovainio M, Kivimaki M, Pulkki LPS, Keltingas-Jarvinen L. The longitudinal effects of social support and hositility on depressive tendencies. Soc Sci Med. 2006;63:1374–1382. doi: 10.1016/j.socscimed.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Hlastala S, Kotler J, McClellan J, McCauley E. Interpersonal and social rhythm therapy for adolescents with bipolar disorder: Treatment development and results from an open trial. Depress Anxiety. 2010;27:457–464. doi: 10.1002/da.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S, Wehr TA. The role of sleep and wakefulness in the genesis of depression and mania. Encephale. 1992;18:45–50. [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The Longitudinal Interval Follow-Up Evaluation: A comprehensive method for outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Keyes M, Maslowsky J, Hamilton A, Schulenberg J. The great sleep recession: changes in sleep duration among US adolescents, 1991–2012. Pediatrics. 2015;135:460–468. doi: 10.1542/peds.2014-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman G, Weissman MM, Rounsaville BJ, Chevron E. Interpersonal psychotherapy of depression. Basic Books; New York: 1984. [Google Scholar]
- Levenson J, Axelson D, Merranko J, Angulo M, Goldstein T, Mullin B, Goldstein B, Brent D, Diler R, Hickey M, Monk K, Sakolsky D, Kupfer D, Birmaher B. Differences in sleep disturbances among offspring of parents with and without bipolar disorder: association with conversion to bipolar disorder. Bipolar Disord. 2015;17:836–848. doi: 10.1111/bdi.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MF, Rueschman M, Winkelman J, Ellenbogen J, Solet J, Dulin H, Berkman LF, Buxton O. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36:1747–1755. doi: 10.5665/sleep.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorry PD, Hickie IB, Yung AR, Pantelis C, Jackson H. Clinical staging of psychiatric disorders: a heuristic framework for choosing earlier, safer and more effective interventions. Aust N Z J Psychiatry. 2006;40:616–622. doi: 10.1080/j.1440-1614.2006.01860.x. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Whitton S, Peckham A, Welge J, Otto M. Patient preference for psychological vs pharmacological treatment of psychiatric disorders: a meta-analytic review. J Clin Psychiatry. 2013;74:595–602. doi: 10.4088/JCP.12r07757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz D, Schneck C, Singh M, Taylor D, George E, Cosgrove V, Howe M, Dickinson M, Garber J, Chang K. Early intervention for symptomatic youth at risk for bipolar disorder: A randomized trial of family-focused therapy. J Am Acad Child and Adolesc Psychiatry. 2013;52:121–131. doi: 10.1016/j.jaac.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Chang K. Prevention of bipolar disorder in at-risk children: Theoretical assumptions and empirical foundations. Dev Psychopathol. 2008;20:881–897. doi: 10.1017/S0954579408000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Otto MW, Frank E, Reilly-Harrington NA, Wisniewski SR, Kogan JN, Nierenberg AA, Calabrese JR, Marangell LB, Gyulai L, Araga M, Gonzalez JM, Shirley ER, Thase ME, Sachs GS. Psychosocial treatments for bipolar depression: A 1-year randomized trial from the systematic treatment enhancement program. Arch Gen Psychiatry. 2007;64:419–426. doi: 10.1001/archpsyc.64.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen PB, Pedersen CB, Melbye M, Mors O, Ewald H. Individual and familial risk factors for bipolar affective disorders in Denmark. Arch Gen Psychiatry. 2003;60:1209–1215. doi: 10.1001/archpsyc.60.12.1209. [DOI] [PubMed] [Google Scholar]
- Nadkarni RB, Fristad M. Clinical course of children with a depressive spectrum disorder and transient manic symptoms. Bipolar Disord. 2010;12:494–503. doi: 10.1111/j.1399-5618.2010.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Henry DB, Devineni B, Carbray JA, Birmaher B. Child Mania Rating Scale: Development, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 2006b;45:550–560. doi: 10.1097/01.chi.0000205700.40700.50. [DOI] [PubMed] [Google Scholar]
- Pellegrini D, Kosisky S, Nackman D, Cytryn L, McKnew DH, Gershon E, Hamovit J, Cammuso K. Personal and social resources in children of patients with bipolar affective disorder and children of normal control subjects. Am J Psychiatry. 1986;143:856–861. doi: 10.1176/ajp.143.7.856. [DOI] [PubMed] [Google Scholar]
- Post RM, Altshuler L, Kupka R, McElroy S, Frye MA, Rowe M, Grunze H, Suppes T, Keck P, Leverich G, Nolen W. Multigenerational positive family history of psychiatric disorders is assocaited with poor prognosis in bipolar disorder. J Neuropsychiatry Clin Neurosci. 2015;27:304–310. doi: 10.1176/appi.neuropsych.14080204. [DOI] [PubMed] [Google Scholar]
- Report of the National Advisory Mental Health Council’s Workgroup. From discovery to cure: Accelerating the development of new and personalized interventions for mental illnesses. 2010 [Google Scholar]
- Scott J, Hickie I, McGorry PD. Pre-emptive psychiatric treatments: pipe dream of realistic outcome of clinical staging models? Neuropsychiatry. 2012;2:263–265. [Google Scholar]
- Scott J, Leboyer M, Hickie I, Berk M, Kapczinski F, Frank E, Kupfer D, McGorry PD. Clinical staging in psychiatry: a cross-cutting model of diagnosis with heuristic and practical value. Br J Psychiatry. 2013;202:243–245. doi: 10.1192/bjp.bp.112.110858. [DOI] [PubMed] [Google Scholar]
- Singh M, DelBello MP, Stanford K, Soutullo C, McDonough-Ryan P, McElroy S, Strakowski SM. Psychopathology in children of bipolar parents. J Affect Disord. 2007;102:131–136. doi: 10.1016/j.jad.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Stoleru S, Nottelmann E, Belmont B, Ronsaville D. Sleep problems in children of affectively ill mothers. J Child Psychol Psychiatry. 1997;38:831–841. doi: 10.1111/j.1469-7610.1997.tb01601.x. [DOI] [PubMed] [Google Scholar]
- Tsuchiyama K, Nagayama H, Kojima K, Yamada K. Discrepancy between subjective and objective sleep in patients with depression. Psychiatry Clin Neurosci. 2003;57:259–264. doi: 10.1046/j.1440-1819.2003.01114.x. [DOI] [PubMed] [Google Scholar]
- University of, Pittsburgh. Structured Clinical Interview for DSM-IV Sleep Disorders 2010 [Google Scholar]
- Vallarino M, Henry C, Etain B, Gehue L, Macneil C, Scott E, Barbato A, Conus P, Hlastala S, Fristad M, Mikloqitz D, Scott J. An evidence map of psychosocial interventions for the earliest stages of bipolar disorder. Lancet Psychiatry. 2015;2:548–563. doi: 10.1016/S2215-0366(15)00156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EF, Frank E, Steiner S. Discriminating maintenance treatments for recurrent depression: Development and implementation of a rating scale. Journal of Psychotherapy Practice and Research. 1992;1:280–290. [PMC free article] [PubMed] [Google Scholar]
- Wing Y, Chan N, Man Y. A school-based sleep education program for adolescents: a cluster randomized trial. Pediatrics. 2015;135:e635–e643. doi: 10.1542/peds.2014-2419. [DOI] [PubMed] [Google Scholar]
- Wolfson A, Carskadon MA, Acebo C, Seifer R, Fallone G, Labyak S, Martin J. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;2:213–216. doi: 10.1093/sleep/26.2.213. [DOI] [PubMed] [Google Scholar]