ABSTRACT
Hepatitis C virus (HCV) infection is a global health problem, with nearly 2 million new infections occurring every year and up to 85% of these infections becoming chronic infections that pose serious long-term health risks. To effectively reduce the prevalence of HCV infection and associated diseases, it is important to understand the intracellular dynamics of the viral life cycle. Here, we present a detailed mathematical model that represents the full hepatitis C virus life cycle. It is the first full HCV model to be fit to acute intracellular infection data and the first to explore the functions of distinct viral proteins, probing multiple hypotheses of cis- and trans-acting mechanisms to provide insights for drug targeting. Model parameters were derived from the literature, experiments, and fitting to experimental intracellular viral RNA, extracellular viral titer, and HCV core and NS3 protein kinetic data from viral inoculation to steady state. Our model predicts higher rates for protein translation and polyprotein cleavage than previous replicon models and demonstrates that the processes of translation and synthesis of viral RNA have the most influence on the levels of the species we tracked in experiments. Overall, our experimental data and the resulting mathematical infection model reveal information about the regulation of core protein during infection, produce specific insights into the roles of the viral core, NS5A, and NS5B proteins, and demonstrate the sensitivities of viral proteins and RNA to distinct reactions within the life cycle.
IMPORTANCE We have designed a model for the full life cycle of hepatitis C virus. Past efforts have largely focused on modeling hepatitis C virus replicon systems, in which transfected subgenomic HCV RNA maintains autonomous replication in the absence of virion production or spread. We started with the general structure of these previous replicon models and expanded it to create a model that incorporates the full virus life cycle as well as additional intracellular mechanistic detail. We compared several different hypotheses that have been proposed for different parts of the life cycle and applied the corresponding model variations to infection data to determine which hypotheses are most consistent with the empirical kinetic data. Because the infection data we have collected for this study are a more physiologically relevant representation of a viral life cycle than data obtained from a replicon system, our model can make more accurate predictions about clinical hepatitis C virus infections.
KEYWORDS: hepatitis C virus, mathematical model, polyprotein cleavage, RNA, hepatitis C virus
INTRODUCTION
Hepatitis C virus (HCV) is one of the most prevalent viruses in the world, with an estimated 71 million people worldwide affected by chronic infection (1). Despite the existence of effective direct-acting antiviral treatments, which can effectively cure more than 90% of HCV cases treated, many people infected with the virus do not have access to these expensive drugs and remain untreated (2–4). It is estimated that approximately 399,000 people die every year due to cirrhosis or hepatocellular carcinoma associated with current or past chronic HCV infection (1). Further study of the virus will help to yield greater understanding of the viral life cycle, which could help to expand treatment options, target resistant strains, and/or limit HCV-associated pathology.
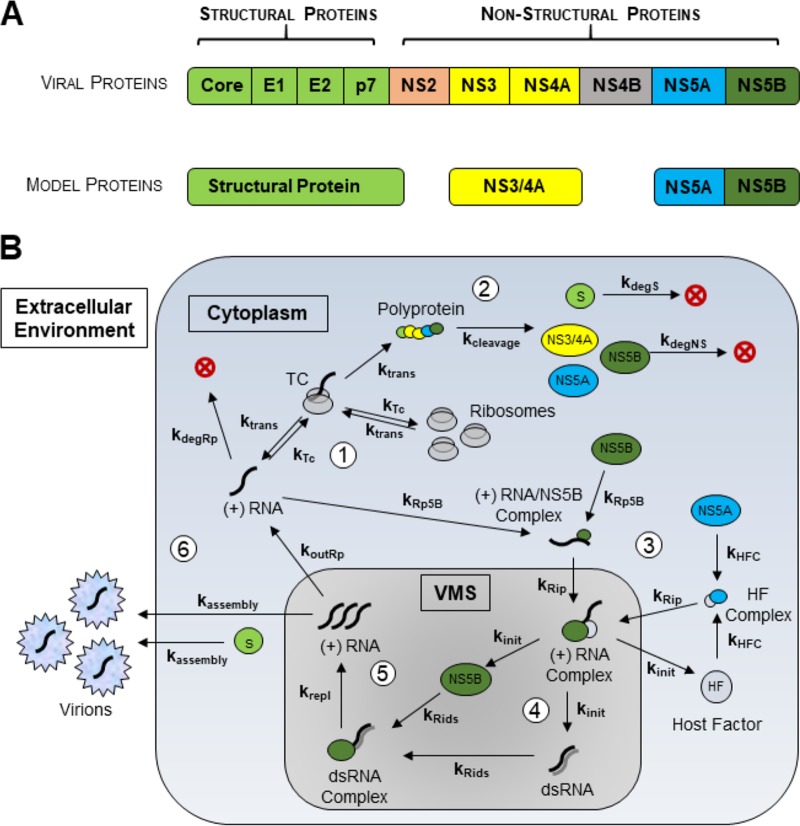
HCV is a blood-borne plus-strand RNA virus of the family Flaviviridae that infects hepatocytes and maintains virion production over the course of years, sustaining a chronic infection that often results in minimal symptoms for much of this period (5). The viral particle consists of an enveloped capsid containing an approximately 9,600-nucleotide genomic RNA. The HCV life cycle begins with endocytosis of a viral particle into the host cell and the subsequent uncoating and release of the viral RNA into the cytoplasm. This viral genome serves as the template for host cell ribosomes to translate the virally encoded polyprotein, which is subsequently cleaved into individual viral proteins by a combination of both viral and host proteases (Fig. 1A). Once synthesized, the viral nonstructural proteins remodel the host cell membranes to form viral replication compartments referred to as the vesicular membranous structure (VMS). Here the viral polymerase, in concert with other nonstructural proteins, amplifies the viral genomic RNA through a negative-strand intermediate. Newly synthesized viral RNA is then either utilized to translate more viral proteins or packaged into capsids made up of the core protein. These RNA-containing capsids are enveloped as they bud into the endoplasmic reticulum, picking up the viral envelope glycoproteins E1 and E2, and are secreted from the cell via the secretory pathway.
FIG 1.
Schematic of the mathematical model of infectious HCV. (A) Diagram of the HCV polyprotein and its representation as various model species. (B) The entire model schematic and reactions involved. The six steps are indicated by white circles with the numbers 1 to 6. In step 1, a single initiator plus-strand RNA [(+) RNA] associates with available ribosomes to form a translation complex (TC), which then synthesizes the viral polyprotein. In step 2, the viral proteins are cleaved in a single step from the polyprotein, releasing a generalized structural protein and each of the nonstructural proteins NS3/4A, NS5A, and NS5B. Generalized viral structural proteins are shown as a green circle labeled S. In step 3, RNA polymerase NS5B reassociates with a cytoplasmic RNA molecule, while NS5A associates with a host factor (HF). Together these complexes are imported into the vesicular membranous structure (VMS) in a second-order reaction. In step 4, the minus strand of the viral RNA is synthesized from the newly imported plus-strand complex, after which it releases NS5B and forms a double-strand RNA molecule. In step 5, the dsRNA is bound once again by the NS5B molecule, which synthesizes new plus-strand RNA from this template. In step 6, VMS plus-strand RNA associates with a structural protein to assemble the viral particle, which is then exported into the extracellular environment.
Development of the HCV subgenomic replicon model in 1999 demonstrated that five of the nonstructural proteins (NS3, NS4A, NS4B, NS5A, and NS5B) are necessary and sufficient for HCV RNA replication (6). While subgenomic replicons do not recapitulate the full viral life cycle, they provide a useful experimental system for studying HCV replication dynamics. Two notable HCV subgenomic replicon mathematical models have been developed in the past decade: the first one built in 2007 by Dahari et al. which was fit to steady-state HCV replicon data (7) and another in 2013 by Binder et al. which expanded upon the first model to capture the early dynamics of the HCV replicon system immediately after transfection of subgenomic RNA into host cells (8). The primary difference between the two models is the import step of RNA into the VMS, a reaction which was updated in the later model to incorporate an interaction with a limited quantity of a host factor molecule and to represent the cis-acting behavior of the viral RNA polymerase NS5B. This behavior has been reported for HCV as well as other flaviviruses and RNA viruses (9–11). The structure of the Binder model (8) has also been integrated into a multiscale infection model by Clausznitzer et al. to study patient-to-patient variation in response to treatment with the NS5A inhibitor daclatasvir (12). The Clausznitzer model added virus entry and egress processes, as well as a generalized structural protein involved in viral particle assembly and egress. However, most parameters in the multiscale model were taken from the Binder model, and new reaction constants were fit only to extracellular viral load data, which makes it difficult to draw direct conclusions about intracellular processes during infection.
Our efforts began with an evaluation of the structures used in these models to determine where more detail or improvements could be made. The previous models lump viral proteins into a single entity, making it impossible to separate individual functions. Furthermore, the assumed cis-acting behavior of the viral polymerase (NS5B) as well as other viral NS proteins in the previous models limited how the activity of these proteins could be represented. We address these issues by incorporating more detail across the replication cycle and focusing on distinctions between HCV nonstructural proteins. In addition, we have expanded upon these replicon models to include viral infection initiation and secretion of progeny HCV particles from the host cell and incorporated new experimental data about protein degradation rates to create a detailed mathematical model of HCV infection in vitro. We then trained and validated this model by fitting to experimental RNA and protein data produced using the JFH-1 HCV cell culture (HCVcc) system. Here, we present this comprehensive model and use it to probe individual viral protein cis- and trans-acting functions, protein regulation, and assess the function of the VMS.
RESULTS
The hepatitis C virus infection model.
Our infection model was developed by expanding and modifying the basic structure of previous models to accommodate observations from the experimental literature and our own experimental data. The final model schematic is shown in Fig. 1B. This model consists of 21 biological reactions with 15 reaction constants (Table 1) and 17 species that yield 17 differential equations to represent their time-dependent concentrations (see Materials and Methods).
TABLE 1.
Model parameter estimates obtained from experiment, literature, and simulation
| Process or category and parameter | Parameter estimate | Descriptiona | Source of model parameter |
|---|---|---|---|
| Translation | |||
| kTC | 1 molecule−1 h−1 | Transcription complex formation | 8 |
| ktranslation | 180 h−1 | Polyprotein translation rate | Fitting |
| kcleavage | 9 h−1 | Structural protein cleavage rate | Fitting |
| Replication | |||
| kHFC | 0.0008 molecule−1 h−1 | Host factor/NS5A complex formation | Fitting |
| kRp5B | 0.1 molecule−1 h−1 | (+)RNA/NS5B complex formation | Fitting |
| kRip | 0.6 molecule−1 h−1 | (+)RNA and NS5B import into VMS | Fitting |
| kinitiation | 1.12 h−1 | (−)RNA synthesis | 6, 51, 52 |
| kRids | 10 molecule−1 h−1 | dsRNA replication complex formation | 8 |
| kreplication | 1.12 h−1 | (+)RNA synthesis | 6, 51, 52 |
| Import/export | |||
| koutRp | 0.307 h−1 | (+)RNA transport from VMS to cytoplasm | 8 |
| kassembly | 6.7 × 10−10 molecule−1 h−1 | Viral particle assembly | Fitting |
| Degradation | |||
| kdegRp | 0.26 h−1 | Cytoplasmic (+)RNA degradation | Fitting |
| kdegS | Initial, 0.61 h−1; final, 0.10 h−1 | Structural protein degradation | Experimental data |
| kdegNS | 0.11 h−1 | NS3/4A degradation | Experimental data |
| kdegVMS | 0.001 h−1 | VMS species degradation | Fitting; 38, 39 |
| Host species | |||
| No. of ribosome molecules | 5,000 molecules | Fitting | |
| No. of host factor molecules | 30 molecules | Fitting | |
| Initial values | |||
| No. of plus-strand RNA | 1 molecule | ||
| No. of core protein | 180 molecules |
(+)RNA, plus-strand RNA; (−)RNA, minus-strand RNA.
To represent a single virus initiating infection, there is one plus-strand RNA molecule at the start of the simulation at 0 h. In step 1 of the model, the cytoplasmic HCV RNA associates with available ribosomes in a second-order reaction to form a translation complex (TC) at a rate kTC. This complex then synthesizes the viral polyprotein in a first-order reaction with rate constant ktranslation. In step 2, the viral structural proteins and the nonstructural proteins (with NS3/4A, NS5A, and NS5B specifically represented in the model) are cleaved simultaneously to represent the rapid processing of the viral polyprotein that has been reported in the literature (13, 14). In step 3, we assume that a single cytoplasmic HCV RNA and NS5B associate to form a 1:1 RNA/NS5B complex at rate kRp5B, and a free host factor (HF) associates with NS5A to form a HF/NS5A complex at rate kHFC, both in second-order reactions according to the reactant concentrations. These two complexes are then imported into the vesicular membranous structure (VMS) in a second-order reaction at rate kRip to produce an Rip (RNA plus-strand intermediate) complex that will make minus-strand RNA. In step 4, inside the VMS, the minus-strand RNA is synthesized—creating a double-strand RNA (dsRNA) molecule—in a first-order reaction with rate constant kinitiation. Next, in step 5, the dsRNA molecule associates with NS5B to form the Rids (RNA double-stranded intermediate) complex, a second-order reaction at rate kRids, which then synthesizes new plus-strand RNA in a first-order reaction with rate constant kreplication. This double-stranded molecule remains in the VMS to continue synthesizing plus-strand RNA. New plus-strand RNA in the VMS can be exported into the cytoplasm in a first-order reaction at rate koutRp for subsequent translation of viral proteins or, in step 6, be assembled into a virus particle and exported from the cell through association with cytoplasmic structural protein, a second-order reaction at rate kassembly (Fig. 1B; Table 1 and 2). This model was finalized after a comparison of several hypothesized mechanisms to find the one able to best fit our experimental data. These variant models were constructed and evaluated to address the main goals of our system as follows.
TABLE 2.
Ordinary differential equations for the final model
| Compartment and species | Equationa | Equation no. |
|---|---|---|
| Cytoplasm | ||
| Plus-strand RNA | 1 | |
| Ribosomes (Ribo) | 2 | |
| Translation complex (TC) | 3 | |
| Polyprotein (PP) | 4 | |
| Core protein | 5 | |
| NS3/4A protein complex | 6 | |
| NS5A protein | 7 | |
| Cytoplasmic NS5B protein (NS5Bcyt) | 8 | |
| Host factor (HF) | 9 | |
| NS5A/host factor complex (HFC) | 10 | |
| RNA/NS5B complex | 11 | |
| VMS | ||
| Plus-strand RNA complex | 12 | |
| Double-strand RNA (dsRNA) | 13 | |
| VMS NS5B | 14 | |
| dsRNA complex | 15 | |
| VMS plus-strand RNA | 16 | |
| Extracellular environment | ||
| Secreted viral particle | 17 |
ktrans, ktranslation; kinit, kinitiation; S, core/structural protein; krepl, kreplication; V, viral particle.
Roles of cis- and trans-acting viral proteins.
A key element of the most recent replicon model's structure is the direct import of translation complexes into the VMS in a third-order reaction with a cellular host factor molecule and a single viral protein. This was meant to recapitulate the proximity of the viral RNA to protein translated from it, as well as the cis-acting behavior of the nonstructural proteins, most of which at the time had yet to be found capable of being trans-complemented (8). However, with more recent data indicating the ability for trans-complementation of the HCV nonstructural proteins (15), one goal of our model was to examine and probe this concept further and to more accurately portray the cis/trans-acting behavior of the HCV nonstructural proteins involved in replication (NS3-NS5B).
Individually, the protein NS3 is acknowledged to assist in several processes throughout the viral life cycle (16, 17), but its most essential and well-defined function is performed in tandem with the cofactor NS4A as a critical viral protease. Therefore, our model treats these two proteins as a single unit, acting only in their protease function and cleaving themselves and the downstream viral proteins from the HCV polyprotein (18). The protease activity of NS3/4A functions only in cis (10, 15), and for this reason, the subsequent cleavage steps were chosen to be first-order reactions, with the activity of NS3/4A encompassed in the reaction constants, as the whole-cell concentration of this protein will not affect the rate of cis-acting activity.
The viral protein NS4B, like its neighbors in the polyprotein, takes part in a number of viral functions, but its most distinct role is in helping to assemble the membranous web architecture of the VMS (19, 20). The functional role of NS4B was not included in the model, because the sequence and process of VMS formation remain largely unknown and likely involve many host molecules as well as NS4B self-interaction (21), which would add complexity without adequate mechanistic understanding or justification.
Research in the past decade has consistently demonstrated the potential for the viral protein NS5A to be trans-complemented when deactivating mutations are introduced into the replicon NS5A sequence (10, 15). Previous models have used a single generalized nonstructural protein and allowed the cytoplasmic role of that protein to be supplied in trans, ostensibly representing the function of the protein NS5A. However, once inside the VMS, the generalized nonstructural protein in these models switches to acting like the viral RNA polymerase NS5B (7, 8, 12). Our model was designed to separate these functions to better assess the individual importance and sensitivity of the system to NS5A and NS5B individually.
The NS5A protein has been implicated in a diverse and complex set of viral processes within the cell, with different domains of the protein associated with viral replication and viral particle assembly (16, 22–25). The various functions of this protein appear to be modulated by the phosphorylation state and binding to an array of cellular host factors, including the host factor cyclophilin A, and these interactions have been explored as a target of HCV treatment (26–31).
To incorporate NS5A individually in our model, we assume that after cleavage from the polyprotein, NS5A associates with a generalized host factor to form a host factor complex. This is meant to encompass the various interactions between NS5A and the host environment. After this step, the NS5A-host factor complex associates with a complex which includes NS5B and HCV plus-strand RNA to create a replication complex, which is imported into the VMS (Fig. 1B, step 4). This is consistent with the activity of cyclophilin A, which associates with both NS5A and NS5B, and localizes to the VMS during HCV replication (32). It also agrees with prior research that has shown that NS5A is required for the formation of double-membrane vesicles within the VMS, which house the HCV replication complexes (33). Finally, it is consistent with studies that have shown that NS5A exhibits RNA-binding activity required for the initiation of replication (34, 35). To simplify our model, the viral particle assembly function of NS5A (36, 37) was omitted, though this mechanism may be of interest in future investigations.
The RNA-dependent RNA polymerase NS5B is critical for a productive hepatitis C virus infection and is an important therapeutic target (22), which makes its role in the viral life cycle important to understand. The primary role of NS5B is in the synthesis of minus- and plus-strand viral RNA within the VMS (16), which is also the primary role it holds within this model. Despite prior belief that NS5B must function in cis, recent trans-complementation studies show that in a system where the polymerase function of NS5B is defective, trans-complementation can succeed if functional NS5B is supplied to the defective system as a member of a separate NS3-NS5B polyprotein construct (10, 15). On the basis of this observation, we constructed models to represent two novel mechanisms for the interaction between viral RNA and NS5B which would allow for differing cis- or trans-activities of NS5B, as alternatives to the mechanism proposed by Binder et al. (8) (Fig. 2A).
FIG 2.
Schematic comparison of two additional cis-acting protein mechanisms. The Binder/Clausznitzer mechanism has been proposed by previous modeling papers. In the Binder/Clausznitzer mechanism, RNA is imported into the VMS as part of an active translation complex in a third-order reaction with NS5B and native host factor. An alternative cis-protein mechanism we propose assumes that the viral RNA stays associated with the polyprotein translated from it throughout the cleavage steps, whereas the final model (Fig. 1) assumes that it is free to release into the greater cytoplasmic RNA pool. This alternative model assumes the final RNA/NS5B complex can be imported to the VMS with the host factor complex or be recycled at rate kTCR (translation complex recycle) for another round of translation.
In alternative mechanism 1, we hypothesize that the plus-strand RNA molecule remains bound to NS5B throughout the cleavage process (Fig. 2B). At the end of the cleavage steps, NS5A is released—as it can be supplied in trans—and a complex of RNA with NS5B remains. This RNA/NS5B complex then associates with the host factor/NS5A complex (see above NS5A section), and these two are imported into the VMS in a second-order reaction. This mechanism suggests that NS5B trans-complementation would occur only if the wild-type NS5B protein were able to replace the defective NS5B that is translated from the RNA. This is one of the possibilities posed by Kazakov et al. in their trans-complementation study (15).
The other possibility proposed by the Kazakov et al. study is that the plus-strand RNA is transferred from the ribosome that produced a defective polyprotein to a functional set of the NS3-NS5B proteins for replication (15). For our model, the implication of this mechanism is that the plus-strand RNA associates with a particular NS5B only immediately prior to trafficking into the VMS. Therefore, in alternative mechanism 2, we hypothesize that the plus-strand RNA molecule is not bound to NS5B through the processing step and can instead continue to translate more polyprotein (Fig. 1B). In this model, the RNA is required to associate with a free NS5B molecule to form the RNA/NS5B complex, which is then imported into the VMS, as in alternative mechanism 1. Of note, alternative mechanism 2 allows for a larger accumulation of excess viral proteins early in the infection, which is consistent with previous research which has shown that most viral proteins do not enter the VMS (38, 39). Both these models were fit to the experimental data to determine which was able to produce a better fit.
VMS structure and replication mechanism.
Early HCV imaging studies established that the structure of the VMS consists of a membranous web that forms a protective double-membrane system around the viral replication complexes (19, 40). Consistent with this, our initial fitting efforts showed a tendency for the VMS degradation rate in our model to drop to zero to achieve a better fit. As this is consistent with prior studies that have shown complete nuclease/protease resistance within the replicative compartment (38, 39), we set the VMS degradation rate to 0.001—a low but nonzero value in the interest of biological accuracy—for our simulation.
Notably, recent imaging and three-dimensional (3D) modeling of HCV-induced membrane alterations have indicated that the VMS contains multiple, smaller individual replication complexes enclosed in distinct vesicles (41) consistent with previous stoichiometry indicating that individual HCV replication complexes contain a single minus-strand RNA and 10 positive-strand RNAs (42). The implication of this hypothesis for an HCV model is that, rather than treating the VMS as a single large pool of viral protein and template RNA for replication, each reaction inside the VMS would be first order and would not depend on the larger VMS concentration of proteins or RNAs. For our model, only one reaction must change to achieve this: the formation of the dsRNA/NS5B complex to produce new plus-strand RNA, which is originally designed as a second-order reaction. The broader implication of this first-order model is that there will be a smaller difference between early life cycle and late life cycle association of the Rids complex, as the total amount of NS5B will have no bearing on this reaction rate.
Experimental monitoring of HCV life cycle kinetics.
To train the mathematical model on the early infection dynamics of HCV, we performed experiments to quantify intracellular and extracellular viral parameters from the initiation of infection to steady state. For these experiments, Huh7 cells were inoculated with JFH-1 HCV cell culture (HCVcc) at a multiplicity of infection (MOI) of 3 for 3 h. Parallel samples were collected at various time points to count the number of cells and measure the levels or titers of extracellular HCV, and intracellular HCV and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA, as well as the viral and cellular proteins core, NS3, and actin. Immunofluorescence staining 24 h postinoculation before progeny virus production is observed indicated that this inoculation resulted in 95% of cells in the culture being infected (see Materials and Methods).
HCV RNA and cellular GAPDH mRNA were measured by reverse transcription-quantitative PCR (RT-qPCR) and used to calculate HCV RNA copies per cell. As typically observed after a high-MOI viral infection, the RNA accumulation kinetics showed a slight decline of HCV RNA from 3 to 6 h postinoculation, indicative of the degradation of excess noninfectious input viral RNA, before evidence of RNA amplification was detected at 9 h. This was followed by an exponential increase in viral RNA thereafter (Fig. 3A). The presence of infectious virus was monitored by titration of the culture medium (Fig. 3B). The infectious virus detected at the 3-h time point is the virus remaining from the inoculum that had not initiated infection prior to its removal. After removal of the inoculum at 3 h, residual infectious virus declined slowly. The first increase in extracellular infectious virus was detected at 24 h, presumably indicating the accumulation of secreted progeny virus, which subsequently increased exponentially until the end of the experiment.
FIG 3.
Acute HCVcc infection RNA and protein kinetic data. A 48-h infection experiment using Huh7 cells with JFH-1 hepatitis C virus at an MOI of 3. (A) Profiles were calculated for the average total viral RNA (plus and minus strand), core protein, and NS3 protein per cell over the course of the experiment, with the early time points not used in fitting due to residual background levels of HCV RNA and core protein. (B) Extracellular titer data were calculated by counting focus-forming units (FFU) in the infection system throughout the 48-h experiment. The FFU data were then converted to virions produced per cell using cell counts and previous data that showed 240 virions per FFU in an experiment with the same virus strain. (C) Western blot images for core, NS3, and actin proteins.
In parallel wells, we measured intracellular core, NS3, and β-actin protein by Western blotting (Fig. 3C). Core was detected at 3 h postinoculation, but levels slowly declined until 15 h postinoculation, consistent with the detection of input core and subsequent degradation before any new accumulation of core could be detected (Fig. 3A). In contrast, NS3 was barely detected at 3 h postinoculation but immediately started to accumulate until ∼12 h postinoculation when HCV RNA amplification becomes exponential. NS3 levels plateaued from 12 to 24 h postinoculation before beginning to increase again coincident with the first detectable accumulation of core and the first detectable progeny virus secretion (Fig. 3B). The levels of β-actin were used to normalize protein levels across all time points.
HCV protein degradation kinetics.
Because core and NS3 did not accumulate with the same kinetics during the viral life cycle (Fig. 3A), we proceeded to determine the degradation rate of these proteins at two time points in the viral life cycle. For this experiment, Huh7 cells were infected with HCVcc at an MOI of 3. Cycloheximide, a protein translational inhibitor, was introduced at either 12 h postinoculation (at the beginning of HCV RNA replication but prior to progeny virion production) or 27 h postinoculation (coincident with active progeny virion assembly) to examine differences between degradation rates at these time points. After the addition of cycloheximide, protein lysates were harvested from parallel cultures at 1-h intervals, and levels of core and NS3 were measured by Western blotting to monitor the decay of the preexisting pool of each of these proteins. In the absence of new protein synthesis, NS3 levels remained relatively stable for 3 h at both time intervals with similar degradation rates of 0.11 h−1 (12 to 15 h postinoculation) and 0.05 h−1 (27 to 30 h postinoculation) (Fig. 4). In contrast, while core protein was detected during the later cycloheximide treatment, it rapidly disappeared during the 12- to 15-h postinoculation time interval corresponding to a rapid degradation rate of 0.61 h−1 early in infection and a significantly slower 0.10 h−1 degradation rate later in infection (Fig. 4). While core protein is known to exist in different functional populations within the cells (e.g., unassembled versus assembled), our measurement of total core degradation rate represents an average of all the core present in the cells and presumably the changes in average degradation observed reflect the relative difference in the size of these different core populations early versus later in the viral life cycle. The NS3 data were incorporated by fitting the degradation rate for the nonstructural proteins (kdegNS) in the model using these calculated rates as upper and lower bounds. To address the core protein degradation rate, we evaluated models with different core degradation rates (as described below) that were consistent with the new degradation data available. The core degradation rate (kdegS) was set at 0.61 h−1 from 0 to 21 h, the first time point where we observe significant viral production, and at 0.10 h−1 from 21 h onward.
FIG 4.

Viral protein degradation during early and late viral life cycle. Western blot images for two 3-h experiments beginning at 12 and 27 h postinfection in the viral life cycle. Infected cells were treated with cycloheximide at the respective time points to halt translation. The levels of core, NS3, and β-actin were measured at hourly intervals after translation inhibition by densitometry image analysis of the Western blot images and normalizing to the β-actin level. Degradation rates at the 12- and 27-h time points for core and NS3 were calculated on the basis of the relative protein levels determined by Western blotting. In both cases, the degradation rates were found to increase over the course of the infection experiment.
Comparison of model mechanism.
To test the alternative proposed HCV life cycle mechanisms incorporated into our model, our empirically measured intracellular HCV RNA, virion production, and NS3 and core protein levels were fit to the model species total viral RNA, virions produced, NS3/4A, and structural protein, respectively. Total viral RNA is defined as the sum of all intracellular HCV RNA molecules contained within the model species , (plus-strand RNA in the cytoplasm) (plus-strand RNA in the VMS), dsRNA (two molecules), TC, Rp5B, Rip, and Rids (two molecules) (Table 2).
Nonstructural protein cis/trans behavior.
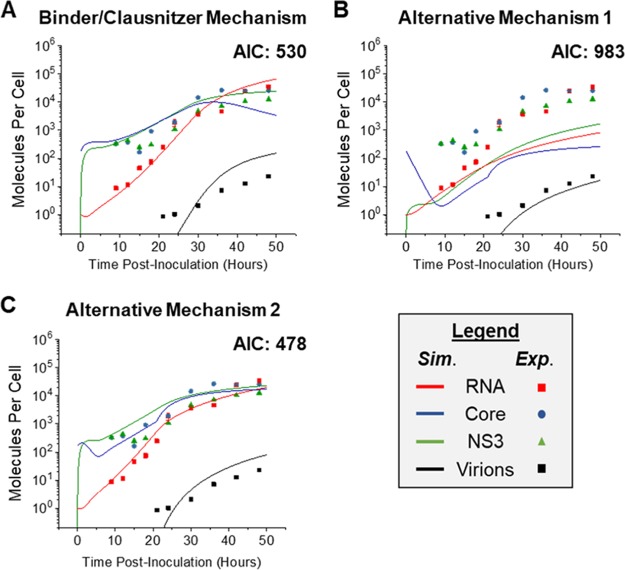
Our two hypothesized model variants for cis/trans-acting nonstructural proteins, described previously as alternative mechanism 1 and alternative mechanism 2, as well as the model based on the cis-acting protein mechanism proposed by Binder et al. (8) and used by Clausznitzer et al. (12) were fit to our experimental kinetic data to assess the separate mechanisms for cis- and trans-acting NS protein functions. The fits of the models were evaluated using the Akaike information criterion (AIC), which evaluates goodness of fit against model complexity, with lower scores indicating a better fit.
For the Binder/Clausznitzer mechanism, the fitted model had an AIC of 530 (Fig. 5A). Alternative mechanism 1 yielded an AIC of 983 (Fig. 5B), and alternative mechanism 2 yielded an AIC of 478 (Fig. 5C). The AIC for alternative mechanism 2 is substantially lower than the AIC for either of the other two models, indicating a much better model, which is apparent in the qualitative comparison of the plots. For this reason, this dual cis/trans-acting protein mechanism was chosen as the best representation for the kinetics of JFH-1 HCVcc infection in Huh7 cells.
FIG 5.
Comparison of three model variants for cis- and trans-acting protein behavior. (A) Fitted profiles for the mechanism used by previous models (Fig. 2) for the involvement of cis-acting behavior of NS5B was proposed during model development and fitting to the experimental data. This model requires that HCV RNA be imported into the VMS as part of an active translation complex (TC), along with NS5B and host factor (HF) in a third-order reaction. The fitted profiles yielded an AIC of 530. (B) Fitted profiles for the alternative cis-protein mechanism proposed by this paper (Fig. 2), which requires plus-strand RNA to be imported into the VMS using only proteins translated from itself. This fit yielded an AIC of 983. (C) Fitted profiles for the final model's mechanism for both cis- and trans-acting behavior of NS5B, which is represented by association of the NS5B protein with cytoplasmic plus-strand RNA after its cleavage, and together the two are imported into the VMS. The fitted profiles yielded an AIC of 478. Simulated (Sim.) and experimental (Exp.) data are shown.
Independent replication complexes in VMS.
To explore the possibility that all replication complex vesicles within the VMS act independently, the reactions within the VMS were modified to be first-order reactions with respect to the number of minus-strand RNAs present. As noted previously, this requires only modifying the reaction for the formation of the dsRNA/NS5B complex, so that it no longer depends on the NS5B concentration. This mechanism was evaluated in the final model against the original pooled VMS model structure. The new model fitting produced an AIC of 479 (Fig. 6), which is similar to the pooled VMS model (AIC of 478), indicating only small differences in qualitative fits. As the fitting parameters for these models are very similar, this structure may warrant further exploration with additional data, despite the fact that we maintained a pooled VMS in the final model.
FIG 6.
VMS model structure comparison. The model was modified to represent the hypothesis that the VMS is segregated into independent vesicular replication complexes, such that the production rate of plus-strand RNA would depend only on the number of minus-strand RNA molecules present. The model yielded an AIC of 479, which is a comparable fit to that for the chosen final model in RNA fitting, with only minimal qualitative differences in the fitting observed.
Variable core degradation rate.
To evaluate whether allowing for a variable core degradation rate in the model (as described above) improves the quality of the fit to the experimental data, the final model was refit using a constant degradation rate. Refitting yields a constant degradation rate of 0.28 h−1, and an AIC of 486 indicating a slightly worse model than the variable rate model (Fig. 7).
FIG 7.
Model fit with a constant core protein degradation rate. For comparison with our final model, the same model structure was refit to a new constant core protein degradation rate, as opposed to the changing core degradation rate that is part of the final model. This produced a slightly reduced fit quality.
Model validation.
To determine whether the model we have constructed is able to predict the infection dynamics of additional experiments, we performed a second set of HCV infection experiments under slightly modified experimental conditions and applied the model. These new experiments were conducted with Huh7 cells infected with a different stock of JFH-1 HCVcc at a higher MOI of 7. Again, intracellular HCV and GAPDH RNA were measured by RT-qPCR, and the number of HCV RNA copies per cell was calculated based on the number of cells.
To account for inherent biological variability between experiments, model parameters for reactions in which virus-derived species interact with each other and for which we do not have literature values, were allowed to vary up or down by 10% of their values from fitting the training data. These parameters are kRp5B, kRip, and kassembly.
Quality of fit was assessed using root mean-squared log error (RMSLE) to allow for comparison across orders-of-magnitude variation in the data and for different numbers of data points for the 36-h versus 48-h experiments. The validation RNA data achieved an RMSLE of 0.14, which is an improvement on the quality of fit achieved in the original RNA data fitting, which produced an RMSLE of 0.24 (Fig. 8). This new fit validates the applicability of our model to experiments independent from our original data set.
FIG 8.
Model validation. The final model structure was validated by fitting to a separate set of intracellular RNA data that was not involved in the original model development or parameter determination. The new model fitting allowed the parameters corresponding to viral self-interaction (kRp5B, kRip, and kassembly) to vary 10% in either direction.
Sensitivity analysis.
A parameter sensitivity analysis was conducted using the complex-step derivative approximation for HCV intracellular RNA as well as core and NS3 proteins relative to all model parameters. This method uses differential sensitivity values of the form d(model species)/d(parameter) at many time points throughout the simulation interval and integrates these values with respect to time to find relative average sensitivity values across the entire simulation (see Materials and Methods). These values can be evaluated on a relative basis, e.g., a sensitivity value of 20 corresponds to twice the influence on a fractional change in RNA or protein levels as a sensitivity of 10, when this effect is averaged across the whole simulation period.
For each species, the highest sensitivity is seen primarily for the polyprotein translation rate (ktranslation) and RNA synthesis (kinitiation and kreplication), which is expected, as each process directly produces more intracellular viral species (Fig. 9). Additionally, we observe elevated sensitivities to the parameters for translation complex formation (kTC), RNA transport to the cytoplasm (koutRp) and, for virion production, particle assembly/egress (kassembly). Of the degradation rates, the structural protein rate has the largest negative sensitivity for its own levels as well as virion production. For the VMS degradation rate (kdegVMS), there is low sensitivity for each species at the low rate we have chosen for our model. The number of virions produced consistently has higher sensitivities than the other model species, indicating that it is the most variable with fluctuations in the viral life cycle kinetics. The degradation rates produce a consistent negative sensitivity, as expected, given that degradation of viral species will slow the viral life cycle.
FIG 9.
Sensitivity analysis for RNA, core, and NS3 relative to reaction constants. Sensitivities calculated for RNA, NS3, core protein (based on the surrogate structural protein), and virions produced relative to reaction rates of our model. Similar patterns are observed across each chart, with translation (ktranslation), minus-strand synthesis (kinitiation), and replication (kreplication) yielding the highest sensitivities. Virion production consistently showed the highest sensitivity to model changes.
DISCUSSION
Evaluating viral protein cis/trans-activities creates a more accurate infection model.
While experimentally testing whether proteins function in cis or trans can be complex, modeling different mechanisms against empirical data provides an alternative strategy for investigating such questions. Our comparison of three possible mechanisms for the cis/trans-acting behavior of HCV nonstructural proteins (Fig. 5) demonstrates that the mechanism that offers the greatest trans-activity potential—referred to as alternative mechanism 2—is the best model for the HCVcc experimental data. In earlier models, the nature of nonstructural viral proteins was represented as a cis function in which active translation complex was imported into the VMS alongside a host factor and generalized nonstructural protein. However, based on more recent observations suggesting that the nonstructural proteins can be complemented as a group in trans (15), we have proposed an alternate mechanism in which the cytoplasmic viral RNA does not necessarily remain associated with the proteins that have been translated from it but can associate with any NS5B protein after cleavage from the viral polyprotein. Fitting this model to experimental data for total viral RNA, virion production, and viral proteins (NS3 and core) demonstrated that this was the best model as quantified by AIC value, suggesting that the assembly of viral replication complexes may exhibit more trans-interaction between the viral RNA and proteins than previously thought.
Introducing core protein regulation creates a more physiologically relevant infection model.
While HCV proteins are by default translated at the same rate as part of the virally encoded polyprotein, our HCVcc infection protein kinetic data revealed that the accumulation of de novo core protein was significantly delayed compared to NS3. Analysis of core and NS3 degradation rates at different times revealed a sharp decrease in core degradation rate between 12 and 27 h postinoculation, corresponding to the start of progeny viral production. Notably, incorporating this change in core degradation into our model allowed for a better model than if the core degradation was fit at a constant rate (AIC of 478 versus 486, respectively). This suggests that this change in core stability may be significant to the overall dynamics of the HCV life cycle which may warrant further theoretical and/or experimental exploration. In accordance with this possibility, the virion production of the model shows a substantial negative sensitivity to the core degradation rate (Fig. 7).
Further experiment is required to clarify the functional structure of the membranous web.
Similar to all known positive-strand RNA viruses (43), HCV induces cellular membrane alterations to create a protected space for replication of its genome with accumulating evidence suggesting that this larger membranous structure consists of smaller individual replication complexes enclosed in distinct vesicles (41). Thus, we used our model to compare the previously proposed pooled VMS structure to one in which all vesicles would act independently to determine whether either mechanism would more accurately represent the experimental data (Fig. 6). This alternate VMS structure produced a model quality virtually equivalent to that of the original mechanism. We cannot conclude which VMS structure may be more biologically relevant, as the first-order reaction for independently acting vesicles does not appear to be a crucial aspect of the model. On the basis of the marginally better model as determined by AIC, as well as the fact that previous replicon models used a pooled VMS, we have kept this pooled mechanism in our final model.
The usefulness of the final model in predicting the dynamics of hepatitis C virus infections is supported by the quality of fit achieved using a separate set of experimental HCV infection data (Fig. 8). Fitting this experimental data, while allowing only unknown parameter values for viral self-interaction to vary slightly, yielded a high-quality fit, which indicates that our model will be applicable to other sets of experimental data outside of our training set.
Fitting infection data show parameter changes from previous models and demonstrates areas for future experiment.
Many of our model parameter values have been repurposed from a prior study by Binder et al. (8), which fit an intracellular HCV model to data from a genotype 2a subgenomic replicon experiment. This system was developed from a JFH-1 isolate, the same virus clone we have used in our experiments. However, upon fitting, some model parameters involved with translation (ktranslation, kcleavage, and number of available ribosomes) were found to vary markedly from the analogous parameters in the previous model, despite the translation mechanism remaining largely intact between models. This observation may reflect a limitation in directly applying HCV replicon model parameter estimates to predict HCV infection dynamics, the significant degree of variability/permissiveness between cell lines used, which has been documented in the HCV literature (8, 44), or perhaps the difference between transfection of large quantities of HCV replicon RNA into cells versus the more authentic HCVcc infections we performed where it is believed that HCV replication begins from a single viral RNA. While such variability might in some cases make it difficult to utilize a single model across experimental systems, when comparing sufficiently similar experimental systems (e.g., replicon-to-replicon or infection-to-infection experimental systems), modeling can potentially help postulate what might make one cell line more permissive (as demonstrated by Binder et al. [8]) or one viral clone more robust.
Sensitivity analysis indicates that viral translation and replication are the critical steps in altering the viral production rate.
In terms of the steady-state levels and accumulation kinetics for total intracellular viral RNA, core, NS3, and virion production, the parameters that exhibited the most influence in this infection model were found to be ktranslation (polyprotein translation rate), kinitiation (minus-strand synthesis), and kreplication (plus-strand synthesis). These sensitivities highlight the major synthesis steps of the viral life cycle, an expected observation, as these are the processes that drive production of the RNA and protein species we are monitoring experimentally. The high sensitivity of model species to the plus- and minus-strand synthesis steps is also consistent with findings presented by Binder et al. in their analysis of the replicon mathematical model, which concluded that the replication vesicles were the most influential component of the system (8). Additional information gained from the expansion of the model to describe the full HCV life cycle indicates that virion production exhibits a high magnitude of sensitivity to several model parameters. Along with translation and replication, we observe a high sensitivity to particle assembly/egress (kassembly) and a low negative sensitivity to degradation of core protein (kdegS). These two parameters would be expected to influence virion production and raise the possibility of therapeutic targets within the viral life cycle to decrease HCV output.
Conclusions.
In this study, we have presented a model of the hepatitis C virus infection life cycle that both accurately represents the dynamics of the viral infection system and may be used to probe the functions and behaviors of individual viral proteins produced by the HCV genome. This model improves on the fitting quality of previous mechanisms, confirms distinct regulation of the levels of the HCV structural versus nonstructural viral proteins during the viral life cycle, and helps to elucidate the functional interaction of different viral proteins with the host cell. While our final model (Fig. 1) describes the infection data better than previous models, there is still room for improvement in the fit. Importantly, the nature of the fit discrepancies observed between the model simulation and the experimental data raises some mechanistic ideas that could be explored in future work. As such, this model can now be used to guide future experimentation and will allow us to continue to improve our understanding of HCV infection dynamics. Future experimental efforts could also help to clarify the precise kinetics and mechanisms that are approximated by the differential equations posed in this mathematical model. Moreover, the experimental testing of model predictions for viral production and life cycle sensitivities may aid in efforts to understand the life cycle and to target antiviral drugs and treat chronic hepatitis C virus infection.
MATERIALS AND METHODS
Experimental measurements of HCV replication. (i) Cells.
Huh7 cells were obtained from Francis Chisari (The Scripps Research Institute, La Jolla, CA) (42) and passaged in complete Dulbecco's modified Eagle's medium (cDMEM) supplemented with 10% fetal bovine serum (FBS) (HyClone), 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mM l-glutamine (Invitrogen). Cells were incubated at 37°C under 5% CO2.
(ii) Virus.
The plasmid containing the full-length HCV JFH-1 genome (pUC-JFH1) was provided by Takaji Wakita (National Institute of Infectious Diseases, Tokyo, Japan) (45). The stocks of JFH-1 HCV cell culture (HCVcc) used for experiments were generated as described previously (46).
(iii) HCVcc infections.
For HCV infection time course experiments, Huh7 cells were plated and grown overnight to a cell count of 8,000 for the training set experiments and 10,000 for the validation experiments. The next day, cultures were inoculated with HCVcc at a multiplicity of infection (MOI) of 3 (training) or an MOI of 7 (validation), defining this moment as time zero. At 3 h postinoculation, the viral inoculum was removed, cells were rinsed, and fresh medium was added. We then harvested the media and cell lysates from triplicate wells for titer analysis and RNA, respectively, and cell lysate from an additional triplicate wells for protein analysis at the indicated time points. To monitor the percentage of cells infected, immunofluorescence staining for HCV NS5A was performed at 24 h postinoculation before any significant progeny virus release was detected.
(iv) RNA isolation and RT-qPCR analysis.
For RNA extraction, cell lysates were harvested in 200 μl nucleic acid purification lysis solution (Applied Biosystems) from three replicate wells. Total cellular RNA was purified using an ABI PRISM 6100 Nucleic Acid PrepStation (Applied Biosystems) per the manufacturer's instructions. Extracellular RNA was isolated by adding a 5/3× guanidine thiocyanate (GTC) solution to the culture medium to obtain a 1× GTC lysate, which was subsequently processed using standard protocols (47). One microgram of RNA was used for cDNA synthesis using random primer reverse transcription (Applied Biosystems), followed by reverse transcription-quantitative PCR (RT-qPCR) using FastStart Universal SYBR green master mix (Roche Applied Sciences, Indianapolis, IN). The following qPCR primers were used: HCV primers, 5′-GCC TAG CCA TGG CGT TAG TA-3′ (sense) and 5′-CTC CCG GGG CACTCG CAA GC-3′ (antisense); human GAPDH primers, 5′-GAA GGT GAA GGT CGG AGT C-3′ (sense) and 5′-GAA GAT GGT GAT GGG ATT TC-3′ (antisense). HCV RNA copies were quantified relative to a standard curve comprised of serial dilutions of the pJFH-1 plasmid and normalize to cellular GAPDH RNA.
(v) RNA per-cell quantification.
Cell counts were performed at a subset of time points, and the relative levels of GAPDH mRNA, a cellular housekeeping gene, were assayed at all points, confirming that cell count did not change over the course of the experiment, confirming that in this experimental model system in the time frame assayed, there is no cell death observed. This constant cell count, multiplied by the 95% of cells determined to be infected based on an HCV NS5A immunofluorescence assay (Fig. 10), was used to determine the approximate number of infected cells throughout the experiment. RT-qPCR data, scaled to a per-well basis and divided by the number of infected cells, was then used to calculate the average number of copies of HCV RNA per infected cell.
FIG 10.

Percent infected cells in cells infected at an MOI of 3. Cells were infected with an MOI of 3 for 3 h before removal of the virus. At 24 h postinoculation, the cells were fixed, stained, and analyzed for percent infected, which was calculated by counting cells with viral protein versus the total number of cells. A blue Hoechst stain was used to stain DNA in all cells, and an anti-NS5A antibody (red) was used to stain infected cells.
(vi) Extracellular infectivity titration assay.
Experimental supernatant samples were serially diluted 10-fold in cDMEM, and 100 μl was used to infect each well of naive Huh7 cells cultured in 96-well plates. The inoculum was incubated with cells for 24 h at 37°C and then overlaid with 150 μl cDMEM containing 0.5% methylcellulose (wt/vol) (Fluka BioChemika, Switzerland) to give a final concentration of 0.3% methylcellulose. Seventy-two hours postinfection (p.i.), medium was removed, cells were fixed with 4% paraformaldehyde (Sigma), and immunohistochemical staining for HCV E2-positive foci was performed as previously described (46).
(vii) Immunofluorescence HCV NS5A staining.
Cells were fixed with 4% paraformaldehyde (Sigma) for 20 min and rinsed with 1× phosphate-buffered saline (PBS). HCV NS5A was detected by incubation at room temperature with 1× PBS containing 0.5% (vol/vol) Triton X-100 and 3% (wt/vol) bovine serum albumin (BSA) and a 1:500 dilution of the monoclonal NS5A antibody 9E10 (a gift from Charles Rice, Rockefeller University, New York, NY), followed by 1 h of incubation with a 1:500 dilution of a horseradish peroxidase (HRP)-conjugated anti-mouse antibody (Vector Laboratories, CA) and Hoechst dye diluted 1:3,000 before subsequent detection with 3-amino-9-ethylcarbazole (AEC) substrate (BD Biosciences). Cells were washed with distilled water (dH2O). Staining was photographed using a Nikon TE2000U microscope (Nikon Instruments).
(viii) Western blotting.
Cells were harvested at various time points from triplicate wells in 1.25% Triton X-100 lysis buffer (Triton X-100, 50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 2 mM EDTA) supplemented with a protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN), and pooled. Thirty-five micrograms of pooled protein was resolved by 10% SDS-PAGE and transferred to Hybond nitrocellulose membranes. The membranes were blocked with 5% nonfat milk for 1 h followed by an overnight incubation with primary antibodies anticore (clone C7-50; Santa Cruz Biotechnologies, Santa Cruz, CA) or anti-NS3 (clone 9-G2; Watertown, MA) each at a 1:500 dilution. The membranes were washed three times with 1× Tris-buffered saline (TBS) containing 0.05% Tween 20 (vol/vol) and then incubated with secondary HRP-conjugated goat anti-mouse (Pierce, Rockford, IL) at a 1:4,000 dilution for 30 min. For a loading control, the membranes were incubated with a HRP-conjugated β-actin monoclonal antibody at a 1:25,000 dilution. SuperSignal West Pico chemiluminescent substrate was used to detect bound antibody complexes (Pierce, Rockford, IL), and the resulting signal was quantified by densitometry using OptiQuant Image Analysis software (PerkinElmer, Waltham, MA).
(ix) Protein per-cell quantification.
The absolute quantities of core protein per cell were estimated based on the initial virus input. Specifically, because replication is not detected until 9 h postinoculation and translation is minimum at 3 h postinoculation, we assumed that the HCV RNA and the vast majority of core protein present at 3 h postinoculation represented input virions. Therefore, using the estimated HCV particle core/RNA ratio from the literature of 180:1 (48), we estimated the number of core molecules based on our quantitative measurement of HCV RNA. The number of core molecules and NS3 molecules at all time points were then scaled to the 3-h time point based on the relative Western blot signal obtained, under the assumption that the measured digital light unit (DLU) quantities for the two proteins correspond to a roughly 1:1 molar ratio.
(x) Protein degradation experiments.
Huh7 cells were infected with HCVcc at an MOI of 3, and cycloheximide was introduced at a concentration of 50 μg/ml to stop protein synthesis at either 12 or 27 h postinoculation. After the addition of cycloheximide, protein was harvested from parallel cultures at 1-h increments for 3 h to monitor protein levels by Western blotting. Western blots were quantified, and core protein and NS3 levels were normalized to cellular actin levels. Degradation rates were calculated by fitting a trend line to the decaying quantities of protein after translation had been halted.
Mathematical model equations.
The mathematical model that describes the infection system of HCV consists of 17 equations, one for each individual species within the system. The system is divided into the general cytoplasmic compartment versus a specific cytoplasmic VMS compartment, which are distinct in their functions as well as degradation rates, with VMS-enclosed species being resistant to protease/nuclease degradation. The cytoplasm contains 11 species of the model, while the VMS contains 5. The defining equations are shown in Table 2. In most cases, species are referred to by the abbreviation presented in the model schematic (Fig. 1B). The exceptions are Rp (plus-strand RNA), HFC (host factor/NS5A complex), Rp5B (RNA/NS5B complex), Rip (minus-strand synthesis complex), Rids (plus-strand synthesis complex), and Vout (extracellular virions). All model parameters are displayed in Table 1.
Model parameter estimation.
Model parameters were obtained through a combination of fitting and estimations based on the literature and previous models. The Dahari study (7) averaged steady-state values from several HCV genotype 1b subgenomic replicons to fit its model, while the Binder model (8) used genotype 2a (JFH-1, as in our study) subgenomic replicon constructs for its experiments.
Translation-phase parameters.
Translation-phase parameters encompass the parameters involved in translation and polyprotein processing (Table 1). The parameter kTC was estimated in previous models (7, 8) simulating subgenomic replicon experiments, and this estimate was used in our model. For ktranslation, the prior models' value of 100 polyproteins per h was used as an initial estimate but was found to be insufficient. An upper bound of 180 polyproteins per h was calculated based on an estimate of 10 amino acids per s, within the range observed for eukaryotic cells (49, 50), and the assumption of polyribosomes distributing across the 9,000-nucleotide (nt) viral RNA open reading frame (ORF) at the same proportion as estimated by Dahari et al. in their replicon model (7). The cleavage parameter kcleavage was calculated based on fitting.
Replication-phase parameters.
Replication-phase parameters encompass the parameters involved in VMS import, replication complex formation, and RNA synthesis (Table 1). The rates for viral RNA synthesis were calculated previously from the literature (6, 51, 52) in earlier HCV models and held constant on a per-nucleotide basis for building this model, as it is a trait inherent to the NS5B polymerase. For the parameter kRids, which exists with perfect analogy in previous models, the prior value was used. The parameter estimate for kRids was found to be adequate for the infection model after fitting. The value for kRip, which changes from a third-order reaction in previous models to a second-order reaction in ours, was calculated with fitting. The new parameters kHFC and kRp5B were also calculated using model fitting to the training data.
Transport parameters.
Transport parameters involve cellular import/export and VMS export of RNA. The VMS export parameter (koutRp) was present in previous models, and this value was used. The viral particle assembly rate constant (kassembly) was initially estimated using the similar second-order virion production parameter used in the previous multiscale model (12). From fitting this parameter guess, however, this was found to be too high of a value, likely due to the fact that our model incorporates 180 structural protein molecules into each secreted particle, while the previous model used only one. We estimated kassembly through data fitting.
Host species.
Ribosomes, host factor, and extracellular RNA were defined as host species. The numbers of available ribosomes and host factors were initially taken to be equal to the quantities used in previous models, but it was soon observed in fitting that a higher quantity of available ribosomes was more appropriate by fitting the experimental data.
Degradation rates.
The degradation rate for nonstructural proteins was estimated using the values calculated for the degradation rate of NS3 at the 12- and 27-h time points in the viral life cycle (Fig. 4). These degradation rates, 0.11 h−1 and 0.05 h−1, were used as the upper and lower bounds for fitting the protein degradation rates, and the fitting algorithm chose 0.11 h−1 as the overall nonstructural protein degradation rate. This estimation of degradation rates was found to be acceptable for our model after fitting and aided in the reduction of degrees of freedom in the fitting process. Degradation rates for all species in the VMS were estimated to be zero based on previous studies showing complete protease/nuclease resistance for species in the replicative compartment (38, 39). Cytoplasmic RNA degradation rate (kdegRp) was fit to our data after using the value from the Binder model as an initial estimate. As described above, two separate core degradation rates were calculated for the first and second phases of the life cycle (prior to and after virion production starting at 21 h).
Simulation procedure.
Simulation and data fitting were carried out using MATLAB 2016b with the SimBiology package to construct a model of ordinary differential equations according to those presented above (https://www.colorado.edu/UCB/chatterjeelab/HCVmodel.html). The “Fit Data” task was used to estimate model parameters using the stiff ode15s solver with absolute and relative tolerances set at 10−7. This fitting was performed in an iterative fashion, as the model contains too many parameters to be efficiently estimated simultaneously. The model was fit to experimental data for RNA, core, and NS3 simultaneously with a proportional error model described by equation 18. This was chosen based on the large order-of-magnitude variation in the data across the experiments.
| (18) |
where yexp is species y experimental response, ysim is species y simulation value, and b is the proportional error parameter. A tolerance of 1E−8 was chosen for termination of the data fitting procedure for the step change in estimated parameters and the function value, and 1E−6 for the first-order optimality.
Sensitivity analysis.
The “Sensitivity Analysis” task of the SimBiology package was used to estimate parameter sensitivities for RNA, NS3, core protein, and virion production. This task uses the complex-step derivative approximation, which is often used for metabolic systems (53–55). Full dedimensionalization of the analysis was used to be able to compare parameters of widely different orders of magnitude. This calculation for complex-step derivative sensitivities can be represented by the following equations for each parameter:
| (19) |
| (20) |
| (21) |
where f′ is the first derivative of the simulation function with respect to a given reaction rate, kreaction, and t is time.
Identifiability analysis.
We used the program GenSSI (56) to test our model for global structural identifiability on the parameters that were subjected to our model fitting to examine the uniqueness of our solution. These parameters are kTC, ktranslation, kcleavage, kHFC, kRp5B, kRip, kassembly, and kdegRp. Parameters for which experimental or literature values were used were fixed in this analysis and were not considered for identifiability. Identifiability analysis by GenSSI, using iterative Lie derivatives to calculate successive identifiability tableaus as described previously by Chiş et al. (56) and Balsa-Canto et al. (57) (Fig. 11), determines that the parameters kTC, ktranslation, kcleavage, kRp5B, and kdegRp are globally identifiable and that kHFC, kRp5B, and kRip are nonidentifiable. The former indicates that a unique solution exists for the parameters, while the latter indicates that we cannot be sure of a unique solution for these parameters. For this reason, we avoid drawing conclusions based on those parameter values. As our model's goal is primarily to evaluate model structure and add biological accuracy to the protein mechanisms, we have concluded that this degree of identifiability is enough to provide a useful model. To calculate values of model parameters with confidence, more experimental data would be necessary.
FIG 11.
Model structural identifiability tableaus. Identifiability analysis was conducted for the eight parameters that did not have a literature or experimental basis in our model: kTC, ktranslation, kcleavage, kHFC, kRp5B, kRip, kassembly, and kdegRp. Our analysis used iterative Lie derivatives to calculate successive identifiability tableaus, i.e., Jacobian matrices, as described previously by Chiş et al. and Balsa-Canto et al. (56, 57). Non-zero elements, marked by filled-in black spaces in the grid, indicate that the Lie derivative component (a coefficient of a power series representative of the system) depends on that parameter. White columns indicate nonidentifiability. The reduced tableau demonstrates identifiable parameters and can be used to create a system of equations for identifying parameters.
ACKNOWLEDGMENTS
Portions of this work were performed under the auspices of the U.S. Department of Energy under contract DE-AC52-06NA25396. This work was funded by National Institutes of Health grants R01-AI078881 (S.L.U.), R01-OD011095, R01-AI028433, R01-AI116868 (A.S.P.), and University of Colorado start-up grant and DARPA Young Faculty Award (D17AP00024) to A.C.
REFERENCES
- 1.World Health Organization. 2017. Global hepatitis report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Burstow NJ, Mohamed Z, Gomaa AI, Sonderup MW, Cook NA, Waked I, Spearman CW, Taylor-Robinson SD. 2017. Hepatitis C treatment: where are we now? Int J Gen Med 10:39–52. doi: 10.2147/IJGM.S127689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatterjee A, Guedj J, Perelson AS. 2012. Mathematical modelling of HCV infection: what can it teach us in the era of direct-acting antiviral agents? Antivir Ther 17:1171–1182. doi: 10.3851/IMP2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee A, Smith PF, Perelson AS. 2013. Hepatitis C viral kinetics. The past, present, and future. Clin Liver Dis 17:13–26. doi: 10.1016/j.cld.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2016. Hepatitis C FAQs for the Public. Division of Viral Hepatitis and National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 6.Lohmann V, Körner F, Koch J, Herian U, Theilmann L, Bartenschlager R. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 7.Dahari H, Ribeiro RM, Rice CM, Perelson AS. 2007. Mathematical modeling of subgenomic hepatitis C virus replication in Huh-7 cells. J Virol 81:750–760. doi: 10.1128/JVI.01304-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binder M, Sulaimanov N, Clausznitzer D, Schulze M, Hüber CM, Lenz SM, Schlöder JP, Trippler M, Bartenschlager R, Lohmann V, Kaderali L. 2013. Replication vesicles are load- and choke-points in the hepatitis C virus lifecycle. PLoS Pathog 9:e1003561. doi: 10.1371/journal.ppat.1003561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novak JE, Kirkegaard K. 1994. Coupling between genomic translation and replication in an RNA virus. Genes Dev 8:1726–1737. doi: 10.1101/gad.8.14.1726. [DOI] [PubMed] [Google Scholar]
- 10.Appel N, Herian U, Bartenschlager R, Appel N, Herian U, Bartenschlager R. 2005. Efficient rescue of hepatitis C virus RNA replication by trans-complementation with nonstructural protein 5A efficient rescue of hepatitis C virus RNA replication by trans-complementation with nonstructural protein 5A. J Virol 79:896–909. doi: 10.1128/JVI.79.2.896-909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khromykh AA, Sedlak PL, Westaway EG. 2000. cis- and trans-Acting elements in flavivirus RNA replication. J Virol 74:3253–3263. doi: 10.1128/JVI.74.7.3253-3263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clausznitzer D, Harnisch J, Kaderali L. 2016. Multi-scale model for hepatitis C viral load kinetics under treatment with direct acting antivirals. Virus Res 218:96–101. doi: 10.1016/j.virusres.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Bartenschlager R, Ahlborn-Laake L, Mous J, Jacobsen H. 1994. Kinetic and structural analyses of hepatitis C virus polyprotein processing. J Virol 68:5045–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin C, Pragai BM, Grakoui A, Xu J, Rice CM. 1994. Hepatitis C virus NS3 serine proteinase: trans-cleavage requirements and processing kinetics. J Virol 68:8147–8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazakov T, Yang F, Ramanathan HN, Kohlway A, Diamond MS, Lindenbach BD. 2015. Hepatitis C virus RNA replication depends on specific cis- and trans-acting activities of viral nonstructural proteins. PLoS Pathog 11:1–30. doi: 10.1371/journal.ppat.1004817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moradpour D, Penin F. 2013. Hepatitis C virus proteins: from structure to function, p 113–142. In Bartenschlager R. (ed), Hepatitis C virus: from molecular virology to antiviral therapy. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 17.Morikawa K, Lange CM, Gouttenoire J, Meylan E, Brass V, Penin F, Moradpour D. 2011. Nonstructural protein 3-4A: the Swiss army knife of hepatitis C virus. J Viral Hepat 18:305–315. doi: 10.1111/j.1365-2893.2011.01451.x. [DOI] [PubMed] [Google Scholar]
- 18.Pietschmann T, Lohmann V, Rutter G, Kurpanek K, Bartenschlager R. 2001. Characterization of cell lines carrying self-replicating hepatitis C virus RNAs. J Virol 75:1252–1264. doi: 10.1128/JVI.75.3.1252-1264.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egger D, Wölk B, Gosert R, Bianchi L, Blum HE, Moradpour D, Bienz K. 2002. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J Virol 76:5974–5984. doi: 10.1128/JVI.76.12.5974-5984.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouttenoire J, Penin F, Moradpour D. 2010. Hepatitis C virus nonstructural protein 4B: a journey into unexplored territory. Rev Med Virol 20:117–129. doi: 10.1002/rmv.640. [DOI] [PubMed] [Google Scholar]
- 21.Paul D, Romero-Brey I, Gouttenoire J, Stoitsova S, Krijnse-Locker J, Moradpour D, Bartenschlager R. 2011. NS4B self-interaction through conserved C-terminal elements is required for the establishment of functional hepatitis C virus replication complexes. J Virol 85:6963–6976. doi: 10.1128/JVI.00502-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartenschlager R, Lohmann V, Penin F. 2013. The molecular and structural basis of advanced antiviral therapy for hepatitis C virus infection. Nat Rev Microbiol 11:482–496. doi: 10.1038/nrmicro3046. [DOI] [PubMed] [Google Scholar]
- 23.Moradpour D, Brass V, Penin F. 2005. Function follows form: the structure of the N-terminal domain of HCV NS5A. Hepatology 42:732–735. doi: 10.1002/hep.20851. [DOI] [PubMed] [Google Scholar]
- 24.Appel N, Zayas M, Miller S, Krijnse-Locker J, Schaller T, Friebe P, Kallis S, Engel U, Bartenschlager R. 2008. Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly. PLoS Pathog 4:e1000035. doi: 10.1371/journal.ppat.1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tellinghuisen TL, Foss KL, Treadaway JC, Rice CM. 2008. Identification of residues required for RNA replication in domains II and III of the hepatitis C virus NS5A protein. J Virol 82:1073–1083. doi: 10.1128/JVI.00328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans MJ, Rice CM, Goff SP. 2004. Phosphorylation of hepatitis C virus nonstructural protein 5A modulates its protein interactions and viral RNA replication. Proc Natl Acad Sci U S A 101:13038–13043. doi: 10.1073/pnas.0405152101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanoulle X, Badillo A, Wieruszeski JM, Verdegem D, Landrieu I, Bartenschlager R, Penin F, Lippens G. 2009. Hepatitis C virus NS5A protein is a substrate for the peptidyl-prolyl cis/trans isomerase activity of cyclophilins A and B. J Biol Chem 284:13589–13601. doi: 10.1074/jbc.M809244200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaneko T, Tanji Y, Satoh S, Hijikata M, Asabe S, Kimura K, Shimotohno K. 1994. Production of two phosphoproteins from the NS5A region of the hepatitis C viral genome. Biochem Biophys Res Commun 205:320–326. doi: 10.1006/bbrc.1994.2667. [DOI] [PubMed] [Google Scholar]
- 29.Reiss S, Rebhan I, Backes P, Romero-Brey I, Erfle H, Matula P, Kaderali L, Poenisch M, Blankenburg H, Hiet M-S, Longerich T, Diehl S, Ramirez F, Balla T, Rohr K, Kaul A, Schirmacher P, Lohmann V, Bartenschlager R. 2011. Recruitment and activation of a lipid kinase by NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe 9:32–45. doi: 10.1016/j.chom.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verdegem D, Badillo A, Wieruszeski JM, Landrieu I, Leroy A, Bartenschlager R, Penin F, Lippens G, Hanoulle X. 2011. Domain 3 of NS5A protein from the hepatitis C virus has intrinsic α-helical propensity and is a substrate of cyclophilin A. J Biol Chem 286:20441–20454. doi: 10.1074/jbc.M110.182436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guedj J, Yu J, Levi M, Li B, Kern S, Naoumov NV, Perelson AS. 2014. Modeling viral kinetics and treatment outcome during alisporivir interferon-free treatment in hepatitis C virus genotype 2 and 3 patients. Hepatology 59:1706–1714. doi: 10.1002/hep.26989. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Yang F, Robotham JM, Tang H. 2009. Critical role of cyclophilin A and its prolyl-peptidyl isomerase activity in the structure and function of the hepatitis C virus replication complex. J Virol 83:6554–6565. doi: 10.1128/JVI.02550-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero-Brey I, Berger C, Kallis S, Kolovou A, Paul D, Lohmann V, Bartenschlager R. 2015. NS5A domain 1 and polyprotein cleavage kinetics are critical for induction of double-membrane vesicles associated with hepatitis C virus replication. mBio 6:e00759. doi: 10.1128/mBio.00759-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang J, Huang L, Cordek DG, Vaughan R, Reynolds SL, Kihara G, Raney KD, Kao CC, Cameron CE. 2010. Hepatitis C virus nonstructural protein 5A: biochemical characterization of a novel structural class of RNA-binding proteins. J Virol 84:12480–12491. doi: 10.1128/JVI.01319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang L, Hwang J, Sharma SD, Hargittai MRS, Chen Y, Arnold JJ, Raney KD, Cameron CE. 2005. Hepatitis C virus nonstructural protein 5A (NS5A) is an RNA-binding protein. J Biol Chem 280:36417–36428. doi: 10.1074/jbc.M508175200. [DOI] [PubMed] [Google Scholar]
- 36.Guedj J, Dahari H, Rong L, Sansone ND, Nettles RE, Cotler SJ, Layden TJ, Uprichard SL, Perelson AS. 2013. Modeling shows that the NS5A inhibitor daclatasvir has two modes of action and yields a shorter estimate of the hepatitis C virus half-life. Proc Natl Acad Sci U S A 110:3991–3996. doi: 10.1073/pnas.1203110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGivern DR, Masaki T, Williford S, Ingravallo P, Feng Z, Lahser F, Asante-Appiah E, Neddermann P, De Francesco R, Howe AY, Lemon SM. 2014. Kinetic analyses reveal potent and early blockade of hepatitis C virus assembly by NS5A inhibitors. Gastroenterology 147:453–462. doi: 10.1053/j.gastro.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinkert D, Bartenschlager R, Lohmann V. 2005. Quantitative analysis of the hepatitis C virus replication complex. J Virol 79:13594–13605. doi: 10.1128/JVI.79.21.13594-13605.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyanari Y, Hijikata M, Yamaji M, Hosaka M, Takahashi H, Shimotohno K. 2003. Hepatitis C virus non-structural proteins in the probable membranous compartment function in viral genome replication. J Biol Chem 278:50301–50308. doi: 10.1074/jbc.M305684200. [DOI] [PubMed] [Google Scholar]
- 40.Gosert R, Egger D, Lohmann V, Bartenschlager R, Blum HE, Bienz K, Moradpour D. 2003. Identification of the hepatitis C virus RNA replication complex in Huh-7 cells harboring subgenomic replicons. J Virol 77:5487–5492. doi: 10.1128/JVI.77.9.5487-5492.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero-Brey I, Merz A, Chiramel A, Lee J-Y, Chlanda P, Haselman U, Santarella-Mellwig R, Habermann A, Hoppe S, Kallis S, Walther P, Antony C, Krijnse-Locker J, Bartenschlager R. 2012. Three-dimensional architecture and biogenesis of membrane structures associated with hepatitis C virus replication. PLoS Pathog 8:e1003056. doi: 10.1371/journal.ppat.1003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. 2005. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci U S A 102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller S, Krijnse-Locker J. 2008. Modification of intracellular membrane structures for virus replication. Nat Rev Microbiol 6:363–374. doi: 10.1038/nrmicro1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye F, Xin Z, Han W, Fan J, Yin B, Wu S, Yang W, Yuan J, Qiang B, Sun W, Peng X. 2015. Quantitative proteomics analysis of the hepatitis C virus replicon high-permissive and low-permissive cell lines. PLoS One 10:e0142082. doi: 10.1371/journal.pone.0142082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kato T, Furusaka A, Miyamoto M, Date T, Yasui K, Hiramoto J, Nagayama K, Tanaka T, Wakita T. 2001. Sequence analysis of hepatitis C virus isolated from a fulminant hepatitis patient. J Med Virol 64:334–339. doi: 10.1002/jmv.1055. [DOI] [PubMed] [Google Scholar]
- 46.Yu X, Uprichard SL. 2010. Cell-based hepatitis C virus infection fluorescence resonance energy transfer (FRET) assay for antiviral compound screening. Curr Protoc Microbiol Chapter 17:Unit 17.5. doi: 10.1002/9780471729259.mc1705s18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chomczynski P, Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 48.Schüttler CG, Thomas C, Discher T, Friese G, Lohmeyer J, Schuster R, Schaefer S, Gerlich WH. 2004. Variable ratio of hepatitis C virus RNA to viral core antigen in patient sera. J Clin Microbiol 42:1977–1981. doi: 10.1128/JCM.42.5.1977-1981.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olofsson S-O, Bostrom K, Carlsson P, Boren J, Wettesten M, Bjursell G, Wikhmd O, Bondjers G. 1987. Structure and biosynthesis of apolipoprotein B. Am Heart J 113:446–452. [DOI] [PubMed] [Google Scholar]
- 50.Siwiak M, Zielenkiewicz P. 2013. Transimulation - protein biosynthesis web service. PLoS One 8:e73943. doi: 10.1371/journal.pone.0073943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh JW, Ito T, Lai MM. 1999. A recombinant hepatitis C virus RNA-dependent RNA polymerase capable of copying the full-length viral RNA. J Virol 73:7694–7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma H, Leveque V, De Witte A, Li W, Hendricks T, Clausen SM, Cammack N, Klumpp K. 2005. Inhibition of native hepatitis C virus replicase by nucleotide and non-nucleoside inhibitors. Virology 332:8–15. doi: 10.1016/j.virol.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 53.Ingalls BP, Sauro HM. 2003. Sensitivity analysis of stoichiometric networks: an extension of metabolic control analysis to non-steady state trajectories. J Theor Biol 222:23–36. doi: 10.1016/S0022-5193(03)00011-0. [DOI] [PubMed] [Google Scholar]
- 54.Martins JRRA, Kroo IM, Alonso JJ. 2000. An automated method for sensitivity analysis using complex variables. AIAA-2000-0689. Proceedings of the 39th AIAA Aerospace Sciences Meeting. American Institute of Aeronautics and Astronautics, Reston, VA. [Google Scholar]
- 55.MathWorks. 2017. SimBiology documentation. MATLAB. MathWorks, Natick, CA. [Google Scholar]
- 56.Chiş O, Banga JR, Balsa-Canto E. 2011. GenSSI: a software toolbox for structural identifiability analysis of biological models. Bioinformatics 27:2610–2611. doi: 10.1093/bioinformatics/btr431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balsa-Canto E, Alonso AA, Banga JR. 2010. An iterative identification procedure for dynamic modeling of biochemical networks. BMC Syst Biol 4:11. doi: 10.1186/1752-0509-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]