Abstract
Objective
The primary objective of this trial was to evaluate the feasibility, safety, and efficacy of a predictive hyperglycemia and hypoglycemia minimization (PHHM) system vs predictive low glucose suspension (PLGS) alone in optimizing overnight glucose control in children 6 to 14 years old.
Research Design and Methods
Twenty-eight participants 6 to 14 years old with T1D duration ≥1 year with daily insulin therapy ≥12 months and on insulin pump therapy for ≥6 months were randomized per night into PHHM mode or PLGS-only mode for 42 nights. The primary outcome was percentage of time in sensor-measured range 70 to 180 mg/dL in the overnight period.
Results
The addition of automated insulin delivery with PHHM increased time in target range (70–180 mg/dL) from 66 ± 11% during PLGS nights to 76 ± 9% during PHHM nights (P<.001), without increasing hypoglycemia as measured by time below various thresholds. Average morning blood glucose improved from 176 ± 28 mg/dL following PLGS nights to 154 ± 19 mg/dL following PHHM nights (P<.001).
Conclusions
The PHHM system was effective in optimizing overnight glycemic control, significantly increasing time in range, lowering mean glucose, and decreasing glycemic variability compared to PLGS alone in children 6 to 14 years old.
Keywords: automated insulin delivery, continuous glucose monitoring, type 1 diabetes
1 | INTRODUCTION
The overnight period is a particularly perilous time for patients with type 1 diabetes (T1D) especially with regard to the dangers from prolonged nocturnal hypoglycemia.1,2 It has been well documented that patients with T1D have decreased counter-regulatory hormone responses during sleep3 as well as increased arousal thresholds4 limiting the ability of many patients to detect and respond to nocturnal hypoglycemia. Prolonged nocturnal hypoglycemia can lead to severe consequences such as hypoglycemic seizures5 and even death from the so-called dead-in-bed syndrome.6 In pediatric patients, in particular, parental fear of hypoglycemia has been linked to increased parental stress which in turn has been linked to worse glycemic control. 7 Overnight continuous glucose monitoring (CGM) used alone or in a sensor augmented pump (SAP) fashion has proven inadequate to prevent overnight hypoglycemia as patients tend to become desensitized to overnight alarms.8
While monitoring alone may not be sufficient to reduce or alleviate the risk and burden of nocturnal hypoglycemia, existing and emerging automated insulin delivery (AID) systems show significant promise to accomplish this aim. AID systems combine a CGM sensor, continuous subcutaneous insulin infusion (CSII) pump, and control algorithm which modulates delivery of insulin and/or other hormones, such as glucagon or amylin.9–11 AID may be limited to suspension of insulin delivery or may involve suspending, reducing, or increasing insulin delivery based on CGM values. Much initial work has focused on the role of AID in the overnight period to mitigate hypoglycemia with studies showing significant reduction in hypoglycemia across multiple system designs.12–15 As AID systems have now progressed to longitudinal outpatient studies, the major consistent theme has been the success of overnight control, particularly the reduction in nocturnal hypoglycemia.16–25
Many trials in the pediatric age group have even targeted diabetes camp settings and found significant hypoglycemia reduction with AID use even in this remarkably challenging setting in children.15,20,21,26–28 In addition, the open-label, non-randomized, pivotal trial of the Medtronic 670G AID system (Medtronic Diabetes, Northridge, CA) showed that use of AID in adolescents was associated with a significant reduction in percentage of CGM values <70 mg/dL from 5.8 ± 5.3% during the run-in phase to 2.9 ± 1.6% during the AID phase (P =.002) with concurrent improvement in percentage time in target range of 70 to 180 mg/dL from 64.2 ± 14.1% to 71.5 ± 10.3% (P<.001).17
The major objective of our study group has been to develop a simple system with minimal alarms which is primarily active at night and allows patients and their parents to have undisturbed sleep. Initial work on this system tested a Kalman filter-based predictive low glucose suspend (PLGS) algorithm in a randomized controlled fashion in 45 participants 15 to 45 years old,29 45 participants 11 to 14 years old, and 36 participants 4 to 10 years old.30 Each study participant used the PLGS system 42 nights, and each night was randomized to have the system active or inactive. These trials showed reduction in hypoglycemia time <60 mg/dL by 68 to 81% with experimental PLGS over control SAP therapy. However, the reduction in hypoglycemia was accompanied by an increase in morning glucose of 4 to 17 mg/dL. To further optimize nocturnal glycemic control, an insulindosing module was added to the existing PLGS system to create a predictive hyperglycemia and hypoglycemia minimization (PHHM) system. The first iteration of this system was tested in 30 participants who were 15 to 45 year olds, with each participant using the system for 42 nights with randomization each night to have hyperglycemia mitigation active or inactive and PLGS active each night. The results demonstrated that PHHM significantly improved time in target range of 70 to 180 mg/dL by 7% and average morning glucose by 21 mg/dL when compared to PLGS alone.31 In this trial, we aim to expand upon this work by evaluating the feasibility, safety, and efficacy of the PHHM system in a younger group of participants, aged 6 to 14 years old.
1.1 | Study design and methods
This study was conducted at 2 clinical centers. The protocol was approved by each institutional review board (IRB) and written informed consent was obtained from each participant or parent, with assent obtained as required. Major eligibility criteria included age range of 6 to 14 years, diagnosis of T1D with use of daily insulin therapy for ≥12 months, and insulin pump therapy for ≥6 months. Participants were required to have an enrollment glycated haemoglobin (HbA1c) <10.0% (86 mmol/mol), to live with a parent/legal guardian available to provide assistance when the study system was in use at night, and to ensure uninterrupted internet access during system use. Participants were excluded if there was a history of diabetic ketoacidosis or severe hypoglycemia within the 6 months preceding study enrollment, or a medical and/or psychiatric condition considered to interfere with ability to complete protocol. Additional eligibility criteria are listed in Table S1 (Supporting information).
1.1.1 | System
Closed-loop control was implemented using a control algorithm on a bedside laptop computer with wireless communication to an insulin pump. The system included a MiniMed Paradigm REAL-Time Veo System and Enlite glucose sensor (Siemens, Malvern, PA). The bedside computer received CGM and insulin history data from the pump and responded with insulin delivery commands (basal suspensions and boluses) based on the algorithm’s output. Audible sensor glucose alerts were set at 60 mg/dL and 300 mg/dL on the pump, but there were no additional alerts for automated pump suspensions or automated correction boluses. There were real-time automated notifications to clinical staff, triggered by glucose values <65 mg/dL for 30 minutes, individual automated boluses >0.5 U, or loss of communication with the remote monitoring system for ≥90 minutes. Participants used the Bayer Contour Next Link meter for capillary blood glucose monitoring (Bayer HealthCare LL, Whippany, New Jersey).
1.1.2 | Algorithm details
Details of the control algorithm’s PLGS component have been previously described in References 29, 32, and 33 and are briefly summarized here. The algorithm suspended basal insulin delivery if the current sensor glucose was ≤70 mg/dL at any time or <230 mg/dL and predicted to fall below 80 mg/dL in the next 50 minutes. Basal insulin was restored on the first sensor rise during insulin suspension, and suspension time could not exceed 120 minutes in a 150-minute window or a cumulative total of 300 minutes/night. The PLGS component of the algorithm functioned during both intervention and control nights enabling us to independently assess the effects of the hyperglycemia minimization component.
Details of the algorithm’s insulin dosing component that enabled the system’s PHHM mode have also been previously described in Reference 31 and are summarized here. The controller used a Kalman filter with a prediction horizon of 30 minutes and could issue a fractional automated correction bolus every 5 minutes when the estimated glucose was predicted to exceed 140 mg/dL. Safety constraints included an insulin-on-board (IOB) limit and limits on the maximum individual bolus or cumulative insulin delivery for the night, accounting for any automated or manual boluses delivered.
1.1.3 | Synopsis of study protocol
A run-in phase preceded the randomized trial. During the initial part of the run-in phase, the sensor was initiated and used for 14 to 21 days to verify that the participant could successfully use the pump and sensor. Successful participants then used the complete system with PHHM mode activated at home for 5 nights to verify the ability to use it successfully. Six participants withdrew without proceeding to the randomized trial due to logistical issues or dissatisfaction with system components during the run-in phase and one due to HbA1c screening failure (Figure S1).
During the randomized trial, the system was used until 42 nights with at least 4 hours of sensor glucose data per night were completed. Each night, following initiation procedures which included verification that the meter-measured blood glucose was between 90 and 270 mg/dL, the system randomly activated either PHHM or PLGS according to a predefined schedule with the aim of completing 21 nights with PHHM and 21 nights with PLGS. Participants were blinded to the assignment. Participants were advised to use the system on consecutive nights if possible but to avoid system use during periods of illness. The maximum number of days allowed by the study to complete the 42 nights was 90 days. Upon waking, the system was stopped, a meter blood glucose was measured and overnight carbohydrate intake was recorded. Participants were instructed to perform a blood ketone test if blood glucose was ≥300 mg/dL for over 1 hour or ≥400 mg/dL at any point during system use. Since participants and their families typically did not follow the ketone testing instructions based on retrospective assessment of blood glucose and ketone meter data, the ketone data were considered incomplete and were not included in analyses. During the day, participants used the Veo pump and Enlite glucose sensor (Medtronic Diabetes, Northridge, CA) in sensor-augmented pump mode only. The thresholdbased LGS feature of the Veo pump was disabled during the study.
During the randomized trial, study visits occurred after 21 days and after completion of the study. HbA1c was measured using a point-of-care device (DCA 2000 or DCA Vantage; Siemens) at enrollment, and at each randomized trial visit. Adverse event reporting included severe hypoglycemia (participant required assistance of another person due to altered consciousness and required administration of carbohydrate, glucagon, or other resuscitative actions), diabetic ketoacidosis (as defined by the Diabetes Control and Complications Trial34), and any study- or device-related event.
1.1.4 | Statistical methods
The statistical methods were similar to the ones previously described31 and are briefly summarized here.
The primary outcome was percentage of time in range 70 to 180 mg/dL pooled across nights. Analyses followed the intention-to-treat principle, with each night analyzed by the treatment arm assigned by randomization, and with all participants and all randomized nights included in the primary analysis. All other efficacy metrics were considered secondary exploratory analyses and no adjustment was made for multiple comparisons.
Repeated-measures regression models with an unstructured covariance structure were used to test the differences between the 2 treatment arms, while adjusting for the averaged bedtime blood glucose value across nights for the primary and all other participant-level outcomes. Logarithmic or square-root transformations were used for secondary outcome variables with a skewed distribution.
Additional analyses were performed for night-level secondary outcomes (eg, proportion of nights with at least 1 sensor glucose concentration <70 mg/dL). These analyses were restricted to nights with at least 4 hours of available sensor data. Generalized linear mixed models with a logistic link function for binary outcomes or identity link function for continuous outcomes were used to test the differences between the 2 treatment arms using random participant effects and a within-participant autocorrelation structure to account for multiple nights from the same participant, while adjusting for the bedtime blood glucose. All P-values are 2-tailed and analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, North Carolina).
Analyses for percentage time glucose below 54 mg/dL, area over curve 54 mg/dL, and proportion of nights with nadir below 54 mg/dL events were added posthoc. The methods for the first two paralleled the pooled analyses, while the last paralleled the night-level analyses.
2 | RESULTS
The trial included 28 participants, 6 to 14 years of age, 46% male, 79% Caucasian, with median T1D duration of 4 years, median HbA1c level of 7.6% (60 mmol/mol) at study enrollment, and a median daily insulin dose of 0.83 U/kg/day (Table S2). The median number of nights to complete the study was 64. Overall, there were 1290 randomized nights included in the primary analysis with a median of 9.3 hours of sensor data per night, for a total of 11 322 hours of sensor data. Night-level analyses, which required 4 or more hours of sensor data, were conducted on 1202 nights (93%, referred to hereafter as analyzable nights).
During analyzable nights, 1 or more pump suspensions occurred on 431 (72%) of the 600 PLGS nights and on 475 (79%) of the 602 PHHM nights. Median total duration of suspension on nights with a pump suspension was 70 minutes (interquartile range [IQR] 35, 111) during PLGS nights and 70 minutes (IQR 35, 125) during PHHM nights. Median sensor glucose at first pump shutoff was 117 mg/dL for PLGS and 116 mg/dL for PHHM nights (Table S3).
One or more automatic boluses occurred during 500 (83%) of the 602 PHHM nights; median total insulin delivery given by automatic bolus was 1.10 (IQR 0.45, 2.01) units per night (Table S4), with a median individual bolus of 0.05 U (range 0.025–0.700 U). Median sensor glucose at the time of the first automatic bolus was 156 mg/dL. With respect to total insulin delivery overnight, there was a median of 6.50 (IQR 4.74, 9.44) units of total manual boluses plus basal insulin delivered during PLGS nights and a median of 7.36 (IQR 5.23, 10.90) units of total automatic, manual boluses, and basal insulin delivered during PHHM nights (P<.001). There were 395 (66%) nights with both pump suspensions and automatic boluses during the 602 PHHM nights, with a representative example shown in Figure S2.
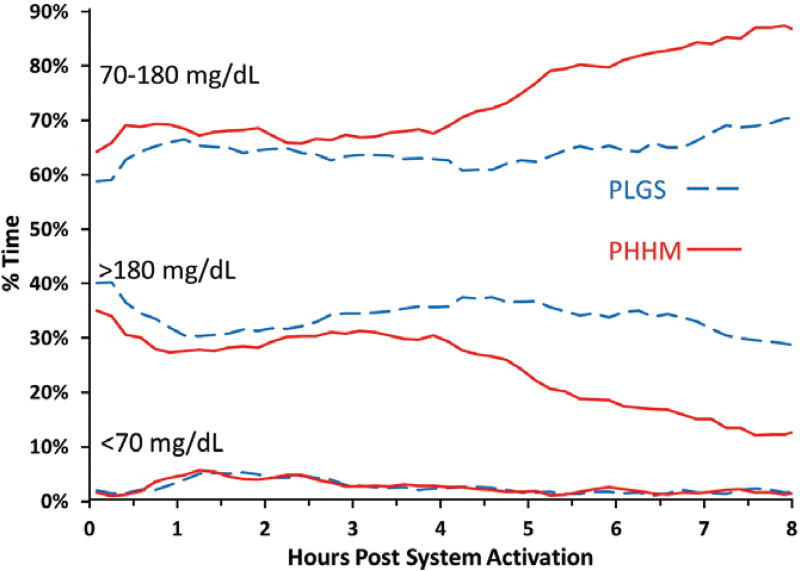
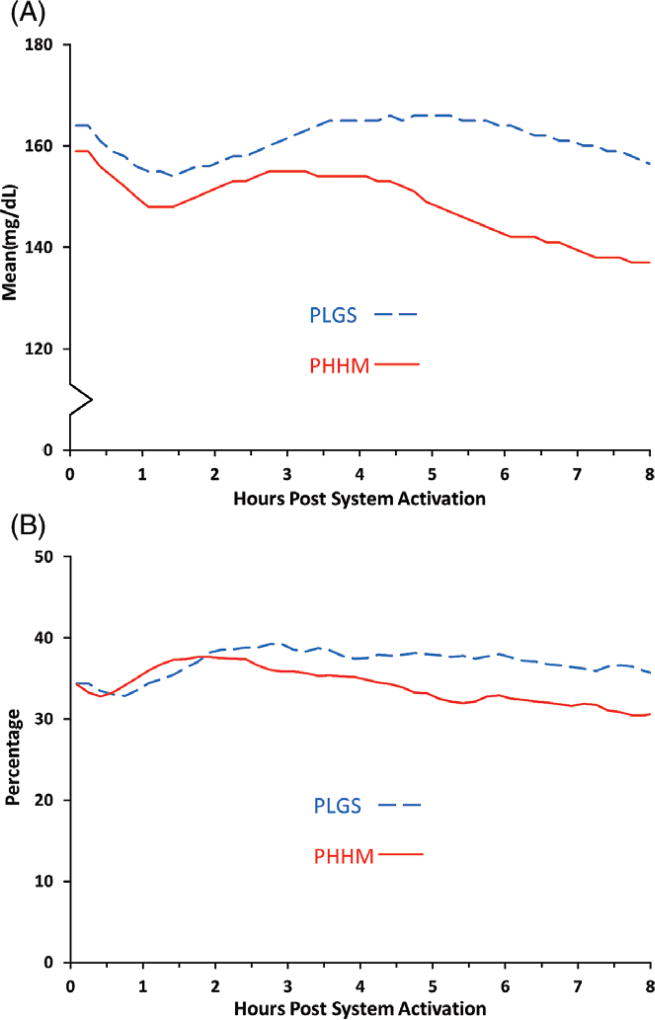
Mean ± SD time in the range of 70 to 180 mg/dL was 66% ± 11% during PLGS nights vs 76% ± 9% during PHHM nights (P<.001, Table 1, Figure 1). Figure S3 shows that all participants, except one, had a higher percentage time 70 to 180 mg/dL on the PHMM nights compared with the PLGS nights. Mean ± SD overnight mean sensor glucose was 160 ± 15 mg/dL during PLGS nights vs 147 ± 13 mg/dL during PHHM nights (P<.001, Figure 2). Median overnight sensor coefficient of variation (CV) was 35% (IQR 30%, 38%) during PLGS nights vs 32% (IQR 28%, 36%) during PHHM nights (P=.01, Figure 2). As shown in Figures 1 and 2, the PHHM system required approximately 3 hours from the time of activation before improvements in glycemic metrics compared with PLGS started to appear, and the curves were still diverging at 8 hours.
TABLE 1.
Efficacy and safety participant-level outcomes (N = 28 participants)a
| PLGS nights | PHHM nights | P-value | |
|---|---|---|---|
| # Randomized nights | |||
| Median (IQR) | 23 (22, 24) | 23 (22, 24) | NA |
| Total number of sensor readings hours | |||
| Median (IQR) | 196 (188, 215) | 201 (192, 217) | Not done |
| Bedtime blood glucose (mg/dL) | |||
| Mean ± SD | 171 ± 16 | 163 ± 18 | Not done |
| Overall outcomes | |||
| Time in range 70–180 mg/dL | |||
| Mean ± SD, primary outcome | 66% ± 11% | 76% ± 9% | <.001 |
| Overnight mean glucose (mg/dL) | |||
| Mean ± SD | 160 ± 15 | 147 ± 13 | <.001 |
| Standard deviation (mg/dL) | |||
| Median (IQR) | 55 (48, 63) | 46 (41, 55) | <.001 |
| Coefficient of variation (SD/mean) | |||
| Median (IQR) | 35% (30%, 38%) | 32% (28%, 36%) | .01 |
| Time in range 70–140 mg/dL | |||
| Mean ± SD | 41% ± 9% | 49% ± 10% | <.001 |
| Hypoglycemia outcomes | |||
| Time spent <70 mg/dL | |||
| Median (IQR) | 1.8% (0.9%, 2.6%) | 1.8% (1.0%, 2.9%) | .76 |
| Time spent <60 mg/dL | |||
| Median (IQR) | 0.7% (0.2%, 0.9%) | 0.6% (0.2%, 1.1%) | .97 |
| Time spent <54 mg/dL | |||
| Median (IQR) | 0.2% (0.0%, 0.5%) | 0.2% (0.0%, 0.6%) | .44 |
| Time spent <50 mg/dL | |||
| Median (IQR) | 0.1% (0.0%, 0.4%) | 0.2% (0.0%, 0.3%) | .30 |
| Area over curve 70 mg/dL | |||
| Median (IQR) | 0.16 (0.08, 0.24) | 0.20 (0.07, 0.28) | .85 |
| Area over curve 54 mg/dL | |||
| Median (IQR) | 0.01 (0.00, 0.04) | 0.01 (0.00, 0.04) | .28 |
| Low blood glucose index | |||
| Median (IQR) | 0.57 (0.36, 0.80) | 0.61 (0.47, 0.88) | .43 |
| Hyperglycemia outcomes | |||
| Time spent >180 mg/dL | |||
| Median (IQR) | 30% (22%, 40%) | 21% (15%, 30%) | <.001 |
| Time spent >250 mg/dL | |||
| Median (IQR) | 7% (4%, 13%) | 2% (1%, 6%) | <.001 |
| Time spent >300 mg/dL | |||
| Median (IQR) | 0.7% (0.2%, 2.9%) | 0.2% (0.0%, 1.2%) | .03 |
| Area over curve 180 mg/dL | |||
| Median (IQR) | 15.58 (9.16, 20.91) | 8.07 (4.32, 12.13) | <.001 |
| High blood glucose index | |||
| Median (IQR) | 6.67 (4.89, 8.88) | 4.86 (3.41, 6.17) | <.001 |
| Morning glucose outcomes | |||
| Mean morning blood glucose (mg/dL) | |||
| Mean ± SD | 176 ± 28 | 154 ± 19 | <.001 |
Glucose results from CGM unless specified as blood glucose. To convert glucose to mmol/L, multiply by 0.0555.
Figure 1.
CGM Metrics for percentage in range, above and below range from system activation by treatment arm (N = 28 participants)
Figure 2.
Mean glucose (A) and glucose coefficient of variation (B) from system activation by treatment arm (N = 28 participants)
Median time below 70 mg/dL was 1.8% (IQR 0.9%, 2.6%) during PLGS nights and 1.8% (IQR 1.0%, 2.9%) during PHHM nights (P=.76). Other sensor-measured hypoglycemic outcomes also were similar between PLGS and PHHM nights (Tables 1 and S5). The above mentioned increase in time 70 to 180 mg/dL was achieved by a corresponding decrease in median time >180 mg/dL: from 30% (IQR: 22%, 40%) during PLGS nights to 21% (IQR 15%, 30%) during PHHM nights (P<.001). Similar improvements were observed for other sensor-measured hyperglycemic outcomes (Tables 1 and S5).
Mean ± SD morning blood glucose was 176 ± 28 mg/dL following PLGS nights vs 154 ± 19 mg/dL following PHHM nights (P<.001, Table 1), with sensor glucose levels equalizing about 2 hours after system deactivation (Figure S4).
Sensor data were available to the controller for an average across participants of 90% of the time the system was running on randomized nights (Table S6). During PHHM nights, the system delivered 92% of the automatic boluses requested by the controller within 5 minutes.
Among 7071 sensor-meter glucose pairs obtained during day and night CGM use, the overall median difference for the Enlite glucose sensor was −6 mg/dL (IQR: −28, +12), median ARD was 13% (IQR: 6%, 24%), mean ARD was 18%, and 59% of pairs met the ISO criteria.35
Prior to randomized system use, 1 participant had prolonged interruption of insulin delivery and associated hyperglycemia after failing to follow manufacturer’s instructions when inserting a new cartridge. Another participant experienced unintended automated insulin delivery leading to hypoglycemia (nadir 51 mg/dL) that required carbohydrate rescue but did not meet study criteria for severe hypoglycemia. The apparent cause was corruption of a wireless bolus command due to electromagnetic interference, with failure of the manufacturer’s cyclic redundancy check (CRC) to detect the corruption and subsequent delivery of a 6.4 U bolus rather than the intended 0.075 U. During the randomized system use period, 1 participant reported an infusion site infection. There were no cases of severe hypoglycemia, diabetic ketoacidosis, or other serious or study-or device-related adverse events during the trial. Median HbA1c levels were 7.9% (IQR 7.2%, 8.8%) [63 mmol/mol (IQR 55, 73)] at the start of randomized system use and 7.9% (IQR 7.3%–8.5%) [63 mmol/mol (IQR 56, 69)] at the end of the trial.
3 | DISCUSSION
The PHHM system was effective in optimizing overnight glycemic control in this double-blind, 6-week at-home study with night-level randomization in children 6 to 14 years old. The PHHM system significantly lowered mean glucose, increased time in target range, and decreased glycemic variability without increasing time spent in hypoglycemia in this age group when compared to PLGS alone. Addition of the hyperglycemia minimization module produced a significant reduction in average morning glucose of 22 mg/dL. The algorithm was safe without any serious adverse events such as diabetic ketoacidosis, severe hypoglycemia, or hospitalizations.
The overnight period has long been identified as an ideal target for improved glycemic control and decreased patient and family burden with the application of AID systems. Fear of hypoglycemia and resulting worsened glycemic control overnight are a major barrier to achieving glycemic targets in the pediatric population.7,36–38 In addition, the parent and family stress associated with fear of hypoglycemia presents a major additional burden for children with T1D and their families.39,40 A recent study by Sharifi et al investigated the role of an Android-based hybrid closed-loop (HCL) system against LGS only overnight in 16 adults and 12 adolescents (12–18 years old) with T1D.41 The study found significant improvement in self-reported sleep quality for the adult participants on HCL compared to LGS but no significant improvement for the adolescents. This project however did not study the parents of children with T1D and did not compare HCL or LGS to conventional SAP therapy alone. Earlier work by Barnard et al comparing overnight HCL therapy with SAP therapy in adolescents 12 to 18 years old found that the psychological benefits of closed-loop systems were significant and improved sleep in a majority of adolescents and their parents.42
The results from this project compare very favorably with other AID trials investigating overnight control in pediatric populations. Across a wide spectrum of AID designs mean overnight percentage of time <70 mg/dL has fallen in the range of 1.4 to 5.4% with average glycemic control falling in the range of 137 to 144 mg/dL and AM glucose in the range of 121 to 149 mg/dL.21,41,43–45 The pivotal trial for the recently approved Medtronic 670G HCL AID system showed for adolescents 14 to 21 years old an overnight % <70 mg/dL of 3.2% with average CGM value of 145.9 mg/dL and 7 AM CGM value of about 142 mg/dL.17,22 This average CGM value is very similar to the 147 mg/dL observed during PHHM nights of the current study, though the 670G system reduces hyperglycemia by increasing basal insulin rather than delivering small correction boluses. The results from our pediatric cohort also compare favorably with our recently published adult cohort which reported that with PHHM the % <70 mg/dL overnight was 1.1%, time in target range 70 to 180 mg/dL was 78%, mean CGM glucose was 143 mg/dL, and mean AM blood glucose was 142 mg/dL.31
The Medtronic 640G is a PLGS system introduced in various parts of the world, but never released in the United States. The 640G system suspends insulin infusion when 2 criteria are met: the sensor glucose is at or within 70 mg/dL above the set low limit and is predicted to be 20 mg/dL above the set low limit in 30 minutes.46 The 640G algorithm thus uses a similar set of threshold and prediction elements to the PLGS portion of the system tested here, but does uses a shorter prediction threshold (30 vs 50 minutes) and does not offer hyperglycemia minimization.47
A significant strength for this study is its scientific design in which the system mode (PLGS vs PHHM) was randomly selected each night and the study participant was blinded to this assignment. The inclusion/exclusion criteria were relatively broad allowing patients with an HbA1c of <10.0% to participate in the trial, helping to improve generalizability.
A limitation of this study is that the AID system did not have an adaptive component in that the algorithm did not “learn” day-to-day based on previous data. Another potential limitation is that participants were remotely monitored overnight by study staff and parents were contacted for prolonged hypoglycemia. However, this safety constraint was present on both PLGS and PHHM nights with a similar incidence rate (Table S6). Prior to randomized system use by any participant, there was 1 bolus command error resulting in over-delivery and subsequent hypoglycemia, for which remote monitoring prevented a potential severe adverse event. Although this prototype system did rely on the pump manufacturer’s unmodified wireless security implementation, the pump itself was not designed or approved for frequent insulin dosing via wireless control. After review of this event with the data safety committee, Food and Drug Administration (FDA), and IRB, a maximum bolus size constraint of 1.0 U was introduced both in software and hardware to mitigate such events for the remainder of the study. Although any AID system with wireless transmission of bolus commands is in principle vulnerable to signal corruption, commercialized systems will generally have very robust error-detecting code to identify and reject corrupted commands.
The per-night randomized nature of this trial prevented assessment of the respective impacts of the PLGS and PHHM components on HbA1c, and use of these components in previous studies was not associated with an increase in HbA1c.29–31 The overall goals of the project were to limit hypoglycemia with hyperglycemia minimization added to help minimize secondary hyperglycemia associated with insulin suspension. Based on these goals, reduction of hypoglycemia without increasing HbA1c may be viewed as a success of the project aims.
In conclusion, the PHHM system performed very well in this pediatric population, achieving 76% overnight time in the range of 70 to 180 mg/dL with no increase in hypoglycemia compared with PLGS alone. This was achieved in a customizable system used at home, overnight with minimal system maintenance in a pediatric population allowing for improved glycemic control during what is for many families the most stressful time for T1D management.
Supplementary Material
Acknowledgments
We would like to recognize the efforts of the participants and their families and thank them. We also would like to recognize Martin Cantwell, BSC, Medtronic Diabetes, Northridge, CA and Chris McCarthy, Jaeb Center for Health Research, Tampa, FL for their significant engineering contributions.
The project described was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK085591), grants from JDRF (22-2013-266), and the JDRF Canadian Clinical Trial Network (CCTN) which is a public-private partnership including JDRF International, JDRF-Canada (JDRF-C) and the Federal Economic Development Agency for Southern Ontario (FedDev Ontario); and is supported by JDRF # 80-2010-585. This work was also supported by the Stanford Clinical and Translational Science Award (CTSA) to Spectrum (UL1 TR001085). The CTSA program is led by the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH).
Continuous glucose monitors and sensors were purchased at a bulk discount price from Medtronic MiniMed, Inc. (Northridge, CA). Home glucose meters and test strips were provided to the study by Bayer HealthCare LLC. Home ketone meters and test strips were provided by Abbott Diabetes Care, Inc. The companies had no involvement in the design, conduct, or analysis of the trial or the manuscript preparation.
Funding information: Juvenile Diabetes Research Foundation, Grant/Award number: 22-2013-266; Juvenile Diabetes Research Foundation Canada, Grant/Award number: 80-2010-585; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award number: R01DK085591; Stanford Clinical and Translational Science Award, Grant/Award number: UL1 TR001085
Abbreviations
- AID
automated insulin delivery
- CGM
continuous glucose monitoring
- CSII
continuous subcutaneous insulin infusion
- HbA1c
glycated hemoglobin
- HCL
hybrid closed loop
- IOB
insulin-on-board
- IQR
interquartile range
- LGS
low glucose suspend
- PHHM
predictive hyperglycemia and hypoglycaemia minimization
- PLGS
predictive low glucose suspend
- SAP
sensor augmented pump
- T1D
type 1 diabetes
Footnotes
Author contributions
The study was designed and conducted by the investigators. The writing group collectively wrote the manuscript and vouch for the data. G.P.F. is the guarantor of this work and, as such, had full access to all the data in the study. B.A.B., D.M.W, P.C., H.P.C, D.M.M., R.P.W., E.J., L.N., L.E., H.M., and T.T.L. researched data, contributed to discussion, and reviewed/edited manuscript. B.W.B., F.C., N.N., and C. K. contributed to discussion, and reviewed/edited manuscript. G.P.F., D.R., J.W.L., and R.W. B. wrote the manuscript, contributed to discussion, and reviewed/edited manuscript.
Disclosure
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
G.P.F. has received grants from NIDDK, during the conduct of the study; grants from Medtronic, grants from Dexcom, grants from Tandem, grants from Insulet, personal fees from Abbott, grants from Bigfoot, grants from Novo Nordisk, outside the submitted work. D.R. has nothing to disclose. F.C. has nothing to disclose. B.W.B. reports personal fees from BD, outside the submitted work. In addition, B.W.B. has a patent US 9227014 issued. HPC has nothing to disclose. R.P.W. reports personal fees from Eli Lilly & Company, personal fees from Novo Nordisk, grants from Novo Nordisk, grants from Lexicon Pharmaceuticals, grants from Dexcom, grants from Bigfoot Biomedical, outside the submitted work. D.M.M. reports grants from Medtronic, Dexcom and other support from Insulet, outside the submitted work. E.J. has nothing to disclose. T.T.L. has nothing to disclose. D.M.W. reports grants from JDRF, grants from NIH, during the conduct of the study; personal fees from Roche, outside the submitted work. In addition, D.M.W. has a patent 9227014 issued. L.N. has nothing to disclose. L.E. has received grants from NIH, during the conduct of the study; grants from Medtronic, grants from Dexcom, grants from Insulet, grants from Bigfoot, grants from Xeris, grants from Tandem, grants from the Helmsley Charitable Trust, outside the submitted work. H.M. has nothing to disclose. H.P.C. has nothing to disclose. N.N. has nothing to disclose. J.W.L. reports grants from NIDDK, grants from JDRF, during the conduct of the study; other from Animas Corporation, other from Bigfoot Biomedical, other from Tandem Diabetes Care, other from Eli Lilly and Company, outside the submitted work. C.K. has nothing to disclose. R.W.B. reports grants to his institution from NIH, during the conduct of the study; also to his institution: grants and non-financial support from Dexcom, grants from Animas, non-financial support from Abbott Diabetes Care, grants and other from Bigfoot, grants and other from Tandem, other from Lilly, outside the submitted work.
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care. 1997;20(1):22–25. doi: 10.2337/diacare.20.1.22. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46(2):271–286. [PubMed] [Google Scholar]
- 3.Jones TW, Porter P, Sherwin RS, et al. Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med. 1998;338(23):1657–1662. doi: 10.1056/NEJM199806043382303. [DOI] [PubMed] [Google Scholar]
- 4.Ly TT, Jones TW, Griffiths A, et al. Hypoglycemia does not change the threshold for arousal from sleep in adolescents with type 1 diabetes. Diabetes Technol Ther. 2012;14(2):101–104. doi: 10.1089/dia.2011.0144. [DOI] [PubMed] [Google Scholar]
- 5.Buckingham B, Wilson DM, Lecher T, Hanas R, Kaiserman K, Cameron F. Duration of nocturnal hypoglycemia before seizures. Diabetes Care. 2008;31(11):2110–2112. doi: 10.2337/dc08-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanenberg RJ, Newton CA, Drake AJ. Confirmation of hypoglycemia in the “dead-in-bed” syndrome, as captured by a retrospective continuous glucose monitoring system. Endocr Pract. 2010;16(2):244–248. doi: 10.4158/EP09260.CR. [DOI] [PubMed] [Google Scholar]
- 7.Viaene AS, Van Daele T, Bleys D, Faust K, Massa GG. Fear of hypoglycemia, parenting stress, and metabolic control for children with type 1 diabetes and their parents. J Clin Psychol Med Settings. 2017;24(1):74–81. doi: 10.1007/s10880-017-9489-8. [DOI] [PubMed] [Google Scholar]
- 8.Buckingham B, Block J, Burdick J, et al. Response to nocturnal alarms using a real-time glucose sensor. Diabetes Technol Ther. 2005;7(3):440–447. doi: 10.1089/dia.2005.7.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kowalski AJ. Can we really close the loop and how soon? Accelerating the availability of an artificial pancreas: a roadmap to better diabetes outcomes. Diabetes Technol Ther. 2009;11(suppl 1):S113–S119. doi: 10.1089/dia.2009.0031. [DOI] [PubMed] [Google Scholar]
- 10.Kowalski A. Pathway to artificial pancreas systems revisited: moving downstream. Diabetes care. 2015;38(6):1036–1043. doi: 10.2337/dc15-0364. [DOI] [PubMed] [Google Scholar]
- 11.Forlenza GP, Buckingham B, Maahs DM. Progress in diabetes technology: developments in insulin pumps, continuous glucose monitors, and progress towards the artificial pancreas. J Pediatr. 2016;169:13–20. doi: 10.1016/j.jpeds.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hovorka R, Kumareswaran K, Harris J, et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ. 2011;d1855:342. doi: 10.1136/bmj.d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Grady MJ, Retterath AJ, Keenan DB, et al. The use of an automated, portable glucose control system for overnight glucose control in adolescents and young adults with type 1 diabetes. Diabetes Care. 2012;35(11):2182–2187. doi: 10.2337/dc12-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown SA, Kovatchev BP, Breton MD, et al. Multinight “bedside” closed-loop control for patients with type 1 diabetes. Diabetes Technol Ther. 2015;17(3):203–209. doi: 10.1089/dia.2014.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368(9):824–833. doi: 10.1056/NEJMoa1206881. [DOI] [PubMed] [Google Scholar]
- 16.Kovatchev B, Cheng P, Anderson SM, et al. Feasibility of long-term closed-loop control: a multicenter 6-month trial of 24/7 automated insulin delivery. Diabetes Technol Ther. 2017;19(1):18–24. doi: 10.1089/dia.2016.0333. [DOI] [PubMed] [Google Scholar]
- 17.Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19:155–163. doi: 10.1089/dia.2016.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol. 2016;4(3):233–243. doi: 10.1016/S2213-8587(15)00489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renard E, Farret A, Kropff J, et al. Day and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: results of a single-arm 1-month experience compared with a previously reported feasibility study of evening and night at home. Diabetes Care. 2016;39:1151–1160. doi: 10.2337/dc16-0008. [DOI] [PubMed] [Google Scholar]
- 20.Ly TT, Buckingham BA, DeSalvo DJ, et al. Day-and-night closed-loop control using the unified safety system in adolescents with type 1 diabetes at camp. Diabetes Care. 2016;39:e106–e107. doi: 10.2337/dc16-0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Favero S, Boscari F, Messori M, et al. Randomized summer camp crossover trial in 5- to 9-year-old children: outpatient wearable artificial pancreas is feasible and safe. Diabetes Care. 2016;39(7):1180–1185. doi: 10.2337/dc15-2815. [DOI] [PubMed] [Google Scholar]
- 22.Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316:1407–1408. doi: 10.1001/jama.2016.11708. [DOI] [PubMed] [Google Scholar]
- 23.Anderson SM, Raghinaru D, Pinsker JE, et al. Multinational home use of closed-loop control is safe and effective. Diabetes Care. 2016;39:1143–1150. doi: 10.2337/dc15-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial Beta cell in type 1 diabetes. N Engl J Med. 2015;373(22):2129–2140. doi: 10.1056/NEJMoa1509351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovatchev BP, Renard E, Cobelli C, et al. Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care. 2014;37(7):1789–1796. doi: 10.2337/dc13-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ly TT, Breton MD, Keith-Hynes P, et al. Overnight glucose control with an automated, unified safety system in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Care. 2014;37(8):2310–2316. doi: 10.2337/dc14-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Troncone A, Bonfanti R, Iafusco D, et al. Evaluating the experience of children with type 1 diabetes and their parents taking part in an artificial pancreas clinical trial over multiple days in a diabetes camp setting. Diabetes Care. 2016;39(12):2158–2164. doi: 10.2337/dc16-1073. [DOI] [PubMed] [Google Scholar]
- 28.Weissberg-Benchell J, Hessler D, Polonsky WH, Fisher L. Psychosocial impact of the bionic pancreas during summer camp. J Diabetes Sci Technol. 2016;10(4):840–844. doi: 10.1177/1932296816640289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maahs DM, Calhoun P, Buckingham BA, et al. A randomized trial of a home system to reduce nocturnal hypoglycemia in type 1 diabetes. Diabetes Care. 2014;37(7):1885–1891. doi: 10.2337/dc13-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckingham BA, Raghinaru D, Cameron F, et al. Predictive low-glucose insulin suspension reduces duration of nocturnal hypoglycemia in children without increasing ketosis. Diabetes Care. 2015;38(7):1197–1204. doi: 10.2337/dc14-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spaic T, Driscoll M, Raghinaru D, et al. Predictive hyperglycemia and hypoglycemia minimization: in-home evaluation of safety, feasibility, and efficacy in overnight glucose control in type 1 diabetes. Diabetes Care. 2017;40(3):359–366. doi: 10.2337/dc16-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckingham BA, Cameron F, Calhoun P, et al. Outpatient safety assessment of an in-home predictive low-glucose suspend system with type 1 diabetes subjects at elevated risk of nocturnal hypoglycemia. Diabetes Technol Ther. 2013;15(8):622–627. doi: 10.1089/dia.2013.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron F, Wilson DM, Buckingham BA, et al. Inpatient studies of a Kalman-filter-based predictive pump shutoff algorithm. J Diabetes Sci Technol. 2012;6(5):1142–1147. doi: 10.1177/193229681200600519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The DCCT Research Group. The diabetes control and complications trial (DCCT) Design and methodologic considerations for the feasibility phase. Diabetes. 1986;35(5):530–545. [PubMed] [Google Scholar]
- 35.Standardization IOf ISO 15197:2013.: in vitro Diagnostic Test Systems—Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus. Geneva, Switzerland: International Organization for Standardization; 2013. [Google Scholar]
- 36.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA. The T1D exchange clinic registry. J Clin Endocrinol Metab. 2012;97(12):4383–4389. doi: 10.1210/jc.2012-1561. [DOI] [PubMed] [Google Scholar]
- 37.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38(6):971–978. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 38.Maahs DM, Hermann JM, DuBose SN, et al. Contrasting the clinical care and outcomes of 2622 children with type 1 diabetes less than 6 years of age in the United States T1D exchange and German/Austrian DPV registries. Diabetologia. 2014;57(8):1578–1585. doi: 10.1007/s00125-014-3272-2. [DOI] [PubMed] [Google Scholar]
- 39.Hatton DL, Canam C, Thorne S, Hughes AM. Parents’ perceptions of caring for an infant or toddler with diabetes. J Adv Nurs. 1995;22(3):569–577. doi: 10.1046/j.1365-2648.1995.22030569.x. [DOI] [PubMed] [Google Scholar]
- 40.Patton SR, Dolan LM, Smith LB, Thomas IH, Powers SW. Pediatric parenting stress and its relation to depressive symptoms and fear of hypoglycemia in parents of young children with type 1 diabetes mellitus. J Clin Psychol Med Settings. 2011;18(4):345–352. doi: 10.1007/s10880-011-9256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharifi A, De Bock MI, Jayawardene D, et al. Glycemia, treatment satisfaction, cognition, and sleep quality in adults and adolescents with type 1 diabetes when using a closed-loop system overnight versus sensor-augmented pump with low-glucose suspend function: a randomized crossover study. Diabetes Technol Ther. 2016;18(12):772–783. doi: 10.1089/dia.2016.0288. [DOI] [PubMed] [Google Scholar]
- 42.Barnard KD, Wysocki T, Allen JM, et al. Closing the loop overnight at home setting: psychosocial impact for adolescents with type 1 diabetes and their parents. BMJ Open Diabetes Res Ccare. 2014;2(1):e000025. doi: 10.1136/bmjdrc-2014-000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol. 2015;3(1):17–26. doi: 10.1016/S2213-8587(14)70226-8. [DOI] [PubMed] [Google Scholar]
- 44.Ly TT, Keenan DB, Roy A, et al. Automated overnight closed-loop control using a proportional-integral-derivative algorithm with insulin feedback in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Technol Ther. 2016;18(6):377–384. doi: 10.1089/dia.2015.0431. [DOI] [PubMed] [Google Scholar]
- 45.Hovorka R, Elleri D, Thabit H, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37(5):1204–1211. doi: 10.2337/dc13-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abraham MB, Nicholas JA, Ly TT, et al. Safety and efficacy of the predictive low glucose management system in the prevention of hypoglycaemia: protocol for randomised controlled home trial to evaluate the suspend before low function. BMJ Open. 2016;6(4):e011589. doi: 10.1136/bmjopen-2016-011589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Messer LH, Forlenza GP, Wadwa RP, et al. The dawn of automated insulin delivery: A new clinical framework to conceptualize insulin administration. Pediatr Diabetes. 2017 doi: 10.1111/pedi.12535. [epub ahead of print] https://doi.org/10.11/pedi.12535. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.