ABSTRACT
Porcine circovirus type 2 (PCV2) capsid protein (Cap) is a unique structure protein that plays pivotal roles in the process of viral replication and pathogenesis. Herein, we characterized a putative porcine Makorin RING finger protein 1 (pMKRN1) variant, an N-terminal-truncated variant of putative full-size porcine MKRN1 which has a unique expression pattern resulting from the porcine mkrn1 gene and which interacts with PCV2 Cap. A domain mapping assay showed that the C terminus of pMKRN1 and fragments (amino acids 108 to 198) of Cap are required for this interaction. PCV2 transiently upregulated pMKRN1 in PK-15 cells, but persistent viral infection downregulated pMKRN1 in major pathological tissues of PCV2-infected piglets. Overexpression of pMKRN1 significantly inhibited the generation of progeny PCV2 via ubiquitination and degradation of Cap, whereas knockout of pMKRN1 blocked Cap degradation and promoted progeny virus replication. pMKRN1 specifically targeted PCV2 Cap lysine residues 164, 179, and 191 to induce polyubiquitination and subsequent degradation. Mutation of either of the three lysine residues in the Cap protein or mutation of the histidine at residue 243 within the RING finger domain of pMKRN1 abrogated the E3 ligase activity of pMKRN1, rendering cells incapable of inducing Cap ubiquitination and degradation. Consistent with this finding, a Cap ubiquitination-deficient PCV2 strain showed enhanced virus replication and produced severe histological lesions in the lung and lymph node tissues compared with wild-type PCV2. Taken together, the results presented here suggest that PCV2 downregulates the pMKRN1 variant to avoid pMKRN1-mediated Cap ubiquitination and degradation, thus promoting viral replication and pathogenesis in its targeted tissues.
IMPORTANCE Porcine circovirus type 2 is the pathogen to which pigs are the most susceptible, causing immense economic losses in the global swine industry, but whether host cells have developed some strategies to prevent viral replication is still unclear. Here, we found that porcine MKRN1 (pMKRN1) was upregulated in the early stage of PCV2 infection and mediated the polyubiquitination and degradation of Cap protein to block PCV2 replication, yet persistent PCV2 infection downregulated pMKRN1 levels to avoid degradation, promoting viral replication and pathogenesis in its targeted tissues. These data present new insight into the molecular mechanisms underlying the antiviral effects of pMKRN1 E3 ligase during PCV2 infection and also suggest potential new control measures for PCV2 outbreaks.
KEYWORDS: PCV2, pMKRN1, replication, ubiquitination
INTRODUCTION
Porcine circovirus type 2 (PCV2), a member of the family Circoviridae, is the major causative agent of porcine circovirus-associated diseases (PCVAD) (1). PCVAD induce a variety of progressive disease syndromes, such as postweaning multisystemic wasting syndrome (PMWS), porcine dermatitis and nephropathy syndrome (PDNS), porcine respiratory disease complex (PRDC), and porcine reproductive disorders (2). PCV2 is a small nonenveloped virus with a circular, single-stranded DNA genome of ∼1.7 kb containing 11 potential open reading frames (ORFs) (3). Among of these ORFs, ORF1 and ORF2 are the major structural and functional proteins required for the replication and pathogenesis of PCV2 (4–7). ORF1 encodes two replication-associated proteins (Rep and Rep′) which are necessary for the rolling-circle replication of viral DNA (5, 8). ORF2 encodes the capsid protein (Cap), a unique structural component of the virion and the dominant immunogenic agent (3, 4, 9). Cap not only is indispensable for encapsulating the viral genome to assemble infectious virions but also is required for transport of the Rep protein and viral DNA between the cytoplasm and nucleus (10–12). Additionally, Cap is a key pathogenic factor of PCV2 (13, 14). It is currently unclear, however, if PCV2-infected host cells have strategies to minimize viral pathogenesis by targeting Cap.
Numerous host cellular proteins have been identified to be the binding partners of PCV2 Cap proteins, including E3 ubiquitin (Ub) ligase family member Makorin RING finger protein 1 (MKRN1), C1q receptor gC1qR, chaperonin Hsp40, the prostate apoptosis response-4 (Par-4) protein, the nucleosome assembly protein-1, and nucleophosmin-1 (NPM1) (15). However, the specific roles of these cellular proteins in interacting with and modulating PCV2 pathogenesis remain largely unknown.
The mkrn1 gene is the intron-containing founder of the intronless mkrn gene family and has a high degree of sequence conservation in species ranging from invertebrates to vertebrates (16). mkrn1 was first identified as a novel RING finger gene encoding E3 ligase in screening for the regulators of the ubiquitination and proteasome-dependent degradation of human telomerase reverse transcriptase (hTERT) (17). Recently, MKRN1 has been shown to mediate the degradation of many substrates through the ubiquitin-proteasome system (UPS), such as host proteins p53, p21, FADD, and PTEN, as well as viral proteins, indicating that MKRN1 is involved in numerous cellular and disease processes (17–21). In eukaryotic cells, UPS is the major protein degradation pathway mediated by the 26S proteasome (22). E3 ligases catalyze the final step of the ubiquitination cascade by transfer of ubiquitin from the E2 enzyme to form an isopeptide bond between the lysine residue of the target protein and the glycine of ubiquitin. The target proteins, including misfolded, typically insoluble, and unfunctional proteins, are usually conjugated to a successive ubiquitin chain and are recognized for their rapid degradation by the 26S proteasome (23).
MKRN1 contains a RING finger domain and functions as a RING finger E3 ligase; it can simultaneously bind both the E2-Ub thioester and the substrate and catalyzes the direct transfer of ubiquitin from the E2 enzyme to the substrate (24). However, the E3 ligase function of MKRN1 is associated with its gene structure in different orthologs. Human MKRN1 includes four isoforms, which are encoded by a single mkrn1 gene and which arise by alternative splicing and differential polyadenylation. MKRN1-long (GenBank accession no. NP_038474) has four C3H-type zinc fingers (ZFs), an MKRN-type ZF (MTZF), and a highly conserved C3HC4-type RING finger domain. C3H-type ZFs are RNA-binding motifs (25, 26), whereas the RING finger domain is a protein/protein interaction module characteristic of RING finger-class E3 ubiquitin ligases (27). Human MKRN1 transcript variants (including those with GenBank accession no. NP_001138597.1, XP_011514299.1, XP_011514300.1, and NP_001278592.1), named MKRN1-short, lack the C-terminal ZF and the last 6 amino acids (aa) of the RING finger domain (RFΔCC), essential for binding the second zinc ion, or the N-terminal fragment (64 aa). Currently, the sequence and isoforms of porcine MKRN1 (pMKRN1) have not been identified, and the effects of porcine MKRN1 on PCV2 replication and pathogenesis remain unknown.
In the present study, we characterized the molecular and cellular expression pattern of porcine MKRN1 and identified a putative porcine MKRN1 variant, pMKRN1-short; this variant is truncated at the N terminus, is expressed in PK-15 cells, and interacts with PCV2 Cap proteins. We also discovered the role of pMKRN1 in mediating the ubiquitination and degradation of the Cap protein and determined the interaction regions of the pMKRN1 and Cap proteins as well as the target sites of pMKRN1 in Cap. In addition, we constructed a Cap ubiquitination-deficient PCV2 strain, which showed enhanced virus replication and severe histological lesions in the infected piglets.
RESULTS
Porcine MKRN1 is a PCV2 Cap-interacting molecule.
MKRN1, as a transcriptional coregulator and an E3 ligase, has previously been identified to interact with the PCV2 core protein (Cap) in a yeast two-hybrid assay (15). To better understand the molecular action of porcine MKRN1 in the process of PCV2 infection, we first characterized the molecular and expression pattern of porcine MKRN1. The putative porcine full-size MKRN1 protein (pMKRN1-long; GenBank accession no. XM_003134608.4), a protein 482 amino acids in length with a molecular mass of 53.1 kDa, showed 93.4% identity with human MKRN1-long (GenBank accession no. NP_038474). In comparison, the putative porcine MKRN1 variant (pMKRN1-short; UniProtKB accession no. A0A287ALB7), an N-terminal-truncated variant of putative full-size porcine MKRN1 418 amino acids in length with a molecular mass of 46.1 kDa, showed 76.6% identity with human MKRN1-short 1 (GenBank accession no. NP_001138597.1; a C-terminal-truncated variant of full-size human MKRN1 329 amino acids in length with a molecular mass of 36.3 kDa), 95.5% identity with human MKRN1-short 2 (GenBank accession no. XP_011514299.1 and XP_011514300.1; an N-terminal-truncated variant of full-size human MKRN1 418 amino acids in length with a molecular mass of 46.1 kDa), and 75% identity with human MKRN1-short 3 (GenBank accession no. NP_001278592.1; an N-terminal-truncated and C-terminal-truncated variant). pMKRN1-long consists of four C3H-type RNA-binding-motif ZFs, an MKRN-type ZF, and a C3HC4-type RING finger domain, whereas pMKRN1-short lacks the N-terminal 64 aa (including 1 cysteine residue of the first C3H-type ZF). The putative pMKRN1-short is the same as human MKRN1-short 2 but differs from human MKRN1-short 1 and human MKRN1-short 3, which lack the N-terminal 64 aa and/or C-terminal ZF4 and part of the RING finger domain that is essential for RING finger-class E3 ubiquitin ligases (Fig. 1A).
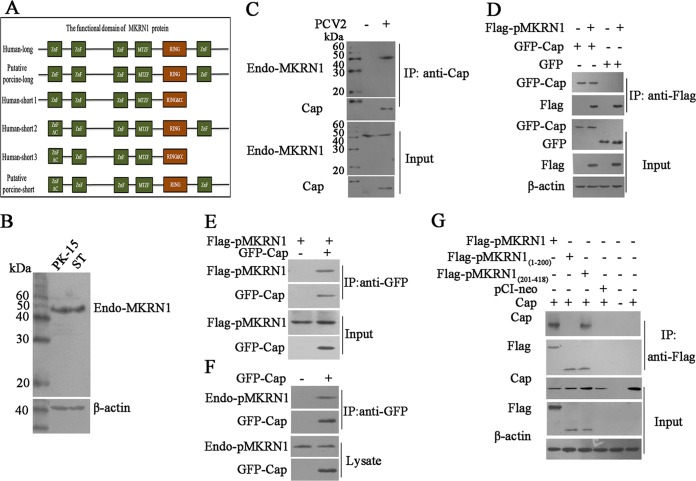
FIG 1.
Porcine MKRN1 interaction with PCV2 Cap. (A) Schematic comparison of the functional domains of MKRN1-long (Human-long; GenBank accession no. NP_038474), putative porcine full-size MKRN1 protein (Porcine-long; GenBank accession no. XM_003134608.4), the human MKRN1 variants human-MKRN1-short 1 (Human-short1; GenBank accession no. NP_001138597.1), human MKRN1-short 2 (Human-short2; GenBank accession no. XP_011514299.1, XP_011514300.1), and human MKRN1-short 3 (Human-short3; GenBank accession no. NP_001278592.1), and the putative porcine MKRN1 variant pMKRN1-short (Putative porcine-short; UniProtKB accession no. A0A287ALB7). (B) Molecular expression patterns of MKRN1 in PK-15 cells and ST cells. PK-15 cells and ST cells were lysed by RIPA, and the lysates were subjected to Western blotting with MKRN1 antibodies. (C) The putative porcine MKRN1 variant (pMKRN1-short) interacts with PCV2 Cap protein. PK-15 cells were infected with PCV2 (MOI = 1) for 24 h, and lysates were immunoprecipitated (IP) using an anti-Cap antibody; an anti-MKRN1 antibody was used for immunoblotting. (D, E) Cap interacts with pMKRN1 in transfected cells. HEK293T cells were cotransfected with GFP-Cap and Flag-pMKRN1 expression plasmids or cotransfected with the pEGFP control vector and the Flag-pMKRN1 expression plasmid. The cells transfected with the pEGFP control vector, the GFP-Cap expression plasmid, or the Flag-pMKRN1 expression plasmid alone served as controls. Immunoprecipitation was performed to detect the GFP-Cap interaction with Flag-pMKRN1 using anti-Flag antibodies or anti-GFP antibodies. (F) Endogenous MKRN1 interacts with GFP-Cap. PK-15 cells were transfected with GFP-Cap, cells were lysed for immunoprecipitation with GFP monoclonal antibodies, and anti-MKRN1 antibodies were used for immunoblotting. The cells untransfected with GFP-Cap served as a control. (G) The C terminus (aa 201 to 418) of pMKRN1 is responsible for interacting with Cap. HEK293T cells were transfected with plasmids to express the indicated proteins, an immunoprecipitation assay was performed using anti-Flag monoclonal antibodies, and anti-Cap antibodies were used for immunoblotting. These results were confirmed by three independent experiments.
To determine if pMKRN1-long and/or pMKRN1-short interacts with PCV2 Cap, we first detected the molecular expression patterns of MKRN1 in PK-15 cells and ST cells and found that only about 46 kDa of pMKRN1 (pMKRN1-short) was detected (Fig. 1B). Furthermore, when PK-15 cells were infected with PCV2 (multiplicity of infection [MOI] = 1) and cell extracts were immunoprecipitated using an anti-PCV2 Cap antibody, only endogenous pMKRN1-short (a 46-kDa band) was detected in the coprecipitated complex (Fig. 1C). Consistently, coimmunoprecipitation analysis using either overexpressed or endogenous pMKRN1-short along with recombinant PCV2 Cap showed that both exogenous pMKRN1-short and endogenous pMKRN1-short were able to interact with the recombinant PCV2 Cap protein (Fig. 1D to F). Domain mapping analysis revealed that the C-terminal fragment (aa 201 to 418) of pMKRN1-short but not the N-terminal fragment (aa 1 to 200) interacted with Cap (Fig. 1G). These results confirmed the interaction between pMKRN1 and Cap and further demonstrated that the C terminus of pMKRN1-short is responsible for its interaction with the Cap of PCV2.
The expression of pMKRN1 is regulated by PCV2 and inversely correlated with the Cap level in PCV2-infected cells and tissues.
To determine the roles of porcine MKRN1 in the process of PCV2 infection, we further investigated the expression profiles of pMKRN1 (referred to as pMKRN1-short here if not specified otherwise) in PCV2-infected PK-15 cells. Time course experiments showed that pMKRN1 mRNA was significantly increased at 12 h postinfection (hpi) and reached the highest levels at 24 hpi (Fig. 2A). Consistent with the changes of pMKRN1 mRNA levels, the pMKRN1 protein also showed a gradual increase at 12 hpi and peaked at 24 hpi and decreased thereafter (Fig. 2B). In contrast to the pattern of pMKRN1, the PCV2 Cap protein level showed a gradual decrease during the first 24 hpi and increased at 36 to 48 hpi (Fig. 2C). These results suggest that PCV2 Cap levels might be modulated by pMKRN1 during viral infection.
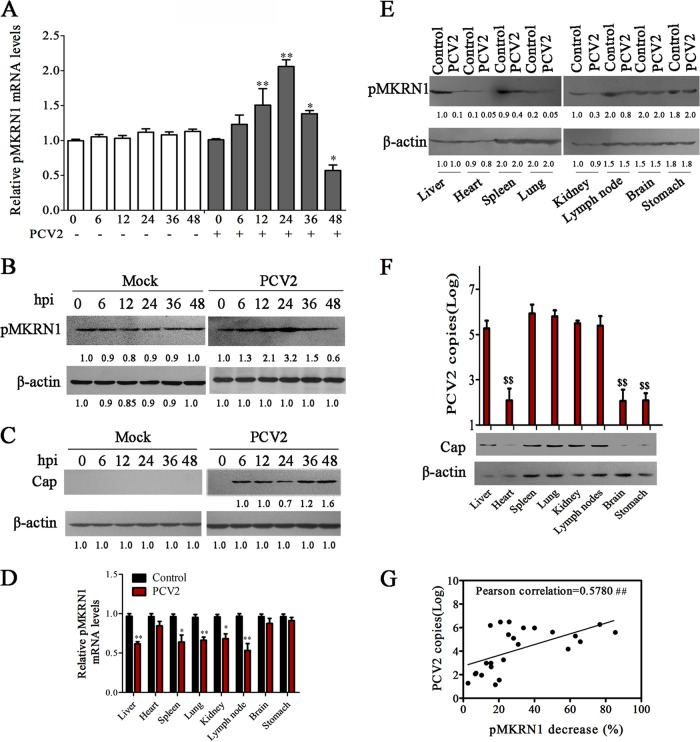
FIG 2.
The expression of pMKRN1 is regulated by PCV2 and inversely associated with the Cap level in PCV2-infected cells and tissues. (A to C) PK-15 cells were infected with PCV2 at an MOI of 1 or mock infected for the indicated times, and the mRNA levels of pMKRN1 (A), the protein levels of pMKRN1 (B), and the levels of Cap (C) were measured. The results are the means ± SEMs from 3 independent experiments. *, P < 0.05 versus mock-infected cells at 0 h; **, P < 0.01 versus mock-infected cells at 0 h. (D to F) Piglets were infected with PCV2 or mock infected for 14 or 28 days. Different tissues were used for detecting pMKRN1 mRNA levels in PCV2-infected or control piglets at 14 days postinfection (D), the pMKRN1 levels (E) and Cap levels (F) were compared at 28 days postinfection by immunoblotting, and quantitative PCR was used to analyze PCV2 copies number (F). The data shown are representatives from three independent experiments. *, P < 0.05 versus the same mock-infected tissues; **, P < 0.01 versus the same mock-infected tissues; $$, P < 0.01 versus other tissues of the PCV2-infected group. (G) The levels of the pMKRN1 protein and the number of PCV2 copies (log) in different tissues of PCV2-infected piglets were measured, the decreased percentages of pMKRN1 in each detected tissue (100% minus the percentage of pMKRN1 in the PCV2-infected group relative to the amount in the mock-infected group [quantity for the PCV2-infected group/quantity for the mock-infected group]) were calculated, and then their associations were evaluated using Pearson's coefficient of correlation analysis, as shown by positive trend lines. The numbers immediately beneath the lanes in panels B, C, and E represent the quantity of the indicated bands normalized to the content in the control lane in each blot (lane 1). ##, P < 0.01, which demonstrates that the reduction of pMKRN1 has a significant positive association with PCV2 copy numbers.
To further confirm whether the viral loads of PCV2 correlate with pMKRN1 expression in different infected tissues, we examined the mRNA and protein levels of pMKRN1 in different tissues of piglets infected with PCV2. The results showed that the mRNA levels of pMKRN1 were decreased in the major targeted tissues (liver, spleen, lung, kidney, and lymph node) of PCV2-infected piglets at 14 days postinfection (dpi) compared with those in the tissues of uninfected control piglets (Fig. 2D). Consistently, pMKRN1 protein levels varied in different tissues of uninfected control piglets and also showed an apparent decrease in liver, spleen, lung, kidney, and lymph node of PCV2-infected piglets at 28 days postinfection (Fig. 2E). Simultaneously, PCV2 viral loads and Cap protein levels were higher in the major pathological tissues (liver, spleen, lung, kidney, and lymph node) than in the nonpathological tissues (heart, brain, and stomach) of PCV2-infected piglets (Fig. 2F). Next, we further analyzed whether the replication levels of PCV2 (Table 1) were correlated with the decreased levels of pMKRN1 in different tissues of PCV2-infected piglets (Table 1) by Pearson's coefficient of correlation analysis. As shown in Fig. 2G, PCV2 copy numbers were positively associated with the decreased percentages of pMKRN1 (the levels in PCV2-infected piglets relative to those in mock-infected piglets) (r = 0.5780, P = 0.0031). These results demonstrate that the expression of pMKRN1 is regulated by PCV2 and inversely correlated with the Cap level in PCV2-infected cells and tissues.
TABLE 1.
Quantification of PCV2 viral loads and decreased percentage of pMKRN1 in different tissues from PCV2-infected pigletsa
| Tissue | Viral load (no. of copies/g) | Decreased % of pMKRN1 |
|---|---|---|
| Liver | 2.84E5 ± 1.22E5 | 55.50 ± 15.97 |
| Heart | 3.68E2 ± 2.96E2 | 13.62 ± 3.28 |
| Spleen | 1.52E6 ± 8.71E5 | 32.93 ± 11.16 |
| Lung | 1.34E6 ± 9.12E5 | 41.59 ± 9.60 |
| Kidney | 4.67E5 ± 2.45E5 | 40.73 ± 11.39 |
| Lymph node | 6.84E5 ± 5.89E5 | 56.91 ± 14.70 |
| Brain | 3.54E2 ± 3.00E2 | 8.660 ± 3.08 |
| Stomach | 2.02E2 ± 1.29E2 | 14.22 ± 3.95 |
Pigs were infected with 5 × 105 TCID50 PCV2 or mock infected, PCV2 viral loads in tissues from PCV2-infected piglets on 28 dpi were determined by qPCR and are presented as the mean DNA copy number divided by gram of tissue, and the protein levels of pMKRN1 in different tissues from PCV2- or mock-infected piglets on 28 dpi were determined by Western blotting. pMKRN1 levels were quantified, and the decrease in the levels of pMKRN1 in the PCV2-infected group relative to the levels in the mock-infected group were calculated and analyzed. Data are presented as the mean ± SD.
Overexpression of pMKRN1 leads to the reduction of Cap protein and viral replication.
Given that the levels of pMKRN1 and Cap are negatively correlated in PCV2-infected cells, we asked if pMKRN1 could regulate the levels of Cap protein. To answer this question, we established a cell line stably expressing Flag-pMKRN1, named PK-15pMKRN1 (Fig. 3A). When Cap recombinant plasmid (pcDNA-Cap) was transfected into either PK-15pMKRN1 or control cell lines, i.e., PK-15PCI or PK-15 cells, the level of Cap protein in PK-15pMKRN1 cells was decreased up to 70% compared to that in control cells that lacked Flag-pMKRN1 expression (Fig. 3B). We further tested whether transient overexpression of pMKRN1 could also decrease Cap levels and found that increasing doses of pMKRN1 expression plasmids (0, 1, 3, 5 μg) gradually decreased the expression of Cap but did not affect the amount of green fluorescent protein (GFP) in PK-15 cells or ST cells cotransfected with the GFP expression vector (Fig. 3C and D). Consistently, Cap protein was also decreased in the cells that overexpressed pMKRN1 (PK-15pMKRN1 cells) compared to the cells without pMKRN1 overexpression (PK-15 and PK-15PCI cells) when these cells were infected with PCV2, whereas PCV2 Rep or the protein encoded by ORF3 did not show any difference in these cells (Fig. 3E). We noted that Cap mRNA was not significantly decreased in the cells that overexpressed pMKRN1 compared to the cells without pMKRN1 overexpression (Fig. 3F), suggesting that pMKRN1 could not regulate Cap expression at the transcriptional level. In line with the reduction of Cap protein levels, the production of progeny virus was also markedly decreased in PK-15pMKRN1 cells compared with that in control PK-15 or PK-15PCI cells after PCV2 infection (Fig. 3G). Together, these results indicate that pMKRN1 overexpression significantly inhibits the generation of progeny PCV2, likely by reducing the levels of Cap.
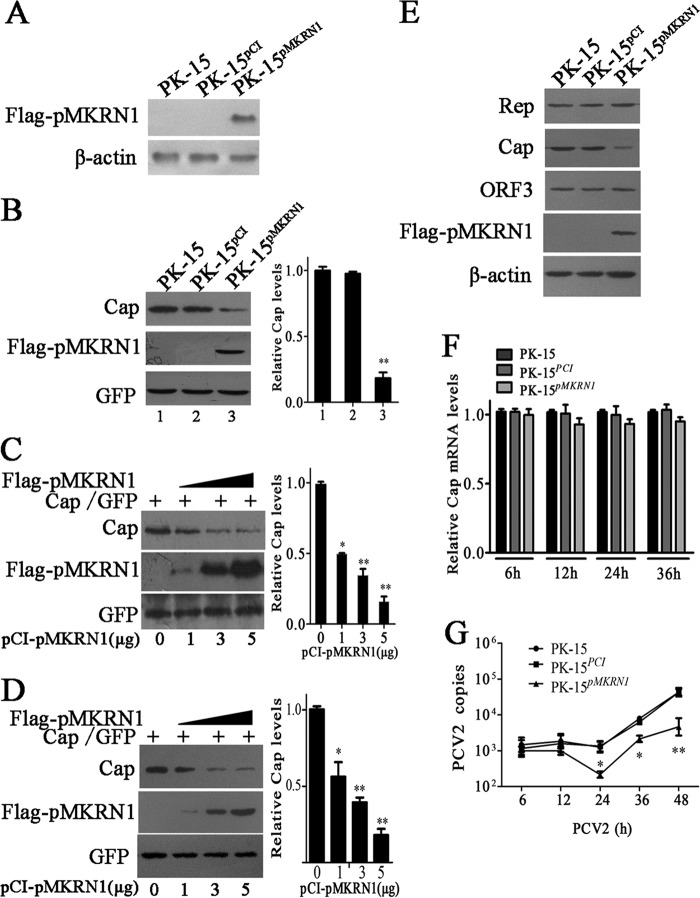
FIG 3.
Overexpression of pMKRN1 decreases ORF2 protein levels and inhibits the replication of PCV2. (A) A stable cell line, PK-15pMKRN1, expresses the pMKRN1 protein. PK-15 cells were transfected with linearized pCI-Flag-pMKRN1 or the control vector, pCI-neo, to establish PK-15pMKRN1 and PK-15PCI cells selected by G418, respectively. The cells were analyzed by immunoblotting using an anti-Flag antibody. β-Actin was detected as an internal control. (B) The level of Cap was decreased in cells overexpressing pMKRN1. (Left) The pcDNA-Cap and pEGFP vectors were cotransfected into PK-15, PK-15PCI, or PK-15pMKRN1 cells, and Cap, pMKRN1, and GFP levels were detected by immunoblotting. (Right) The ImageJ program was used to quantify the relative intensities of the bands. **, P < 0.01 versus the Cap level of PK-15 cells. (C and D) Increased pMKRN1 expression is correlated with decreased Cap. (Left) Different doses of pCI-Flag-pMKRN1 plasmids (0, 1, 3, 5 μg) were transfected with a fixed amount of Cap expression vectors into PK-15 cells (C) or ST cells (D), and Western blotting was used to examine Cap expression. GFP was used as a control. (Right) Relative intensities of the bands. *, P < 0.05 versus Cap levels in PK-15 cells or ST cells with 0 μg of pCI-Flag-pMKRN1 transfection; **, P < 0.01 versus Cap levels in PK-15 cells or ST cells with 0 μg of pCI-Flag-pMKRN1 transfection. (E to G) PK-15, PK-15PCI, and PK-15pMKRN1 cells were infected with PCV2 at an MOI of 1 for the indicated periods, and PCV2 Rep, Cap, and ORF3 protein levels were determined by Western blotting (E); the mRNA levels of Cap (F) and PCV2 copy numbers (G) were determined by quantitative PCR. The Cap mRNA levels of PK-15 cells at each time point were defined as 1 in panel F. The data shown are representatives from three independent experiments. *, P < 0.05 versus PCV2-infected PK-15 cells at the same time points; **, P < 0.01 versus PCV2-infected PK-15 cells at the same time points.
Knockout of pMKRN1 increases Cap protein and promotes progeny virus production.
In order to define the physiological role of pMKRN1 in inhibition of the Cap protein level and PCV2 replication, we utilized the clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9 genome-editing system to generate pMKRN1 knockout PK-15 cells. Three guide RNAs (gRNAs; gRNA-64, gRNA-266, gRNA-2211) were designed to target exon 1, exon 1, and exon 8 in the pMKRN1 genome, respectively (Fig. 4A). Three single-cell clones, MKRN1(64), MKRN1(266), and MKRN1(2211), were selected from cells transfected with the Cas9 vector encoding gRNA-64, gRNA-266, and gRNA-2211, respectively. Western analysis showed that pMKRN1 expression was deficient in MKRN1(64) and MKRN1(266) cells but was unaffected in the MKRN1(2211) cell clone (Fig. 4B). To examine the deletion or insertion mutation of the targeted locus, genomic DNA was extracted for amplification using primers flanking three gRNA loci. Genomic sequencing of the gRNA-64 cell clone (64PKpmkrn1−/−) showed that 302 nucleotides of locus 64 were replaced by an unknown sequence, leading to termination of protein translation, while the gRNA-266 cell clone (266PKpmkrn1−/−) showed a 2-nucleotide deletion, resulting in an early stop codon in locus 266 (data not shown). In contrast, the gRNA-2211 cell clone (2211PKpmkrn1+/+) did not show any changes in the sequence of locus 2211 compared with the wild-type (WT) DNA sequence (data not shown). It should be mentioned that pMKRN1 knockout did not appreciably affect the viability and proliferation of cells (data not shown).
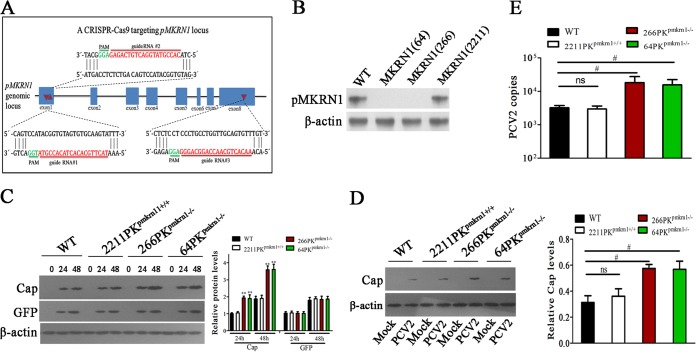
FIG 4.
Knockout of pMKRN1 by CRISPR-Cas9 increases Cap protein and promotes progeny virus replication. (A) Schematic diagram of gRNA targeting sites at the pMKRN1 genomic region. The gRNA-targeting sites (sites 1, 2, and 3) located on exon 1, exon 1, and exon 8 are shown. Red arrows indicate gRNA-targeting sites, the gRNA-targeting sequences are in red, and the protospacer adjacent motif (PAM) sequences are shown in green. (B) Examination of pMKRN1 expression in PK-15 cells with a CRISPR-Cas9 system targeting the pMKRN1 locus. Three single-cell clones [MKRN1(64), MKRN1(266), MKRN1(2211)] were derived from cells infected with lentiviral pseudotypes expressing gRNAs 1, 2, and 3, respectively. (C) Knockout of pMKRN1 increases Cap expression. Wild-type PK-15, 2211PKpmkrn1+/+, 266PKpmkrn1−/−, and 64PKpmkrn1−/− were transfected with Cap expression vectors or pEGFP control vectors, and Cap and GFP expression levels were detected by Western blotting at the indicated times (in hours). **, P < 0.01 versus the indicated protein levels in wild-type PK-15 cells at the same time. (D, E) Knockout of pMKRN1 promotes PCV2 progeny virion production. Wild-type PK-15, 2211PKpmkrn1+/+, 266PKpmkrn1−/−, and 64PKpmkrn1−/− cells were infected with PCV2 at an MOI of 1 for 24 h, and Western blotting was performed to detect PCV2 Cap (D) and quantitative PCR analysis was used to determine the number of PCV2 copies (E). #, P < 0.05; ns, not significant.
Next, we determined the effects of pMKRN1 knockout on Cap protein expression. Wild-type PK-15, 64PKpmkrn1−/−, 266PKpmkrn1−/−, and 2211PKpmkrn1+/+ cells were cotransfected with the Cap-expressing vector and the pEGFP control vector; at 24 h or 48 h posttransfection, the Cap protein level in 64PKpmkrn1−/− and 266PKpmkrn1−/− cells was higher than that in wild-type PK-15 and 2211PKpmkrn1+/+ cells. In contrast, the level of the GFP control did not show any difference among these cells (Fig. 4C). Consistently, when wild-type PK-15, 64PKpmkrn1−/−, 266PKpmkrn1−/−, and 2211PKpmkrn1+/+ cells were infected with PCV2 at an MOI of 1 for 24 h, PCV2 Cap expression and progeny virion production were higher in 64PKpmkrn1−/− and 266PKpmkrn1−/− cells than that in wild-type PK-15 and 2211PKpmkrn1+/+ cells (Fig. 4D and E). These results demonstrate that knockout of pMKRN1 increases the Cap protein level and promotes progeny virion production.
pMKRN1 mediates ubiquitination and degradation of PCV2 Cap.
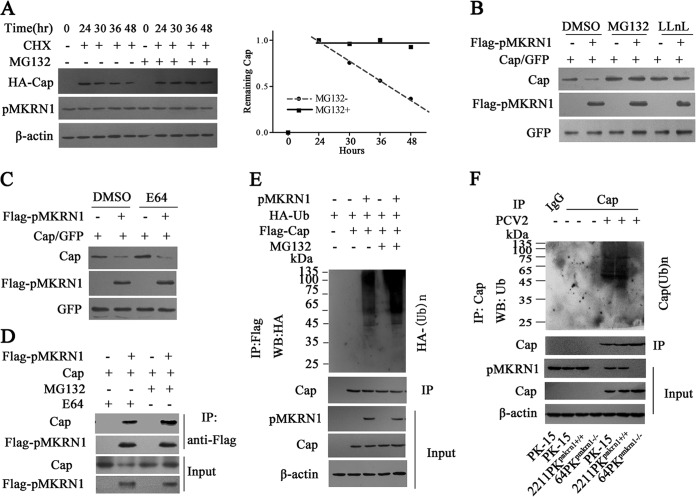
Several studies have reported that MKRN1 can serve as an E3 ligase to degrade viral proteins or host molecules (18–20, 28). Here, we first monitored the stability of Cap in cells treated with the protein synthesis inhibitor cycloheximide (CHX) and the roles of the proteasome pathway in the reduction of Cap protein. The results showed that the Cap level was gradually decreased in the CHX-treated cells in the absence of MG132 (a specific inhibitor of the proteasome pathway) and decreased up to 70% at 24 h after CHX treatment, but the levels of Cap proteins did not significantly change in CHX-treated cells in the presence of MG132 (Fig. 5A). Furthermore, addition of MG132 or N-acetyl-l-leucinyl-l-leucinyl-norleucinal (LLnL) to inhibit the proteasome pathway could prevent the reduction of Cap induced by exogenous pMKRN1 coexpression (Fig. 5B), whereas addition of E64, a lysosome inhibitor, had no effect (Fig. 5C). Consistent with these findings, we found that pMKRN1 could bind more Cap protein in the presence of MG132 than in the presence of E64 (Fig. 5D). These results suggest that PCV2 Cap is degraded by a proteasome-dependent pathway.
FIG 5.
pMKRN1 induces Cap ubiquitination, resulting in its degradation via the proteasome pathway. (A to D) pMKRN1 promotes the degradation of Cap via the proteasome pathway. PK-15 cells were transfected with HA-Cap; at 24 h posttransfection, the cells were treated with CHX (100 μg/ml) for another 24 h; and the expression of HA-Cap was tested at the indicated times by immunoblotting (A). 64PKpmkrn1−/− cells were transfected with pCI-Flag-pMKRN1, pcDNA-Cap, and pEGFP, as indicated; the cells were treated with 10 μM MG132 (a proteasome inhibitor), 10 μM LLnL (an MG132 analogue) (B), or 10 μM E64 (a lysosome inhibitor) (C); and the expression of Flag-pMKRN1, Cap, or GFP was analyzed by Western blotting (WB). The lysates were immunoprecipitated with anti-Flag antibodies, and Western blotting was used to detect Cap (D). (E) pMKRN1 induces Cap ubiquitination. 64PKpmkrn1−/− cells were transfected with the indicated expression plasmids, followed by treatment of the cells with MG132 (10 μM) for 6 h. Cap proteins were immunoprecipitated using an anti-Flag antibody, and ubiquitinated proteins were immunoblotted using an anti-HA antibody. (F) The ubiquitination of Cap is lost in pMKRN1 knockout cells. Wild-type PK-15, 2211PKpmkrn1+/+, or 64PKpmkrn1−/− cells were infected with PCV2, cell lysates were immunoprecipitated using anti-Cap antibodies, and polyubiquitinated Cap [Cap(Ub)n] was detected by immunoblotting using anti-ubiquitin antibodies. These results were confirmed in three independent experiments.
As pMKRN1 is an E3 ligase, we further tested whether the degradation of Cap is dependent on polyubiquitination. Ubiquitination assays showed that exogenous pMKRN1 was able to induce the polyubiquitination of Cap in 64PKpmkrn1−/− cells, and this ubiquitination was enhanced in the presence of MG132 (Fig. 5E). Accordingly, ubiquitination of Cap was detected in PCV2-infected (Fig. 5F) or Flag-Cap-transfected (data not shown) wild-type PK-15 or 2211PKpmkrn1+/+ cells but not in PCV2-infected or Flag-Cap-transfected 64PKpmkrn1−/− cells, suggesting that pMKRN1 is necessary for the ubiquitination process of Cap protein during PCV2 infection.
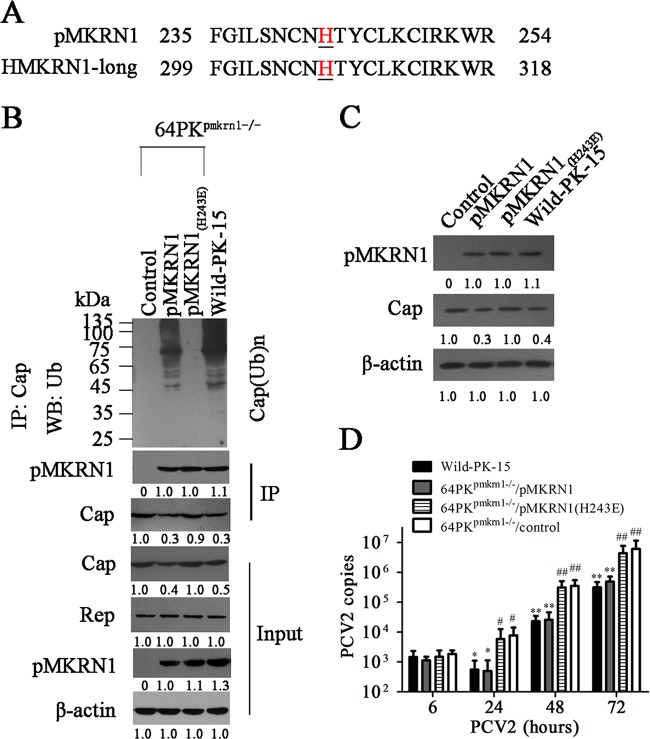
For human MKRN1, mutation of the histidine at residue 307 within the RING finger domain to aspartate abolishes its E3 ligase activities (18). Since the sequence of pMKRN1 shows a higher identity to human MKRN1 in this region and all histidine residues within this region are conserved between human and porcine homologs of pMKRN1 (Fig. 6A), we further determined whether the histidine 243 residue is critical for the E3 ligase activities of pMKRN1. In MKRN1-deficient (64PKpmkrn1−/−) cells, expression of wild-type pMKRN1 restored both the ubiquitination and degradation activities, whereas expression of the pMKRN1(H243E) mutant rendered to the cell loss of the ability to induce Cap ubiquitination and degradation, even though it was expressed at the WT level and still able to bind to Cap protein (Fig. 6B). Consequently, the pMKRN1(H243E) mutant also markedly enhanced PCV2 Cap protein levels and the production of progeny PCV2 in MKRN1-deficient (64PKpmkrn1−/−) cells compared with wild-type pMKRN1 (Fig. 6C and D). These data demonstrate that pMKRN1 functions as a specific E3 ligase for PCV2 Cap and regulates the replication of progeny PCV2 through induction of polyubiquitination and degradation of Cap.
FIG 6.
The histidine 243 residue is critical for the E3 ligase activity of pMKRN1. (A) The histidine residue is conserved between pMKRN1 and human MKRN1-long. (B to D) Cap ubiquitination can be restored by wild-type pMKRN1 but not mutant pMKRN1(H243E) in pMKRN1 knockout cells. 64PKpmkrn1−/− cells were transfected with plasmids to express wild-type pMKRN1 (64PKpmkrn1−/−/pMKRN1) or mutated pMKRN1 [64PKpmkrn1−/−/pMKRN1(H243E)] or transfected with control plasmids (64PKpmkrn1−/−/control); the cells were then infected with PCV2 (MOI = 1), and ubiquitination of Cap (B), the expression of Cap and pMKRN1 (C), and the production of progeny PCV2 were measured (D). The data shown are representatives from three independent experiments. *, P < 0.05 versus PCV2-infected 64PKpmkrn1−/−/control cells; **, P < 0.01 versus PCV2-infected 64PKpmkrn1−/−/control cells; #, P < 0.05 versus PCV2-infected 64PKpmkrn1−/−/pMKRN1 cells; ##, P < 0.01 versus PCV2-infected 64PKpmkrn1−/−/pMKRN1 cells. The numbers under the gels represent quantification of indicated bands, normalized to the first visible band in each blot (C and D). DMSO, dimethyl sulfoxide.
pMKRN1 targets PCV2 Cap lysine residues 164, 179, and 191 for ubiquitination and degradation.
To identify the regions of PCV2 Cap required for pMKRN1 binding and ubiquitination, 10 deletion mutants of Cap were constructed (Fig. 7A). Coimmunoprecipitation assay showed that mutants Cap(1-41), Cap(1-83), Cap(1-107), and Cap(199-234) were not able to bind to pMKRN1, whereas all the other mutants could interact with pMKRN1, suggesting that two fragments (aa 108 to 161 and aa 162 to 198) are required for interaction with pMKRN1 (Fig. 7B). Furthermore, we observed that the fragment from aa 162 to 198 was degraded by pMKRN1, whereas the fragments from aa 1 to 107, 108 to 161, and 199 to 234 were not, suggesting that the fragment from aa 162 to 198 is required for pMKRN1-mediated degradation of Cap (Fig. 7C). In this region (aa 162 to 198), three lysine residues (164, 179, and 191) were noted (Fig. 7D). When these three lysine residues were replaced by alanine, we observed that the corresponding mutants, Cap(K164A), Cap(K179A), and Cap(K191A), were still able to bind to pMKRN1 and degraded by 70 to 90% in the presence of pMKRN1 (Fig. 7E). However, when all these three lysine residues were replaced by alanine, the degradation of Cap was almost completely blocked (Fig. 7E). Consistent with these observations, ubiquitination of mutants Cap(K164A), Cap(K179A), and Cap(K191A) was modestly decreased, whereas the Cap(3M) (K164A, K179A, K191A) mutant was barely polyubiquitinated by pMKRN1 (Fig. 7F). Taken together, these results demonstrate that pMKRN1 specifically targets PCV2 Cap lysine residues 164, 179, and 191 for ubiquitination and subsequent degradation.
FIG 7.
PCV2 Cap lysine residues 164, 179, and 191 are the sites of ubiquitination by pMKRN1. (A) Schematic diagram of N- and C-terminal truncation mutants of PCV2 Cap. (B) Mapping the regions of PCV2 Cap that interact with pMKRN1. HEK293T cells were transfected with plasmids to express Flag-pMKRN1 and Cap truncation mutants fused with the GFP tag, and lysates were immunoprecipitated with an anti-GFP antibody, followed by immunoblotting using an anti-MKRN1 antibody, anti-GFP antibody, or anti-β-actin antibody. The middle white space divides the samples that were resolved by two SDS-PAGEs due to the limit of the gel lanes. (C) PCV2 Cap containing amino acid residues 162 to 198 is degraded by pMKRN1. HEK293T cells were transfected with plasmids to express Flag-pMKRN1 and the indicated Cap mutants, and Western blotting was used to detect the levels of Cap mutant expression with anti-HA antibodies. (D to F) K164, K179, and K191 were the ubiquitination sites of the PCV2 Cap protein. The Cap lysine residues at residues 164, 179, and 191 were replaced with alanine (D). HEK293T cells were transfected to express Flag-pMKRN1 and the indicated Cap lysine mutants, Western blotting was used to detect the expression levels of Cap or mutants using anti-Cap antibodies (E), and the ubiquitination of Cap mutants was detected (F). These results were confirmed in three independent experiments.
Mutation of ubiquitination site of Cap protein enhances the replication and pathogenesis of PCV2.
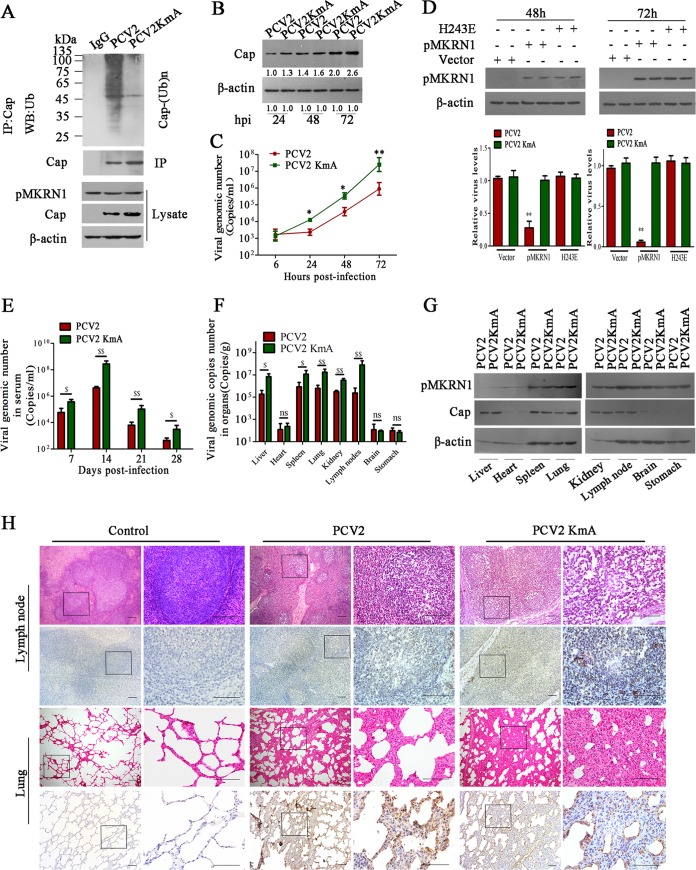
As Cap-3M (K164A, K179A, K191A) cannot be polyubiquitinated and degraded in cells expressing pMKRN1, we hypothesized that mutation of the ubiquitination site of Cap protein might enhance the replication and pathogenesis of PCV2 in vivo. We thus constructed a PCV2 mutant strain (PCV2KmA) in which all three lysine residues (164, 179, and 191) of Cap were replaced by alanine. As would be expected, the polyubiquitination of Cap protein was not detected in PCV2KmA-infected PK-15 cells (Fig. 8A) or in PCV2KmA-infected ST cells (data not shown). The Cap protein levels and progeny virion production in PCV2KmA-infected PK-15 (Fig. 8B and C) or ST (data not shown) cells were significantly higher than those in wild-type PCV2-infected PK-15 or ST cells. In addition, in MKRN1-deficient (64PKpmkrn1−/−) cells, wild-type pMKRN1 expression could markedly suppress the replication of PCV2 but did not affect PCV2KmA replication (Fig. 8D). However, the production of progeny PCV2 and PCV2KmA was not significantly different in either pMKRN1-deficient cells or pMKRN1(H243E) mutant-expressing cells (Fig. 8D). This result further confirms that PCV2 production is associated with the E3 ligase activities of pMKRN1. Next, to explore whether Cap ubiquitination could affect the replication and pathogenesis of PCV2 in vivo, we tested viral loads, Cap protein levels, and pathological lesions of different tissues and compared them between piglets infected with wild-type and mutant PCV2. The results showed that the viral loads in the sera of piglets infected with PCV2KmA were significantly higher than those in the sera of wild-type PCV2-infected piglets from 7 to 28 dpi (Fig. 8E). Consistently, in the liver, spleen, lung, kidney, and lymph node tissues, the relative viral genomic copy numbers and Cap levels of PCV2KmA-infected piglets were significantly higher than those of PCV2-infected piglets, but the levels of pMKRN1 in the same tissues did not show any difference between wild-type PCV2-infected piglets and mutated PCV2-infected piglets (Fig. 8F and G). Consequently, PCV2KmA infection induced more severe histological lesions in the lung and lymph node tissues than wild-type PCV2 infection (Fig. 8H). PCV2KmA-infected piglets showed more severe interstitial pneumonia (characterized by thicker alveolus walls and inflammatory cell infiltration) and lymphoid depletion (characterized by lymphocyte necrosis and a reduction in the cortex and paracortex) than wild-type PCV2-infected piglets and showed decreased amounts of germinal centers or lost the germinal centers (Fig. 8H). The mean lung histological lesion scores and mean lymphoid depletion scores in the PCV2KmA-infected group were significantly higher than those in the wild-type PCV2-infected group (Table 2). Simultaneously, PCV2 was detected in the lung and lymph node from the piglets infected with PCV2 or PCV2KmA by immunohistochemistry using anti-ORF1 antibodies. The numbers of PCV2-positive cells were higher in the tissues of PCV2KmA-infected piglets than in the tissues of PCV2-infected piglets (Fig. 8H). These results revealed that mutation of the ubiquitination site of the Cap protein enhances the replication and pathogenesis of PCV2 in piglets.
FIG 8.
Mutation of ubiquitination sites of Cap protein enhances the replication and pathogenesis of PCV2. (A to D) Mutant PCV2 (PCV2KmA) containing the three lysine residues (164, 179, and 191) replaced by alanine shows increased Cap levels and viral reproduction due to the loss of ubiquitination. PK-15 cells were infected with PCV2 or PCV2KmA, and Cap ubiquitination was examined (A), Cap expression levels were determined (B), and viral copy numbers were measured by quantitative PCR (C). *, P < 0.05 versus PCV2-infected cells; **, P < 0.01 versus PCV2-infected cells. (D) 64PKpmkrn1−/− cells were transfected with wild-type pMKRN1, the pMKRN1(H243E) mutant, or the pCI-neo vector, and then the cells were infected by PCV2 or the PCV2KmA mutant for 48 h and 72 h. Relative PCV2 or PCV2KmA levels were measured by quantitative PCR. **, P < 0.01 versus pCI-neo vector-transfected cells. (E to G) Piglets were infected with PCV2 or PCV2KmA (5 × 105 TCID50) by intranasal injection. The numbers of PCV2 copies in serum at the indicated times (E) and at 28 days postinfection of tissues (F) were measured by quantitative PCR. The pMKRN1 expression levels in tissues at 28 days postinfection were measured by Western blotting (G). $, P < 0.05; $$, P < 0.01; ns, not significant. Results were confirmed in three independent experiments. (H) Hematoxylin and eosin staining of lung and lymph node tissues and immunohistochemical staining with anti-ORF1 antibody of lung and lymph node tissues derived from PCV2- or PCV2KmA-infected piglets at 28 days postinfection. The streptavidin-biotin-peroxidase complex method with counterstaining with hematoxylin was used. The regions with black rectangles were amplified, and the images are shown on the right. Bars, 100 μm.
TABLE 2.
Comparison of histological lesion score of lungs and lymph nodes from PCV2- and PCV2KmA-infected piglets
| Groupa | Piglet label | Score |
|
|---|---|---|---|
| Lungb | Lymph nodesc | ||
| PBS | A | 0 | 0 |
| B | 0 | 0 | |
| C | 0 | 0 | |
| PCV2 | D | 4 | 2 |
| E | 2 | 1 | |
| F | 3 | 2 | |
| PCV2KmA | G | 5 | 3 |
| H | 4 | 2 | |
| I | 6 | 3 | |
PCV2, porcine circovirus type 2; PCV2KmA, a PCV2 mutant.
The score ranged from 0 (normal) to 6 (severe).
The score ranged from 0 (normal) to 3 (severe).
DISCUSSION
During viral infection, the virus is recognized by pathogen recognition receptors of infected cells, which trigger signaling cascades to initiate innate intracellular antiviral defenses and to restrict viral replication (29–32). Three protein degradation systems (the ubiquitin-proteasome system [UPS], lysosomes, and the autophagy system) contribute to the maintenance of protein homeostasis, as well as antiviral defenses against different viral infections (33, 34). Previous studies have shown that E3 ligase-mediated ubiquitin is crucial for viral degradation, such as in the process of Japanese encephalitis virus (JEV), HIV, and West Nile virus (WNV) infections (20, 35, 36). For example, Nedd4, an E3 ubiquitin ligase that is highly expressed in the central nervous system, is upregulated in response to JEV infection, which in turn negatively regulates JEV replication through activation of autophagy. BCA2 (Rabring7, RNF115, or ZNF364), a RING finger E3 ubiquitin ligase, interacts with the matrix region of the retroviral Gag protein of HIV-1, resulting in its ubiquitination and subsequent lysosomal degradation (36). MKRN1, a RING finger E3 ubiquitin ligase, could serve as a switch in innate intracellular antiviral defense systems. In WNV-infected cells, human full-size MKRN1 interacts with the WNV capsid and mediates its ubiquitination and degradation to attenuate the cytotoxic effects of viral infection (20). In this study, we showed that pMKRN1-short has a unique expression pattern and is encoded by the porcine mkrn1 gene. In the early stage of PCV2 infection, the expression of pMKRN1 was upregulated to degrade PCV2 Cap protein to reduce virus replication, but in the late phase of PCV2 infection, the expression of pMKRN1 was efficiently downregulated, which might be regulated by PCV2 or induced by self-ubiquitination, as in human adenovirus infection (37). Knockout of the entire pMKRN1 gene, mutations that abolish the E3 ligase activity of pMKRN1, or mutation of the ubiquitination residues of PCV2 Cap protein all increase Cap expression levels and enhance progeny virus production and viral pathogenesis.
The putative porcine pMKRN1-short is an N-terminal-truncated variant of full-size porcine MKRN1 418 amino acids in length, and it shares 95.5% identity with human MKRN1-short 2 (GenBank accession no. XP_011514299.1 and XP_011514300.1), which is an N-terminal-truncated variant of full-size human MKRN1 418 amino acids in length with a molecular mass of 46.1 kDa. In this study, we did not detect the putative porcine full-size MKRN1 protein (pMKRN1-long) in PK-15 cells or in most porcine tissues; rather, pMKRN1-short was identified to have the unique expression pattern of the porcine mkrn1 gene in most porcine tissues or cells. Although pMKRN1-short lacks the N-terminal 64 aa (including 1 cysteine residue of the first C3H-type ZF), it is intact in the RING finger E3 ubiquitin ligase domain. Thus, pMKRN1-short could interact with PCV2 Cap protein to mediate its ubiquitination and subsequent proteasomal degradation through its RING finger E3 ubiquitin ligase activity. Notably, human MKRN1-short has not been reported to modulate the ubiquitination of intracellular or viral proteins, because it lacks the C-terminal ZF4 and part of the RING finger domain essential for RING finger-class E3 ubiquitin ligases.
MKRN1 is first identified as a RING finger E3 ligase that regulates the degradation of hTERT through UPS (17). Many other E3 ligases are also involved in UPS, like MARCH9, cIAP1/2, FAF1, and TRIM25 (38–41), which bind to E2 and substrate, primarily provide the Ub chain to the substrate, facilitating the formation of an isopeptide; finally, the target protein is degraded into small peptide fragments by the 26S proteasome (23, 42). E3s mediate the degradation of substrates associated with conserved structural domains, including two classes, the really interesting new gene (RING) family and the homology to the E6AP C terminus (HECT), although a few have a U box, which is structurally and functionally similar to the RING finger domain (43, 44). CBL proteins (members of the CBL family) have a HECT domain, which could interact with activated receptor tyrosine kinase (RTK) and traffic it to the lysosome for degradation (45). However, RING finger type E3 ligase contains a Zn2+-coordinating domain that consists of a series of specifically spaced cysteine and histidine residues. The function of RING finger (or RING-like) is as a scaffold binding E2 and substrate (46). Human MKRN1 has been reported to regulate numerous cellular molecules through the ubiquitin-proteasome system (UPS), including p53, p21, FADD, and PTEN, as well as some viral proteins (17–21). In the current study, we showed that porcine MKRN1 (pMKRN1-short) regulates the degradation of PCV2 Cap through activating UPS and not the lysosome system. This activity of pMKRN1-short is similar to that of human MKRN1-long, which targets WNV capsid protein (20). pMKRN1-short specifically targets PCV2 Cap lysine residues 164, 179, and 191 to induce polyubiquitination and subsequent degradation. Of note, sequence alignment of PCV2 2a type Cap and 2b type Cap revealed that lysine residues 164 and 179 are highly conserved, but K191 is quite diverse (data not shown). We demonstrated that mutation of all three lysine residues in Cap protein or mutation of the histidine at residue 243 within the RING finger domain of pMKRN1, which abolishes its E3 ligase activity, rendered to the cells loss of the ability to induce Cap ubiquitination and degradation. Hence, MKRN1 not only regulates flavivirus capsid protein but also plays a similar role in regulation of circovirus capsid protein.
pMKRN1 is the first circovirus E3 ligase to be identified during the PCV2 infection process. Overexpression of pMKRN1 significantly inhibited the generation and propagation of progeny PCV2 via ubiquitination and degradation of Cap protein, whereas knockout of pMKRN1 or mutation of key residues to abolish its E3 ligase activity promoted progeny virus production. Importantly, mutation of three lysine residues of Cap protein in PCV2 promoted progeny virus production, resulting in enhanced histological lesions in mutated PCV2-infected piglets compared with wild-type PCV2-infected piglets. These data indicate that pMKRN1 functions as an intrinsic host defense factor against PCV2 infection and reveal a new protective mechanism of porcine host cells that is through posttranslational modification of proteasomal degradation of PCV2 capsid protein.
Now that it is known that pMKRN1 suppresses viral replication during PCV2 infection, why are pigs still susceptible to PCV2? The data presented here partially answer this question. We observed that pMKRN1 expression levels were increased in the earlier stage of PCV2 infection, which correlated with a relatively lower level of PCV2 infection, whereas prolonged PCV2 infection downregulated pMKRN1 levels, which promoted the later stage of PCV2 infection in infected cells. Our in vivo data showed that the expression of pMKRN1 is inversely correlated with the Cap level as well as the viral loads in PCV2-infected tissues. These data confirmed that pMKRN1 functions as a host defense factor to inhibit PCV2 replication and also demonstrate that PCV2 has evolved a mechanism to evade the pMKRN1-mediated host defensive strategy to benefit its replication. However, it is currently unclear how exactly PCV2 regulates pMKRN1 expression during infection, and our ongoing work is trying to tackle these issues.
In summary, the data presented here demonstrate that a putative porcine Makorin RING finger protein 1 (pMKRN1) variant, which shows a unique expression pattern of the porcine mkrn1 gene, interacts with PCV2 Cap to modulate PCV2 replication through E3 ligase-medicated ubiquitination and degradation of Cap and elucidate the regulation patterns of pMKRN1 in this process. Our findings provide one possible reason for the slower virus replication in the earlier stage of PCV2 infection and will help us to further understand the antiviral mechanisms of MKRN1 in pigs.
MATERIALS AND METHODS
Cell culture and virus.
PK-15 and HEK293T cells purchased from the American Type Culture Collection (ATCC) were cultured in Dulbecco's modified Eagle medium (DMEM) (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone) in a 5% CO2 culture. The PCV2 strain (GenBank accession no. EU366323) was isolated and stocked in the Tong lab (47).
Plasmids and reagents.
PCV2 open reading frame 2 (ORF2), coding for Cap protein, and nine deletion mutant fragments were amplified from the PCV2 genome template and subcloned into the pEGFP-N1 vector. Cap fragments were also subcloned into the pCDNA3.1 vector with or without a Flag tag sequence, and the Cap lysine mutants [Cap(K164), Cap(K179), and Cap(K191) fragments and the Cap(3M) mutant] were amplified by overlap PCR. Other Cap deletion mutant fragments for analysis of degradation were subcloned into the pCI-neo vector with a hemagglutinin (HA) tag sequence. The ubiquitination plasmid (HA-ubiquitin) was donated by Rui Zhang. The porcine MKRN1 cDNA was amplified from the testes of pigs and then was subcloned into the pCI-neo vector. All sequences were confirmed by sequencing analysis (Sangon Biotech).
Antibodies included monoclonal mouse anti-green fluorescent protein (anti-GFP) antibody (catalog number CSB-MA000051M0m; Cusabio), monoclonal rabbit anti-GFP antibody (catalog number G10362; Invitrogen), mouse monoclonal anti-Flag (M2) antibody (catalog number F1804; Sigma-Aldrich), rabbit monoclonal anti-Flag antibody (catalog number 701629; Invitrogen), rabbit polyclonal anti-MKRN1 antibody (catalog number A300-990A; Bethyl Laboratories, Inc.), mouse monoclonal anti-ubiquitin antibody (Cell Signaling Technology, 3936), and mouse monoclonal anti-HA tag antibody (catalog number AE008; ABclonal). PCV2 Rep, Cap, and ORF3 protein antibodies were produced in the Tong lab (48). Horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG was purchased from Invitrogen (catalog numbers 31430 and 31460, respectively). MG132 and E64 were purchased from Sigma-Aldrich.
Western blotting.
Cells were lysed by radioimmunoprecipitation assay (RIPA) buffer containing 1 mM phenylmethylsulfonyl fluoride and protease inhibitors (Sigma-Aldrich) on ice for 30 min. The protein concentration was determined by using a bicinchoninic acid protein assay kit (Thermo Fisher), resolved by 12% SDS-PAGE, and transferred to Immobilon transfer membranes (Millipore). The process of immunoblotting in detail was performed as described in a previous study with the indicated antibodies (49). Immunoreactive bands were visualized using enhanced chemiluminescence reagents (Bio-Rad). β-Actin (catalog number A00702-100; GenScript) was utilized as a control.
qPCR.
Virus DNA was extracted by use of a MiniBEST viral DNA extraction kit (Bio Teke) according to the manufacturer's instructions. The method of absolute quantification of PCV2 was previously described (50), with the concentrations of viral DNA in the samples being calculated. Total RNA was isolated by the TRIzol reagent according to the manufacturer's instructions (Invitrogen). Reverse transcription was performed using Moloney murine leukemia virus reverse transcriptase (Invitrogen) with random primers. Quantitative PCR (qPCR) analyses were performed on an iQ5 real-time PCR system (Bio-Rad) using SYBR Premix Ex Taq II DNA polymerase (TaKaRa). The pMKRN1 mRNA transcription levels were normalized against those of porcine β-actin by the 2−ΔΔCT threshold cycle (CT) method, and the relative fold change in the amount of the pMKRN1 gene at different times of PCV2 infection or mock infection of PK-15 cells was then calculated. All test samples were run in three independent experiments, and data are representative of the mean values. The following primers were used: PCV2-qPCR-F (TTGAATGTGGAGCTCCTAGAT), PCV2-qPCR-R (GCAAGGTACTCACAGCAGTAGACA), pMKRN1-qPCR-F (CAAACTGCAGTGGAGACGAA), pMKRN1-qPCR-R (CACACATGTCACAGGGGTCT), β-actin-F (GGACTTCGAGCAGGAGATGG), and β-actin-R (AGGAAGGAGGGCTGGAAGAG).
CRISPR-Cas9-mediated MKRN1 knockout in PK-15 cells.
CRISPR-Cas9 design and the analysis method were described in a previous study (51). In detail, three pairs of gRNAs specifically targeting the porcine MKRN1 sequence were designed using the optimized CRISPR design tool (http://crispr.mit.edu/). Oligonucleotides were annealed and ligated into the Lenti-CRISPRv2 plasmid (catalog number 52961; Addgene) by use of the BsmBI sites, respectively, and confirmed by sequencing analysis (Sangon Biotech). HEK293T cells were cotransfected with recombinant plasmids accompanied with psPAX2 (catalog number 12260; Addgene) and pMD2.G (catalog number 12259; Addgene) to obtain recombinant lentiviruses at 72 h. Then, the culture supernatant was collected and used to infect PK-15 cells. After 48 h, the cells were selected by puromycin (InvivoGen) at a concentration of 5 μg per ml. Positive cells were obtained after about 2 weeks and then subcloned into 96-well plates for single-clone growth and saved as cell stocks.
Immunoprecipitation assay.
Cells were cultured in a 100-mm-diameter dish, plasmids were cotransfected using the Lipofectamine 3000 reagent (Inventrogen) according to the manufacturer's instructions; 36 h later, the cells were lysed with lysis buffer (150 mM NaCl, 50 mM Tris-HCl [pH 7.4], 1% Nonidet P-40, 0.5% Triton X-100, 1 mM EDTA, 0.1% sodium deoxycholate, 1 mM dithiothreitol, 0.2 mM phenylmethylsulfonyl fluoride, protease inhibitor protease inhibitor cocktail [Sigma-Aldrich]) on ice for 30 min. The cell lysate supernatant was collected by centrifuging for 15 min at 13,000 × g and incubated with protein G-agarose/protein A-agarose (Santa Cruz) for precleaning of nonspecific proteins for 1 h at 4°C. The samples were centrifuged, and the supernatant was incubated with the indicated antibodies overnight at 4°C. The complex was incubated with protein G-agarose–protein A-agarose again for 30 min at room temperature and then centrifuged at 2,000 × g for 10 s and washed three times with phosphate-buffered saline (PBS). Finally, the bound proteins were eluted by boiling for 10 min in 2× loading buffer and analyzed by Western blotting using the indicated antibodies.
Animal experiments.
Eighteen 5-week-old piglets, free of PCV2, porcine reproductive and respiratory syndrome virus, classical swine fever virus, pseudorabies virus, porcine parvovirus, swine influenza virus, and Mycoplasma hyopneumoniae infection, were randomly assigned to 3 groups of 6 piglets each and housed separately. The pigs in group 1 were inoculated with PBS (2.5 ml in each nostril) as an uninfected control. Group 2 pigs were each inoculated with 5 ml of 105 median (50%) tissue culture infective doses (TCID50)/ml of PCV2 (2.5 ml in each nostril). Group 3 pigs were each inoculated with 5 ml of 105 TCID50/ml of PCV2KmA (2.5 ml in each nostril). After challenge, the pigs were monitored for 14 days or 28 days for rectal temperatures and clinical signs. Blood was collected from all animals on 0, 7, 14, 21, and 28 days postinfection (dpi) for detection of PCV2 using real-time PCR. Pigs were individually weighed from 0 to 28 dpi, and relative daily weight gains (RDWG; expressed as daily weight gain/primary body weight) were determined.
Pathological examination.
Necropsies of piglets were performed on 14 and 28 dpi, and superficial inguinal lymph node, lung, liver, heart, spleen, kidney, brain, and stomach tissues were collected. Then, samples were fixed by 10% formalin, embedded in paraffin wax, sliced in a microtome (Leica) to 4 μm, affixed onto slides, and then subjected to hematoxylin and eosin (HE) staining for microscopic examination and immunohistochemistry (IHC) staining for detection of PCV2 or the PCV2 mutant as described previously (52).
The histological lesions were scored as described in a previous study (53). Lung sections were scored for the presence and severity of type 2 pneumocyte hypertrophy and hyperplasia, alveolar septal infiltration with inflammatory cells, peribronchial lymphoid hyperplasia, the amount of alveolar exudate, and the amount of inflammation in the lamina propria of the bronchi and bronchioles, with the scores ranging from 0 to 6 (0, normal; 1, mild multifocal; 2, mild diffuse; 3, moderate multifocal; 4, moderate diffuse; 5, severe multifocal; 6, severe diffuse). Lymph nodes were evaluated for the presence of lymphoid depletion and given scores ranging from 0 to 3 (0, normal; 1, mild lymphoid depletion with loss of overall cellularity; 2, moderate lymphoid depletion; 3, severe lymphoid depletion with loss of lymphoid follicle structure).
Ethics statement.
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the Fourth Military Medical University (permit number 20150406) and were performed according to the Animal Ethics Procedures and Guidelines of the People's Republic of China. No other specific permissions were required for these activities. This study did not involve endangered or protected species.
ImageJ quantification and statistics.
Image J software was used to quantify band intensity. Degradation rates of Cap were calculated by use of the pEGFP vector. Pearson's correlation coefficients were calculated to examine the correlations between decreased levels of pMKRN1 with PCV2 production. Data are expressed as the mean ± standard error of the mean (SEM) or the mean ± standard deviation (SD). Differences among groups were analyzed by one-way analysis of variance followed by the Bonferroni post hoc test or unpaired t tests. P values of <0.05 or less were considered significant.
ACKNOWLEDGMENTS
We thank Rui Zhang of the Fourth Military Medical University for providing HA-ubiquitin vectors (Addgene). We also thank other members of the Shan-Lu Liu laboratory and the Dewen Tong laboratory for their help in this work.
This work was supported by the National Natural Science Foundation of China (31672535, 31372401, 31372411) and U.S. NIH grants (1R01AI112381 and 1R21AI109464) to S.-L.L. This work was also supported by the Science and Technology Innovation Project in Shaanxi Province (2016KTCL02-13), Central Project of Major Agricultural Technology Promotion funds (K3360217060), and Fundamental Research Funds for the Central Universities (2452017023).
REFERENCES
- 1.Opriessnig T, Meng XJ, Halbur PG. 2007. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest 19:591–615. doi: 10.1177/104063870701900601. [DOI] [PubMed] [Google Scholar]
- 2.Thomson J, Smith B, Allan G, McNeilly F, McVicar C. 2000. PDNS, PMWS and porcine circovirus type 2 in Scotland. Porcine dermatitis and nephropathy syndrome. Post-weaning multisystemic wasting syndrome. Vet Rec 146:651–652. [PubMed] [Google Scholar]
- 3.Pineyro PE, Kenney SP, Gimenez-Lirola LG, Opriessnig T, Tian D, Heffron CL, Meng XJ. 2016. Evaluation of the use of non-pathogenic porcine circovirus type 1 as a vaccine delivery virus vector to express antigenic epitopes of porcine reproductive and respiratory syndrome virus. Virus Res 213:100–108. doi: 10.1016/j.virusres.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Nawagitgul P, Morozov I, Bolin SR, Harms PA, Sorden SD, Paul PS. 2000. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J Gen Virol 81:2281–2287. doi: 10.1099/0022-1317-81-9-2281. [DOI] [PubMed] [Google Scholar]
- 5.Mankertz A, Mankertz J, Wolf K, Buhk HJ. 1998. Identification of a protein essential for replication of porcine circovirus. J Gen Virol 79(Pt 2):381–384. [DOI] [PubMed] [Google Scholar]
- 6.Mankertz A, Mueller B, Steinfeldt T, Schmitt C, Finsterbusch T. 2003. New reporter gene-based replication assay reveals exchangeability of replication factors of porcine circovirus types 1 and 2. J Virol 77:9885–9893. doi: 10.1128/JVI.77.18.9885-9893.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burbelo PD, Ragheb JA, Kapoor A, Zhang Y. 2013. The serological evidence in humans supports a negligible risk of zoonotic infection from porcine circovirus type 2. Biologicals 41:430–434. doi: 10.1016/j.biologicals.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mankertz A, Hillenbrand B. 2001. Replication of porcine circovirus type 1 requires two proteins encoded by the viral rep gene. Virology 279:429–438. doi: 10.1006/viro.2000.0730. [DOI] [PubMed] [Google Scholar]
- 9.Niederwerder MC, Bawa B, Serao NV, Trible BR, Kerrigan MA, Lunney JK, Dekkers JC, Rowland RR. 2015. Vaccination with a porcine reproductive and respiratory syndrome (PRRS) modified live virus vaccine followed by challenge with PRRS virus and porcine circovirus type 2 (PCV2) protects against PRRS but enhances PCV2 replication and pathogenesis compared to results for nonvaccinated cochallenged controls. Clin Vaccine Immunol 22:1244–1254. doi: 10.1128/CVI.00434-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timmusk S, Fossum C, Berg M. 2006. Porcine circovirus type 2 replicase binds the capsid protein and an intermediate filament-like protein. J Gen Virol 87:3215–3223. doi: 10.1099/vir.0.81785-0. [DOI] [PubMed] [Google Scholar]
- 11.Trible BR, Rowland RR. 2012. Genetic variation of porcine circovirus type 2 (PCV2) and its relevance to vaccination, pathogenesis and diagnosis. Virus Res 164:68–77. doi: 10.1016/j.virusres.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Van Renne N, Liu C, Nauwynck HJ. 2015. A sequence of basic residues in the porcine circovirus type 2 capsid protein is crucial for its co-expression and co-localization with the replication protein. J Gen Virol 96:3566–3576. doi: 10.1099/jgv.0.000302. [DOI] [PubMed] [Google Scholar]
- 13.Fenaux M, Opriessnig T, Halbur PG, Elvinger F, Meng XJ. 2004. Two amino acid mutations in the capsid protein of type 2 porcine circovirus (PCV2) enhanced PCV2 replication in vitro and attenuated the virus in vivo. J Virol 78:13440–13446. doi: 10.1128/JVI.78.24.13440-13446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiederkehr DD, Sydler T, Buergi E, Haessig M, Zimmermann D, Pospischil A, Brugnera E, Sidler X. 2009. A new emerging genotype subgroup within PCV-2b dominates the PMWS epizooty in Switzerland. Vet Microbiol 136:27–35. doi: 10.1016/j.vetmic.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Finsterbusch T, Steinfeldt T, Doberstein K, Rodner C, Mankertz A. 2009. Interaction of the replication proteins and the capsid protein of porcine circovirus type 1 and 2 with host proteins. Virology 386:122–131. doi: 10.1016/j.virol.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Gray TA, Hernandez L, Carey AH, Schaldach MA, Smithwick MJ, Rus K, Marshall Graves JA, Stewart CL, Nicholls RD. 2000. The ancient source of a distinct gene family encoding proteins featuring RING and C(3)H zinc-finger motifs with abundant expression in developing brain and nervous system. Genomics 66:76–86. doi: 10.1006/geno.2000.6199. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Park SM, Kang MR, Oh SY, Lee TH, Muller MT, Chung IK. 2005. Ubiquitin ligase MKRN1 modulates telomere length homeostasis through a proteolysis of hTERT. Genes Dev 19:776–781. doi: 10.1101/gad.1289405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee EW, Lee MS, Camus S, Ghim J, Yang MR, Oh W, Ha NC, Lane DP, Song J. 2009. Differential regulation of p53 and p21 by MKRN1 E3 ligase controls cell cycle arrest and apoptosis. EMBO J 28:2100–2113. doi: 10.1038/emboj.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee EW, Kim JH, Ahn YH, Seo J, Ko A, Jeong M, Kim SJ, Ro JY, Park KM, Lee HW, Park EJ, Chun KH, Song J. 2012. Ubiquitination and degradation of the FADD adaptor protein regulate death receptor-mediated apoptosis and necroptosis. Nat Commun 3:978. doi: 10.1038/ncomms1981. [DOI] [PubMed] [Google Scholar]
- 20.Ko A, Lee EW, Yeh JY, Yang MR, Oh W, Moon JS, Song J. 2010. MKRN1 induces degradation of West Nile virus capsid protein by functioning as an E3 ligase. J Virol 84:426–436. doi: 10.1128/JVI.00725-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MS, Jeong MH, Lee HW, Han HJ, Ko A, Hewitt SM, Kim JH, Chun KH, Chung JY, Lee C, Cho H, Song J. 2015. PI3K/AKT activation induces PTEN ubiquitination and destabilization accelerating tumourigenesis. Nat Commun 6:7769. doi: 10.1038/ncomms8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craiu A, Gaczynska M, Akopian T, Gramm CF, Fenteany G, Goldberg AL, Rock KL. 1997. Lactacystin and clasto-lactacystin beta-lactone modify multiple proteasome beta-subunits and inhibit intracellular protein degradation and major histocompatibility complex class I antigen presentation. J Biol Chem 272:13437–13445. doi: 10.1074/jbc.272.20.13437. [DOI] [PubMed] [Google Scholar]
- 23.Pickart CM. 2001. Mechanisms underlying ubiquitination. Annu Rev Biochem 70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 24.Al-Gindan Y, Omer AH, A-Humaidan Y, Peters W, Evans DA. 1983. A case of mucocutaneous leishmaniasis in Saudi Arabia caused by Leishmania major and its response to treatment. Clin Exp Dermatol 8:185–188. doi: 10.1111/j.1365-2230.1983.tb01763.x. [DOI] [PubMed] [Google Scholar]
- 25.Lunde BM, Moore C, Varani G. 2007. RNA-binding proteins: modular design for efficient function. Nat Rev Mol Cell Biol 8:479–490. doi: 10.1038/nrm2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang J, Song W, Tromp G, Kolattukudy PE, Fu M. 2008. Genome-wide survey and expression profiling of CCCH-zinc finger family reveals a functional module in macrophage activation. PLoS One 3:e2880. doi: 10.1371/journal.pone.0002880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter T. 2007. The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol Cell 28:730–738. doi: 10.1016/j.molcel.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Ko A, Shin JY, Seo J, Lee KD, Lee EW, Lee MS, Lee HW, Choi IJ, Jeong JS, Chun KH, Song J. 2012. Acceleration of gastric tumorigenesis through MKRN1-mediated posttranslational regulation of p14ARF. J Natl Cancer Inst 104:1660–1672. doi: 10.1093/jnci/djs424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altfeld M, Gale M Jr. 2015. Innate immunity against HIV-1 infection. Nat Immunol 16:554–562. doi: 10.1038/ni.3157. [DOI] [PubMed] [Google Scholar]
- 30.Reddy SS, Foreman HC, Sioux TO, Park GH, Poli V, Reich NC, Krug LT. 2016. Ablation of STAT3 in the B cell compartment restricts gammaherpesvirus latency in vivo. mBio 7:e0723-16. doi: 10.1128/mBio.00723-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Pan Q, Ding S, Wang Z, Yu J, Finzi A, Liu SL, Liang C. 2017. The V3 loop of HIV-1 Env determines viral susceptibility to IFITM3 impairment of viral infectivity. J Virol 91:e02441-16. doi: 10.1128/JVI.02441-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azab W, Osterrieder K. 2017. Initial contact: the first steps in herpesvirus entry. Adv Anat Embryol Cell Biol 223:1–27. doi: 10.1007/978-3-319-53168-7_1. [DOI] [PubMed] [Google Scholar]
- 33.Castro A, Bernis C, Vigneron S, Labbe JC, Lorca T. 2005. The anaphase-promoting complex: a key factor in the regulation of cell cycle. Oncogene 24:314–325. doi: 10.1038/sj.onc.1207973. [DOI] [PubMed] [Google Scholar]
- 34.Rogov V, Dotsch V, Johansen T, Kirkin V. 2014. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol Cell 53:167–178. doi: 10.1016/j.molcel.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Xu Q, Zhu N, Chen S, Zhao P, Ren H, Zhu S, Tang H, Zhu Y, Qi Z. 2017. E3 ubiquitin ligase Nedd4 promotes Japanese encephalitis virus replication by suppressing autophagy in human neuroblastoma cells. Sci Rep 7:45375. doi: 10.1038/srep45375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nityanandam R, Serra-Moreno R. 2014. BCA2/Rabring7 targets HIV-1 Gag for lysosomal degradation in a tetherin-independent manner. PLoS Pathog 10:e1004151. doi: 10.1371/journal.ppat.1004151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inturi R, Mun K, Singethan K, Schreiner S, Punga T. 2018. Human adenovirus infection causes cellular E3 ubiquitin ligase MKRN1 degradation involving the viral core protein pVII. J Virol 92:01154-17. doi: 10.1128/JVI.01154-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Angelis Rigotti F, De Gassart A, Pforr C, Cano F, N′Guessan P, Combes A, Camossetto V, Lehner P, Pierre P, Gatti E. 2017. MARCH9-mediated ubiquitination regulates MHC I export from the TGN. Immunol Cell Biol 95:753–764. doi: 10.1038/icb.2017.44. [DOI] [PubMed] [Google Scholar]
- 39.Bertrand MJ, Lippens S, Staes A, Gilbert B, Roelandt R, De Medts J, Gevaert K, Declercq W, Vandenabeele P. 2011. cIAP1/2 are direct E3 ligases conjugating diverse types of ubiquitin chains to receptor interacting proteins kinases 1 to 4 (RIP1-4). PLoS One 6:e22356. doi: 10.1371/journal.pone.0022356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng X, Wang X, Tu F, Wang Q, Fan Z, Gao G. 2017. TRIM25 is required for the antiviral activity of zinc finger antiviral protein. J Virol 91:e00088-17. doi: 10.1128/JVI.00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JH, Park ME, Nikapitiya C, Kim TH, Uddin MB, Lee HC, Kim E, Ma JY, Jung JU, Kim CJ, Lee JS. 2017. FAS-associated factor-1 positively regulates type I interferon response to RNA virus infection by targeting NLRX1. PLoS Pathog 13:e1006398. doi: 10.1371/journal.ppat.1006398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hershko A, Ciechanover A. 1998. The ubiquitin system. Annu Rev Biochem 67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 43.Deshaies RJ, Joazeiro CA. 2009. RING domain E3 ubiquitin ligases. Annu Rev Biochem 78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 44.Ohi MD, Vander Kooi CW, Rosenberg JA, Chazin WJ, Gould KL. 2003. Structural insights into the U-box, a domain associated with multi-ubiquitination. Nat Struct Biol 10:250–255. doi: 10.1038/nsb906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Acconcia F, Sigismund S, Polo S. 2009. Ubiquitin in trafficking: the network at work. Exp Cell Res 315:1610–1618. doi: 10.1016/j.yexcr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 46.Ozkan E, Yu H, Deisenhofer J. 2005. Mechanistic insight into the allosteric activation of a ubiquitin-conjugating enzyme by RING-type ubiquitin ligases. Proc Natl Acad Sci U S A 102:18890–18895. doi: 10.1073/pnas.0509418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Y, Shao M, Xu X, Zhang X, Du Q, Zhao X, Zhang W, Lyu Y, Tong D. 2013. Evidence for different patterns of natural inter-genotype recombination between two PCV2 parental strains in the field. Virus Res 175:78–86. doi: 10.1016/j.virusres.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Du Q, Huang Y, Wang T, Zhang X, Chen Y, Cui B, Li D, Zhao X, Zhang W, Chang L, Tong D. 2016. Porcine circovirus type 2 activates PI3K/Akt and p38 MAPK pathways to promote interleukin-10 production in macrophages via Cap interaction of gC1qR. Oncotarget 7:17492–17507. doi: 10.18632/oncotarget.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cui B, Liu W, Wang X, Chen Y, Du Q, Zhao X, Zhang H, Liu SL, Tong D, Huang Y. 2017. Brucella Omp25 upregulates miR-155, miR-21-5p, and miR-23b to inhibit interleukin-12 production via modulation of programmed death-1 signaling in human monocyte/macrophages. Front Immunol 8:708. doi: 10.3389/fimmu.2017.00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li D, Xu D, Wang Z, Du Q, Chang L, Zhao X, Huang Y, Tong D. 2017. Immunogenicity evaluation of modified adenovirus vaccines expressing porcine circovirus type 2 capsid protein in pigs. Viral Immunol 30:111–119. doi: 10.1089/vim.2016.0086. [DOI] [PubMed] [Google Scholar]
- 51.Tan R, Nakajima S, Wang Q, Sun H, Xue J, Wu J, Hellwig S, Zeng X, Yates NA, Smithgall TE, Lei M, Jiang Y, Levine AS, Su B, Lan L. 2017. Nek7 protects telomeres from oxidative DNA damage by phosphorylation and stabilization of TRF1. Mol Cell 65:818–831.e5. doi: 10.1016/j.molcel.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorden SD, Harms PA, Nawagitgul P, Cavanaugh D, Paul PS. 1999. Development of a polyclonal-antibody-based immunohistochemical method for the detection of type 2 porcine circovirus in formalin-fixed, paraffin-embedded tissue. J Vet Diagn Invest 11:528–530. doi: 10.1177/104063879901100607. [DOI] [PubMed] [Google Scholar]
- 53.Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng XJ, Halbur PG. 2004. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol 41:624–640. doi: 10.1354/vp.41-6-624. [DOI] [PubMed] [Google Scholar]