Abstract
OBJECTIVE:
The aim of this study was to assess the relationship between the degree of unilateral spatial neglect during the acute phase of stroke and long-term functional independence.
METHODS:
This was a prospective study of right ischemic stroke patients in which the independent variable was the degree of spatial neglect and the outcome that was measured was functional independence. The potential confounding factors included sex, age, stroke severity, topography of the lesion, risk factors, glycemia and the treatment received. Unilateral spatial neglect was measured using the line cancellation test, the star cancellation test and the line bisection test within 48 hours of the onset of symptoms. Functional independence was measured using the modified Rankin and Barthel scales at 90 days after discharge. The relationship between unilateral spatial neglect and functional independence was analyzed using multiple logistic regression that was corrected for confounding factors.
RESULTS:
We studied 60 patients with a median age of 68 (34–89) years, 52% of whom were male and 74% of whom were Caucasian. The risk for moderate to severe disability increased with increasing star cancellation test scores (OR=1.14 [1.03–1.26], p=0.01) corrected for the stroke severity, which was a confounding factor that had a statistically positive association with disability (OR=1.63 [1.13–2.65], p=0.01). The best chance of functional independence decreased with increasing star cancellation test scores (OR=0.86 [0.78–0.96], p=0.006) corrected for the stroke severity, which was a confounding factor that had a statistically negative association with independence (OR=0.66 [0.48–0.92], p=0.017).
CONCLUSION:
The severity of unilateral spatial neglect in acute stroke worsens the degree of long-term disability and functional independence.
Keywords: Stroke, Unilateral Spatial Neglect, Functional Independence, Incapacity, Autonomy
INTRODUCTION
Stroke is both the second leading cause of death worldwide and the primary cause of chronic disability in adults 1–5. Unilateral spatial neglect (USN) is characterized by the inability to respond to people or objects that are presented contralateral to the lesioned side of the brain and is also a symptom that cannot be accounted for by either motor or sensory deficits. USN is more common in right- than left-hemisphere strokes and can contribute to disability 6,7. The incidence of USN varies widely, ranging from 10–82% in right-hemisphere stroke patients 8-10. The main areas involved in USN are related to the right hemisphere, such as lesions in the right posterior parietal lobe 11-12, and individuals with USN after stroke present with major functional disabilities as well as decreased rates of adherence to rehabilitation programs 13-15. Furthermore, USN can decrease a patient’s ability to return to work and thus has socioeconomic impacts on a community’s public health status 16,17. The aim of this study was to evaluate the relationship between the degree of USN during the acute phase of stroke with disability and long-term functional independence. The main hypothesis of this study was that a higher degree of USN during the acute phase of a stroke with disability would predict greater long-term disability.
MATERIALS AND METHODS
Study design and participants
This observational study was conducted in accordance with the principles of the Declaration of Helsinki. Patients were selected after the study protocol was approved by the institutional review board of the Botucatu Medical School (Of. 122/11). All participants or their legal representatives were aware of the study objectives and provided written informed consent.
This study included 60 individuals who had suffered a right-hemisphere stroke, as confirmed by a CT or MRI scan, with a cut-off Mini-Mental State Examination score of >24. The individuals were admitted to the stroke unit of the Botucatu Medical School between March 2015 and April 2016. We excluded patients with previous cranial trauma, hemorrhagic stroke, dementia, prior changes in vision, hemianopsia or other associated neurological diseases.
Measurement of unilateral spatial neglect and long-term outcomes
The degree of USN was measured during the acute phase of stroke, which was between 48 and 72 hours after the stroke onset, using three tests.
1) The line cancelation test (LCT): the degree of USN was determined by the proportion of lines that were omitted from a total of 40 lines randomly distributed on one sheet of paper 18. A greater omission of lines indicated more severe USN.
2) The star cancelation test (SCT): the degree of neglect was determined by the proportion of stars that were omitted from a total of 56 stars that were associated with distractors 19. A greater omission of stars indicated more severe USN.
3) The line bisection test (LBT): each patient was asked to detect and indicate the point corresponding to the midlines of 18 transverse lines that were arranged in three columns (at the left, center, and right of the page) of six lines each. The degree of neglect was determined by the location of the selected point relative to the midline 20. A greater deviation of the selected point from the midline indicated more severe USN.
In all the USN tests, the examiner placed the examination sheet in front of the patient with a distance of 60 cm between the glabella and the center of the paper 21.
The following are the potential confounding factors that could have affected the outcome of this study: age, sex, race, years of education, risk factors, topography (LACS=lacunar stroke; PACS=partial anterior circulation; TACS=total anterior circulation; POCS=posterior circulation), etiology (large-vessel occlusion, small-vessel occlusion, cardioembolism, other causes, or indeterminate), National Institutes of Health Stroke Scale (NIHSS) score at admission, blood glucose level at admission and treatment received. The risk factors evaluated included hypertension, smoking, obesity, diabetes mellitus (DM), alcohol consumption, dyslipidemia, prior stroke, congestive heart failure (CHF), prior acute myocardial infarction (AMI), atrial fibrillation and depression. Additional data, including any previous use of antihypertensive medications, oral hypoglycemic agents, parenteral insulin or oral lipid-lowering drugs, were either collected from the clinical history of each patient or were confirmed clinically by laboratory tests administered during hospitalization. Hypertension was indicated when the systolic blood pressure was ≥140/90 mmHg, dyslipidemia was indicated when cholesterol levels were ≥240 mg/dL, DM was indicated when the glycated hemoglobin level was >7%, obesity was indicated when the body mass index was ≥30 kg/m2, and depression was indicated by a score >8 on the Hospital Anxiety and Depression Scale (HADS) 22-25.
The long-term outcomes examined in this study were functional independence, as measured by the modified Rankin scale (mRS), and autonomy, as evaluated by the Barthel scale 26. The outpatient follow-up time was 90 days, and the outcomes were evaluated by the principal investigator of the study. Outcomes were classified as favorable if the patient presented an mRS score of 0–2 or unfavorable if the patient presented an mRS score of 3–5. The autonomy outcome was considered favorable if the Barthel index score was greater than 95 27. All evaluated patients underwent physiotherapy, which consisted of conventional exercises, with the rehabilitation service at the Botucatu Medical School.
Sample size
Because a sample of the target population was selected from a specific source, the sample type was considered nonprobabilistic. A previous study by our research group determined that it would be necessary to evaluate 50 patients to achieve a statistical power of 80% (beta error 0.2 and alpha error 0.05) 28.
Statistical analysis
Multiple logistic regression was used to analyze the effect of USN on disability and autonomy, and potential confounders were adjusted for by backward selection of data with a value of p>0.1. In the adjusted multiple regression model, statistical significance was set at p<0.05. Statistical analyses were performed using SPSS software version 21.0 (IBM®, Chicago, Illinois, USA).
RESULTS
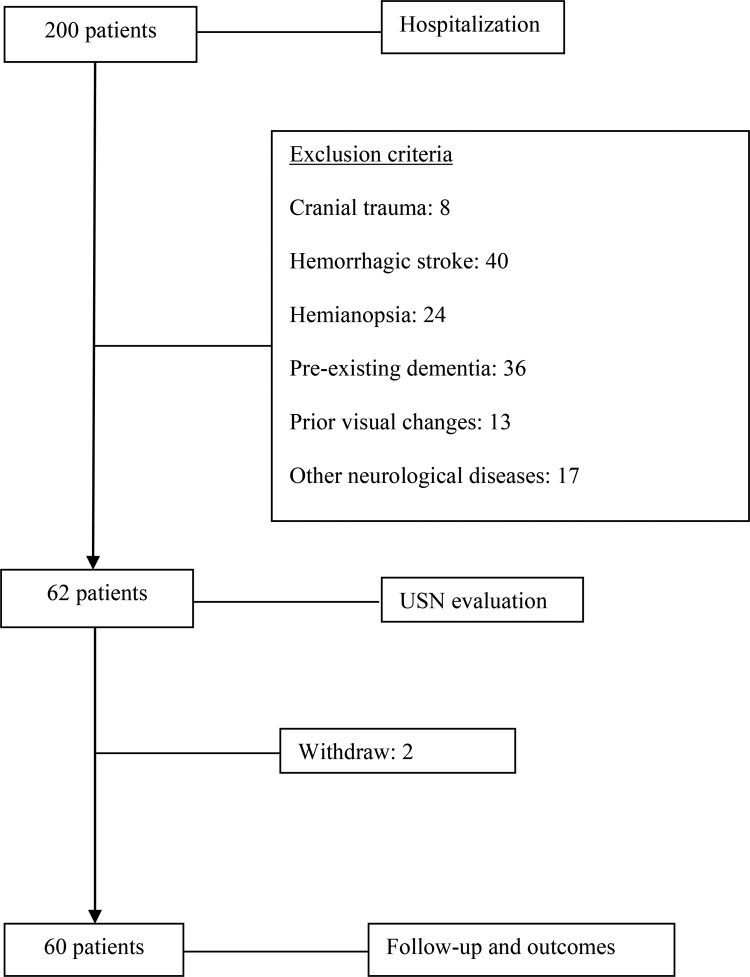
Sixty (60) of the 200 individuals recruited for the study met the eligibility criteria. The main reasons for exclusion of an individual were presentation of cranial trauma (n=8), previous hemorrhagic stroke (n=40), hemianopsia (n=24), previous presentation of dementia (n=36), prior changes in vision (n=13) and other neurological diseases (n=17). Two patients discontinued participation in the study during the follow-up period (Figure 1).
Figure 1.
Flow diagram of the patients included in the study.
The clinical and demographic data of the patients are displayed in Table 1. The median age was 68 years, and the patients were predominantly male and white. Hypertension was the main risk factor, and the clinical-topographic classification was predominantly PACS with cardioembolic etiology. The mean blood glucose level at entry was 114 mg/dL, and the average NIHSS score was 11. Among the patients studied, 21 received conservative treatment, 9 underwent intravenous thrombolysis, and 5 underwent a decompressive hemicraniectomy.
Table 1.
Description of the sample.
| Variable | N=60 | % |
|---|---|---|
| Demographic | ||
| Male | 31 | 51.6 |
| Age (years)1 | 68 (34–89) | |
| Race | ||
| Caucasian | 48 | 80.0 |
| Non-Caucasian | 12 | 20.0 |
| Years of education | 7 (1 – 10) | |
| Risk factors | ||
| Hypertension | 45 | 75.0 |
| Diabetes | 18 | 30.0 |
| Smoking | 22 | 36.6 |
| Obesity | 8 | 13.3 |
| Alcoholic | 8 | 13.3 |
| Prior stroke | 5 | 8.3 |
| CHF | 2 | 5.7 |
| Prior AMI | 4 | 3.3 |
| AF | 8 | 13.3 |
| Depression | 8 | 13.3 |
| BAMFORD | ||
| LACS | 6 | 10.0 |
| PACS | 30 | 50.0 |
| TACS | 20 | 33.3 |
| POCS | 4 | 6.7 |
| TOAST | ||
| Large-artery atherosclerosis | 15 | 25.0 |
| Cardioembolism | 21 | 35.0 |
| Small-vessel occlusion | 6 | 10.0 |
| Other determined etiology | 6 | 10.0 |
| Undetermined etiology | 12 | 20.0 |
| Glycemia at admission1 | 114 (71–140) | |
| NHISS at admission1 | 11 (3–24) | |
| Treatment | ||
| Conservative | 35 | 58.3 |
| Thrombolysis | 16 | 26.6 |
| Surgery | 9 | 15.0 |
Values are presented as the median; CHF=congestive heart failure; AMI=acute myocardial infarction; AF=atrial fibrillation; LACS=lacunar stroke; PACS=partial anterior circulation; TACS=total anterior circulation; POCS=posterior circulation; NIHSS=National Institutes of Health Stroke Scale.
In the univariate analyses of the mRS (Table 2) and the Barthel index (Table 3), the only variables that were correlated with the outcome were the NIHSS score and the SCT score.
Table 2.
Univariate analyses comparing patients with favorable vs. unfavorable modified Rankin scale scores.
| Variables | Modified Rankin Scale cut-off | ||
|---|---|---|---|
| 0-2 (n=19) | 3-5 (n=41) | p | |
| Demographic | |||
| Median Age | 66.0 (44.0 - 88.0) | 68.0 (34.0 - 89.0) | 0.503 |
| Non-Caucasian | 3 (15.8%) | 9 (22.0%) | 0.735 |
| Median years of education | 6 (1-9) | 7 (1-10) | 0.335 |
| Risk factors | |||
| Hypertension | 12 (63.2%) | 33 (80.5%) | 0.103 |
| Diabetes | 6 (31.6%) | 12 (29.3%) | 0.856 |
| Smoking | 7 (36.8%) | 15 (36.6%) | 0.985 |
| Obesity | 4 (21.1%) | 4 (9.8%) | 0.249 |
| Alcoholic | 2 (10.5%) | 6 (14.6%) | 1.000 |
| Prior stroke | 1 (5.3%) | 4 (9.8%) | 1.000 |
| CHF | 1 (5.3%) | 1 (2.4%) | 0.537 |
| Prior AMI | 1 (5.3%) | 3 (7.3%) | 1.000 |
| AF | 4 (21.1%) | 4 (9.8%) | 0.249 |
| Depression | 3 (15.8%) | 5 (12.2%) | 0.699 |
| Glycemia at admission | 114.0 (87.0 - 140.0) | 114.0 (71.0 - 140.0) | 0.663 |
| NIHSS at admission | 6.0 (3.0 - 11.0) | 13.0 (4.0 - 24.0) | <0.001 |
| LCT | 10.0 (0.0 - 28.0) | 30.0 (6.0 - 40.0) | 0.056 |
| SCT | 22.0 (6.0 - 40.0) | 40.0 (16.0 - 52.0) | <0.001 |
| LBT | 42.3 (15.7 - 72.7) | 78.3 (29.8 - 98.8) | 0.068 |
CHF=congestive heart failure; AMI=acute myocardial infarction; AF=atrial fibrillation; NIHSS=National Institutes of Health Stroke Scale; LCT=line cancellation test; SCT=star cancellation test; LBT=line bisection test.
Table 3.
Univariate analyses comparing patients with favorable vs. unfavorable Barthel index scores.
| Variables | Barthel Cut-off | ||
|---|---|---|---|
| <95 (n=6) | ≥95 (n=54) | ||
| Demographic | |||
| Median age | 61.0 (44.0 - 76.0) | 68.5 (34.0 - 89.0) | 0.056 |
| Non-Caucasian | 0 (0%) | 12 (22.2%) | 0.333 |
| Median years of education | 6 (1-9) | 7 (1-10) | 0.399 |
| Risk factors | |||
| Hypertension | 4 (66.7%) | 41 (77.4%) | 0.620 |
| Diabetes | 2 (33.3%) | 16 (29.6%) | 1.000 |
| Smoking | 1 (16.7%) | 21 (38.9%) | 0.400 |
| Obesity | 0 (0.0%) | 8 (14.8%) | 0.585 |
| Alcoholic | 1 (16.7%) | 7 (13.0%) | 1.000 |
| Prior stroke | 1 (16.7%) | 4 (7.4%) | 0.421 |
| CHF | 0 (0.0%) | 2 (3.7%) | 1.000 |
| Prior AMI | 0 (0.0%) | 4 (7.4%) | 1.000 |
| AF | 1 (16.7%) | 7 (13.0%) | 1.000 |
| Depression | 0 (0.0%) | 8 (14.8%) | 0.585 |
| Glycemia at admission | 96.0 (87.0 - 137.0) | 114.0 (71.0-140.0) | 0.056 |
| NIHSS at admission | 8.0 (.0 - 11.0) | 11.0 (3.0 - 24.0) | <0.001 |
| LCT | 11.5 (2.0 - 28.0) | 25.0 (0.0 - 40.0) | 0.074 |
| SCT | 23.0 (14.0 - 40.0) | 37.0 (6.0 - 52.0) | 0.048 |
CHF=congestive heart failure; AMI=acute myocardial infarction; AF=atrial fibrillation; NIHSS=National Institutes of Health Stroke Scale; LCT=line cancellation test; SCT=star cancellation test; LBT=line bisection test.
The risk of moderate to severe 3–5 disability, as measured by the mRS, increased with increasing SCT scores (OR=1.14 [1.03–1.26], p=0.010); this result was corrected for the effect of potential confounders. The NIHSS score was a confounder with a significantly positive association with disability (OR=1.63 [1.13–2.65], p=0.010; Table 4).
Table 4.
Model adjusted to explain the chance of moderate to severe disability 90 days after a stroke, as measured by the mRS as a function of the star cancellation test.
| Variable | β | SE | p | OR | 95% CI | |
|---|---|---|---|---|---|---|
| NIHSS | 0.49 | 0.19 | 0.010 | 1.63 | 1.13 | 2.35 |
| SCT | 0.13 | 0.05 | 0.010 | 1.14 | 1.03 | 1.26 |
| Constant | -8.41 | 2.89 | 0.004 | 0.00 | ||
NIHSS=National Institutes of Health Stroke Scale; SCT=star cancellation test; β=beta estimate; SE=standard error; OR=odds ratio; CI=confidence interval.
The likelihood of functional independence, as measured by the Barthel scale, decreased with increasing SCT scores (OR=0.86 [0.78–0.96], p=0.006); this result was corrected for the effect of potential confounders. The NIHSS score was a confounder with a significantly negative association with independence (OR=0.66 [0.48–0.92], p=0.017; Table 5).
Table 5.
Model adjusted to explain the chance of autonomy 90 days after a stroke, as measured by the Barthel index as a function of the star cancellation test.
| Variable | β | SE | p | OR | 95% CI | |
|---|---|---|---|---|---|---|
| NIHSS | 0.40 | 0.17 | 0.017 | 0.66 | 0.48 | 0.92 |
| SCT | 0.14 | 0.05 | 0.006 | 0.86 | 0.78 | 0.95 |
| Constant | -7.63 | 2.65 | 0.004 | 0.00 | ||
NIHSS=National Institutes of Health Stroke Scale; SCT=star cancellation test; β=beta estimate; SE=standard error; OR=odds ratio; CI=confidence interval.
DISCUSSION
This study demonstrated that the degree of acute-stage USN is an important predictor of long-term disability in patients who have experienced a right-sided stroke. It is well established in the literature that the NIHSS score on admission affects the functional outcome of patients with ischemic stroke. However, in the subgroup of patients with USN, several factors may interfere with the functional outcome 29,30. The degree of USN is related to the region of the ischemic or hemorrhagic lesion, and higher degrees of USN have been reported in patients with lesions in the posterior parietal region, which interfere with the attention network and, consequently, diminish the performance of functional activities 31,32.
Our study is novel in three ways. First, we adjusted our data for the NIHSS confounder, whereas authors of previous studies have not tested for this correlation 33-34. Higher NIHSS scores are associated with extensive brain damage in the acute phase and a poorer prognosis in the chronic phase of stroke. High NIHSS scores in our study were associated with poor outcomes, and this result suggests that stroke severity affects recovery. Second, we used the mRS to determine the functional prognosis. This scale is widely used to measure functional outcomes in large clinical trials of stroke patients. Third, we tested the correlation between LCT or LBT scores and the functional prognosis of individuals with a right-hemispheric stroke.
The SCT was the best predictor of long-term disability in our study. Several authors have reported that cancelation tasks are generally the most sensitive tests and that the SCT is the most reliable test for measuring the degree of USN at any stage of stroke because of its high sensitivity and specificity, whereas the LBT has relatively poor sensitivity 13,35. The SCT is the strongest predictor of disability in patients who have experienced a stroke in the right hemisphere, with an efficacy similar to that of the NIHSS; this finding is internationally recognized by the scientific community.
In this study, we did not investigate the mechanisms underlying the unfavorable prognostic role of USN in the chronic phase, such as those involved in trunk control, postural balance and stroke volume, because the role of USN in this phase could be related to highly neglected findings and could influence the observed results. However, this work emphasizes the need for physicians to consider USN as an important prognostic factor in ischemic stroke. USN is neglected by the major neurological scales; if a patient with only USN presents during the acute phase of ischemic stroke, the treatment protocols do not consider initiating thrombolytic therapy as they do for aphasic patients. The importance of USN should be considered in protocols for the evaluation and treatment of the acute phase of stroke.
In conclusion, patients presenting with more severe USN during the acute phase of a right-hemisphere ischemic stroke have a poorer prognosis in terms of functional independence and long-term autonomy than do patients with less severe USN.
AUTHOR CONTRIBUTIONS
Luvizutto GJ was responsible for the literature search, data collection, and manuscript writing. Moliga AF and Rizzatti GR were responsible for manuscript writing and data collection. Neto EM and Fogaroli MO were responsible for the data interpretation. Nunes HR was responsible for the data analysis and interpretation. Resende LA was responsible for the data interpretation and study design. Bazan R was responsible for the study design.
ACKNOWLEDGMENTS
The authors would like to thank the Clinics Hospital of the Botucatu Medical School for supporting this research. This work was supported by the [FAPESP] under grant [2015-14231-0].
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Bonita R. Epidemiology of stroke. Lancet. 1992;339((8789)):342–4. doi: 10.1016/0140-6736(92)91658-U. [DOI] [PubMed] [Google Scholar]
- 2.Bray BD, Ayis S, Campbell J, Hoffman A, Roughton M, Tyrrell PJ, et al. Associations between the organisation of stroke services, process of care, and mortality in England: prospective cohort study. BMJ. 2013;346:f2827. doi: 10.1136/bmj.f2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hata J, Kiyohara Y. Epidemiology of stroke and coronary artery disease in Asia. Circ J. 2013;77((8)):1923–32. doi: 10.1253/circj.CJ-13-0786. [DOI] [PubMed] [Google Scholar]
- 4.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44((3)):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 5.Pontes-Neto OM, Silva GS, Feitosa MR, de Figueiredo NL, Fiorot JA, Jr, Rocha TN, et al. Stroke awareness in Brazil: alarming results in a community-based study. Stroke. 2008;39((2)):292–6. doi: 10.1161/STROKEAHA.107.493908. [DOI] [PubMed] [Google Scholar]
- 6.Gorgoraptis N, Mah YH, Machner B, Singh-Curry V, Malhotra P, Hadji-Michael M, et al. The effects of the dopamine agonist rotigotine on hemispatial neglect following stroke. Brain. 2012;135((Pt 8)):2478–91. doi: 10.1093/brain/aws154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P, Hreha K, Fortis P, Goedert KM, Barrett AM. Functional assessment of spatial neglect: a review of the Catherine Bergego Scale and an introduction of the Kessler Foundation neglect assessment process. Top Stroke Rehabil. 2012;19((5)):423–35. doi: 10.1310/tsr1905-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone SP, Halligan PW, Greenwood RJ. The incidence of neglect phenomena and related disorders in patients with an acute right or left hemisphere stroke. Age Ageing. 1993;22((1)):46–52. doi: 10.1093/ageing/22.1.46. [DOI] [PubMed] [Google Scholar]
- 9.Vanier M, Gauthier L, Lambert J, Pepin EP, Robillard A, Dubouloz CJ, et al. Evaluation of left visuospatial neglect: norms and discrimination power of two tests. Neuropsychology. 1990;4:87–96. doi: 10.1037/0894-4105.4.2.87. [DOI] [Google Scholar]
- 10.Plummer P, Morris ME, Dunai J. Assessment of unilateral neglect. Phys Ther. 2003;83((8)):732–40. [PubMed] [Google Scholar]
- 11.Tanaka T, Ifukube T, Sugihara S, Izumi T. A case study of new assessment and training of unilateral spatial neglect in stroke patients: effect of visual image transformation and visual stimulation by using a Head Mounted Display system (HMD) J Neuroeng Rehabil. 2010;7:20. doi: 10.1186/1743-0003-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azouvi P, Olivier S, de Montety G, Samuel C, Louis-Dreyfus A, Tesio L. Behavioral assessment of unilateral neglect: study of the psychometric properties of the Catherine Bergego Scale. Arch Phys Med Rehabil. 2003;84((1)):51–7. doi: 10.1053/apmr.2003.50062. [DOI] [PubMed] [Google Scholar]
- 13.Agrell BM, Dehlin OI, Dahlgren CJ. Neglect in elderly stroke patients: a comparison of five tests. Psychiatry Clin Neurosci. 1997;51((5)):295–300. doi: 10.1111/j.1440-1819.1997.tb03201.x. [DOI] [PubMed] [Google Scholar]
- 14.Gottesman RF, Kleinman JT, Davis C, Heidler-Gary J, Newhart M, Kannan V, et al. Unilateral neglect is more severe and common in older patients with right hemispheric stroke. Neurology. 2008;71((18)):1439–44. doi: 10.1212/01.wnl.0000327888.48230.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey M, Muir K, Reeves I, Duncan G, Birschel P, Roberts M, et al. Long term improvements in activities of daily living in patients with hemispatial neglect. Behav Neurol. 2010;23((4)):237–9. doi: 10.1155/2010/253161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown DL, Boden-Albala B, Langa KM, Lisabeth LD, Fair M, Smith MA, et al. Projected costs of ischemic stroke in the United States. Neurology. 2006;67((8)):1390–5. doi: 10.1212/01.wnl.0000237024.16438.20. [DOI] [PubMed] [Google Scholar]
- 17.Treger I, Shames J, Giaquinto S, Ring H. Return to work in stroke patients. Disabil Rehabil. 2007;29((17)):1397–403. doi: 10.1080/09638280701314923. [DOI] [PubMed] [Google Scholar]
- 18.Albert ML. A simple test of visual neglect. Neurology. 1973;23((6)):658–64. doi: 10.1212/WNL.23.6.658. [DOI] [PubMed] [Google Scholar]
- 19.Halligan PW, Burn JP, Marshall JC, Wade DT. Visuo-spatial neglect: qualitative differences and laterality of cerebral lesion. J Neurol Neurosurg Psychiatry. 1992;55((11)):1060–8. doi: 10.1136/jnnp.55.11.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schenkenberg T, Bradford DC, Ajax ET. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology. 1980;30((5)):509–17. doi: 10.1212/WNL.30.5.509. [DOI] [PubMed] [Google Scholar]
- 21.Keller I, Schindler I, Kerkhoff G, von Rosen F, Golz D. Visuospatial neglect in near and far space: dissociation between line bisection and letter cancellation. Neuropsychologia. 2005;43((5)):724–31. doi: 10.1016/j.neuropsychologia.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31((7)):1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 23.Jellinger PS, Smith DA, Mehta AE, Ganda O, Handelsman Y, Rodbard HW, et al. American Association of Clinical Endocrinologists’ guidelines for management of dyslipidemia and prevention of atherosclerosis. Endocr Pract. 2012;18(Suppl 1):1–78. doi: 10.4158/EP.18.S1.1. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association. Standards of medical care in diabetes--2008. Diabetes Care. 2008;31(Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 25.Wirth A, Wabitsch M, Hauner H. The prevention and treatment of obesity. Dtsch Arztebl Int. 2014;111((42)):705–13. doi: 10.3238/arztebl.2014.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cincura C, Pontes-Neto OM, Neville IS, Mendes HF, Menezes DF, Mariano DC, et al. Validation of the National Institutes of Health Stroke Scale, modified Rankin Scale and Barthel Index in Brazil: the role of cultural adaptation and structured interviewing. Cerebrovasc Dis. 2009;27((2)):119–22. doi: 10.1159/000177918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balu S. Differences in psychometric properties, cut-off scores and outcomes between the Barthel Index and Modified Rankin Scale in pharmacotherapy-based stroke trials: systematic literature review. Curr Med Res Opin. 2009;25((6)):1329–41. doi: 10.1185/03007990902875877. [DOI] [PubMed] [Google Scholar]
- 28.Luvizutto GJ, Monteiro TA, Braga GP, Bazan SG, Resende LA, Bazan R. Low haemoglobin levels increase unilateral spatial neglect in acute phase of stroke. Arq Neuropsiquiatr. 2014;72((10)):757–61. doi: 10.1590/0004-282X20140112. [DOI] [PubMed] [Google Scholar]
- 29.Lyden P, Lu M, Jackson C, Marler J, Kothari R, Brott T, Zivin J. Underlying structure of the National Institutes of Health Stroke Scale: results of a factor analysis. NINDS tPA Stroke Trial Investigators. Stroke. 1999;30((11)):2347–54. doi: 10.1161/01.STR.30.11.2347. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed R, Zuberi BF, Afsar S. Stroke scale score and early prediction of outcome after stroke. J Coll Physicians Surg Pak. 2004;14((5)):267–9. [PubMed] [Google Scholar]
- 31.Paolucci S, Antonucci G, Grasso MG, Pizzamiglio L. The role of unilateral spatial neglect in rehabilitation of right brain-damaged ischemic stroke patients: a matched comparison. Arch Phys Med Rehabil. 2001;82((6)):743–9. doi: 10.1053/apmr.2001.23191. [DOI] [PubMed] [Google Scholar]
- 32.Funahashi S, Kubota K. Working memory and prefrontal cortex. Neurosci Res. 1994;21((1)):1–11. doi: 10.1016/0168-0102(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 33.Di Monaco M, Schintu S, Dotta M, Barba S, Tappero R, Gindri P. Severity of unilateral spatial neglect is an independent predictor of functional outcome after acute inpatient rehabilitation in individuals with right hemispheric stroke. Arch Phys Med Rehabil. 2011;92((8)):1250–6. doi: 10.1016/j.apmr.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Farnè A, Buxbaum LJ, Ferraro M, Frassinetti F, Whyte J, Veramonti T, et al. Patterns of spontaneous recovery of neglect and associated disorders in acute right brain-damaged patients. J Neurol Neurosurg Psychiatry. 2004;75((10)):1401–10. doi: 10.1136/jnnp.2002.003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferber S, Karnath HO. How to assess spatial neglect-line bisection or cancellation tasks. J Clin Exp Neuropsychol. 2001;23((5)):599–607. doi: 10.1076/jcen.23.5.599.1243. [DOI] [PubMed] [Google Scholar]