Abstract
Coffee’s long-term effect on cognitive function remains unclear with studies suggesting both benefits and adverse effects. We used Mendelian randomization to investigate the causal relationship between habitual coffee consumption and cognitive function in mid- to later life. This included up to 415,530 participants and 300,760 coffee drinkers from 10 meta-analysed European ancestry cohorts. In each cohort, composite cognitive scores that capture global cognition and memory were computed using available tests. A genetic score derived using CYP1A1/2 (rs2472297) and AHR (rs6968865) was chosen as a proxy for habitual coffee consumption. Null associations were observed when examining the associations of the genetic score with global and memory cognition (β = −0.0007, 95% C.I. −0.009 to 0.008, P = 0.87; β = −0.001, 95% C.I. −0.005 to 0.002, P = 0.51, respectively), with high consistency between studies (Pheterogeneity > 0.4 for both). Domain specific analyses using available cognitive measures in the UK Biobank also did not support effects by habitual coffee intake for reaction time, pairs matching, reasoning or prospective memory (P ≥ 0.05 for all). Despite the power to detect very small effects, our meta-analysis provided no evidence for causal long-term effects of habitual coffee consumption on global cognition or memory.
Introduction
Coffee is one of the most widely consumed beverages worldwide1. Its health benefits and risks have long been debated, with some studies suggesting reductions in the risk of various diseases2–5 and mortality6–8, while other studies have suggested a potential increase in cardiovascular risk9.
Comprised of over 1,000 chemical compounds, coffee gains its popularity through its acute stimulatory effects, which are attributed to the pharmacological activity of caffeine1. Caffeine can readily cross the blood-brain barrier, and is believed to exert its neurocognitive effects by antagonizing adenosine receptors in the central nervous system1. Large-scale meta-analyses of prospective studies suggested that higher coffee consumption is associated with a lower risk for Alzheimer’s disease10,11, while a recent genetic study using summary data from large consortia did not support a causal association12. Studies on the effects of coffee consumption on specific cognitive domains have yielded mixed results for executive function and memory, with some suggesting benefits, and others showing null or adverse effects13–15. Inconsistent findings may reflect methodological problems commonly seen with dietary exposures including confounding and reverse causation, the latter being particularly important in this case given that caffeine intake is typically one of the first behaviors to be altered when the individual’s health status declines16.
Randomised clinical trials are the gold standard for testing causal effects, however, these are expensive to undertake and it is difficult to examine influences of prolonged exposures. An alternative is Mendelian randomization, also called “nature’s randomised trial”, which is a non-invasive and cost-effective observational approach to identify possible causal associations17. In Mendelian randomization, causality is inferred from the association between exposure-related genetic variants and the outcome of interest18,19. As genotypes are assigned (at random) at conception they will not be generally affected by disease or social and lifestyle factors, allowing us to overcome some of the common problems with observational epidemiology. For a commonly used addictive stimulant such as caffeine, which has been proposed to have both adverse and beneficial effects on cognitive outcomes14,20, Mendelian randomization is therefore an attractive approach to use as it allows us to address issues both in relation to safety and preventative potential.
Genome-wide association studies to date have identified 8 loci influencing habitual coffee intakes21,22. Two common genetic variants have the strongest effects and known biological mechanisms, one located between the cytochrome P450 1A1 (CYP1A1) and CYP1A2 gene regions (rs2472297) and the other 51 kb upstream the aryl-hydrocarbon receptor (AHR) gene (rs6968865)23. CYP1A2 accounts for ~95% of hepatic caffeine clearance, and AHR plays a key role in metabolizing xenobiotics and inducing the transcription of CYP1A224. In this study, including individual level data for up to 415,530 European ancestry participants, we examined the causal association between genetically instrumented habitual coffee intake, and cognitive function in mid- to later-life, with the aim of establishing causal evidence for potential long-term cognitive effects of coffee consumption. Given the variants influencing patterns of coffee consumption also affect caffeine intake and clearance23–25, secondary analyses were conducted with respect to habitual tea and caffeine intakes.
Methods
Study population
Data came from 10 European cohorts including the 1958 British birth cohort (1958BC), UK Biobank, Mothers of Avon Longitudinal Study of Parents and Children (ALSPAC-M), Northern Finland Birth Cohorts 1966 (NFBC1966), Cardiovascular Risk in Young Finns Study (YFS), Helsinki Birth Cohort Study (HBCS), Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS), Uppsala Longitudinal Study of Adult Men (ULSAM), Swedish twin registry (STR) and TwinGene studies. Participants in each study have provided an informed consent, and each study is covered by ethical approvals from the relevant ethics committees. A more detailed description of each cohort is provided in the supplementary text. Data analysis in each cohort was restricted to participants with complete information on AHR and CYP1A1/2 genetic variants, who also had information on habitual coffee intake and cognitive measures.
Genetic variants
Genome-wide association studies to date have identified eight loci influencing habitual coffee intake patterns21,22. For our primary analyses we chose the two strongest loci that have well-characterized biological functions affecting caffeine metabolism, CYP1A1/2 and AHR24. The remaining six loci (POR, EFCAB5, GCKR, ABCG2, MLXIPL, and BDNF) were used in secondary analyses. Our strategy for the selection of instrumental variables is expanded upon in Supplementary Fig. S1.
The primary variant for CYP1A1/2 was rs2472297, and for AHR rs6968865. In cohorts where rs6968865 was unavailable, its proxy, rs6968554 (r2 = 1.0) or rs4410790 (r2 = 0.97) was used (Supplementary Table S1). As the two variants had very similar effects on habitual coffee intake in the original GWAS21 we used an unweighted genetic score constructed by summing the number of intake-increase alleles in CYP1A1/2 (T in rs2472297) and AHR (T in rs6968865; G in rs6968554; C in rs4410790)23. Information on genotyping and related quality control is provided in the supplementary text (Supplementary Table S1). In secondary analyses we used rs17685, rs9902453, rs1260326, rs1481012, rs7800944, and rs6265 for POR, EFCAB5, GCKR, ABCG2, MLXIPL, and BDNF, respectively.
Habitual coffee, tea and caffeine consumption
Coffee intake was the primary exposure of interest, with tea and caffeine consumption only used in the secondary analyses. Habitual coffee consumption (cups/day) in each cohort was computed using the available self-reported intake information, and in cohorts where the intake was indicated in an interval (e.g. 2 to 4 cups a day), the median was used. A categorical indicator for the intake level (<1, 1–4 and ≥4 cups/day) was also created for the purpose of stratification in subgroup analyses. Habitual tea consumption (cups/day) was computed the same way as the habitual coffee consumption, and habitual caffeine intake (mg/day) was approximated by combining information on coffee and tea consumption and assuming that one cup of coffee and tea contained approximately 75 mg and 40 mg of caffeine, respectively23. Information on other caffeinated drinks (e.g. energy drinks, hot chocolate) was not available for most of the studies, and hence was not included in the computation for habitual caffeine intake. Detailed descriptions for coffee and tea intake information in each cohort can be found in the supplementary text (Supplementary Table S2).
Global cognition and memory scores
Cognitive measures included in the cohorts ranged from tests of global cognition such as the Mini-Mental State Examination (MMSE) to domain specific tests such as the Trail Making Tests, Digit Symbol Coding and the Paired Associates Learning test (Supplementary Table S3). Given the diversity of cognitive measures across cohorts, we computed composite global cognition and memory scores and used them as our primary outcomes. Those composite scores were constructed within each cohort by summing the standardized scores of the relevant cognitive tests. Such composite scores are regularly used as a way of producing a more stable representation of cognitive function, harmonizing outcomes between cohorts, and maximizing interpretability26. Individual cognitive tests used in the global cognitive and memory scores were all coded to have higher scores as indicative of better cognitive function. Aside from the composite cognitive scores in the primary analyses, domain-specific cognitive measures in the UK Biobank, including reaction time (N = 288,905), pairs matching (N = 290,574), reasoning (N = 93,512) and prospective memory (N = 95,340) were used in the secondary analyses for domain-specific effects.
Statistical analyses
Three primary association analyses were performed, including (1) the phenotypic associations between the habitual coffee intake patterns and global cognition and memory function, (2) genetic associations between the genetic score indexing higher coffee intakes and global and memory cognition, and (3) instrument validation assessing the association between the genetic instruments and coffee intake patterns. For the genetic associations, since the coffee-cognition relationship may vary by intake level, we performed the association analyses under different levels of habitual coffee intake (1–4 and ≥4 cups/day). As a negative control, we also examined the genetic associations among non-coffee drinkers (<1 cup/day). For all three association analyses we used the linear regression and fitted two models, with model 1 including sex and age as covariates, and model 2 adjusted for sex, age, smoking (non-current and current smokers), education attainment and depression. In the genetic analyses we assumed an additive inheritance model, and additionally adjusted for principal components (PCs) to account for population stratification.
We performed secondary subgroup analyses to test if the genetic and phenotypic associations varied by age (<65 or ≥65 years) or sex. Since the genetic instruments indexing habitual coffee intake likely influence the intake patterns through affecting caffeine clearance23–25, we conducted secondary analyses for habitual tea and caffeine intakes (similar to the analyses performed for habitual coffee intake), with analyses on tea consumption further restricting the data to participants who did not drink coffee. Further, in the UK Biobank with a sample size up to 290,574, we performed domain-specific analyses to complement our primary findings using composite cognitive scores. Additional sensitivity analyses were conducted using alternative genetic instruments (POR and EFCAB5) to index habitual coffee intakes. Furthermore, we conducted analyses using an alternative MR approach, MR-Egger regression, which is suggested to be robust to pleiotropy27 and which therefore allowed us to use all eight genome-wide significant variants associated with habitual coffee intake patterns (CYP1A1/2, AHR, POR, EFCAB5, GCKR, ABCG2, MLXIPL, and BDNF)21.
Data analyses were conducted in each participating cohort, and the results were sent to the University of South Australia for quality control and meta-analyses. To ensure the uniformity in data analysis across cohorts, a detailed analysis plan and related statistical script was supplied to all cohorts. Random-effects models were used for the meta-analyses, with sensitivity analyses using fixed effects in the absence of heterogeneity. Meta-regression was used to assess variation by cohort characteristics, including age, sex or country. Further, power analyses28 were performed to determine the minimal effect size we were powered to detect in the primary and domain specific genetic association analyses. All data analyses were performed using Stata (StataCorp LP, College Station, Texas, USA).
Results
Information for coffee intake was available for 415,530 participants from 10 European cohorts, including 300,760 coffee consumers. Basic characteristics for participants in different studies are shown in Table 1. Median daily coffee consumption ranged from 2 to 4 cups/day (Table 1), with more coffee being consumed daily in Finland and Sweden than in the UK. Allele frequencies for AHR and CYP1A1/2 were consistent with those reported for the respective populations in the 1000 Genomes project. The genotype frequencies of both variants were in Hardy-Weinberg equilibrium in all cohorts (P > 0.07).
Table 1.
Characteristics of the participating cohorts.
| Country | N* | Males (%) | Age, yrs | Coffee intake, cups/day | Global scores Median (IQR) | Memory scores Median (IQR) | AHR MAF (%) | CYP1A1/2 MAF (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥65 yrs (%) | Median (IQR) | 1–4 cups/day (%) | ≥4 cups/day (%) | Median (IQR) | ||||||||
| 1958BC | UK | 4,416 | 50.7 | 0.0 | 50 (0) | 48.1 | 18.6 | 3 (1) | 0 (1.3) | 0.04 (1.3) | 38.7 | 26.4 |
| ALSPAC-M | UK | 4,734 | 0.0 | 0.0 | 51 (6) | 53.1 | 21.3 | 2 (3) | 0.03 (1.4) | 0.13 (1.3) | 36.2 | 26.5 |
| UK Biobank | UK | 404,620 | 46.0 | 19.6 | 58 (12) | 51.4 | 20.4 | 2 (3) | 0.2 (1.1) | 0.34 (1.2) | 36.4 | 26.7 |
| HBCS | Finland | 1,653 | 41.5 | 90.0 | 68 (4) | 73.2 | 17.0 | 2.5 (0.4) | 0.08 (1.3) | 0.12 (1.3) | 35.6 | 19.6 |
| NFBC1966 | Finland | 3,488 | 43.5 | 0.0 | 47 (1) | 32.2 | 63.4 | 4 (3) | −0.06 (1.2) | −0.06 (1.2) | 31.6 | 24.6 |
| YFS | Finland | 2,323 | 45.7 | 0.0 | 43 (9) | 38.2 | 44.1 | 4 (3) | 0.14 (1.3) | 0.22 (0.9) | 31.4 | 25.0 |
| PIVUS | Sweden | 882 | 49.9 | 100.0 | 70 (0) | 62.4 | 30.1 | 3.1 (1.9) | 0.18 (1) | 0.17 (1.2) | 35.9 | 28.7 |
| ULSAM | Sweden | 1,081 | 100.0 | 100.0 | 71 (1) | 63.2 | 32.4 | 3.4 (1.9) | −0.16 (1.2) | — | 34.7 | 26.8 |
| STR | Sweden | 1,073 | 45.0 | 76.1 | 72 (10) | 45.3 | 51.1 | 4 (2) | 0.05 (1.4) | −0.09 (1.5) | 37.7 | 28.7 |
| TwinGene | Sweden | 2,370 | 51.3 | 100.0 | 69 (6) | 53.9 | 40.1 | 3 (2) | 0.09 (1.2) | 0.16 (1.2) | 34.8 | 27.7 |
IQR: interquartile range; MAF: minor allele frequency. *Numbers reflect the maximal sample of individuals from each cohort who had genetic data.
Phenotypic associations
There was no evidence for an overall phenotypic association between habitual coffee intake and global cognition or memory function (Table 2). Although higher coffee intake appeared to be associated with lower global cognitive scores in the sex and age adjusted analyses (Table 2, P = 0.04), this tentative association was no longer present after further adjustment for smoking, education level and depression (Table 2, P = 0.26).
Table 2.
Phenotypic and genetic associations of habitual coffee intake with cognition.
| Global cognition | Memory cognition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | β* (95% C.I.) | P | I2 | Pheterogeneity | N | β* (95% C.I.) | P | I2 | Pheterogeneity | |
| Phenotypic effect (cups/day) | 300,806 | −0.02 (−0.039, −0.001) |
0.04 | 85 | <0.001 | 301,850 | −0.012 (−0.029, 0.005) |
0.16 | 80 | <0.001 |
| Adjusted phenotypic effect (cups/day) | 295,823 | −0.008 (−0.023, 0.006) |
0.26 | 74 | <0.001 | 296,777 | −0.004 (−0.017, 0.01) |
0.58 | 66 | 0.003 |
| Genetic effect (intake-increase-allele)# | 300,760 | −0.0007 (−0.009, 0.008) |
0.87 | 4 | 0.40 | 301,804 | −0.001 (−0.005, 0.002) |
0.51 | 0 | 0.64 |
*All models adjusted for sex and age, with the adjusted phenotypic model controlling further on smoking, education and depression and the genetic models additionally adjusting for principal components. #Among coffee drinkers.
There was considerable variability in the phenotypic associations across studies, with I2 ranging from 66% to 85% (Pheterogeneity ≤ 0.003 for all comparisons, Table 2). However, there were no systematic study level differences in the phenotypic associations by age, sex or country (Pmeta-regression > 0.05 for all).
Genetic associations and instrument validation
Genetic associations were not affected by adjustment for smoking, education level and depression. Results are presented for the simpler model with adjustment for sex, age, and PCs to minimize the risk of collider bias potentially introduced by the statistical adjustment29.
CYP1A1/2, AHR and the genetic score showed the expected patterns with habitual coffee consumption (Fig. 1), with each allele on the genetic score associated with an average of 0.16 additional cups of coffee/day (95% C.I. 0.12–0.20, P = 3 × 10−15).
Figure 1.
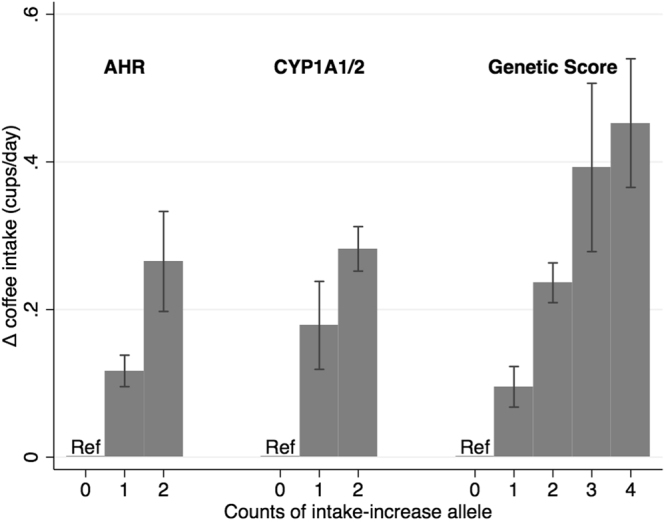
Association of AHR, CYP1A1/2 and genetic score with habitual coffee intake (cups/day) among coffee drinkers. Error bars are the 95% confidence intervals.
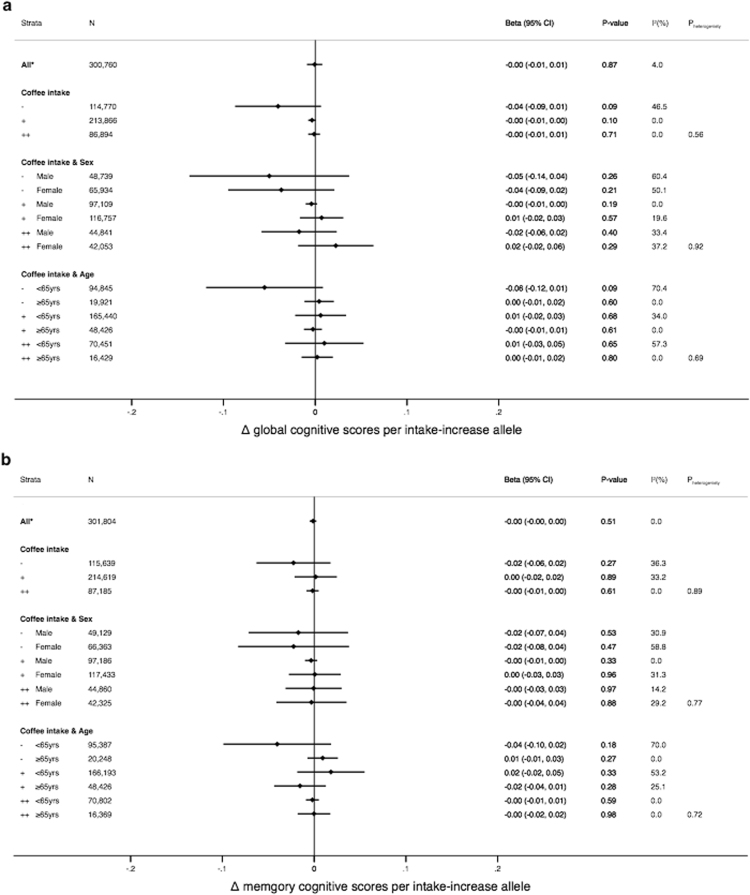
There was no evidence for an association between the genetic score with global cognition or memory among habitual coffee drinkers (P > 0.5 for both, Fig. 2), with high consistency between the cohorts (I2 ≤ 4% for both, Table 2 and Supplementary Fig. S6). Further, there was also no evidence for variation in the genetic effect by the degree of habitual coffee intake (Fig. 2, Pheterogeneity ≥ 0.56 for both global cognition and memory). As expected, in analyses restricted to participants who did not drink coffee as the negative control, the genetic score was not associated with global cognition or memory (P > 0.09 for both, Fig. 2).
Figure 2.
Association of genetic score with global (a) and memory (b) cognition among coffee drinkers and in subgroups stratified by coffee intake (<1, 1–4 or ≥4 cups/day), sex and age (<65 yrs or ≥65 yrs). *Among coffee consumers; −, + and ++ denote <1, 1–4 and ≥4 cups/day, respectively. Error bars are the 95% confidence intervals.
Secondary analyses
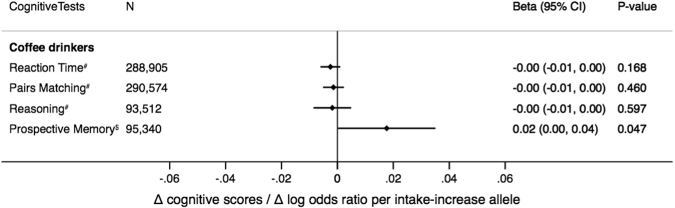
Phenotypic and genetic effects did not vary by age (<65 yrs or ≥65 yrs) or sex (Pheterogeneity > 0.19 for all comparisons). Sensitivity analyses using two additional variants associated with coffee consumption, POR and EFCAB521, provided no consistent evidence for association with global or memory cognition (Supplementary Fig. S2). An isolated association with memory function was seen for EFCAB5 (P = 0.004), a variant which had previously been suggested to be “non-pleiotropic” indicator for coffee consumption12, but which is located near the serotonin transporter21. MR-Egger regression, using all eight genome-wide significant variants associated with habitual coffee intake21, also provided no evidence for association with global or memory cognition (−0.0096 [95% C.I. −0.056 to 0.036], and 0.0054 [95% C.I. −0.048 to 0.058], respectively). Domain specific information was available in the UK Biobank for reaction time (N = 288,905), pairs matching (N = 290,574), reasoning (N = 93,512) and prospective memory (N = 95,340). The genetic score was not associated with reaction time, pairs matching, or reasoning among coffee drinkers (P ≥ 0.05 for all, Fig. 3). For prospective memory the results were inconclusive (P = 0.047), however, this nominal benefit was not consistently seen in across caffeine intake variants (Supplementary Fig. S3).
Figure 3.
Association of the genetic score with different cognitive tests among coffee consumers in the UK Biobank. For all cognitive tests, positive effect sizes indicate improvements in cognitive function. For the prospective memory (i.e. a binary outcome), odds = probability of being correct/probability of being incorrect at the first attempt. Error bars are the 95% confidence intervals. #Effect size is change in cognitive scores per intake-increase allele; $Effect size is change in log odds ratio per intake-increase allele.
Intake-increase alleles of CYP1A1/2, AHR and the genetic scores associated with increased habitual tea and caffeine intake in a dose-dependent matter (Supplementary Fig. S4). We examined the association of the genetic score with cognition among tea/caffeine consumers, including corresponding subgroup analyses, with null associations found in all these analyses (Supplementary Fig. S5). Sensitivity analyses on tea consumption restricting data to participants who did not drink coffee (N = 103,064) also obtained null associations (P = 0.35 and 0.57 for global cognition and memory function, respectively). Detailed results can be found in the supplementary text.
Instrument validation in the UK Biobank
In the UK Biobank, the genetic score explained 0.33% of variation in coffee consumption patterns (F-statistic = 952.6). There were no significant imbalances in allele distribution of CYP1A1/2, AHR or genetic score by background covariates (P ≥ 0.05 for all, Supplementary Table S5).
Power calculations
For the genetic association, with a sample size of 300,760 (the sample size for global cognitive function) and a two-sided α level of 0.05, at 80% of power we would sufficiently detect a 0.0055 standard deviation (SD) change in the cognitive score for every coffee-intake-increase allele. Approximating from the distribution of coffee intake in the UK Biobank (SD = 2 cups/day), this translates into a causal effect of 0.045 SD by each cup of coffee. For the domain specific analyses in the UK Biobank, with the lowest sample size of 93,512 (the sample size for the reasoning test), the effect size that we would be sufficiently powered to detect is 0.0099 SD for a continuous outcome and 0.024 log odds ratio (log OR, OR 1.02) for a binary outcome. This translates into a 0.081 SD and 0.20 log OR (OR 1.22) by each cup of coffee, respectively.
Discussion
Our large-scale genetic analyses including over 300,000 coffee drinkers did not find any evidence to support beneficial or adverse long-term effects of coffee intake on global cognition or memory. Our findings are in line with a recent Mendelian randomization study on Alzheimer’s disease12, but contrasts with earlier observational studies14, alleviating concerns about potentially adverse effects on memory function. Our null finding is convincing given the power to detect even very small effects, and as the results were remarkably consistent between cohorts, and also across different genetic variants used to index habitual coffee intake. While our analyses did not provide evidence for long-term benefits, these data suggest that there are no adverse effects on memory or cognitive function.
Mendelian randomization has become an increasingly popular approach for examining causal relationships, and it has begun to show its potential by clarifying a number of previously misconceived associations30,31. For studies to investigate the effects of coffee intake, it has the obvious attraction in the ability to test the preventative potential before advancing to more costly intervention studies. However, as used in the present study, Mendelian randomization has great prospects in addressing safety concerns with exposures considered to be potentially harmful.
Global cognitive function encompasses several domains with each domain itself being a complex entity32. It is possible that the effect of habitual coffee consumption may vary from one domain to another. If this is true, then composite cognitive scores capturing different cognitive domains may potentially dilute any domain-specific effects. Domain-specific analyses were conducted using smaller sub-samples of the UK Biobank with available information, with no evidence for either benefit or harm by coffee intake. Some uncertainty remains with prospective memory, where results were borderline, retaining the possibility of some nominal benefit. Given that this effect was not consistently observed across all instruments for habitual coffee intake it is most likely to be a chance finding.
In Mendelian randomization the ability to make causal inferences directly depends on the availability and quality of genetic instruments. Genome-wide association studies to date have identified eight loci influencing habitual coffee intakes21,22, including CYP1A1/2, AHR, POR, EFCAB5, GCKR, ABCG2, MLXIPL, and BDNF. We chose to use the two strongest variants to construct our primary instrument, as both consistently associate with habitual coffee intake21–23 and have well-characterized biological mechanisms influencing caffeine metabolism which plausibly explain their association with coffee intake24. However, pleiotropic effects cannot be fully excluded given genome-wide association analyses on Parkinson’s disease, blood pressure and bladder cancer have shown signals both for AHR and CYP1A1/233–35, although these weak associations could reflect downstream causal effects of caffeine or coffee consumption36. To exclude the possibility that the null association with cognitive function which we observed in this study is driven by opposing patterns induced by pleiotropy in AHR and/or CYP1A1/2, we conducted sensitivity analyses in the UK Biobank using other variants to index habitual coffee consumption21 and replicated our main analyses including POR and EFCAB5, which have unknown effects on caffeine metabolism but which did not have previously reported pleiotropic effects. Also these analyses provided no support for causal effects of habitual coffee intake on cognition, although there appeared to be an association between memory function and rs9902453, located in the intron of the EFCAB5. There is very limited information on EFCAB5 in the literature, however, the variant is in close proximity to SLC6A4, the gene encoding serotonin transporter21. Serotonin affects cognitive abilities, in particular memory consolidation37, hence, the observed rs9902453–memory function association is likely a reflection of a pleiotropic effect than a true causal effect of coffee intake. Pleiotropy was also considered to be a problem with four other variants identified in the earlier GWAS on coffee consumption (notably GCKR, ABCG2, MLXIPL, and BDNF)21, which according to the GWAS catalogue, had shown primary associations with traits including BMI, smoking initiation, and gout12. In further sensitivity analyses we included all eight coffee intake associated variants21 in the MR-Egger regression which is robust to pleiotropy27. However, any change in our instrument selection strategy did not affect our conclusions, and also these analyses provided no support for any causal association between habitual coffee intake and cognition.
As secondary analyses, we examined the association of the genetic instruments with habitual tea and caffeine intakes. While people who consume more coffee tend to drink less tea, our analyses suggest that the genetic drivers for coffee and tea consumption are similar. This, together with the known functions of CYP1A1/2 and AHR, confirm that influences on caffeine metabolism likely explains the association between the genetic instruments and habitual coffee intake. It can be argued as a limitation with our approach that the genetic instruments, which were used to index habitual coffee intake, can also index habitual tea intake. However, it is unlikely that a causal coffee-cognition relationship would have been confounded by habitual tea intakes in our study, given the notably weaker genetic influences on tea than on coffee consumption. Further, it needs to be noted that, any inferences relating to caffeine will need to be drawn with caution as the instruments indexing greater caffeine consumption may reflect a faster rate of caffeine clearance, and hence a lower (rather than higher) circulating levels of bioactive caffeine38.
As a further limitation, it can be argued that our genetic association results among coffee drinkers could have been affected by the collider bias as the genetic score indexing the extent of habitual coffee intake is also associated with the probability of being a coffee drinker39. However, sensitivity analyses in the UK Biobank study conducted in the coffee and non-coffee drinkers combined, also showed a null association (results not shown). Also, while the test for the association between the genetic score and the outcome is a valid test for a causal relationship40, it cannot estimate the magnitude of the causal effect if it exists. However, given that null associations were observed in our study, there would have been little gain in applying additional methods (such as instrumental variable analyses)41 to quantify these ‘null’ effects.
In conclusion, we found no evidence that habitual coffee consumption is causally associated with global and memory cognition in mid- to later-life, despite the power to detect very small effects. This suggests that interventions to protect cognition or slow cognitive decline using coffee are unlikely to be successful and should not be prioritized in future trials. That said, there was no evidence for any adverse effect, contrary to some previous observational studies, and hence it appears safe to consume coffee at least with respect to preserving memory function.
Electronic supplementary material
Acknowledgements
This study was financially supported by J.J. Mason and H.S. Williams Memorial Foundation CT23158. For full acknowledgement and study specific funding information, see the supplementary materials.
Author Contributions
Wrote the manuscript: A.Z., E.H. Study supervision: K.M., L.L., J.G.E., O.T.R., S.H., N.L.P., J.V., M.R.J., M.R.M., E.I., E.H. Data analysis: A.Z., A.E.T., V.K., Y.Q.Z., S.P.R., J.L., P.S., L.B., A.M., O.T.R., M.R.J., E.H. Provision of administrative technical or material support: S.P.R., J.A., T.L., M.K., N.H.K., M.P., K.M., L.L., O.T.R., S.H., N.L.P., M.R.J., E.I., D.J.L., E.H. Data collection: S.P.R., J.L., J.A., T.L., M.K., N.H.K., M.P., K.M., L.L., C.P., J.G.E., O.T.R., N.L.P., E.I., E.H. Interpretation and manuscript revision: A.Z., A.E.T., V.K., Y.Q.Z., S.P.R., J.L., P.S., L.B., D.L., J.A., T.L., M.K., N.H.K., K.M., A.M., L.L., C.P., J.G.E., O.T.R., S.H., N.L.P., J.V., M.R.J., M.R.M., E.I., D.J.L., E.H.
Competing Interests
A.E.T. receives grant from Pfizer (unrelated to present project). E.I. is a scientific advisor for Precision Wellness, Cellink and Olink Proteomics for work unrelated to the present project. D.L. is supported by the UK National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South West Peninsula (PenCLAHRC). The views expressed in this article are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health. All other authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25919-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacological reviews. 1999;51:83–133. [PubMed] [Google Scholar]
- 2.Santos C, Costa J, Santos J, Vaz-Carneiro A, Lunet N. Caffeine intake and dementia: systematic review and meta-analysis. Journal of Alzheimer’s disease: JAD. 2010;20(Suppl 1):S187–204. doi: 10.3233/JAD-2010-091387. [DOI] [PubMed] [Google Scholar]
- 3.Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129:643–659. doi: 10.1161/CIRCULATIONAHA.113.005925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes care. 2014;37:569–586. doi: 10.2337/dc13-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross GW, et al. Association of coffee and caffeine intake with the risk of Parkinson disease. Jama. 2000;283:2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 6.Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of coffee drinking with total and cause-specific mortality. The New England journal of medicine. 2012;366:1891–1904. doi: 10.1056/NEJMoa1112010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding M, et al. Association of Coffee Consumption With Total and Cause-Specific Mortality in 3 Large Prospective Cohorts. Circulation. 2015;132:2305–2315. doi: 10.1161/CIRCULATIONAHA.115.017341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loftfield E, et al. Association of Coffee Consumption With Overall and Cause-Specific Mortality in a Large US Prospective Cohort Study. American journal of epidemiology. 2015;182:1010–1022. doi: 10.1093/aje/kwv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. Jama. 2006;295:1135–1141. doi: 10.1001/jama.295.10.1135. [DOI] [PubMed] [Google Scholar]
- 10.Liu QP, et al. Habitual coffee consumption and risk of cognitive decline/dementia: A systematic review and meta-analysis of prospective cohort studies. Nutrition (Burbank, Los Angeles County, Calif.) 2016;32:628–636. doi: 10.1016/j.nut.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Wu, L., Sun, D. & He, Y. Coffee intake and the incident risk of cognitive disorders: A dose-response meta-analysis of nine prospective cohort studies. Clinical nutrition(Edinburgh, Scotland) (2016). [DOI] [PubMed]
- 12.Kwok MK, Leung GM, Schooling CM. Habitual coffee consumption and risk of type 2 diabetes, ischemic heart disease, depression and Alzheimer’s disease: a Mendelian randomization study. Scientific reports. 2016;6:36500. doi: 10.1038/srep36500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng L, Gwee X, Kua EH, Ng TP. Cognitive function and tea consumption in community dwelling older Chinese in Singapore. The journal of nutrition, health & aging. 2010;14:433–438. doi: 10.1007/s12603-010-0095-9. [DOI] [PubMed] [Google Scholar]
- 14.Araujo LF, et al. Association of Coffee Consumption with MRI Markers and Cognitive Function: A Population-Based Study. Journal of Alzheimer’s disease: JAD. 2016;53:451–461. doi: 10.3233/JAD-160116. [DOI] [PubMed] [Google Scholar]
- 15.Araujo LF, et al. Inconsistency of Association between Coffee Consumption and Cognitive Function in Adults and Elderly in a Cross-Sectional Study (ELSA-Brasil) Nutrients. 2015;7:9590–9601. doi: 10.3390/nu7115487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soroko S, Chang J, Barrett-Connor E. Reasons for changing caffeinated coffee consumption: the Rancho Bernardo Study. Journal of the American College of Nutrition. 1996;15:97–101. doi: 10.1080/07315724.1996.10718571. [DOI] [PubMed] [Google Scholar]
- 17.Davey SG, Ebrahim S. What can mendelian randomisation tell us about modifiable behavioural and environmental exposures? BMJ. 2005;330:1076–1079. doi: 10.1136/bmj.330.7499.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey SG. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat.Med. 2008;27:1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 19.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Human molecular genetics. 2014;23:R89–98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haller S, et al. Acute caffeine administration effect on brain activation patterns in mild cognitive impairment. Journal of Alzheimer’s disease: JAD. 2014;41:101–112. doi: 10.3233/JAD-132360. [DOI] [PubMed] [Google Scholar]
- 21.Cornelis MC, et al. Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Molecular psychiatry. 2015;20:647–656. doi: 10.1038/mp.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornelis MC, et al. Genome-wide meta-analysis identifies regions on 7p21 (AHR) and 15q24 (CYP1A2) as determinants of habitual caffeine consumption. PLoS genetics. 2011;7:e1002033. doi: 10.1371/journal.pgen.1002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon G, Taylor AE, Davey Smith G, Munafo MR. Phenotype refinement strengthens the association of AHR and CYP1A1 genotype with caffeine consumption. PloS one. 2014;9:e103448. doi: 10.1371/journal.pone.0103448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josse AR, Da Costa LA, Campos H, El-Sohemy A. Associations between polymorphisms in the AHR and CYP1A1-CYP1A2 gene regions and habitual caffeine consumption. The American journal of clinical nutrition. 2012;96:665–671. doi: 10.3945/ajcn.112.038794. [DOI] [PubMed] [Google Scholar]
- 25.Cornelis MC, et al. Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Human molecular genetics. 2016;25:5472–5482. doi: 10.1093/hmg/ddw334. [DOI] [PubMed] [Google Scholar]
- 26.Llewellyn, D. J., Lang, I. A., Langa, K. M., Naughton, F. & Matthews, F. E. Exposure to secondhand smoke and cognitive impairment in non-smokers: national cross sectional study with cotinine measurement. BMJ (Clinical research ed.)338, b462 (2009). [DOI] [PMC free article] [PubMed]
- 27.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. International journal of epidemiology. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgess S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. International journal of epidemiology. 2014;43:922–929. doi: 10.1093/ije/dyu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole SR, et al. Illustrating bias due to conditioning on a collider. International journal of epidemiology. 2010;39:417–420. doi: 10.1093/ije/dyp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye Z, et al. Association between circulating 25-hydroxyvitamin D and incident type 2 diabetes: a mendelian randomisation study. The lancet. Diabetes & endocrinology. 2015;3:35–42. doi: 10.1016/S2213-8587(14)70184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmes, M. V. et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ (Clinical research ed.)349, g4164 (2014). [DOI] [PMC free article] [PubMed]
- 32.Nehlig A. Is caffeine a cognitive enhancer? Journal of Alzheimer’s disease: JAD. 2010;20(Suppl 1):S85–94. doi: 10.3233/JAD-2010-091315. [DOI] [PubMed] [Google Scholar]
- 33.Levy D, et al. Genome-wide association study of blood pressure and hypertension. Nature genetics. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacArthur J, et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog) Nucleic acids research. 2017;45:D896–d901. doi: 10.1093/nar/gkw1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamza TH, et al. Genome-wide gene-environment study identifies glutamate receptor gene GRIN2A as a Parkinson’s disease modifier gene via interaction with coffee. PLoS genetics. 2011;7:e1002237. doi: 10.1371/journal.pgen.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor AE, et al. Investigating the possible causal role of coffee consumption with prostate cancer risk and progression using Mendelian randomization analysis. International journal of cancer. 2017;140:322–328. doi: 10.1002/ijc.30462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowen P, Sherwood AC. The role of serotonin in cognitive function: evidence from recent studies and implications for understanding depression. Journal of psychopharmacology (Oxford, England) 2013;27:575–583. doi: 10.1177/0269881113482531. [DOI] [PubMed] [Google Scholar]
- 38.Guessous I, et al. Caffeine intake and CYP1A2 variants associated with high caffeine intake protect non-smokers from hypertension. Human molecular genetics. 2012;21:3283–3292. doi: 10.1093/hmg/dds137. [DOI] [PubMed] [Google Scholar]
- 39.Taylor, A. E., Davey Smith, G. & Munafo, M. R. Associations of coffee genetic risk scores with consumption of coffee, tea and other beverages in the UK Biobank. Addiction(Abingdon, England) (2017). [DOI] [PMC free article] [PubMed]
- 40.Hernan MA, Robins JM. Instruments for causal inference: an epidemiologist’s dream? Epidemiology (Cambridge, Mass.) 2006;17:360–372. doi: 10.1097/01.ede.0000222409.00878.37. [DOI] [PubMed] [Google Scholar]
- 41.Burgess, S., Small, D. S. & Thompson, S. G. A review of instrumental variable estimators for Mendelian randomization. Statistical methods in medical research (2015). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.