A CTLA-4-specific antibody, ipilimumab, is clinically used in anticancer immunotherapy, yet induces severe autoimmune side effects. Two papers by Du et al. published recently in Cell Research now suggest strategies for potentially uncoupling toxicity and efficacy of CTLA-4-targeting antibodies.
Although modern pharmacological agents are designed to target specific molecules and processes, it appears that they often (always?) mediate clinical efficacy by other or additional mechanisms than those they have been initially designed for. Ipilimumab, a clinically-used so-called immune checkpoint inhibitor (ICI) is probably not an exception to this rule. Ipilimumab has been FDA approved for the treatment of metastatic melanoma and might get clinical approval for additional oncological indications, most likely in combination with other ICIs targeting PD-1 or its ligand PD-L1.1 Ipilimumab is thought to bind to CTLA-4 expressed by T lymphocytes and to block its interaction with CTLA-4 ligands, in particular B7–1 (CD80) and B7–2 (CD86), expressed by antigen presenting cells (APCs), such as dendritic cells (DCs), within the tumor microenvironment or lymphoid organs.2 By blocking the interaction between CTLA-4 and B7–1 or B7–2, ipilimumab then would inhibit the transmission of inhibitory signals to CTLA-4-expressing T lymphocytes (Fig. 1a), thereby favoring desirable anticancer immune responses1 and also undesirable autoimmune side effects.3 Two papers published in Cell Research by Du et al. challenge this mode of action of ipilimumab and indeed suggest a strategy for uncoupling antitumor efficacy and pro-autoimmune toxicity.4,5
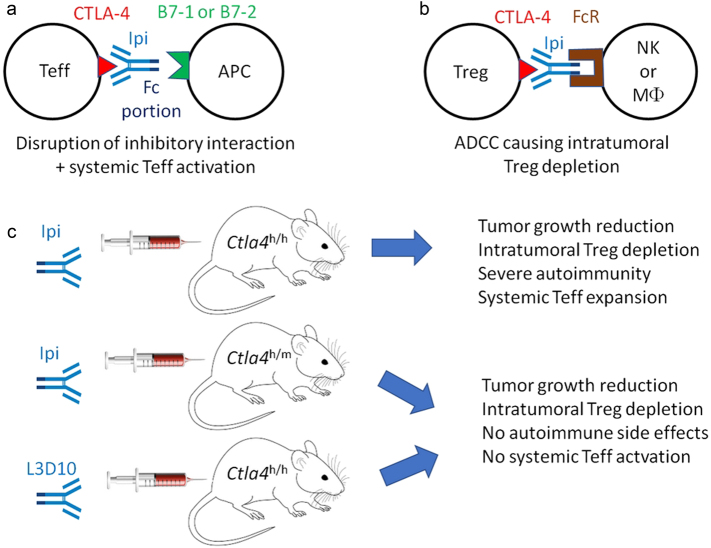
Fig. 1.
Mechanisms of action of CTLA-4-targeting antibodies. a Traditional view suggesting that optimal antibodies must block the interaction between the co-inhibitory receptor CTLA-4 on effector T cells (Teff) and its ligand B7–1 or B7–2 expressed on APCs. b Alternative view suggesting that CTLA-4 antibodies must eliminate Tregs from the tumor bed by bridging CTLA-4 expressed on the Treg surface to Fc receptors on NK cells or macrophages (MΦ) to mediate ADCC. c Uncoupling efficacy and toxicity of ipilimumab in mouse models of cancer. When administered to mice with a fully ‘humanized’ Ctla4h/h genotype, ipilimumab combined with PD-1-specific antibodies induces autoimmune disease, but no such effect is detected in heterozygous Ctla4h/m mice. In contrast, ipilimumab has similar anticancer effects against tumors implanted in Ctla4h/h and Ctla4h/m mice. Moreover, alternative anti-CTLA4 antibodies may mediate reduced pro-autoimmune effects yet have similar anticancer efficacy. Note that the effects shown in c are particularly strong when anti-CTLA4 antibodies are combined with antibodies targeting PD-1
In the first paper, Du et al.5 provide evidence that ipilimumab does not block the interaction between plastic-immobilized B7–1 or B7–2 and soluble CTLA-4 protein. This finding is at odds with a recent crystallographic study showing that ipilimumab contacts the front β-sheet of CTLA-4 and intersects with the CTLA-4:Β7 recognition surface, indicating that direct steric overlap between ipilimumab and the B7 ligands is a major mechanistic contributor to ipilimumab function.6 Du et al. use ‘humanized’ mice in which the mouse Ctla4 gene has been replaced by human CTLA4 to produce evidence suggesting that ipilimumab does not efficiently block the interaction between CTLA-4 and B7–1 or B7–2, whereas another anti-CTLA-4 antibody generated by their laboratory (L3D10) is able to do so and hence to upregulate B7–1 and B7–2 on DC in vivo.5 However, both ipilimumab and L3D10 display a similar efficacy in suppressing the growth of tumors implanted in immunocompetent mice in which both alleles of Ctla4 have been replaced by human CTLA4 (Ctla4h/h mice). An L3D10 antibody that has been genetically modified to suppress its capacity to interfere with the interaction between CTLA-4 and B7–1 or B7–2 fully conserves its antitumor efficacy in this setting, again underscoring the possibility that targeting CTLA-4 might have anticancer effects independently of the blockade of its interaction with B7–1 or B7–2.5
Importantly, several papers studying anti-mouse CTLA-4 antibodies have come up with the suggestion that ipilimumab (which is an IgG1 antibody) may actually act through its capacity to interact with CTLA-4 on regulatory T cells (Tregs) within the tumor bed (on which CTLA-4 expression is particularly high), thus facilitating Treg depletion by antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis (ADCP) by NK cells and macrophages, respectively (Fig. 1b). Thus, altering the Fc regions of existing anti-mouse CTLA-4 antibodies (to switch them to isotypes that cannot bind to Fc receptors (FcR) on natural killer (NK) cells and macrophages),7 as well as knockout of FcRIV or its signaling transducer Fcer1 from the host genome,8,9 prevent intratumoral Treg depletion and abolish the anticancer effects of CTLA-4-specific antibodies in mouse models. In vitro, ipilimumab can mediate ADCC when added to Treg cells that are co-cultured with human FcγRIIIA (CD16)-expressing monocytes.10 The antitumor effect of ipilimumab detectable in Ctla4h/h mice is lost upon injection of FcR-blocking antibodies, which prevent local Treg depletion by ipilimumab.5 Moreover, although anti-CTLA-4 antibodies are usually administered to patients or mice through the systemic route, local injection of such antibodies is fully competent in suppressing tumor growth in mouse models.11 That said, there are several important arguments against ADCC as the sole mechanism of ipilimumab’s clinical activity in patients. First, tremelimumab, a non-FcR binding IgG2 antibody specific for CTLA-4 does mediate anticancer effects in patients, along with a similar profile of adverse events.1 In addition, polymorphisms affecting FcRIII affinity for IgG1 do not affect the antimelanoma efficacy of ipilimumab.12 Hence, there may be major differences in the immunobiology of CTLA-4 targeting antibodies in mice and humans.
The clinical (systemic) use of ipilimumab is overshadowed by severe autoimmune side effects that are further exacerbated when ipilimumab is combined with other immunotherapeutic agents targeting the PD-1/PD-L1 interaction.3 Until recently, it has been thought that these side effects are ‘mechanism-related’ and hence difficult to be uncoupled from anticancer efficacy.2 Du et al.4 now unravel novel strategies for dissociating the antitumoral and pro-autoimmune effects of CTLA-4-targeting antibodies. For this, they developed a novel model of immune toxicity of ipilimumab and anti-PD1 antibodies, by injecting such agents repeatedly into very young Ctla4h/h mice (on days 10, 13, 16 and 19 after birth). This procedure leads to the induction of severe autoimmunity characterized by growth reduction, anemia and immune infiltration of all major organs. Du et al. then set out to compare the immunotoxicity and tumor growth-suppressive activity of ipilimumab in mice in which either both alleles of endogenous Ctla4 have been replaced by human CTLA4 (Ctla4h/h mice) or only one of the two alleles has been ‘humanized’ (heterozygous Ctla4h/m mice). Surprisingly, tumors implanted in Ctla4h/h and Ctla4h/m mice were equally susceptible to the anticancer effects of ipilimumab, correlating with a similar degree of local Treg depletion. However, the autoimmune effects of ipilimumab were radically different in Ctla4h/h and Ctla4h/m mice. While Ctla4h/h mice developed clinical and histological signs of severe autoimmunity correlating with the expansion of effector memory T cells (at the expense of naïve T cells) and autoreactive Foxp3–CD4+ T lymphocytes (more so than their Foxp3+CD4+ equivalents), Ctla4h/m mice were refractory to this side effect.4 This suggests that the mouse allele of Ctla4 (which is not engaged by the antibody) may transmit sufficient inhibitory signals to maintain immune homeostasis. Moreover, it implies that general T cell activation outside of the tumor is not required for the tumor growth-restraining effect of ipilimumab that remains intact in Ctla4h/m mice.
Another, hitherto unexplained observation concerns the lower pro-autoimmune effect of L3D10 compared to ipilimumab in Ctla4h/h mice.4 At this point, it remains to be determined whether these disparities may be explained by subtle differences in the interaction of these antibodies with CTLA-4, hence impacting intracellular CTLA-4-transmitted signals or interactions with CTLA-4-binding partners such as B7–1, B7–2 and perhaps other yet-to-be-elucidated ligands. These pending questions must be addressed in future studies to generate ever more efficient and less toxic immunotherapeutic agents.
References
- 1.Sharma P, Allison JP. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Annu. Rev. Immunol. 2016;34:539–573. doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 3.Fecher LA, Agarwala SS, Hodi FS, Weber JS. Oncologist. 2013;18:733–743. doi: 10.1634/theoncologist.2012-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du, X. et al. Cell Res.10.1038/s41422-018-0012-z (2018).
- 5.Du, X. et al. Cell Res.10.1038/s41422-018-0011-0 (2018).
- 6.Ramagopal UA, et al. Proc. Natl Acad. Sci. USA. 2017;114:E4223–E4232. doi: 10.1073/pnas.1617941114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selby MJ, et al. Cancer Immunol. Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 8.Simpson TR, et al. J. Exp. Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulliard Y, et al. J. Exp. Med. 2013;210:1685–1693. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romano E, et al. Proc. Natl Acad. Sci. USA. 2015;112:6140–6145. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marabelle A, et al. J. Clin. Invest. 2013;123:2447–2463. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A. J. Hematol. Oncol. 2013;6:1. doi: 10.1186/1756-8722-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]