ABSTRACT
The apolipoprotein B editing complex 3 (APOBEC3) proteins are potent retroviral restriction factors that are under strong positive selection, both in terms of gene copy number and sequence diversity. A common feature of all the members of the APOBEC3 family is the presence of one or two cytidine deamination domains, essential for cytidine deamination of retroviral reverse transcripts as well as packaging into virions. Several studies have indicated that human and mouse APOBEC3 proteins restrict retrovirus infection via cytidine deaminase (CD)-dependent and -independent means. To understand the relative contribution of CD-independent restriction in vivo, we created strains of transgenic mice on an APOBEC3 knockout background that express a deaminase-dead mouse APOBEC3 due to point mutations in both CD domains (E73Q/E253Q). Here, we show that the CD-dead APOBEC3 can restrict murine retroviruses in vivo. Moreover, unlike the wild-type protein, the mutant APOBEC3 is not packaged into virions but acts only as a cell-intrinsic restriction factor that blocks reverse transcription by incoming viruses. Finally, we show that wild-type and CD-dead mouse APOBEC3 can bind to murine leukemia virus (MLV) reverse transcriptase. Our findings suggest that the mouse APOBEC3 cytidine deaminase activity is not required for retrovirus restriction.
IMPORTANCE APOBEC3 proteins are important host cellular restriction factors essential for restricting retrovirus infection by causing mutations in the virus genome and by blocking reverse transcription. While both methods of restriction function in vitro, little is known about their role during in vivo infection. By developing transgenic mice with mutations in the cytidine deamination domains needed for enzymatic activity and interaction with viral RNA, we show that APOBEC3 proteins can still restrict in vivo infection by interacting with reverse transcriptase and blocking its activity. These studies demonstrate that APOBEC3 proteins have evolved multiple means for blocking retrovirus infection and that all of these means function in vivo.
KEYWORDS: APOBEC3, MLV, cytidine deamination domains, retrovirus, transgenic mice, virus pathogenesis
INTRODUCTION
Mammals have developed multiple mechanisms to defend themselves against retroviruses. One of these mechanisms involves members of the apolipoprotein B editing complex 3 (APOBEC3) family of genes (1, 2). APOBEC3 genes encode DNA-editing enzymes and are under strong positive selection (3, 4). Humans have seven APOBEC3 genes, while mice have only one (5). APOBEC3 proteins are cytidine deaminases (CDs) characterized by the presence of one or two cytidine deamination domains essential for both nucleic acid binding and enzymatic activity (5). APOBEC3 proteins are incorporated in budding virions via interaction with both nucleocapsid and RNA (6–9). When these virions infect new cells and undergo reverse transcription, APOBEC3 binds to single-stranded DNA reverse transcripts and deaminates cytidines, resulting in uracils (5, 10). This results in G-to-A transitions in the positive coding strand, a process termed hypermutation (10–12). APOBEC3-mediated hypermutation of retroviral DNA is detrimental to virus replication. Apolipoprotein B editing complex 3G (APOBEC3G), APOBEC3F, and mouse APOBEC3 have also all been shown to restrict retroviruses in a cytidine deamination-independent manner by blocking the generation of early reverse transcripts (10, 13–17). Previous reports have shown that APOBEC3G interacts with the reverse transcriptase (RT) of HIV-1 and thus inhibits replication (18, 19). Other reports have shown that APOBEC3G blocked the elongation step of reverse transcription (13, 20, 21); it has been proposed that APOBEC3G utilizes a “roadblock” mechanism to inhibit RT-dependent DNA elongation by binding to the RNA with a higher affinity than RT, which consequently leads to the steric hindrance of RT progression (21, 22).
The mouse genome encodes a single Apobec3 gene. Mouse APOBEC3 can restrict ΔVif HIV-1 infection as robustly as APOBEC3G by inducing high levels of G-to-A mutations in the viral DNA (10, 23, 24). Mouse APOBEC3 also restricts infection by exogenous murine gammaretroviruses, such as Friend murine leukemia virus (FMLV), Moloney MLV (MMLV), and AKR MLV, and betaretroviruses, such as mouse mammary tumor virus (MMTV), both in vivo and in vitro (8, 25–27). However, mouse APOBEC3 restricts FMLV, MMLV, or MMTV only by blocking reverse transcription and not by causing cytidine deamination of the viral DNA (15, 17, 23, 24); an exception to this is AKR MLV, where low levels of hypermutation have been observed in virus sequences isolated from infected mice (25). Recent studies have shown that a glycosylated alternate translation product of gag found in gammaretroviruses, glyco-Gag, is essential in counteracting the deleterious effects of APOBEC3 (28–30). Glyco-Gag stabilizes the core of the incoming virion and prevents APOBEC3 from accessing the reverse transcription intermediate (30, 31).
APOBEC3 proteins are comprised of either one or two conserved zinc-coordinating domains, referred to as the CD domains (5). Each domain has an H-X-E sequence, where E is the active site for APOBEC3-mediated deamination (when the domains are enzymatically active), and a P-C-X2.4-C domain important for RNA binding and APOBEC3 incorporation into the budding virion (32, 33). In the case of human APOBEC3G, the C-terminal domain has deaminase activity, and the N-terminal domain is essential for encapsidation (32). For mouse APOBEC3, the C-terminal domain is essential for encapsidation, while the N-terminal domain possesses the cytidine deaminase activity (23, 32). Early reports showed that both CD domains were essential for APOBEC3G's antiretroviral function as mutating either the C- or N-terminal domain abrogated APOBEC3-mediated restriction. In contrast, mutations of the active sites of either or both CD domains of mouse APOBEC3 did not affect its ability to restrict retroviruses; however, only certain amino acid changes (E to Q) in the mouse APOBEC3 active site inhibited the ability of APOBEC3 to be packaged inside budding virions (24, 34), while other changes (E to A) in the active site of mouse APOBEC3 allowed incorporation into the virions (27, 32). Nevertheless, all previous reports on the role of the cytidine deamination domains during retrovirus infection were performed in vitro. What the relative contribution is of deaminase-dependent and -independent mechanisms on retrovirus restriction in vivo is currently unknown.
Here, we examine the relative contribution of deaminase-dependent and -independent mechanisms of restriction in vivo, using transgenic mice that express mouse APOBEC3 lacking CD activity due to point mutations in both CD domains (E73Q/E253Q). This study demonstrates that the CD-dead mouse APOBEC3 protein restricted wild-type (WT) MMLV, glyco-Gag mutant MMLV (MLVgGag), and MMTV in vivo. We also show that the mutant APOBEC3 is not packaged into MMLV virions and that WT and CD-dead APOBEC3 can still bind to the MMLV RT. Thus, the mouse APOBEC3-mediated access to the MMLV reverse transcription complex (RTC) is not CD dependent. Cell-intrinsic CD-dead mouse APOBEC3 did restrict incoming MMLV in primary dendritic cells (pDCs) isolated from the transgenic mice. Given the similar levels of restriction by CD-dead and WT APOBEC3 proteins, we conclude that mouse APOBEC3 restricts exogenous retroviruses in vivo mainly in a CD-independent manner.
RESULTS
Generation of mice expressing CD-dead mouse APOBEC3.
To study the role(s) of the cytidine deamination domains of mouse APOBEC3 in vivo, we used the chicken β-actin regulatory region to drive expression of a myc-tagged mouse APOBEC3 gene with mutations in the active sites of the two cytidine deamination domains (E73Q/E253Q) and created transgenic mice on a C57BL/6 background (Fig. 1A and B). These mutations were previously shown to abrogate both the CD activity and the ability of APOBEC3 to be packaged into MMLV virions (24). Two independent transgenic strains that each transmitted the mouse APOBEC3 (E73Q/E253Q) transgene were obtained. Each of these strains was then back-crossed onto the APOBEC3 knockout (KO) background (also C57BL/6) to generate mice containing functional copies of only the mutant mouse APOBEC3 (mA3CDmut) gene.
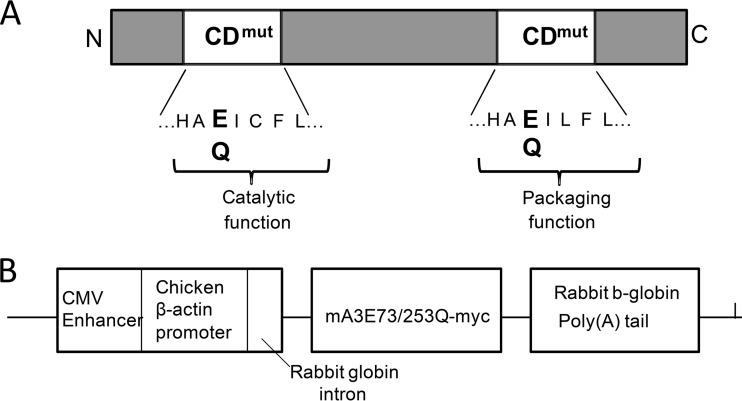
FIG 1.
Schematic diagram of the mouse APOBEC3 protein and the amino acids mutated in the CD domains (A) and the transgenic construct used to generate the mice (B). The mA3CDmut transgene was crossed onto an APOBEC3 KO background to generate mice that express only the mA3CDmut transgene and not the endogenous mouse APOBEC3. CMV, cytomegalovirus.
To determine the level of transgene expression, we first isolated RNA from different tissues, including peripheral blood mononuclear cells (PBMCs), and performed reverse transcriptase quantitative PCR (RT-qPCR). RNA from C57BL/6 mouse PBMCs served as controls. For each transgene, there was one strain expressing high levels and one expressing low levels of mA3CDmut (mA3CDhigh and mA3CDlow, respectively), as defined by their relative expression levels in lymphoid tissues. The levels of the transgene expressed by the mA3CDhigh strain were slightly lower than those of the endogenous mouse gene expressed by the WT strain in spleen and thymus, organs that are the sites of infection for MMLV and MMTV (Fig. 2A). The mA3CDlow strain had very low but detectable levels of expression of the transgene in various tissues and was expressed at approximately 100-fold-lower levels in the spleen and thymus (Fig. 2A and B). As the β-actin regulatory region was used, transgene expression was seen in many tissues and in several at levels higher than those of endogenous mouse APOBEC3 (e.g., heart and muscle) (Fig. 2A). We also performed Western blotting on the splenocytes from the WT, APOBEC3 KO, mA3CDhigh, and mA3CDlow mice and found similar results (see below).
FIG 2.
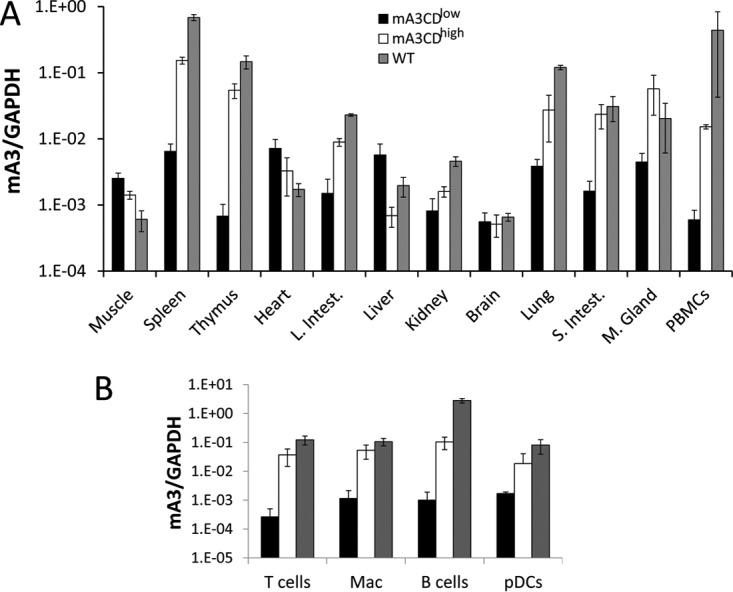
Expression of the mA3CDmut transgene. (A) RT-qPCR analysis of RNA isolated from different tissues of the mA3CDhigh and mA3CDlow strains. Shown for comparison are the endogenous APOBEC3 levels in nontransgenic C57BL/6 mice (WT). The mice used for this analysis were uninfected. RNA levels are the average of four individual mice. Error bars denote standard deviations. L. Intest, large intestine; S. Intest, small intestine; M. Gland, mammary gland; mA3, mouse APOBEC3. (B) Transgene expression in MMLV and MMTV in vivo targets of infection. T cells, B cells, peripheral dendritic cells (pDCs), and macrophages (Mac) were purified from mice of each genotype by cell sorting. RNA isolated from the purified cells was analyzed by RT-qPCR for transgene expression. Shown are the averages for cells isolated from four individual mice. Error bars denote standard deviations.
Both MMLV and MMTV initially infect dendritic and other sentinel cells and then B and T lymphocytes during in vivo infection (26, 35–37). To determine if the target sentinel/lymphoid cells expressed the transgene, we sorted PBMCs from the transgenic mice into different populations and tested each for the expression of the transgene. B and T cells, DCs, and macrophages from the mA3CDhigh strain all expressed the transgene (Fig. 2B). The mA3CDlow strain expressed the transgene in T and B cells, macrophages, and DCs at very low levels (Fig. 2B). Thus, the transgenes were expressed in the appropriate MMTV and MLV target cell types, sentinel cells and lymphocytes.
Mouse APOBEC3 (E73Q/E253Q) restricts infection by murine retroviruses.
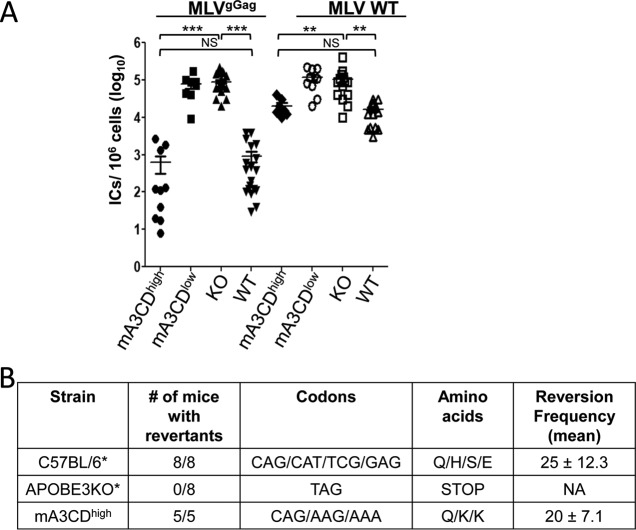
We next tested whether the mA3CDmut protein (E73Q/E253Q) would function as an antiviral restriction factor in vivo. Newborn pups from each of the transgenic strains were infected with MMLV at 1 day after birth and harvested 6 days later; we showed previously that infection levels between the mouse APOBEC3 WT mice and the mouse APOBEC3 KO mice differ the most at 6 days postinfection (dpi) (26). Virus titers were obtained from splenocyte cultures of individual mice. The mA3CDhigh mice had levels of infection similar to those of the WT mice but had viral titers, on average, 2 logs lower than those of the KO mice (Fig. 3A). In contrast, the mA3CDlow mice had infection levels similar to those of the KO mice (Fig. 3A). Similar results were obtained with MMTV-infected mice subcutaneously inoculated with virus at 5 days and examined for infection 4 days later (Fig. 3B). In conclusion, both mA3CDhigh and WT mice restrict murine retrovirus infection in vivo.
FIG 3.

Mouse APOBEC3 (E73Q/E253Q) restricts murine retrovirus infection in vivo. (A) Newborn mice were infected with MMLV, and at 6 dpi, virus titers in spleens were measured. Each point represents the titer obtained from an individual mouse. The horizontal bar in each group represents the average. The transgenic mice were derived from four litters each; the knockout mice are the littermates of the transgenic mice. (B) Four APOBEC3 KO, WT, mA3CDlow, and mA3CDhigh mice were infected with MMTV subcutaneously, and at 4 dpi DNA was isolated from the draining lymph nodes and subjected to RT-qPCR with MMTV-specific primers as previously described (8). Error bars represent standard deviations. ***, P ≤ 0.0001; NS, not significant (one-way analysis of variance).
Glyco-Gag mutant MMLV is restricted by mouse APOBEC3 (E73Q/E253Q).
Previous reports have shown that the MLVgGag replicates more poorly in WT mice than in KO mice (28, 30). We also demonstrated that the core of MLVgGag was more unstable than that of the WT virus, which allows mouse APOBEC3 to access and subsequently restrict the reverse transcription complex (30). Hence, it is possible that the nucleic acid binding ability of mouse APOBEC3 is required for the mouse APOBEC3-mediated restriction of MLVgGag. To examine whether mutant mouse APOBEC3 (E73Q/E253Q) can restrict MLVgGag as well as mouse APOBEC3 WT, we infected WT, KO, mA3CDhigh, and mA3CDlow pups with WT and MLVgGag and harvested spleens at 18 dpi. WT and mA3CDhigh mice infected with MLVgGag showed significantly lower levels of infectious virus in the spleens than the KO and mA3CDlow mice (Fig. 4A). In contrast, WT virus replicated to slightly lower levels in the WT and mA3CDhigh mice than in the KO mice, as indicated by the virus titers (Fig. 4A). Moreover, in the case of the KO mice, MLVgGag and WT MLV replicated to the same levels, similar to results previously reported (28, 30).
FIG 4.
MMLV WT and MLVgGag virus loads in infected mice. (A) Mice were infected with equal amounts of virus and killed at 18 dpi. Levels of ICs in cells isolated from the spleens of APOBEC3 KO, WT, mA3CDlow, and mA3CDhigh mice infected with MMLV WT and MLVgGag virus were determined. The transgenic mice were derived from two to three litters each; the knockout mice are the littermates of the transgenic mice. (***, P ≤ 0.0001; **, P ≤ 0.001; NS, not significant; Mann Whitney t test). (B) Glyco-Gag mutant virus reverts in mA3CDhigh mice. Mice of the indicated genotype were infected with MLVgGag, and at 6 weeks postinfection, MMLV DNA was isolated from spleens and thymuses of the infected mice, PCR-amplified, and sequenced. WT MMLV has a TAT(Y) codon. Reversion frequency shows the average percentage of sequenced clones in each mouse that showed reversion. The data for the C57BL/6 group and 6/8 of the APOBEC3 KO mice (asterisks) were taken from Stavrou et al. (30).
The glyco-Gag mutant virus has a stop codon in the gPr80Gag reading frame (UAU to UAG) at nucleotide (nt) 608, which is 12 nt 5′ of the canonical Gag AUG (38). MLV glyco-Gag-negative viruses revert to WT in mice (39–41). It was previously shown that MLVgGag reverted to WT at 6 weeks postinfection in WT mice that express APOBEC3 and not in the KO mice (30). Thus, a key role of the glyco-Gag protein is to counteract APOBEC3 during infection. As MLVgGag was also inhibited by mouse APOBEC3 (E73Q/E253Q), we examined virus isolated from the mA3CDhigh transgenic mice for reversion. We infected neonatal KO and mA3CDhigh mice with MLVgGag, and at 6 weeks after infection, DNA was isolated from the spleens and thymuses. PCR was performed using primers that target the glyco-Gag region with the stop codon, and the PCR products were sequenced. As previously described, MLVgGag does not revert to WT MLV in the KO mice (30). On the other hand, MLVgGag viral sequences isolated at 6 weeks after infection reverted to WT in 5/5 mA3CDhigh mice (Fig. 4B). The reversion frequencies in the mA3CDhigh and C57BL/6 mice were not significantly different (Fig. 4B). Thus, the glyco-Gag-dependent anti-APOBEC3 activity is not dependent on the cytidine deaminase activity.
MMLV produced in vivo does not package APOBEC3 (E73Q/E253Q).
Human APOBEC3G and mouse APOBEC3, both potent antiretroviral proteins, are packaged inside budding virions (8, 23, 24, 27). It was previously shown that while mutations in the glutamates (E67Q and E259Q) of the cytidine deamination domains of APOBEC3G did not affect packaging of the protein inside the budding virions, the same mutations in mouse APOBEC3 (E73Q and E253Q) led to diminished packaging inside the budding virions in vitro (24). To determine whether APOBEC3 packaging in MMLV virions in vivo is affected by mutations in the cytidine deamination domains (E73Q/E253Q), 1- to 2-day-old KO, mA3CDhigh, mA3CDlow, and WT pups were infected with MMLV. At 16 days postinfection (dpi), we examined virions produced by these splenocytes. Virus released into the supernatants was recovered, and viral RNA was quantified by reverse-transcribed RT-qPCR, and equal amounts of virions were subjected to Western blot analysis. The presence of mature virions was demonstrated by Western blotting with anti-MLV antiserum; similar amounts of p30 capsid (p30CA) were found in the viruses, which were also analyzed by Western blotting with anti-mouse APOBEC3 antiserum (Fig. 5). While APOBEC3 protein was detected in the spleen extracts from the mA3CDhigh and WT mice, only endogenous mouse APOBEC3 was incorporated in MMLV virions (30); the catalytically inactive mouse APOBEC3 (E73Q/E253Q) was not detected in MMLV virions isolated from either of the transgenic mouse strains (mA3CDhigh or mA3CDlow) (Fig. 5). Therefore, in the mA3CDhigh mice, while CD-dead APOBEC3 (E73Q/E253Q) is expressed in the splenocytes of the infected mice, it does not get incorporated into the budding virions.
FIG 5.
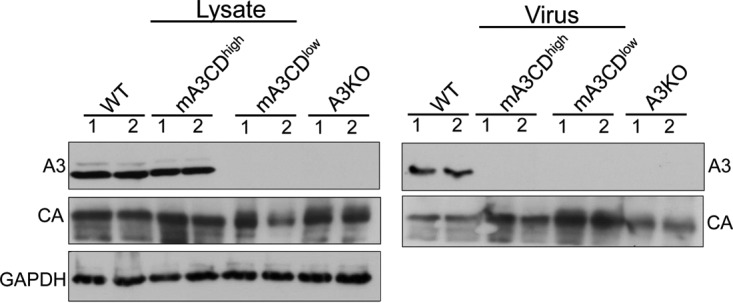
mA3CDmut is not packaged inside MLV virions. Newborn WT, mA3CDhigh, mA3CDlow, and KO mice were infected with MMLV. Splenic extracts and isolated virions were analyzed by Western blotting. mA3CDmut was detected with precleared anti-mouse APOBEC3 antiserum. Shown is a representative Western blot from two mice per genotype, as indicated, infected with MMLV. This experiment was repeated three times using virions and lysates from different infected litters and gave similar results. The knockout mice are the littermates of the transgenic mice. CA, capsid; A3, APOBEC3.
Cell-intrinsic mouse APOBEC3 (E73Q/E253Q) can restrict incoming virions.
Another means by which both mouse and human A3 proteins restrict infection is by targeting incoming virus, particularly in sentinel cells of the immune system (15, 17, 26, 30, 42–45). To determine if this mechanism was in effect in the transgenic mice, particularly in the mA3CDhigh transgenic mice, which restricted infection without virion incorporation, we isolated bone marrow-derived dendritic cells (BMDCs) from the different transgenic, WT, and KO mice and infected them with MMLV. DNA was isolated at different times postinfection, and qPCR was performed to measure the MMLV DNA levels. These time points represent the time of maximal reverse transcription (2 h) while the 4-h and 8-h time points are when DNA integration begins and ends, respectively (46). MMLV DNA levels in the BMDCs of the mA3CDhigh mice were significantly reduced compared to those of the mA3CDlow and KO BMDCs at 4 and 8 h postinfection but were higher than and statistically different from the MMLV DNA levels seen in the WT BMDCs (Fig. 6). The lack of target cell restriction in the mA3CDlow strain probably reflects a lower level of mA3CDmut expression in the transgenic BMDCs than of endogenous APOBEC3 expression in WT BMDCs (Fig. 6B). Thus, the mutant mouse APOBEC3 (E73Q/E253Q) functions as a target cell restriction factor.
FIG 6.

Expression of mA3CDmut in BMDCs restricts incoming MLV. (A) Infection of BMDCs isolated from KO, WT, mA3CDlow, and mA3CDhigh mice with MMLV isolated from KO mice. RT-qPCR analysis of genomic DNA was performed with MMLV-specific primers and normalized to GAPDH levels. BMDCs were isolated according to standard procedures. Shown are the results of four independent experiments with three technical replicates in each experiment. Error bars indicate standard deviations. *, P ≤ 0.05; **, P ≤.0.0001 (Mann Whitney t test). (B) Mouse APOBEC3 expression levels in BMDCs of the different mice. Shown is RT-PCR analysis of mouse APOBEC3 normalized to GAPDH from BMDCs isolated from two mice of each genotype. Error bars show variations between the two samples.
Mouse APOBEC3 interaction with MMLV RT does not require functional CD domains.
Human APOBEC3G has been previously shown to interact with the HIV RT, and this interaction was independent of binding to the viral RNA or other cellular or viral proteins (19). We examined whether MMLV RT can bind to mouse APOBEC3 and whether this interaction required the CD domains of mouse APOBEC3. Cells were cotransfected with plasmids expressing either wild-type or CD-dead mouse APOBEC3 and MMLV RT. Coimmunoprecipitations were performed, and we found that mouse APOBEC3 bound very potently to MLV RT (Fig. 7A). This MMLV RT-mouse APOBEC3 interaction was not solely dependent on the CD domains of mouse APOBEC3 as CD-dead APOBEC3 also bound to MMLV RT, albeit at slightly lower levels (Fig. 7A and B). The interaction of wild-type or CD-dead mouse APOBEC3 was not dependent on RNA since RNase A treatment of the extracts prior to immunoprecipitation did not affect the interaction (Fig. 7C). Thus, the ability of mouse APOBEC3 to inhibit MMLV reverse transcription may not be dependent on its nucleic acid ability but on its ability to bind to RT itself.
FIG 7.
Coimmunoprecipitation of APOBEC3 and MLV RT. 293T cells were cotransfected with MMLV-RT-myc and mA3/mA3CDmut-HA. (A) Lysates were immunoprecipitated with an anti-myc antibody, and Western blots were probed with an anti-HA antibody. Red fluorescent protein (RFP) was used as a control. Shown is a representative of three different experiments. (B) Quantitative analysis of the amount of immunoprecipitated protein relative to that of the input protein, using ImageJ analysis software (NIH), from three independent experiments. (C) Prior to immunoprecipitation as described for panel A, the lysates were treated with RNase A. Shown is a representative Western blot of three different experiments. IP, immunoprecipitation.
DISCUSSION
APOBEC3-dependent deamination of cytidines to uracils is specific to single-stranded DNA and occurs through a zinc-binding domain, in which a conserved glutamic acid is essential for the enzymatic function of the protein (47, 48). The cytidine deamination domains of human APOBEC3 proteins have been studied extensively in vitro. APOBEC3G, APOBEC3F, and APOBEC3B have two cytidine deamination domains: the C-terminal CD domain of these enzymes is the catalytically active domain responsible for cytidine deamination, while the N-terminal CD domain is responsible for the incorporation of APOBEC3 into budding virions (32, 49). However, in the case of mouse APOBEC3, which also has two CD domains, the order is reversed: the N-terminal cytidine deamination domain has catalytic activity, while the C-terminal cytidine deamination domain is important for APOBEC3 packaging (32, 49). In previous in vitro studies, when the cytidine deamination domains of the human APOBEC3G were mutated, APOBEC3G incorporation remained for the most part unaffected, but the antiretroviral function was severely abrogated (24). In contrast, for mouse APOBEC3, mutations in the cytidine deamination domains had little effect on the antiretroviral function of the protein, but certain mutations (E73/253Q) abrogated its packaging inside the budding retroviral virion in vitro (24). To this end, we generated transgenic mice expressing mouse APOBEC3 with mutations in both CD domains (E73Q/E253Q). Two mouse APOBEC3 cytidine deamination mutant lines were generated, mA3CDhigh and mA3CDlow, expressing levels of the transgene similar to or lower than the levels of the endogenous mouse APOBEC3. This allowed us to examine the dose-dependent effect of the transgene during retrovirus infection.
In vitro studies have shown that while mutations in the cytidine deamination domains resulted in low levels of APOBEC3 incorporation in the budding virions, they did not affect the antiviral function of APOBEC3 (8, 24). The transgenic mice we developed showed that CD-dead APOBEC3 restricts MMTV and MMLV infection in vivo almost as well as the WT APOBEC3, demonstrating that CD catalytic activity is dispensable during infection in vivo. We also examined whether the CD-dead APOBEC3 was packaged inside the budding virions made during in vivo infection and did not detect any CD-dead APOBEC3 inside the budding virions; however, endogenous mouse APOBEC3 was incorporated into MMLV virions, as we previously described (15, 30). Thus, this confirms our previous finding that APOBEC3-mediated restriction of MMTV and MMLV in vivo is not exclusively due to APOBEC3 incorporated into the budding virions (15, 17, 44, 45). To determine how this packaging-independent restriction occurs, we generated BMDCs from the transgenic mice and showed that they were less infected than APOBEC3 KO BMDCs, albeit more infected than WT cells. We conclude that APOBEC3 expressed in target cells plays an important role in the restriction of murine retroviruses. Thus, our studies provide strong support to the argument that the CD domains of mouse APOBEC3 are not essential for restriction of murine retroviruses in vivo.
A common feature among gammaretroviruses is an additional glycosylated form of the Gag protein, termed gPr80gag or glyco-Gag, which uses a CUG initiation codon upstream and in frame with the Gag polyprotein AUG (50–52). Glyco-Gag has 88 amino acids more in the N terminus than the Gag protein and is cleaved by cellular proteases to two proteins of 55 and 40 kDa each (53, 54). The N-terminal fragment, composed of the glyco-Gag unique sequence, matrix, and pp12gag, is also found in MMLV virions, while the C-terminal fragment gets secreted by the cell (28, 55, 56). It was recently shown that the glycosylation sites of glyco-Gag are essential for its role in capsid stability, as they interact with the Pr65Gag structural polyprotein, and that glyco-Gag is incorporated in the MLV envelope as a type I transmembrane protein (31). The preservation of the glyco-Gag protein in the gammaretroviruses indicates its significance in virus replication. Glyco-Gag is dispensable for virus replication in vitro (38, 40, 57) but is essential for in vivo infectivity (38, 39, 41, 57), and in WT mice, glyco-Gag-deficient MLV reverts to WT virus (39, 41, 57). We previously showed that glyco-Gag plays an essential role in counteracting the deleterious effect of APOBEC3 by stabilizing the core and by preventing APOBEC3 from accessing the reverse transcription complex (30) and that APOBEC3 exerts selective pressure for the maintenance of glyco-Gag in gammaretroviruses. In this study, we show that the glyco-Gag mutant virus is restricted by the CD-dead protein at levels similar to those of wild-type mouse APOBEC3, and reversion to wild-type virus was observed in both the WT and mA3CDmut transgenic mice. These findings further confirm that APOBEC3 does not require the cytidine deamination domains to counteract glyco-Gag in vivo and that it is likely the ability of mouse APOBEC3 to inhibit reverse transcription that puts in vivo selective pressure on the virus to retain the glyco-Gag protein. Which regions of mouse APOBEC3 are critical for its in vivo restriction are currently not known although phosphorylation sites outside the CD domains have been shown to be important for the antiretroviral function of mouse APOBEC3 in vitro (58).
This study shows (i) that murine retroviruses are restricted primarily in a cytidine deamination-independent manner, (ii) that both packaged and cell-intrinsic APOBEC3 are important for restriction of murine retroviruses in vitro, and (iii) that MMLV RT can interact with mouse APOBEC3 and that this interaction is only partially dependent on the CD domains, suggesting that mouse APOBEC3 may inhibit reverse transcription in MLV by blocking the reverse transcriptase itself. MMLV, similar to MMTV, is thought to be transmitted from mothers to offspring via the milk, and we showed previously that APOBEC3 packaged into milk-borne MMTV was less infectious than virus lacking APOBEC3 (59). Thus, it is possible that a major role of packaged APOBEC3 is to prevent transmission of the virus from mother to offspring, while the function of the cell-intrinsic APOBEC3 is to prevent virus spread in the infected individual.
MATERIALS AND METHODS
Ethics statement.
All mice were housed according to the policies of the Institutional Animal Care and Use Committee of the University of Pennsylvania (UPENN) and the Animal Care Committee (ACC) of the University of Illinois at Chicago (UIC), and all studies were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (60). The experiments performed with mice in this study were approved by these committees (UPENN IACUC protocol 801594 and UIC ACC protocol 15-222).
Transgene construction.
The mouse APOBEC3 (E73Q/E253Q) construct was kindly provided to us by David Derse (deceased). The mouse APOBEC3 (E73/253Q) fragment from this construct was subcloned into pcDNA3.1(-)/myc-His (Invitrogen) and then into the pCAGGs vector (courtesy of Yongwon Choi) (Fig. 1A). Construction details are available upon request. All clones were sequenced to verify the inserts.
Generation of mice.
The pCAGGs vector carrying the myc-tagged mouse APOBEC3 (E73Q/E253Q) gene was cut with SalI and PstI to remove vector sequences, and the insert was gel purified prior to microinjection (Fig. 1B). Fertilized eggs of C57BL/6 mice were injected with purified DNA fragments by the Transgenic and Chimeric Mouse Facility of the University of Pennsylvania. The transgenic mice were then backcrossed with mouse APOBEC3 KO mice to generate animals containing functional copies of only the transgene. All transgenic lines were maintained by breeding with mouse APOBEC3 KO mice, so the transgenes were carried in heterozygotes. This allowed us to generate nontransgenic, matched controls for infection studies. Mice were genotyped for mouse APOBEC3 using primers previously described (45).
Transgene RNA analysis.
Tissues were harvested from 3-month-old mice, and RNA was isolated with the use of TRIzol (Invitrogen). RNA was further processed for the elimination of any residual phenol according to the RNA cleanup method per the manufacturer's recommendation (Qiagen) and then treated with DNase I to eliminate any contaminating genomic DNA (Qiagen). cDNA was produced from the purified RNA using a SuperScript III First Strand Synthesis System for RT-PCR (Invitrogen). RT-PCR was performed using a Power SYBR green PCR master mix kit (Applied Biosystems) with the mouse APOBEC3 primers, as previously described (17). For the transcriptional profile, a standard curve was made using known quantities of PCR product DNA and then used to calculate the absolute copy numbers for mouse APOBEC3 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as previously described (17). The mouse APOBEC3 values were then normalized to the levels of GAPDH in each sample.
Western blotting.
Spleens were lysed for 30 min on ice in protein lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% deoxycholate, 0.1% SDS, and protease inhibitor cocktail) and then sonicated and centrifuged at 10,000 × g for 10 min. To quantify the levels of mouse APOBEC3 expression, 100 μg of spleen lysates from infected mice was analyzed on 10% SDS-polyacrylamide gels. For Western blot analysis of virions, viral RNA was quantified by RT-qPCR, as previously described (30), and equal amounts were loaded on gels. The following antibodies were used: polyclonal goat anti-MLV antibody (NCI Repository), rabbit anti-GAPDH (Cell Signaling Technology), and precleared rabbit anti-mouse APOBEC3 (15, 30). Horseradish peroxidase (HRP)-conjugated anti-rabbit immunoglobulin (Ig) (Cell Signaling Technology) and anti-goat Ig were used for detection, using a SuperSignal West Femto chemiluminescent substrate (Thermo Scientific) and ECL Western blot detection reagent (GE Healthcare).
Coimmunoprecipitation of APOBEC3 and MLV RT.
HEK293 cells were transfected with plasmids expressing Myc-RT and hemagglutinin (HA)-tagged APOBEC3 proteins. Twenty-four hours after transfection, cells were lysed in cell lysis buffer (Cell Signaling) containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and Halt protease and phosphatase inhibitor cocktail (Thermo Scientific). Supernatants were incubated with monoclonal anti-myc antibody (Cell Signaling) at 4°C with gentle rotation for 4 h, and then protein A/G Plus-agarose (Santa Cruz Biotechnology) was added and incubated at 4°C with gentle rotation overnight. Following immunoprecipitation, beads were washed four times with lysis buffer. Immunoprecipitated proteins were analyzed by immunoblot analysis using rabbit anti-HA or mouse anti-myc antibodies (Cell Signaling). Bands were quantified using ImageJ (NIH). In the case of the RNase A-treated samples, prior to immunoprecipitation, cell lysates were incubated with RNase A (50 μg/ml) at 37°C for 30 min and then processed as described above.
Primary cell isolation.
BMDCs were generated from the mA3CDhigh, mA3CDlow, WT, and APOBEC3 KO mice as previously described (17). The cells were differentiated with recombinant murine granulocyte-macrophage colony-stimulating factor (20 ng/ml; Invitrogen). Macrophages, peripheral dendritic cells (pDCs), and B and T cells were obtained from the peripheral blood of mouse APOBEC3 (E73Q/E253Q) transgenic mice and C57BL/6 mice of similar ages. The blood was treated with ammonium-chloride-potassium (ACK) lysis buffer to remove red blood cells. After cells were washed with phosphate-buffered saline (PBS)–2% fetal bovine serum (FBS), they were incubated with anti-CD3-phycoerythrin (PE)-Cy5, anti-B220-allophycocyanin (APC), anti-F4/80-fluorescein isothiocyanate (FITC), and anti-CD11c-PE for 30 min on ice. Stained cells were washed two times with PBS–2% FBS and sorted using a Beckman-Coulter EPICs Elite ESP instrument. The sorted cells were lysed for RNA isolation using a PicoPure RNA purification kit (Thermo Fisher Scientific) according to the manufacturer's instructions.
Virus isolation.
MMLV and gGagmut MLV were isolated from the supernatants of NIH 3T3 fibroblasts stably infected with virus, as previously described (38). For virus isolation from spleen, splenocytes were collected and incubated in RPMI 1640 medium, 10% fetal calf serum (FCS), nonessential amino acids, and penicillin-streptomycin for 48 h. The medium was passed through a 0.4-μm-pore-size filter, treated with 20 U/ml DNase I (Roche) at 37°C for 30 min, and pelleted through a 30% sucrose cushion. After resuspension, titers of WT and gGagmut MLV were determined on NIH 3T3 cells, and virus was quantified by reverse-transcribed RT-qPCR and analyzed by Western blotting with anti-MLV antiserum. The primers used for virus quantification are located in the env genes (MMLV, 5′-CCTACTACGAAGGGGTTG-3′ and 5′-CACATGGTACCTGTAGGGGC-3′). MMTV(RIII) virus was isolated as previously described from tumors of APOBEC3 KO mice (17). Real-time PCR was used to calculate approximate MMTV virion RNA levels in the preparation, as previously described (15).
Infectivity assays.
MMLV and gGagmut MLV infection levels in the spleens of the infected mice were determined by infectious center (IC) assays using a focal immunofluorescence assay, as previously described (26). ICs were counted using a Nikon Diaphot 300 fluorescence microscope.
In vivo infections.
For all experiments, newborn mice were generated by crossing mice heterozygous for the transgene, homozygous for mouse APOBEC3 KO with APOBEC3 KO mice, generating both transgenic and nontransgenic mouse APOBEC3 KO controls. Infections were done without genotyping the mice; genotyping was performed after virus titers were determined. Newborn C57BL/6 mice were also infected with MMLV and served as WT controls. For MMLV infections, 2-day-old mice were infected by intraperitoneal (i.p.) injection of 105 ICs of WT MMLV or MLVgGag and harvested at either 6 dpi or 18 dpi, as previously described (26, 30). For MMTV(RIII) infections, 1 × 106 virions were injected subcutaneously into 5-day-old mice. Infected mouse draining lymph nodes were harvested at 4 days postinfection (45). To measure the amounts of viral DNA in the spleens of the infected mice, splenic DNA was isolated using a DNeasy blood and tissue kit (Qiagen). RT-PCR was performed using a Power SYBR green PCR master mix kit (Applied Biosystems). For MMLV, the env primers described above in “Virus isolation” were used (61). The primers used for MMTV detection were 5′-CGTGAAAGACTCGCCAGAGCTA-3′ and 5′-GAAGATGATCTTCAAGGGCAATGCCT-3′. GAPDH primers were used in both cases for normalization (5′-CCCCTTCATTGACCTCAACTACA-3′ and 5′-CGCTCCTGGAGGATGGTGAT-3′).
Target cell assays.
BMDCs were infected by the spinoculation method as previously described (35). Briefly, BMDCs were seeded in a 96-well plate at a cell density of 105/100 μl. MMLV (multiplicity of infection [MOI] of 1) was added to the cells, and the plates were centrifuged at 1,200 × g for 120 min at room temperature. After centrifugation, the cells were incubated for 0, 2, 4, and 8 h at 37°C. DNA was extracted using a DNeasy kit (Qiagen) according to the manufacturer's instructions. RT-qPCR was performed to examine the infection levels, as described above in “In vivo infections.”
Revertant analysis.
Mouse APOBEC3 (E73Q/E253Q) transgenic mice (2 days old) were infected with MLVgGag and sacrificed at 6 weeks. DNA was isolated from spleens, and the glyco-Gag region was PCR amplified and sequenced as previously described (30).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism software. Tests used to determine significance are described in the figure legends.
ACKNOWLEDGMENTS
We thank Yongwon Choi, Hung Fan, Takayuki Nitta, and David Derse for reagents used in this study. We also thank Alexya Aguilera and the University of Illinois at Chicago Flow Cytometry core for help with the primary cell sorting experiments. Finally, we thank Swathi Kotla for developing a precleared mouse APOBEC3 antibody that was used in this study.
This work was supported by National Institutes of Health grant R01-AI-085015. S.S. was supported by F32-AI100512 and a Mathilde Krim Fellowship in Basic Biomedical Research, American Foundation for AIDS Research (108993-57-RKHF).
We declare that we have no competing interests.
REFERENCES
- 1.Goff SP. 2004. Retrovirus restriction factors. Mol Cell 16:849–859. doi: 10.1016/j.molcel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Zheng YH, Jeang KT, Tokunaga K. 2012. Host restriction factors in retroviral infection: promises in virus-host interaction. Retrovirology 9:112. doi: 10.1186/1742-4690-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanville B, Dolan MA, Wollenberg K, Yan Y, Martin C, Yeung ML, Strebel K, Buckler-White A, Kozak CA. 2010. Adaptive evolution of Mus Apobec3 includes retroviral insertion and positive selection at two clusters of residues flanking the substrate groove. PLoS Pathog 6:e1000974. doi: 10.1371/journal.ppat.1000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawyer SL, Emerman M, Malik HS. 2004. Ancient adaptive evolution of the primate antiviral DNA-editing enzyme APOBEC3G. PLoS Biol 2:E275. doi: 10.1371/journal.pbio.0020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris RS, Dudley JP. 2015. APOBECs and virus restriction. Virology 479–480:131–145. doi: 10.1016/j.virol.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apolonia L, Schulz R, Curk T, Rocha P, Swanson CM, Schaller T, Ule J, Malim MH. 2015. Promiscuous RNA binding ensures effective encapsidation of APOBEC3 proteins by HIV-1. PLoS Pathog 11:e1004609. doi: 10.1371/journal.ppat.1004609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cen S, Guo F, Niu M, Saadatmand J, Deflassieux J, Kleiman L. 2004. The interaction between HIV-1 Gag and APOBEC3G. J Biol Chem 279:33177–33184. doi: 10.1074/jbc.M402062200. [DOI] [PubMed] [Google Scholar]
- 8.Okeoma CM, Lovsin N, Peterlin BM, Ross SR. 2007. APOBEC3 inhibits mouse mammary tumour virus replication in vivo. Nature 445:927–930. doi: 10.1038/nature05540. [DOI] [PubMed] [Google Scholar]
- 9.Svarovskaia ES, Xu H, Mbisa JL, Barr R, Gorelick RJ, Ono A, Freed EO, Hu WS, Pathak VK. 2004. Human apolipoprotein B mRNA-editing enzyme-catalytic polypeptide-like 3G (APOBEC3G) is incorporated into HIV-1 virions through interactions with viral and nonviral RNAs. J Biol Chem 279:35822–35828. doi: 10.1074/jbc.M405761200. [DOI] [PubMed] [Google Scholar]
- 10.Bishop KN, Holmes RK, Sheehy AM, Davidson NO, Cho SJ, Malim MH. 2004. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr Biol 14:1392–1396. doi: 10.1016/j.cub.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 11.Liddament MT, Brown WL, Schumacher AJ, Harris RS. 2004. APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr Biol 14:1385–1391. doi: 10.1016/j.cub.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 12.Wiegand HL, Doehle BP, Bogerd HP, Cullen BR. 2004. A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. EMBO J 23:2451–2458. doi: 10.1038/sj.emboj.7600246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes RK, Koning FA, Bishop KN, Malim MH. 2007. APOBEC3F can inhibit the accumulation of HIV-1 reverse transcription products in the absence of hypermutation. Comparisons with APOBEC3G. J Biol Chem 282:2587–2595. doi: 10.1074/jbc.M607298200. [DOI] [PubMed] [Google Scholar]
- 14.Luo K, Wang T, Liu B, Tian C, Xiao Z, Kappes J, Yu XF. 2007. Cytidine deaminases APOBEC3G and APOBEC3F interact with human immunodeficiency virus type 1 integrase and inhibit proviral DNA formation. J Virol 81:7238–7248. doi: 10.1128/JVI.02584-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacMillan AL, Kohli RM, Ross SR. 2013. APOBEC3 inhibition of mouse mammary tumor virus infection: the role of cytidine deamination versus inhibition of reverse transcription. J Virol 87:4808–4817. doi: 10.1128/JVI.00112-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman EN, Holmes RK, Craig HM, Klein KC, Lingappa JR, Malim MH, Sheehy AM. 2005. Antiviral function of APOBEC3G can be dissociated from cytidine deaminase activity. Curr Biol 15:166–170. doi: 10.1016/j.cub.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 17.Stavrou S, Crawford D, Blouch K, Browne EP, Kohli RM, Ross SR. 2014. Different modes of retrovirus restriction by human APOBEC3A and APOBEC3G in vivo. PLoS Pathog 10:e1004145. doi: 10.1371/journal.ppat.1004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollpeter D, Parsons M, Sobala AE, Coxhead S, Lang RD, Bruns AM, Papaioannou S, McDonnell JM, Apolonia L, Chowdhury JA, Horvath CM, Malim MH. 2018. Deep sequencing of HIV-1 reverse transcripts reveals the multifaceted antiviral functions of APOBEC3G. Nat Microbiol 3:220–233. doi: 10.1038/s41564-017-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Ao Z, Chen L, Kobinger G, Peng J, Yao X. 2012. The cellular antiviral protein APOBEC3G interacts with HIV-1 reverse transcriptase and inhibits its function during viral replication. J Virol 86:3777–3786. doi: 10.1128/JVI.06594-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bishop KN, Verma M, Kim EY, Wolinsky SM, Malim MH. 2008. APOBEC3G inhibits elongation of HIV-1 reverse transcripts. PLoS Pathog 4:e1000231. doi: 10.1371/journal.ppat.1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwatani Y, Chan DS, Wang F, Maynard KS, Sugiura W, Gronenborn AM, Rouzina I, Williams MC, Musier-Forsyth K, Levin JG. 2007. Deaminase-independent inhibition of HIV-1 reverse transcription by APOBEC3G. Nucleic Acids Res 35:7096–7108. doi: 10.1093/nar/gkm750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belanger K, Savoie M, Rosales Gerpe MC, Couture JF, Langlois MA. 2013. Binding of RNA by APOBEC3G controls deamination-independent restriction of retroviruses. Nucleic Acids Res 41:7438–7452. doi: 10.1093/nar/gkt527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Browne EP, Littman DR. 2008. Species-specific restriction of APOBEC3-mediated hypermutation. J Virol 82:1305–1313. doi: 10.1128/JVI.01371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rulli SJ Jr, Mirro J, Hill SA, Lloyd P, Gorelick RJ, Coffin JM, Derse D, Rein A. 2008. Interactions of murine APOBEC3 and human APOBEC3G with murine leukemia viruses. J Virol 82:6566–6575. doi: 10.1128/JVI.01357-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langlois MA, Kemmerich K, Rada C, Neuberger MS. 2009. The AKV murine leukemia virus is restricted and hypermutated by mouse APOBEC3. J Virol 83:11550–11559. doi: 10.1128/JVI.01430-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low A, Okeoma CM, Lovsin N, de las Heras M, Taylor TH, Peterlin BM, Ross SR, Fan H. 2009. Enhanced replication and pathogenesis of Moloney murine leukemia virus in mice defective in the murine APOBEC3 gene. Virology 385:455–463. doi: 10.1016/j.virol.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda E, Tsuji-Kawahara S, Sakamoto M, Langlois MA, Neuberger MS, Rada C, Miyazawa M. 2008. Mouse APOBEC3 restricts friend leukemia virus infection and pathogenesis in vivo. J Virol 82:10998–11008. doi: 10.1128/JVI.01311-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolokithas A, Rosenke K, Malik F, Hendrick D, Swanson L, Santiago ML, Portis JL, Hasenkrug KJ, Evans LH. 2010. The glycosylated Gag protein of a murine leukemia virus inhibits the antiretroviral function of APOBEC3. J Virol 84:10933–10936. doi: 10.1128/JVI.01023-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosales Gerpe MC, Renner TM, Belanger K, Lam C, Aydin H, Langlois MA. 2015. N-linked glycosylation protects gammaretroviruses against deamination by APOBEC3 proteins. J Virol 89:2342–2357. doi: 10.1128/JVI.03330-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stavrou S, Nitta T, Kotla S, Ha D, Nagashima K, Rein AR, Fan H, Ross SR. 2013. Murine leukemia virus glycosylated Gag blocks apolipoprotein B editing complex 3 and cytosolic sensor access to the reverse transcription complex. Proc Natl Acad Sci U S A 110:9078–9083. doi: 10.1073/pnas.1217399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renner TM, Belanger K, Lam C, Gerpe MR, McBane JE, Langlois MA. 2018. Full-length glycosylated Gag of murine leukemia virus can associate with the viral envelope as a type I integral membrane protein. J Virol 92:e01530-17. doi: 10.1128/JVI.01530-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hakata Y, Landau NR. 2006. Reversed functional organization of mouse and human APOBEC3 cytidine deaminase domains. J Biol Chem 281:36624–36631. doi: 10.1074/jbc.M604980200. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Ma J, Zhang Q, Zhou J, Yin X, Zhai C, You X, Yu L, Guo F, Zhao L, Li Z, Zeng Y, Cen S. 2011. Functional analysis of the two cytidine deaminase domains in APOBEC3G. Virology 414:130–136. doi: 10.1016/j.virol.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Nair S, Sanchez-Martinez S, Ji X, Rein A. 2014. Biochemical and biological studies of mouse APOBEC3. J Virol 88:3850–3860. doi: 10.1128/JVI.03456-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Courreges MC, Burzyn D, Nepomnaschy I, Piazzon I, Ross SR. 2007. Critical role of dendritic cells in mouse mammary tumor virus in vivo infection. J Virol 81:3769–3777. doi: 10.1128/JVI.02728-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dzuris JL, Golovkina TV, Ross SR. 1997. Both T and B cells shed infectious mouse mammary tumor virus. J Virol 71:6044–6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finstad SL, Rosenberg N, Levy LS. 2007. Diminished potential for B-lymphoid differentiation after murine leukemia virus infection in vivo and in EML hematopoietic progenitor cells. J Virol 81:7274–7279. doi: 10.1128/JVI.00250-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan H, Chute H, Chao E, Feuerman M. 1983. Construction and characterization of Moloney murine leukemia virus mutants unable to synthesize glycosylated gag polyprotein. Proc Natl Acad Sci U S A 80:5965–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chun R, Fan H. 1994. Recovery of glycosylated gag virus from mice infected with a glycosylated gag-negative mutant of Moloney murine leukemia virus. J Biomed Sci 1:218–223. doi: 10.1007/BF02253305. [DOI] [PubMed] [Google Scholar]
- 40.Corbin A, Prats AC, Darlix JL, Sitbon M. 1994. A nonstructural gag-encoded glycoprotein precursor is necessary for efficient spreading and pathogenesis of murine leukemia viruses. J Virol 68:3857–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Low A, Datta S, Kuznetsov Y, Jahid S, Kothari N, McPherson A, Fan H. 2007. Mutation in the glycosylated gag protein of murine leukemia virus results in reduced in vivo infectivity and a novel defect in viral budding or release. J Virol 81:3685–3692. doi: 10.1128/JVI.01538-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berger G, Durand S, Fargier G, Nguyen XN, Cordeil S, Bouaziz S, Muriaux D, Darlix JL, Cimarelli A. 2011. APOBEC3A is a specific inhibitor of the early phases of HIV-1 infection in myeloid cells. PLoS Pathog 7:e1002221. doi: 10.1371/journal.ppat.1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koning FA, Goujon C, Bauby H, Malim MH. 2011. Target cell-mediated editing of HIV-1 cDNA by APOBEC3 proteins in human macrophages. J Virol 85:13448–13452. doi: 10.1128/JVI.00775-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okeoma CM, Low A, Bailis W, Fan HY, Peterlin BM, Ross SR. 2009. Induction of APOBEC3 in vivo causes increased restriction of retrovirus infection. J Virol 83:3486–3495. doi: 10.1128/JVI.02347-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okeoma CM, Petersen J, Ross SR. 2009. Expression of murine APOBEC3 alleles in different mouse strains and their effect on mouse mammary tumor virus infection. J Virol 83:3029–3038. doi: 10.1128/JVI.02536-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfeiffer JK, Topping RS, Shin NH, Telesnitsky A. 1999. Altering the intracellular environment increases the frequency of tandem repeat deletion during Moloney murine leukemia virus reverse transcription. J Virol 73:8441–8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS, Malim MH. 2003. DNA deamination mediates innate immunity to retroviral infection. Cell 113:803–809. doi: 10.1016/S0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 48.Harris RS, Petersen-Mahrt SK, Neuberger MS. 2002. RNA editing enzyme APOBEC1 and some of its homologs can act as DNA mutators. Mol Cell 10:1247–1253. doi: 10.1016/S1097-2765(02)00742-6. [DOI] [PubMed] [Google Scholar]
- 49.Jonsson SR, Hache G, Stenglein MD, Fahrenkrug SC, Andresdottir V, Harris RS. 2006. Evolutionarily conserved and non-conserved retrovirus restriction activities of artiodactyl APOBEC3F proteins. Nucleic Acids Res 34:5683–5694. doi: 10.1093/nar/gkl721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edwards SA, Fan H. 1979. gag-Related polyproteins of Moloney murine leukemia virus: evidence for independent synthesis of glycosylated and unglycosylated forms. J Virol 30:551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans LH, Dresler S, Kabat D. 1977. Synthesis and glycosylation of polyprotein precursors to the internal core proteins of Friend murine leukemia virus. J Virol 24:865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prats AC, De Billy G, Wang P, Darlix JL. 1989. CUG initiation codon used for the synthesis of a cell surface antigen coded by the murine leukemia virus. J Mol Biol 205:363–372. doi: 10.1016/0022-2836(89)90347-1. [DOI] [PubMed] [Google Scholar]
- 53.Edwards SA, Fan H. 1980. Sequence relationship of glycosylated and unglycosylated gag polyproteins of Moloney murine leukemia virus. J Virol 35:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujisawa R, McAtee FJ, Zirbel JH, Portis JL. 1997. Characterization of glycosylated Gag expressed by a neurovirulent murine leukemia virus: identification of differences in processing in vitro and in vivo. J Virol 71:5355–5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fujisawa R, McAtee FJ, Favara C, Hayes SF, Portis JL. 2001. N-terminal cleavage fragment of glycosylated Gag is incorporated into murine oncornavirus particles. J Virol 75:11239–11243. doi: 10.1128/JVI.75.22.11239-11243.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pillemer EA, Kooistra DA, Witte ON, Weissman IL. 1986. Monoclonal antibody to the amino-terminal L sequence of murine leukemia virus glycosylated gag polyproteins demonstrates their unusual orientation in the cell membrane. J Virol 57:413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwartzberg P, Colicelli J, Goff SP. 1983. Deletion mutants of Moloney murine leukemia virus which lack glycosylated gag protein are replication competent. J Virol 46:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nair S, Rein A. 2014. Antiretroviral restriction factors in mice. Virus Res 193:130–134. doi: 10.1016/j.virusres.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okeoma CM, Huegel AL, Lingappa J, Feldman MD, Ross SR. 2010. APOBEC3 proteins expressed in mammary epithelial cells are packaged into retroviruses and can restrict transmission of milk-borne virions. Cell Host Microbe 8:534–543. doi: 10.1016/j.chom.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC. [Google Scholar]
- 61.Stavrou S, Blouch K, Kotla S, Bass A, Ross SR. 2015. Nucleic acid recognition orchestrates the anti-viral response to retroviruses. Cell Host Microbe 17:478–488. doi: 10.1016/j.chom.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]