ABSTRACT
Antibody Fc-dependent functions are linked to prevention and control of HIV-1 infection. Basic NK cell biology is likely key to understanding the contributions that anti-HIV-1 antibody-dependent NK cell activation and cytolysis make to HIV-1 susceptibility and disease progression. The importance of NK cell education through inhibitory receptors specific for self-HLA-I in determining the potency of anti-HIV-1 antibody-mediated NK cell activation and cytolysis is controversial. To address this issue more definitively, we utilized HLA-I genotyping, flow cytometry staining panels, and cytolysis assays to assess the functionality of educated and noneducated peripheral blood NK cells. We now demonstrate that educated NK cells are superior in terms of their capacity to become activated and/or mediate cytolysis following anti-HIV-1 antibody-dependent stimulation. The profiles of activation observed were similar to those observed upon direct stimulation of NK cells with target cells devoid of HLA-I. Noneducated NK cells make significantly lower contributions to total NK cell activation than would be expected from their frequency within the total NK cell population (i.e., they are hypofunctional), and educated NK cells make contributions similar to or higher than their frequency in the total NK cell population. Finally, NK cells educated through at least one killer immunoglobulin-like receptor and NKG2A exhibited the most significant difference between actual and expected contributions to the total NK cell response, based on their frequency within the total NK cell population, suggesting that summation of NK cell education through inhibitory receptors determines overall NK cell functionality. These observations have potential implications for understanding HIV-1 vaccine efficacy and disease progression.
IMPORTANCE NK cells are major mediators of anti-HIV-1 antibody-dependent functions, including cytokine production and cytolysis. The mechanisms controlling the capacity of individual NK cells to mediate antibody-dependent functions remain poorly defined. We now show that NK cell education determines the capacity of NK cells to exhibit anti-HIV-1 antibody-dependent activation and mediate antibody-dependent cellular cytotoxicity. These observations suggest that the process of NK cell education could be of importance for understanding HIV-1 pathogenesis and designing immune-based prophylactics or therapeutics.
KEYWORDS: ADCC, human immunodeficiency virus, natural killer cells
INTRODUCTION
Novel vaccines and immunotherapeutics are needed to help prevent new HIV-1 infections and/or achieve an HIV-1 cure. Cellular immune responses capable of eliminating HIV-1-infected target cells are likely to play a role in strategies to prevent or cure HIV-1 infection. One potentially important response, antibody-dependent cellular cytotoxicity (ADCC), is mediated by several effector cells, including natural killer (NK) cells, monocytes, and neutrophils, upon recognition of antigen-bound IgG on infected target cells via Fc receptors (1). The potential importance of ADCC in the context of HIV-1 exposure and infection is highlighted through a series of studies linking the response to protective outcomes against HIV-1 and surrogate viruses in animal models. Indeed, antibodies capable of triggering ADCC were associated with the partial protection conferred by the RV144 vaccine trial in Thailand (2–4). Similarly, ADCC responses have been linked to slower HIV-1 disease progression in elite controllers, protection of infants from exposure to HIV-1 within breast milk, and live-attenuated vaccine-conferred protection of nonhuman primates from challenge with pathogenic virus (5–7). Given the putative importance of ADCC in preventing and controlling HIV-1 infection, much effort has been directed toward characterizing the specificities and constant-region (Fc) features of antibodies capable of triggering ADCC (8–10).
In addition to efforts to understand the nature of the antibody responses involved in anti-HIV-1 ADCC, the characteristics of the effector cells involved in such responses are a topic of much interest (1, 11–18). Among peripheral blood effector cells capable of mediating ADCC, NK cells appear to be responsible for much of the cytolytic activity (1). In addition to cytolysis, NK cells activated by antibody-coated target cells produce cytokines, such as gamma interferon (IFN-γ), and beta chemokines, such as CCL4 (14, 19). The capacity of NK cells to mediate functions following stimulation is regulated by the process of NK cell education (11, 12, 16, 17, 20–24).
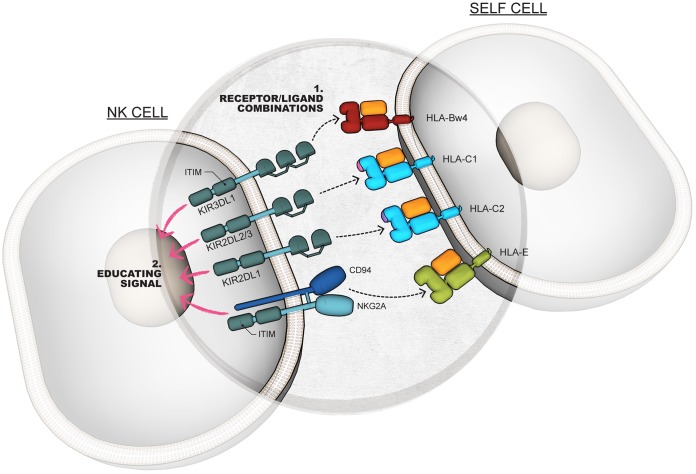
During NK cell education, cells expressing inhibitory receptors capable of binding to self major histocompatibility complex class I (MHC-I or HLA-I) ligands are “educated” to give them the capacity to mediate functions upon later stimulation. Cells not carrying inhibitory receptors for self-HLA-I remain “noneducated” and are hypofunctional upon later stimulation. The education of human NK cells has been shown to occur through several inhibitory receptor/ligand combinations, including KIR3DL1/HLA-Bw4, KIR2DL1/HLA-C2, KIR2DL2/3/HLA-C1, and NKG2A/HLA-E (Fig. 1) (11, 20–24). With regard to anti-HIV-1 antibody-dependent activation of NK cells, evidence has been demonstrated that educated NK cells are more likely to exhibit activation (i.e., cytokine and degranulation marker expression) than noneducated NK cells upon stimulation with HIV-1 envelope-coated target cells (11, 12, 16, 17). The role of educated and noneducated NK cells in anti-HIV-1 antibody-dependent killing (i.e., ADCC) of envelope-coated target cells, however, remains controversial. In an assessment of anti-HIV-1 ADCC using granzyme B delivery as a marker, Isitman et al. observed no differences between ADCC responses by NK cells from donors carrying education-competent KIR3DL1/HLA-Bw4 receptor/ligand combinations and those from donors lacking KIR3DL1/HLA-Bw4 combinations (14). This observation raises the prospect that NK cell education is not important in determining the capacity of NK cells to mediate anti-HIV-1 ADCC. Alternatively, it is possible that education-competent receptor/ligand combinations other than KIR3DL1/HLA-Bw4 either are more important in determining ADCC potential or can compensate for missing KIR3DL1/HLA-Bw4 combinations.
FIG 1.
NK cell education. The education of NK cells occurs via the interaction of inhibitory NK cell receptors with self-HLA-I molecules expressed on autologous cells. This process involves ligation of inhibitory NK cell receptors by self-HLA-I ligands (1) and signaling through the inhibitory NK cell receptors (2).
Given the outstanding questions regarding the contribution of the process of NK cell education to the potential of NK cells to mediate ADCC, we initiated a rigorous investigation to determine the role of this process in regulating the functional potential of NK cells for anti-HIV-1 antibody-dependent functions. As a comparison, we simultaneously evaluated the role of NK cell education in direct NK cell activation against target cells devoid of HLA-I. We now present novel data showing that education has a marked influence on the capacity of NK cells to exhibit both anti-HIV-1 antibody-dependent activation and ADCC. Our data demonstrate a larger contribution for educated than noneducated NK cells in anti-HIV-1 antibody-dependent NK cell functions. Furthermore, NK cells expressing NKG2A in combination with at least one KIR that recognizes self-HLA-I appear to exceed expectations, based on the frequency with which they occur in the total NK cell population, in their ability to mediate direct and anti-HIV-1 antibody-dependent functions. These data are discussed in the context of their implications for the prevention and pathogenesis of HIV-1 infection.
RESULTS
Identifying educated and noneducated NK cells.
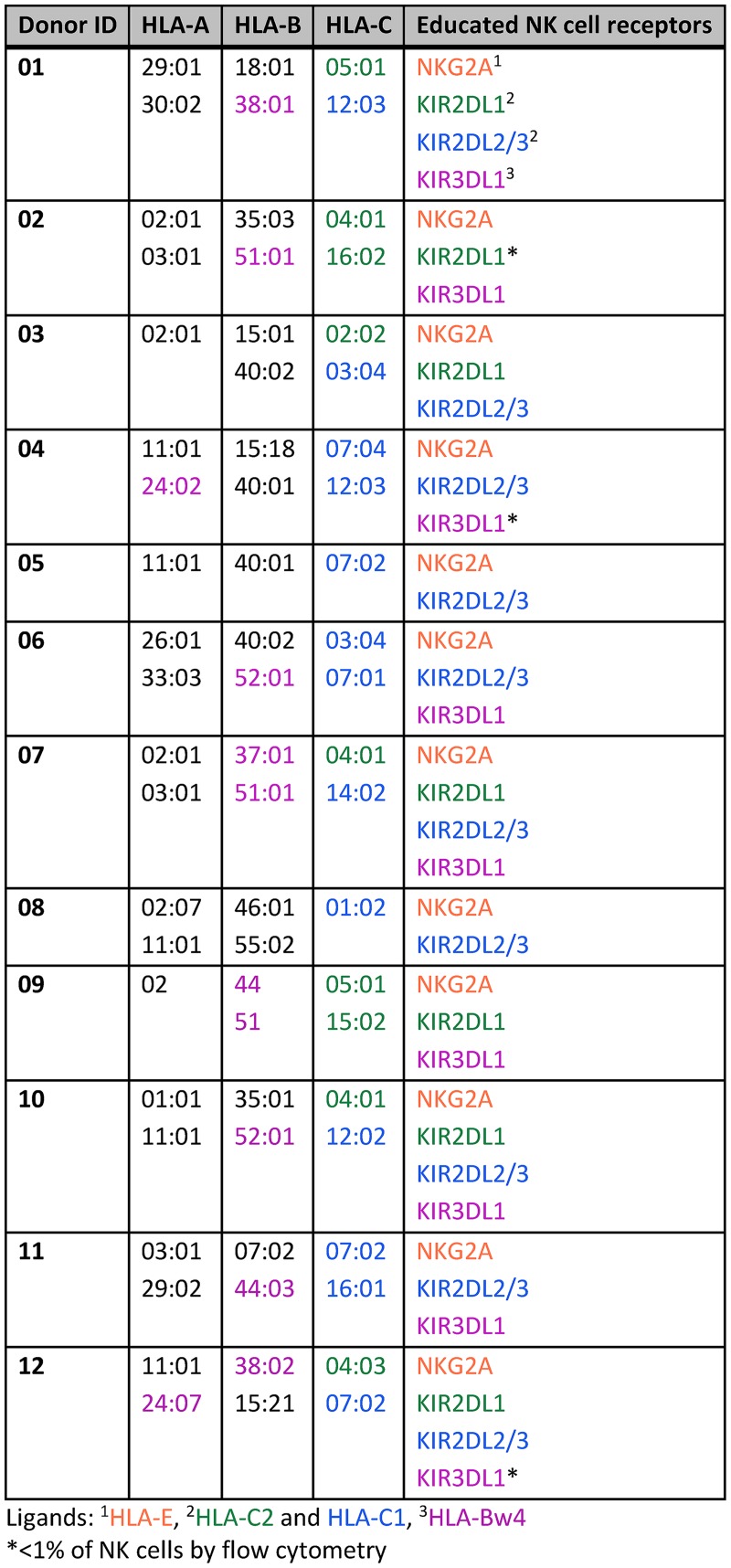
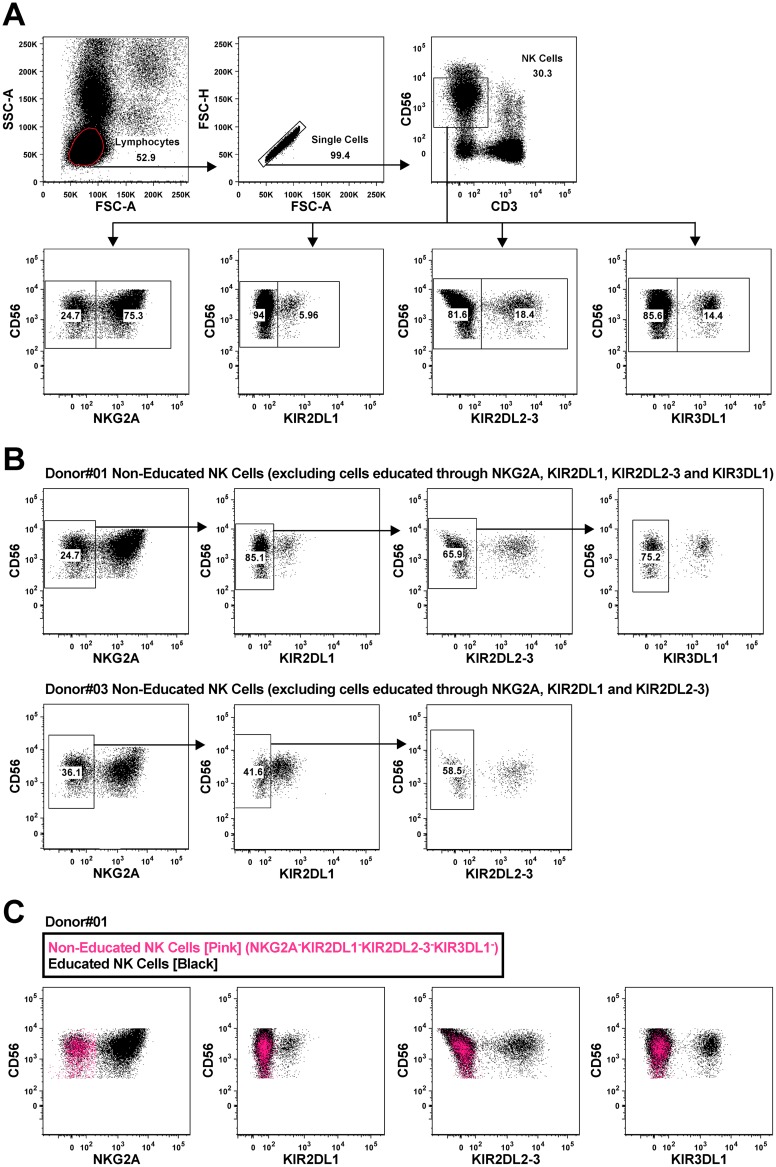
Educated NK cells are broadly defined through their expression of inhibitory receptors capable of binding self-HLA-I. This includes cells expressing NKG2A, KIR3DL1, KIR2DL1, and/or KIR2DL2-3 in individuals carrying the HLA-E, HLA-Bw4, HLA-C2, and/or HLA-C1 ligands, respectively (Fig. 1). Indeed, all of these receptor/ligand combinations have been demonstrated to educate NK cells in previous studies (11, 12, 16, 17, 20–24). Noneducated NK cells are characterized by a lack of expression of inhibitory NK cell receptors or by the expression of inhibitory receptors that are incapable of interacting with self-HLA-I. To define educated and noneducated NK cells within our cohort, we typed each donor for HLA-A, B and C (Table 1). These HLA-I types were used to characterize each donor as carrying HLA-Bw4, HLA-C1, and/or HLA-C2 ligands. All donors were considered to carry HLA-E. Next, peripheral blood mononuclear cells (PBMC) from donors were stained with fluorescence-conjugated antibodies to detect expression of NKG2A, KIR3DL1, KIR2DL1, and KIR2DL2-3 within the NK cell population (Fig. 2A). Table 1 lists the inhibitory NK cell receptors through which each donor's NK cells should be educated, as well as denotation of any educating receptors that were poorly detected by flow cytometry. These staining patterns were used to identify noneducated NK cells as those lacking expression of any of the receptors studied that could educate NK cells given the donor's HLA-I type. This gating is highlighted for two representative donors in Fig. 2B. Boolean gating was used to identify educated NK cells as the product of subtracting the noneducated NK cells from the total NK cell gate (Fig. 2C). This rigorous characterization of educated and noneducated NK cells within the total NK cell population allowed for experiments to (i) compare functionalities of bulk NK cell populations simultaneously after antibody-mediated and direct activation and (ii) sort by fluorescence-activated cell sorting (FACS) populations enriched for educated and noneducated NK cells to assess their role in antibody-mediated killing.
TABLE 1.
Donor characteristics
FIG 2.
Flow cytometry gating strategy to analyze educated and noneducated NK cells. (A) Top, gating on lymphocytes, single cells, and CD3− CD56dim NK cells. Bottom, gating on NKG2A+/−, KIR2DL1+/−, KIR2DL2-3+/−, and KIR3DL1+/− cells within the NK cell population. (B) Gating on noneducated NK cells for two representative donors (donor 01, NKG2A− KIR2DL1− KIR2DL2-3− KIR3DL1− NK cells; donor 03, NKG2A− KIR2DL1− KIR2DL2-3− NK cells). (C) Boolean gating was used to identify educated NK cells by subtracting noneducated NK cells from total NK cells. The FACS plots depict NKG2A, KIR2DL1, KIR2DL2-3, and KIR3DL1 expression in noneducated (pink) and educated (black) NK cells for a donor carrying the HLA-E, HLA-Bw4, HLA-C2, and HLA-C1 ligands.
Frequency of activated educated and noneducated NK cells following direct and antibody-dependent activation.
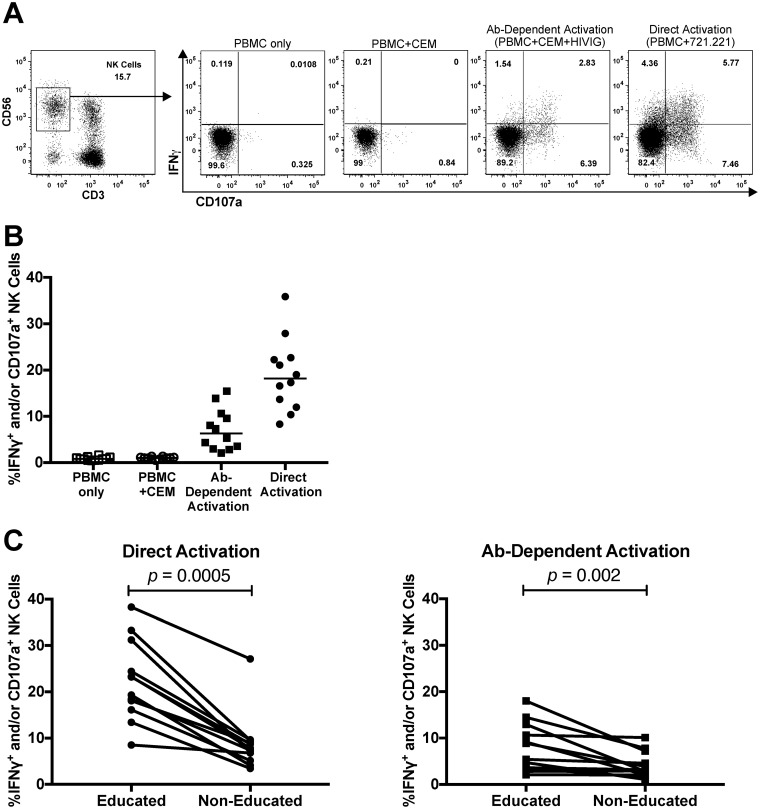
Having demonstrated a method to identify educated and noneducated NK cells, we proceeded to assess the relative activation of educated and noneducated NK cells upon direct or antibody-dependent stimulation. While the anti-HIV-1 antibody-dependent functions of NK cells educated through individual inhibitory receptors have been previously assessed (11, 12, 14, 16, 17), to our knowledge this is the first attempt to evaluate the activation of the whole educated NK cell population relative to that of the noneducated NK cell population. Incubating bulk NK cells within PBMC with the HLA-I-devoid 721.221 cell line assessed direct NK cell activation, which was measured as NK cell CD107a expression and/or IFN-γ production via flow cytometry. We assessed antibody-dependent activation by incubating PBMC with HIV-1BaL gp120-coated CEM.NKr-CCR5 cells in the presence of anti-HIV-1 immunoglobulin (HIVIG) and measuring NK cell CD107a expression and/or IFN-γ production via flow cytometry (12, 25). Gating used to assess NK cell activation is shown in Fig. 3A. Both direct and antibody-dependent stimulation of NK cells triggered robust activation within total NK cells (Fig. 3B). Assessment of the frequency of activated NK cells within the educated and noneducated populations revealed higher percentages of activated cells in the educated population after both direct (21.3% [8.5% to 38.3%] [median and range] versus 7.6% [3.5% to 27.1%], P = 0.0005) and antibody-dependent (7.2% [2.1% to 18.0%] versus 3.1% [1.1% to 10.1%], P = 0.002) stimulations (Fig. 3C), consistent with previous work evaluating the capacity of individual inhibitory NK cell receptors to educate anti-HIV-1 antibody-dependent NK cell activation (11, 12, 16, 17).
FIG 3.
Assessment of direct and antibody-dependent NK cell activation using flow cytometry. NK cell activation is measured as the percentage of CD3− CD56dim NK cells that express the degranulation marker CD107a and/or IFN-γ. (A) Gating on IFN-γ+ and/or CD107a+ NK cells in a representative donor. (B) The graph depicts NK cell activation in unstimulated NK cells (“PBMC only” and “PBMC+CEM”) and stimulated NK cells (“Ab-Dependent Activation” [PBMC + CEM + HIVIG] and “Direct Activation” [PBMC + 721.221]). Lines indicate medians. (C) The graphs show the percentages of activated NK cells within the educated and noneducated populations following direct and antibody-dependent stimulation. Educated and noneducated data were compared using the Wilcoxon matched-pairs test. A P value of <0.05 was considered significant.
While these data provide evidence that educated NK cells exhibit more activation following both direct and antibody-dependent stimulation, they do not address whether the enhanced activation of educated NK cells reflects an increased capacity to degranulate (as measured by CD107a expression), produce cytokine, or both. Indeed, the measure of total NK cell activation introduced the possibility that enhanced activation in educated NK cells could be driven by an increased capacity to mediate only one of these functions. Therefore, we compared educated and noneducated NK cells for their total degranulation and total IFN-γ production following direct and antibody-dependent stimulation. As shown in Fig. 4, educated NK cells exhibited higher levels of total CD107a expression and total IFN-γ production following both direct (16.9% [3.1% to 33.5%] versus 6.4% [2.1% to 25.3%] [P = 0.001] and 10.6% [5.7% to 22.9%] versus 1.6% [0.5% to 4.8%] [P = 0.0005], respectively) and antibody-dependent (5.1% [1.7% to 12.3%] versus 2.8% [1.1% to 9.0%] [P = 0.04] and 2.2% [0.5% to 13.1%] versus 0.4% [0.0% to 4.5%] [P = 0.0005], respectively) stimulations. Collectively, these data provide evidence supportive of the notion that educated NK cells have a higher potential to exhibit both direct and antibody-dependent activation-induced profiles.
FIG 4.
Total degranulation (right) and total IFN-γ production (left) in educated and noneducated NK cells after direct (top) and antibody-dependent (bottom) stimulation. Educated and noneducated data were compared using the Wilcoxon matched-pairs test. A P value of <0.05 was considered significant.
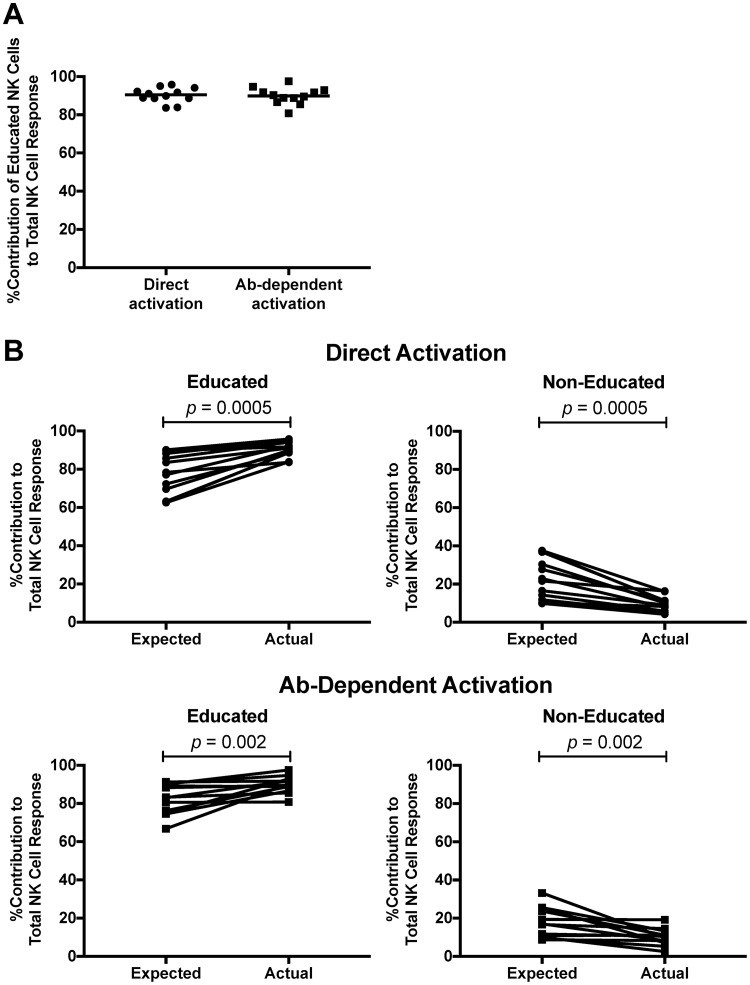
Percent contributions of educated and noneducated NK cells to total NK cell activation.
Although educated NK cells represented a more functional population of NK cells than noneducated NK cells, these data do not portray the contribution that educated NK cells make to the total NK cell response. Therefore, we calculated the percent contributions that educated and noneducated NK cells made to the total NK cell response by dividing the number of activated NK cells within the educated or noneducated population by the number of total activated NK cells. We found educated NK cells to account for a majority of responding cells after both direct (90.5% [83.6% to 95.8%]) and antibody-dependent (89.9% [80.8% to 97.5%]) stimulation (Fig. 5A). The observation that educated NK cells exhibit higher levels of activation after direct and antibody-dependent stimulation supports the hypothesis that NK cell education confers functional potential (26). Further corroborating these data, we determined that the percent contribution of educated NK cells to total NK cell activation was higher than their frequency within the total NK cell population. The NK cell education hypothesis predicts that noneducated NK cells will exhibit hypofunctional responses following stimulation, which might be represented by percent contributions to the total NK cell response that are lower than the frequency with which these cells occur in the total NK cell population. Alternatively, as educated NK cells have acquired functional potential through the education process, the NK cell education hypothesis would predict these cells to make percent contributions to the total NK cell response that are equal to or greater than the frequency with which these cells occur in the total NK cell population. Indeed, we observed the actual percent contribution of educated NK cells to activation following direct (P = 0.0005) and antibody-dependent (P = 0.002) stimulation to be significantly higher than the expected percent contribution (Fig. 5B). Also consistent with the NK cell education hypothesis, we noted that the actual percent contribution of noneducated NK cells to the total NK cell response was significantly lower than their expected percent contribution following direct and antibody-dependent stimulation (P = 0.0005 and P = 0.002, respectively). Again, these data support the notion that the process of NK cell education confers on NK cells functional potential that determines responses to both direct and antibody-dependent stimulation, as well as supporting the concept that noneducated NK cells are hypofunctional following stimulation.
FIG 5.
Contributions of educated and noneducated NK cells to total NK cell response. (A) The graph depicts the percent contribution of educated NK cells to total NK cell activation after direct and antibody-dependent stimulation. Lines indicate medians. (B) The expected contributions of educated and noneducated NK cells to total NK cell activation were calculated as the frequency of these subpopulations within the total NK cell population. The actual contribution was calculated as the frequency of activated educated/noneducated NK cells within the total activated NK cell population. The graphs show the expected and actual percent contributions of educated (left) and noneducated (right) NK cells to the total NK cell response following direct (top) and antibody-dependent (bottom) stimulation. Data were compared using the Wilcoxon matched-pairs test. A P value of <0.05 was considered significant.
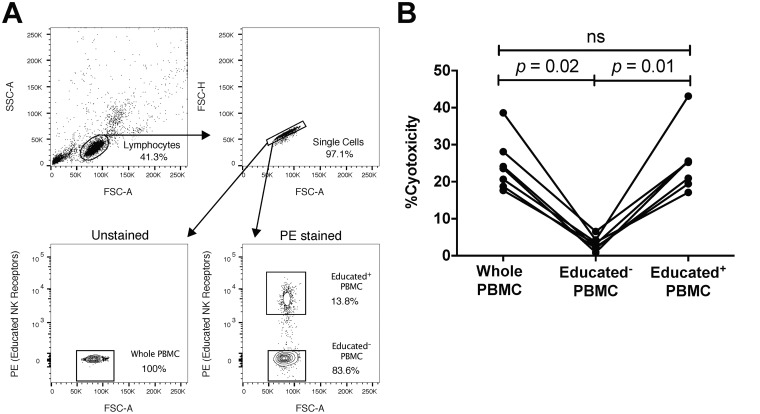
Contribution of educated NK cells to the ADCC mediated by NK cells within PBMC.
Given that educated NK cells exhibited higher percentages of activated cells and accounted for the majority of the total number of NK cells activated, we next assessed if this functional advantage extended to antibody-dependent killing (i.e., ADCC). As educated cells from the utilized donors exhibited a clear functional advantage in terms of degranulation following stimulation with antibody-coated CEM.NKr-CCR5 target cells, we hypothesized that educated cells would be major contributors to anti-HIV-1 ADCC. Previous research, however, has called the role of educated NK cells in antibody-dependent cytolysis into question, as the amount of granzyme B delivered by NK cells to anti-HIV-1 antibody-coated target cells was not predicted by the presence or absence of the KIR3DL1/HLA-Bw4 education-competent receptor/ligand combination (14). To more formally address whether this previous observation reflects a lack of a role for educated NK cells in driving the anti-HIV-1 ADCC response or simply a compensation for a missing receptor/ligand combination by other education-competent receptor/ligand combinations, we used phycoerythrin (PE)-conjugated antibodies to individually relevant combinations of NKG2A, KIR3DL1, KIR2DL1, and KIR2DL2/3 to identify educated NK cells within PBMC. Using the resultant staining, we sorted lymphocytes into a population containing educated NK cells (i.e., Educated+) and a population lacking educated NK cells (i.e., Educated−) (Fig. 6A). The PE-conjugated antibodies employed were individually based on the educating inhibitory receptors expressed on each utilized donor's NK cells, as listed in Table 1. Next, the whole lymphoid PBMC (i.e., PBMC), Educated+, and Educated− populations were assessed for their capacity to kill gp120-coated CEM.NKr-CCR5 target cells in the presence of anti-HIV-1 antibody in a lactate dehydrogenase (LDH) release assay. This was accomplished by implementing a 20:1 effector-to-target cell ratio of PBMC to gp120-coated CEM.NKr-CCR5 target cells, or an amount of Educated+ and Educated− effector cells that would be present in the 4 × 105 total PBMC used to achieve a 20:1 effector-to-target cell ratio. As shown in Fig. 6B, elimination of the educated NK cells in the Educated− population almost completely abrogated the capacity of cells to mediate antibody-dependent cytolysis (3.2% [0.9% to 6.6%]). Alternatively, the whole PBMC and Educated+ populations mediated similar robust levels of anti-HIV-1 antibody-dependent cytolysis (23.6% [17.7% to 38.6%] and 25.2% [17.1% to 43.1%], respectively), which were both significantly higher than that mediated by the Educated− population (P = 0.02 and P = 0.01, respectively). It should be noted that this assay measures total cytolysis (i.e., antibody dependent and direct) of gp120-coated CEM.NKr-CCR5 target cells. To exclude the possibility that the observed differences between cytotoxicity mediated by different effector cell populations were due to differences in direct cytolysis, we subtracted background direct cytolysis and compared the remaining antibody-dependent cytolysis. Background direct cytolysis of gp120-coated CEM.NKr-CCR5 target cells was negligible, and cytotoxicity attributable to anti-HIV-1 antibody was significantly lower in Educated− PBMC (2.6% [0.9% to 6.5%]) than in whole PBMC (21.7% [17.1% to 35.4%], P = 0.02) or in Educated+ PBMC (23.6% [16.6% to 38.5%], P = 0.01).
FIG 6.
Contribution of educated NK cells to ADCC against gp120-coated CEM.NKr-CCR5 cells. PBMC were stained with a donor-dependent combination of PE-conjugated antibodies to enable the separation of PBMC into a population containing educated NK cells (Educated+) and a population lacking educated NK cells (Educated−) using FACS. (A) Gating strategy used to define and collect whole PBMC (unstained PBMC), Educated+ PBMC (PE-positive gated lymphocytes), and Educated− PBMC (PE-negative gated lymphocytes). (B) The levels of anti-HIV-1 ADCC mediated by whole, Educated+, and Educated− PBMC against gp120-coated CEM.NKr-CCR5 target cells were assessed using the LDH cytotoxicity assay. Data were compared using the Friedman test followed by Dunn's post hoc test. A P value of <0.05 was considered significant.
Education through NKG2A and/or KIR and the activation potential of NK cells.
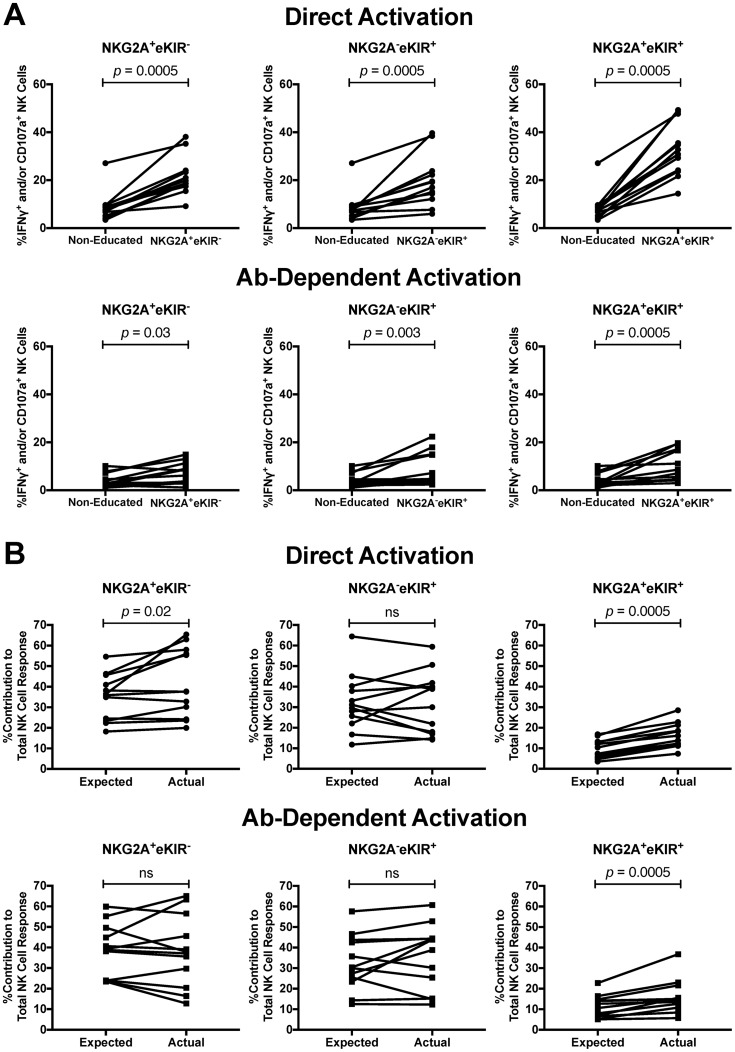
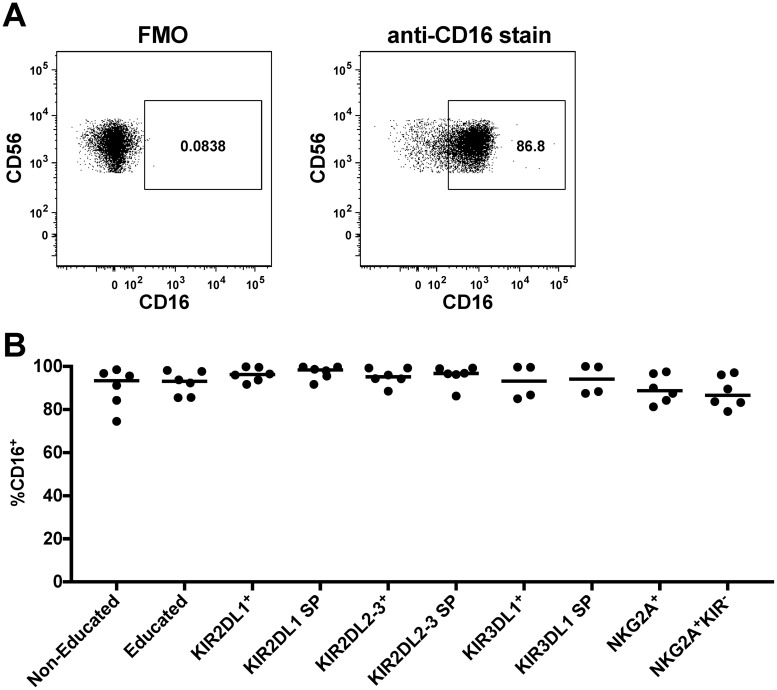
It is now clear that NK cells can become educated through either interactions between NKG2A and HLA-E or interactions between inhibitory KIR and HLA-I ligands (11, 12, 16, 17, 20–24). Our HLA-I-typed donor cells stained for NKG2A, KIR3DL1, KIR2DL1, and KIR2DL2/3 allowed us to address the relative contributions of NK cells educated through these two pathways to anti-HIV-1 antibody-dependent and direct activation. Initially we compared the frequency of bulk NK cells within PBMC activated following anti-HIV-1 antibody-dependent or direct stimulation in NK cell populations containg cells educated through NKG2A only (i.e., NKG2A+ educating KIR−), educated through KIR only (i.e., NKG2A− educating KIR+), or educated through both NKG2A and KIR (i.e., NKG2A+ educating KIR+) to noneducated NK cells. For these analyses we focused on total activated NK cells (i.e., CD107a+ and/or IFN-γ+), as the analyses in Fig. 4 demonstrated that both CD107a expression and IFN-γ production contribute to the functional advantages of educated NK cells. As depicted in Fig. 7A, NK cells educated through NKG2A only (19.3% [9.2% to 38.1%]), KIR only (18.1% [6.0% to 39.7%]), or both NKG2A and KIR (31.8% [14.4% to 49.3%]) were significantly more activated than noneducated NK cells (7.6% [3.5% to 27.1%]) (P = 0.0005 for all three comparisons) upon direct stimulation with 721.221 target cells. Similarly, following anti-HIV-1 antibody-dependent stimulation, a significantly higher level of activation than in noneducated NK cells (3.1% [1.1% to 10.1%]) was observed in NK cells educated through NKG2A (7.1% [1.1% to 14.9%], P = 0.03), KIR (4.6% [2.4% to 22.4%], P = 0.003), and both NKG2A and KIR (7.8% [3.0% to 19.6%], P = 0.0005). Following either direct stimulation with 721.221 or anti-HIV-1 antibody-dependent activation, we noted that NK cells educated through all three permutations of KIR and NKG2A were significantly more functional than noneducated NK cells. That the KIR+ NKG2A− subset was significantly more functional than noneducated NK cells for antibody-dependent functions might be surprising in that this subset could contain NK cells expressing a single KIR, which have previously been suggested to express low levels of CD16 (15). Therefore, we next assessed the CD16 expression levels on NK cells expressing different permutations of KIR and NKG2A in a subset of our donors to see if we could recapture these previously reported differences in CD16 expression. We did not, however, observe low levels of CD16 expression on NK cells expressing any of the assessed KIRs or on NK cells expressing single KIR (Fig. 8).
FIG 7.
Activation potential of NK cells educated through NKG2A and/or KIR. (A) The graphs depict the percentages of activated NK cells within the noneducated population and populations containing cells educated through NKG2A only (i.e., NKG2A+ educating KIR− [NKG2A+ eKIR−]) (left), KIR only (NKG2A− eKIR+) (center), and both NKG2A and KIR (NKG2A+ eKIR+) (right) following direct (top) and antibody-dependent (bottom) stimulation. (B) The expected contribution of NKG2A+/− eKIR+/− NK cells to total NK cell activation was calculated as the frequency of these subpopulations within the total NK cell population. The graphs show expected and actual percent contributions of NKG2A+ eKIR− (left), NKG2A− eKIR+ (center), and NKG2A+ eKIR+ (right) educated NK cells to total NK cell activation after direct (top) and antibody-dependent (bottom) stimulation. Data were compared using the Wilcoxon matched-pairs test. A P value of <0.05 was considered significant.
FIG 8.
CD16 expression in different NK cell populations. (A) Gating on CD16+ NK cells (CD3− CD56dim lymphocytes) in samples with and without anti-CD16 stain. (B) The graph depicts the frequencies of CD16+ cells within the noneducated NK cell population, the educated NK cell population, and NK cell populations expressing different permutations of KIR and NKG2A. A superscript plus represents NK cells that are positive for a specific receptor regardless of the expression of other receptors (e.g., KIR2DL1+). SP represents NK cells that are single positive for a specific receptor and are negative for the three other receptors regardless of whether they are educated through the receptor or not (e.g., KIR2DL1 SP). Lines indicate medians.
While data regarding the frequency of activated NK cells within each subset provide information about the feasibility of activating NK cells of different phenotypes, these data do not provide insight into the contribution that each of these subsets makes to the total level of NK cell activation or into whether these NK cell subsets are hyper- or hypofunctional in their actual, compared to expected, contributions to NK cell function. Therefore, we next calculated the percent contribution of each of these three NK cell populations to the total NK cell response. We noted that while noneducated NK cells made significantly lower (i.e., hypofunctional) actual responses, compared to expected responses (Fig. 5B), following both direct and anti-HIV-1 antibody-dependent stimulation (9.6% [4.2% to 16.4%] versus 22.2% [9.9% to 37.5%] [P = 0.0005] and 10.1% [2.5% to 19.2%] versus 16.9% [8.7% to 33.2%] [P = 0.002], respectively), all three subsets of educated NK cells either did not significantly differ in their actual and expected contributions or exhibited actual contributions that were significantly higher than their expected contributions following direct (NKG2A+ KIR−, P = 0.02; NKG2A+ KIR+, P = 0.0005) and antibody-dependent (NKG2A+ KIR+, P = 0.0005) stimulation (Fig. 7B). The educated NK cell subset with the most significant difference between expected and actual percent contributions for both direct and antibody-dependent activation was the subset containing cells educated through both KIR and NKG2A. These observations suggest that NK cell education through two or more inhibitory NK cell receptors (i.e., NKG2A+ KIR+) can increase the chances of an NK cell contributing to the total NK cell response.
DISCUSSION
Highly functional NK cells will be critical in maximizing the effect of Fc-functional antibodies against HIV-1. However, recent research has raised questions about the role of NK cell education in anti-HIV-1 antibody-dependent functions against HIV-1 gp120-coated target cells (14, 15). We now present a more definitive data set demonstrating that educated NK cells exhibit higher levels of antibody-dependent activation than noneducated cells upon stimulation with gp120-coated target cells in the presence of anti-HIV-1 immunoglobulin. We also demonstrate that educated NK cells make a higher contribution to the total NK activation following anti-HIV-1 antibody-dependent stimulation, and this percent contribution is higher than would be expected based on the frequency with which educated NK cells occur in the total NK cell population. Furthermore, using FACS to obtain populations of PBMC enriched or depleted for educated NK cells, we present evidence that educated NK cells mediate most of the anti-HIV-1 ADCC observed against gp120-coated target cells. Collectively, these data demonstrate an important role for educated NK cells in anti-HIV-1 antibody-dependent responses to gp120-coated targets.
While the presented data are in agreement with much of what has been published by others and us regarding antibody-dependent NK cell activation to HIV-1 envelope or other antigens (11, 12, 16, 17, 20, 23), the cytolysis data (Fig. 6) are in apparent disagreement with the observation by Isitman et al. (14) that KIR3DL1/HLA-Bw4 combinations did not determine NK cell ADCC potential. Indeed, on the basis of their observation that donors carrying or lacking the KIR3DL1/HLA-Bw4 receptor/ligand combination exhibited similar levels of granzyme B delivery to gp120-coated target cells in the presence of HIVIG, Isitman et al. claimed that the potency of ADCC was not determined by NK education. We now demonstrate that a series of inhibitory NK cell receptor/ligand combinations can educate NK cells to become activated upon anti-HIV-1 antibody-dependent stimulation. From this observation, we proceeded to use PE-conjugated antibodies to sort PBMC enriched or depleted for educated NK cells. These sorted populations were implemented in LDH release cytolysis assays to demonstrate that educated NK cells trigger the majority of ADCC mediated by effector cells within PBMC. The experiments reported by Isitman et al. were analyzed with comparisons of the total NK cell population within PBMC between donors. As such, there was no way of determining the relative responsiveness of the uneducated NK cells or those educated through other inhibitory NK cell receptor/ligand combinations. As our experimental system directly compares cell fractions containing or lacking educated NK cells, we feel that our results might offer an alternative explanation for the observations of Isitman et al. We hypothesize that instead of NK cell education playing no role in the ADCC reported by Isitman et al., alternative education-competent receptor/ligand combinations could compensate for the lack of education through KIR3DL1 in donors lacking HLA-Bw4. Future experiments implementing the experimental conditions from the current study in the granzyme B release assay utilized by Isitman et al. would further test this hypothesis.
Several limitations of the current study should be noted. First, the current study solely utilized PBMC samples from HIV-1-uninfected donors. These samples provide an idea of how NK cells from HIV-1-uninfected individuals might utilize vaccine-induced antibodies or antibodies available within HIV-1-infected bodily fluids on exposure (7, 25) to respond to infected cells established early after HIV-1 exposure. Samples from HIV-1-uninfected donors, however, cannot replace samples from HIV-1-infected individuals. Indeed, numerous alterations in NK cell differentiation, function, and phenotype accompany HIV-1 infection (27, 28). Therefore, future studies are required to understand the relative contributions of educated and noneducated NK cells from HIV-1-infected donors to the antibody-dependent responses of their total NK cell population. Second, the current study classified educated KIR2DL2/3 NK cells as being derived solely from HLA-C1-carrying donors. While KIR2DL2/3-expressing NK cells are educated within HLA-C1-carrying donors (20, 24), these receptors have been shown to interact with two HLA-B molecules exhibiting HLA-C1 characteristics and HLA-C2 molecules (29). Additional research is required to address whether KIR2DL2/3 interactions with HLA-B and HLA-C2 contribute to NK cell education. This information will be valuable for further refining the definition of educated and noneducated NK cells. Lastly, it should be noted that the utilized DX27 (i.e., anti-KIR2DL2/3) and 143211 (i.e., anti-KIR2DL1) antibody clones cross-react with the activating KIR2DS2 and KIR2DS5 receptors, respectively (30). As such, the educated NK cell population identified in some donors might include cells expressing these activating receptors, which would be noneducated in the absence of additional inhibitory receptor expression. Given the low functionality of gated noneducated NK cells, the potential inclusion of noneducated NK cells within the educated NK cell population might have diluted the functional advantages observed for educated NK cells.
In addition to addressing questions regarding the role of NK cell education in determining anti-HIV-1 antibody-dependent functions, the presented data are relevant to discussions of the role of anti-HIV-1 antibody-dependent NK cell functions in the prevention and pathogenesis of HIV-1 infection. Although our utilized experimental system evaluated anti-HIV-1 antibody responses directed to CD4-induced epitopes on HIV-1 envelope that are not normally exposed on cells infected with most wild-type HIV-1 strains, recent evidence suggests that HIV-1 CRF A/E naturally samples conformations that expose these epitopes (31). Given that the RV144 trial targeted CRF A/E, antibodies that target CD4-induced envelope epitopes might have played a role in the modest protection observed (2–4). As such, the characteristics of NK cells that respond to CD4-induced epitopes on HIV-1 envelope could be significant for understanding RV144-conferred protection. RV144 trial participants have been screened for diversity at the HLA-I and KIR loci (32). We hypothesize that contributions of specific HLA-I/KIR combinations to RV144-conferred protection would be difficult to reveal, because in the absence of any given education-competent receptor/ligand combinations, alternative combinations likely compensate and improve the functional potential of the total NK cell population. Data supporting this hypothesis would suggest that HIV-1 vaccines inducing nonneutralizing antibodies could be successful in the general population and not restricted to individuals carrying certain education-competent receptor/ligand combinations.
The conformation of the HIV-1 envelope and the abundance of ADCC-competent antibodies directed to epitopes revealed upon envelope binding to CD4 might also be of significance for understanding HIV-1 disease progression (9, 33). It has been demonstrated that the envelope on cells infected with many HIV-1 strains does not assume the CD4-bound conformation naturally and is prevented from binding cell surface CD4 due to the downregulation of CD4 by HIV-1 accessory proteins, Nef and Vpu. Thus, ADCC antibodies to CD4-induced epitopes, which are commonly observed in HIV-1-infected donors, will not be able to bind to their cognate epitopes on HIV-1-infected cells. Recently, however, Richard et al. (33) demonstrated that gp120 shed from in vitro-infected cells binds to uninfected cells within the same culture. Furthermore, addition of plasma from HIV-1-infected donors or monoclonal antibodies recognizing CD4-induced epitopes resulted in preferential antibody binding and killing of HIV-1-uninfected cells. These results were supportive of a previously published hypothesis by Lyerly et al. (34). Although much evidence supports a role for anti-HIV-1 antibody-dependent NK cell responses in slowing disease progression (6, 35), it is possible that ADCC of uninfected bystander cells could contribute to CD4+ T-cell depletion in vivo in areas of high viral replication. Indeed, the majority of cell death in HIV-1-infected lymph nodes occurs in noninfected bystander cells (36). Further, the components required for anti-HIV-1 ADCC of bystander cells are likely present within lymph nodes during HIV-1 infection. While lymph nodes in a nondisease state generally contain fewer mature CD16+ NK cells, a study in simian immunodeficiency virus (SIV)-infected macaques demonstrated in situ differentiation of mature and cytolytic CD16+ NK cells (37, 38). Additionally, gp120 is detectable within lymph nodes of simian/human immunodeficiency virus (SHIV)-infected macaques and HIV-1-infected individuals (39, 40). If the phenomenon of bystander cell death through ADCC contributes to HIV-1 disease progression, our data suggest that educated NK cells could make a large contribution to the process.
The potential for educated NK cells to contribute to killing of uninfected bystander cells via ADCC raises questions regarding the ability of educated NK cell-mediated ADCC to be inhibited by interactions between inhibitory NK cell receptors and their HLA-I ligands. The literature contains conflicting information with regard to this matter. Others and we have demonstrated that antibody-dependent activation allows NK cells to at least partially overcome inhibition through inhibitory KIR to mediate antibody-dependent responses to HIV-1 and other antigens (12, 17, 41, 42). Additional research, however, has demonstrated that inhibitory KIR/HLA-I interactions can decrease antibody-dependent responses to HIV-1 and antigens detected by monoclonal antibodies targeting malignancy targets (42, 43). One study demonstrated that the ability of inhibitory KIR/HLA-I interactions to influence anti-CD20 antibody-dependent responses was largely dependent on which monoclonal antibody was used during the stimulation (42). This suggests that the degree to which NK cells can overcome inhibitory signals depends on achieving an optimal level of stimulation. Along similar lines of reasoning, it could be proposed that the degree to which inhibitory NK cell receptor/HLA-I interactions can inhibit antibody-dependent responses is linked to the strength of the interaction between the particular allelic variants of the receptor/ligand combination involved. Indeed, this might be interesting in terms of understanding how allelic combinations of KIR3DL1 and HLA-Bw4 contribute to slowing HIV-1 disease progression (44). Assessment of the relative abilities of protective and nonprotective allelic combinations of receptors and ligands to inhibit killing of bystander cells via anti-HIV-1 ADCC could provide clues to how KIR3DL1/HLA-Bw4 allelic combinations influence HIV-1 disease progression. It is plausible that potent KIR3DL1/HLA-Bw4 combinations would inhibit antibody-dependent NK cell-mediated killing of uninfected bystander cells expressing constitutively expressed levels of HLA-B. HIV-1-infected cells, however, express reduced levels of HLA-B, due to Nef-mediated downregulation (45). As such, HIV-1-infected cells might be more susceptible to ADCC mediated by NK cells from donors with potent allelic combinations of KIR3DL1 and HLA-Bw4 if antibodies recognizing appropriate epitopes are present. These experiments would best be done using autologous CD4+ T cells expressing the full complement of autologous self-HLA-I molecules and/or cell lines designed to express single HLA-I molecules.
There is currently much interest in anti-HIV-1 antibody-dependent responses and their ability to contribute to protection from HIV-1 infection and/or slow disease progression. In the presented data we demonstrate that NK cells educated through inhibitory NK cell receptor interactions with self-HLA-I ligands account for most of the anti-HIV-1 antibody-dependent activation and cytolysis observed in the utilized experimental system. Future research is needed to determine the ability of inhibitory NK cell receptor/ligand combinations to act as regulators of antibody-dependent NK cell responses to uninfected bystander target cells and HIV-1-infected target cells expressing envelopes in different conformations.
MATERIALS AND METHODS
Subjects.
Whole blood from 12 HIV-1-uninfected donors was collected by venipuncture into Vacutainers containing sodium heparin. The Victorian Transplantation and Immunogenetics Services (Parkville, Victoria, Australia) typed donors to four-digit resolution for HLA-A, -B, and -C (Table 1). Donor 09 was typed for HLA-A and -B as previously described (23). PBMC were isolated from whole blood by Ficoll density gradient and utilized for functional assays. Informed consent was obtained from all donors prior to sample collection. The ethics committees of all participating institutions approved the conducted studies.
Cell lines and anti-HIV-1 antibodies.
The CEM.NKr-CCR5 CD4+ human T-cell line was obtained from the NIH AIDS Reagent Program. The HLA-I-devoid 721.221 human B-cell line was a kind gift from Andrew Brooks (University of Melbourne). HIVIG (NIH AIDS Reagent Program) was used to assess anti-HIV-1 antibody-dependent NK cell activation and ADCC. For anti-HIV-1 antibody-dependent NK cell activation and ADCC assays, a 1:1,000 dilution of HIVIG was used to obtain robust readouts, as this dilution of HIVIG avoids the prozone effect of higher concentrations and suboptimal responses of lower concentrations (46).
Antibody-dependent NK cell activation assay.
An NK cell activation intracellular cytokine staining assay was performed to assess the functional importance of NK cell education in direct and antibody-dependent NK cell activation, as previously described (11, 12, 25). Briefly, CEM.NKr-CCR5 target cells were prepared by coating with HIV-1BaL gp120 (3 μg/ml/106 cells; NIH AIDS Reagent Program) for 90 min at 4°C. To assess antibody-dependent NK cell activation, freshly isolated PBMC (1 × 106) were combined with CEM.NKr-CCR5 cells (1 × 105) at a 10:1 ratio in the absence or presence of a 1:1,000 dilution of pooled HIV immunoglobulin. Control experiments were conducted only in the absence of anti-HIV-1 antibody, as we have previously reported that NK cell responsiveness to gp120-coated CD4+ target cells is not detected in the presence of HIV-1-uninfected antibody sources (12). To assess direct NK cell activation, PBMC (1 × 106) were combined with 721.221 cells (1 × 106) at a 1:1 ratio. As a control, PBMC (1 × 106) were incubated alone. All incubations included allophycocyanin (APC) H7-conjugated anti-CD107a antibody (clone H4A3; BD Biosciences), brefeldin A (Sigma) (5 μg/ml), and monensin (BD Biosciences) (6 μg/ml) and were conducted for 5 h at 37°C. After incubation, cells were surface stained with peridinin chlorophyll protein (PerCP)-conjugated anti-CD3 (clone SK7; BD Biosciences), PE-Cy7-conjugated anti-CD56 (clone NCAM16.2; BD Biosciences), APC-conjugated anti-NKG2A (clone Z199; Beckman Coulter), fluorescein isothiocyanate (FITC)-conjugated anti-KIR2DL1 (clone 143211; R&D Systems), BV711-conjugated anti-KIR3DL1 (clone DX9; BD Biosciences), and PE-conjugated anti-KIR2DL2/3 (clone DX27; BD Biosciences) antibodies for 30 min at room temperature in the dark. Next, cells were fixed in 1% formaldehyde (Polysciences Inc.), permeabilized with permeabilization buffer (BD Biosciences), and stained with Alexa Fluor 700-conjugated anti-IFN-γ antibody (clone B27; BD Biosciences) for 1 h at room temperature. Lastly, cells were acquired on an LSR Fortessa flow cytometer (BD Biosciences). Flow cytometry data were analyzed using FlowJo software.
LDH release cytotoxicity assay.
The CytoTox 96 nonradioactive cytotoxicity assay kit (Promega) was used to measure antibody-dependent cytotoxicity via LDH release, as previously described (12). Briefly, CEM.NKr-CCR5 target cells were prepared by coating with HIV-1BaL gp120 (3 μg/ml/106 cells) for 90 min at 4°C. All assay conditions were conducted in triplicate in 96-well round-bottom tissue culture plates (Greiner Bio-One) with freshly isolated PBMC as effector cells. The spontaneous LDH release from target and effector cells was assessed by incubation of each cell population alone. Maximum LDH release from target cells was assessed by the addition of lysis solution (Promega) to wells containing only target cells. Additional wells containing culture medium alone were also included. Effector and target cells were combined at a 20:1 ratio for whole PBMC, with each well containing 2 × 104 targets combined with 4 × 105 effectors. In experiments assessing the antibody-dependent cytotoxicity potential of PBMC enriched for educated NK cells or depleted of educated NK cells by FACS, the number of Educated+ PBMC or Educated− PBMC that would be present in whole PBMC at a 20:1 effector-to-target cell ratio was combined with 2 × 104 targets. To measure anti-HIV-1 ADCC, experimental conditions included assessment of LDH release upon the addition of a 1:1,000 dilution of HIVIG to wells containing effectors and CEM.NKr-CCR5 targets. After the addition of all reagents to wells, plates were spun at 250 × g for 4 min and incubated for 4 h at 37°C. Following incubation, plates were spun at 250 × g for 4 min, and 50 μl/well of supernatant was transferred to an enzyme-linked immunosorbent assay (ELISA) plate (Thermo Fisher Scientific). Next, 50 μl of substrate (Promega) was added to each well containing supernatant and incubated for 30 min at room temperature in the dark. Finally, the reaction was stopped by the addition of 50 μl/well of stop solution (Promega), and absorbance was recorded at 492 nm. The optical density values for wells containing medium only were subtracted from all other optical density values. The remaining values were used to calculate the percentage of cytotoxicity with the following formula: percent cytotoxicity = [(experimental − effector spontaneous − target spontaneous)/(target maximum − target spontaneous)] × 100.
FACS of Educated+ PBMC and Educated− PBMC.
Cell separation of PBMC into Educated+ PBMC and Educated− PBMC was performed with FACS. First, 20 × 106 to 30 × 106 PBMC were stained with PE-conjugated anti-NKG2A antibody (clone Z199; Beckman Coulter) and a donor-dependent combination of PE-conjugated anti-KIR2DL1 (clone 143211; R&D Systems), anti-KIR3DL1 (clone DX9; BD Biosciences), and anti-KIR2DL2/3 (clone DX27; BD Biosciences) antibodies for 30 min at room temperature in the dark (donor relevant combinations of educating receptors are listed in Table 1). As a control, unstained PBMC were concurrently incubated. Following incubation, stained and unstained PBMC were washed and resuspended in phosphate-buffered saline (PBS) plus 1% fetal calf serum (FCS) and acquired on the BD FACSAria III cell sorter (BD Biosciences) located within the flow cytometry platform at the Peter Doherty Institute for Infection and Immunity. Educated+ PBMC and Educated− PBMC (stained PBMC) were defined as PE-positive and PE-negative gated lymphocytes, respectively. As a control, whole PBMC (unstained PBMC) were sorted as PE-negative gated lymphocytes. All three PBMC populations were collected and washed in RF10 medium prior to use in functional assays.
Data analyses.
Data collation and statistical analyses were completed with GraphPad PRISM version 7.0a. Paired comparisons of educated and noneducated NK cell subsets within donors were completed using Wilcoxon matched-pairs tests. Comparisons between multiple matched groups were performed using the Friedman test followed by Dunn's post hoc test. Differences were considered statistically significant at a P value of <0.05. Data throughout are reported in the following format: (median [range]).
ACKNOWLEDGMENTS
We acknowledge the blood donors for making this research possible. We acknowledge Jason Thean Kit Ooi for the artwork in Fig. 1.
We have no conflicts of interest to declare.
This work was funded by a program grant (1052979) from the National Health and Medical Research Council of Australia (NHMRC). M.S.P. is supported by a fellowship from the Canadian Institutes of Health Research (CIHR).
REFERENCES
- 1.Smalls-Mantey A, Connors M, Sattentau QJ. 2013. Comparative efficiency of HIV-1-infected T cell killing by NK cells, monocytes and neutrophils. PLoS One 8:e74858. doi: 10.1371/journal.pone.0074858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, MOPH-TAVEG Investigators. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 4.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. 2013. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A 110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alpert MD, Harvey JD, Lauer WA, Reeves RK, Piatak M Jr, Carville A, Mansfield KG, Lifson JD, Li W, Desrosiers RC, Johnson RP, Evans DT. 2012. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PLoS Pathog 8:e1002890. doi: 10.1371/journal.ppat.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambotte O, Ferrari G, Moog C, Yates NL, Liao HX, Parks RJ, Hicks CB, Owzar K, Tomaras GD, Montefiori DC, Haynes BF, Delfraissy JF. 2009. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 23:897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. 2012. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog 8:e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung AW, Crispin M, Pritchard L, Robinson H, Gorny MK, Yu X, Bailey-Kellogg C, Ackerman ME, Scanlan C, Zolla-Pazner S, Alter G. 2014. Identification of antibody glycosylation structures that predict monoclonal antibody Fc-effector function. AIDS 28:2523–2530. doi: 10.1097/QAD.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veillette M, Coutu M, Richard J, Batraville LA, Dagher O, Bernard N, Tremblay C, Kaufmann DE, Roger M, Finzi A. 2015. The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. J Virol 89:545–551. doi: 10.1128/JVI.02868-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Bredow B, Arias JF, Heyer LN, Moldt B, Le K, Robinson JE, Zolla-Pazner S, Burton DR, Evans DT. 2016. Comparison of antibody-dependent cell-mediated cytotoxicity and virus neutralization by HIV-1 Env-specific monoclonal antibodies. J Virol 90:6127–6139. doi: 10.1128/JVI.00347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gooneratne SL, Center RJ, Kent SJ, Parsons MS. 2016. Functional advantage of educated KIR2DL1(+) natural killer cells for anti-HIV-1 antibody-dependent activation. Clin Exp Immunol 184:101–109. doi: 10.1111/cei.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gooneratne SL, Richard J, Lee WS, Finzi A, Kent SJ, Parsons MS. 2015. Slaying the Trojan horse: natural killer cells exhibit robust anti-HIV-1 antibody-dependent activation and cytolysis against allogeneic T cells. J Virol 89:97–109. doi: 10.1128/JVI.02461-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isitman G, Lisovsky I, Tremblay-McLean A, Kovacs C, Harris M, Routy JP, Bruneau J, Wainberg MA, Tremblay C, Bernard NF. 2016. Antibody-dependent cellular cytotoxicity activity of effector cells from HIV-infected elite and viral controllers. AIDS Res Hum Retroviruses 32:1079–1088. doi: 10.1089/aid.2016.0157. [DOI] [PubMed] [Google Scholar]
- 14.Isitman G, Lisovsky I, Tremblay-McLean A, Parsons MS, Shoukry NH, Wainberg MA, Bruneau J, Bernard NF. 2015. Natural killer cell education does not affect the magnitude of granzyme B delivery to target cells by antibody-dependent cellular cytotoxicity. AIDS 29:1433–1443. doi: 10.1097/QAD.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 15.Isitman G, Tremblay-McLean A, Lisovsky I, Bruneau J, Lebouche B, Routy JP, Bernard NF. 2016. NK cells expressing the inhibitory killer immunoglobulin-like receptors (iKIR) KIR2DL1, KIR2DL3 and KIR3DL1 are less likely to be CD16+ than their iKIR negative counterparts. PLoS One 11:e0164517. doi: 10.1371/journal.pone.0164517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parsons MS, Loh L, Gooneratne S, Center RJ, Kent SJ. 2014. Role of education and differentiation in determining the potential of natural killer cells to respond to antibody-dependent stimulation. AIDS 28:2781–2786. doi: 10.1097/QAD.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 17.Parsons MS, Wren L, Isitman G, Navis M, Stratov I, Bernard NF, Kent SJ. 2012. HIV infection abrogates the functional advantage of natural killer cells educated through KIR3DL1/HLA-Bw4 interactions to mediate anti-HIV antibody-dependent cellular cytotoxicity. J Virol 86:4488–4495. doi: 10.1128/JVI.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wren LH, Stratov I, Kent SJ, Parsons MS. 2013. Obstacles to ideal anti-HIV antibody-dependent cellular cytotoxicity responses. Vaccine 31:5506–5517. doi: 10.1016/j.vaccine.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 19.Chung AW, Rollman E, Center RJ, Kent SJ, Stratov I. 2009. Rapid degranulation of NK cells following activation by HIV-specific antibodies. J Immunol 182:1202–1210. doi: 10.4049/jimmunol.182.2.1202. [DOI] [PubMed] [Google Scholar]
- 20.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E. 2006. Human NK cell education by inhibitory receptors for MHC class I. Immunity 25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Yawata M, Yawata N, Draghi M, Partheniou F, Little A-M, Parham P. 2008. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood 112:2369–2380. doi: 10.1182/blood-2008-03-143727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Sunwoo JB, Yang L, Choi T, Song YJ, French AR, Vlahiotis A, Piccirillo JF, Cella M, Colonna M, Mohanakumar T, Hsu KC, Dupont B, Yokoyama WM. 2008. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci U S A 105:3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons MS, Zipperlen K, Gallant M, Grant M. 2010. Killer cell immunoglobulin-like receptor 3DL1 licenses CD16-mediated effector functions of natural killer cells. J Leukoc Biol 88:905–912. doi: 10.1189/jlb.1009687. [DOI] [PubMed] [Google Scholar]
- 24.Sim MJ, Stowell J, Sergeant R, Altmann DM, Long EO, Boyton RJ. 2016. KIR2DL3 and KIR2DL1 show similar impact on licensing of human NK cells. Eur J Immunol 46:185–191. doi: 10.1002/eji.201545757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons MS, Madhavi V, Ana-Sosa-Batiz F, Center RJ, Wilson KM, Bunupuradah T, Ruxrungtham K, Kent SJ. 2016. Seminal plasma anti-HIV antibodies trigger antibody-dependent cellular cytotoxicity: implications for HIV transmission. J Acquir Immune Defic Syndr 71:17–23. doi: 10.1097/QAI.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 26.Boudreau JE, Hsu KC. 2018. Natural killer cell education and the response to infection and cancer therapy: stay tuned. Trends Immunol 39:222–239. doi: 10.1016/j.it.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heath J, Newhook N, Comeau E, Gallant M, Fudge N, Grant M. 2016. NKG2C(+)CD57(+) natural killer cell expansion parallels cytomegalovirus-specific CD8(+) T cell evolution towards senescence. J Immunol Res 2016:7470124. doi: 10.1155/2016/7470124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, Marcenaro E, Bottino C, Moretta L, Moretta A, Fauci AS. 2003. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A 100:15011–15016. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moesta AK, Parham P. 2012. Diverse functionality among human NK cell receptors for the C1 epitope of HLA-C: KIR2DS2, KIR2DL2, and KIR2DL3. Front Immunol 3:336. doi: 10.3389/fimmu.2012.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czaja K, Borer AS, Schmied L, Terszowski G, Stern M, Gonzalez A. 2014. A comprehensive analysis of the binding of anti-KIR antibodies to activating KIRs. Genes Immun 15:33–37. doi: 10.1038/gene.2013.58. [DOI] [PubMed] [Google Scholar]
- 31.Prevost J, Zoubchenok D, Richard J, Veillette M, Pacheco B, Coutu M, Brassard N, Parsons MS, Ruxrungtham K, Bunupuradah T, Tovanabutra S, Hwang KK, Moody MA, Haynes BF, Bonsignori M, Sodroski J, Kaufmann DE, Shaw GM, Chenine AL, Finzi A. 2017. Influence of the envelope gp120 Phe 43 cavity on HIV-1 sensitivity to antibody-dependent cell-mediated cytotoxicity responses. J Virol 91:e02452–16. doi: 10.1128/JVI.02452-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prentice HA, Ehrenberg PK, Baldwin KM, Geretz A, Andrews C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, O'Connell RJ, Robb ML, Kim JH, Michael NL, Thomas R. 2014. HLA class I, KIR, and genome-wide SNP diversity in the RV144 Thai phase 3 HIV vaccine clinical trial. Immunogenetics 66:299–310. doi: 10.1007/s00251-014-0765-6. [DOI] [PubMed] [Google Scholar]
- 33.Richard J, Veillette M, Ding S, Zoubchenok D, Alsahafi N, Coutu M, Brassard N, Park J, Courter JR, Melillo B, Smith AB 3rd, Shaw GM, Hahn BH, Sodroski J, Kaufmann DE, Finzi A. 2016. Small CD4 mimetics prevent HIV-1 uninfected bystander CD4 + T cell killing mediated by antibody-dependent cell-mediated cytotoxicity. EBioMedicine 3:122–134. doi: 10.1016/j.ebiom.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyerly HK, Matthews TJ, Langlois AJ, Bolognesi DP, Weinhold KJ. 1987. Human T-cell lymphotropic virus IIIB glycoprotein (gp120) bound to CD4 determinants on normal lymphocytes and expressed by infected cells serves as target for immune attack. Proc Natl Acad Sci U S A 84:4601–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wren LH, Chung AW, Isitman G, Kelleher AD, Parsons MS, Amin J, Cooper DA, ADCC Study Collaboration Investigators, Stratov I, Navis M, Kent SJ. 2013. Specific antibody-dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology 138:116–123. doi: 10.1111/imm.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med 1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 37.Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA, Moretta A, Munz C. 2004. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol 172:1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 38.Schafer JL, Li H, Evans TI, Estes JD, Reeves RK. 2015. Accumulation of cytotoxic CD16+ NK cells in simian immunodeficiency virus-infected lymph nodes associated with in situ differentiation and functional anergy. J Virol 89:6887–6894. doi: 10.1128/JVI.00660-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santosuosso M, Righi E, Lindstrom V, Leblanc PR, Poznansky MC. 2009. HIV-1 envelope protein gp120 is present at high concentrations in secondary lymphoid organs of individuals with chronic HIV-1 infection. J Infect Dis 200:1050–1053. doi: 10.1086/605695. [DOI] [PubMed] [Google Scholar]
- 40.Stevceva L, Yoon V, Carville A, Pacheco B, Santosuosso M, Korioth-Schmitz B, Mansfield K, Poznansky MC. 2008. The efficacy of T cell-mediated immune responses is reduced by the envelope protein of the chimeric HIV-1/SIV-KB9 virus in vivo. J Immunol 181:5510–5521. doi: 10.4049/jimmunol.181.8.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lang P, Pfeiffer M, Handgretinger R, Schumm M, Demirdelen B, Stanojevic S, Klingebiel T, Kohl U, Kuci S, Niethammer D. 2002. Clinical scale isolation of T cell-depleted CD56+ donor lymphocytes in children. Bone Marrow Transplant 29:497–502. doi: 10.1038/sj.bmt.1703406. [DOI] [PubMed] [Google Scholar]
- 42.Terszowski G, Klein C, Stern M. 2014. KIR/HLA interactions negatively affect rituximab- but not GA101 (obinutuzumab)-induced antibody-dependent cellular cytotoxicity. J Immunol 192:5618–5624. doi: 10.4049/jimmunol.1400288. [DOI] [PubMed] [Google Scholar]
- 43.Ward JP, Bonaparte MI, Barker E. 2004. HLA-C and HLA-E reduce antibody-dependent natural killer cell-mediated cytotoxicity of HIV-infected primary T cell blasts. AIDS 18:1769–1779. doi: 10.1097/00002030-200409030-00005. [DOI] [PubMed] [Google Scholar]
- 44.Martin MP, Qi Y, Gao X, Yamada E, Martin JN, Pereyra F, Colombo S, Brown EE, Shupert WL, Phair J, Goedert JJ, Buchbinder S, Kirk GD, Telenti A, Connors M, O'Brien SJ, Walker BD, Parham P, Deeks SG, McVicar DW, Carrington M. 2007. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet 39:733–740. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661–671. doi: 10.1016/S1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 46.Pollara J, Hart L, Brewer F, Pickeral J, Packard BZ, Hoxie JA, Komoriya A, Ochsenbauer C, Kappes JC, Roederer M, Huang Y, Weinhold KJ, Tomaras GD, Haynes BF, Montefiori DC, Ferrari G. 2011. High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A 79:603–612. doi: 10.1002/cyto.a.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]