Abstract
Immune checkpoint blockade therapy (ICBT) uses drugs to interrupt signaling pathways that inhibit antitumor immune responses. Although ICBT has provided clinical benefits in certain cancer patients, a large number of patients do not respond to ICBT. Therefore, it is necessary to find other efficient targets to promote the effects of ICBT. Renal cell carcinoma (RCC) is one of the leading causes of cancer-associated mortality worldwide. To date, there is no efficient treatment for patients with advanced RCC. The present study aimed to evaluate the prognostic value of CD103+ cells in patients with RCC and their potential role in enhancing the effect of ICBT in RCC. A total of 200 tumor tissue samples were collected from patients with RCC. The CD103+ cell count and survival of these patients was assessed, and the role of CD103+ cells in combination with ICBT was evaluated in an RCC mouse model. It was identified that a high CD103+ cell count was an independent favorable prognosticator in patients with RCC. The expansion of CD103+ cells promoted the effects of ICBT in the RCC xenograft mouse model, while depletion of CD103+ cells had the opposite effect. Furthermore, the expansion of CD103+ cells enhanced the count and activation of tumor infiltrating CD8+ T cells in RCC tumor tissue. These results indicate that a high CD103+ cell count is an independent favorable prognosticator in RCC patients. Thus, the expansion of CD103+ cells may increase the efficacy of ICBT in patients with RCC.
Keywords: renal cell carcinoma, CD103+ cells, immune checkpoint blockade therapy, prognosis, survival
Introduction
Tumor development depends on numerous reciprocal interactions between the tumor environment and tumor cells. Tumor immune cells are among the major components of the tumor microenvironment (1,2). The antitumor immune response consists of a series of stepwise events that are regulated by stimulatory and inhibitory factors (3). Previous evidence has suggested that the immune response to tumors typically does not function correctly, which results in tumor cells escaping from the surveillance of the immune system (3). Immune checkpoint proteins, which include inhibitory receptors and ligands such as programmed cell death (PD) 1/PD-ligand 1 (L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4)/B7, are key inhibitors in suppressing the initiation and function of tumor immunity. Immune checkpoint blockade therapy (ICBT) uses drugs that can interrupt the inhibitory checkpoints to enhance tumor immunity and induce cancer regression. ICBT has increased the five-year survival rate by 15% from 30% in certain cancer patients, including patients with melanoma (4,5). However, certain patients with renal cancer do not respond to ICBT (5,6), indicating the urgency to decrease intrinsic tumor resistance to therapeutic agents.
Cluster of differentiation (CD) 103 (αE integrin) is a subunit of the heterodimeric integrin molecule αEβ7. CD103 is widely expressed in intraepithelial lymphocytes, tumor infiltrating lymphocytes and certain dendritic cells (7–9). Previous studies have demonstrated that CD103 serves an important role in the cell lysis caused by tumor-specific infiltrating lymphocytes via interacting with its ligand, E-cadherin, on the tumor cells, triggering lytic granule polarization and exocytosis (10,11). Furthermore, the ligation of CD103 and E-cadherin promotes the adhesion of T cells to tumor cells and induces co-stimulation in activated cytotoxic T cells (12). These findings suggest that CD103 may be a target for enhancing tumor immunity.
Renal cell carcinoma (RCC) is ranked as the seventh most common cancer type in males and the ninth most common cancer type in females worldwide, accounting for 2–3% of all adult malignancies (13). The primary treatments for RCC remain surgery-oriented and these strategies are not optimal for patients with advanced RCC. To date, no adjuvant therapies have been identified to be of a significant benefit to patients with RCC (14). Previously, ICBT drugs have been tested in clinical trials; however, <30% of patients with RCC responded to these treatments (15). Thus, there is a requirement to enhance the sensitivity of RCC to ICBT. In the current study, the prognostic value of CD103 in patients with RCC was examined, and the potential therapeutic value of combining CD103 with ICBT was evaluated in a RCC mouse model.
Materials and methods
Patient samples
A total of 200 RCC tumor samples were obtained from the archive of Tianjin Nankai Hospital (Tianjin, China). Samples collected between August 2015 and January 2016 were formalin-fixed and paraffin-embedded (FFPE). The patients were diagnosed between April 2004 and April 2010. Informed consent was provided by all patients or their legal representatives. None of the patients received chemotherapy or radiotherapy prior to the collection of tissue samples by laparoscopic radical nephrectomy. In the present study, all patients were randomly assigned to a training group (n=100) or testing group (n=100) to increase the accuracy of the results. The training group underwent relaxation training, including mindfulness and music relaxation for 1 h per day. For many cancer patients, physical symptoms, including sleep difficulties or level of fatigue are severely affected by surgery and chemotherapy, which may be influenced by the immune system (16). Patients were divided into two groups and only patients aged between 30–75 with no signs of diseases of the immune system were included. The clinicopathological features of the patients included in the present study are summarized in Table I. The tumor-node-metastasis (TNM) system of the American Joint Committee on Cancer and the histological grading criteria of the World Health Organization were used to evaluate the tumors (16).
Table I.
Clinicopathological features and CD103+ cell count of the patients with renal cell carcinoma included in the present study.
| A, Training group (n=100) | ||||
|---|---|---|---|---|
| CD103+ cell count | ||||
| Parameter | Low, n (%) | High, n (%) | P-value | |
| Age, years | ||||
| <61 | 26 (38.2) | 42 (61.8) | 0.161 | |
| ≥61 | 17 (53.1) | 15 (46.9) | ||
| Gender | ||||
| Male | 14 (36.8) | 24 (63.2) | 0.330 | |
| Female | 29 (46.8) | 33 (53.2) | ||
| Grade | ||||
| I+II | 17 (41.5) | 24 (58.5) | 0.796 | |
| III+IV | 26 (44.1) | 33 (55.9) | ||
| T stage | ||||
| T1+T2 | 16 (50.0) | 16 (50.0) | 0.332 | |
| T3+T4 | 27 (39.7) | 41 (60.3) | ||
| Lymph node | ||||
| N0-N2 | 17 (37.0) | 29 (63.0) | 0.260 | |
| N3-N4 | 26 (48.1) | 28 (51.9) | ||
| Metastasis | ||||
| Negative | 36 (41.9) | 50 (58.1) | 0.568 | |
| Positive | 7 (50.0) | 7 (50.0) | ||
| TNM stage | ||||
| I+II | 13 (28.9) | 32 (71.1) | 0.010 | |
| III+IV | 30 (54.5) | 25 (45.5) | ||
| Patient status | ||||
| Alive | 5 (13.5) | 32 (86.5) | P<0.001 | |
| Succumbed | 38 (60.3) | 25 (39.7) | ||
| B, Testing group (n=100) | ||||
| CD103+ cell count | ||||
| Parameter | Low, n (%) | High, n (%) | P-value | |
| Age, years | ||||
| <62 | 28 (43.7) | 36 (56.3) | 0.148 | |
| ≥62 | 12 (33.3) | 24 (66.7) | ||
| Sex | ||||
| Male | 19 (41.3) | 27 (58.7) | 0.307 | |
| Female | 21 (39.6) | 32 (60.4) | ||
| Grade | ||||
| I+II | 15 (33.3) | 30 (66.7) | 0.218 | |
| III+IV | 25 (45.5) | 30 (54.5) | ||
| T stage | ||||
| T1+T2 | 22 (47.8) | 24 (52.2) | 0.140 | |
| T3+T4 | 18 (33.3) | 36 (66.7) | ||
| Lymph node | ||||
| N0-N2 | 20 (37.7) | 33 (62.3) | 0.624 | |
| N3-N4 | 20 (42.6) | 27 (57.4) | ||
| Metastasis | ||||
| Negative | 32 (38.6) | 51 (61.4) | 0.514 | |
| Positive | 8 (47.1) | 7 (52.9) | ||
| TNM stage | ||||
| I+II | 15 (30.0) | 35 (70.0) | 0.041 | |
| III+IV | 25 (50.0) | 25 (50.0) | ||
| Patient status | ||||
| Alive | 13 (25.0) | 39 (75.0) | 0.001 | |
| Succumbed | 27 (56.2) | 21 (43.8) | ||
TNM, tumor-node-metastasis; CD, cluster of differentiation.
Follow-up data for all patients was collected by reviewing archive information, or by making visits or phone calls. Survival time was defined as the interval between the date of surgery and date of RCC-associated mortality. Patients who succumbed due to reasons other than RCC were excluded from the present study. The present study was approved by the Ethics Committee of Tianjin Nankai Hospital.
RCC cell culture
A murine RCC cell line, Renca, was obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in complete RPMI-1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin. Cells were maintained in a humidified incubator with 5% CO2 at 37°C.
Mouse model of RCC
A total of 30 female BALB/c mice (age, 5 weeks old; weight, 17–19 g) were purchased from Shanghai Laboratory Animal Center (Shanghai, China). An RCC xenograft mouse model was established by injecting Renca cells (1×106) into the flank of the mice subcutaneously. A total of 1 week later, the mice were treated with anti-PD-L1 (200 µg/mouse; cat. no. ab213480) and anti-CTLA4 antibodies (200 µg/mouse, cat. no. ab134090) in combination with PBS (n=10), anti-CD103 antibody (n=10; 200 µg/mouse; cat. no. ab25198; all Abcam, Cambridge, UK) or the CD103+ cell growth factor Fms-related tyrosine kinase 3 ligand (FLT3L; n=10; 25 mg/kg; cat. no. bs5905; BIOSS, Beijing, China) via tail vein injection. The treatment was performed once a week for 4 weeks. The tumor volume and mice survival status were checked and recorded once a day. Mice were sacrificed once tumor volume exceeded 3,000 mm3. All mice were housed at a temperature of 22–24°C in a specific pathogen-free room with a 12 h light/dark cycle and free access to clean water and standard food.
Immunostaining
Following standard procedures, the expression of CD103 and CD8 in RCC FFPE samples was evaluated using immunohistochemistry (IHC) and immunofluorescent staining. Briefly, the 4-µm thick sample sections were deparaffinized with xylene and rehydrated with ethanol, followed by microwave antigen retrieval at 95°C for 10 min. Following cooling, they were washed with distilled water and blocked in normal 10% goat serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at room temperature for 30 min. Subsequently, primary antibodies were added and the sections were incubated at 4°C overnight. The primary antibodies used were as follows: Anti-CD103 (1:100; cat. no. 14-1031-85) and anti-CD8 (1:100; cat. no. RB-9009-P1; both Thermo Fisher Scientific, Inc). The goat anti-mouse secondary antibody (1:500; cat. no. ab6785; Abcam) was added and incubated at 37°C for 10 min, followed by washing with PBS three times for 5 min. All slides were observed under a fluorescent microscope (BZ-9000; Keyence Corporation, Osaka, Japan) by two people independently. Five fields of view from each section were selected randomly to calculate the average percentage of CD8+ and CD103+ cells. Then, the groups were given a score for CD8+ and CD103+ expression depending on the percentage of CD8+ and CD103+ cells, which was calculated following cell counts and gave the following values: 1, <35% positive; 2, 35–70% positive; and 3, >70% positive.
ELISA
ELISA was performed to evaluate the level of interferon (IFN)-γ (cat. no. ab100690), granzyme B (cat. no. ab46142), and PD-L1 (cat. no. ab214565) in RCC tumor tissue from the xenograft mouse model following treatment. All ELISA kits were purchased from Abcam. Tumor tissues harvested from the mice were immediately lysed using radioimmunoprecipitation assay buffer supplemented with protease inhibitor (cat. no. ab156034, Abcam). Standard ELISA procedures were conducted according to the manufacturer's protocol. Data were normalized to the control group.
Fluorescence-activated cell sorting (FACS)
FACS analysis was performed to identify the number of CD103+ cells in the tumor tissue of the RCC mouse model following treatment. Standard procedures were followed. Briefly, tumor tissue harvested from the mice were cut into small pieces and dissociated into single cells by enzymatic digestion. Cells were carefully suspended in the residual volume (200 µl) of staining buffer and then 200 µl freshly prepared cold fixation buffer (Shanghai Haoran Biotechnology Co., Ltd., Shanghai, China) was added. Cells were fixed with 70% ethyl alcohol (Shanghai Haoran Biological Technology Co., Ltd.) for 30 min at 4°C in the dark. Then, the sample was centrifuged at 1,000 × g for 5 min at room temperature and the fixative was removed. To permeabilize the cells, the cell pellet was suspended in 200 µl of freshly prepared, pre-warmed (37°C) 0.1% Triton X-100 (Shanghai Haoran Biological Technology Co., Ltd.) and cells were then incubated for 30 min at 37°C in the dark. Cells were subsequently centrifuged at 1,000 × g for 5 min at room temperature and the buffer was removed. Then, cells were incubated with anti-CD103 antibodies for 1 h on ice and washed with PBS. Subsequently, the cells were incubated with fluorophore-labeled goat anti-mouse secondary antibody (1:300, cat. no. ab150117; Abcam) for 15 min at room temperature. The stained cells were analyzed using the FACSCanto II (BD Biosciences, San Jose, CA, USA). FlowJo software 8.0 (FlowJo, LLC, Ashland, OR, USA) was used to analyze the data.
Statistical analysis
Data are presented as the mean ± standard deviation of ≤3 independent experiments. All data analysis was performed using SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). One-way analysis of variance followed by a Dunnett's test, and a Student's t-test or the Chi-squared test was used to analyze the statistical significance of differences between groups. The Kaplan-Meier estimator method was used for survival analysis and the log-rank test was used to compare survival curves between different groups. The proportional hazards (Cox) regression method was used to determine the independent prognostic impact of factors on the survival of patients with RCC. A receiver operating characteristic (ROC) curve was used to determine the point at which CD103+ cell numbers were no longer positive indicators at a high sensitivity and specificity in RCC patients and areas under the curves (AUCs) and confidence interval (CI) were also reported. Two-tailed P<0.05 was considered to indicate a statistically significant difference.
Results
A higher number of CD103+ cells is associated with a later TNM stage and poorer survival in RCC
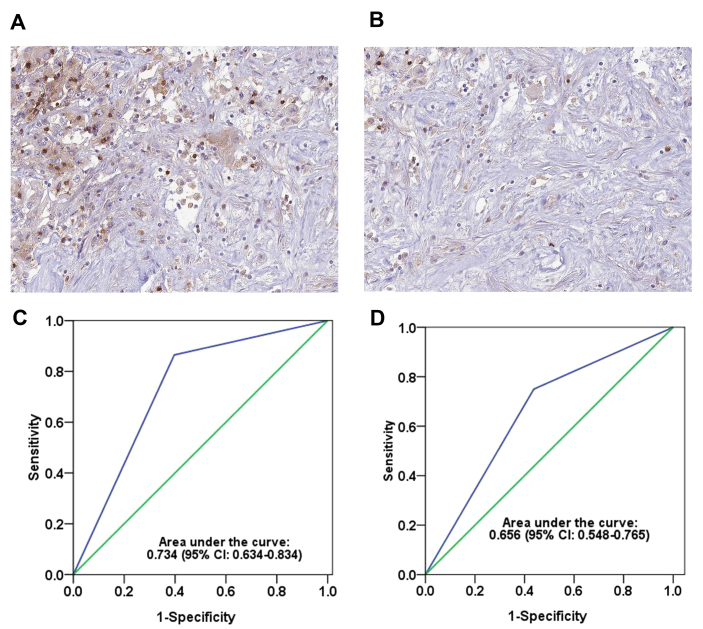
In order to explore the role of CD103+ cells in RCC development, RCC tissue samples were collected from patients and the number of CD103+ cells was evaluated by IHC. As demonstrated in Fig. 1A and B, different levels of CD103+ cells were observed among these patients. In order to further measure the difference between a high and low number of CD103+ cells, patients with RCC were randomly distributed into two groups, a training group (n=100; average age, 61 years old) and a testing group (n=100; average age, 62 years old) in order to eliminate any other factors that may have effected the immune system. The proportion of patients with advanced-stage RCC was 55% in the training cohort and 50% in the testing cohort.
Figure 1.
CD103+ cells in RCC tumor tissue. Representative images indicating (A) high and (B) low numbers of CD103+ cells in the immunohistochemistry stained RCC tumor tissues. Magnification, ×400. Receiver operating characteristic curves demonstrating the area under the curve of CD103+ cells in the (C) training and (D) testing groups. CI, confidence interval; RCC, renal cell carcinoma; CD, cluster of differentiation.
According to the ROC curve analysis, a score of 2 was chosen as the cutoff point for a ‘high’ or ‘low’ number of CD103+ cells (≥2 was determined as ‘high’; <2 was determined as ‘low’). This cutoff point had high sensitivity and specificity in the training [area under the curve (AUC), 0.734; 95% CI, 0.634–0.834; Fig. 1C] and testing (AUC, 0.656; 95% CI, 0.548–0.765; Fig. 1D) groups. Furthermore, the association between the number of CD103+ cells and other clinicopathological parameters was analyzed using the Chi-squared test (Table I). The number of CD103+ cells was similar in the training group compared with the testing group. In the testing and training groups, an early TNM stage was associated with a significantly higher number of CD103+ cells compared with a later TNM stage (P=0.041 and P=0.010, respectively). Notably, the number of CD103+ cells was also associated with the survival of RCC patients: Patients who succumbed to RCC had a significantly lower number of CD103+ cells compared with patients who survived at the end of our research (P<0.001 in the training group; P=0.001 in the testing group).
A high number of CD103+ cells is a favorable prognosticator for patients with RCC
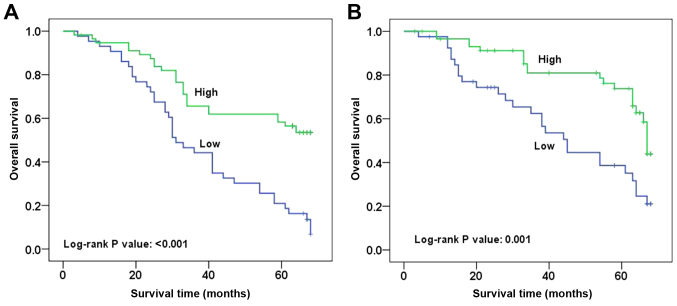
The difference in the number of CD103+ cells among patients with RCC and its association with outcome suggested that CD103+ cells could serve a key role in the progression of RCC. Thus, follow-up data was obtained for these patients and survival analysis was performed. As shown in Fig. 2, typical survival curves were observed for patients with RCC with high or low numbers of CD103+ cells. Patients with a high number of CD103+ cells had a significantly longer survival rate compared with those with a low number of CD103+ cells in the training and testing groups (P<0.001 and P=0.001, respectively). Furthermore, Cox regression analysis indicated that a high number of CD103+ cells was an independent predictor of good survival in patients with RCC when other clinicopathological features were not predictors of survival (P=0.001 in the training group, P=0.003 in the testing group; Table II). Consistent results for CD103+ expression were observed in the training [hazard ratio (HR), 0.365; 95% CI, 0.205–0.648] and testing (HR, 0.391; 95% CI, 0.210–0.728) groups (Table II).
Figure 2.
Survival curves of patients with RCC patients in the training and testing groups based on CD103+ cell count. (A) Survival curve of patients with RCC in the training group. Patients with a high CD103+ cell count had a significantly longer survival time compared with those with a low CD103+ cell count (log-rank test, P<0.001). (B) Survival curve of patients with RCC in the testing group. Patients with a high CD103+ cell count had a significantly longer survival time compared with those with a low CD103+ cell count (log-rank test, P=0.001). RCC, renal cell carcinoma; CD, cluster of differentiation.
Table II.
Multivariate Cox regression analysis of the overall survival of patients with renal cell carcinoma.
| A, Training group (n=100) | ||
|---|---|---|
| Clinicopathological feature | P-value | HR (95% CI) |
| Age (≥60 vs. <60 years) | 0.120 | 1.532 (0.895–2.621) |
| Gender (male vs. female) | 0.267 | 0.734 (0.425–1.267) |
| T stage (T3-T4 vs. T1-T2) | 0.097 | 1.664 (0.911–3.040) |
| Lymph node (N3-N4 vs. N0-N2) | 0.092 | 1.601 (0.926–2.767) |
| Metastasis (positive vs. negative) | 0.368 | 1.399 (0.673–2.906) |
| TNM stage (III–IV vs. I–II) | 0.619 | 1.163 (0.641–2.112) |
| Grade (III+IV vs. I+II) | 0.064 | 1.6681 (0.970–2.913) |
| CD103expression (high vs. low) | 0.001 | 0.365 (0.205–0.648) |
| B, Testing group (n=100) | ||
| Clinicopathological feature | P-value | HR (95% CI) |
| Age (≥60 vs. <60 years) | 0.995 | 0.998 (0.535–1.861) |
| Gender (male vs. female) | 0.555 | 1.211 (0.641–2.288) |
| T stage (T3-T4 vs. T1-T2) | 0.946 | 1.000 (0.549–1.820) |
| Lymph node (N3-N4 vs. N0-N2) | 0.063 | 1.801 (0.968–3.349) |
| Metastasis (positive vs. negative) | 0.595 | 0.804 (0.360–1.795) |
| TNM stage (III–IV vs. I–II) | 0.776 | 0.910 (0.475–1.743) |
| Grade (III+IV vs. I+II) | 0.233 | 1.460 (0.784–2.718) |
| CD103 expression (high vs. low) | 0.003 | 0.391 (0.210–0.728) |
HR, hazard ratio; CI, confidence interval; TNM, tumor-node-metastasis; CD, cluster of differentiation.
CD103 depletion inhibits the effects of ICBT in an RCC animal model
In order to further explore the role of CD103+ cells in RCC development, a RCC xenograft mouse model was established using a murine RCC cell line, Renca. In this animal model, the tumors of the mice administered with ICBT (anti-PD-L1 and anti-CTL40a antibodies) and anti-CD103 antibodies grew faster compared with the mice administered with ICBT and PBS (Fig. 3A). Notably, following administration with ICBT and FLT3L, tumor growth was significantly inhibited compared with the group that received ICBT and anti-CD103 antibodies (P<0.05; Fig. 3A). Furthermore, mice that received ICBT and anti-CD103 antibodies had a significantly shorter survival time compared with the mice administered ICBT treatment with PBS or those administered ICBT and FLT3L (both P<0.01; Fig. 3B). In addition, a decreased number of CD103+ cells were observed within the tumor tissue of mice that received ICBT and anti-CD103 antibodies, and a significantly increased number of CD103+ cells were observed in the mice administered ICBT and FLT3L treatment, compared with mice administered with ICBT treatment with PBS (both P<0.01; Fig. 3C). These data suggest that CD103+ cells serve a key role in suppressing the development of RCC in an immune response-dependent manner.
Figure 3.
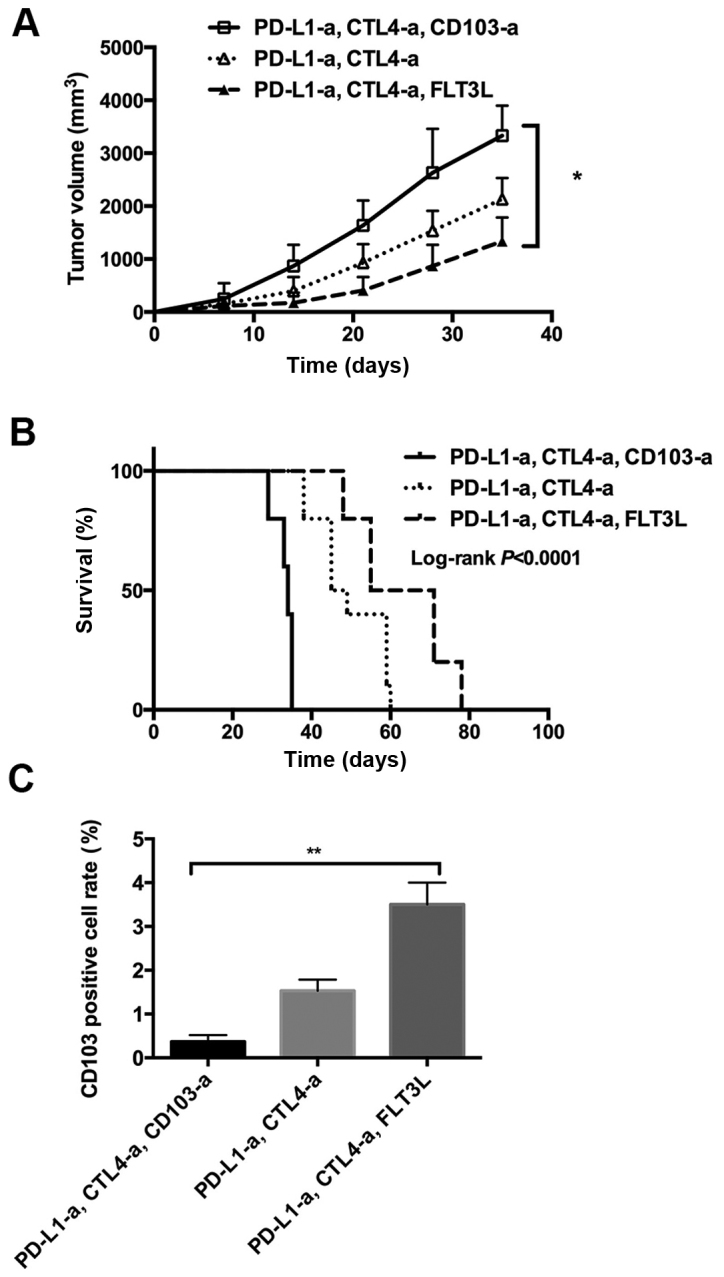
Expansion of CD103+ cells increases the response to immune checkpoint blockade therapy in a RCC xenograft mouse model. (A) Tumor volume in RCC xenograft mice. (B) Survival curve of RCC xenograft mice. (C) Quantitative analysis of CD103+ cells in the tumor tissue of RCC xenograft mice. RCC, renal cell carcinoma; -a, antibody; PD-L1, programmed cell death ligand 1; FLT3L, Fms-related tyrosine kinase 3 ligand; CTLA4, cytotoxic T-lymphocyte-associated protein 4; CD, cluster of differentiation. *P<0.05, **P<0.01.
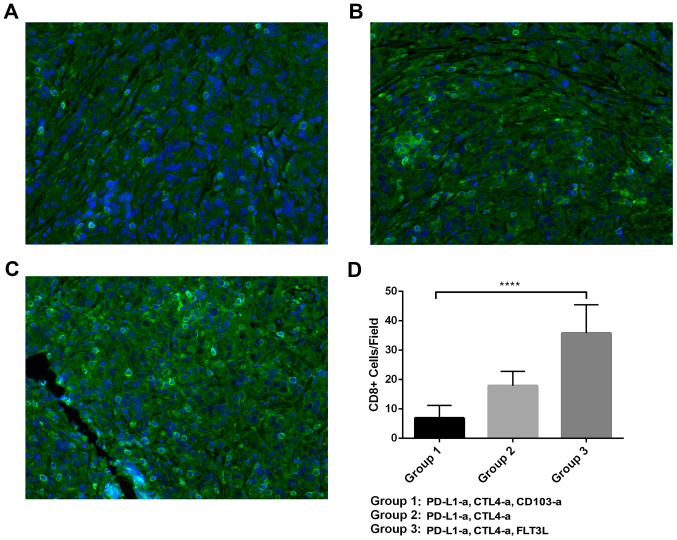
CD103+ cells promote the function of CD8+ T cells
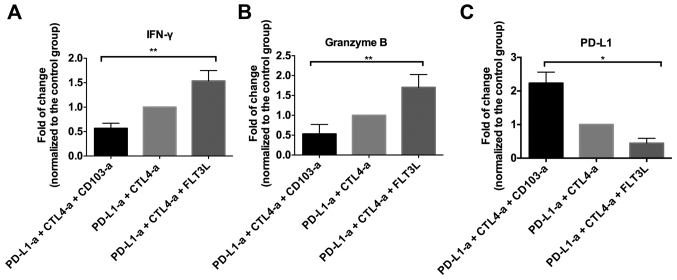
Based on the proposal that CD103+ cells may work synergistically with tumor immunity to suppress the development of RCC in an animal model, T cell functions were investigated further in these mice. As demonstrated in Fig. 4, varying levels of CD8+ T cell infiltration were identified in different groups of mice. The quantitative analysis indicated that the mice that received ICBT and CD103 depletion had a significantly decreased number of CD8+ T-cells, while the mice administered FLT3L and ICBT treatment had a significantly increased number of CD8+ T-cells, compared with the group administered with ICBT treatment and PBS (both P<0.0001; Fig. 4D). Similar trends were observed in the level of IFN-γ and granzyme B in tumor tissue (Fig. 5A and B). In addition, a significantly increased PD-L1 level was observed in the tumor tissue of mice administered ICBT and anti-CD103 treatment, but a significantly decreased level of PD-L1 was observed in mice administered with ICBT and FLT3L treatment, compared with the group administered with ICBT treatment and PBS (P<0.05; Fig. 5C).
Figure 4.
Tumor infiltrating CD8+ T cells in a RCC xenograft mouse model. (A-C) Representative images of CD8+ T cells in the tumor tissue of RCC mice treated with ICBT and anti-CD103 antibodies, ICBT alone, or ICBT and FLT3L. Magnification, ×400. (D) Quantitative analysis of CD8+ T cells in the RCC xenograft mouse model. RCC, renal cell carcinoma; ICBT, immune checkpoint blockade therapy; -a, antibody; PD-L1, programmed cell death ligand 1; CTLA4, cytotoxic T-lymphocyte-associated protein 4; FLT3L, Fms-related tyrosine kinase 3 ligand; CD, cluster of differentiation. ****P<0.0001.
Figure 5.
Expansion of CD103+ cells enhances the activation of T cells in the tumor tissue of RCC xenograft mice. RCC tumor tissue was lysed and the supernatant was used to measure the level of (A) IFN-γ, (B) granzyme B and (C) PD-L1. RCC, renal cell carcinoma; IFN, interferon; -a, antibody; PD-L1, programmed cell death ligand 1; CTLA4, cytotoxic T-lymphocyte-associated protein 4; FLT3L, Fms-related tyrosine kinase 3 ligand; CD, cluster of differentiation. *P<0.05, **P<0.01.
Discussion
The tumor microenvironment influences tumor initiation and progression (17). Among the large spectrum of tumor stromal cells, immune cells are critical in restricting cancer development (11). However, tumors have been indicated to suppress the antitumor immune response via stimulating inhibitory immune checkpoints (6). Thus, targeting these inhibitory immune checkpoints to release inhibition of tumor immunity may benefit patients with cancer. An example of a strategy that uses immune checkpoints is ICBT, which has achieved significant clinical benefits. However, the majority of patients with cancer such as glioma do not respond to ICBT (5–6). Previous studies have demonstrated that CD103+ dendritic cells are the only antigen-presenting cells that transport intact antigens to the lymph nodes and prime tumor-specific CD8+ T cells in melanoma (18–20). Furthermore, previous studies have revealed that a high CD103+ cell count is a favorable marker for patients with various types of cancer, including lung (11), ovarian (21) and bladder (21) cancer. However, the role of CD103+ cells in RCC remains unknown.
The current study identified that CD103+ cell count was associated with the outcome of patients with RCC. Patients with RCC with adverse outcomes tended to have a low CD103+ cell count in the tumor stroma. Survival analysis based on the follow-up data of a large number of patients with RCC was consistent with this, indicating that patients with high CD103 expression had longer survival times. In addition, multivariate analysis identified that high CD103 expression was an independent favorable prognostic factor for patients with RCC. To the best of our knowledge, this is the first report to reveal the prognostic value of CD103+ cells in RCC. These findings are consistent with previous studies that reported that CD103+ cells are protective in various cancer types (11,20,21). These results suggest a potential therapeutic benefit of expanding CD103+ cells in an RCC animal model.
In order to further explore the role of CD103+ cells in tumor immunity in RCC, CD103+ cells were expanded or depleted in an RCC mouse model. The results demonstrated that the expansion of CD103+ cells could enhance the response to ICBT, which is consistent with the results of previous studies in other tumor types, including melanoma and breast cancer (19,20,22). Notably, the number of tumor infiltrating CD8+ T cells was increased after treatment with a growth factor of CD103+ cells. Furthermore, activation of these CD8+ T cells was observed, indicated by significantly increased levels of IFN-γ and granzyme B. As CD8+ T cells are the major effector cells of tumor immunity, these results suggest that CD103+ cells are required for the activation of tumor-specific cytotoxic T cells in RCC. This is consistent with previous reports that demonstrated that CD103+ cells have unique antigen processing and presentation capabilities, and act as superior stimulators of naïve and activated CD8+ T cells (10,23). In addition, in the present study, anti-CD103 treatment inhibited the effects of ICBT, decreased the number of tumor infiltrating CD8+ T cells, and decreased IFN-γ and granzyme B levels. These results indicate that CD103+ cells may act as a key ‘switch’ for regulating tumor immunity.
Advances in cancer immunology make this an exciting time to research and treat cancer. However, the insensitivity of certain tumors, including RCC, to immune responses restricts the use of immunotherapies. The results of the present study indicate that a high CD103+ cell count is an independent favorable prognostic factor in patients with RCC. Thus, the expansion of CD103+ cells may increase the efficacy of ICBT in patients with RCC.
Acknowledgements
The present study was supported by Tianjin Nankai Hospital (Tianjin, China).
References
- 1.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee S, Modi S, McGinn O, Zhao X, Dudeja V, Ramakrishnan S, Saluja AK. Impaired synthesis of stromal components in response to minnelide improves vascular function, drug delivery and survival in pancreatic cancer. Clin Cancer Res. 2016;22:415–425. doi: 10.1158/1078-0432.CCR-15-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: New immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 5.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, Hadley GA. TGF-{beta}-dependent CD103 expression by CD8+ T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med. 2005;201:1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kilshaw PJ, Murant SJ. A new surface antigen on intraepithelial lymphocytes in the intestine. Eur J Immunol. 1990;20:2201–2207. doi: 10.1002/eji.1830201008. [DOI] [PubMed] [Google Scholar]
- 9.Evers BD, Engel DR, Böhner AM, Tittel AP, Krause TA, Heuser C, Garbi N, Kastenmuller W, Mack M, Tiegs G, et al. CD103+ kidney dendritic cells protect against crescentic GN by maintaining IL-10-producing regulatory t cells. J Am Soc Nephrol. 2016;27:3368–3382. doi: 10.1681/ASN.2015080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Floc'h AL, Jalil A, Vergnon I, Le Maux Chansac B, Lazar V, Bismuth G, Chouaib S, Mami-Chouaib F. Alpha E beta 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med. 2007;204:559–570. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpréville V, Validire P, Besse B, Mami-Chouaib F. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194:3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 12.Le Floc'h A, Jalil A, Franciszkiewicz K, Validire P, Vergnon I, Mami-Chouaib F. Minimal engagement of CD103 on Cytotoxic T lymphocytes with an E-cadherin-Fc molecule triggers lytic granule polarization via a phospholipase Cgamma-dependent pathway. Cancer Res. 2011;71:328–338. doi: 10.1158/0008-5472.CAN-10-2457. [DOI] [PubMed] [Google Scholar]
- 13.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 14.Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ. 2014;349:g4797. doi: 10.1136/bmj.g4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: Recent successes and next steps. Nat Rev Cancer. 2011;11:805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen M. Comparing relaxation training and cognitive-behavioral group therapy for women with breast cancer. Res Social Work Pract. 2007;17:313–323. doi: 10.1177/1049731506293741. [DOI] [Google Scholar]
- 17.Chan AC, Fan ST, Poon RT, Cheung TT, Chok KS, Chan SC, Lo CM. Evaluation of the seventh edition of the American Joint Committee on Cancer tumour-node-metastasis (TNM) staging system for patients undergoing curative resection of hepatocellular carcinoma: Implications for the development of a refined staging system. HPB (Oxford) 2013;15:439–448. doi: 10.1111/j.1477-2574.2012.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, He Y, Chen H. Autophagic tumor stroma: Mechanisms and roles in tumor growth and progression. Int J Cancer. 2013;132:1–8. doi: 10.1002/ijc.27664. [DOI] [PubMed] [Google Scholar]
- 19.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J, Tung N, et al. Expansion and activation of CD103+ dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity. 2016;44:924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webb JR, Milne K, Nelson BH. Location, location, location: CD103 demarcates intraepithelial, prognostically favorable CD8+ tumor-infiltrating lymphocytes in ovarian cancer. Oncoimmunology. 2014;3:e27668. doi: 10.4161/onci.27668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B, Wu S, Zeng H, Liu Z, Dong W, He W, Chen X, Dong X, Zheng L, Lin T, Huang J. CD103+ tumor infiltrating lymphocytes predict a favorable prognosis in urothelial cell carcinoma of the bladder. J Urol. 2015;194:556–562. doi: 10.1016/j.juro.2015.02.2941. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Cao X. Intratumoral dendritic cells in the anti-tumor immune response. Cell Mol Immunol. 2015;12:387–390. doi: 10.1038/cmi.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]