Abstract
Traditional chemotherapeutic agents non-selectively eliminate cancer cells at the expense of normal tissue; in an attempt to minimize such effects, a new class of targeted agents, immunotherapy, was introduced in the late 1950s with the discovery of interferons and the development of the first cancer vaccine. Ever since, immunotherapy evolved, exploiting different cellular mechanisms including dendritic cell therapy, monoclonal antibodies, and cytokines. Immune checkpoint inhibitors (ICPI) are the most recent subclass of this family and we herein review the basis of exploiting this new subclass of immunotherapy with radiotherapy in the context of studies evaluating their effects on human subjects and focusing on the synergism between the molecular pathways operating in the background. PubMed was searched for studies evaluating the combined use of ICPI and radiotherapy among human subjects. The majority of studies noted an increased response rate in patients receiving combined therapy with no significant increase in toxicity. Outcomes varied among the different ICPI, and treatment with combined anti-PD-1 and anti-CTLA-4 had a higher response rate compared to either modality alone. Synergistic use of ICPI and radiotherapy has the potential to improve survival, however the specifics regarding treatment plan is dependent on a myriad of factors including the genetic and molecular makeup of the tumor as well as the patient.
Keywords: Immune checkpoint inhibitors (ICPI), radiation therapy
Background
Immune checkpoint inhibitors (ICPI) mechanism of actions
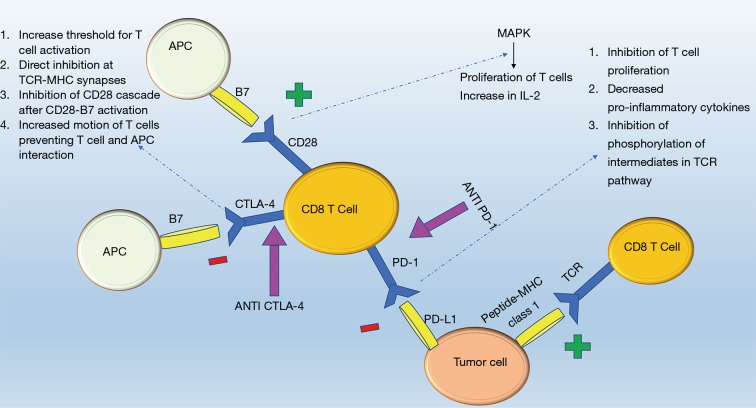
Immune checkpoints serve as “suppressors” (1) targeting T cells (2) in a tightly regulated system relying on antigen specificity and signal amplification to modify the behavior of the immune system in response to foreign versus self-antigens (3). Antigen presenting cells (APC) process and express antigens on major histocompatibility complexes (MHC) recognized by T cell receptors. The T-cell receptor recognizes and interlocks with specific peptide-MHC combinations triggering a cascade either to kill the cell expressing the peptide (cytotoxic T cells) or to recruit other components of the immune to link adaptive and innate immunity (helper T cells) (4). The overall immune response is amplified culminating in the release of cytokines, recruitment of immune effectors cells, and strengthening subsequent T cell receptor interactions (3). Checkpoints suppress this activation helping healthy cells evade from the immune system. The same mechanism is exploited by cancer cells through expression of inhibitory ligands and receptors that suppress T-cell effector function; thus building cancer cell mediated immune tolerance (3). ICPI helps overcome this tolerance and in particular three molecules part of the ICPI pathway currently under investigation for pharmacological intervention include: CTLA-4, PD-1, and PD-L1 inhibitor (Figure 1).
Figure 1.
Visualization of interactions between different immune system players and the role of immune checkpoint inhibitors in this pathway.
CTLA-4 inhibitors
CTLA-4, a down regulator of the immune response, is a protein receptor homologous to CD28 that provides the second activation signal following antigen recognition (5). Although, both CTLA-4 and CD28 receptors are located on T cells and interact with B7 receptors on APCs, the CTLA-4 receptor has 500–2,500 times higher affinity for the B7 receptor in comparison to the CD28 receptor (6). Despite this high affinity, two mechanisms regulate its activity, first its expression only follows activation of T cells and is proportional to the strength of the TCR stimulus (5,7,8) and second CTLA-4 resides in an endosomal compartment and surface expression is restricted until activation (8). Once CTLA-4 interlocks with the B7 receptor, a tyrosine kinase signal cascade is triggered (7,8) leading to an increased threshold of stimulatory signals required for T cell activation, thereby decreasing the replicability of T cells (8) and interfering with CD28:B7 and TCR:MHC binding via direct inhibition at the TCR immune synapse, interruption of the CD28 cascade, and increased motion of T cells limiting the interaction between T cells and APCs (8-11).
In contrast, a powerful immune response is induced when an antibody blocks the CTLA-4:B7 interaction and favors B7:CD28 mediated mitogen activated protein kinase pathways resulting in proliferation of T cells, increased T cell survival, and production of growth cytokines such as IL-2 to activate T effector cells (8,12) and increase the diversity of the T cell response (13,14).
PD-1 inhibitors
Similar to CTLA-4, the PD-1 receptor interacts with two ligands within the B7 family, PD-L1 and PD-L2 (12) through which T cell proliferation is inhibited and expression of pro-inflammatory markers (IFN- γ, TNF-α, and IL-2) is decreased. In T cells whose receptors have recognized their respective antigen on the T cell receptor, simultaneous stimulation of the PD-1 receptor inhibits phosphorylation of intermediates in the TCR pathway inhibiting activation of T cell (12). On a molecular level, activation of PD-1 receptor switches on the tyrosine based motif, recruiting the Src homology 2-containing tyrosine phosphatase (SHP-2) which serves to dephosphorylate and inhibit phosphoinositide 3-kinase (PI3K) activity, decreasing glucose metabolism and IL-2 secretion (15). PD-1 receptor activation on T-regulatory cells increases transformation of CD4 positive T cells to T regulatory cells further enhancing the anti-inflammatory environment (15). This receptor is important in states of chronic inflammation when T cells have been constantly stimulated (16), however, in instances such as tumors and chronic infections, this safety mechanism against auto-immunity can result in inadequate immune support.
The two ligands, PD-L1 and PD-L2, differ in expression and function: PD-L1 has a wider distribution on leukocytes, tumor cells, nonlymphoid tissue, and its expression can be triggered on parenchymal cells via the local presence of cytokines such as IFN-γ and TNF-α (17,18). Increased expression of PD-L1 by healthy tissue in response to these pro-inflammatory markers allows for peripheral immune tolerance. In contrast, PD-L2 has more limited expression on dendritic cells, monocytes, and mast cells (15), and while PD-L1 modulates CD8+ T cell function, PD-L2 modulates CD4+ function (19). Selectively blocking PD-L1 ligand maintains the interaction between PD-1 and PD-L2 providing self-tolerance and minimizing side effects (3) which otherwise would occur if PD-1 receptor blockade occurs disrupting the interaction between PD-1 receptor and both the PD-L1 and PD-L2 ligands. Decreased PD-1 and PD-L1 interaction increases the number of T cells and pro-inflammatory markers at the tumor site creating an environment more suitable for tumor suppression (20). In order for these interactions to function tumor markers such as PD-1 on tumor infiltrating lymphocytes and PD-L1 on tumor cells have to be present, highlighting the importance of biomarkers to predict effective response to ICPI (21,22).
Combining and differentiating PD-1 and CTLA-4
Although both PD-1 and CTLA-4 interact with the same family of B7 ligands and suppress T cell function, their distinct functions play an important role in their corresponding clinical use. In contrast to CTLA-4’s role early in the immune response by preventing T cell activation, PD-1 inhibits peripheral tolerance by suppressing effector T cell function further down the immune cascade. Clinically, the combination of CTLA-4 and PD-1 antagonists has shown to enhance antitumor response via activation of different pathways indicating distinct, but synergistic mechanisms of action (23,24).
Synergy between radiotherapy and check point inhibitors
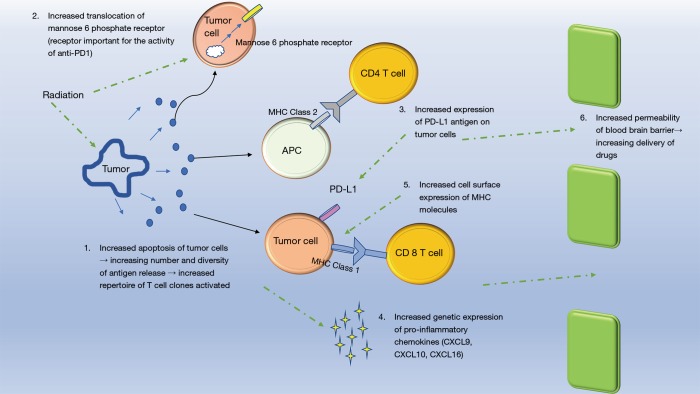
Radiotherapy may potentiate the efficacy of immunotherapy via several mechanisms (Figure 2). First, radiotherapy induces apoptosis of tumor cells, thereby increasing cross presentation of tumor antigens via APCs (25) and direct T cell activation (26). Radiotherapy induced tumoricidal effect results in release of more tumor antigens leading to clonal expansion of activated T cells (27) through which both the diversity of T cell populations and the rate at which they are activated are enhanced (4). Radiotherapy also creates a pro-inflammatory milieu by upregulating cell surface molecules such as MHC class 1 and CD95 (Fas) and increasing the secretion of IFN-1 via accumulation of cytoplasmic double stranded DNA (26,28). The enhanced immune response following radiotherapy may explain the abscopal effect of radiation in which metastatic lesions outside the field of radiation regress following radiotherapy (29,30), however, radiotherapy also induces expression of repair mechanisms such as TREX1 to alleviate radiotherapy induced damage thereby requiring an external mechanism to alter the existing immune response to radiotherapy (28). ICPI has the potential to tip the balance towards a more pro-inflammatory environment to enhance the tumoricidal effects of radiotherapy while decreasing immunosuppressive cytokines such as TGF-β and cells such as T-regs (29,31).
Figure 2.
Visualization of the interplay between radiotherapy and immunotherapy accounting for their synergistic outcomes.
Since the interplay between these two modalities vis-a-vis dosage, sequence of therapy administration, and potential toxicities have considerable impact on the overall outcome, this review will delve into clinical studies focusing exclusively on human subjects that have examined the combined use of these modalities to evaluate for: optimal dosage, interval of dosage, tumor response rate, the effect of biomarkers on response rate, and toxicity in the context of specific immune checkpoint blocking agents and tumor types (29).
Methods
From December 1990 to November 2017, PubMed was searched using the following queries:
“CTLA-4 Blockade AND radiotherapy” (54 results);
“Ipilimumab (Ipi) AND radiotherapy” (147 results);
“PD-1 Blockade AND radiotherapy” (58 results);
“PD-L1 blockade AND radiotherapy” (44 results);
“Nivolumab AND radiotherapy” (81 results);
“Pembrolizumab AND radiotherapy” (64 results);
“Atezolizumab AND radiotherapy” (6 results).
The abstracts of these 442 hits were screened for relevance of which 20 were selected for in-depth review. Inclusion criteria were the following: (I) more than 10 patients in the study; (II) median follow up length of at least 6 weeks; (III) combined treatment of radiotherapy and ICPI; (IV) objective measurement of outcome consisting of the following: overall response rate (ORR), overall survival (OS), metastasis-free survival (MFS) or metastasis-free progression rate (MFP) with significance reported as P value, hazard ratios (HR), or confidence interval (CI).
Results
Response rate
Overall, combination of ICPI and radiotherapy has improved outcomes in comparison to either modality alone (Table online: http://atm.amegroups.com/public/system/atm/supp-atm.2018.03.09-1.pdf) (32-51). In a study by Tazi et al., patients treated with ICPI were split into two cohorts based on the presence (A) or absence (B) of brain metastasis (39). The 3-year survival rates from the first cycle of ICPI were 50% (95% Cl, 27–93%) and 39% (95% Cl, 19–81%) for cohort A and B respectively (39); however, combination therapy [including stereotactic radiosurgery (SRS)] had improved outcomes among patients with brain metastasis to achieve survival rates comparable to those without brain metastasis. Using historical control patients receiving radiotherapy alone for unresectable melanoma brain metastasis, Ahmed et al. reported on improved survival with the addition of Nivolumab either before or after radiotherapy (46). Although the combination of SRS and Ipilimumab achieved 1-year-local control rates comparable with those achieved with Nivolumab (~80%), the distant control rates were higher with Nivolumab suggesting that anti-PD1 may lead to better outcomes when compared to anti-CTLA-4 therapy especially for tumors with higher degrees of PD-L1 immunohistochemical staining (46). Similar findings were reported in a study done by Qian et al. where patients either received concurrent therapy for all lesions, non-concurrent therapy for all lesions, or a combination of concurrent therapy and non-concurrent therapy that varied based on lesion (47). The median percent reduction of tumor mass was higher in patients receiving anti-PD-1 ICPI versus those treated with anti-CTLA-4 therapy at 3 months (89.3% vs. 66.2%, P<0.0001), and 6 months (95.1% vs. 75.9%, P=0.0004), respectively (47).
When evaluating outcome in the context of ICPI, the variable chosen to represent the data is important as in studies where metastasis progression free survival (MPFS) was not statistically different among patients receiving ICPI alone versus combination therapy, OS was noted to be superior in the combination group (48,51), highlighting the inadequacies of MPFS as a reliable surrogate to measure the effectiveness of ICPI as the treatment effect is not large enough to be statistically significant predisposing to a type 2 error (52). To improve the reliability of MPFS, the duration of treatment response examined can be increased as highlighted in Kwon et al.’s examination of men diagnosed with castration resistant prostate cancer and at least one bone metastasis receiving bone directed radiotherapy (8 Gy in 1 fraction) with or without Ipilimumab where the MPFS HR before 5 months was 1.46 (95% CI, 1.10–1.95), from 5 to 12 months 0.65 (95% CI, 0.50–0.85), and beyond 12 months 0.60 (95% CI, 0.43–0.86) (37). Appropriate statistical measures need to be utilized to capture the mechanistic design of ICPI as treatment results take time to manifest and achieve statistical significance reflecting the slower onset of ICPI’s effects and their reliance on stimulating existing immune pathways.
Treatment sequence
The timing between ICPI administration and radiation may influence the overall outcome. Silk et al. noted that patients who received Ipilimumab before radiation had a lower OS of 8.1 months compared to an OS of 18.4 months among those who received Ipilimumab after radiation therapy, however, patients who received ICPI prior to radiation therapy had a higher response rate (40%) compared to patients who received ICPI after radiation therapy (17%) (34). In this study, response rate is a better reflection of treatment outcome as patients who received radiation therapy first were treated with radiation therapy in 2009–2010, therefore, those who lived long enough after radiation therapy to receive Ipilimumab after it became available in 2011 already had a predisposition to improved survival, resulting in a potential overestimation of OS and were more probable to receive alternate therapies interfering with the observed results. On the contrary, by measuring response rate immediately following initiation of either radiation or ICPI restricts the cumulative effects of alternate therapies on the outcome measured suggesting ORR to be a more precise indicator. Similar findings were reported by Qin et al. where patients who received ICPI before radiation therapy had an increased duration of tumor response to radiation compared to patients who received ICPI after radiation therapy (74.7% vs. 44.8% at 12 months; P=0.01, log-rank test) (41). In contrast, Keiss et al. noted patients who received SRS prior to Ipilimumab or concurrent with Ipilimumab had better OS and less regional recurrence than patients treated with SRS after Ipilimumab (1-year OS 65% vs. 56% vs. 40%, P=0.008; 1 year regional recurrence 69% vs. 64% vs. 92% P=0.003) (40), however, in Keiss et al.’s study patients who received SRS after Ipilimumab had disease refractory to Ipilimumab, and thus had worse prognostic outcome and only patients with stable disease and a positive response to Ipilimumab induction therapy progressed to receive maintenance Ipilimumab; therefore, these patients were predisposed to a more favorable prognosis. Contrary to the preceding studies, Knisely et al. found no statistical difference in median survival between patients who received Ipilimumab before SRS and patients who received Ipilimumab after SRS; 19.8 months (95% Cl, 1.5 months—not yet reached upper limit) versus 21.3 months (95% Cl, 15.7 months–not yet reached upper limit) (P=0.58) respectively (38), however, limitations of this study include a smaller sample size compared to the previous studies, thereby reducing the statistical significance of the study and a possible difference in the baseline characteristics of those who received Ipilimumab before radiation and those who received Ipilimumab after radiation, as these baseline characteristics were not discussed.
Appropriate timing of radiation and ICPI is important to optimize treatment response. An et al. compared responses among patients who received SRS before and after 5.5 months of treatment with Ipilimumab and noted those who received early SRS, within 5.5 months of Ipilimumab, had significantly better intracranial control of brain metastasis of melanoma in comparison to patients who received late SRS (HR=2.07; 95% CI, 1.03–4.16) (32). Late SRS treatment had efficacy levels comparable to isolated SRS treatment, whereas, early SRS treatment had a similar efficacy to whole brain irradiation (WBRT), without the irreversible, cognitive deficits associated with WBRT (32), furthermore, patients who developed brain metastasis earlier had a more severe disease presentation and required earlier treatment with SRS compared to those who received SRS 5.5 months after Ipilimumab treatment undermining the association between early initiation of treatment and treatment response. A similar study by Patel et al. noted improved OS among patients who started Ipilimumab within 14 days of SRS (42.9% at both the 1-year and 2-year time point) compared to those who received Ipilimumab after 14 days (33.8% at 1 year and 16.9% at 2 years) (42). On comparison of concurrent vs. sequential use of these therapies, Liniker et al. noted irradiated lesions receiving concurrent therapy had a higher response rate at 64% in comparison to irradiated lesions receiving sequential anti-PD-1 therapy. These studies highlight timing is critical to how these two modalities complement each other and reflects their mechanisms of action as immunotherapy alters immune cell profiles and the local cytokine environment resulting in enhanced efficacy of radiotherapy (53-55), and radiation increases the expression of mannose 6 phosphate (M6P) receptor, a prerequisite receptor for Ipilimumab’s activity, within 3 days of radiation before it returns to baseline within 7 to 14 days, therefore, to maximize therapy efficacy, concurrent administration of both modalities needs to take place within a limited time frame that depends on tumor characteristics (such as specific mutations) as well as the overall treatment plan (42).
Location of radiation and radiation dosage
Mouse model examinations of dose response rates showed conflicting findings regarding radiation dose and fractionation. Regimens with more fractions and less dose per fraction (8 Gy ×3, 6 Gy ×5) had increased IFN-1 levels and prevented the induction of TREX1, a protein expressed to degrade cytosolic DNA. On the contrary, higher doses in the vicinity of 30 Gy were required to eliminate myeloid cells within the tumors before T cells were able to infiltrate the tumor (28,56). Human subjects’ studies displayed similar conflicting conclusions. In a study by Qin et al., patients receiving ablative doses of extracranial radiation therapy (median dose of 16 Gy per fraction in 1 fraction) had double the median OS as those who received conventionally fractionation (33 Gy in 11 fractions) (19.6 vs. 10.2 months; 95% CI, (14.0–38.1 months vs. 6.7 months to not reached), furthermore (41), on the contrary in studies by Chandra et al. and Patel et al., increased fractions at lower dose per fraction resulted in improved outcomes (50,57). Chandra et al. noted among patients with metastatic melanoma radiation fraction size was the only variable that remained significant for improved rate of index response in univariate and multivariate analysis; specifically, a radiation fraction size of ≤3 Gy was associated with an improved rate of index lesion (50), similarly in Patel et al.’s examination patients who received 2–5 fractions were noted to have a higher response rate in comparison to those receiving a single fraction (57).
Many studies on concurrent ICPI and radiotherapy focus on metastatic brain melanoma. Few studies have examined other malignancies and the efficacy of combined treatment at other sites. Tang et al. examined 34 patients who received either concurrent or sequential treatment with Ipilimumab and radiotherapy at either the lung or liver for the following primaries; non-small cell lung cancer (NSCLC), colorectal carcinoma, sarcoma and renal cell carcinoma (43). Clinical benefit correlated with increased ratios of CD8+/CD4+ T cells and increased peripheral cells expressing ICOS, GITR, and 4-1BB (43). In comparison to combined hepatic and lung radiation, hepatic radiation was associated with higher levels of CD8+ T cells, increased expression of ICOS, GITR, and LAG3 among those CD8+ cells, and increased expression of 4-1BB, GITR, TIM-3 and PD1 among CD4+ Treg cells (43), all pro-inflammatory changes, however, since multiple tumor specimens were studied and there was an unequal representation of the different histologies at liver and lung sites, the inherent differences among these primaries can confound the results (43).
Toxicity
Both modalities have inherent toxicities that have the potential to amplify when combined. Radiation has systemic adversities including dermatitis, pneumonitis, esophagitis, emesis (58), and specific to intracranial tumors necrosis and hemorrhage resulting in inflammation and mass effect (59). Immune therapy can cause gastrointestinal effects including vomiting and liver injury as well as other systemic manifestations including dermatitis, fatigue, and anemia (60). No difference in adverse effects were noted among patients in most of the studies examined who received combination therapy compared to those who received either modality alone, therefore it is important to highlight the studies that noted a difference (34,36,37,39,42). Kiess et al. noted an increase in brain mass diameter due to edema and hemorrhage of 150% in half the patients treated with radiation during or before Ipilimumab, but not present in patients treated with radiation after Ipilimumab (40). In particular, patients treated with radiation before Ipilimumab, had an increase in mass diameter after the initiation of Ipilimumab, indicating that the inflammation, hemorrhage, and edema associated with radiation was exacerbated by the addition of Ipilimumab and these changes can clinically manifest as headaches, seizures and other neurologic symptoms (40). In this study, patients were only monitored for 1 year, however, as inflammation subsides these symptoms should minimize and follow up past 1 year can determine whether these changes are reversible. Steroids have thus far been used to manage these cerebral effects, however as patients receiving concurrent therapy have improved survival rates, long term use of steroids to minimize inflammation can cause systemic effects, necessitating the need for alternative modalities to reduce the use of steroids including the potential use of surgical intervention to reduce the size of the brain mass (38). In contrast to Kiess et al.’s study, Ahmed et al., reported only 11% of cases with an increase in brain mass volume due to hemorrhage and edema when concurrent therapy of anti-PD1 and radiation therapy was used, this difference questions whether there is a difference in the interplay between the different ICPI agents and radiation that could account for their distinct adverse effect profiles (46).
Future studies
Biomarkers
Biomarkers are critical to predict tumor response to ICPI (61,62) as studies have noted improved efficacy of ICPI targeted at tumors with increased mutations (63) and specific markers (64). In vitro studies have noted that cGAS and STING expression is important in the transcription of factors required for the release of IFN-1 whereas in vivo studies have yet to identify specific mutations and genetic markers that prognosticate good treatment response to ICPI (32,36,37,56). Further examination for these markers is critical to help predict prior to treatment patient response to both help design treatment plans constructed for the patient’s particular tumor composition and to minimize unnecessary financial costs for patients who would not benefit from ICPI.
Conclusions
ICPI and radiotherapy enhance the capacity of the intrinsic immune system to overcome the anti-inflammatory properties inherent to tumor cells and create a pro-inflammatory milieu that has potential to maintain sustained anti-tumorigenic properties via immunological memory. Studies show improved outcomes with minimal adverse effects, however, more clinical trials are required to establish a better understanding of how elements such as radiation dosage, tumor histology, and treatment schedule interact to effect treatment response. Although, the purpose of this review is to clarify these points, it is evident that with only a limited number of studies involving human subjects examining combination therapy, definitive answers are difficult to conclude emphasizing the complexity of this relationship and suggesting the future of these modalities is perhaps not to have stringent guidelines, but rather be individually catered to complement both the genetic makeup of the tumor and the patient.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Batlevi CL, Matsuki E, Brentjens RJ, et al. Novel immunotherapies in lymphoid malignancies. Nature reviews Clinical oncology 2016;13:25-40. 10.1038/nrclinonc.2015.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanson HL, Donermeyer DL, Ikeda H, et al. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity 2000;13:265-76. 10.1016/S1074-7613(00)00026-1 [DOI] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frank SA. Immunology and Evolution of Infectious Disease. Princeton (NJ): Princeton University Press, 2002. [PubMed] [Google Scholar]
- 5.Wolchok JD, Saenger Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist 2008;13 Suppl 4:2-9. 10.1634/theoncologist.13-S4-2 [DOI] [PubMed] [Google Scholar]
- 6.Peggs KS, Quezada SA, Korman AJ, et al. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol 2006;18:206-13. 10.1016/j.coi.2006.01.011 [DOI] [PubMed] [Google Scholar]
- 7.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity 2002;16:23-35. 10.1016/S1074-7613(01)00259-X [DOI] [PubMed] [Google Scholar]
- 8.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 2002;3:611-8. 10.1038/ni0702-611 [DOI] [PubMed] [Google Scholar]
- 9.Fallarino F, Fields PE, Gajewski TF. B7-1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J Exp Med 1998;188:205-10. 10.1084/jem.188.1.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masteller EL, Chuang E, Mullen AC, et al. Structural analysis of CTLA-4 function in vivo. J Immunol 2000;164:5319-27. 10.4049/jimmunol.164.10.5319 [DOI] [PubMed] [Google Scholar]
- 11.Schneider H, Downey J, Smith A, et al. Reversal of the TCR stop signal by CTLA-4. Science 2006;313:1972-5. 10.1126/science.1131078 [DOI] [PubMed] [Google Scholar]
- 12.Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol 2016;39:98-106. 10.1097/COC.0000000000000239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev 2008;224:166-82. 10.1111/j.1600-065X.2008.00662.x [DOI] [PubMed] [Google Scholar]
- 14.Robert L, Tsoi J, Wang X, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res 2014;20:2424-32. 10.1158/1078-0432.CCR-13-2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohaegbulam KC, Assal A, Lazar-Molnar E, et al. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med 2015;21:24-33. 10.1016/j.molmed.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wherry EJ. T cell exhaustion. Nat Immunol 2011;12:492. 10.1038/ni.2035 [DOI] [PubMed] [Google Scholar]
- 17.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 2004;4:336-47. 10.1038/nri1349 [DOI] [PubMed] [Google Scholar]
- 18.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodhankar S, Chen Y, Lapato A, et al. Targeting immune co-stimulatory effects of PD-L1 and PD-L2 might represent an effective therapeutic strategy in stroke. Front Cell Neurosci 2014;8:228. 10.3389/fncel.2014.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res 2007;13:5271-9. 10.1158/1078-0432.CCR-07-1030 [DOI] [PubMed] [Google Scholar]
- 21.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014;20:5064-74. 10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005;25:9543-53. 10.1128/MCB.25.21.9543-9553.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callahan MK, Postow MA, Wolchok JD. CTLA-4 and PD-1 Pathway Blockade: Combinations in the Clinic. Front Oncol 2015;4:385. 10.3389/fonc.2014.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golden EB, Frances D, Pellicciotta I, et al. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 2014;3:e28518. 10.4161/onci.28518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilones KA, Vanpouille-Box C, Demaria S. Combination of radiotherapy and immune checkpoint inhibitors. Semin Radiat Oncol 2015;25:28-33. 10.1016/j.semradonc.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 27.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res 2005;11:3353-62. 10.1158/1078-0432.CCR-04-2062 [DOI] [PubMed] [Google Scholar]
- 28.Vanpouille-Box C, Formenti SC, Demaria S. Toward Precision Radiotherapy for Use with Immune Checkpoint Blockers. Clin Cancer Res 2018;24:259-65. 10.1158/1078-0432.CCR-16-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teng F, Kong L, Meng X, et al. Radiotherapy combined with immune checkpoint blockade immunotherapy: Achievements and challenges. Cancer Lett 2015;365:23-9. 10.1016/j.canlet.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 30.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013;105:256-65. 10.1093/jnci/djs629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.An Y, Jiang W, Kim BYS, et al. Stereotactic radiosurgery of early melanoma brain metastases after initiation of anti-CTLA-4 treatment is associated with improved intracranial control. Radiother Oncol 2017;125:80-8. 10.1016/j.radonc.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 33.Barker CA, Postow MA, Khan SA, et al. Concurrent Radiotherapy and Ipilimumab Immunotherapy for Patients with Melanoma. Cancer Immunol Res 2013;1:92-8. 10.1158/2326-6066.CIR-13-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silk AW, Bassetti MF, West BT, et al. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2013;2:899-906. 10.1002/cam4.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerber NK, Young RJ, Barker CA, et al. Ipilimumab and whole brain radiation therapy for melanoma brain metastases. J Neurooncol 2015;121:159-65. 10.1007/s11060-014-1617-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathew M, Tam M, Ott PA, et al. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res 2013;23:191-5. 10.1097/CMR.0b013e32835f3d90 [DOI] [PubMed] [Google Scholar]
- 37.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:700-12. 10.1016/S1470-2045(14)70189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knisely JP, Yu JB, Flanigan J, et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg 2012;117:227-33. 10.3171/2012.5.JNS111929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tazi K, Hathaway A, Chiuzan C, et al. Survival of melanoma patients with brain metastases treated with ipilimumab and stereotactic radiosurgery. Cancer Med 2015;4:1-6. 10.1002/cam4.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiess AP, Wolchok JD, Barker CA, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys 2015;92:368-75. 10.1016/j.ijrobp.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin R, Olson A, Singh B, et al. Safety and Efficacy of Radiation Therapy in Advanced Melanoma Patients Treated With Ipilimumab. Int J Radiat Oncol Biol Phys 2016;96:72-7. 10.1016/j.ijrobp.2016.04.017 [DOI] [PubMed] [Google Scholar]
- 42.Patel KR, Shoukat S, Oliver DE, et al. Ipilimumab and Stereotactic Radiosurgery Versus Stereotactic Radiosurgery Alone for Newly Diagnosed Melanoma Brain Metastases. Am J Clin Oncol 2017;40:444-50. 10.1097/COC.0000000000000199 [DOI] [PubMed] [Google Scholar]
- 43.Tang C, Welsh JW, de Groot P, et al. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clin Cancer Res 2017;23:1388-96. 10.1158/1078-0432.CCR-16-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaudy-Marqueste C, Dussouil AS, Carron R, et al. Survival of melanoma patients treated with targeted therapy and immunotherapy after systematic upfront control of brain metastases by radiosurgery. Eur J Cancer 2017;84:44-54. 10.1016/j.ejca.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 45.Liniker E, Menzies AM, Kong BY, et al. Activity and safety of radiotherapy with anti-PD-1 drug therapy in patients with metastatic melanoma. Oncoimmunology 2016;5:e1214788. 10.1080/2162402X.2016.1214788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed KA, Stallworth DG, Kim Y, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol 2016;27:434-41. 10.1093/annonc/mdv622 [DOI] [PubMed] [Google Scholar]
- 47.Qian JM, Yu JB, Kluger HM, et al. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer 2016;122:3051-8. 10.1002/cncr.30138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aboudaram A, Modesto A, Chaltiel L, et al. Concurrent radiotherapy for patients with metastatic melanoma and receiving anti-programmed-death 1 therapy: a safe and effective combination. Melanoma Res 2017;27:485-91. 10.1097/CMR.0000000000000386 [DOI] [PubMed] [Google Scholar]
- 49.Ribeiro Gomes J, Schmerling RA, Haddad CK, et al. Analysis of the Abscopal Effect With Anti-PD1 Therapy in Patients With Metastatic Solid Tumors. J Immunother 2016;39:367-72. 10.1097/CJI.0000000000000141 [DOI] [PubMed] [Google Scholar]
- 50.Chandra RA, Wilhite TJ, Balboni TA, et al. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology 2015;4:e1046028. 10.1080/2162402X.2015.1046028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koller KM, Mackley HB, Liu J, et al. Improved survival and complete response rates in patients with advanced melanoma treated with concurrent ipilimumab and radiotherapy versus ipilimumab alone. Cancer Biol Ther 2017;18:36-42. 10.1080/15384047.2016.1264543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan A, Porcher R, Crequit P, et al. Differences in Treatment Effect Size Between Overall Survival and Progression-Free Survival in Immunotherapy Trials: A Meta-Epidemiologic Study of Trials With Results Posted at ClinicalTrials.gov. J Clin Oncol 2017;35:1686-94. 10.1200/JCO.2016.71.2109 [DOI] [PubMed] [Google Scholar]
- 53.Grob TJ, Novak U, Maisse C, et al. Human delta Np73 regulates a dominant negative feedback loop for TAp73 and p53. Cell Death Differ 2001;8:1213-23. 10.1038/sj.cdd.4400962 [DOI] [PubMed] [Google Scholar]
- 54.Bergamaschi D, Samuels Y, O’Neil NJ, et al. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet 2003;33:162-7. 10.1038/ng1070 [DOI] [PubMed] [Google Scholar]
- 55.Pandolfi S, Montagnani V, Lapucci A, et al. HEDGEHOG/GLI-E2F1 axis modulates iASPP expression and function and regulates melanoma cell growth. Cell Death Differ 2015;22:2006-19. 10.1038/cdd.2015.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Filatenkov A, Baker J, Mueller AM, et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin Cancer Res 2015;21:3727-39. 10.1158/1078-0432.CCR-14-2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel KR, Oliver DE, Okwan-Dadu D, et al. Abstract 247: Comparing radiation therapy and ipilimumab to ipilimumab alone in metastatic melanoma patients. Cancer Res 2015;75:247 10.1158/1538-7445.AM2015-247 [DOI] [Google Scholar]
- 58.Berkey FJ. Managing the adverse effects of radiation therapy. Am Fam Physician 2010;82:381-8, 94. [PubMed]
- 59.Tomura N, Izumi J, Sakuma I, et al. Radiation-induced changes in the brain following stereotactic irradiation evaluated by sequential MRI. CMIG Extra Cases 2004;28:73-9. 10.1016/j.compmedimag.2004.10.003 [DOI] [Google Scholar]
- 60.Long J, Lin J, Wang A, et al. PD-1/PD-L blockade in gastrointestinal cancers: lessons learned and the road toward precision immunotherapy. J Hematol Oncol 2017;10:146. 10.1186/s13045-017-0511-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marginean EC, Melosky B. Is There a Role for Programmed Death Ligand-1 Testing and Immunotherapy in Colorectal Cancer With Microsatellite Instability? Part I, Colorectal Cancer: Microsatellite Instability, Testing, and Clinical Implications. Arch Pathol Lab Med 2018;142:17-25. 10.5858/arpa.2017-0040-RA [DOI] [PubMed] [Google Scholar]
- 62.Clark DP. Biomarkers for immune checkpoint inhibitors: The importance of tumor topography and the challenges to cytopathology. Cancer Cytopathol 2018;126:11-9. 10.1002/cncy.21951 [DOI] [PubMed] [Google Scholar]
- 63.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agostini LP, Stur E, Garcia FM, et al. ATM, BCL2, and TGFbeta Gene Polymorphisms as Radiotherapy Outcome Biomarkers in Head and Neck Squamous Cell Carcinoma Patients. Genet Test Mol Biomarkers 2017;21:727-35. 10.1089/gtmb.2017.0180 [DOI] [PubMed] [Google Scholar]