Abstract
Reducing the plasma levels of low-density lipoprotein-cholesterol (LDL-C) is critical for patients with coronary heart disease (CHD). Conventional treatment with statins alone may not achieve the goal of lowering LDL-C due to drug intolerance or resistance. The present study evaluated the effectiveness and safety of combining statin with another lipid-lowering agent in the management of dyslipidemia in CHD patients. A total of 180 patients with CHD were divided into three therapeutic groups (n=60 in each): Statin/colesevelam group (20 mg atorvastatin and 10 mg colesevelam daily), statin/ezetimibe group (20 mg atorvastatin and 10 mg ezetimibe daily) and high-intensity statin monotherapy group (30 mg atorvastatin daily). The baseline plasma lipid levels were measured. The duration of the treatment was eight weeks and the side effects were noted at one year's follow-up. After eight weeks' treatment, the mean plasma level of LDL-C was reduced by 45.2, 44.8 and 30.0% in the statin/colesevelam, statin/ezetimibe and statin monotherapy group, respectively. The reduction of LDL-C in the combinational therapy groups was greater than that in the statin monotherapy group (P<0.05). The proportion of patients achieving the goal of lowering LDL-C in the combinational therapy groups was higher than that in the statin monotherapy group. The effectiveness of reducing lipids was similar in the two combinational statin/colesevelam and statin/ezetimibe groups. Rates of adverse events were not significantly different among the three groups. In conclusion, statins combined with colesevelam or ezetimibe were more effective in reducing plasma LDL-C levels than high-intensity statin monotherapy. This combinational therapeutic strategy may be an alternative for patients that are resistant or intolerant to statins.
Keywords: coronary heart disease, statin, colesevelam, ezetimibe, effectiveness
Introduction
It is well established that dyslipidemia is one of the most important risk factors for coronary heart disease (CHD) (1,2). For patients with established CHD, the use of lipid-lowering drugs is important in order to reduce the risk of recurrent cardiovascular events (3). Based on a series of clinical trials over the last two decades, statins block the rate-limiting step in the biosynthesis of cholesterol and are the first-line treatments used to decrease low-density lipoprotein-cholesterol (LDL-C) levels (4,5). The United States guidelines recommend using high-intensity statin therapy in coronary patients in order to achieve a lowering of LDL-C by at least 50% (6). European guidelines recommend an LDL-C goal of <1.8 mmol/l (70 mg/dl) or at least a 50% reduction of LDL-C in patients with established CHD (2,7).
Certain patients are resistant or poorly tolerant to statin treatment. Other lipid-lowering agents may be combined with statins to reduce lipid levels. Ezetimibe and colesevelam hydrocholoride are two cholesterol-lowering agents often used as add-on therapy to statins to further lower LDL-C levels when therapeutic goals are not achieved with statins alone. Colesevelam is the second-generation medication of bile acid sequestrant, which has a high binding affinity for specific bile acids (8,9). Studies have demonstrated that colesevelam in combination with other lipid-lowering drugs effectively lowers plasma lipid levels (10,11). By contrast, ezetimibe lowers LDL-C levels by inhibiting intestinal cholesterol absorption when used alone or with statin therapy (12,13). The results of the IMProved Reduction of Outcomes: Vytorin Efficacy International Trial indicated that significantly more CHD patients treated with a combination of a statin and ezetimibe met LDL-C goals than patients treated with statin alone (14,15). Ezetimibe-statin combination therapy reduces cardiovascular outcomes in patients following vascular surgery or acute coronary syndrome (16). The effectiveness and safety of combined therapy with statins and other lipid-lowering agents is worthy of further investigation.
Given the importance of LDL-C reduction in influencing long-term risks of cardiovascular events, it's important to investigate the clinical effectiveness of cholesterol-targeted agents (17). Only few studies have been performed to investigate the use combinational therapy in CHD patients. The present study investigated the effectiveness and safety of statin/colesevelam combination therapy, statin/ezetimibe combination therapy and high-intensity statin monotherapy in patients with CHD. The present study may provide guidance for the management of dyslipidemia in CHD patients.
Patients and methods
Enrollment of participants
The enrolled CHD patients with hypercholesterolemia were hospitalized and received percutaneous coronary intervention (PCI) at the Department of Cardiovascular Medicine of Linyi Central Hospital (Linyi, China) between January 2016 and June 2016. All of the participants were Chinese. The study was approved by the Ethics Committee of Linyi Central Hospital (Linyi, China). Patients provided written informed consent prior to the study commencing. None of the patients took any lipid-lowering agents within one month prior to admission and those with plasma LDL-C levels of >100 mg/dl were eligible for inclusion. Patients were required to have liver alanine aminotransferase (ALT) or aspartate aminotransferase (AST) and creatine phosphokinase (CK) of <50% above the upper limit of normal (ULN). Pregnant or lactating patients, patients with kidney or liver diseases, and patients with malignant tumors, autoimmune diseases or hypothyroidism were excluded from the study. In total, 180 patients were enrolled in the study.
Patient grouping
After receiving standard treatments for CHD, patients were randomly divided into three groups that received different lipid-lowering therapies: Statin/colesevelam combined therapy (statin/col; 20 mg atorvastatin and 10 mg colesevelam daily), statin/ezetimibe combined therapy (statin/eze; 20 mg atorvastatin and 10 mg ezetimibe daily) and high-intensity statin monotherapy (statin mono; 30 mg atorvastatin daily). The drugs were taken once a day. Plasma levels of lipids and enzymes were measured on admission and eight weeks after lipid-lowering therapy. Patients were followed up for one year after treatment for monitoring of adverse events.
No differences in the demographic data were noted among the three groups of patients. The baseline characteristics of the patients are summarized in Table I. The majority of the patients were male. The age (mean ± standard deviation) was 61±9.1 years in the statin/col group, 60±8.7 years in the statin/eze group and 60±8.5 years in the statin monotherapy group.
Table I.
Baseline characteristics of patients.
| Characteristics | Statin/col (n=60) | Statin/eze (n=60) | Statin mono (n=60) |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 61±9.1 | 60±8.7 | 60±8.5 |
| Males | 38 (63.3) | 40 (66.7) | 37 (61.7) |
| Hypertension | 34 (56.7) | 33 (55) | 35 (58.3) |
| Smoking history | 27 (45) | 29 (48.3) | 28 (46.7) |
| Clinical presentation | |||
| STEMI | 19 (31.7) | 20 (33.3) | 17 (28.3) |
| NSTE-ACS | 41 (68.3) | 40 (66.7) | 43 (71.7) |
Values are expressed as the mean ± standard deviation or as n (%). STEMI, ST-elevation myocardial infarction; NSTE-ACS, non-ST-segment elevation acute coronary syndrome; col, colesevelam; eze, ezetimibe; mono, monotherapy.
Effectiveness and safety measurements
Treatments were considered as effective when the LDL-C-lowering goals were achieved. The goal was defined as LDL-C <70 mg/dl (1.8 mmol/l) or a reduction of LDL-C by at least 50%. The effectiveness rates in the three groups of patients were compared. The percentage changes of LDL-C from baseline after eight weeks of treatment were recorded and compared. Plasma lipid levels were measured. Safety was assessed by recording the occurrence of adverse cardiovascular events, including all-cause death, recurrence of myocardial infarction, coronary revascularization and stroke. Adverse cardiovascular events were measured at one year after treatment of CHD.
Statistical analyses
Categorical variables were presented as absolute values or percentages. The Chi-square test was used for comparisons among three groups of patients. Continuous variables were described as the mean ± standard deviation and differences between groups were assessed by one-way analysis of variance followed by a Least Significant Difference test. P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed with SPSS 20 (IBM Corp., Armonk, NY, USA) or GraphPad prism 5 software (GraphPad, Inc., La Jolla, CA, USA).
Results
Baseline information of patients
The mean baseline LDL-C was 3.1±0.6, 2.9±0.4 and 3.0±0.6 mmol/l in the statin/col, statin/eze and statin mono group, respectively. Baseline values of plasma lipids and safety parameters were similar among the three groups (Table II).
Table II.
Baseline levels of plasma lipids and enzymes of patients in the three groups.
| Items | Statin/col (n=60) | Statin/eze (n=60) | Statin mono (n=60) |
|---|---|---|---|
| Baseline plasma lipid values (mmol/l) | |||
| LDL-C | 3.1±0.6 | 2.9±0.4 | 3.0±0.6 |
| Total C | 4.7±0.7 | 4.6±0.5 | 4.7±0.7 |
| HDL-C | 1.2±0.3 | 1.2±0.2 | 1.3±0.3 |
| Triglycerides | 1.8±0.5 | 1.9±0.5 | 1.9±0.6 |
| Baseline plasma enzyme levels (U/l) | |||
| AST | 25.6±6.2 | 26.3±5.7 | 27.1±6.9 |
| ALT | 26.1±7.8 | 27.0±8.1 | 25.3±8.4 |
| CK | 105.1±40.0 | 117.2±45.3 | 110.4±39.5 |
Values are expressed as the mean ± standard deviation. No significant differences were present between the groups for all parameters. LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CK, creatine phosphokinase; col, colesevelam; eze, ezetimibe; mono, monotherapy.
Effectiveness of the three therapeutic strategies
After eight weeks of treatment, the levels of LDL-C in the three groups of participants were all reduced (Table III). Combinational treatments resulted in significantly greater reductions in mean LDL-C levels as compared with those achieved by high-intensity statin monotherapy (Fig. 1). The reductions of the mean LDL-C values in the two combinational treatment groups were not significantly different (Fig. 1). The proportions of patients achieving the LDL-C-lowering goal were 68, 72 and 50% in the statin/col, statin/eze and statin monotherapy group, respectively (data not shown). In the combinational treatment groups, a higher percentage of patients achieved the LDL-C goals.
Table III.
Plasma lipid levels (mmol/l) after eight weeks of treatment.
| Plasma lipid | Statin/col (n=60) | Statin/eze (n=60) | Statin mono (n=60) |
|---|---|---|---|
| LDL-C | 1.7±0.4a | 1.9±0.3a | 2.1±0.4 |
| Total C | 3.1±0.6 | 3.3±0.4 | 3.5±0.6 |
| HDL-C | 1.3±0.3 | 1.1±0.3 | 1.2±0.3 |
| Triglycerides | 1.4±0.4 | 1.4±0.4 | 1.5±0.5 |
The plasma levels of LDL-C, total C and triglycerides were reduced by the three treatments. The plasma levels of HDL-C were increased by statin/col treatment and were reduced by statin/eze and statin monotherapy. Values are expressed as the mean ± standard deviation.
P<0.05 vs. the statin mono group. col, colesevelam; eze, ezetimibe; mono, monotherapy; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
Figure 1.
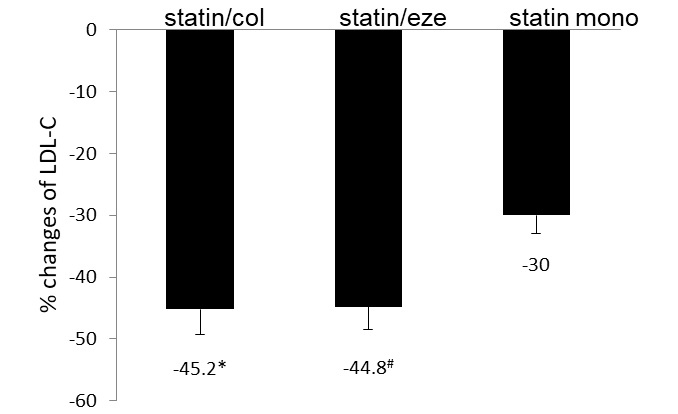
Percentage changes of LDL-C from baseline in the three groups after eight weeks of treatment. Patients in the statin/col and statin/eze groups achieved higher percentages of LDL-C reductions compared with those in the statin monotherapy group. Values are expressed as the ± standard error of the mean (n=60). *P=0.03 and #P=0.04 vs. the statin mono group. LDL-C, low-density lipoprotein cholesterol; col, colesevelam; eze, ezetimibe; mono, monotherapy.
The percentage reductions of total cholesterols were 34.0, 34.8 and 25.5% in the statin/col, statin/eze and statin mono group, respectively. Combinational treatments achieved higher effectiveness in reducing total cholesterol (Fig. 2). Changes in triglycerides were similar among the three treatment groups. Levels of HDL-C were increased in the statin/col group and decreased in statin/eze and statin mono groups compared with the baseline levels. The difference was not significant among the three groups.
Figure 2.
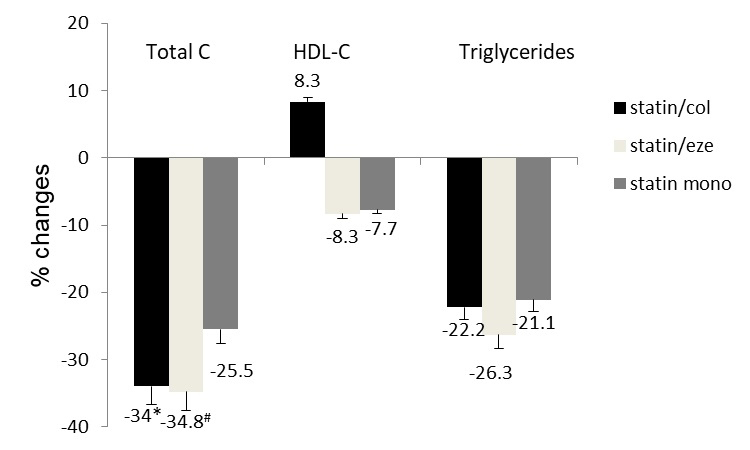
Percentage changes of total C, HDL-C and triglycerides from baseline after eight weeks of treatment. Patients in the statin/col and statin/eze groups achieved higher percentages of total C reduction compared with those in the statin monotherapy group. Reductions of HDL-C and triglycerides were similar among the three groups. Values are expressed as the ± standard error of the mean (n=60). *P=0.043 and #P=0.035 vs. statin mono group. total C, total cholesterol; HDL-C, high-density lipoprotein cholesterol; col, colesevelam; eze, ezetimibe; mono, monotherapy.
Safety assessment
The proportion of patients reporting serious adverse events, including strokes, coronary artery diseases and mortalities, was similar among the three treatment groups. During the 12-month follow-up, one case of stroke was reported in the statin mono group, one coronary artery bypass grafting in the statin/col group and one transient ischemic attack in the statin/eze group. These serious events were considered not to associated with the drugs. The number and rate of other observed drug-associated events, which were mainly muscle-associated adverse events, were similar among the three groups (P=0.73). No patient was reported to have increased ALT or AST ≥3X ULN. Two patients had CK ≥5X ULN. These two patients also had symptoms of myalgia. A summary of the safety assessment is presented in Table IV.
Table IV.
Summary of safety data.
| Item | Statin/col (n=60) | Statin/eze (n=60) | Statin mono (n=60) |
|---|---|---|---|
| Serious adverse events | 1 (1.7) | 1 (1.7) | 1 (1.7) |
| Drug-associated adverse events | 4 (6.7) | 3 (5) | 3 (5) |
| Serious drug-associated adverse events | 0 | 0 | 0 |
| ALT/AST ≥3X ULN | 0 | 0 | 0 |
| CK ≥5X ULN | 1 (1.7) | 0 | 1 (1.7) |
Values are expressed as n (%). One serious adverse events, including strokes, coronary artery diseases and mortalities, was reported in each of the three groups. Drug-associated adverse events were similar in the three groups. No significant differences were present among the three groups. ALT, AST and CK levels were not significantly different among the three groups. AST, aspartate aminotransferase; ALT, alanine aminotransferase; CK, creatine phosphokinase; ULN, upper limit of normal; col, colesevelam; eze, ezetimibe; mono, monotherapy.
Discussion
The importance of reducing the levels of LDL-C in CHD patients has been well recognized. When the first-line statin medication is insufficient or poorly tolerated, a second-line treatment option, including ezetimibe or colesevelam, may be considered. These second-line treatments are known to reduce LDL-C levels. However, studies investigating the effectiveness and side effects of combinational treatment in CHD patients, particularly in Asian populations, are currently lacking. The present study compared the effectiveness of statin/ezetimibe, statin/colesevelam and high-intensity statin monotherapy in the management of plasma lipids in CHD patients, and the side effects were also observed. The present study indicated that the combined therapies were more effective in reducing LDL-C than high-dose statin monotherapy in CHD patients. Furthermore, a larger percentage of patients achieved lowering LDL-C goals in the combined therapy groups than in the statin monotherapy group. The safety was similar among the three therapeutic methods.
It has been reported that in patients with hypercholesterolemia and CHD, achieving LDL-C targets often fails (18). Moderate dosages of statin therapy may not be sufficient for achieving the LDL-C treatment goals (19). Physicians often prescribe low or moderate doses of statins instead of high-intensity statins in order to reduce adverse effects (20,21). Indeed, certain patients are intolerant or resistant to high doses of statin therapy. Statins combined with other lipid-lowering agents may be a choice for those patients. Several studies have investigated the effects of statin/eze as compared with high-intensity statin monotherapy (22–25). Although the dosages of agents used may differ between various studies, the present results were consistent or similar to those of previous studies. As atorvastatin is usually prescribed at a daily dosage of 20 mg in China, patients in the high-intensity statin group received 30 mg atorvastatin daily and patients in the combined therapy groups received 20 mg atorvastatin plus 10 mg ezetimibe or colesevelam. A review estimated that compared with high-intensity statin monotherapy, mid-intensity statin combined with ezetimibe may achieve a further decrease of LDL-C by 5–15% (26). The results of the present study indicated that statin combined with ezetimibe reduced LDL-C by 44.8% as compared with 30% by statin alone. The proportion of patients reaching the LDL-C-lowering goal was also higher in the statin/eze group, indicating that combining ezetimibe with a moderate dose of statin is more effective in reducing LDL-C than high-intensity statin therapy alone. Statins inhibit the production of cholesterol, which in turn may upregulate the absorption of cholesterol, whereas ezetimibe reduces the absorption of cholesterol by inhibiting the Niemann-Pick C1-like 1 protein. This may reflect that the two agents have different mechanisms by which they reduce LDL. Colesevelam is another type of lipid-lowering agent, which acts as a bile acid sequestrant (27,28). Combining colesevelam with statins is an alternative to high-intensity statin therapy. Several trials compared the effect of statin monotherapy to combination therapy with bile acid sequestrant in patients with hyperlipidemia (10,29–32). In high-risk hyperlipidemic patients, low-intensity statin combined with bile acid sequestrant decreased LDL-C levels 0–14% more than moderate-intensity statin monotherapy (26). However, there is currently a lack of studies investigating the lipid-lowering effects of colesevelam plus statin in CHD patients. The results of the present study indicated that colesevelam combined with moderate-intensity statin therapy was more effective in lowering LDL-C than high-intensity statin monotherapy. Colesevelam has been reported to improve the lipoprotein particles; in the present study, HDL was increased by colesevelam, which was consistent with the results of previous studies (33,34). The proportion of patients achieving the LDL-C lowering goal was 68% in the statin/col group as compared with 50% in the statin monotherapy group. The effects of the two combinational treatment methods were similar, suggesting that administration of colesevelam or ezetimibe combined with moderate-dose statin may be an alternative method for the management of hyperlipidemia.
The safeties and tolerability of the three treatment methods were studied and compared. There was no report of drug-associated serious side effects. A total of 10 patients (5.6%) developed musculoskeletal side effects and the symptoms were mainly myalgia. The rate is consistent with that reported in a previous study (35). None of the patients had any increased ALT or AST by ≥3X ULN; however, two patients had CK ≥5X ULN. The incidence of side effects was similar among the three groups of patients. The major side effects of colesevelam are reported to be gastrointestinal discomfort, including nausea, abdominal cramps and impaired absorption of other medications (8,36). None of these side effects were observed in the present study. The treatments were generally well tolerated and safe in the whole population of participants.
In conclusion, atorvastatin combined with colesevelam or ezetimibe was more effective than high-intensity statin monotherapy in reducing plasma LDL-C levels in patients with CHD. The combined therapies are safe and well-tolerated. The combinational therapeutic strategy may be an alternative to high-intensity statin monotherapy for patients that are resistant or intolerant to statins. One limitation of the present study is that the number of patients is moderate. Multi-centered, large-scale and randomized clinical trials are warranted to confirm the results of the present study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CHL gathered the patients' clinical information, and analyzed and interpreted the patient data. QWL helped to design the study and was a major contributor in writing the study. XHX designed the study and carried out the statistical analysis. All authors read and approved the final study.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Linyi Central Hospital (Linyi, China). Patients provided written informed consent prior to the study commencing.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Graham I, Cooney MT, Bradley D, Dudina A, Reiner Z. Dyslipidemias in the prevention of cardiovascular disease: Risks and causality. Curr Cardiol Rep. 2012;14:709–720. doi: 10.1007/s11886-012-0313-7. [DOI] [PubMed] [Google Scholar]
- 2.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren WM, Albus C, Benlian P, Boysen G, Cifkova R, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Atherosclerosis. 2012;223:1–68. doi: 10.1016/j.atherosclerosis.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Cholesterol Treatment Trialists' (CTT) Collaboration, corp-author. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. National Heart, Lung, and Blood Institute, et al: Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 5.Davidson MH, Toth PP. Combination therapy in the management of complex dyslipidemias. Curr Opin Lipidol. 2004;15:423–431. doi: 10.1097/01.mol.0000137221.16160.b9. [DOI] [PubMed] [Google Scholar]
- 6.Stone NJ, Robinson JG, Lichtenstein AH, Merz Bairey CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 7.European Association for Cardiovascular Prevention & Rehabilitation, corp-author. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, et al. ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 8.Davidson MH, Dillon MA, Gordon B, Jones P, Samuels J, Weiss S, Isaacsohn J, Toth P, Burke SK. Colesevelam hydrochloride (cholestagel): A new, potent bile acid sequestrant associated with a low incidence of gastrointestinal side effects. Arch Intern Med. 1999;159:1893–1900. doi: 10.1001/archinte.159.16.1893. [DOI] [PubMed] [Google Scholar]
- 9.Insull W, Jr, Toth P, Mullican W, Hunninghake D, Burke S, Donovan JM, Davidson MH. Effectiveness of colesevelam hydrochloride in decreasing LDL cholesterol in patients with primary hypercholesterolemia: A 24-week randomized controlled trial. Mayo Clin Proc. 2001;76:971–982. doi: 10.4065/76.10.971. [DOI] [PubMed] [Google Scholar]
- 10.Jones MR, Nwose OM. Role of colesevelam in combination lipid-lowering therapy. Am J Cardiovasc Drugs. 2013;13:315–323. doi: 10.1007/s40256-013-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katsiki N, Athyros VG, Karagiannis A. Exploring the management of statin intolerant patients: 2016 and beyond. Curr Vasc Pharmacol. 2016;14:523–533. doi: 10.2174/1570161114666160226150028. [DOI] [PubMed] [Google Scholar]
- 12.Engelking LJ, McFarlane MR, Li CK, Liang G. Blockade of cholesterol absorption by ezetimibe reveals a complex homeostatic network in enterocytes. J Lipid Res. 2012;53:1359–1368. doi: 10.1194/jlr.M027599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toth PP, Davidson MH. Cholesterol absorption blockade with ezetimibe. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:455–462. doi: 10.2174/156800605774962086. [DOI] [PubMed] [Google Scholar]
- 14.Reiner Z. Combined therapy in the treatment of dyslipidemia. Fundam Clin Pharmacol. 2010;24:19–28. doi: 10.1111/j.1472-8206.2009.00764.x. [DOI] [PubMed] [Google Scholar]
- 15.Špinar J, Špinarová L, Vitovec J. IMProved reduction of outcomes: Vytorin efficacy international trial (studie IMPROVE-IT) Vnitr Lek. 2014;60:1095–1101. (In Czech) [PubMed] [Google Scholar]
- 16.Sando KR, Knight M. Nonstatin therapies for management of dyslipidemia: A review. Clin Ther. 2015;37:2153–2179. doi: 10.1016/j.clinthera.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Otokozawa S, Ai M, Asztalos BF, White CC, Demissie-Banjaw S, Cupples LA, Nakajima K, Wilson PW, Schaefer EJ. Direct assessment of plasma low density lipoprotein and high density lipoprotein cholesterol levels and coronary heart disease: Results from the Framingham Offspring Study. Atherosclerosis. 2010;213:251–255. doi: 10.1016/j.atherosclerosis.2010.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foley KA, Simpson RJ, Jr, Crouse JR, III, Weiss TW, Markson LE, Alexander CM. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol. 2003;92:79–81. doi: 10.1016/S0002-9149(03)00474-0. [DOI] [PubMed] [Google Scholar]
- 19.Kotseva K, Wood D, De Backer G, De Bacquer D, Pyörälä K, Keil U. EUROASPIRE Study Group: EUROASPIRE III: A survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur J Cardiovasc Prev Rehabil. 2009;16:121–137. doi: 10.1097/HJR.0b013e3283294b1d. [DOI] [PubMed] [Google Scholar]
- 20.Reiner Ž, Tedeschi-Reiner E. Prevalence and types of persistent dyslipidemia in patients treated with statins. Croat Med J. 2013;54:339–345. doi: 10.3325/cmj.2013.54.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Šimić I, Reiner Ž. Adverse effects of statins-myths and reality. Curr Pharm Des. 2015;21:1220–1226. doi: 10.2174/1381612820666141013134447. [DOI] [PubMed] [Google Scholar]
- 22.Averna M, Zaninelli A, Le Grazie C, Gensini GF. Ezetimibe/simvastatin 10/20 mg versus simvastatin 40 mg in coronary heart disease patients. J Clin Lipidol. 2010;4:272–278. doi: 10.1016/j.jacl.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Conard SE, Bays HE, Leiter LA, Bird SR, Rubino J, Lowe RS, Tomassini JE, Tershakovec AM. Efficacy and safety of ezetimibe added on to atorvastatin (20 mg) versus uptitration of atorvastatin (to 40 mg) in hypercholesterolemic patients at moderately high risk for coronary heart disease. Am J Cardiol. 2008;102:1489–1494. doi: 10.1016/j.amjcard.2008.09.075. [DOI] [PubMed] [Google Scholar]
- 24.Barrios V, Amabile N, Paganelli F, Chen JW, Allen C, Johnson-Levonas AO, Massaad R, Vandormael K. Lipid-altering efficacy of switching from atorvastatin 10 mg/day to ezetimibe/simvastatin 10/20 mg/day compared to doubling the dose of atorvastatin in hypercholesterolaemic patients with atherosclerosis or coronary heart disease. Int J Clin Pract. 2005;59:1377–1386. doi: 10.1111/j.1368-5031.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- 25.Ballantyne CM, Blazing MA, King TR, Brady WE, Palmisano J. Efficacy and safety of ezetimibe co-administered with simvastatin compared with atorvastatin in adults with hypercholesterolemia. Am J Cardiol. 2004;93:1487–1494. doi: 10.1016/j.amjcard.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 26.Gudzune KA, Monroe AK, Sharma R, Ranasinghe PD, Chelladurai Y, Robinson KA. Effectiveness of combination therapy with statin and another lipid-modifying agent compared with intensified statin monotherapy: A systematic review. Ann Intern Med. 2014;160:468–476. doi: 10.7326/M13-2526. [DOI] [PubMed] [Google Scholar]
- 27.Tziomalos K, Karagiannis A, Mikhailidis DP, Athyros VG. Colesevelam: A new and improved bile acid sequestrant? Curr Pharm Des. 2013;19:3115–3123. doi: 10.2174/1381612811319170019. [DOI] [PubMed] [Google Scholar]
- 28.Wong NN. Colesevelam: A new bile acid sequestrant. Heart Dis. 2001;3:63–70. doi: 10.1097/00132580-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Knapp HH, Schrott H, Ma P, Knopp R, Chin B, Gaziano JM, Donovan JM, Burke SK, Davidson MH. Efficacy and safety of combination simvastatin and colesevelam in patients with primary hypercholesterolemia. Am J Med. 2001;110:352–360. doi: 10.1016/S0002-9343(01)00638-6. [DOI] [PubMed] [Google Scholar]
- 30.Schrott HG, Stein EA, Dujovne CA, Davidson MH, Goris GB, Oliphant TH, Phillips JC, Shawaryn GG. Enhanced low-density lipoprotein cholesterol reduction and cost-effectiveness by low-dose colestipol plus lovastatin combination therapy. Am J Cardiol. 1995;75:34–39. doi: 10.1016/S0002-9149(99)80523-2. [DOI] [PubMed] [Google Scholar]
- 31.Bays HE, Davidson M, Jones MR, Abby SL. Effects of colesevelam hydrochloride on low-density lipoprotein cholesterol and high-sensitivity C-reactive protein when added to statins in patients with hypercholesterolemia. Am J Cardiol. 2006;97:1198–1205. doi: 10.1016/j.amjcard.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 32.Hunninghake D, Insull W, Jr, Toth P, Davidson D, Donovan JM, Burke SK. Coadministration of colesevelam hydrochloride with atorvastatin lowers LDL cholesterol additively. Atherosclerosis. 2001;158:407–416. doi: 10.1016/S0021-9150(01)00437-3. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg RB, Rosenson RS, Hernandez-Triana E, Misir S, Jones MR. Initial combination therapy with metformin plus colesevelam improves lipoprotein particles in patients with early type 2 diabetes mellitus. J Clin Lipidol. 2012;6:318–324. doi: 10.1016/j.jacl.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Davidson MH, Donovan JM, Misir S, Jones MR. A 50-week extension study on the safety and efficacy of colesevelam in adults with primary hypercholesterolemia. Am J Cardiovasc Drugs. 2010;10:305–314. doi: 10.2165/11584310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Ramkumar S, Raghunath A, Raghunath S. Statin therapy: Review of safety and potential side effects. Acta Cardiol Sin. 2016;32:631–639. doi: 10.6515/ACS20160611A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobson TA, Armani A, McKenney JM, Guyton JR. Safety considerations with gastrointestinally active lipid-lowering drugs. Am J Cardiol. 2007;99:47C–55C. doi: 10.1016/j.amjcard.2006.11.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


