Abstract
Disturbances in the gut microbiota composition are associated with chronic inflammatory diseases of the intestine and the liver. In a preliminary study, Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 could inhibit Escherichia coli growth and lipopolysaccharide-induced NF-κB activation linked to gut inflammation. Here, we investigated their effects on 2,4,6-trinitrobenzesulfonic acid (TNBS)-induced colitis and liver damage in mice. First, oral administration of LC27 or LC67 (1 × 109 CFU/mouse) inhibited TNBS-induced colon shortening [F(5,30) = 100.66, P < 0.05] and myeloperoxidase activity [F(5,30) = 56.48, P < 0.05]. These probiotics restored TNBS-induced disturbance of gut microbiota, leading to the suppression of Proteobacteria to Bacteroidetes ratio and fecal and blood lipopolysaccharide levels. Second, LC27 and LC67 inhibited TNBS-induced NF-κB activation, reversed TNBS-suppressed tight junction protein expression, and restored Th17/Treg balance. Also, treatment with LC27 or LC67 significantly decreased TNBS-induced alanine transaminase [ALT, F(5,30) = 3.50, P < 0.05] and aspartate transaminase [AST, F(5,30) = 12.81, P < 0.05] levels in the blood, as well as t-butylhydroperoxide-induced ALT and AST levels. Finally, the mixture of LC27 and LC67 (0.5 × 109 CFU/mouse, respectively) synergistically attenuated TNBS- or t-butylhydroperoxide-induced colitis and liver damage. The capability of LC27 and LC67 to reverse TNBS-mediated microbiota shift and damage signals suggests that these probiotics may synergistically attenuate colitis and liver injury by alleviating gut microbiota imbalance.
Introduction
The gut microbiota consists of populations of bacteria, viruses, fungi, and protozoa in the gastrointestinal tract of hosts1. The composition of gut microbiota is affected by intrinsic and extrinsic factors, such as diet, drugs, hormones, and stress2,3. The majority of gut microbiota consists of bacteria, which play important roles in the health status of hosts4. For example, experimental colitis can be established in conventional laboratory animals, however, it does not significantly progress in germ-free animals. The gastrointestinal tract is the first organ affected by gut microbiota-generated byproducts such as endotoxins (lipopolysaccharide [LPS]), as well as and bacterial DNA and metabolites, followed by the liver5,6. The overexpression of LPS, as well as disturbances in the gut microbiota, can lead to inflammation in the gastrointestinal tract (e.g., colitis), and disruption in gut wall integrity and permeability; this further accelerates the absorption of byproducts, including endotoxins, into the liver. Excessive exposure to LPS also causes inflammation in the liver, and accelerates non-alcoholic and alcoholic liver diseases5,7. Dysregulated responses of the innate and adaptive immune systems against the gut microbiota are essential for the progression of colitis and liver injury in susceptible individuals8,9. The innate immune response is activated by many immune cells including macrophages10. These immune cells detect gut bacteria and their byproducts such as endotoxins, present their antigens to T cells, and induce adaptive immune responses11. Adaptive responses, such as activation of Th17 cells and regulatory T cells (Tregs), are thought to play major roles in the pathogenesis of colitis12. Th17 cells are promoted by IL-23, which is secreted by macrophages, and produce interleukin (IL)-1713. Th17 cells and IL-17 are involved in colitis14. Tregs suppresses Th17 cell proliferation and differentiation by regulating the expression of anti-inflammatory cytokines such as IL-10 and TGFβ15,16. Previous studies have shown that IL-10-deficient mice spontaneously develop colitis17. Therefore, immune modulators that regulate macrophage activation and Th17 cell and Treg differentiation via the gut microbiota-liver axis could simultaneously inhibit colitis and prevent liver injury.
Numerous studies have shown that functional foods including probiotics are beneficial for reducing the risks of metabolic and degenerative diseases and promoting good health18,19. Of these, lactobacilli and bifidobacteria have been reported to be beneficial microbes, as they support the maintenance of gut microbiota homeostasis in humans and animals20,21. These probiotics restore balance to gut microbiota composition22, induce host immune systems23, and have anti-obesity24, anti-hepatoprotective25, and anti-colitic effects26. Bifidobacterium longum alleviates dextran sulfate sodium (DSS)-induced colitis by suppressing IL-17A responses27. Bifidobacterium infantis inhibits colitis in mice by inducing Treg differentiation28. Lactobacillus plantarum C29 ameliorates age-dependent colitis in aged mice via inhibition of the NF-κB signaling pathways29. Lactobacillus rhamnosus GG also attenuates ethanol-induced liver injury in mice by restoring the gastrointestinal barrier via regulation of tight junction proteins and miR122a expression30. Lactobacillus casei MYL01 attenuates ethanol-induced liver damage in vitro by regulating the expression of proinflammatory cytokines such as tumor necrosis factor (TNF)-α and anti-inflammatory cytokine such as IL-1031. However, the effects of probiotics against both colitis and liver injury have not been thoroughly investigated.
Therefore, to understand the simultaneous effects of probiotics against colitis and liver injury, we screened for probiotics that can potently suppress bacterial growth and LPS production in Escherichia coli, and inhibited NF-κB activation in LPS-stimulated macrophages in vitro. Here, two probiotics, Bifidobacterium longum LC67 isolated from human fecal microbiota, and Lactobacillus plantarum LC27 isolated from kimchi, were selected to investigate their effects against 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis and liver injury in mice.
Results
Effects of LC67 and LC27 on growth and LPS production of E. coli, and the innate and adaptive immune response in vitro
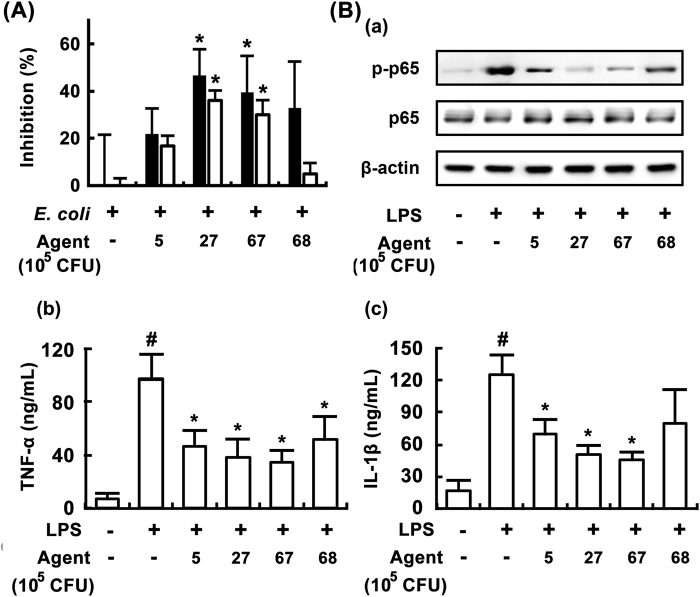
In order to screen lactobacilli and bifidobacteria that were capable of inhibiting growth and LPS production of E. coli, we isolated probiotics strains from the human gut microbiota and kimchi. Of the 100 isolated lactobacilli and bifidobacteria, LC27 and LC67 most potently exhibited the growth [F(4,15) = 4.12, P < 0.05] and LPS production [F(4,15) = 30.23, P < 0.05), followed by LC68 and LC5 (Fig. 1A). The other isolated bacteria did not inhibit them (Supplement Fig. S1). LC5, LC27, LC67, and LC68 also significantly inhibited NF-κB activation as well as TNF-α [F(5,18) = 13.43, P < 0.05] and IL-1β expression [F(5,18) = 11.36, P < 0.05] in LPS-stimulated macrophages (Fig. 1B). However, no cytotoxic effects against peritoneal macrophages under the experimental conditions were observed (data not shown).
Figure 1.
Inhibitory effects of bifidobacteria and lactobacilli isolated from human fecal microbiota and kimchi on the LPS production and growth of Escherichia coli and the NF-κB activation in LPS-stimulated macrophages. (A) Effects on bifidobacteria and lactobacilli on LPS production and growth of E. coli. Lactobacillus brevis L5, L. plantarum LC27, Bifidobacterium longum LC67, and B. longum LC68 (1 × 106 CFU/mL) was anaerobically cultured in the presence of E. coli (1 × 106 CFU/mL) in GAM (10 mL) and measured the number of E. coli growth (black bar) and level of LPS (white bar). The number of E. coli alone cultured for 24 h was 3.8 × 109 CFU/mL and its LPS level was 8.2 ng/mL. (B) Effects on LPS-stimulated NF-κB (a) and TNF-α (b) and IL-1β expression (c). Peritoneal macrophages (0.5 × 106 cells) were treated with 100 ng/mL of LPS in the absence or presence of probiotics (1 × 103 or 1 × 105 CFU/well) for 90 min (for NF-κB) or 24 h (for TNF-α and IL-1β). All data are shown as the mean ± SD (n = 4). #p < 0.05 vs. normal control group. *p < 0.05 vs. group treated with LPS alone.
Next, we evaluated the anti-colitic effects of these probiotics in mice with TNBS-induced colitis (Fig. 2). Of these, LC27 and LC67 most potently inhibited TNBS-induced colitis markers, such as reduction in colon length [F(6,35) = 21.36, P < 0.05] and macroscopic score [F(6,35) = 21.54, P < 0.05]. LC27 and LC67 were identified as Lactobacillus plantarum and Bifidobacterium longum, respectively, based on results of Gram staining, sugar utilization testing (API 50 CHL Kit), and 16 S rRNA sequencing (ABI 3730XL DNA analysis).
Figure 2.
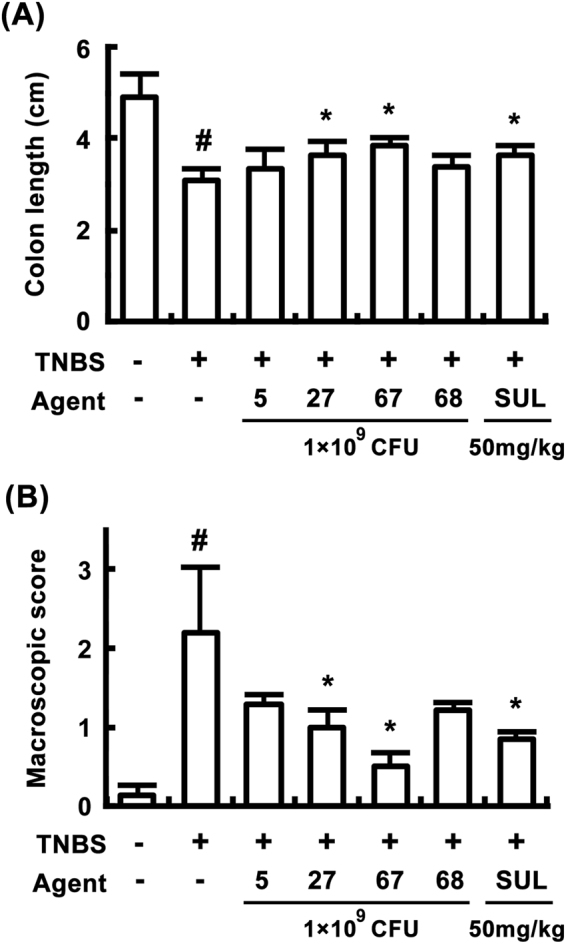
Anti-colitic effects of bifidobacteria and lactobacilli in mice. (A) Effects on colon length. (B) Effects on macroscopic score. TNBS, except in the normal control group, was intrarectally administered to mice and test agents [saline, LC5, LC27, LC67, LC68 (2 × 109 CFU/mouse), or sulfasalazine (SUL; 50 mg/kg)] were orally administered for 3 days. All data are shown as the mean ± SD (n = 6). #p < 0.05 vs. normal control group. *p < 0.05 vs. group treated with TNBS alone.
Anti-inflammatory effects of LC27 and LC67 in mice with TNBS-induced colitis
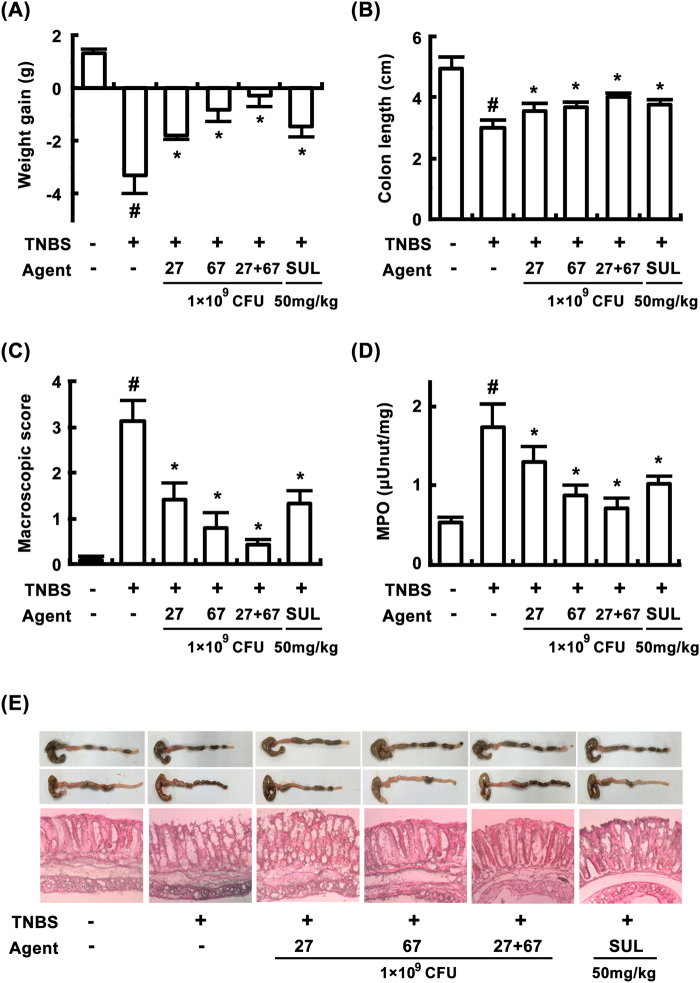
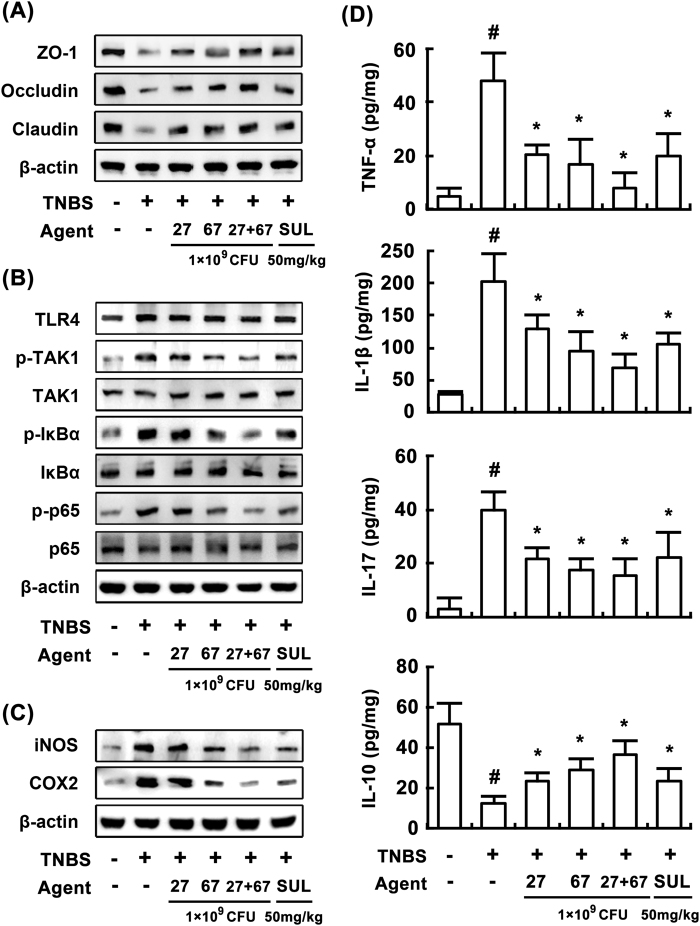
In order to understand the anti-colitic properties of LC27 and LC67, we investigated the anti-inflammatory effects of LC27 and LC67 in mice with TNBS-induced colitis. TNBS caused severe inflammation in the colon, and resulted in colon shortening, increased myeloperoxidase activity, edema, and epithelial cell disruption by ulcerations (Fig. 3). Treatment with LC27 or LC67 (1 × 109 CFU/mouse) inhibited TNBS-induced colon shortening [F(5,30) = 100.66, P < 0.05] and myeloperoxidase activity [F(5,30) = 56.48, P < 0.05] and suppressed edema and epithelial cell disruption in the colon (Fig. 3B,D,E). TNBS treatment also suppressed the expression of the colonic tight junction proteins such as claudin-1, occludin, and zonula occludens (ZO)-1 (Fig. 4A), whereas treatment with probiotics restored expression of tight junction proteins. Treatment with LC27 or LC67 inhibited TNBS-induced NF-κB activation, TAK1 and IκBα phosphorylation, as well as COX-2 and iNOS expression (Fig. 4B,C). In addition, they inhibited TNBS-induced expression of IL-1β [F(5,30) = 28.31, P < 0.05], IL-17 [F(5,30) = 21.84, P < 0.05], and TNF-α [F(5,30) = 29.49, P < 0.05], and increased TNBS-suppressed IL-10 expression [F(5,30) = 28.47, P < 0.05] (Fig. 4D).
Figure 3.
Effects of LC27, LC67, and PM on body weight (A), colon length (B), macroscopic disease (C), colonic myeloperoxidase (MPO) activity (D), and histological examination (E) in the colon of mice with TNBS-induced colitis. TNBS, except in the normal control group, was intrarectally administered to mice and test agents [saline, LC5, LC27, LC67, LC68 (2 × 109 CFU/mouse), or sulfasalazine (SUL; 50 mg/kg)] were orally administered for 3 days. The mice were sacrificed 18 h after the final administration of test agents. All data are shown as the mean ± SD (n = 6). #p < 0.05 vs. normal control group. *p < 0.05 vs. group treated with TNBS alone.
Figure 4.
Effects of LC27, LC67, and PM on the expression of tight junction proteins (A), activation of NF-κB (B), iNOS and COX-2 (C), and expression of inflammatory cytokines (D) in mice with TNBS-induced colitis. TNBS, except in the normal control group, was intrarectally administered to mice and test agents [saline, LC5, LC27, LC67, LC68 (2 × 109 CFU/mouse), or sulfasalazine (SUL; 50 mg/kg)] were orally administered for 3 days. Cytokines were determined by ELISA. iNOS, COX-2, and NF-κB signaling molecules were determined by immunoblotting. All values are shown as the mean ± SD (n = 6). #p < 0.05 vs. normal control group. *p < 0.05 vs. group treated with TNBS alone.
To understand whether the LC27 and LC67 mixture has synergistic or antagonistic effects against colitis in vivo, we treated mice with LC27 (0.5 × 109 CFU/mouse) and LC67 (0.5 × 109 CFU/mouse) in combination, and compared the result to that of mice treated with LC27 or LC67 alone. Treatment with LC27 and LC67 mixture (PM) synergistically attenuated TNBS-induced colon shortening [F(5,30) = 100.66, P < 0.05] and myeloperoxidase activity [F(5,30) = 56.48, P < 0.05], and increased expression of tight junction proteins. LC27 and LC67 also synergistically inhibited NF-κB activation and reduced expressions of iNOS, COX-2, TNF-α, and IL-1β.
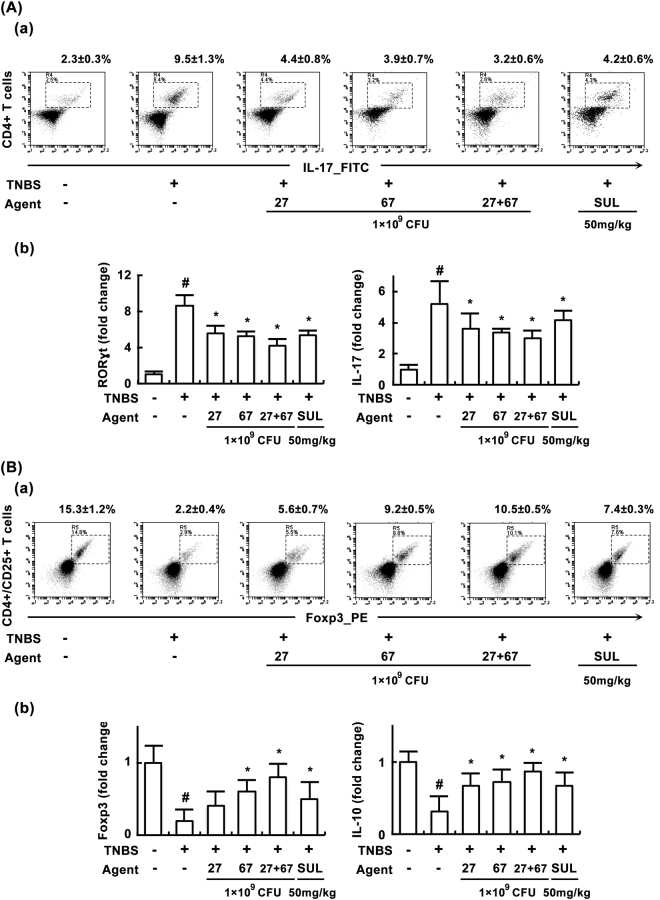
In order to understand the effects of probiotics on the Th cell differentiation, we examined the effect of LC27 and LC67 on the differentiation of Th17 cells and Tregs in mice with TNBS-induced colitis (Fig. 5A,B). Treatment with TNBS significantly increased and decreased Th17 and Treg differentiation in the lamina propria of colons, respectively. Treatment with LC27 or LC67 inhibited TNBS-induced Th17 cell differentiation (Fig. 5A), and increased Treg cell differentiation (Fig. 5B). qPCR anlaysis revealed that TNBS significantly upregulated RORγt and IL-17 expression, and suppressed Foxp3 and IL-10 expression. However, treatment with LC27 or LC67 inhibited the TNBS-induced expression of RORγt [F(5,30) = 124.83, P < 0.05] and IL-17 [F(5,30) = 19.10, P < 0.05], and increased the TNBS-suppressed expression of Foxp3 [F(5,30) = 12.32, P < 0.05] and IL-10 [F(5,30) = 11.64, P < 0.05]. As expected, PM down-regulated TNBS-induced Th17 cell differentiation and RORγt and IL-17 expressions, and upregulated Treg cell differentiation and FoxP3 and IL-10 expressions.
Figure 5.
Effects of LC27, LC67, and PM on Th17 and Treg differentiation in the colon of mice with TNBS-induced colitis. (A) Effects on Th17 cell differentiation. (a) Effects on Th17 cell differentiation assessed by FACS. (b) Effects on ROTγt and IL-17 expression assessed by qRT-PCR. Cells isolated from the lamina propria were stained for cell surface CD4 and intracellular IL-17 and analyzed by flow cytometry. (B) Effects on Th17 cell differentiation. (a) Effects on Th17 cell differentiation assessed by FACS. (b) Effects on Foxp3 and IL-10 expression assessed by qRT-PCR. Cells isolated from the lamina propria were stained for the cell surface CD4 and CD25 and intracellular Foxp3 and analyzed by flow cytometry. TNBS, except in the normal control group, was intrarectally administered to mice and test agents [saline, LC5, LC27, LC67, LC68 (2 × 109 CFU/mouse), or sulfasalazine (SUL; 50 mg/kg)] were orally administered for 3 days. All data are shown as the mean ± SD (n = 6). All values are shown as the mean ± SD. (n = 6). #p < 0.05 vs. normal control group. *p < 0.05 vs. group treated with TNBS alone.
Ameliorating effects of LC27, LC67, and PM against TNBS-induced liver injury in mice
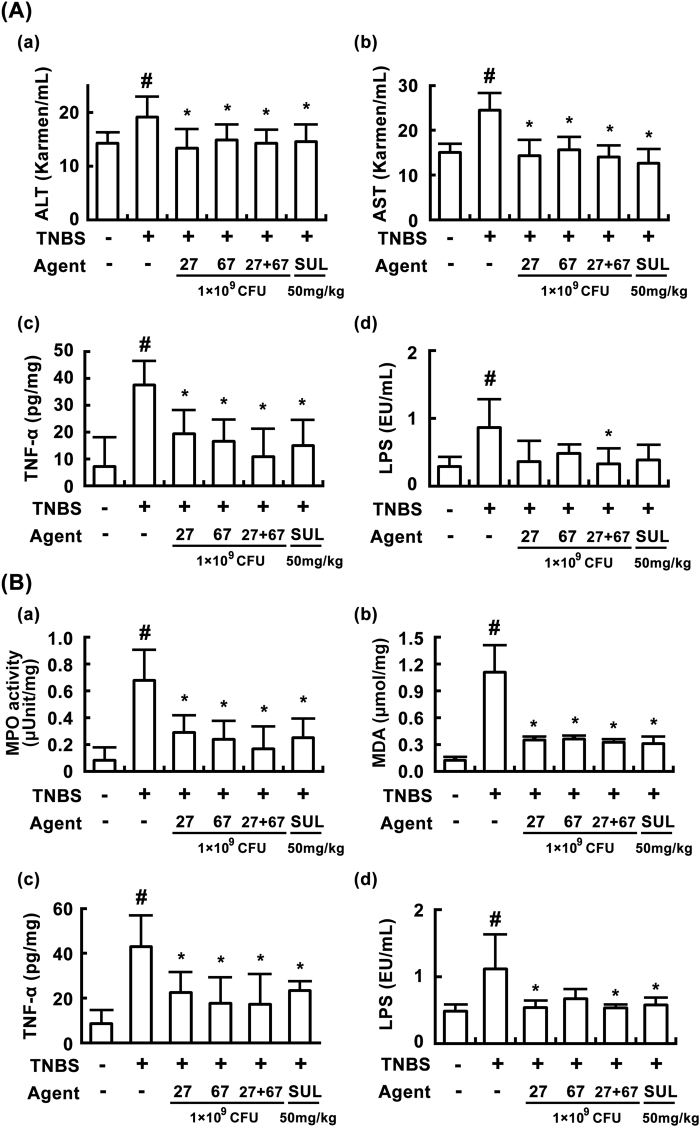
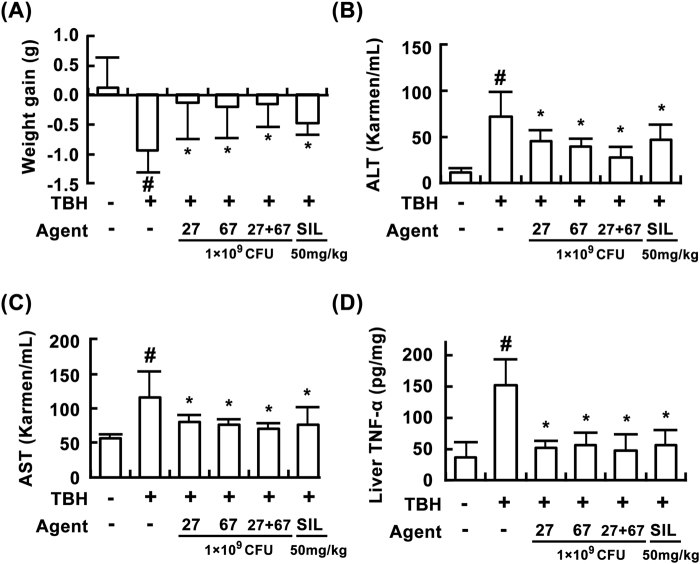
In order to investigate whether TNBS treatment could induce liver damage in mice, we measured biomarkers for the liver damage in mice with TNBS-induced colitis. TNBS treatment increased levels of alanine transaminase (ALT) and aspartate transaminase (AST) in the blood, as well as levels of TNF-α in the blood and the liver: it caused liver damage (Fig. 6A). TNBS treatment also increased myeloperoxidase activity and MDA level in the liver (Fig. 6B). However, oral administration of LC27, LC67, or PM for 3 days significantly decreased TNBS-induced ALT [F(5,30) = 3.50, P < 0.05], AST [F(5,30) = 12.81, P < 0.05], TNF-α [F(5,30) = 8.45, P < 0.05], and LPS level [F(5,30) = 4.29, P < 0.05], in the blood, as well as myeloperoxidase activity and MDA and TNF-α levels in the liver (Fig. 6A,B). Additionally, these probiotics attenuated body weight (F = 4.07, P < 0.05), blood ALT (F = 10.97, P < 0.05), AST (F = 6.45, P < 0.05), and liver TNF-α levels (F = 16.15, P < 0.05) in mice with tert-butyl hydroxyperoxide (t-BHP)-induced liver injury (Fig. 7).
Figure 6.
Effects of LC27, LC67, and PM on TNBS-induced liver injury in mice. (A) Effects on blood ALT (a), AST (b), TNF-α (c), and LPS levels (d). (B) Effects on liver myeloperoxidase (MPO) (a), malondialdehyde (MDA) (b), TNF-α (c), and LPS levels (d). TNBS, except in the normal control group, was intrarectally administered to mice and test agents [saline, LC5, LC27, LC67, LC68 (2 × 109 CFU/mouse), or sulfasalazine (SUL; 50 mg/kg)] were orally administered for 3 days. LPS was determined using LAL assay kit. All values are shown as the mean ± SD (n = 6). #p < 0.05 vs. normal control group. *p < 0.05 vs. group treated with TNBS alone.
Figure 7.
Effects of LC27, LC67, and PM on t-BHP-induced liver injury in mice. Effects on body weight (A), blood ALT (B), AST (C), and liver TNF-α levels (D). t-BHP, except in the normal control group, was intraperitoneally injected to mice and test agents [saline, LC27 (2 × 109 CFU/mouse), LC67 (2 × 109 CFU/mouse), PM (the mixture of LC27 and LC67 [1:1]; 1 × 109 CFU/mouse), or silymarin (SIL, 50 mg/kg)] were orally administered for 3 days. All values are shown as the mean ± SD (n = 6). #p < 0.05 vs. normal control group. *p < 0.05 vs. group treated with TNBS alone.
Effects of LC27, LC67, and PM on TNBS-induced gut microbiota disturbance in mice
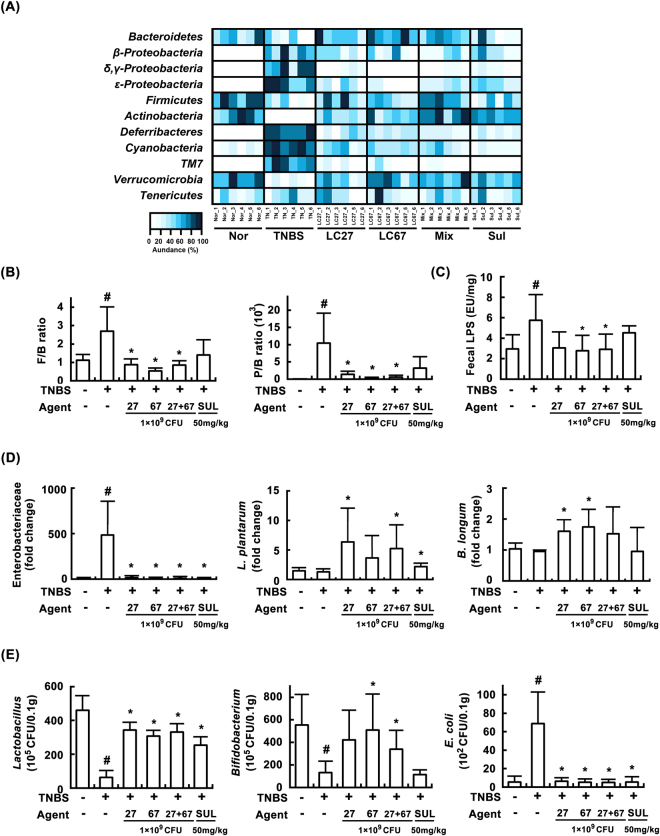
Previous studies have shown that TNBS treatment increases the ratio of Firmicutes/Bacteroidetes, and elevates LPS production in the gut microbiota32. Moreover, the overexpression of LPS in the gut microbiota leads to gastrointestinal inflammation24 and excessive exposure to LPS causes inflammation in the liver5,7. Therefore, we investigated the effects of LC27, LC67, and PM on the gut microbiota composition and LPS production in mice with TNBS-induced colitis and liver injury (Fig. 8). TNBS treatment increased the ratio of Firmicutes/Bacteroidetes, like previously reported32. Furthermore, TNBS treatment increased the number of Proteobacteria but reduced the number of Bacteroidetes, resulting in a reduced Proteobacteria/Bacteroidetes ratio [F(5,30) = 5.22, P < 0.05] (Fig. 8B). Treatment with LC27, LC67, or PM significantly inhibited the TNBS-induced Proteobacteria level, and increased the TNBS-suppressed Bacteroidetes level (Fig. 8A). Moreover, TNBS treatment significantly increased the LPS level [F(5,30) = 3.85, P < 0.05] in the colonic fluid and blood of mice, whereas LC27, LC67, or their mixture significantly decreased the TNBS-induced LPS production (Fig. 8C). TNBS treatment also increased Enterobacteriaceae levels including Escherichia coli. However, these probiotics reversed the suppression of lactobacilli [F(5,30) = 31.36, P < 0.05] and bifidobacteria [F(5,30) = 4.08, P < 0.05] by TNBS, and reduced the amount of Enterobacteriaceae and Escherichia coli [F(5,30) = 18.34, P < 0.05], which belong to phylum Proteobacteria (Fig. 8B,C). Furthermore, these treatments increased the populations of Lactobacillus plantarum and Bifidobacterium longum. Collectively, these data suggest that LC27 and LC67 can alleviate TNBS-induced gut microbiota imbalance.
Figure 8.
Effects of LC27, LC67, and PM on gut microbiota composition in mice with TNBS-induced colitis and liver injury. (A) Effects on gut microbiota, assessed by qPCR. (B) Effects on the ratio of Firmicutes to Bacteroidetes (F/B) and Proteobacteria to Bacteroidetes ratio (P/B). (C) Effects on gut microbiota LPS levels, assessed by LAL assay kit. (D) Effects on the populations of Enterobacteriaceae, Lactobacillus plantarum, and Bifidobacterium longum, assessed by qPCR. (E) Effects on the levels of Lactobacillus sp., Bifidobacterium sp., E. coli, assessed by the culture of selective media. Test agents and saline were orally administered for 3 days after TNBS treatment. TNBS, except in the normal control group, was intrarectally administered to mice and test agents [saline, LC27, LC67, PM (1 × 109 CFU/mouse), or sulfasalazine (SS; 50 mg/kg)] were orally administered for 3 days. All values are shown as mean ± SD (n = 6). #p < 0.05 vs. normal control group. *p < 0.05 vs. group treated with TNBS alone.
Discussion
Acute and chronic inflammations are the body’s response to injuries and infections33,34. Acute inflammation is a normal and beneficial response to injury, whereas chronic inflammation is persistent and excessive. Inflammatory reactions in the gastrointestinal tract can be activated by a variety of stresses such as excessive ROS, alcohol, and LPS owing to disturbance of gut microbiota34. Exposure to alcohol or high fat diet causes dysbiosis via alteration in the gut microbiota, including an increase in Proteobacteria and a decrease in Bacteroidetes5. An increase in the Gram-negative Proteobacteria reduces the expression of cellular tight junctions and increases gut permeability through overexpression of LPS, resulting in increased absorption of LPS into the blood. LPS, a major driver of systemic inflammation35, increases blood TNF-α levels via Toll-like receptor 4-associated NF-κB signaling pathway to cause inflammation, even though blood TNF-α level is barely detectable in mice in absence of any stimuli or treatment36. Therefore, chronic inflammatory responses lead to progressive damage to the body, resulting in a variety of chronic inflammatory diseases, such as colitis, hepatitis, and rheumatoid arthritis37. To regulate these inflammatory diseases, probiotics may be used to control TNF-α expression via regulation of NF-κB in immune cells, as well as to inhibit LPS production induced by the gut microbiota imbalance.
In the present study, we found that LC27 and LC67 inhibited TNBS-induced colitis via inhibition of NF-κB in macrophages and epithelial cells, similar to previous reports38. Furthermore, treatment with these probiotics restored TNBS-disturbed gut microbiota composition: they suppressed the population of Enterobacteriaceae, particularly Escherichia coli, which is belonging to Proteobacteria, and gut microbiota LPS levels and increased the populations of lactobacilli and bifidobacteria, including Lactobacillus plantarum and Bifidobacterium longum. Additionally, it has been shown that Lactobacillus brevis G-101 inhibits TNF-α and IL-1β expression in macrophages, leading to attenuation of colitis39. In addition, Bifidobacterium longum CH57 was found to attenuate colitis by inhibiting NF-κB signaling pathways and TNF-α expression. Lactobacillus plantarum C29 also ameliorates colitis in aged mice by inhibiting NF-κB signaling29. Another study has demonstrated that Lactobacillus casei DN-114001 inhibits DSS-induced colitis by inhibiting gut membrane permeability and NF-κB activation40. Furthermore, some probiotics were also shown to restore composition of gut microbiota and fecal LPS level in mice with colitis29,40. These results suggest that probiotics can inhibit NF-κB activation and restore disrupted gut microbiota composition to attenuate colitis. Furthermore, treatment with these probiotics significantly suppressed blood LPS and TNF-α levels in mice with TNBS-induced colitis. These treatments also reduced liver MDA and myeloperoxidase activity, and blood AST and ALT levels, resulting in attenuation of TNBS-induced liver injury in mice. In spite of short-term treatment with these probiotics, their effects were comparable to those of sulfasalazine, a positive agent. Another study showed that L. rhamnosus CCFM1107 reduces oxidative stress and restores intestinal flora in ethanol-treated mice, which ameliorates liver injury41. In addition, L. rhamnosus GG reestablishes the gastrointestinal barrier via the suppression of tight junction proteins and miR122a in mice, leading to the alleviation of ethanol-induced liver injury30. Lastly, L. acidophilus CSG exhibited hepatoprotective effects in mice during CCl4 and t-BHP-induced oxidative stress by restoring the disturbed gut microbiota26. These results suggest that probiotics such as LC27 and LC67 are effective against colitis, and could attenuate oxidative stress-induced liver injury and colitis by inhibiting NF-κB activation, scavenging ROS, and alleviating gut microbiota imbalance.
Activated macrophages secrete IL-23, which induces expression of proinflammatory cytokines such as TNF-α and IL-6, and also promotes the differentiation and activation of Th17 cells10,33,34. Th17 cells suppress Treg the differentiation, resulting in the onset of chronic inflammatory diseases such as colitis13. Conversely, activated Tregs inhibit Th17 cell the differentiation via secretion of IL-10 and TGF-β, which results in attenuation of chronic colitis. Therefore, the Th17/Treg cell balance is important for the development of colitis.
In the present study, we found that LC27 and LC67 inhibited Th17 cell differentiation and RORγt expression, and enhanced Treg differentiation and Foxp3 expression, leading to attenuation of colitis and liver injury. Furthermore, an increase in IL-10 expression was also observed both in vitro and in vivo. It has been previously shown that Lactobacillus brevis CH23 restores Th17/Treg balance via regulation of the transcription factors Foxp3 and RORγt, as well as the cytokines IL-17 and IL-10, resulting in recovery from colitis28. Similarly, Lactobacillus casei MYL01 attenuates ethanol-induced liver damage in vitro via regulation of TNF-α and IL-10 expression31. Lactobacillus casei also suppresses the development of rheumatoid arthritis by upregulating IL-10 expression42. Bifidobacterium longum ameliorates inflammatory diseases by suppressing IL-17 expression27. These results suggest that LC23 and LC67 may inhibit colitis and liver injury by correcting the imbalance of Th17/Treg cells involved in adaptive immunity, via regulation of innate immune cells and gut microbiota composition, and by increasing the expression of colonic tight junction proteins.
Although commercial probiotic products contain a combination of various probiotics such as Lactobacilli, Bifidobacteria, and Streptococci the combined effects of these probiotic mixtures have not been thoroughly investigated20. For example, Bifidobacterium longum CH57 and Lactobacillus brevis CH23 synergistically inhibit colitis by inhibiting macrophage activation and restoring Th17/Treg balance26. In the present study, the LC27 and LC67 mixture PM, synergistically rather than additively, attenuated TNBS-induced colitis such as colon shortening, myeloperoxidase activity, TNF-α and IL-10 expression, and Th17 and Treg cell differentiation. However, TNBS-induced liver damage was significantly attenuated by treatment with PM. This involved correcting the Th17/Treg imbalance via regulation of innate immune cells and gut microbiota composition and increasing the expression of colonic tight junction proteins. Furthermore, these probiotics also attenuated t-BHP-induced liver injury. These results suggest that synergistic probiotic products containing a combination of bacterial species, such as PM, may be more effective in protecting diseased caused by imbalance of the gut microbiota.
In conclusion, Lactobacillus plantarum LC27, isolated from kimchi, suppressed gut bacterial LPS production and NF-κB activation in macrophages. Bifidobacterium longum LC67, isolated from the human gut microbiota, inhibited gut bacterial LPS production and differentiation of splenic T cells into Th17 cells. It also increased Treg cell differentiation via up-regulation of IL-10 and Foxp3 expression. These probiotics synergistically attenuated TNBS-induced colitis and liver injury as well as t-BHP-induced liver injury by correcting the gut microbiota composition and inhibiting inflammatory responses involved in innate and adaptive immunity. Collectively, our study supports that LC27 and LC67 could be effective tools to control colitis and liver damage induced by altered gut microbiota landscape.
Materials and Methods
Materials
LPS purified from Escherichia coli O111:B4, TNBS, t-BHP, collagenase type VIII, RPMI 1640 were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Antibodies for COX-2, ERK, p-ERK, IκBα, p- IκBα, IRAK1, p-IRAK1, iNOS, p65, p-p65, TAK1 and p-TAK1 were purchased from Cell Signaling Technology (Beverly, MA, U.S.A.). Radioimmuno-precipitation assay (RIPA) buffer, tetramethyl benzidine was purchased from Sigma (St Louis, MO, U.S.A.). ELISA kits for IL-1β, IL-6, IL-10, and TNF-α were purchased from R&D Systems (Minneapolis, MN, U.S.A.). mRNA isolation kit was purchased from Qiagen (Hilden, Germany). A diazo-coupled limulus amoebocyte lysate (LAL) assay kit was purchased from Cape Cod Inc. (E. Falmouth, MA, USA). Pan T Cell Isolation Kit II was purchased from MiltenyiBiotec GmbH (Bergisch Gladbach, Germany). Anti-CD28, anti-CD3, recombinant IL-6, and recombinant TGF-β were purchased from BioGems International Inc. (Westlake Village, CA, U.S.A.). Fetal bovine serum (FBS) and heat-inactivated fetal calf serum (FCS) purchased from Panbiotech GmbH (Aidenbach, Germany). de Man, Rogosa and Sharpe (MRS) medium for probiotics was purchased from BD (Sparks, MD, USA). General anaerobic medium (GAM) for probiotics and other bacteria were purchased from Nissui Pharmaceutical Co (Tokyo, Japan). Other chemicals used were of the highest grade available.
Probiotics preparation
Probiotics including Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 were cultured in general media for probiotics. Briefly, these probiotics were grown to the density of 2 - 4 × 109 CFU/mL and centrifuged to harvest cells. The collected cells (1 × 1010 CFU/mL) were suspended in phosphate buffered saline (inactivated at 80 °C for 30 min, used for in vitro experiments) or 1% glucose (for in vivo experiments).
Animals
Male C57BL/6 (21–23 g, 6-weeks old) were supplied from RaonBio Inc. (Seoul, Korea). All animals were housed in wire cages at 20–22 °C and 50 ± 10% humidity, fed standard laboratory chow and water ad libitum. After the acclimation for 7 days, mice were used in experiments.
All animal experiments were approved by the Committee for the Care and Use of Laboratory Animals in the Kyung Hee University and performed in accordance with the Kyung Hee University Guidelines for Laboratory Animals Care and Usage (IRB No., KHUASP(SE)-16-049).
Preparation of macrophages
Macrophages were prepared according to the method of Jeong et al.29. Mice were intraperitoneally injected with 4% (w/v) thioglycolate solution (2 mL) and killed 4 days after the injection29. Cells were removed with RPMI 1640 in the peritoneal cavity, centrifuged (300 × g, 10 min), and washed with RPMI 1640 twice. Collected cells (1.5 × 106 cells/well) were incubated in RAF at 37 °C for 20 h and washed three times. The attached cells were used as macrophages. To evaluate the anti-inflammatory effect of probiotics, macrophages (1 × 106 cells/well) were treated with LPS (100 ng/mL) in the absence or presence of each probiotic (1 × 103 or 1 × 105 CFU/mL) for 90 min (for p65 and p-p65) or 24 h (for TNF-α and IL-1β).
Preparation of mice with experimental colitis and liver injury
First, mice were randomly divided into 7 groups: normal control, TNBS-induced colitic control groups treated with vehicle, four probiotics (1 × 109 CFU), or sulfasalazine (50 mg/kg). Each group consisted of six mice.
Second, mice were randomly divided into 6 groups: normal control, TNBS-induced colitic control groups treated with vehicle, LC27 (1 × 109 CFU/mouse), LC67 (2 × 109 CFU/mouse), and their mixture (1:1, each 1 × 109 CFU/mouse), or sulfasalazine (50 mg/kg). Each group consisted of six mice. Colitis was induced by the intrarectal injection of 2.5% (w/v) TNBS solution (100 μL, dissolved in 50% ethanol) into the colon of mice anesthetized with ether43. Normal control group was treated with vehicle alone instead of TNBS. To entirely distribute TNBS within the colon, mice were held in a vertical position for 30 s after the TNBS injection. Test agents (probiotics or sulfasalazine dissolved in 1% glucose) were orally administered once a day for 3 days after TNBS treatment. Mice were killed 18 h after the final administration of test agents. Normal control group was treated with vehicle alone instead of test agents. Whole-blood samples were immediately withdrawn from carotid artery. Sera were prepared by centrifugation (10 min, 250 × g) and ALT, AST, and TNF-α levels were then determined according to the method of Lee et al.44. The colon was removed and opened longitudinally. The colitis grade was macroscopically scored (0, no ulcer and no inflammation; 1, no ulceration and local hyperemia; 2, ulceration with hyperemia; 3, ulceration and inflammation at one site only; 4, two or more sites of ulceration and inflammation; 5, ulceration extending more than 2 cm). The colons were gently washed by ice-cold phosphate buffered saline (PBS) and were stored at −80 °C until used in the experiment for, myeloperoxidase activity assay, ELISA, and immunoblotting.
Preparation of mice with t-BHP-induced hepatic injury
Mice with t-BHP-induced hepatic injury were prepared according to the method of Lee et al.44. Firs, mice were randomly divided into 6 groups: normal control, t-BHP-treated control groups treated with vehicle, probiotics (LC27, LC67, or their mixture (1:1): 1 × 109 CFU/mouse), or silymarin (50 mg/kg). Each group consisted of six mice. Mice were intraperitoneally treated with 1.5 mmol t-BHP/kg. Test agents were orally administered once a day for 3 days from 24 h after treatment with t-BHP. Control group was given with saline instead of the sample compounds. Blood samples were collected 18 h after the final administration of test agents by cardiac puncture under ether anesthesia. Sera were obtained by centrifugation (1000 × g, 15 min) and ALT, AST, and TNF-α levels were then determined according to the method of Lee et al.44.
Histological examination
Colons or livers were fixed in 50 mM phosphate buffer (pH 7.4) containing 4% paraformaldehyde overnight, frozen in optimal cutting temperature solution, cut into 15 μm section using a cryostat, stained with hematoxylin-eosin, and then observed under a light microscopy43.
Assay of myeloperoxidase (MPO) activity
Mouse colon or liver tissues were homogenized in 10 mM potassium phosphate buffer (pH 7.0) containing 0.5% hexadecyl trimethyl ammonium bromide, and centrifuged for 10 min at 20,000 × g at 4 °C44. The resulting supernatants (50 μL) were added to the reaction mixture containing 0.1 mM H2O2 and 1.6 mM tetramethyl benzidine preincubated at 37 °C for 2 min, and sequentially monitored the absorbance (650 nm) at 37 °C for 5 min. Myeloperoxidase activity was calculated as the quantity of enzyme degrading 1 μmol/mL of peroxide, and expressed in unit/mg protein protein43. The amount of protein was determined by the method of Bradford.
Quantitative real time - polymerase chain reaction (qPCR)
Reverse transcription was performed with 2 μg of total RNA isolated from the colon. Real time PCR for IL-10, IL-17, Foxp3, RAR-related orphan receptor gamma t (RORγt), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was performed as described previously43, utilizing Qiagen thermal cycler, which used SYBER premix agents. Thermal cycling conditions were as follows: activation of DNA polymerase at 95 °C for 5 min, followed by 36 cycles of denaturation and amplification at 95 °C for 5 s and 63 °C for 30 s, respectively. The normalized expression of the assayed genes, with respect to GAPDH, was computed for all samples by using the Microsoft Excel data spreadsheet. Primers were used as follows43: IL-10 forward, 5′-ATG CTG CCT GCT CTT ACT GAC TG-3′, reverse, 5′-CCC AAG TAA CCC TTA AAG TCC TGC-3′; IL-17 forward, 5′-TTT AAC TCC CTT GGC GCA AAA-3′ reverse, 5′-CTT TCC CTC CGC ATT GAC AC-3′; RORγt forward, 5′-ACAGCCACTGCATTCCCA GTTT-3′, reverse, 5′- TCTCGGAAGGACTTGCAGACAT-3′; Foxp3 forsward, 5′-CCC ATC CCC AGG AGT CTT-3′, reverse, 5′-ACC ATG ACT AGG GGC ACT GTA-3′; and GAPDH forward, 5′-TGC AGT GGC AAA GTG GAG AT-3′, reverse, 5′-TTT GCC GTG AGT GGA GTC AT-3′.
Real time PCR for Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes was performed with 100 ng of total DNA isolated from the colon fluid, utilizing Takara thermal cycler, which used SYBER premix agents45. Thermal cycling conditions were as follows: activation of DNA polymerase for 30 s at 95 °C, followed by 35 cycles of denaturation and amplification at 95 °C for 5 s and 63 °C for 30 s, respectively. The normalized expression of the assayed genes, with respect to bacterial rRNA, was computed for all samples using the Microsoft Excel data spreadsheet. Primers were used as follows45,46: Firmicutes forward, 5′-GGA GYA TGT GGT TTA ATT CGA AGC A-3′, reverse, 5′-AGC TGA CGA CAA CCA TGC AC-3′; Bacteroidetes forward, 5′-GTT TAA TTC GAT GAT ACG CGA G-3′ reverse, 5′-TTA ASC CGA CAC CTC ACG G-3′; Actinobacteria forward, 5′-TGT AGC GGT GGA ATG CGC-3′, reverse, 5′-AAT TAA GCC ACA TGC TCC GCT-3′; δ/γ-Proteobacteria forward, 5′-GCT AAC GCA TTA AGT RYC CCG-3′, reverse 5′-GCC ATG CRG CAC CTG TCT-3′; Enterobacteriaceae forward, 5-TGC CGT AAC TTC GGG AGA AGG CA-3′, reverse, 5′-TCA AGG ACC AGT GTT CAG TGT C-3′; Bifidobacterium longum forward, 5′-CAG TTG ATC GCA TGG TCT T-3′, reverse, 5′-TAC CCG TCG AAG CCA C-3′; Lactobacillus plantarum forward, 5′-TCA TGA TTT ACA TTT GAG TG-3′, reverse, 5′-GAC CAT GCG GTC CAA GTT GTT-3′; and bacterial 16S rRNA forward, 5′-AGA GTT TGA TCC TGG CTC AG-3′, reverse 5′-AAG GAG GTG WTC CAR CC-3′.
Determination of LPS
The content of LPS was determined using a LAL assay kit according to manufacturer’s protocol29. For the assay of culture medial LPS contents, each probiotic (1 × 106 CFU/mL) and E. coli (1 × 106 CFU/mL) was simultaneously inoculated in GAM (5 mL) and anaerobically cultured for 37 °C for 24 h. The cultured suspension was sonicated for 1 h on ice, centrifuged at 5,000 × g for 10 min, filtrated through a 0.45 μm filter followed by re-filtration through a 0.22 μm filter, and inactivated at 70 °C for 10 min.
For the assay of fecal LPS contents, colon content from mice (100 mg) were placed in 50 mL of PBS in a pyrogen-free tube and sonicated for 1 h on ice. After centrifugation at 400 × g for 10 min, the supernatant was collected, sterilized by filtration through a 0.45 μm filter followed by re-filtration through a 0.22 μm filter, and inactivated at 70 °C for 10 min.
For the assay of blood LPS contents, serum (5 μL) was diluted 1:10 in pyrogen-free water, inactivated for 10 min at 70 °C, and incubated with LAL solution for 30 min at 37 °C.
Each filterate or serum (50 μl) was incubated with LAL solution at 37 °C for 30 min, added additional reagents to formation of a magenta derivative, and measured the absorbance at 545 nm.
Flow cytometry of Th17 and Treg cells in the lamina propria of colons
For the assay of Th17 cells and Tregs, colons were cut into small pieces, incubated with 2.5 mM EDTA at 37 °C with agitation for 20 min, minced, and digested for 20 min with RPMI containing 1 mg/mL collagenase type VIII at 37 °C. Lamina propria cells were then prepared44. T cells were isolated using a Pan T cell Isolation Kit II, fixed and stained with anti-FoxP3 or anti-IL-17A antibodies, and then analyzed by flow cytometry (C6 Flow Cytometer® System, San Jose, CA, USA).
ELISA and immunoblotting
Colon, liver tissues, and cultured cells were homogenized in the RIPA lysis buffer (1 mL) containing 1% phosphatase inhibitor cocktail and 1% protease inhibitor cocktail at 4 °C and centrifuged at 15,000 × g for 15 min.
For the determination of cytokines, the supernatants of tissue homogenates and cultured cells were transferred to a 96-well microplate. IL-1β, IL-10, IL-17, and TNF-α expression levels were determined using ELISA kits44.
For the immunoblotting, the supernatants of tissue and cultured cell homogenates were subjected to electrophoresis on sodium dodecyl sulfate-polyacrylamide gel, transferred to nitrocellulose membrane, blocked with non-fat dried-milk proteins, probed with antibodies, and washed with PBS with tween 2043. Proteins were detected with horseradish peroxidase-conjugated secondary antibodies. Protein bands were visualized with an enhanced chemiluminescence detection kit.
Statistical analysis
All data are indicated as the mean ± standard deviation (SD), with statistical significance analyzed using one-way ANOVA followed by Duncan’s multiple range test (P < 0.05).
Electronic supplementary material
Acknowledgements
This research was supported by the the Commercializations Promotion Agency for R&D Outcomes (COMPA 2016K000104) funded by the Ministry of Science, ICT and Future Planning (MSIP), Korea.
Author Contributions
D.-H.K., S.-E.J. and M.J.H. designed the study and wrote the protocol. J.-J.J., S.-E.J. and J.-K.K. performed all the pharmacological experiments and statistical analysis. D.-H.K., M.J.H. and S.-E.J. wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25775-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sankar SA, Lagier JC, Pontarotti P, Raoult D, Fournier PE. The human gut microbiome, a taxonomic conundrum. Syst Appl Microbiol. 2015;38:276–86. doi: 10.1016/j.syapm.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Kataoka K. The intestinal microbiota and its role in human health and disease. J Med Invest. 2016;63:27–37. doi: 10.2152/jmi.63.27. [DOI] [PubMed] [Google Scholar]
- 3.Marchesi JR, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirschner R, Hsu T, Tuan NN, Chen CL, Huang SL. Characterization of fungal and bacterial components in gut/fecal microbiome. Curr Drug Metab. 2015;16:272–283. doi: 10.2174/1389200216666150812124625. [DOI] [PubMed] [Google Scholar]
- 5.Haque TR, Barritt AS., 4th Intestinal microbiota in liver disease. Best Pract Res Clin Gastroenterol. 2016;30:133–142. doi: 10.1016/j.bpg.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Elson CO, Alexander KL. Host-microbiota interactions in the intestine. Dig Dis. 2015;33:131–136. doi: 10.1159/000369534. [DOI] [PubMed] [Google Scholar]
- 7.Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol. 2013;28(Suppl 1):77–84. doi: 10.1111/jgh.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y, Chen Z. Inflammatory bowel disease related innate immunity and adaptive immunity. Am J Transl Res. 2016;8:2490–2497. [PMC free article] [PubMed] [Google Scholar]
- 9.Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6–19. doi: 10.1038/mi.2013.73. [DOI] [PubMed] [Google Scholar]
- 10.Mowat AM, Bain CC. Mucosal macrophages in intestinal homeostasis and inflammation. J Innate Immun. 2011;3:550–564. doi: 10.1159/000329099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander KL, Targan SR, Elson CO., 3rd Microbiota activation and regulation of innate and adaptive immunity. Immunol Rev. 2014;260:206–220. doi: 10.1111/imr.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Sundrud MS, Skepner J, Yamagata T. Targeting Th17 cells in autoimmune diseases. Trend Pharmacol Sci. 2014;35:493–500. doi: 10.1016/j.tips.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Leppkes M, et al. RORgamma-expressing TH17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Kanai T, Mikami Y, Sujino T, Hisamatsu T, Hibi T. RORγt-dependent IL-17A-producing cells in the pathogenesis of intestinal inflammation. Mucosal Immunol. 2012;5:240–224. doi: 10.1038/mi.2012.6. [DOI] [PubMed] [Google Scholar]
- 15.Gibson DJ, Ryan EJ, Doherty GA. Keeping the bowel regular: the emerging role of Treg as a therapeutic target in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2716–2724. doi: 10.1097/MIB.0b013e31829ed7df. [DOI] [PubMed] [Google Scholar]
- 16.O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 17.Goettel JA, et al. KSR1 protects from interleukin-10 deficiency-induced colitis in mice by suppressing T-lymphocyte interferon-γ production. Gastroenterology. 2011;140:265–274. doi: 10.1053/j.gastro.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behnsen J, Deriu E, Sassone-Corsi M, Raffatellu M. Probiotics: Properties, examples, and specific applications. Cold Spring Harb Perspect Med. 2013;3:a010074. doi: 10.1101/cshperspect.a010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammadmoradi S, Javidan A, Kordi J. Boom of probiotics: This time non-alcoholic fatty liver disease – A mini review. J Funct Foods. 2014;11:30–35. doi: 10.1016/j.jff.2014.08.022. [DOI] [Google Scholar]
- 20.Vijaya Kumar B, Vijayendra SV, Reddy OV. Trends in dairy and non-dairy probiotic products - a review. J Food Sci Technol. 2015;52:6112–6124. doi: 10.1007/s13197-015-1795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emge JR, et al. Modulation of the microbiota-gut-brain axis by probiotics in a murine model of inflammatory bowel disease. Am J Physiol Gastrointestinal Liver Physiol. 2016;310:G989–998. doi: 10.1152/ajpgi.00086.2016. [DOI] [PubMed] [Google Scholar]
- 22.Petrof EO. Probiotics and Gastrointestinal Disease: Clinical Evidence and BasicScience. Antiinflamm Antiallergy Agents Med Chem. 2009;8:260–269. doi: 10.2174/187152309789151977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang HJ, Im SH. Probiotics as an immune modulator. J Nutr Sci Vitaminol (Tokyo) 2015;61:Suppl, S103–105. doi: 10.3177/jnsv.61.S103. [DOI] [PubMed] [Google Scholar]
- 24.Kim KA, Jeong JJ, Kim DH. Lactobacillus brevis OK56 ameliorates high-fat diet-induced obesity in mice by inhibiting NF-κB activation and gut microbial LPS production. J Funct Foods. 2015;13:183–191. doi: 10.1016/j.jff.2014.12.045. [DOI] [Google Scholar]
- 25.Han SY, et al. Hepatoprotective effect of lactic acid bacteria, inhibitors of beta-glucuronidase production against intestinal microflora. Arch Pharm Res. 2005;28:325–329. doi: 10.1007/BF02977800. [DOI] [PubMed] [Google Scholar]
- 26.Lim SM, Jeong JJ, Jang SE, Han MJ, Kim DH. A mixture of the probiotic strains Bifidobacterium longum CH57 and Lactobacillus brevis CH23 ameliorates colitis in mice by inhibiting macrophage activation and restoring the Th17/Treg balance. J Funct Foods. 2016;27:295–309. doi: 10.1016/j.jff.2016.09.011. [DOI] [Google Scholar]
- 27.Miyauchi E, et al. Bifidobacterium longum alleviates dextran sulfate sodium-induced colitis by suppressing IL-17A response: involvement of intestinal epithelial costimulatory molecules. PLoS One. 2013;8:e79735. doi: 10.1371/journal.pone.0079735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuo L, et al. Bifidobacterium infantis attenuates colitis by regulating T cell subset responses. World J Gastroenterol. 2014;20:18316–18329. doi: 10.3748/wjg.v20.i48.18316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong JJ, et al. Orally administrated Lactobacillus pentosus var. plantarum C29 ameliorates age-dependent colitis by inhibiting the nuclear factor-kappa B signaling pathway via the regulation of lipopolysaccharide production by gut microbiota. PLoS One. 2015;10:e0116533. doi: 10.1371/journal.pone.0116533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao H, et al. Inhibition of miR122a by Lactobacillus rhamnosus GG culture supernatant increases intestinal occludin expression and protects mice from alcoholic liver disease. Toxicol Lett. 2015;234(3):194–200. doi: 10.1016/j.toxlet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Chiu YH, Tsai JJ, Lin SL, Lin MY. Lactobacillus casei MYL01 modulates the proinflammatory state induced by ethanol in an in vitro model. J Dairy Sci. 2014;97:2009–2016. doi: 10.3168/jds.2013-7514. [DOI] [PubMed] [Google Scholar]
- 32.Hawiger J. Innate immunity and inflammation: a transcriptional paradigm. Immunol Res. 2001;23:99–109. doi: 10.1385/IR:23:2-3:099. [DOI] [PubMed] [Google Scholar]
- 33.Bistrian B. Systemic response to inflammation. Nutr Rev. 2007;65:S170–172. doi: 10.1301/nr.2007.dec.S170-S172. [DOI] [PubMed] [Google Scholar]
- 34.Bawa M, Saraswat VA. Gut-liver axis: role of inflammasomes. J Clin Exp Hepatol. 2013;3:141–149. doi: 10.1016/j.jceh.2013.03.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seeley JJ, Ghosh S. Molecular mechanisms of innate memory and tolerance to LPS. J Leukoc Biol. 2017;3:107–119. doi: 10.1189/jlb.3MR0316-118RR. [DOI] [PubMed] [Google Scholar]
- 36.Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation. Rheumatology. 2008;47:584–590. doi: 10.1093/rheumatology/kem298. [DOI] [PubMed] [Google Scholar]
- 37.Katz JP, Lichtenstein GR. Rheumatologic manifestations of gastrointestinal diseases. Gastroenterol Clin North Am. 1998;27:533–562. doi: 10.1016/S0889-8553(05)70020-0. [DOI] [PubMed] [Google Scholar]
- 38.Dotan I, Rachmilewitz D. Probiotics in inflammatory bowel disease: possible mechanisms of action. Curr Opinion Gastroenterol. 2005;21:426–430. [PubMed] [Google Scholar]
- 39.Jang SE, et al. Lactobacillus brevis G-101 ameliorates colitis in mice by inhibiting NF-κB, MAPK and AKT pathways and by polarizing M1 macrophages to M2-like macrophages. J Appl Microbiol. 2013;115:888–896. doi: 10.1111/jam.12273. [DOI] [PubMed] [Google Scholar]
- 40.Zakostelska Z, et al. Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS One. 2011;6:e27961. doi: 10.1371/journal.pone.0027961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian F, et al. Lactobacillus rhamnosus CCFM1107 treatment ameliorates alcohol-induced liver injury in a mouse model of chronic alcohol feeding. J Microbiol. 2015;53:856–863. doi: 10.1007/s12275-015-5239-5. [DOI] [PubMed] [Google Scholar]
- 42.So, J. S. et alLactobacillus casei suppresses experimental arthritis by down-regulating T helper 1 effector functions. Mol Immunol45, 2690–2699 (008). [DOI] [PubMed]
- 43.Lim SM, Kang GD, Jeong JJ, Choi HS, Kim DH. Neomangiferin modulates the Th17/Treg balance and ameliorates colitis in mice. Phytomedicine. 2016;23:131–140. doi: 10.1016/j.phymed.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Lee HU, Bae EA, Han MJ, Kim NJ, Kim DH. Hepatoprotective effect of ginsenoside Rb1 and compound K on tert-butyl hydroperoxide-induced liver injury. Liver Int. 2005;25:1069–1073. doi: 10.1111/j.1478-3231.2005.01068.x. [DOI] [PubMed] [Google Scholar]
- 45.Yang YW, et al. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR Analysis of predominant bacteria in mouse feces. Appl Environ Microbiol. 2015;81:6749–6756. doi: 10.1128/AEM.01906-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim SM, Choi HS, Jeong JJ, Han SW, Kim DH. The rhizome mixture of Anemarrhena asphodeloides and Coptis chinensis attenuates mesalazine-resistant colitis in mice. Evid Based Complement Alternat Med. 2016;2016:5895184. doi: 10.1155/2016/5895184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.