ABSTRACT
Zaire and Sudan ebolavirus species cause a severe disease in humans and nonhuman primates (NHPs) characterized by a high mortality rate. There are no licensed therapies or vaccines against Ebola virus disease (EVD), and the recent 2013 to 2016 outbreak in West Africa highlighted the need for EVD-specific medical countermeasures. Here, we generated and characterized head-to-head the immunogenicity and efficacy of five vaccine candidates against Zaire ebolavirus (EBOV) and Sudan ebolavirus (SUDV) based on the highly attenuated poxvirus vector modified vaccinia virus Ankara (MVA) expressing either the virus glycoprotein (GP) or GP together with the virus protein 40 (VP40) forming virus-like particles (VLPs). In a human monocytic cell line, the different MVA vectors (termed MVA-EBOVs and MVA-SUDVs) triggered robust innate immune responses, with production of beta interferon (IFN-β), proinflammatory cytokines, and chemokines. Additionally, several innate immune cells, such as dendritic cells, neutrophils, and natural killer cells, were differentially recruited in the peritoneal cavity of mice inoculated with MVA-EBOVs. After immunization of mice with a homologous prime/boost protocol (MVA/MVA), total IgG antibodies against GP or VP40 from Zaire and Sudan ebolavirus were differentially induced by these vectors, which were mainly of the IgG1 and IgG3 isotypes. Remarkably, an MVA-EBOV construct coexpressing GP and VP40 protected chimeric mice challenged with EBOV to a greater extent than a vector expressing GP alone. These results support the consideration of MVA-EBOVs and MVA-SUDVs expressing GP and VP40 and producing VLPs as best-in-class potential vaccine candidates against EBOV and SUDV.
IMPORTANCE EBOV and SUDV cause a severe hemorrhagic fever affecting humans and NHPs. Since their discovery in 1976, they have caused several sporadic epidemics, with the recent outbreak in West Africa from 2013 to 2016 being the largest and most severe, with more than 11,000 deaths being reported. Although some vaccines are in advanced clinical phases, less expensive, safer, and more effective licensed vaccines are desirable. We generated and characterized head-to-head the immunogenicity and efficacy of five novel vaccines against EBOV and SUDV based on the poxvirus MVA expressing GP or GP and VP40. The expression of GP and VP40 leads to the formation of VLPs. These MVA-EBOV and MVA-SUDV recombinants triggered robust innate and humoral immune responses in mice. Furthermore, MVA-EBOV recombinants expressing GP and VP40 induced high protection against EBOV in a mouse challenge model. Thus, MVA expressing GP and VP40 and producing VLPs is a promising vaccine candidate against EBOV and SUDV.
KEYWORDS: Ebola, poxvirus, MVA, GP, VP40, mice, immunogenicity, protection
INTRODUCTION
Ebola virus disease (EVD) is a serious zoonotic hemorrhagic fever in humans and nonhuman primates (NHP) caused by filoviruses of the genus Ebolavirus that were discovered in 1976 during two simultaneous outbreaks in the Democratic Republic of Congo and Sudan (1). The Ebolavirus genus includes 5 different species, which, in decreasing order of virulence, are Zaire ebolavirus, Sudan ebolavirus, Bundibugyo ebolavirus, Taï Forest ebolavirus, and Reston ebolavirus, with differences in their amino acid sequences of about 40% (2). The Zaire ebolavirus species includes the Ebola virus (EBOV), and the Sudan ebolavirus species includes the Sudan virus (SUDV) as the only members. The case fatality rates of EBOV, SUDV, and Bundibugyo virus (BDBV) infections range from 20% to 90%, while Reston virus (RESTV) is presumably nonpathogenic for humans but does cause EVD in NHPs (3). EVD can be transmitted directly to humans from fruit bats, which are considered putative reservoir species of the Ebolavirus genus, or indirectly through intermediate reservoirs, such as NHPs (1, 4). EVD usually spreads between humans through the exchange of body fluids and secretions (1, 4).
Since its discovery in 1976, EBOV and SUDV have caused several sporadic outbreaks of hemorrhagic fever mainly in East and Central Africa (5). However, the recent outbreak from 2013 to 2016 in West Africa, which was caused by the Makona variant of EBOV, was the largest and most severe epidemic, being the first time that EVD was localized mainly in urban areas with a global spread (4, 6). Since the beginning of the outbreak (December 2013) to the end (June 2016), a total of 28,616 cases of EBOV infection were reported in Guinea, Liberia, and Sierra Leone, with 11,310 deaths and also with some imported cases being reported in other parts of the world, including Nigeria, Senegal, Spain, the United States, Mali, and the United Kingdom (7).
Like other members of the family Filoviridae, EBOV and SUDV are enveloped, nonsegmented, single-stranded, negative-sense RNA viruses. EBOV and SUDV particles are filamentous with a uniform diameter (80 nm) but a variable length, reaching up to 14,000 nm (5). The 19-kb viral genome encodes 8 proteins: nucleoprotein (NP), viral protein 35 (VP35), VP40, virion envelope glycoprotein (GP), sGP, VP30, VP24, and the RNA-dependent viral polymerase (L) (1). Four of the genes encode structural proteins, including GP, NP, and two matrix proteins, VP24 and VP40, while the nonstructural proteins include VP30 and VP35 and the RNA-dependent viral polymerase (L) (8). The GP is responsible for attachment and viral entry and plays an important role in inducing binding and neutralizing antibodies against ebolavirus, which are important correlates of protection (9). The VP40 matrix protein plays a key role in virus assembly and budding, being capable of driving budding of filamentous virus-like particles (VLPs) from mammalian cells in the absence or presence of other ebolavirus proteins (10, 11). For example, it has been described that coexpression of VP40 and GP proteins results in the release of ebolavirus VLPs, which have been shown to confer effective protection against lethal challenges in animal models (12–16). Therefore, both the GP and VP40 proteins are excellent targets to be included as immunogens in novel vaccine candidates.
Several vaccine candidates against ebolavirus have been developed based on different platforms for the expression of ebolavirus antigens, such as DNA, recombinant proteins, VLPs, and replicating and nonreplicating viral vectors, with most of them expressing the GP as the main immunogen (17). Their efficacy and protection have been evaluated in NHP models for ebolavirus infection (8, 18–20), and several vaccine candidates have shown promising results in human clinical trials (21–25). The three leading vaccine candidates are the replicating vesicular stomatitis virus (VSV)-vector vaccine encoding EBOV GP (rVSV-ZEBOV), the replication-defective chimpanzee adenovirus type 3-vector vaccine (ChAd3-EBO-Z), and the replication-defective human adenovirus type 26-vector vaccine (Ad26-ZEBOV) (26). In rVSV-ZEBOV, the VSV GP has been replaced by the GP from the EBOV Kikwit-95 variant, attenuating the pathogenicity of the VSV and enabling infection of cells and replication using the EBOV GP (18). It showed a good safety and immunogenicity profile in several human clinical trials (24, 27), including a phase III clinical trial in Guinea, where safety and efficacy have been tested with promising results (22). ChAd3-EBO-Z also encodes the EBOV GP in the monovalent or bivalent form, and its safety and immunogenicity have been tested in several human clinical trials (21, 23, 28). A phase II clinical trial in Liberia, where safety and efficacy are being evaluated, is currently ongoing (29), and another phase II clinical trial in children from Nigeria, Mali, and Senegal has finished recently (ClinicalTrials.gov registration no. NCT02548078). Ad26-ZEBOV also encodes the EBOV GP, and its safety and immunogenicity have been tested in human clinical trials (30, 31). Furthermore, to further assess its long-term safety and immunogenicity, two phase III clinical trials are currently ongoing in Sierra Leone (ClinicalTrials.gov registration no. NCT02661464 and NCT02509494). However, despite the advances in the development of vaccines against ebolavirus, there are no approved vaccines for treatment and prevention. Moreover, there are a number of safety concerns with these vaccines, and whether these vaccines will be licensed is unclear.
One of the most promising viral vectors is the highly attenuated poxvirus strain modified vaccinia virus Ankara (MVA), which was generated by passaging the Turkish smallpox vaccine chorioallantoic vaccinia virus Ankara (CVA) strain more than 570 times in primary chicken embryo fibroblast (CEF) cells (32). MVA has been widely used in several preclinical and clinical trials as a vaccine vector against multiple infectious diseases and cancer (33–36), showing that MVA vectors are safe, express high levels of the heterologous antigens, and are strongly immunogenic. Thus, the use of MVA as a vector to generate a vaccine candidate against ebolavirus could be a useful approach to counteract the disease. Recently, the generation of MVA-based vaccines against ebolavirus has been reported (22, 37), and one vaccine candidate has been tested only for immunogenicity in clinical trials as a boost of ChAd3-EBO-Z (21, 26, 37) or Ad26-ZEBOV (30).
As the immunogenicity and efficacy of MVA vectors expressing different levels of ebolavirus antigens have not been compared head-to-head, in this study we developed and characterized novel MVA-based vaccine candidates against EBOV and SUDV (termed MVA-EBOVs and MVA-SUDVs, respectively) expressing either GP or GP together with VP40 from the Zaire and Sudan ebolavirus species, respectively. MVA-EBOV recombinants expressing GP and VP40 were able to produce VLPs. Differences in the innate immune responses in human macrophages and in the recruitment of immune cells in the peritoneal cavity of inoculated mice, as well as in the extent of humoral ebolavirus-specific immune responses in immunized mice, were observed between the MVA vectors. Remarkably, compared to the other vectors, MVA-EBOVs expressing GP and VP40 together and producing VLPs were able to induce the highest protection against EBOV in a mouse challenge model.
RESULTS
Generation of recombinant MVA-EBOV/SUDVs expressing the GP or GP and VP40 genes from Zaire and Sudan ebolaviruses.
To develop novel vaccines against EBOV and SUDV, we generated different MVA-based vaccine candidates, termed MVA-EBOV/SUDVs, (i) expressing only the GP gene from EBOV or SUDV (termed MVA-GP Zaire or MVA-GP Sudan, respectively), (ii) expressing GP fused to VP40 from EBOV and SUDV (termed MVA-GP-2A-VP40 Zaire and MVA-GP-2A-VP40 Sudan, respectively), or (iii) coexpressing from different viral loci GP and VP40 from EBOV (termed MVA-GP-VP40 Zaire). All these genes were inserted in the vector backbone of an optimized parental MVA, which also contained deletions in the vaccinia virus (VACV) immunomodulatory genes C6L, K7R, and A46R (termed MVA-GFP) (see Materials and Methods). We have previously described that an MVA vector lacking those VACV genes and expressing chikungunya virus genes encoding the structural virus proteins is able to fully protect mice and NHPs after challenge with chikungunya virus (38, 39).
A diagram of the different recombinant MVA-EBOV/SUDVs is shown in Fig. 1A, which shows the corresponding VACV deletions, the GP or GP-2A-VP40 Zaire or Sudan genes inserted into the VACV thymidine kinase (TK) locus, and the VP40 Zaire gene inserted into the VACV hemagglutinin (HA) locus, with all genes being under the transcriptional control of the synthetic early/late (sE/L) viral promoter driving the constitutive expression of the EBOV or SUDV GP and VP40 proteins. The correct insertion and purity of recombinant MVA-EBOV/SUDVs were confirmed by PCR and DNA sequence analysis. PCR using primers annealing in the VACV TK-flanking regions confirmed the presence of the GP gene in MVA-GP Zaire, MVA-GP Sudan, and MVA-GP-VP40 Zaire and the GP-2A-VP40 gene in MVA-GP-2A-VP40 Zaire and MVA-GP-2A-VP40 Sudan, no wild-type (WT) contamination in the preparation, and amplification of the green fluorescent protein (GFP) and the VACV TK genes in the parental virus MVA-GFP and in wild-type attenuated MVA (MVA-WT), respectively (Fig. 1B). Furthermore, PCR using primers annealing in the VACV HA-flanking regions confirmed the presence of the EBOV VP40 gene in MVA-GP-VP40 Zaire, with the VACV HA locus being amplified in MVA-GFP or MVA-GP Zaire (Fig. 1B). Moreover, PCRs using primers annealing in the C6L-, K7R-, and A46R-flanking regions confirmed the deletion of the VACV genes C6L, K7R, and A46R, respectively, from all MVA-EBOV/SUDVs (data not shown). The right insertion and correct sequence of the GP and GP-2A-VP40 genes inserted in the TK locus and the VP40 gene inserted in the HA locus and the correct deletions of the VACV genes C6L, K7R, and A46R were also confirmed by DNA sequencing (data not shown).
FIG 1.
Generation and in vitro characterization of recombinant MVA-EBOV/SUDVs. (A) Scheme of the genome map of recombinant MVA-EBOV/SUDVs expressing GP and VP40 from Zaire and Sudan ebolavirus species. The different regions are indicated by capital letters. The right and left terminal regions are shown. Below the map, the deleted or fragmented VACV genes are depicted as black boxes. The GP Zaire, GP Sudan, GP-2A-VP40 Zaire, and GP-2A-VP40 Sudan genes driven by the sE/L virus promoter and inserted within the VACV TK viral locus (J2R) are indicated. The VP40 Zaire gene driven by the sE/L virus promoter and inserted within the VACV HA viral locus (A56R) is also indicated. The deleted VACV C6L, K7R, and A46R genes are also indicated. TK-L, TK left; TK-R, TK right. (B) PCR analysis of VACV TK and HA loci. Viral DNA was extracted from DF-1 cells mock infected or infected at 5 PFU/cell with MVA-EBOV/SUDVs, parental MVA-GFP, or MVA-WT. Primers spanning the TK locus-flanking regions were used for PCR analysis of the GP and GP-2A-VP40 Zaire and Sudan genes inserted within the TK locus, and primers spanning the HA locus-flanking regions were used for PCR analysis of the VP40 Zaire gene. Amplified DNA products are indicated by arrows on the right. A molecular size marker (1-kb ladder) with the corresponding sizes (base pairs) is indicated on the left. (C) Expression of GP and VP40 Zaire or Sudan proteins. DF-1 cells were mock infected or infected at 5 PFU/cell with MVA-EBOV/SUDVs and parental MVA-GFP. At 24 hpi, cells were lysed in Laemmli buffer, fractionated by 10% SDS-PAGE, and analyzed by Western blotting using mouse polyclonal antibodies against GP-Zaire, GP-Sudan, and VP40 Sudan, and a rabbit polyclonal antibody against VP40 Zaire. Rabbit antibodies against the VACV E3 protein and β-actin were used as a loading control for the quantity of virus antigen and of host cell protein, respectively. Arrows on the right indicate the GP and VP40 proteins, together with VACV E3 and β-actin. The sizes of standards (in kilodaltons; Precision Plus protein standards; Bio-Rad Laboratories) are indicated on the left.
MVA-EBOV/SUDVs express the GP and VP40 Zaire and Sudan ebolavirus genes.
In order to confirm that the MVA-EBOV/SUDVs constitutively express and correctly process the GP, GP-2A-VP40, and VP40 genes, we performed a Western blot analysis of cell extracts from DF-1 cells mock infected or infected with MVA-EBOV/SUDVs (MVA-GP Zaire, MVA-GP Sudan, MVA-GP-2A-VP40 Zaire, MVA-GP-2A-VP40 Sudan, and MVA-GP-VP40 Zaire) or MVA-GFP using specific antibodies that recognize the GP and VP40 proteins from EBOV and SUDV. The results demonstrated that all the MVA-EBOV/SUDVs correctly expressed the GP and VP40 Zaire or Sudan proteins (Fig. 1C). In particular, MVA-GP-2A-VP40 recombinants expressed a GP protein of a higher molecular size that corresponded to the expression of a fused GP-2A-VP40 insert without processing (Fig. 1C).
Additionally, at 3, 7, and 24 h postinfection (hpi), we collected cell extracts or supernatants from DF-1 cells mock infected or infected with MVA-EBOV/SUDVs, MVA-GFP, and MVA-WT, in order to analyze by Western blotting the expression of GP and VP40 proteins at early times postinfection and the secretion of GP and VP40 proteins to the medium (Fig. 2A and B). The results showed that expression of the GP protein in cell extracts from MVA-GP and MVA-GP-2A-VP40 recombinants was detected at early times postinfection, while expression of GP from MVA-GP-VP40 Zaire was mainly detected at 24 hpi (Fig. 2A). Moreover, VP40 was expressed at early times postinfection from MVA-GP-VP40 Zaire and MVA-GP-2A-VP40 Sudan, while the expression of VP40 from MVA-GP-2A-VP40 Zaire was detected at 24 hpi (Fig. 2A). Furthermore, all MVA-EBOV/SUDVs were able to secrete the GP protein, while secretion of the VP40 protein was observed with MVA-GP-VP40 Zaire and MVA-GP-2A-VP40 Sudan but poorly with MVA-GP-2A-VP40 Zaire (Fig. 2B).
FIG 2.
GP and VP40 protein expression and viral growth kinetics of MVA-EBOV/SUDVs. (A and B) Expression kinetics of GP and VP40 proteins of Zaire and Sudan ebolaviruses present in cellular pellets (A) and supernatants (B) from cells infected with MVA-EBOV/SUDVs. DF-1 cells were mock infected or infected at 5 PFU/cell with MVA-EBOV/SUDVs, parental MVA-GFP, and MVA-WT, and at 3, 7, and 24 hpi, cellular pellets (A) and cell supernatants (B) were collected (the supernatants had previously been precipitated with 10% TCA), lysed in Laemmli buffer, fractionated by 10% SDS-PAGE, and analyzed by Western blotting using mouse polyclonal antibodies against GP-Zaire, GP-Sudan, and VP40 Sudan and a rabbit polyclonal antibody against VP40 Zaire. Rabbit antibody against β-actin was used as a loading control for the quantity of cells. Arrows on the right indicate the GP and VP40 proteins and β-actin. The sizes of standards (in kilodaltons) are indicated on the left. (C) Flow cytometry analysis of GP expression levels in cells infected with MVA-EBOV/SUDVs. HeLa cells were mock infected or infected at 5 PFU/cell with MVA-EBOV/SUDVs and MVA-WT, and at 24 hpi, cells were collected and analyzed by flow cytometry using mouse polyclonal antibodies against GP Zaire and Sudan proteins, followed by an anti-mouse FITC-conjugated antibody. The cell surface expression of GP is represented in the histogram plots. (D) Viral growth kinetics of MVA-EBOV/SUDVs. Monolayers of DF-1 cells were infected at 0.01 PFU/cell with parental MVA-GFP and the different MVA-EBOV/SUDVs. At different times postinfection (0, 24, 48, and 72 h), cells were collected and virus titers in cell lysates were quantified by a plaque immunostaining assay with anti-VACV antibodies. The means of the results from two independent experiments are shown.
To further quantify and determine differences in GP expression levels between the MVA-EBOV/SUDV recombinants, we next analyzed by flow cytometry the GP Zaire and Sudan expression at 24 hpi in HeLa cells mock infected or infected with MVA-EBOV/SUDVs and MVA-WT (Fig. 2C). The results showed that MVA-GP-2A-VP40 recombinants expressed higher GP levels than the other MVA-EBOV/SUDV recombinants, with the differences being greater between the MVA-EBOVs than between the MVA-SUDVs. Additionally, MVA-GP Zaire expressed slightly higher GP levels than MVA-GP-VP40 Zaire. These results are in accordance with the expression levels detected by Western blotting (Fig. 1C and 2A and B). Thus, the MVA-GP-2A-VP40 Zaire and Sudan recombinants express higher GP levels than the MVA-GP Zaire and Sudan recombinants or MVA-GP-VP40 Zaire; clearly, the MVA vectors express different levels of GP.
MVA-EBOV/SUDVs replicate in cell cultures.
To determine whether expression of GP and VP40 Zaire or Sudan proteins affects MVA replication in cell culture, we next analyzed the growth kinetics in DF-1 cells of MVA-GP Zaire, MVA-GP Sudan, MVA-GP-2A-VP40 Zaire, MVA-GP-2A-VP40 Sudan, MVA-GP-VP40 Zaire, and parental MVA-GFP. The results showed that the kinetics of viral growth were similar between all these viruses (Fig. 2D), demonstrating that the expression of the GP and VP40 proteins does not impair MVA vector replication under permissive conditions.
MVA-EBOV/SUDVs are stable in cell culture.
To certify that MVA-EBOV/SUDVs are stable and can be maintained in cultured cells without the loss of the GP and VP40 genes, the MVA-EBOV/SUDVs were further grown in DF-1 cells infected at a low multiplicity of infection (MOI) during 5 consecutive passages, and expression of the GP and VP40 proteins was determined by Western blotting. The results showed that all the MVA-EBOV/SUDVs efficiently expressed the GP and VP40 proteins after serial passages, demonstrating that the recombinant MVA-EBOV/SUDVs are genetically stable (Fig. 3A). This was also confirmed by Western blotting after the isolation of 20 individual plaques at passage 5 (data not shown).
FIG 3.
Genetic stability of MVA-EBOV/SUDVs and analysis of apoptosis. (A) Stability of MVA-EBOV/SUDVs. Passage 2 (P2) stocks of MVA-EBOV/SUDVs were continuously grown at a low MOI until passage 5 in DF-1 cells. Then, DF-1 cells were mock infected or infected with MVA-EBOV/SUDVs from the different passages or with MVA-WT. At 24 hpi, cells were lysed in Laemmli buffer, fractionated by 10% SDS-PAGE, and analyzed by Western blotting using mouse polyclonal antibody against GP-Zaire, GP-Sudan, and VP40 Sudan and a rabbit polyclonal antibody against VP40 Zaire. Rabbit anti-VACV early E3 protein antibody was used as a VACV loading control. Arrows on the right indicate the positions of the GP and VP40 proteins and the VACV E3 protein. The sizes of the standards (in kilodaltons) are indicated on the left. (B) Apoptosis in MVA-EBOV/SUDV-infected cells. HeLa cells were mock infected or infected at 5 PFU/cell with MVA-EBOV/SUDVs, parental MVA-GFP, and MVA-WT. At 18 hpi, cells were lysed in Laemmli buffer, fractionated by 10% SDS-PAGE, and analyzed by Western blotting using a mouse anti-human cleaved PARP antibody. Rabbit antibody against β-actin was used as a loading control for the quantity of cells. Arrows on the right, with their corresponding sizes (in kilodaltons), indicate the positions of the full-length PARP, cleaved PARP, and β-actin. The sizes of standards (in kilodaltons) are indicated on the left.
Apoptosis in MVA-EBOV/SUDV-infected cells.
It has been reported that filovirus GP proteins expressed in the cell surface reduced Fas-mediated apoptotic signals by the steric shielding effect of the mucin-like region of the GP protein (40). Thus, in order to determine whether expression of GP and VP40 Zaire or Sudan proteins impacts apoptosis, we performed a Western blot analysis of cell extracts collected at 18 hpi from HeLa cells mock infected or infected with MVA-EBOV/SUDVs, MVA-GFP, and MVA-WT, using specific antibodies against cleaved poly(ADP-ribose) polymerase (PARP) protein. The results showed that apoptosis levels were similarly induced between all these viruses, indicating that the constitutive expression of the GP and VP40 proteins does not influence the apoptosis levels induced by the parental virus MVA-GFP (Fig. 3B).
Intracellular distribution of GP and VP40 proteins in MVA-EBOV/SUDV-infected cells.
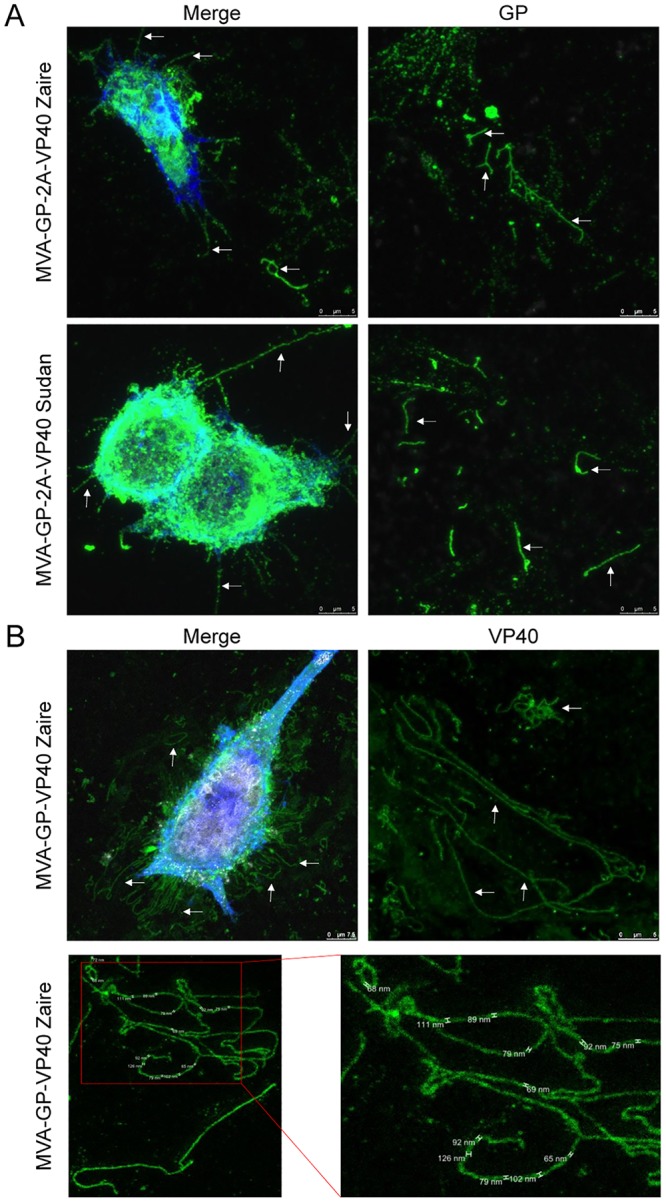
The expression and intracellular localization of GP and VP40 proteins were examined by confocal immunofluorescence microscopy in HeLa cells mock infected or infected at an MOI of 0.5 PFU/cell with MVA-EBOV/SUDVs and MVA-WT, using specific antibodies against the EBOV and SUDV GP and VP40 proteins and specific probes to detect polymerized actin (phalloidin) and DNA (4′,6-diamidino-2-phenylindole [DAPI]). The results showed that the GP Zaire or Sudan proteins were expressed mainly in the cytoplasm and cellular membrane of cells infected with the corresponding MVA-EBOV/SUDVs, being also present in cellular protrusions (Fig. 4). Moreover, the GP protein formed aggregates in the absence of VP40, but it formed filamentous structures protruding from the cell surface in the presence of VP40 (Fig. 4). On the other hand, the VP40 Zaire or Sudan proteins were expressed mainly in the cytoplasm and cellular membrane of cells infected with MVA-GP-2A-VP40 Zaire, MVA-GP-2A-VP40 Sudan, and MVA-GP-VP40 Zaire, being also present in cellular protrusions (Fig. 5).
FIG 4.
Immunofluorescence analysis of GP proteins produced in MVA-EBOV/SUDV-infected cells. Subconfluent HeLa cells cultured on glass coverslips were mock infected or infected at 0.5 PFU/cell with MVA-EBOV/SUDVs or MVA-WT. At 18 hpi, cells were fixed with 3% PFA, quenched in the presence of NH4Cl, permeabilized, blocked with saponin-FCS, and labeled with antibodies against the GP Zaire and GP Sudan proteins. Then, cells were treated with secondary antibodies conjugated with the fluorochrome Alexa Fluor 488 (green), the probe phalloidin (to stain F-actin, shown in blue), and DAPI (to mark DNA, shown in gray). Coverslips were mounted on glass slides, conserved in ProLong Gold antifade reagent, and visualized by confocal microscopy. Detection of GP proteins is shown in the first column, detection of F-actin is shown in the second column, detection of nuclei is shown in the third column, and merged images are shown in the last column. Bars, 7.5 μm.
FIG 5.
Immunofluorescence analysis of VP40 proteins produced in MVA-EBOV/SUDV-infected cells. Subconfluent HeLa cells cultured on glass coverslips were mock infected or infected at 0.5 PFU/cell with MVA-EBOV/SUDVs or MVA-WT. At 18 hpi, cells were fixed with 3% PFA, quenched in the presence of NH4Cl, permeabilized, blocked with saponin-FCS, and labeled with antibodies against VP40 Zaire or VP40 Sudan proteins. Then, cells were treated with secondary antibodies conjugated with the fluorochrome Alexa Fluor 488 (green), the probe phalloidin (to stain F-actin, shown in blue), and DAPI (to mark DNA, shown in gray). Coverslips were mounted on glass slides, conserved in ProLong Gold antifade reagent, and visualized by confocal microscopy. Detection of VP40 proteins is shown in the first column, detection of F-actin is shown in the second column, detection of nuclei is shown in the third column, and merged images are shown in the last column. Bars, 7.5 μm.
Additionally, in order to increase GP and VP40 Zaire and Sudan protein production and secretion, we infected HeLa cells at a high MOI (50 PFU/cell) with MVA-GP-2A-VP40 Zaire, MVA-GP-2A-VP40 Sudan, and MVA-GP-VP40 Zaire, and expression of GP and VP40 proteins was analyzed by confocal microscopy. The results showed that these recombinant MVA-EBOV/SUDVs formed VLP-like structures similar in size and morphology to the characteristic VLPs described for ebolavirus that were detected around the cellular membrane (Fig. 6A and B, left) and in the extracellular space (Fig. 6A and B, right, and 6B, bottom). VLPs formed by MVA-GP-2A-VP40 recombinants were detected using specific antibodies against GP Zaire or Sudan (Fig. 6A) and not detected using antibodies against VP40 (data not shown), while VLPs formed by MVA-GP-VP40 Zaire were detected using specific antibodies against VP40 Zaire (Fig. 6B) and not detected using antibodies against GP (data not shown). As expected, in MVA-WT-infected cells, these VLP forms were not produced and, hence, not detected using antibodies against GP or VP40 (data not shown). These results reveal that VLPs formed by the MVA-GP-2A-VP40 recombinants contain more GP and less VP40 protein, while VLPs formed by MVA-GP-VP40 Zaire contain more VP40 and less GP protein (Fig. 6A and B).
FIG 6.
Filamentous structures of Zaire and Sudan VLPs produced in MVA-EBOV/SUDV-infected cells. Subconfluent HeLa cells cultured on glass coverslips were mock infected or infected at 50 PFU/cell with MVA-GP-2A-VP40 Zaire (A), MVA-GP-2A-VP40 Sudan (A), and MVA-GP-VP40 Zaire (B), fixed at 18 hpi with 3% PFA, quenched in the presence of NH4Cl, permeabilized, blocked with saponin-FCS, and labeled with antibodies against the GP Zaire and GP Sudan proteins (A) or against the VP40 Zaire protein (B). Then, cells were treated with secondary antibodies conjugated with the fluorochrome Alexa Fluor 488 (green), the probe phalloidin (to stain F-actin, shown in blue), and DAPI (to mark DNA, shown in gray). Coverslips were mounted on glass slides, conserved in ProLong Gold antifade reagent, and visualized by confocal microscopy. Detection of GP or VP40 proteins (in green) around the cellular membrane is shown in the merge pictures of the first column, and extracellular detection of GP or VP40 proteins is shown in the second column. Zaire and Sudan VLPs are indicated with white arrows. Bars, 5 μm. In panel B (bottom), a superresolution image taken using stimulated emission depletion (STED) microscopy is shown. The diameters of the filamentous VLPs are indicated.
MVA-GP-VP40 Zaire expressing GP and VP40 forms VLPs, as confirmed by electron microscopy.
To further characterize the VLPs formed by the recombinant MVA-EBOV/SUDVs expressing GP and VP40, HeLa cells were infected with MVA-GP-2A-VP40 Zaire, MVA-GP-2A-VP40 Sudan, MVA-GP-VP40 Zaire, and MVA-WT, and the supernatants were partially purified by a sucrose cushion. We detected by Western blotting the expression of GP in supernatants obtained from the MVA-EBOV/SUDVs tested, while VP40 was detected only in supernatants derived from MVA-GP-VP40 Zaire and MVA-GP-2A-VP40 Sudan and not in supernatants derived from MVA-GP-2A-VP40 Zaire (Fig. 7A). To confirm the VLP nature, supernatants obtained from MVA-GP-VP40 Zaire were analyzed by immunoelectron microscopy using gold-labeled antibodies against GP. The results showed that MVA-GP-VP40 Zaire formed VLPs of a filamentous morphology that resembled those of EBOV (Fig. 7B to G), while VLPs were not detected in supernatants derived from MVA-WT (Fig. 7H). However, we could not detect VLPs in supernatants derived from MVA-GP-2A-VP40 recombinants, suggesting very low levels of VLP production by the former MVA vector (data not shown).
FIG 7.
Identification by electron microscopy of Zaire and Sudan VLPs in purified supernatants of MVA-EBOV/SUDV-infected cells. (A) Expression of GP and VP40 proteins in purified supernatants of MVA-EBOV/SUDV-infected cells. HeLa cells were infected at 10 PFU/cell with MVA-GP-2A-VP40 Zaire, MVA-GP-2A-VP40 Sudan, MVA-GP-VP40 Zaire, or MVA-WT. At 24 hpi, supernatants were collected and purified by ultracentrifugation through a 20% sucrose cushion, and pellets were resuspended in Laemmli buffer, fractionated by 10% SDS-PAGE, and analyzed by Western blotting using antibodies against the GP and VP40 proteins. Arrows on the right indicate the positions of the GP and VP40 proteins. The sizes of standards (in kilodaltons) are indicated on the left. (B to H) Detection by electron microscopy of EBOV VLPs in purified supernatants of MVA-GP-VP40 Zaire-infected cells. HeLa cells were infected at 10 PFU/cell with MVA-GP-VP40 Zaire (B to G) or MVA-WT (H). At 24 hpi, supernatants were collected, purified by ultracentrifugation through a 20% sucrose cushion, stabilized with PFA, dialyzed, adsorbed to nickel grids, and treated with a rabbit antibody against GP Zaire. Then, the grids were treated with secondary antibodies conjugated with gold beads and stained with 2% uranyl acetate, and the VLPs were visualized by transmission electron microscope. Bars, 0.2 μm (B and C), 100 nm (D, E, and H), and 50 nm (F and G).
MVA-EBOV/SUDVs trigger an innate immune response in THP-1 cells.
Type I interferon (IFN) innate immune responses play a critical role in controlling ebolavirus replication (41). Moreover, deletion of the VACV C6, K7, and A46 proteins from MVA-based vaccine candidates enhanced the beta interferon (IFN-β) responses (42). Thus, to study whether the presence of the GP and VP40 Zaire or Sudan genes in the MVA genome impairs the response of innate immune cells to MVA infection, we analyzed by real-time PCR the levels of expression of type I IFN (IFN-β), proinflammatory cytokines (tumor necrosis factor alpha [TNF-α] and interleukin-6 [IL-6]), chemokines (macrophage inflammatory protein 1α [MIP-1α] and RANTES), IFN-inducible genes (IFIT1 and IFIT2), and a key cytosolic sensor that leads to antiviral IFN production (MDA-5) in human THP-1 macrophages mock infected or infected for 6 h with 5 PFU/cell of MVA-WT, MVA-GFP, and the different MVA-EBOV/SUDVs (Fig. 8). The results showed that MVA-EBOV/SUDVs significantly upregulated the mRNA levels of most of these genes in comparison to MVA-WT but not in comparison to parental MVA-GFP, which contains deletions of the VACV C6, K7, and A46 proteins (Fig. 8). MVA-EBOV/SUDVs expressing GP and VP40 proteins significantly upregulated the mRNA levels of most of these genes in comparison to MVA-EBOV/SUDVs expressing only the GP protein. In particular, MVA-GP-2A-VP40 Zaire or MVA-GP-VP40 Zaire significantly upregulated IFN-β, TNF-α, MDA-5, IFIT1, IFIT2, IL-6, and RANTES mRNA levels in comparison to MVA-GP Zaire, with MVA-GP-VP40 Zaire being the inducer of higher levels of IFN-β, IL-6, and RANTES than the other MVA-EBOV/SUDVs. Furthermore, MVA-GP-2A-VP40 Sudan significantly upregulated the IFN-β, TNF-α, MIP-1α, IL-6, and RANTES mRNA levels in comparison to MVA-GP Sudan, with MVA-GP-2A-VP40 Sudan being the inducer of higher levels of MIP-1α than the other MVA-EBOV/SUDVs. In conclusion, MVA-EBOV/SUDVs, particularly those expressing GP and VP40 proteins, promote a robust innate immune response in human THP-1 macrophages by inducing the expression of IFN-β, IFN-inducible genes, proinflammatory cytokines, and chemokines.
FIG 8.
Innate immune responses triggered by MVA-EBOV/SUDVs in human macrophages. Human THP-1 macrophages were mock infected or infected with MVA-WT, parental MVA-GFP, and MVA-EBOV/SUDVs at 5 PFU/cell. At 6 hpi, RNA was extracted and the mRNA levels of IFN-β, TNF-α, MIP-1α, MDA-5, IFIT1, IFIT2, IL-6, RANTES, and HPRT were analyzed by quantitative reverse transcription-PCR. Results are expressed as the ratio of the level of the gene of interest to the HPRT mRNA level. A.U., arbitrary units. Data are means ± standard deviations for duplicate samples from one experiment and are representative of two independent experiments. P values indicate significantly higher responses between different groups (*, P < 0.05; **, P < 0.005; ***, P < 0.001; ****, P < 0.0001).
MVA-EBOVs elicit the recruitment of immune cells to the peritoneal cavity of infected mice.
In order to determine whether the GP and VP40 proteins expressed from the MVA vectors differentially impact the innate immune responses in vivo, we intraperitoneally (i.p.) inoculated BALB/c mice with MVA-EBOVs, MVA-GFP, or phosphate-buffered saline (PBS), and at 12 h postinoculation, peritoneal exudate cells were collected and the presence of different immune cells was analyzed by flow cytometry (Fig. 9). The results showed that mice injected with MVA-GP-2A-VP40 Zaire exhibited a significant increase in the absolute numbers of neutrophils, natural killer (NK) cells, NK T (NKT) cells, and dendritic cells compared to those that received MVA-GP Zaire, MVA-GP-VP40 Zaire, or the parental MVA-GFP (Fig. 9). Furthermore, MVA-GP Zaire recruited significantly more NK cells and NKT cells and induced a trend toward a higher number of neutrophils and dendritic cells than MVA-GP-VP40 Zaire. In addition, there were no significant differences between the different recombinant viruses in the total numbers of B cells, CD4 T cells, and CD8 T cells, except for MVA-GP-2A-VP40 Zaire, which recruited significantly more CD4 T cells than MVA-GP Zaire and more CD8 T cells than MVA-GP-VP40 Zaire. Moreover, no macrophages were detected in mice injected with MVA-EBOVs and parental MVA-GFP, indicating that macrophage migration may occur at early times postinoculation. In conclusion, MVA-EBOVs, in particular, MVA-GP-2A-VP40 Zaire, promotes a robust innate immune response in BALB/c mice characterized by strong immune cell migration to the peritoneal cavity.
FIG 9.
Recruitment of immune cells in the peritoneal cavity of mice inoculated with MVA-EBOVs expressing GP and VP40. Groups of BALB/c mice (n = 4) were inoculated i.p. with 107 PFU of parental MVA-GFP, MVA-GP Zaire, MVA-GP-2A-VP40 Zaire, MVA-GP-VP40 Zaire, or PBS. At 12 h postinoculation, peritoneal exudate cells were collected and the presence of different immune cells was analyzed by flow cytometry. The absolute numbers of neutrophils, macrophages, NK cells, NK T cells, dendritic cells, B cells, CD4 T cells, and CD8 T cells are shown. Graphs show the mean ± SEM, with each point representing an individual mouse. P values indicate significantly higher responses between different groups (*, P < 0.05; **, P < 0.005; ***, P < 0.001; ****, P < 0.0001).
MVA-EBOV/SUDVs induce differential levels of antibodies against GP Zaire and Sudan.
Antibodies against GP protein are crucial to control ebolavirus infection (8, 43–46). Therefore, to study the ability of MVA-EBOV/SUDVs to induce humoral immune responses against GP proteins, we analyzed by enzyme-linked immunosorbent assay (ELISA) at 21 days postboost the total IgG levels (Fig. 10A) and subclass IgG1, IgG2a, and IgG3 levels (Fig. 10B) of GP Zaire- and Sudan-specific antibodies present in the sera of BALB/c mice immunized with two doses each of MVA-EBOV/SUDV (MVA-GP Zaire, MVA-GP Sudan, MVA-GP Zaire + Sudan, MVA-GP-2A-VP40 Zaire, MVA-GP-2A-VP40 Sudan, MVA-GP-2A-VP40 Zaire + Sudan, MVA-GP-VP40 Zaire) (see Materials and Methods). Animals immunized with two doses of MVA-WT were used as a control group. The results showed that immunization with MVA-GP-2A-VP40 Zaire or MVA-GP-2A-VP40 Zaire + Sudan elicited significantly higher titers of total IgG antibodies against the GP Zaire protein than immunization with the other MVA-EBOV/SUDVs and that immunization with MVA-GP-2A-VP40 Sudan or MVA-GP-2A-VP40 Zaire + Sudan induced higher titers of total IgG antibodies against the GP Sudan protein than immunization with the other MVA-EBOV/SUDVs (Fig. 10A), with the immunization group MVA-GP-2A-VP40 Zaire + Sudan being able to induce antibodies against both GP Zaire and Sudan. Furthermore, immunization with MVA-GP Zaire or MVA-GP-2A-VP40 Zaire elicited significantly higher titers of antibodies against GP Zaire than immunization with MVA-GP-VP40 Zaire.
FIG 10.
Humoral immune response against GP Zaire and GP Sudan induced by MVA-EBOV/SUDVs. Groups of BALB/c mice (n = 5) were immunized intramuscularly with two doses of MVA-GP Zaire, MVA-GP Sudan, MVA-GP Zaire + Sudan, MVA-GP-2A-VP40 Zaire, MVA-GP-2A-VP40 Sudan, MVA-GP-2A-VP40 Zaire + Sudan, MVA-GP-VP40 Zaire, or MVA-WT at weeks 0 and 4. Three weeks after the last immunization serum samples were collected and the titers of total IgG antibody against GP Zaire and GP Sudan proteins (A) or IgG1, IgG2a, and IgG3 isotype antibodies against GP Zaire and GP Sudan proteins (B) in serum samples obtained from each individual mouse (A) or in pooled sera from each immunization group (B) were analyzed by ELISA. Data are from one experiment and are representative of two independent experiments. Antibody titers were calculated as the serum dilution that gave an absorbance at least 3 times higher than that for a naive serum sample. In panel A, the graphs show the mean ± SEM, with each point representing an individual mouse. The detection limit was 40. In panel B, bars represent the mean ± SEM for duplicate samples from pooled sera. P values indicate significantly higher responses between different groups (*, P < 0.05; **, P < 0.005; ***, P < 0.001; ****, P < 0.0001).
Regarding the IgG subclasses of antibodies, MVA-EBOV/SUDVs elicited higher levels of IgG1 antibodies than IgG2 and IgG3 antibodies against GP Zaire and GP Sudan (Fig. 10B). Again, immunization with MVA-GP-2A-VP40 Zaire and MVA-GP-2A-VP40 Zaire + Sudan induced higher IgG1, IgG2a, and IgG3 antibody levels against GP Zaire than the other groups, and immunization with MVA-GP-2A-VP40 Sudan and MVA-GP-2A-VP40 Zaire + Sudan also triggered higher IgG1, IgG2a, and IgG3 antibody levels against GP Sudan than the other groups. The IgG2a/IgG1 and IgG3/IgG1 ratios were below 1 in all the immunization groups.
MVA-EBOV/SUDVs induce high titers of antibodies against VP40 Zaire and Sudan.
To study the ability of MVA-EBOV/SUDVs to elicit humoral immune responses against VP40 proteins, we analyzed by ELISA at 21 days postboost the total IgG levels of VP40 Zaire- and Sudan-specific antibodies present in the sera of BALB/c mice immunized with two doses of each MVA-EBOV/SUDV (see Materials and Methods) (Fig. 11). The results showed that MVA-EBOVs expressing GP and VP40 proteins elicited higher titers of antibodies against VP40 Zaire than MVA-GP-2A-VP40 Sudan, with MVA-GP-VP40 Zaire being the virus inducing higher titers of VP40 Zaire-specific antibodies (Fig. 11, left). Moreover, MVA-EBOVs expressing GP and VP40 were able to induce cross-binding antibodies against the VP40 Sudan protein (Fig. 11, right). Furthermore, immunization with MVA-GP-2A-VP40 Zaire + MVA-GP-2A-VP40 Sudan also induced antibodies to VP40 that were cross-reactive between Zaire and Sudan. In conclusion, MVA-EBOV/SUDVs elicited a robust humoral immune response by inducing specific antibodies against GP and VP40 proteins from Zaire or Sudan ebolavirus species.
FIG 11.
Humoral immune response against VP40 Zaire and VP40 Sudan induced by MVA-EBOV/SUDVs. Groups of BALB/c mice (n = 5) were immunized intramuscularly with two doses of MVA-EBOV/SUDVs, following the protocol described in Materials and Methods. The titers of total IgG antibody against VP40 Zaire and VP40 Sudan proteins are indicated. Data are from one experiment and are representative of two independent experiments. Antibody titers were calculated as the serum dilution that gave an absorbance at least 3 times higher than that for a naive serum sample. Graphs show the mean ± SEM, with each point representing an individual mouse. The detection limit was 40. P values indicate significantly higher responses between different groups (***, P < 0.001; ****, P < 0.0001).
MVA-EBOVs induced protection in a mouse challenge model.
To test the capacity of recombinant MVA-EBOVs to confer protection against an exposure of EBOV, bone marrow chimeric WT → IFNAR−/− C57BL/6 mice, which reproduce important features of human EVD (47), were immunized with an i.p. dose of MVA-GP Zaire, MVA-GP-2A-VP40 Zaire, MVA-GP-VP40 Zaire, or MVA-WT, and 4 weeks later, the mice were challenged i.p. with a lethal dose of EBOV (the Mayinga variant). The results showed that immunization with one single dose of MVA-GP-VP40 Zaire conferred 80% protection against EBOV, while immunization with MVA-GP-2A-VP40 Zaire conferred about 50% protection (Fig. 12). However, immunization with MVA-GP Zaire conferred only 20% protection, similarly to parental MVA-WT. In conclusion, a single dose of MVA-EBOVs expressing GP and VP40 protected up to 80% of chimeric mice after challenge with live pathogenic EBOV with statistically significant differences compared to the other groups.
FIG 12.
Efficacy of MVA-EBOVs expressing GP and VP40 proteins in a mouse challenge model. Groups of bone marrow chimeric WT → IFNAR−/− C57BL/6 mice (n = 5) were immunized i.p. with one dose of MVA-GP Zaire, MVA-GP-2A-VP40 Zaire, MVA-GP-VP40 Zaire, or MVA-WT and challenged i.p. 4 weeks later with a lethal dose of EBOV (1,000 PFU), following the protocol described in Materials and Methods. After challenge, mice were monitored daily for signs of disease and body weight for 11 days, and those mice with a weight loss higher than 20% of the initial weight were euthanized. The percent survival is represented. P values indicate significantly higher responses when the group infected with MVA-GP-VP40 Zaire was compared with the other groups (*, P = 0.0055).
DISCUSSION
EBOV and SUDV are important emerging viruses that cause a severe disease in humans. There have been several outbreaks in Africa during the last few decades, but the outbreak from 2013 to 2016 was the most severe, producing more cases and deaths than all previous epidemics together (4, 6). Thus, finding and developing effective, safe, stable, inexpensive, and easy-to-administer vaccines that can protect the global community against future epidemics are necessary (4). One of the most promising vaccine vectors is the poxvirus MVA, which has been used as a vaccine candidate against several infectious diseases (34–36). Here, we generated and characterized the immunogenicity and efficacy of novel MVA-based vaccine candidates against EBOV and SUDV (termed MVA-EBOV/SUDVs MVA-GP Zaire, MVA-GP Sudan, MVA-GP-2A-VP40 Zaire, MVA-GP-2A-VP40 Sudan, and MVA-GP-VP40 Zaire) expressing GP or GP together with VP40 of Zaire and Sudan ebolavirus species.
The immunogens inserted in the MVA-EBOV/SUDVs included the edited human codon-optimized GP gene and the human codon-optimized VP40 gene, both from EBOV and SUDV. The edited GP protein allows the expression of the full-length GP protein and avoids the expression of the secreted GP protein, as previously reported for other EBOV vaccine candidates (48). Furthermore, the use of human codon-optimized VP40 proteins has been recently reported for other EBOV vaccines (49). As the sole membrane protein encoded by the ebolavirus species, the GP plays a critical role in the entry and pathogenicity of EBOV and SUDV (50, 51) and is a highly immunogenic protein containing both T- and B-cell epitopes able to activate T- and B-cell ebolavirus-specific immune responses that are associated with survival or asymptomatic infection (52, 53). Moreover, other viral vector-based EBOV vaccines expressing GP have demonstrated effective protection against lethal challenge with EBOV in animal models (mice, guinea pigs, and NHPs), such as recombinant human adenovirus serotype 5-vector vaccines (rAd5-GP) (8, 48, 54), chimpanzee adenovirus serotype type 3-vector vaccines (ChAd3-EBO-Z) (23, 37, 44, 46), recombinant vesicular stomatitis virus-vector vaccines (rVSV-GP) (27, 55–57), rabies virus-vector vaccines (RVΔG-GP) (43, 58), virus-like particles (59), and other vectors (4, 60). On the other hand, the major matrix protein VP40 is responsible for the assembly, budding, and release of virion particles and triggers budding of filamentous particles that incorporate other proteins, such as GP, in a more native structure (10, 11). Viral vector-based EBOV vaccines expressing VP40, such as Venezuelan equine encephalitis virus replicons (61, 62), and VLPs (63) have been reported to confer effective protection against lethal challenge with EBOV in animal models.
In an effort to further optimize the MVA-EBOV vaccine candidates and to define to what extent the immunogenicity of the vectors can be modulated to improve efficacy, we have used as the parental virus an MVA that contains deletions in 3 VACV immunomodulatory genes (C6L, K7R, and A46R), previously reported to enhance the immunogenic capacity of the MVA-based vaccine candidates (42). Hence, to define the best-in-class vector, in this investigation we compared head-to-head the immunogenicity profile and efficacy of MVA vectors expressing different levels of GP and VP40 antigens from EBOV and SUDV. The successful generation of the different MVA-EBOV/SUDV vaccine candidates was tested by gene sequence analysis (PCR and DNA sequencing) and protein expression analysis (Western blotting) (Fig. 1). The expression patterns obtained for the GP and VP40 proteins were similar to those observed with other ebolavirus vaccine candidates (8, 49, 64). GP Zaire is expressed as a single protein product of about 150 kDa, whereas GP Sudan is expressed as different-size proteins with molecular masses of between 75 and 150 kDa, which could correspond to different proteolytic products of the full-length GP protein. Moreover, MVA-GP-2A-VP40 recombinants also expressed a GP product larger than 150 kDa that corresponded to a fusion protein of GP and VP40 (Fig. 1 to 3). It has been reported that foot-and-mouth disease virus (FMDV) 2A peptides have different efficiencies of peptide bond skipping that depend on the experimental context, with the cleavage efficiency ranging from 40% to 90% (65–67). Furthermore, VP40 proteins are also expressed as several different proteins with molecular masses of between 25 and 50 kDa, which could correspond to different proteolytic products of the full-length VP40 protein. Interestingly, the GP and VP40 expression levels analyzed by Western blotting (Fig. 1C and 2A and B) and flow cytometry (Fig. 2C) varied between the different MVA-EBOV/SUDVs, with MVA-GP-2A-VP40 recombinants expressing higher levels of GP than MVA-GP recombinants and MVA-GP-VP40 Zaire. These differences could be attributable to the distinct nature of the immunogens or to the distinct insertion of VP40 Zaire in another VACV locus. Thus, while MVA-GP-2A-VP40 Zaire contains the GP-2A-VP40 sequence in the VACV TK locus and GP and VP40 are expressed at an estimated ratio of 1:1, MVA-GP-VP40 Zaire contains the GP in the VACV TK locus and the VP40 in the VACV HA locus, not expressing the same quantity of GP and VP40 proteins. In fact, while the ebolavirus genes are under the control of the same VACV early/late promoter, insertion of VP40 Zaire in the HA locus of MVA-GP Zaire seems to decrease GP expression, and this may be due to a translational competition of both GP and VP40 mRNAs, the stability of the proteins, or higher expression of VP40 in the HA locus versus the TK locus. Moreover, a higher GP expression in the membrane of cells infected with MVA-GP-2A-VP40 recombinants could be also associated with a lower secretion of GP according to GP quantification in the supernatants of MVA-EBOV/SUDV-infected cells (data not shown); unprocessed GP-2A-VP40 protein is likely not secreted.
Robust replication in appropriate cell cultures is critical for the development of cost-effective vaccines. Thus, we showed that recombinant MVA-EBOV/SUDV vaccine candidates replicate at the same level as the parental virus MVA-GFP in DF-1 cells (Fig. 2D), like other vaccines reported previously (58, 64). Moreover, the different MVA-EBOV/SUDVs are stable and expressed GP and VP40 proteins (Fig. 3A); 20 viral plaques picked from each recombinant MVA-EBOV/SUDV at passage 5 correctly expressed GP and VP40 (data not shown).
Since it has been reported that filovirus GP proteins expressed in the cell surface reduced Fas-mediated apoptotic signals by the steric shielding effect of the mucin-like region of the GP protein (40), we analyzed the apoptosis effect of the recombinant MVA-EBOV/SUDVs. We did not find apoptotic differences between the recombinant MVA-EBOV/SUDVs and parental MVA-GFP or MVA-WT, suggesting that there is a basal apoptotic effect mediated by MVA and that the GP contribution to that apoptotic effect is rather low (Fig. 3B).
To further assess the cellular distribution of GP and VP40 Zaire or Sudan proteins from recombinant MVA-EBOV/SUDVs, their expression was analyzed by confocal immunofluorescence microscopy (Fig. 4 to 6). All recombinant MVA-EBOV/SUDVs expressed the GP and VP40 proteins in the cytoplasm and also associated with the cell membrane. GP and VP40 proteins colocalize with the cytoskeleton, and the cellular actin could interact with VP40, playing a functional role in the budding of VLPs (68). The expression pattern of GP differs from that of the distinct MVA-EBOV/SUDVs, forming pleomorphic structures when expressed alone or filamentous structures that resemble VLPs when expressed together with VP40. These results are in accordance with previous reports where different GP morphologies were analyzed by electron microscopy (13). When cells were infected at a high MOI (50 PFU/cell) with MVA-EBOV/SUDVs expressing GP together with VP40, we detected protrusions at the cell membrane that resulted from budding of VP40 together with GP, forming VLPs (Fig. 6). Differences in the expression levels of the GP and VP40 proteins from the MVA-GP-2A-VP40 and MVA-GP-VP40 recombinants may influence the budded VLP levels, length, morphology, and protein composition. In fact, we found that the higher that the expression levels of VP40 were, the higher that the proportion of VP40 in budded VLPs with higher levels and lengths was, while a greater proportion of GP in the VLPs was correlated with a higher GP expression level. By electron microscopy of purified supernatants from HeLa cells infected with MVA-GP-VP40 Zaire (Fig. 7), we observed the presence of filamentous structures with a size and morphology similar to those of the EBOV particles, confirmed by immune gold staining with an antibody against GP. Surprisingly, we could not observed VLPs in purified supernatants from cells infected with MVA-GP-2A-VP40 recombinants (data not shown), probably due to the low levels of VLPs produced from these vectors. An MVA generating VLPs has been described recently, showing some differences in the VLP morphology attributable to the expression of NP together with GP and VP40 proteins (49). Thus, our MVA-EBOV/SUDV vectors coexpressing GP and VP40 produced VLPs of different sizes and distinct GP/VP40 ratios and could act as potent immunogens.
Because type I IFN plays a critical role in controlling EBOV replication (41) and the VACV C6, K7, and A46 proteins (deleted in the MVA-EBOV/SUDV recombinants) inhibited the IFN-β responses (42), we analyzed in infected human macrophages the mRNA transcript levels of IFN-β and IFN-β-related genes, as well as those of the genes for other cytokines and chemokines, such as TNF-α, IL-6, MIP-1α, and RANTES. The results showed that the mRNA levels of those genes induced by MVA-EBOV/SUDVs and parental MVA-GFP were significantly higher than the levels induced by MVA-WT, mainly attributed to the deletion of VACV immunomodulatory genes C6L, K7R, and A46R (Fig. 8). It has been reported that ebolavirus gene products, such as VP24 and VP35, inhibit IFN-β responses (62), but in our case, the combinations of GP plus VP40 proteins did not inhibit these responses. In fact, MVA recombinants expressing GP and VP40 proteins triggered higher innate immune responses than MVA recombinants expressing only GP, probably due to the higher expression of GP or to the formation of VLPs. It has been described that ebolavirus VLPs are able to stimulate early innate immune responses through Toll-like receptor and type I IFN signaling pathways to protect the host from EBOV infection (69). Analyzing the immune cell populations recruited in the peritoneal cavity of infected mice, we observed that MVA-GP-2A-VP40 Zaire significantly triggered a higher absolute number of neutrophils and NK, NKT, and dendritic cells than the other MVA-EBOV recombinants (Fig. 9); this effect could be mediated by the higher expression levels of GP induced by this recombinant than by MVA-GP Zaire and MVA-GP-VP40 Zaire. In fact, it has been described that VLPs formed by coexpression of GP and VP40 enhanced the number of NK cells (16, 70) and promoted the maturation and activation of bone marrow-derived dendritic cells (15, 71), but there are no studies related to the recruitment of neutrophils in ebolavirus infections, although it has been described that neutrophils may play a prominent role in the immune and inflammatory responses to filovirus infections (72).
Most of the experimental evidence in animal models suggests that the protection and survival of immunized animals against EBOV are largely dependent on induction of antibody responses against EBOV GP and particularly associated with the presence of IgG anti-GP antibodies in the serum of vaccinated macaques (8, 43–46, 73–75). Evaluation of humoral immune responses against GP in mice vaccinated with the MVA-EBOVs or MVA-SUDVs showed that these vectors induced high titers of binding IgG antibody against GP Zaire and Sudan, respectively, being also able to induce cross-binding antibodies against GP Sudan and Zaire, respectively (Fig. 10A). Interestingly, higher levels of total anti-GP antibodies were observed in mice immunized with MVA-GP-2A-VP40 recombinants than in mice immunized with the other MVA-GP recombinants. This finding may correlate with the greater expression of GP from the MVA-GP-2A-VP40 recombinants. Our results are different from those of other studies with MVAs expressing only GP and MVAs coexpressing GP and VP40 proteins, in which no differences in the specific antibodies elicited against the GP protein were observed between the two vectors (49). The differences could be due to the nature of viral promoters and the site of insertion of the ebolavirus genes, clearly distinct from ours. Surprisingly, immunization with MVA-GP-VP40 Zaire induced low levels of antibodies against GP Zaire, which could have been due to the low GP expression observed during cell infections (Fig. 1 to 3).
It has been described that antibody-dependent cell-mediated cytotoxicity (ADCC) might play a major role in protection against EBOV infection (45). While in NHPs and humans ADCC depends on IgG1 antibody responses, in mice ADCC depends on IgG2a antibodies, which are critical in protecting mice from EBOV challenge (76). A recent study showed that an MVA expressing GP and VP40 proteins elicited more IgG2 than IgG1 antibodies, but the correlation with protection was not determined (49). In our study, MVA-EBOV/SUDVs elicited IgG2/IgG1 anti-GP antibody ratios of between 0.5 and 1, indicating an induction of more IgG1 antibodies, although their role in protection is unknown. These results are in accordance with those of experiments describing that protected NHPs immunized with a rabies virus EBOV vaccine had an IgG2/IgG1 ratio of less than 1 (43). These discrepancies in the IgG2/IgG1 ratios could be due to the intrinsic properties of the animal models used or to the characteristics of the vector itself. Moreover, in terms of the significance of antibodies against VP40, previous studies showed immunogenicity in rabbits immunized with a purified VP40 protein (77), but efficacy was not studied. We showed that all MVA-EBOV/SUDVs expressing GP and VP40 proteins elicited high titers of antibodies against VP40, and particularly, of all vectors, MVA-GP-VP40 Zaire induced the highest titers of antibodies against the VP40 Zaire and VP40 Sudan proteins (Fig. 11). Our results suggest that GP and VP40 expression levels are directly related to the titers of antibodies against these proteins.
An important consideration of the MVA-EBOV/SUDVs described here was to test their protective efficacy in an animal model. This was performed in a biosafety level 4 (BSL-4) laboratory using chimeric mice susceptible to EBOV infection that had previously been vaccinated with the MVA-EBOVs. The results showed that MVA-GP-VP40 Zaire conferred a high level of protection (80%) against EBOV challenge in mice receiving a single dose; a single dose of MVA-GP-2A-VP40 induced 50% protection, while MVA-GP Zaire was poorly protective against EBOV (Fig. 12). As protection in this human chimeric mouse model could be related to T-cell responses (47), the protection observed in mice immunized with MVA-GP-VP40 Zaire might be explained, in part, by the fact that VLPs containing GP and VP40 proteins displayed more active CD8+ T-cell epitopes. More studies to further understand the role of GP and VP40 humoral and cellular immune responses against EBOV and correlates of protection are needed.
Overall, in the present work we generated 5 MVA-based vaccine candidates against EBOV and SUDV expressing GP and/or VP40 antigens at different levels. Some of them produced VLPs, triggered innate immune responses, and in immunized mice elicited high levels of IgG antibodies against GP and/or VP40 proteins. Interestingly, efficacy of up to 80% protection was observed when chimeric mice vaccinated with a single dose of the vector MVA-GP-VP40 Zaire were challenged with EBOV. These results support the consideration of MVA-EBOV/SUDVs expressing GP and VP40 and producing VLPs as potential vaccine candidates against EBOV and SUDV.
MATERIALS AND METHODS
Ethics statement.
The immunogenicity animal studies were approved by the Ethical Committee of Animal Experimentation (CEEA) of the Centro Nacional de Biotecnología (CNB; Madrid, Spain), in accordance with national and international guidelines and with a Royal Decree (RD 53/2013) (permit number PROEX 331/14), and were conducted at the CNB in a pathogen-free barrier area. The efficacy animal study was approved by the Committee on the Ethics of Animal Experiments of the city of Hamburg, Germany (permit number 125/12), and was conducted at the biosafety level 4 (BSL-4) laboratory at the Bernhard Nocht Institute for Tropical Medicine in Hamburg, Germany. Animal experiments were done in strict accordance with the recommendations of the German Society for Laboratory Animal Science and under the supervision of a veterinarian. We minimized the number of animals used in every experiment and reduced the suffering of the animals in the experimental procedures. All staff that carried out animal experiments passed an education and training program according to category B or C of the Federation of European Laboratory Animal Science Associations.
Cells and viruses.
HeLa cells (human epithelial cervix adenocarcinoma cells; ATCC CCL-2), DF-1 cells (spontaneously immortalized CEF cells; ATCC CRL-12203), human embryonic kidney 293T (HEK293T) cells, and primary CEF cells (obtained from specific-pathogen-free 11-day-old eggs; MSD, Salamanca, Spain) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with penicillin (100 units/ml; Sigma-Aldrich), streptomycin (100 μg/ml; Sigma-Aldrich), l-glutamine (2 mM; Sigma-Aldrich), nonessential amino acids (0.1 mM; Sigma-Aldrich), gentamicin (50 μg/ml; Sigma-Aldrich), amphotericin B (Fungizone; 0.5 μg/ml; Gibco-Life Technologies), and 10% heat-inactivated fetal calf serum (FCS) (Gibco-Life Technologies). The human monocytic THP-1 cell line (ATCC TIB-202) was cultured in RPMI 1640 medium containing 2 mM l-glutamine, 50 μM 2-mercaptoethanol, 100 units/ml penicillin, 100 μg/ml streptomycin (complete medium; all from Sigma-Aldrich), and 10% FCS, as previously described (42, 78). THP-1 cells were differentiated into macrophages by treatment with 0.5 mM phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) for 24 h before usage. Cell cultures were maintained at 37°C in a humidified incubator containing 5% CO2. Cell lines were infected with the MVA recombinants following the methodology previously described (79, 80).
The poxvirus strain used in this study as the parental virus for the generation of the MVA-EBOV and MVA-SUDV recombinants is a wild-type attenuated MVA (MVA-WT) that was modified by inserting the gene encoding the green fluorescent protein (GFP) into the thymidine kinase (TK) locus and by deleting the immunomodulatory vaccinia virus (VACV) genes C6L, K7R, and A46R (the parental virus is termed MVA-GFP) (38). Then, the GFP cassette present in the TK locus of MVA-GFP was replaced by the EBOV or SUDV genes to generate the different MVA-EBOV/SUDV vaccine candidates (see below). In our studies, we also included as a control the MVA-WT. All viruses were grown in primary CEF cells, purified by centrifugation through two 36% (wt/vol) sucrose cushions in 10 mM Tris-HCl (pH 9), and titrated at least two times in DF-1 cells by a plaque immunostaining assay, as previously described (81). All viruses were free of contamination with mycoplasma (checked by a PCR specific for mycoplasma), bacteria (checked by growth in LB plates without ampicillin), or fungi (checked by growth in Columbia blood agar plates [Oxoid]).
EBOV and SUDV antigens.
The EBOV and SUDV antigens inserted in the MVA-EBOV and MVA-SUDV vaccine candidates were the GP gene from EBOV (the Mayinga variant) and SUDV (the Boniface variant), a synthetic gene construct containing GP-2A-VP40 from EBOV and SUDV, or the EBOV VP40 gene. GP and VP40 genes were codon optimized for human use, and the GP was edited (adding an additional A residue at position 885) to render the full-length GP protein and avoid the presence of the secreted GP protein. The GenBank accession numbers of the EBOV and SUDV genomes are AF086833.2 and FJ968794.1, respectively. 2A is a self-cleaving protein (18 amino acids) derived from the foot-and-mouth disease virus (FMDV) that it is inserted between the GP and VP40 genes and allows the expression of GP and VP40 antigens separately after cleavage.
Construction of EBOV and SUDV plasmid transfer vectors.
To generate the different recombinant MVA-EBOV/SUDVs, first we constructed several plasmid transfer vectors that allow the insertion by recombination of the desired ebolavirus antigens in the TK or HA locus of MVA. Thus, the EBOV and SUDV plasmid transfer vectors generated were pCyA-GP Zaire, pCyA-GP Sudan, pCyA-GP-2A-VP40 Zaire, pCyA-GP-2A-VP40 Sudan, and pHA-VP40 Zaire. The plasmid pCyA-20, used for the generation of these plasmid transfer vectors, was previously described (82) and comprises the viral synthetic early/late (sE/L) promoter, a multiple-cloning site, and the selectable marker genes for ampicillin and β-galactosidase (LacZ gene).
Plasmid transfer vectors pCyA-GP Zaire and pCyA-GP Sudan were synthesized by GeneArt (Thermo Fisher Scientific) and contain the edited human codon-optimized GP Zaire or Sudan genes that were introduced between the VACV TK flanking regions. These plasmid transfer vectors were used for the generation of MVA-GP Zaire and MVA-GP Sudan, in which the GP Zaire or GP Sudan genes, respectively, instead of the GFP gene were inserted into the VACV TK locus of the parental MVA-GFP.
Plasmid transfer vectors pCyA-GP-2A-VP40 Zaire and pCyA-GP-2A-VP40 Sudan were constructed and used for the generation of MVA-GP-2A-VP40 Zaire and MVA-GP-2A-VP40 Sudan, respectively, in which the GP-2A-VP40 sequences from Zaire or Sudan ebolavirus species, respectively, instead of the GFP gene were inserted into the VACV TK locus of the parental MVA-GFP. In detail, GP-2A-VP40 Zaire and Sudan sequences were amplified from the pSFV-DREP GP-2A-VP40 Zaire and pSFV-DREP GP-2A-VP40 Sudan plasmids (P. Öhlund, J. García-Arriaza, E. Zusinaite, I. Szurgot, A. Männik, A. Kraus, M. Ustav, A. Merits, M. Esteban, P. Liljeström, and K. Ljungberg, submitted for publication), using oligonucleotides GP-Zaire-BglII (5′-GCAGATCTGCCACCATGGGAGTGACAGGCATCC-3′) (the BglII site is underlined) and VP40-Zaire-NotI (3′-GGGCGGCCGCTTATTTCTCGATCACGGCGGGC-5′) (the NotI site is underlined) to amplify GP-2A-VP40 Zaire and oligonucleotides GP-Sudan-BglII (5′-GCAGATCTGCCACCATGGAAGGCCTGTCTCTGC-3′) (the BglII site is underlined) and VP40-Sudan-NotI (3′-GGGCGGCCGCTCATTTCTCGCTCAGGTAGCTAC-5′) (the NotI site is underlined) to amplify GP-2A-VP40 Sudan (all oligonucleotides were from Sigma-Aldrich). The amplification reactions were performed with Platinum Pfx DNA polymerase (Invitrogen) according to the manufacturer's recommendations. Then, GP-2A-VP40 Zaire and GP-2A-VP40 Sudan were digested with BglII and NotI and cloned into plasmid pCyA-20, which had previously been digested with the same restriction enzymes, to generate pCyA-GP-2A-VP40 Zaire (10,578 bp) and pCyA-GP-2A-VP40 Sudan (10,578 bp), respectively.
Additionally, we generated a novel plasmid transfer vector (termed pHA) to allow the insertion of antigens in the HA locus of MVA. This plasmid was generated by inserting the left and right HA-flanking regions of MVA in the previously described plasmid pCAR-2 (83), using the Gibson assembly technology (New England BioLabs). Thus, pHA comprises the viral sE/L promoter, a multiple-cloning site, the HA-flanking regions of MVA, and the selectable marker genes for ampicillin and β-glucuronidase (the β-Gus gene). Next, we cloned the Zaire VP40 gene in pHA between the HA-flanking regions to generate the plasmid transfer vector pHA-VP40 Zaire. A human codon-optimized VP40 Zaire gene synthetized by GeneArt (Thermo Fisher Scientific) was digested with BamHI and NotI and cloned into plasmid pHA, which had previously been digested with the same restriction enzymes, to generate pHA-VP40 Zaire (7,614 bp).
The correct generation of the different plasmid transfer vectors was further confirmed by DNA sequence analysis. The plasmid transfer vectors were used in transient-infection/transfection assays for the insertion of the EBOV and SUDV GP or GP-2A-VP40 genes into the TK locus of the MVA genome or the EBOV VP40 gene into the HA locus of the MVA genome, all under the transcriptional control of the viral sE/L promoter. All plasmids contain a β-galactosidase (β-Gal) or β-glucuronidase (β-Gus) reporter gene sequence between two repetitions of the TK left-flanking arm or HA left-flanking arm, respectively, which allows the reporter gene to be deleted from the final recombinant virus by homologous recombination.
Generation of recombinant MVA-EBOV/SUDVs.
In this study, we generated several vaccine candidates against Ebola virus, termed MVA-EBOVs (MVA-GP Zaire, MVA-GP-2A-VP40 Zaire, and MVA-GP-VP40 Zaire) and MVA-SUDVs (MVA-GP Sudan and MVA-GP-2A-VP40 Sudan). DF-1 cells (3 × 106 cells) were infected with parental MVA-GFP at a multiplicity of infection (MOI) of 0.05 PFU/cell and transfected 1 h later with 10 μg of the DNA of plasmid pCyA-GP Zaire, pCyA-GP Sudan, pCyA-GP-2A-VP40 Zaire, or pCyA-GP-2A-VP40 Sudan, using the Lipofectamine reagent according to the manufacturer's recommendations (Invitrogen). At 48 h postinfection (hpi), cells were harvested, lysed by freeze-thaw cycling, sonicated, and used for recombinant virus screening by several consecutive rounds of plaque purification in DF-1 cells following the same methodology previously described (38). The resulting recombinant viruses obtained are termed MVA-GP Zaire, MVA-GP-2A-VP40 Zaire, MVA-GP Sudan, and MVA-GP-2A-VP40 Sudan.
For the generation of MVA-GP-VP40 Zaire we used MVA-GP Zaire as the parental virus and pHA-VP40 Zaire as the DNA plasmid transfer vector. After infection/transfection of DF-1 cells, recombinant viruses containing the VP40 Zaire gene inserted in the VACV HA locus and transiently coexpressing the β-Gus marker gene were selected by three consecutive rounds of plaque purification in DF-1 cells stained with X-Gluc (5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid; 800 μg/ml). Then, we isolated recombinant MVA-GP-VP40 Zaire viruses with GP Zaire in the VACV TK locus and VP40 Zaire in the VACV HA locus and with the β-Gus gene deleted by a method similar to the previously described method of generation of the other recombinant MVA-EBOV/SUDVs. The resulting recombinant virus obtained is termed MVA-GP-VP40 Zaire.
The recombinant MVA-EBOV/SUDVs obtained were grown in CEF cells, purified through two 36% (wt/vol) sucrose cushions, and titrated by plaque immunostaining assay.
PCR analysis of recombinant MVA-EBOV/SUDVs.
The correct generation and purity of recombinant MVA-EBOV/SUDVs were confirmed by PCR with primers spanning the junction regions of the GP, VP40, or GP-2A-VP40 inserts and by DNA sequence analysis. Thus, to test the identity and purity of the recombinant MVA-EBOV/SUDVs, viral DNA was extracted from DF-1 cells mock infected or infected at 5 PFU/cell with MVA-WT, MVA-GFP, or the different MVA-EBOV/SUDV vaccine candidates, as previously described (80). Primers TK-L and TK-R (82), annealing in the TK gene-flanking regions, were used for PCR analysis of the TK locus, and primers HA-MVA (5′-TGACACGATTACCAATAC-3′) and HA-2 (5′-GATCCGCATCATCGGTGG-3′), annealing in the HA gene-flanking regions, were used for PCR analysis of the HA locus (all oligonucleotides were from Sigma-Aldrich). Furthermore, to verify that deletion of the VACV genes C6L, K7R, and A46R was correctly present, primers RFC6L-AatII-F and LFC6L-BamHI-R (previously described [78]), LFK7R-AatII-F and RFK7R-BamHI-R (previously described [42]), and LFA46R-AatII-F and RFA46R-BamHI-R (sequences will be provided upon request), spanning the VACV C6L-, K7R-, and A46R-flanking regions, respectively, were used for PCR analysis of the C6L, K7R, and A46R loci, respectively. All the amplification reactions were performed with Platinum Taq DNA polymerase (Invitrogen) according to the manufacturer's recommendations, and the amplification protocol was previously described (84). PCR products were run in a 1% agarose gel and visualized by SYBR Safe staining (Invitrogen). The EBOV and SUDV GP, VP40, and GP-2A-VP40 insertions and VACV C6L, K7R, and A46R deletions were also confirmed by DNA sequence analysis. No mutations were incorporated into the EBOV and SUDV antigens during the PCR.
Expression of EBOV and SUDV proteins from recombinant MVA-EBOV/SUDVs by Western blotting.
To check for the correct expression of the EBOV and SUDV GP, VP40, and GP-2A-VP40 antigens by the different recombinant MVA-EBOV/SUDVs, monolayers of DF-1 cells were mock infected or infected at 5 PFU/cell with MVA-WT, MVA-GFP, or the different MVA-EBOV/SUDVs. At 4, 7, and 24 hpi, supernatants were precipitated with 10% trichloroacetic acid (TCA) and centrifuged at 13,000 rpm for 15 min, and the pellets were resuspended in 1× Laemmli buffer plus β-mercaptoethanol. At those times, cells were collected by scraping and centrifuged at 3,000 rpm for 5 min, and the cellular pellets were lysed in 1× Laemmli buffer plus β-mercaptoethanol. Then, the cell extracts and supernatants were fractionated in 10% SDS-polyacrylamide gels and analyzed by Western blotting with mouse polyclonal antibodies against GP Zaire and GP Sudan (diluted 1:2,000; IBT Bioservices), mouse polyclonal antibody against VP40 Sudan (diluted 1:10,000; IBT Bioservices), or rabbit polyclonal antibody against VP40 Zaire (diluted 1:10,000; IBT Bioservices) to evaluate the expression of the EBOV and SUDV GP and VP40 proteins. As a VACV loading control, a rabbit anti-VACV E3 protein antibody (diluted 1:1,000; Centro Nacional de Biotecnología) was used. As a cell loading control, a rabbit anti-β-actin protein antibody (diluted 1:1,000; Cell Signaling) was used. Anti-mouse horseradish peroxidase (HRP)-conjugated antibody (diluted 1:2,000; Sigma-Aldrich) or anti-rabbit HRP-conjugated antibody (diluted 1:5,000; Sigma) was used as the secondary antibody. The immunocomplexes were detected using an enhanced HRP-luminol chemiluminescence system (ECL Plus; GE Healthcare).
Expression of EBOV and SUDV GP proteins from recombinant MVA-EBOV/SUDVs by flow cytometry.
Monolayers of HeLa cells were mock infected or infected at 5 PFU/cell with MVA-WT, MVA-GFP, or the different MVA-EBOV/SUDVs. At 24 hpi, cells were collected by scraping and centrifuged at 2,000 rpm for 5 min, and the cellular pellets were resuspended in phosphate-buffered saline (PBS) staining solution (1× PBS, 0.5% bovine serum albumin [BSA], 1% FCS, 0.065% sodium azide, 2 mM EDTA) and added to a 96-well plate at a rate of 200,000 cells per well. Then, Fc block (BD Pharmingen) was added for 20 min at 4°C. For the staining, mouse polyclonal antibodies against EBOV and SUDV GP proteins (diluted 1:100; IBT Bioservices) were added to the cells for 30 min at 4°C, and then anti-mouse IgG1 or IgG2a fluorescein isothiocyanate (FITC)-conjugated antibodies (diluted 1:100; eBioscience) were added to the cells as secondary antibodies for 20 min in the dark at 4°C. As controls, cells were stained with (i) unspecific mouse IgG1 or IgG2a antibodies and then anti-mouse IgG1 or IgG2a FITC-conjugated antibodies, (ii) only anti-mouse IgG1 or IgG2a FITC-conjugated antibodies, or (iii) PBS. Finally, cells were fixed with 4% paraformaldehyde (PFA) for 20 min in the dark at 4°C and were acquired using a Gallios flow cytometer (Beckman Coulter). Data were analyzed using FlowJo software (version 8.5.3; Tree Star, Ashland, OR).
Analysis of recombinant MVA-EBOV/SUDV growth.
To determine the virus growth profile of the different MVA-EBOV/SUDVs in comparison to that of parental MVA-GFP, monolayers of DF-1 cells grown in 12-well plates were infected in duplicate at 0.01 PFU/cell with MVA-GFP or with the different recombinant MVA-EBOV/SUDVs. Following virus adsorption for 1 h at 37°C, the inoculum was removed and the infected cells were washed with DMEM and incubated with fresh DMEM containing 2% FCS at 37°C in a 5% CO2 atmosphere. At different times postinfection (0, 24, 48, and 72 hpi), cells were harvested by scraping, frozen and thawed three times, and briefly sonicated. Virus titers in cell lysates were determined by a plaque immunostaining assay in DF-1 cells, as previously described (81), using rabbit polyclonal antibody anti-VACV strain WR (diluted 1:1,000; Centro Nacional de Biotecnología), followed by an anti-rabbit HRP-conjugated antibody (diluted 1:1,000; Sigma).
Genetic stability of recombinant MVA-EBOV/SUDVs.
The genetic stability of recombinant MVA-EBOV/SUDVs was analyzed as previously described (80), with some modifications. Monolayers of DF-1 cells were infected at 0.05 PFU/cell with the different recombinant MVA-EBOV/SUDVs. At 72 hpi, cells were collected by scraping, frozen and thawed three times, and briefly sonicated. Next, cellular extracts were centrifuged at 1,500 rpm for 5 min, and the supernatant was used for a new round of infection at a low MOI. The same procedure was repeated five times. Expression of the EBOV and SUDV proteins was detected by Western blotting (as described above), after infection of DF-1 cells with virus stocks from each passage.
Apoptosis analysis of recombinant MVA-EBOV/SUDVs by Western blotting.
Monolayers of HeLa cells were mock infected or infected at 5 PFU/cell with MVA-WT, MVA-GFP, or the different recombinant MVA-EBOV/SUDVs. At 18 hpi, cells were collected by scraping and centrifuged at 2,000 rpm for 5 min, and cellular pellets were lysed in 1× Laemmli buffer plus β-mercaptoethanol. Then, cellular extracts were fractionated in 10% SDS-polyacrylamide gels and analyzed by Western blotting with a mouse anti-human cleaved PARP antibody (diluted 1:500; Cell Signaling) to evaluate apoptosis levels. An anti-mouse HRP-conjugated antibody (diluted 1:2,000; Sigma) was used as the secondary antibody. The immunocomplexes were detected using an enhanced HRP-luminol chemiluminescence system (ECL Plus; GE Healthcare).
Expression of GP and VP40 proteins from recombinant MVA-EBOV/SUDVs by confocal immunofluorescence microscopy.
Immunofluorescence studies were done as previously described (82), with some modifications. Briefly, HeLa cells cultured on glass coverslips at a confluence of 50% were mock infected or infected with MVA-WT or with the different MVA-EBOV/SUDVs at an MOI of 0.5 or 50 PFU/cell. At 18 hpi, cells were washed with PBS and fixed with 3% PFA in PBS at room temperature (RT) for 15 min. Then, the cells were quenched for 15 min in the presence of 50 mM NH4Cl, permeabilized, and blocked with 0.05% saponin and 5% FCS in PBS. Mouse polyclonal antibodies against GP Zaire and GP Sudan proteins (diluted 1:2,000; IBT Bioservices) and against VP40 Sudan protein (diluted 1:10,000; IBT Bioservices) or rabbit polyclonal antibody against VP40 Zaire protein (diluted 1:10,000; IBT Bioservices) were used to stain the cells for 1 h at RT in the presence of 0.05% saponin–5% FCS in PBS. Next, the coverslips were washed with PBS and the cells were blocked again with 0.05% saponin–5% FCS in PBS for 15 min and stained for 1 h in the dark at RT with specific mouse or rabbit secondary antibodies conjugated with the fluorochrome Alexa Fluor 488 (green) or Alexa Fluor 594 (red) (Invitrogen). Coverslips were then washed with PBS, and cell nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole; Sigma) in PBS for 15 min, along with the high-affinity F-actin phalloidin probe conjugated to the near-infrared fluorescent dye Alexa Fluor 660 (Sigma). After staining, the coverslips were mounted on glass slides and conserved in ProLong Gold antifade reagent (Invitrogen). Images of cell sections were acquired using a Leica TCS SP8 microscope and were recorded and processed by the specialized software LasAF (Leica Microsystems). High-resolution images were taken using stimulated emission depletion (STED) microscopy.
Preparation of EBOV and SUDV VLPs and immunoelectron microscopy.
HeLa cells (1 × 107 cells) were infected with MVA-WT, MVA-GP-2A-VP40 Zaire, MVA-GP-2A-VP40 Sudan, or MVA-GP-VP40 Zaire at an MOI of 10 PFU/cell. Supernatants were collected at 18 hpi and centrifuged at 2,000 rpm for 5 min to eliminate suspension cells. Then, VLPs were concentrated and purified by ultracentrifugation through a 20% sucrose cushion at 25,000 rpm in a Beckmann SW28 rotor for 2 h. The pellet was thoroughly resuspended in ice-cold PBS containing 4% PFA and incubated for 30 min at 4°C. Next, VLP samples were dialyzed to eliminate sucrose and salts through a 0.025-μm-pore-size membrane filter (Merck Millipore) for 1.5 h. After dialysis, 20 μl of each sample was adsorbed to carbon-coated collodion films mounted on 400-mesh/inch nickel grids (Aname, Spain), floated two times in NH4Cl drops, washed several times with 1× PBS, and blocked with PBS containing 0.1% BSA and 5% goat serum (Sigma-Aldrich) for 10 min. Then, samples were incubated with mouse anti-GP Zaire or anti-GP Sudan primary antibodies (diluted 1:10 in PBS containing 0.1% BSA; IBT Bioservices) for 15 min, washed several times with 1× PBS, incubated with goat anti-IgG and anti-IgM secondary antibodies conjugated to 10-nm colloidal gold beads (diluted 1:40 in PBS containing 0.1% BSA and 5% goat serum; BBInternational) for 10 min, and washed several times with 1× PBS and H2O. Finally, samples were stained with 2% uranyl acetate (Aname, Spain) for 1 min and were analyzed in a transmission electron microscope (JEOL JEM-1011; Centro Nacional de Biotecnología, Spain) equipped with a ES1000W Erlangshen charge-coupled-device (CCD) camera (Gatan Inc.) at an acceleration voltage of 40 to 100 kV.
RNA analysis by quantitative real-time PCR.
THP-1 cells were mock infected or infected at 5 PFU/cell with MVA-WT, MVA-GFP, or the different MVA-EBOV/SUDVs. Total RNA was isolated at 6 hpi using an RNeasy kit (Qiagen GmbH, Hilden, Germany). Reverse transcription of at least 500 ng of RNA was performed using a QuantiTect reverse transcription kit (Qiagen GmbH, Hilden, Germany). Quantitative PCR was performed with a 7500 real-time PCR system (Applied Biosystems) using the Power SYBR green PCR master mix (Applied Biosystems), as previously described (79). The expression levels of the IFN-β, TNF-α, MIP-1α, MDA-5, IFIT1, IFIT2, IL-6, RANTES, and hypoxanthine phosphoribosyltransferase (HPRT) genes were analyzed by real-time PCR using specific oligonucleotides (the sequences will be provided upon request). Gene-specific expression was expressed relative to the expression of HPRT in arbitrary units (A.U.). All samples were tested in duplicate, and three different experiments were performed.
Recruitment of immune cells in the peritoneal cavity of BALB/c mice inoculated with recombinant MVA-EBOVs.
Groups of female BALB/c mice (6 to 8 weeks old; n = 4) were injected intraperitoneally (i.p.) with 107 PFU per mouse of MVA-GFP, MVA-GP Zaire, MVA-GP-2A-VP40 Zaire, MVA-GP-VP40 Zaire, or PBS. At 12 h postinoculation, peritoneal exudate cells were collected in 6 ml of PBS–2% FCS and the presence of different immune cells was analyzed by flow cytometry, as previously described (83). Briefly, cells were seeded in a 96-well plate and washed twice with PBS staining solution (1× PBS, 0.5% BSA, 1% FCS, 0.065% sodium azide, 2 mM EDTA). Then, Fc block (BD Pharmingen) was added to the cells for 15 min in the dark at 4°C. For staining, the following antibodies were used: CD11c-FITC (1:50; clone N418; Bioscience), Ly6G-phycoerythrin (PE; 1:100; clone 1A8; BD), CD11b-PE-Cy7 (1:100; clone M1/70; BD), F4/80-allophycocyanin (APC; 1:50; clone BM8; Bioscience), CD19-APC-Cy7 (1:100; clone 1D3; BD), major histocompatibility complex class II (MHC-II; 1:100; clone 1-A/1-E biotin; 2G9; BD) plus avidin-Pacific blue (1:100; Invitrogen), CD45-BV570 (1:100; clone 30-F11; BioLegend), CD4-PE (1:100; clone GK1.5; BD), CD8-PE-Cy7 (1:200; clone 53-6.7; BD), NKP46-APC (1:100; clone 29A1.4; BioLegend), and CD3-APC-Cy7 (1:50; clone 145-2C11; BD). Cells were acquired using a Gallios flow cytometer (Beckman Coulter), and the data were analyzed using FlowJo software (version 8.5.3; Tree Star, Ashland, OR). The different cell populations were gated as follows: dendritic cells (CD11c+, MHC-II+), neutrophils (CD11b+, Ly6G+), macrophages (F4/80 high, CD11b high), B cells (CD19+, CD11b+), CD8+ T cells (CD3+, CD8+), CD4+ T cells (CD3+, CD4+), natural killer (NK) cells (NKP46+, CD3−), and NKT cells (NKP46+, CD3+). The absolute numbers of immune cell populations for each mouse were determined by flow cytometry after extrapolation to the number of cells counted after the peritoneal washes.
Mouse immunogenicity study.
A homologous MVA prime/MVA boost immunization protocol was performed to assay the immunogenicity of MVA-EBOV/SUDV vaccine candidates. Groups of female BALB/c mice (6 to 8 weeks old; n = 5) were immunized with 1 × 107 PFU of MVA-WT, MVA-GP Zaire, MVA-GP Sudan, MVA-GP Zaire + Sudan, MVA-GP-2A-VP40 Zaire, MVA-GP-2A-VP40 Sudan, MVA-GP-2A-VP40 Zaire + Sudan, or MVA-GP-VP40 Zaire by the intramuscular (i.m.) route in 50 μl of PBS. Four weeks later, the animals received a homologous dose of 2 × 107 PFU by the i.m. route in 50 μl of PBS. At 3 weeks after the last immunization, the mice were sacrificed using carbon dioxide (CO2), and the serum of each individual mouse was collected to analyze the levels of specific humoral responses against the GP, VP40, and VACV antigens by an enzyme-linked immunosorbent assay (ELISA). Two independent experiments were performed.
Preparation of VP40 Zaire and VP40 Sudan cell extracts.
To express VP40 proteins in mammalian cell lines, we cloned VP40 Zaire and VP40 Sudan in the commercial vector pcDNA-3 (Life Technologies). Thus, human codon-optimized VP40 Zaire and VP40 Sudan genes synthesized by GeneArt (Life Technologies) were digested with BamHI and NotI and cloned into plasmid pcDNA-3, which had previously been digested with the same restriction enzymes, to generate pcDNA-VP40 Zaire (6,447 bp) and pcDNA-VP40 Sudan (6,447 bp), respectively. Next, in order to express the VP40 Zaire and VP40 Sudan proteins in mammalian cells, we performed a transient-transfection assay using calcium phosphate. Briefly, we treated HEK293T cells (7 × 105 cells/plate) at 70% confluence with 20 μl of 25 mM chloroquine in DMEM and incubated the cells at 37°C for 1 h. Meanwhile, 25 μg of plasmids pcDNA-VP40 Zaire and pcDNA-VP40 Sudan was incubated in 2× Hanks balanced salt solution buffer (50 mM HEPES, 280 mM NaCl, 1.5 mM anhydrous Na2HPO4, H2O2), 1 M CaCl2, and deuterated H2O for 20 min. Then, DNA mixes were added drop by drop to the corresponding plate and incubated for 6 h at 37°C. Next, the medium was removed, new Opti-MEM–3% FCS medium (2 ml/plate) was added, and the plates were incubated at 37°C for 72 h. After 72 h, the cells were collected by scraping and centrifuged at 1,500 rpm for 5 min, and the cellular pellets were lysed in radioimmunoprecipitation assay (RIPA) buffer (30 × 106 cells/ml) and incubated for 15 min on ice. Suspensions were centrifuged at 2,000 rpm for 10 min and then at 10,000 rpm for 10 min. Finally, we determined the total protein concentration in the supernatants by use of a bicinchoninic acid protein assay kit (Thermo Scientific) according to the manufacturer's instructions.
ELISA.
The levels of total binding IgG, IgG1, IgG2a, and IgG3 antibodies against GP Zaire, GP Sudan, VP40 Zaire, and VP40 Sudan present in single or pooled serum samples from immunized mice were measured by enzyme-linked immunosorbent assay (ELISA), as previously described (84). Ninety-six-well plates (Nunc MaxiSorp) were coated with 50 μl of purified recombinant GP Zaire or GP Sudan proteins (Icosagen) at a concentration of 2 μg/ml or with 50 μl of VP40 Zaire and VP40 Sudan cell extracts at a total protein concentration of 200 μg/ml, both in 0.05 M carbonate-bicarbonate buffer (pH 9.6) at 4°C overnight. The plates were then washed three times with 1× PBS supplemented with 0.05% Tween 20 (PBS-Tween) and blocked with 200 μl of 1× PBS containing 5% milk (blocking solution) for 2 h at RT. Individual or pooled serum samples were diluted by 2.5-fold dilutions from 1/40 to 1/61,035 in PBS containing 1% milk and 0.1% Tween 20, added to the plates in a volume of 100 μl/well, and incubated for 1.5 h at RT. Then, the plates were washed three times with PBS-Tween, secondary HRP-conjugated goat anti-mouse or anti-rabbit IgG, IgG1, IgG2a, or IgG3 antibodies (diluted 1:1,000 in PBS containing 1% milk and 0.1% Tween 20; all from Southern Biotechnology Associated, Birmingham, AL) were added in a volume of 100 μl/well, and the plates were incubated for 1 h at RT. The plates were washed again three times with PBS-Tween, and then 100 μl/well of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Sigma-Aldrich) was added. The reaction was stopped after 10 to 15 min by adding 50 μl/well of 1 M H2SO4, and the absorbance at 450 nm was measured on an EZ Read 400 microplate reader (Biochrom). Antibody titers were calculated as the serum dilution that gave an absorbance at least three times higher than that of a naive serum sample.
EBOV challenge study.
An EBOV challenge study to evaluate the efficacy of the recombinant MVA-EBOVs was performed in the BSL-4 laboratory at the Bernhard Nocht Institute for Tropical Medicine in Hamburg, Germany, using bone marrow chimeric WT → IFNAR−/− C57BL/6 mice that were generated as previously described (47). Briefly, 4- to 8-week-old female recipient IFNAR−/− mice were irradiated with a lethal dose (2 doses of 7 Gy given 2 h apart) in a 137Cs irradiator and then transplanted with 3 × 106 bone marrow cells from donor WT mice. Engraftment of donor cells in peripheral blood was analyzed at 4 weeks posttransplantation. Bone marrow chimeric mice with engraftments of 85% and higher were used for the experiments. Groups of bone marrow chimeric female WT → IFNAR−/− C57BL/6 mice (n = 5) were immunized with 1 × 107 PFU of MVA-GP Zaire, MVA-GP-2A-VP40 Zaire, MVA-GP-VP40 Zaire, or MVA-WT by the i.p. route. Four weeks later, the mice were challenged with a lethal dose of 103 PFU of EBOV (the Mayinga variant) via i.p. injection. The mice were monitored daily after challenge for signs of disease and body weight for 11 days, and those with severe signs of disease or a considerable weight loss (more than 20% of the initial weight) were euthanized. At 11 days postchallenge, the mice were euthanized. Percent survival was obtained for each immunized group.
Data analysis and statistical procedures.
The statistical significance of innate immune analysis was determined by an ordinary one-way analysis of variance (ANOVA) test followed by Tukey's multiple-comparison test. The statistical significance of immune cell recruitment was determined by an ordinary one-way ANOVA test followed by Bonferroni's multiple-comparison test with a single pooled variance. The statistical significance of the ELISAs was determined by a two-way ANOVA test followed by Tukey's multiple-comparison test. The statistical significance of the EBOV challenge study was determined by a log-rank (the Mantel-Cox) test.
ACKNOWLEDGMENTS
This research was supported by Spanish grants SAF2013-45232-R and SAF2017-88089-R (AEI/MINECO/FEDER-EU) and partially supported by the German Center for Infection Research (DZIF-TTU01.702).
We thank Cristina Sánchez Corzo and Victoria Jiménez for expert technical assistance in cell culture, virus growth, and purification. We thank the electron and confocal microscopy services at CNB for their valuable support in the electron and confocal microscopy experiments.
REFERENCES
- 1.Feldmann H, Geisbert TW. 2011. Ebola haemorrhagic fever. Lancet 377:849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bukreyev AA, Chandran K, Dolnik O, Dye JM, Ebihara H, Leroy EM, Muhlberger E, Netesov SV, Patterson JL, Paweska JT, Saphire EO, Smither SJ, Takada A, Towner JS, Volchkov VE, Warren TK, Kuhn JH. 2014. Discussions and decisions of the 2012-2014 International Committee on Taxonomy of Viruses (ICTV) Filoviridae Study Group, January 2012-June 2013. Arch Virol 159:821–830. doi: 10.1007/s00705-013-1846-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye L, Yang C. 2015. Development of vaccines for prevention of Ebola virus infection. Microbes Infect 17:98–108. doi: 10.1016/j.micinf.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Ohimain EI. 2016. Recent advances in the development of vaccines for Ebola virus disease. Virus Res 211:174–185. doi: 10.1016/j.virusres.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Leroy EM, Gonzalez JP, Baize S. 2011. Ebola and Marburg haemorrhagic fever viruses: major scientific advances, but a relatively minor public health threat for Africa. Clin Microbiol Infect 17:964–976. doi: 10.1111/j.1469-0691.2011.03535.x. [DOI] [PubMed] [Google Scholar]
- 6.Gatherer D. 2014. The 2014 Ebola virus disease outbreak in West Africa. J Gen Virol 95:1619–1624. doi: 10.1099/vir.0.067199-0. [DOI] [PubMed] [Google Scholar]
- 7.WHO. 2016. Ebola situation report—10 June 2016. World Health Organization, Geneva, Switzerland: http://who.int/csr/disease/ebola/situation-reports/archive/en/. [Google Scholar]
- 8.Sullivan NJ, Geisbert TW, Geisbert JB, Shedlock DJ, Xu L, Lamoreaux L, Custers JH, Popernack PM, Yang ZY, Pau MG, Roederer M, Koup RA, Goudsmit J, Jahrling PB, Nabel GJ. 2006. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med 3:e177. doi: 10.1371/journal.pmed.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhama K, Malik YS, Malik SV, Singh RK. 2015. Ebola from emergence to epidemic: the virus and the disease, global preparedness and perspectives. J Infect Dev Ctries 9:441–455. doi: 10.3855/jidc.6197. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RF, Bell P, Harty RN. 2006. Effect of Ebola virus proteins GP, NP and VP35 on VP40 VLP morphology. Virol J 3:31. doi: 10.1186/1743-422X-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Licata JM, Johnson RF, Han Z, Harty RN. 2004. Contribution of Ebola virus glycoprotein, nucleoprotein, and VP24 to budding of VP40 virus-like particles. J Virol 78:7344–7351. doi: 10.1128/JVI.78.14.7344-7351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jasenosky LD, Neumann G, Lukashevich I, Kawaoka Y. 2001. Ebola virus VP40-induced particle formation and association with the lipid bilayer. J Virol 75:5205–5214. doi: 10.1128/JVI.75.11.5205-5214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noda T, Sagara H, Suzuki E, Takada A, Kida H, Kawaoka Y. 2002. Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP. J Virol 76:4855–4865. doi: 10.1128/JVI.76.10.4855-4865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swenson DL, Warfield KL, Negley DL, Schmaljohn A, Aman MJ, Bavari S. 2005. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine 23:3033–3042. doi: 10.1016/j.vaccine.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 15.Warfield KL, Bosio CM, Welcher BC, Deal EM, Mohamadzadeh M, Schmaljohn A, Aman MJ, Bavari S. 2003. Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci U S A 100:15889–15894. doi: 10.1073/pnas.2237038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warfield KL, Perkins JG, Swenson DL, Deal EM, Bosio CM, Aman MJ, Yokoyama WM, Young HA, Bavari S. 2004. Role of natural killer cells in innate protection against lethal Ebola virus infection. J Exp Med 200:169–179. doi: 10.1084/jem.20032141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavot V. 2016. Ebola virus vaccines: where do we stand? Clin Immunol 173:44–49. doi: 10.1016/j.clim.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, Daddario KM, Hensley LE, Jahrling PB, Geisbert TW. 2005. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med 11:786–790. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 19.Marzi A, Robertson SJ, Haddock E, Feldmann F, Hanley PW, Scott DP, Strong JE, Kobinger G, Best SM, Feldmann H. 2015. Ebola vaccine. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science 349:739–742. doi: 10.1126/science.aab3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan NJ, Martin JE, Graham BS, Nabel GJ. 2009. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat Rev Microbiol 7:393–400. doi: 10.1038/nrmicro2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ewer K, Rampling T, Venkatraman N, Bowyer G, Wright D, Lambe T, Imoukhuede EB, Payne R, Fehling SK, Strecker T, Biedenkopf N, Krahling V, Tully CM, Edwards NJ, Bentley EM, Samuel D, Labbe G, Jin J, Gibani M, Minhinnick A, Wilkie M, Poulton I, Lella N, Roberts R, Hartnell F, Bliss C, Sierra-Davidson K, Powlson J, Berrie E, Tedder R, Roman F, De Ryck I, Nicosia A, Sullivan NJ, Stanley DA, Mbaya OT, Ledgerwood JE, Schwartz RM, Siani L, Colloca S, Folgori A, Di Marco S, Cortese R, Wright E, Becker S, Graham BS, Koup RA, Levine MM, Volkmann A, Chaplin P, et al. 2016. A monovalent chimpanzee adenovirus Ebola vaccine boosted with MVA. N Engl J Med 374:1635–1646. doi: 10.1056/NEJMoa1411627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, Carroll MW, Dean NE, Diatta I, Doumbia M, Draguez B, Duraffour S, Enwere G, Grais R, Gunther S, Gsell PS, Hossmann S, Watle SV, Konde MK, Keita S, Kone S, Kuisma E, Levine MM, Mandal S, Mauget T, Norheim G, Riveros X, Soumah A, Trelle S, Vicari AS, Rottingen JA, Kieny MP. 2017. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet 389:505–518. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledgerwood JE, DeZure AD, Stanley DA, Novik L, Enama ME, Berkowitz NM, Hu Z, Joshi G, Ploquin A, Sitar S, Gordon IJ, Plummer SA, Holman LA, Hendel CS, Yamshchikov G, Roman F, Nicosia A, Colloca S, Cortese R, Bailer RT, Schwartz RM, Roederer M, Mascola JR, Koup RA, Sullivan NJ, Graham BS, VRC 207 Study Team . 2017. Chimpanzee adenovirus vector Ebola vaccine. N Engl J Med 376:928–938. doi: 10.1056/NEJMoa1410863. [DOI] [PubMed] [Google Scholar]
- 24.Regules JA, Beigel JH, Paolino KM, Voell J, Castellano AR, Hu Z, Munoz P, Moon JE, Ruck RC, Bennett JW, Twomey PS, Gutierrez RL, Remich SA, Hack HR, Wisniewski ML, Josleyn MD, Kwilas SA, Van Deusen N, Mbaya OT, Zhou Y, Stanley DA, Jing W, Smith KS, Shi M, Ledgerwood JE, Graham BS, Sullivan NJ, Jagodzinski LL, Peel SA, Alimonti JB, Hooper JW, Silvera PM, Martin BK, Monath TP, Ramsey WJ, Link CJ, Lane HC, Michael NL, Davey RT Jr, Thomas SJ, rVSVΔG-ZEBOV-GP Study Group . 2017. A recombinant vesicular stomatitis virus Ebola vaccine. N Engl J Med 376:330–341. doi: 10.1056/NEJMoa1414216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu FC, Hou LH, Li JX, Wu SP, Liu P, Zhang GR, Hu YM, Meng FY, Xu JJ, Tang R, Zhang JL, Wang WJ, Duan L, Chu K, Liang Q, Hu JL, Luo L, Zhu T, Wang JZ, Chen W. 2015. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet 385:2272–2279. doi: 10.1016/S0140-6736(15)60553-0. [DOI] [PubMed] [Google Scholar]
- 26.Venkatraman N, Silman D, Folegatti PM, Hill AVS. 2 August 2017. Vaccines against Ebola virus. Vaccine. doi: 10.1016/j.vaccine.2017.07.054. [DOI] [PubMed] [Google Scholar]
- 27.Agnandji ST, Huttner A, Zinser ME, Njuguna P, Dahlke C, Fernandes JF, Yerly S, Dayer JA, Kraehling V, Kasonta R, Adegnika AA, Altfeld M, Auderset F, Bache EB, Biedenkopf N, Borregaard S, Brosnahan JS, Burrow R, Combescure C, Desmeules J, Eickmann M, Fehling SK, Finckh A, Goncalves AR, Grobusch MP, Hooper J, Jambrecina A, Kabwende AL, Kaya G, Kimani D, Lell B, Lemaitre B, Lohse AW, Massinga-Loembe M, Matthey A, Mordmuller B, Nolting A, Ogwang C, Ramharter M, Schmidt-Chanasit J, Schmiedel S, Silvera P, Stahl FR, Staines HM, Strecker T, Stubbe HC, Tsofa B, Zaki S, Fast P, Moorthy V, et al. 2016. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med 374:1647–1660. doi: 10.1056/NEJMoa1502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Santis O, Audran R, Pothin E, Warpelin-Decrausaz L, Vallotton L, Wuerzner G, Cochet C, Estoppey D, Steiner-Monard V, Lonchampt S, Thierry AC, Mayor C, Bailer RT, Mbaya OT, Zhou Y, Ploquin A, Sullivan NJ, Graham BS, Roman F, De Ryck I, Ballou WR, Kieny MP, Moorthy V, Spertini F, Genton B. 2016. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis 16:311–320. doi: 10.1016/S1473-3099(15)00486-7. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy SB, Bolay F, Kieh M, Grandits G, Badio M, Ballou R, Eckes R, Feinberg M, Follmann D, Grund B, Gupta S, Hensley L, Higgs E, Janosko K, Johnson M, Kateh F, Logue J, Marchand J, Monath T, Nason M, Nyenswah T, Roman F, Stavale E, Wolfson J, Neaton JD, Lane HC, PREVAIL I Study Group. 2017. Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med 377:1438–1447. doi: 10.1056/NEJMoa1614067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milligan ID, Gibani MM, Sewell R, Clutterbuck EA, Campbell D, Plested E, Nuthall E, Voysey M, Silva-Reyes L, McElrath MJ, De Rosa SC, Frahm N, Cohen KW, Shukarev G, Orzabal N, van Duijnhoven W, Truyers C, Bachmayer N, Splinter D, Samy N, Pau MG, Schuitemaker H, Luhn K, Callendret B, Van Hoof J, Douoguih M, Ewer K, Angus B, Pollard AJ, Snape MD. 2016. Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia Ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA 315:1610–1623. doi: 10.1001/jama.2016.4218. [DOI] [PubMed] [Google Scholar]
- 31.Shukarev G, Callendret B, Luhn K, Douoguih M, EBOVAC1 Consortium . 2017. A two-dose heterologous prime-boost vaccine regimen eliciting sustained immune responses to Ebola Zaire could support a preventive strategy for future outbreaks. Hum Vaccin Immunother 13:266–270. doi: 10.1080/21645515.2017.1264755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayr A, Stickl H, Muller HK, Danner K, Singer H. 1978. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author's transl). Zentralbl Bakteriol B 167:375–390. (In German.) [PubMed] [Google Scholar]
- 33.Gomez CE, Najera JL, Perdiguero B, Garcia-Arriaza J, Sorzano CO, Jimenez V, Gonzalez-Sanz R, Jimenez JL, Munoz-Fernandez MA, Lopez Bernaldo de Quiros JC, Guardo AC, Garcia F, Gatell JM, Plana M, Esteban M. 2011. The HIV/AIDS vaccine candidate MVA-B administered as a single immunogen in humans triggers robust, polyfunctional, and selective effector memory T cell responses to HIV-1 antigens. J Virol 85:11468–11478. doi: 10.1128/JVI.05165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez CE, Perdiguero B, Garcia-Arriaza J, Esteban M. 2013. Clinical applications of attenuated MVA poxvirus strain. Expert Rev Vaccines 12:1395–1416. doi: 10.1586/14760584.2013.845531. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Sampedro L, Perdiguero B, Mejias-Perez E, Garcia-Arriaza J, Di Pilato M, Esteban M. 2015. The evolution of poxvirus vaccines. Viruses 7:1726–1803. doi: 10.3390/v7041726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volz A, Sutter G. 2017. Modified vaccinia virus Ankara: history, value in basic research, and current perspectives for vaccine development. Adv Virus Res 97:187–243. doi: 10.1016/bs.aivir.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tapia MD, Sow SO, Lyke KE, Haidara FC, Diallo F, Doumbia M, Traore A, Coulibaly F, Kodio M, Onwuchekwa U, Sztein MB, Wahid R, Campbell JD, Kieny MP, Moorthy V, Imoukhuede EB, Rampling T, Roman F, De Ryck I, Bellamy AR, Dally L, Mbaya OT, Ploquin A, Zhou Y, Stanley DA, Bailer R, Koup RA, Roederer M, Ledgerwood J, Hill AV, Ballou WR, Sullivan N, Graham B, Levine MM. 2016. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 16:31–42. doi: 10.1016/S1473-3099(15)00362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Arriaza J, Cepeda V, Hallengard D, Sorzano CO, Kummerer BM, Liljestrom P, Esteban M. 2014. A novel poxvirus-based vaccine, MVA-CHIKV, is highly immunogenic and protects mice against chikungunya infection. J Virol 88:3527–3547. doi: 10.1128/JVI.03418-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roques P, Ljungberg K, Kummerer BM, Gosse L, Dereuddre-Bosquet N, Tchitchek N, Hallengard D, Garcia-Arriaza J, Meinke A, Esteban M, Merits A, Le Grand R, Liljestrom P. 2017. Attenuated and vectored vaccines protect nonhuman primates against chikungunya virus. JCI Insight 2:e83527. doi: 10.1172/jci.insight.83527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noyori O, Nakayama E, Maruyama J, Yoshida R, Takada A. 2013. Suppression of Fas-mediated apoptosis via steric shielding by filovirus glycoproteins. Biochem Biophys Res Commun 441:994–998. doi: 10.1016/j.bbrc.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 41.Smith LM, Hensley LE, Geisbert TW, Johnson J, Stossel A, Honko A, Yen JY, Geisbert J, Paragas J, Fritz E, Olinger G, Young HA, Rubins KH, Karp CL. 2013. Interferon-beta therapy prolongs survival in rhesus macaque models of Ebola and Marburg hemorrhagic fever. J Infect Dis 208:310–318. doi: 10.1093/infdis/jis921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Arriaza J, Arnaez P, Gomez CE, Sorzano CO, Esteban M. 2013. Improving adaptive and memory immune responses of an HIV/AIDS vaccine candidate MVA-B by deletion of vaccinia virus genes (C6L and K7R) blocking interferon signaling pathways. PLoS One 8:e66894. doi: 10.1371/journal.pone.0066894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blaney JE, Marzi A, Willet M, Papaneri AB, Wirblich C, Feldmann F, Holbrook M, Jahrling P, Feldmann H, Schnell MJ. 2013. Antibody quality and protection from lethal Ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine. PLoS Pathog 9:e1003389. doi: 10.1371/journal.ppat.1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanley DA, Honko AN, Asiedu C, Trefry JC, Lau-Kilby AW, Johnson JC, Hensley L, Ammendola V, Abbate A, Grazioli F, Foulds KE, Cheng C, Wang L, Donaldson MM, Colloca S, Folgori A, Roederer M, Nabel GJ, Mascola J, Nicosia A, Cortese R, Koup RA, Sullivan NJ. 2014. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 20:1126–1129. doi: 10.1038/nm.3702. [DOI] [PubMed] [Google Scholar]
- 45.Wong G, Richardson JS, Pillet S, Patel A, Qiu X, Alimonti J, Hogan J, Zhang Y, Takada A, Feldmann H, Kobinger GP. 2012. Immune parameters correlate with protection against Ebola virus infection in rodents and nonhuman primates. Sci Transl Med 4:158ra146. doi: 10.1126/scitranslmed.3004582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Y, Sullivan NJ. 2015. Immunology and evolvement of the adenovirus prime, MVA boost Ebola virus vaccine. Curr Opin Immunol 35:131–136. doi: 10.1016/j.coi.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Ludtke A, Ruibal P, Wozniak DM, Pallasch E, Wurr S, Bockholt S, Gomez-Medina S, Qiu X, Kobinger GP, Rodriguez E, Gunther S, Krasemann S, Idoyaga J, Oestereich L, Munoz-Fontela C. 2017. Ebola virus infection kinetics in chimeric mice reveal a key role of T cells as barriers for virus dissemination. Sci Rep 7:43776. doi: 10.1038/srep43776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson JS, Dekker JD, Croyle MA, Kobinger GP. 2010. Recent advances in Ebolavirus vaccine development. Hum Vaccin 6:439–449. doi: 10.4161/hv.6.6.11097. [DOI] [PubMed] [Google Scholar]
- 49.Schweneker M, Laimbacher AS, Zimmer G, Wagner S, Schraner EM, Wolferstatter M, Klingenberg M, Dirmeier U, Steigerwald R, Lauterbach H, Hochrein H, Chaplin P, Suter M, Hausmann J. 2017. Recombinant modified vaccinia virus Ankara generating Ebola virus-like particles. J Virol 91:e00343-. doi: 10.1128/JVI.00343-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gene OG, Julia BE, Vanessa MR, Victoria WJ, Thomas GW, Lisa HE. 2009. Drug targets in infections with Ebola and Marburg viruses. Infect Disord Drug Targets 9:191–200. doi: 10.2174/187152609787847730. [DOI] [PubMed] [Google Scholar]
- 51.Yang ZY, Duckers HJ, Sullivan NJ, Sanchez A, Nabel EG, Nabel GJ. 2000. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat Med 6:886–889. doi: 10.1038/78645. [DOI] [PubMed] [Google Scholar]
- 52.Becquart P, Mahlakoiv T, Nkoghe D, Leroy EM. 2014. Identification of continuous human B-cell epitopes in the VP35, VP40, nucleoprotein and glycoprotein of Ebola virus. PLoS One 9:e96360. doi: 10.1371/journal.pone.0096360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warfield KL, Olinger G, Deal EM, Swenson DL, Bailey M, Negley DL, Hart MK, Bavari S. 2005. Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J Immunol 175:1184–1191. doi: 10.4049/jimmunol.175.2.1184. [DOI] [PubMed] [Google Scholar]
- 54.Choi JH, Jonsson-Schmunk K, Qiu X, Shedlock DJ, Strong J, Xu JX, Michie KL, Audet J, Fernando L, Myers MJ, Weiner D, Bajrovic I, Tran LQ, Wong G, Bello A, Kobinger GP, Schafer SC, Croyle MA. 2015. A single dose respiratory recombinant adenovirus-based vaccine provides long-term protection for non-human primates from lethal Ebola infection. Mol Pharm 12:2712–2731. doi: 10.1021/mp500646d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falzarano D, Geisbert TW, Feldmann H. 2011. Progress in filovirus vaccine development: evaluating the potential for clinical use. Expert Rev Vaccines 10:63–77. doi: 10.1586/erv.10.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marzi A, Engelmann F, Feldmann F, Haberthur K, Shupert WL, Brining D, Scott DP, Geisbert TW, Kawaoka Y, Katze MG, Feldmann H, Messaoudi I. 2013. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci U S A 110:1893–1898. doi: 10.1073/pnas.1209591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mire CE, Matassov D, Geisbert JB, Latham TE, Agans KN, Xu R, Ota-Setlik A, Egan MA, Fenton KA, Clarke DK, Eldridge JH, Geisbert TW. 2015. Single-dose attenuated Vesiculovax vaccines protect primates against Ebola Makona virus. Nature 520:688–691. doi: 10.1038/nature14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papaneri AB, Wirblich C, Cann JA, Cooper K, Jahrling PB, Schnell MJ, Blaney JE. 2012. A replication-deficient rabies virus vaccine expressing Ebola virus glycoprotein is highly attenuated for neurovirulence. Virology 434:18–26. doi: 10.1016/j.virol.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warfield KL, Aman MJ. 2011. Advances in virus-like particle vaccines for filoviruses. J Infect Dis 204(Suppl 3):S1053–S1059. doi: 10.1093/infdis/jir346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friedrich BM, Trefry JC, Biggins JE, Hensley LE, Honko AN, Smith DR, Olinger GG. 2012. Potential vaccines and post-exposure treatments for filovirus infections. Viruses 4:1619–1650. doi: 10.3390/v4091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olinger GG, Bailey MA, Dye JM, Bakken R, Kuehne A, Kondig J, Wilson J, Hogan RJ, Hart MK. 2005. Protective cytotoxic T-cell responses induced by Venezuelan equine encephalitis virus replicons expressing Ebola virus proteins. J Virol 79:14189–14196. doi: 10.1128/JVI.79.22.14189-14196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson JA, Bray M, Bakken R, Hart MK. 2001. Vaccine potential of Ebola virus VP24, VP30, VP35, and VP40 proteins. Virology 286:384–390. doi: 10.1006/viro.2001.1012. [DOI] [PubMed] [Google Scholar]
- 63.Warfield KL, Dye JM, Wells JB, Unfer RC, Holtsberg FW, Shulenin S, Vu H, Swenson DL, Bavari S, Aman MJ. 2015. Homologous and heterologous protection of nonhuman primates by Ebola and Sudan virus-like particles. PLoS One 10:e0118881. doi: 10.1371/journal.pone.0118881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garbutt M, Liebscher R, Wahl-Jensen V, Jones S, Moller P, Wagner R, Volchkov V, Klenk HD, Feldmann H, Stroher U. 2004. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol 78:5458–5465. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daniels RW, Rossano AJ, Macleod GT, Ganetzky B. 2014. Expression of multiple transgenes from a single construct using viral 2A peptides in Drosophila. PLoS One 9:e100637. doi: 10.1371/journal.pone.0100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim JH, Lee SR, Li LH, Park HJ, Park JH, Lee KY, Kim MK, Shin BA, Choi SY. 2011. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One 6:e18556. doi: 10.1371/journal.pone.0018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. 2004. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol 22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 68.Han Z, Harty RN. 2005. Packaging of actin into Ebola virus VLPs. Virol J 2:92. doi: 10.1186/1743-422X-2-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Falasca L, Agrati C, Petrosillo N, Di Caro A, Capobianchi MR, Ippolito G, Piacentini M. 2015. Molecular mechanisms of Ebola virus pathogenesis: focus on cell death. Cell Death Differ 22:1250–1259. doi: 10.1038/cdd.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fuller CL, Ruthel G, Warfield KL, Swenson DL, Bosio CM, Aman MJ, Bavari S. 2007. NKp30-dependent cytolysis of filovirus-infected human dendritic cells. Cell Microbiol 9:962–976. doi: 10.1111/j.1462-5822.2006.00844.x. [DOI] [PubMed] [Google Scholar]
- 71.Bosio CM, Moore BD, Warfield KL, Ruthel G, Mohamadzadeh M, Aman MJ, Bavari S. 2004. Ebola and Marburg virus-like particles activate human myeloid dendritic cells. Virology 326:280–287. doi: 10.1016/j.virol.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 72.Mohamadzadeh M, Coberley SS, Olinger GG, Kalina WV, Ruthel G, Fuller CL, Swenson DL, Pratt WD, Kuhns DB, Schmaljohn AL. 2006. Activation of triggering receptor expressed on myeloid cells-1 on human neutrophils by Marburg and Ebola viruses. J Virol 80:7235–7244. doi: 10.1128/JVI.00543-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olal D, Kuehne AI, Bale S, Halfmann P, Hashiguchi T, Fusco ML, Lee JE, King LB, Kawaoka Y, Dye JM Jr, Saphire EO. 2012. Structure of an antibody in complex with its mucin domain linear epitope that is protective against Ebola virus. J Virol 86:2809–2816. doi: 10.1128/JVI.05549-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiu X, Audet J, Wong G, Pillet S, Bello A, Cabral T, Strong JE, Plummer F, Corbett CR, Alimonti JB, Kobinger GP. 2012. Successful treatment of Ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci Transl Med 4:138ra181. doi: 10.1126/scitranslmed.3003876. [DOI] [PubMed] [Google Scholar]
- 75.Takada A, Ebihara H, Jones S, Feldmann H, Kawaoka Y. 2007. Protective efficacy of neutralizing antibodies against Ebola virus infection. Vaccine 25:993–999. doi: 10.1016/j.vaccine.2006.09.076. [DOI] [PubMed] [Google Scholar]
- 76.Wilson JA, Hevey M, Bakken R, Guest S, Bray M, Schmaljohn AL, Hart MK. 2000. Epitopes involved in antibody-mediated protection from Ebola virus. Science 287:1664–1666. doi: 10.1126/science.287.5458.1664. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y, Li J, Hu Y, Liang Q, Wei M, Zhu F. 2017. Ebola vaccines in clinical trial: the promising candidates. Hum Vaccin Immunother 13:153–168. doi: 10.1080/21645515.2016.1225637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garcia-Arriaza J, Najera JL, Gomez CE, Tewabe N, Sorzano CO, Calandra T, Roger T, Esteban M. 2011. A candidate HIV/AIDS vaccine (MVA-B) lacking vaccinia virus gene C6L enhances memory HIV-1-specific T-cell responses. PLoS One 6:e24244. doi: 10.1371/journal.pone.0024244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delaloye J, Roger T, Steiner-Tardivel QG, Le Roy D, Knaup Reymond M, Akira S, Petrilli V, Gomez CE, Perdiguero B, Tschopp J, Pantaleo G, Esteban M, Calandra T. 2009. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog 5:e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Gomez CE, Najera JL, Jimenez EP, Jimenez V, Wagner R, Graf M, Frachette MJ, Liljestrom P, Pantaleo G, Esteban M. 2007. Head-to-head comparison on the immunogenicity of two HIV/AIDS vaccine candidates based on the attenuated poxvirus strains MVA and NYVAC co-expressing in a single locus the HIV-1BX08 gp120 and HIV-1(IIIB) Gag-Pol-Nef proteins of clade B. Vaccine 25:2863–2885. doi: 10.1016/j.vaccine.2006.09.090. [DOI] [PubMed] [Google Scholar]
- 81.Ramirez JC, Gherardi MM, Esteban M. 2000. Biology of attenuated modified vaccinia virus Ankara recombinant vector in mice: virus fate and activation of B- and T-cell immune responses in comparison with the Western Reserve strain and advantages as a vaccine. J Virol 74:923–933. doi: 10.1128/JVI.74.2.923-933.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gomez CE, Perdiguero B, Cepeda MV, Mingorance L, Garcia-Arriaza J, Vandermeeren A, Sorzano CO, Esteban M. 2013. High, broad, polyfunctional, and durable T cell immune responses induced in mice by a novel hepatitis C virus (HCV) vaccine candidate (MVA-HCV) based on modified vaccinia virus Ankara expressing the nearly full-length HCV genome. J Virol 87:7282–7300. doi: 10.1128/JVI.03246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Di Pilato M, Mejias-Perez E, Zonca M, Perdiguero B, Gomez CE, Trakala M, Nieto J, Najera JL, Sorzano CO, Combadiere C, Pantaleo G, Planelles L, Esteban M. 2015. NFkappaB activation by modified vaccinia virus as a novel strategy to enhance neutrophil migration and HIV-specific T-cell responses. Proc Natl Acad Sci U S A 112:E1333–E1342. doi: 10.1073/pnas.1424341112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garcia-Arriaza J, Najera JL, Gomez CE, Sorzano CO, Esteban M. 2010. Immunogenic profiling in mice of a HIV/AIDS vaccine candidate (MVA-B) expressing four HIV-1 antigens and potentiation by specific gene deletions. PLoS One 5:e12395. doi: 10.1371/journal.pone.0012395. [DOI] [PMC free article] [PubMed] [Google Scholar]