Abstract
Existing explanations of obesity-associated cancer emphasise direct mutagenic effects of dietary components or hormonal imbalance. Some of these hypotheses are reviewed briefly, but recent evidence suggests a major role for chronic inflammation in cancer risk, possibly involving dietary content. These ideas include the inflammation-induced activation of the kynurenine pathway and its role in feeding and metabolism by activation of the aryl hydrocarbon receptor (AHR) and by modulating synaptic transmission in the brain. Evidence for a role of the kynurenine pathway in carcinogenesis then provides a potentially major link between obesity and cancer. A second new hypothesis is based on evidence that serine proteases can deplete cells of the tumour suppressors Deleted in Colorectal Cancer (DCC) and neogenin. These enzymes include mammalian chymotryptic proteases released by pro-inflammatory neutrophils and macrophages. Blood levels of chymotrypsin itself increase in parallel with food intake. The mechanistically similar bacterial enzyme subtilisin is widespread in the environment, animal probiotics, meat processing and cleaning products. Simple public health schemes in these areas, with selective serine protease inhibitors and AHR antagonists and could prevent a range of intestinal and other cancers.
Keywords: Obesity, Serine proteases, Chymotrypsin, Subtilisin, Dependence receptors, DCC, Kynurenine
Abbreviations: Akt, protein kinase B; AHR, aryl hydrocarbon receptor; CNS, central nervous system; CRP, C-reactive protein; DCA, deoxycholic acid; DCC, Deleted in Colorectal Cancer; FAS, fatty acid synthase; GCN2, General Control Non-derepressible-2; IGF-1, insulin-like growth factor -1; IGFBP, insulin-like growth factor binding protein; IL-6, interleukin-6; IRE1α, Inositol Requiring Enzyme-1α; JAK2, janus kinase 2; MAPK, mitogen-activated protein kinase; mTOR, mechanistic (formerly mammalian) target of rapamycin; NF, nuclear factor; ObR, leptin receptor; PI3 kinase, phosphoinositide 3-kinase; STAT3, signal transducer and activator of transcription 3; TNF-α, tumor necrosis factor- α; VEGF-A, vascular endothelial growth factor-A
Highlights
-
•
Inflammation is probably a key link between obesity and cancer.
-
•
Inflammatory activation of the kynurenine pathway affects feeding, metabolism and promotes carcinogenesis.
-
•
Endogenous, environmental and dietary serine proteases deplete cells of tumour suppressors such as DCC.
1. Introduction
The current impact of obesity on public health is a headline concern worldwide, especially since obesity is a significant risk factor for several types of cancer. Obesity is characterized by an excess of body fat considered to be harmful to health and defined by the World Health Organisation as a body mass index (BMI; body weight [kg]/height [m2]) >30 kg/m2 (WHO, 2016) (class 1, 30–35, class 35–40, class 3 > 40) with normal values considered as 18.5–24.9 kg/m2 and overweight as intermediate values of 25–29.9 kg/m2. The term ‘lean’ is occasionally used to refer to weights below 18.5 kg/m2. By these criteria around two-thirds of adults aged over 20 years in the USA are currently overweight with a prevalence of obesity of approximately 35% (Ogden et al., 2012), predicted to reach 42% by 2030 in people over 18 years (Finkelstein et al., 2012).
The main driver for obesity is believed to be an overall rise in caloric intake (Swinburn et al., 2009) with a shift towards snacking patterns of eating and increased consumption of high carbohydrate beverages and dietary fat. Low levels of physical activity increase the problem (Cameron et al., 2003) with a significant but poorly understood role of genetic factors (Thorleifsson et al., 2009). Significant consequences of obesity include the medical, economic and social burdens of obesity-related comorbidities such as coronary heart disease, type-2 diabetes mellitus, respiratory disease and cancer (Renehan et al., 2008). Many of the global concerns around the links between environmental factors and diet, nutrition, obesity and cancer are addressed by the World Cancer Research Fund (WCRF) and their various publications (http://www.wcrf.org/int/policy/our-publications).
2. Obesity and Cancer
Tumor development involves a local microenvironment which promotes cell proliferation, partly through release of mitogenic signals, and induces cell survival mechanisms as well as the induction of tolerance in cytotoxic host T cells (Hanahan and Weinberg, 2011; Prendergast et al., 2010). Parkin and Boyd (2011) estimated that 5.5% of cancer cases in the UK were related to overweight and obesity while others have claimed that the relative risk of mortality from cancer, attributable to obesity, was approximately 14.2% in men and 19.8% in women (Calle et al., 2003).
The association between obesity and cancer is quite secure in human populations (Arslan et al., 2009; Pischon et al., 2008; Xu et al., 2003) especially with respect to tumors of the gastrointestinal (GI) tract (Zeng and Lazarova, 2012; Zeng et al., 2014) where being overweight carries a 1.5–2.4-fold increase in cancer risk (Moore et al., 2005). The link has also been supported by animal experiments in which obesity and cancer have been modified by dietary means (Nogueira et al., 2012a, Nogueira et al., 2012b). Several studies have attempted to define the types of cancer most highly associated with obesity, which include breast cancer in postmenopausal women, colon cancer (especially in men), endometrial, esophageal adenocarcinoma, gall bladder and renal cancers (Bhaskaran et al., 2014; Price et al., 2012; Renehan et al., 2008).
Awareness of the role of lifestyle factors in the relationship between obesity and cancer is gaining prominence and will be addressed in a later section. For example, high red and processed meat consumption has been identified as a risk factor for colorectal cancer (Alexander et al., 2011; Huxley et al., 2009; Magalhaes et al., 2012; Norat et al., 2005) and with an increased risk of obesity (Wang and Beydoun, 2009). A role for adipose tissue is also relevant in the case of breast cancer, where a strong association exists between the amount of mammary adipose tissue and collagen (broadly equating with overall breast size), breast cell density and lifetime risk for mammary cancer (Boyd et al., 2007; DeFilippis et al., 2012). This may be related to the inverse expression of CD36, a common membrane protein which plays a role in cell development and intercellular interactions. Lower levels of CD36 in breast tissue lead to an increase in collagen deposition at the expense of adipose tissue, which declines in quantity. The increased collagen to adipose ratio, as seen normally with aging, results in breasts of higher tissue density and increased cancer risk (Boyd et al., 2007; DeFilippis et al., 2012). Further factors influencing breast cancer development via obesity and breast adiposity have included the possible influence of estrogens produced in adipose tissue. These steroids can promote carcinogenesis and add to the lifetime total of estrogen stimulation from oral contraceptives, hormone replacement therapy, and pregnancies (Gerard and Brown, 2017).
2.1. Insulin Resistance
Adipose tissue is an important site of insulin activity, promoting triglyceride storage and inhibiting lipolysis (Choi et al., 2010). There is a strong positive relationship between fasting insulin levels and postmenopausal cancer risk, specifically in non-users of hormone therapy (Gunter et al., 2009) consistent with the view that postmenopausal breast cancer is analogous to obesity-associated cancer resulting from insulin resistance (Bhaskaran et al., 2014; Renehan et al., 2008). Insulin resistance is a feature of obese individuals, accompanied by a high circulating insulin level which is a well-established risk factor for cancer (Kim et al., 2004; Goodwin et al., 2002; Hsing et al., 2001) and which is associated with marked changes in the levels of inflammatory markers (Lee and Lee, 2014).
In a sample of 208 healthy non-obese volunteers, insulin sensitivity was correlated with cancer development over a 6-year period (Facchini et al., 2001). The insulin resistance associated with obesity may be symptomatic of a more profound dysfunction of the insulin/insulin-like growth factor-1 (IGF-1) axis (Kim et al., 2004; Cohen and LeRoith, 2012). Obesity-related insulin resistance and hyperinsulinemia are associated with elevated blood levels of unbound, but not total, IGF-1 protein (Frystyk et al., 1995; Nam et al., 1997) with activation of the insulin and IGF-1 receptors (IGF-1R) triggering transduction pathways which promote tumor growth (Kulik et al., 1997; Parrizas et al., 1997). Obesity-associated insulin resistance gives rise to increased free IGF-1 levels in the postprandial state whereas a reduction of free IGF-1 is observed in lean insulin-sensitive subjects (Ricart and Fernández-Real, 2001). High levels of insulin could dysregulate IGF-1 signalling by their ability to reduce expression of the hepatic IGF Binding Proteins (IGFBP) (Nam et al., 1997) resulting in increased levels of free IGF. Whether the chronic pattern of postprandial IGF-1 levels is an important factor in the relevance of this protein to obesity remains unresolved, but it could clearly contribute to the obesogenic (and diabetogenic) propensity of modern ‘snacking’ behavior with the frequent consumption of small quantities of foodstuffs, especially those solid and liquid varieties providing high doses of carbohydrate.
Colorectal cancer risk has been associated with increased levels of circulating IGF-1 in men (Ma et al., 1999) although Wolpin et al. (2009) found no link between IGF-1 and colorectal-cancer specific mortality. In addition, a case-control study discovered no association between IGF-1 and premenopausal or postmenopausal breast cancer (Petridou et al., 2000). However, the relative amounts of bound and free IGF present were not clear.
Up-regulation of the insulin and IGF-1Rs has been indicated in cancer (Hellawell et al., 2002; Papa et al., 1990). Both receptors interact with the intracellular insulin receptor substrate 1, which subsequently promotes the phosphoinositide 3-kinase (PI3 kinase)/protein kinase B (Akt) cascade (Myers et al., 1994). This pathway ultimately inhibits programmed cell death (Datta et al., 1997; Kulik et al., 1997). Upon insulin and IGF-1R activation, the intracellular protein Ras stimulates the mitogen-activated protein kinase (MAPK) pathway, which also plays a vital role in cell proliferation and inhibition of apoptosis (Parrizas et al., 1997; Menu et al., 2004) (Fig. 1).
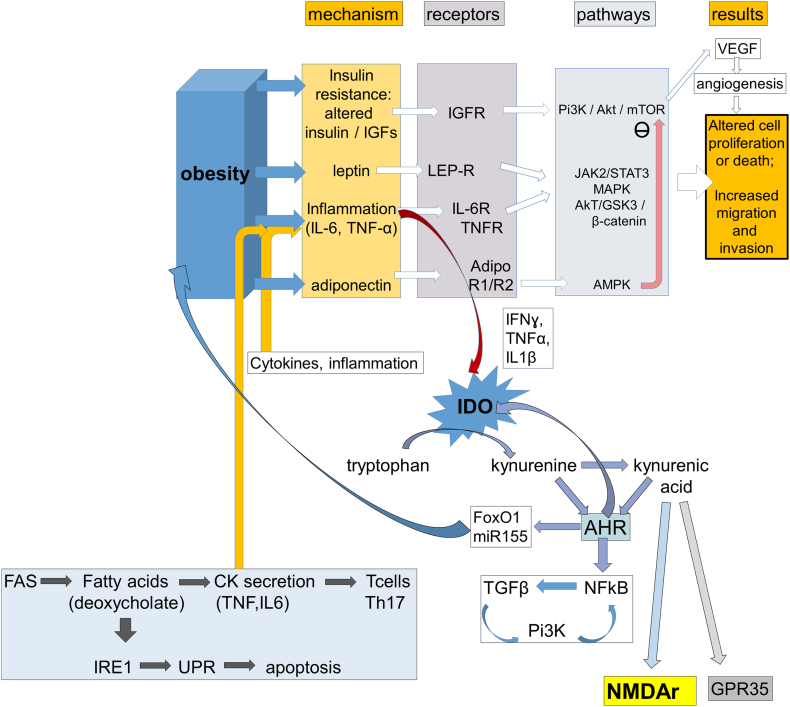
Fig. 1.
A schematic illustrating some of the factors proposed to affect obesity and carcinogenesis. Obesity is associated with hormonal and inflammatory changes summarised under “Mechanism” and including insulin resistance. These factors often act via specific “Receptors” which regulate key “Pathways” responsible for the control of cell viability, proliferation, migration and death. The kynurenine pathway is activated by inflammation and acts via the Aryl Hydrocarbon Receptor (AHR) to modulate transcription factors and microRNAs relevant to BMI regulation as well as regulating feedback on inflammation. Kynurenines can affect feeding directly via glutamate receptors (NMDAr) in the brain and possibly via the G-protein coupled receptor GPR35. Fatty acid metabolism can affect inflammatory cytokine production and T cell balance in the immune system.
2.2. Glucagon
Glucagon, the peptide hormone from pancreatic α-cells opposes the actions of insulin by mobilising glucose and inhibiting its utilisation. Analogues of the natural glucagon-mimetic Glucagon-Like Peptide-1 (GLP-1) reduce body mass and help to prevent type-2 diabetes mellitus and cancer development, partly by inhibiting glycogen synthase kinase-3 (GSK-3). This has led to the introduction of non-peptide compounds such as liraglutide into clinical use (Tomlinson et al., 2016). GLP-1 agonists are also likely to act by suppressing the invasion of pro-inflammatory macrophages into adipose tissue (Lee et al., 2012).
The potential links between obesity and carcinogenesis are exemplified by the finding that GLP-1R agonists can inhibit cancer development as well as type-2 diabetes mellitus. Cancer is one of the main causes of death in patients with type-2 diabetes (Nomiyama and Yanase, 2016; Yorifuji et al., 2016). GLP-1 agonists can promote cell apoptosis in some tumors and cell lines, thus exhibiting anti-cancer activity, in some cases by inhibiting glycogen synthase kinase-3 (GSK-3) (Koehler et al., 2011). This activity may stem from the ability of GLP-1 agonists to suppress invasion of adipose tissue by pro-inflammatory macrophages (Lee et al., 2012).
2.3. Leptin
The ability of adipose tissue to generate a factor or factors that increase cell susceptibility to cancer initiation or progression had been supported by a variety of studies on different forms of cancer. Leptin is an adipocyte specific hormone, a product of the ob gene involved in regulating food intake and body weight via its actions on the central nervous system (CNS) and adipocytes to suppress appetite and promote metabolism (Halaas et al., 1995). In obese individuals, this hormone is present at higher levels than in their leaner counterparts, positively correlating with an increased proportion of body fat and often associated with leptin resistance.
Epidemiological evidence indicates that high leptin levels are associated with an increased risk of colon cancer (Stattin et al., 2004) and breast cancer (Han et al., 2005; Wu et al., 2009). In agreement with the finding that postmenopausal breast cancer is strongly associated with obesity (Renehan et al., 2008), postmenopausal women with the highest waist circumference and leptin concentration are recognized to have the greatest risk of breast cancer (Wu et al., 2009). However, menopausal status has also been deemed irrelevant to hyperleptinemia-associated breast cancer in one study since there was no correlation between them (Han et al., 2005). Furthermore, Mantzoros et al. (1999) disagreed with the notion that leptin is involved in the etiology of breast cancer, although this study was only conducted on premenopausal women. An increased leptin receptor expression has been identified in several types of cancer (Attoub et al., 2000; Kim, 2009).
Leptin is a stimulator of cell proliferation and tumor growth (Chen et al., 2013; Gonzalez et al., 2006, Gonzalez et al., 2009; Hardwick et al., 2001; Takahashi et al., 1997) probably attributable to MAPK phosphorylation and there is an increased expression of leptin receptors in several types of cancer (Dieudonne et al., 2002; Hardwick et al., 2001). Leptin is a promotor of cyclin D1 (Gonzalez et al., 2006), an important contributor to cell cycle progression, and suppresses apoptosis in ovarian cancer cells (Chen et al., 2013). The activation by leptin of PI3K and MAPK also promotes angiogenesis, contributing to tumor growth (Gonzalez et al., 2006). Additional carcinogenic actions of leptin include increasing aromatase expression leading to enhanced pro-estrogenic pathways, estradiol production and estrogen receptor-α signalling (Catalano et al., 2003, Catalano et al., 2004), all of which are of particular significance in estrogen-responsive cancers. Postmenopausal breast cancer is strongly associated with obesity (Renehan et al., 2008). Postmenopausal women with high waist circumference and leptin concentration have the greatest risk of breast cancer (Wu et al., 2009). High leptin levels are also associated with an increased risk of colon cancer.
2.4. Adipokines
In addition to leptin several other adipose-derived factors - adipokines - have subsequently been recognized including adiponectin, tumor necrosis factor- α (TNF-α) and interleukin-6 (IL-6) (Fain et al., 2004). The expansion of adipose tissue in obesity leads to a rise in the plasma levels of these factors with a reduction in adiponectin production (Arita et al., 1999; Hotamisligil et al., 1995; Vendrell et al., 2004). The incidence of several cancers is increased with elevated circulating leptin and IL-6 levels. (Stattin et al., 2004; Wu et al., 2009) and the risk of colorectal adenomas, which have the potential to develop into carcinomas, have been associated with an increased secretion of TNF-α and IL-6 (Kim et al., 2008).
2.4.1. Adiponectin
Adiponectin is a peptide hormone which has a physiological role in glucose metabolism, enhancing insulin sensitivity and glucose uptake (Berg et al., 2001) as well as stimulating fatty acid oxidation (Yamauchi et al., 2002). In contrast to other adipokines, adiponectin concentrations are significantly lower in obese individuals compared to those in a normal BMI range (Arita et al., 1999). There is a negative association between circulating adiponectin levels with cancer risk and disease severity (Dal Maso et al., 2004; Goktas et al., 2005; Malvi et al., 2015; Miyoshi et al., 2003; Wei et al., 2005). The reduction in adiponectin secretion seen in obese individuals may contribute to insulin resistance (Yamauchi et al., 2001).
Adiponectin acts on two receptors. AdipoR1 might play a significant role in mediating adiponectin's anti-cancer effects (Nakayama et al., 2008; Pfeiler et al., 2010) although both are expressed in greater quantities in invasive compared with non-invasive breast cancer (Pfeiler et al., 2010). The investigators suggested that low adiponectin levels could induce a feedback loop causing an up-regulation of AdipoR1 expression. In contrast, AdipoR1 levels are lower in several prostate cancer cell lines compared with healthy prostate tissue (Gao et al., 2015). Similarly, primary tumor progression and differentiation of colorectal cancer cells were associated with a reduced expression of AdipoR1 and AdipoR2 (Byeon et al., 2010). Interestingly, Gialamas et al. (2011) found opposing results, demonstrating that AdipoR2 expression was enhanced in advanced tumors and metastatic colorectal cancer cells, with no relationship to AdipoR1 expression. Any mechanistic relationship is therefore complex or influenced by factors not yet identified or understood.
In support of the concept that adiponectin has anti-cancer activity, it has been reported that the hormone has anti-angiogenic properties in vitro (Brakenhielm et al., 2004). Adiponectin inhibits Vascular Endothelial Growth Factor-A (VEGF-A) modulated cancer neo-vascularisation in prostate cancer cells via AdipoR1 and AMPK activation (Gao et al., 2015), strengthening the hypothesis that adiponectin inhibits cancer growth by suppressing angiogenesis (Fig. 1). Sugiyama et al. (2009) discovered that adiponectin inhibits colorectal cancer cell growth in vitro, probably by down-regulating the mechanistic target of rapamycin (mTOR) via AMPK phosphorylation. The dual action of the AMPK up-regulation and Akt inhibition has been considered an important feature of adiponectin's anti-proliferative and pro-apoptotic receptor-mediated effects in malignant cells (Medina et al., 2014). Correlating with these results, adiponectin inhibits the proliferation of breast cancer cells by hindering cell cycle progression, although there is some controversy surrounding the hormone's ability to induce apoptosis (Nakayama et al., 2008).
Adiponectin may also prevent cancer growth by increasing insulin sensitivity (Berg et al., 2001; Yamauchi et al., 2002) as discussed above. Induction of insulin sensitivity would reduce the circulating levels of insulin and IGF-1 which, at high levels of the free form, is believed to be carcinogenic (Goodwin et al., 2002).
2.4.2. Ceruloplasmin
One of the most recently identified adipokines is ceruloplasmin which is highly concentrated in adipose tissue from obese individuals and is synthesized and released at higher rates than in control subjects (Arner et al., 2014). It was estimated that adipose tissue secretion accounted for almost one quarter of the circulating level of the protein. As ceruloplasmin is involved in angiogenesis, its increase presence in obese subjects may facilitate or promote the development of several cancers.
2.5. Fatty Acid Metabolism
Fatty Acid Synthase (FAS) is responsible for catalysing de novo synthesis of long-chain fatty acids which are crucial for cellular energy metabolism and membrane function (Wakil, 1989). There is a relationship between increased FAS expression and poor patient prognosis in prostate, colon, breast, gastrointestinal and ovarian tumors (Gansler et al., 1997; Keshk et al., 2014; Rossi et al., 2006). Conversely, inhibiting FAS has proven efficacy in cancer therapy (Kridel et al., 2004; Seguin et al., 2012).
Nguyen et al. (2010) identified a FAS polymorphism which was common in males with higher BMI ranges (BMI ≥ 25 kg/m2) and was associated with a greater prostate cancer risk and mortality. Importantly, this correlation was only observed in overweight and obese men, with no association among men of normal weight who possessed this polymorphism. In line with this, tumoral FAS overexpression in obese patients was associated with worse colon cancer mortality rates, in contrast with tumoral FAS overexpression being a sign of improved survival in non-obese patients (Ogino et al., 2008). It was speculated that energy balance might alter the oncogenic influence of FAS upregulation in colon cancer cells, as a hyper-energy state (reflected as the level of adiposity) could augment tumor growth. In contrast, one study concluded that FAS-negative colorectal cancer risk was greater in female patients with a higher BMI, indicating no correlation between BMI and FAS-positive colorectal cancer risk (Kuchiba et al., 2012).
Fatty acids and related microbial products have also been linked with both obesity and cancer (Stone and Darlington, 2017). The compound receiving most attention is deoxycholic acid (DCA), which has been reviewed in previous reports (Balaban et al., 2017; Hara, 2015; Yoshimoto et al., 2013). As noted above, the ability of fatty acids to activate cytokine secretion from macrophages provides a mechanistic link between obesity and inflammation which may be crucial. However, since macrophage and neutrophil activation also enhances the secretion of serine proteases such as chymase, chymotrypsin and cathepsin G, the hypothesis proposed in the following section may also be highly relevant.
2.6. Chronic Inflammation
Chronic inflammation is associated with several non-infective physiological conditions, including obesity (Calle and Kaaks, 2004; Musso et al., 2010; Cottam et al., 2010; George et al., 2017). Local and systemic chronic inflammation have been recognized as states favoring tumor initiation and progression, largely through the generation of pro-inflammatory cytokines, such as TNF-α and IL-6 (Grivennikov et al., 2009; Morris et al., 2013; Howe et al., 2013). Correlations have been made between local chronic inflammatory conditions, such as inflammatory bowel disease, and an increased risk of developing cancers (Bernstein et al., 2001) while systemic inflammation has been correlated with an increased prevalence of colorectal adenomas. In addition, the presence of obesity was correlated with increased levels of IL-6, TNF-α and the inflammatory biomarker C-reactive protein (CRP) (Yudkin et al., 1999; Kim et al., 2008). Both TNF-α and IL-6 are produced by adipose cells (Hotamisligil et al., 1995; Mohamed-Ali et al., 1997) and by macrophages, which typically accumulate in tissues with increased adiposity (Sopasakis et al., 2005; Weisberg et al., 2003). These pro-inflammatory cytokines may then explain the tumor resistance which can be induced by activated macrophages in white adipose tissue (Xu et al., 2003). Adipose tissue contains high concentrations of pro-inflammatory CD4+ Th1 and CD8+ cells together with B cells and dendritic cells (DCs) but in addition has high levels of anti-inflammatory Th2 and Treg cells. The net balance is increasingly shifted towards a pro-inflammatory state in tissue from obese individuals (Lee et al., 2014), promoting an oncogenic environment.
There is an apparent paradox here since, despite the recognition that obesity is accompanied by a chronic low-grade state of inflammation, the evidence for a relationship between systemic inflammatory mediators and the occurrence of cancer is less than compelling. Some general links have been identified, especially in colorectal cancer (Ghuman et al., 2017). It is likely, however, that a resolution of this question will be found in a more specific characterisation and categorisation of the mediators and tumors. Thus, associations have been demonstrated between mediators and the type, location, stability and rate of progression of some cancers (Il'yasova et al., 2005). C Reactive Protein (CRP), TNFa and IL-6 were all correlated with aspects of lung cancer, while CRP and IL6 were correlated with the presence of colorectal cancer and only CRP showed any relationship to breast cancer, with none of these markers having any association with prostate cancer (Il'yasova et al., 2005). As the range of useful markers of inflammation is expanded, more specific relationships are likely to be revealed with different aspects and properties of cancers (Rasmussen et al., 2017). Concentrations of inflammatory markers are, of course, increased in a range of non-cancerous conditions which may dilute any association with cancer.
There are several areas of overlap between the inflammatory hypothesis and those presented above. A recent examination of visceral adipose tissue (Frasca et al., 2017) has revealed high densities of pro-inflammatory B cells in that tissue, expressing elevated levels of inflammatory markers higher than in splenic B cells. Adipocyte-conditioned medium promoted the increased formation of the inflammatory cells, which also secreted increased amounts of adipogenic factors and chemoattractant chemokines. Together these results imply a substantial pro-inflammatory environment associated with visceral adipocytes which would favor carcinogenesis in an obese host.
Adipose tissue is not merely a resting, storage tissue for excess carbohydrates but it secretes a range of compounds with profound effects on metabolic regulation. Obesity is associated with hyperplasia of the adipocytes which generate abnormally high quantities of free fatty acids. The latter are potent activators of macrophages which generate pro-inflammatory cytokines including IL-1β, IL-6 and TNF-α (Howe et al., 2013; Iyengar et al., 2015; Morris et al., 2013). This leads to activation of NFkB via Akt and ultimately to the activation of aromatase and increased synthesis of estrogen, both of which promote estrogen-dependent breast cancer.
Other molecules able to activate inflammatory T cells are continually being identified. The co-activator molecule OX40, for example, enhances the expression of pro-inflammatory genes and exists at high levels on CD4+ T cells located within adipose tissue (Liu et al., 2017). The degree of expression on human T cells was correlated with body weight, whereas induced deficiency of OX40 led to weight loss in experimental mice. OX40 may therefore represent a novel inflammatory regulator relevant to obesity and linking this disorder with inflammation-induced carcinogenesis.
2.6.1. TNF-α
TNF-α expression is up-regulated in parallel with an increase in BMI (Hotamisligil et al., 1995; Kim et al., 2008) although Kern et al. (1995) reported that this relationship did not exist for people in the morbidly obese range. There is also a correlation between circulating TNF-α levels and the prevalence of colorectal adenomata (Kim et al., 2008). The action of TNF-α is thought to be limited to the local adipose tissue microenvironment, where it may function in an autocrine and paracrine fashion since it is not released systemically into the vascular system (Mohamed-Ali et al., 1997). This may explain why local changes in TNF-α levels do not necessarily correspond to variations in systemic TNF-α concentrations (Hotamisligil et al., 1995).
Functionally, TNF-α regulates other adipokines and can induce cell survival., promoting oncogenesis. In vitro studies have demonstrated a reduction of adiponectin mRNA levels within adipose tissue in response to TNF-α, an action that would promote tumor progression (Bruun et al., 2003). On the other hand, although it is widely recognized that TNF-α plays a vital role in inducing tumor necrosis, it is now understood to have anti-apoptotic potential in some tumors, at least partly through the stimulation of nuclear factor-κB (NFκB) (Rubio et al., 2006). The precise role of TNF-α may, therefore, as with other molecules implicated in cancer, be dependent on cell type, cancer stage, local microenvironment and many other factors.
2.6.2. IL-6
The plasma levels of IL-6 in the systemic circulation of morbidly obese patients - a population at risk for cancer related mortality - are significantly greater than control healthy volunteer subjects (Mohamed-Ali et al., 1997; Vendrell et al., 2004; Calle et al., 2003). Higher levels of IL-6 occur in the blood of patients with ovarian and hepatocellular carcinoma, compared with healthy controls (Porta et al., 2008).
The carcinogenic properties of IL-6 seem to be related to its action via the Janus kinase-2 (JAK2)/Signal transducer and activator of transcription-3 (STAT3) signalling pathway (Fig. 1). This is an essential anti-apoptotic and proliferative mechanism in tumor cells, directly activated by the IL-6 receptor (IL-6R) (Loffler et al., 2007; Wang et al., 2013). In addition, IL-6 stimulation of the PI3K/Akt signal transduction pathway leads to the expression of the cell survival factor cyclin D1 (Wegiel et al., 2008), as well as modulating other intracellular proteins which support tumor growth (Fig. 1). IL-6 also inhibits dendritic immune cell differentiation and promotes immune tolerance, reducing T cell immune-surveillance and cytotoxicity (Menetrier-Caux et al., 1998).
Finally, IL-6 provides intriguing links between insulin and the occurrence of inflammation, since insulin is able to promote IL-6 release into the circulation and induces TNF- α gene expression within adipose tissue (Krogh-Madsen et al., 2004).
2.6.3. IRE1
It is likely that with the recognition of inflammation as a significant precursor of cancerous cell behavior, more factors will be identified which encourage or initiate a local inflammatory response, since they will then become suspects for oncogenesis. A recently described example is the Inositol Requiring Enzyme-1 (IRE-1), an endoplasmic reticular enzyme which responds to imposed cellular stress and high fat content feeding by contributing to activation of the Unfolded Protein Response, a co-ordinated reaction to stress which can lead to macromolecular degradation and apoptosis. It has been shown that in adipose tissue-resident macrophages IRE1α activation induces a shift in the biomolecular profile of those cells towards a more highly pro-inflammatory (M1) state of polarization (Bujisic and Martinon, 2017; Shan et al., 2017). If this activation is maintained chronically, it could well contribute to the initiation of tumor formation.
2.6.4. Th17 T Cells
The importance of the relative numbers and activity of Th1 and Th2 helper T cells in determining overall inflammatory status is well established, but the discovery of Th17 cells as a subtype of CD4+ effector T cells related to Th1 cells has introduced a new dimension to the field. Th17 cells contribute to the development of inflammation and hyperglycemia, potentiated by B cell activity in obesity-related diabetes (Ip et al., 2016). Using assays on monocytes from subjects with type-2 diabetes mellitus, it has been found that Th17 cells provide the most robust characterisation of the disorder, especially when associated with B cells, while levels of TNF-α were increased in a range of T cell subsets in addition to Th17 cells. Thus, Th17 cells may represent a crucial link between inflammatory status, type-2 diabetes, elevated BMI values, and carcinogenesis (De Simone et al., 2013; Alizadeh et al., 2013). As noted below, the ability of kynurenine catabolites to suppress activation of pro-inflammatory T cells is also likely to make a significant contribution to regulating immune function and cancer risk.
2.6.5. Inflammasomes
The prominent link between inflammation and obesity has been further emphasized by finding that the NLRP3 inflammasome may contribute to the pathology (Stienstra et al., 2010; Vandanmagsar et al., 2011). The NLRP3 proteins are expressed by adipose tissue macrophages whereas mice deficient in the inflammasome exhibit reduced numbers of activated T cells and lower levels of inflammatory cytokines in adipose tissue. Feeding a high-fat diet activates the inflammasome while mice lacking NLRP3 are relatively resistant to the development of obesity following a high fat diet, and show improved insulin efficacy and glucose metabolic control. Inhibitors of NLRP3 might, therefore, be suitable anti-obesity agents.
2.6. Summary: Existing Hypotheses
Overall, the majority of hypotheses proposed over the past 20–30 years have been based around the physiological functions and pathological correlations of compounds intimately involved in general metabolism of adipose tissue or its regulation by systemic factors and the relevance of those compounds to cell proliferation or development that could contribute to abnormal proliferation and migration leading to oncogenesis. The more recently developed concepts to be described below adopt a wider perspective in which the interface between adipose metabolism, inflammation and carcinogenesis is mediated by newly uncovered links involving biochemical pathways which open new perspectives on the obesity/cancer relationship in a more holistic, biologically integrated manner.
3. New Concepts
3.1. Kynurenines
The kynurenine pathway represents the dominant pathway of tryptophan catabolism, accounting for the disposal of around 95% of the tryptophan not used for protein synthesis. It is initiated by the oxidative enzymes indoleamine-2,3-dioxygenase (IDO) and tryptophan-2,3-dioxygenase (TDO) (Chen and Guillemin, 2009; Stone and Darlington, 2002; Schwarcz and Stone, 2017) but, while TDO is primarily a constitutive hepatic enzyme, IDO is induced and activated by interferon-ɣ which drives the pathway as part of the response to infection and immune system stimulation (Prendergast et al., 2014).
The kynurenine pathway is also driven physiologically by eating. Most foods contain tryptophan and excessive food intake will amplify normal tryptophan catabolism. There is some evidence that tryptophan might mediate feedback changes in food intake, since diets supplemented with tryptophan increase food intake in pigs and show a strong trend to do so in dogs (Fragua et al., 2011). A role for this pathway in feeding behavior is supported by the correlation between levels of kynurenine, kynurenic acid and quinolinic acid with the BMI (Favennec et al., 2015) as well as with increased expression of enzymes along the pathway in adipose tissue from obese human subjects. Enzyme expression was greatest in activated macrophages, consistent with the finding that pathway activation could be induced in adipocytes by pro-inflammatory cytokines. There is strong evidence implicating the kynurenine pathway in the ‘metabolic syndrome’ and insulin resistance which is one of its major features in many obese individuals (Filho et al., 2018; Oh et al., 2017; Oxenkrug et al., 2017; Rebnord et al., 2017). There is a clear correlation between plasma levels of tryptophan and its metabolites, leptin and BMI (Samad et al., 2017). The altered biochemistry appears to develop with chronic obesity since a high kynurenine:tryptophan ratio is seen in adults but not subjects aged 18 or less (Mangge et al., 2014). The increased tryptophan oxidation correlated with abdominal adiposity rather than overall BMI, suggesting that it specifically involved an aspect of fat metabolism – the basis of the metabolic syndrome. The inflammation-induced activation of IDO and its metabolism of tryptophan to kynurenine has been proposed as the major mechanism linking inflammation, depression, type-1 diabetes and obesity (Engin and Engin, 2017; Murfitt et al., 2017; Zhong et al., 2017), partly attributable to the effects of tryptophan metabolites on food craving (Dalkner et al., 2017).
Intriguingly, kynurenine represents an important link with recent studies of the Aryl Hydrocarbon Receptor (AHR) and obesity. The AHR is known to influence food intake and metabolism sufficiently to control body mass in animals fed a diet similar to that of European and North American (“Western”) populations – a concept related to the ‘cafeteria’ diets popular in earlier literature. Conversely, blocking the AHR using specific inhibitors such as CH223191 reduced the development of ‘Western diet’-induced obesity in mice, as did deletion of IDO-1 (Moyer et al., 2016, Moyer et al., 2017).
Kynurenine itself is an important endogenous activator of the AHR, which also responds to exogenous chemicals (notably the dioxin family of toxins) and is activated by both the TGFβ induced NFkB pathway and by Toll-Like Receptors (TLR2/4) activated by oxidized Low Density Lipoprotein (LDL), which also induces IDO-1. Under the influence of TGFβ, the non-canonical activation of NFkB induces IDO-1 expression as well as increased expression of TGFβ. The result is an increased expression of IDO-1 maintained by positive feedback in plasmacytoid DCs (Pallotta et al., 2014a). It has therefore been proposed that it is primarily the kynurenine generated by the TGFβ or TLR activation of IDO-1 which activates the AHR and maintains food intake at obesogenic levels (Moyer et al., 2016). Direct activation of the AHR by dioxins and related xenobiotics increase adipocyte proliferation and differentiation as well as the release of inflammatory compounds by mature adipocytes (Arsenescu et al., 2008). This places the AHR in a powerful position to regulate the immune system (Gutierrez-Vazquez and Quintana, 2018). It has been suggested that xenobiotic compounds maintain ongoing activation of the AHR tending to promote diabetes, glucose intolerance and aspects of the metabolic syndrome (Park et al., 2013).
Paradoxically, IDO-1 expression is greatly reduced or absent in the mouse Non-Obese Diabetic (NOD) model of autoimmune diabetes (type-1), but artificial transfection of IDO-1 inhibited the development of diabetes in parallel with a reduced generation of pro-inflammatory cytokines including IL-6 and TNF-α (Pallotta et al., 2014b). This may indicate that the overall relevance of the kynurenine pathway in type-1 diabetes depends on inflammatory status.
Obesity and some associated risk factors such as hypertension, may involve the effects of kynurenines in the central control of adipose metabolism via the modulation of neuronal glutamate receptors, to be discussed next. Adipose tissue activation initiates sympathetic reflexes which are modulated by glutamate receptors and which include responses to leptin, implying that glutamatergic neural control is located downstream of leptin receptor activation (Cui et al., 2013). Glutamate receptors, especially those sensitive to synthetic N-methyl-D-aspartate (NMDA) may be relevant to obesity since they are present in cerebral regions responsible for appetitive and metabolic regulation. The only known selective endogenous agonist at NMDA receptors is quinolinic acid (Stone and Perkins, 1981) a product of kynurenine metabolism (Stone and Darlington, 2002; Badawy, 2017) (Fig. 2) which has been shown to induce a range of physiological and degenerative changes via the NMDA receptors and which has consequently been implicated in several clinical disorders of metabolism or neuronal function including depression, stroke (Stone et al., 2012a) Huntington's disease (Stone et al., 2012b; Forrest et al., 2010) and cognitive disorders such as schizophrenia (Stone and Darlington, 2002; Stone and Darlington, 2013; Schwarcz and Stone, 2017). The pathway is also involved intimately in early brain development and, as a result, interference with the pathway during embryogenesis can affect brain structure and function (Forrest et al., 2013a, Forrest et al., 2013b; Khalil et al., 2014), cognitive and behavioral performance of the postnatal offspring (Notarangelo and Pocivavsek, 2017) as well as functions of other organ systems (Song et al., 2017). Adipose tissue activation initiates sympathetic reflexes which are modulated by glutamate receptors and which include responses to leptin, implying that glutamatergic neural control is located downstream of leptin receptor activation (Cui et al., 2013) and is an important component of the appetitive neural network. In this context, it may be relevant that the glutamate-induced animal model of obesity has proved valuable in explaining several aspects of over-eating and obesity and has helped to understand the role of positive influences such as exercise (Gobatto et al., 2002). The presence of glutamate receptors as a key factor in obesity also explains the anti-obesity activity of glutamate antagonists such as memantine (Hermanussen and Tresguerres, 2005).
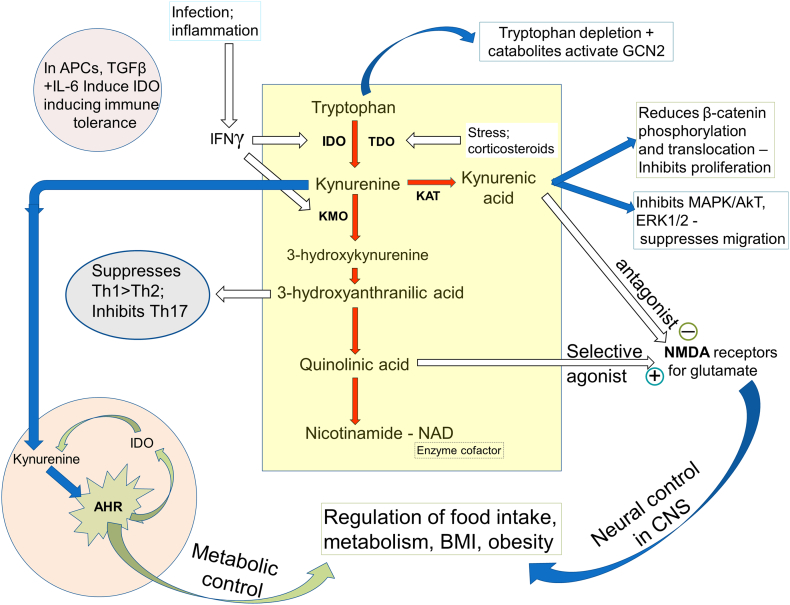
Fig. 2.
The kynurenine pathway is initiated by the oxidation of tryptophan via the enzymes IDO and TDO, with downstream catabolites having toxic, antioxidant and cell-protective activity. Several feedback circuits involving the kynurenines, regulation of the Aryl Hydrocarbon Receptor (AHR), T cell production and balance and activity of the cell survival modulator GCN2 place the pathway in a central position to integrate many aspects of metabolic and feeding control. Quinolinic acid as an agonist and kynurenic acid as an antagonist at NMDA receptors contribute to the activity of feeding regulatory systems in the hypothalamus. Effects on β-catenin activation and translocation as well as key enzymes in the determination of cell viability, such as MAPK and ERK1/2 account for the effects of kynurenines on carcinogenesis.
Importantly, NMDA receptors are blocked by another kynurenine metabolite, kynurenic acid (Perkins and Stone, 1982; Stone et al., 2013). The quinolinic acid activation of NMDAR or their blockade by kynurenic acid could account for part of the regulatory function of kynurenines in feeding behavior and body mass regulation.
3.2. Kynurenines and Cancer
The relevance of the kynurenine pathway is that not only do its components affect the regulation of metabolism, feeding and body mass, largely via the modulation of NMDA receptor activity, but they are also implicated in aspects of carcinogenesis. Expression of the central enzyme of the pathway - kynurenine-3-monooxygenase (KMO) is greater in human hepatic carcinoma cells than controls (Jin et al., 2015) and is known to influence cell proliferation and migration (Lucarelli et al., 2017). A key factor implicating kynurenines in cancer was the discovery that kynurenine was a major endogenous activator of the AHR (Opitz et al., 2011a). Activation of the AHR has been linked to several types of cancer (DiNatale et al., 2010a, DiNatale et al., 2010b) since it promotes Treg development, suppressing effector T cell activity and promoting tumor development and progression. The importance of this kynurenine-AHR interaction lies partly in its positive feedback nature, since AHR activation induces IDO expression which produces more kynurenine and thus initiates a potentially explosive generation of IDO and kynurenine metabolites.
The kynurenine pathway is known to be up-regulated in triple negative breast cancer (TNBC) cells. This seems to include not only IDO but also the recently described TDO2 whose expression is dependent on NFkB. The increased generation of kynurenine is sufficient to activate the AHR and could contribute to cancer progression and metastasis as noted above. Removal or inhibition of the AHR reduces metastasis, as does the inhibition, in vivo, of TDO2 (D'Amato et al., 2015).
The scratch or wound injury assay in culture or in vivo is often used to reflect cell migration and is associated with a significant increase in the expression of IDO in the wounded and adjacent cells. However, wound healing has been reported to improve in the absence of IDO or after its inhibition by 1-methyl-D-tryptophan (1MT) (Ito et al., 2015). Tryptophan but not kynurenine improved wound closure, perhaps indicating a role for kynurenic acid. One caveat to consider in all work employing 1MT is that this compound also inhibits tryptophan uptake and upregulates the expression of IDO-1, an action that would counteract enzyme inhibition (Opitz et al., 2011b). On the other hand it has been shown that the transfection of ectopic IDO into fibroblasts, or their treatment with kynurenine, induced less scar tissue than in normal cells (Li et al., 2014). Part of this phenomenon may be explained by kynurenine's ability to induce matrix metalloprotease activity, an action which, interestingly, is dependent on MAPK activity which has also been linked to oncogenesis.
These considerations account for the widespread interest in the development of IDO inhibitors to suppress Treg (and cancer cell) suppression of T effector cells consistent with data that mice develop fewer tumors in the absence of IDO activity (Thaker et al., 2013).
Kynurenic acid levels are lower in many cancer cells than naïve cells, a factor possibly contributing to tumor development since kynurenic acid inhibits cancer cell migration and proliferation albeit at high, micromolar, concentrations (Walczak et al., 2011, Walczak et al., 2012, Walczak et al., 2014a) probably via inhibition of MAPK, Akt and ERK1/2 (Walczak et al., 2014b). Also, kynurenic acid, as with kynurenine, can suppress inflammation and inhibit the excessive proliferation and overgrowth of regenerating tissue which normally leads to scar formation (Elizei et al., 2015; Poormasjedi-Meibod et al., 2014). Despite these results, kynurenic acid is said to be a good indicator of cancerous tissue with lymphatic metastases (Sagan et al., 2015) although it is often difficult to attribute such correlations to having direct relevance to the cancer itself, rather than the associated inflammation that exists with advanced, malignant disease.
A different perspective on the kynurenine pathway which is relevant to both the control of body mass and cancer progression is its ability to regulate cells of the immune system. It is well established that kynurenine itself can affect the production of Treg cells as noted above, but it is also metabolized to compounds with marked anti-inflammatory activity. Notably, 3-HAA suppresses the mainly pro-inflammatory Th1 subtype of effector T cells as well as Th17 cells, (Fallarino et al., 2002; Stephens et al., 2013; Criado et al., 2009) with little effect on anti-inflammatory Th2 cells. The generation of tryptophan catabolites such as these, in addition to the metabolic effects of tryptophan depletion can activate General Control Non-derepressible-2 (GCN2) and affect cell proliferation and migration (Eleftheriadis et al., 2015). Overall, the kynurenine pathway is believed to promote a net anti-inflammatory balance in the immune system which would reduce the inflammatory driving force converting some normal or potentially cancerous cells to an actively aggressive state.
Finally, the kynurenine pathway plays an important role in most cells as the synthetic route for nicotinamide and NAD. Depletion of the latter enzymic cofactor is known to suppress cell proliferation and motility by disrupting many fundamental metabolic pathways (Kennedy et al., 2016). Thus, any interference with the activity of IDO or subsequent enzymes in the kynurenine pathway, or a loss of the individual catabolites, is likely to yield similar overall negative effects on cell function. While it is not clear whether such interference would be sufficient to exert significant anti-cancer activity, it is likely that a combination of kynurenine pathway disruption leading to lowered levels of NAD, together with conventional chemotherapy, may have therapeutic advantages.
A major challenge remains in relating kynurenine pathway activity to the initiation of cancer. Some growth factors such as TGFβ are thought to be critical inducers of cell motility and invasion. Certainly TGFβ is a factor initiating EMT (Brito et al., 2015) although equating EMT with cell migration has been cast in to doubt by work indicating a clearer relationship with cancer cell viability and chemoresistance (Fischer et al., 2015; Zheng et al., 2015). Inhibition of IDO (using 1MT) potentiated the induction of EMT by TGFβ, promoting cell migration. This was associated with the recognized molecular markers of EMT such as a loss of E-cadherin and increased expression of N-cadherin.
3.3. Serine Proteases
Serine proteases have been reported to affect a myriad of cellular functions including proliferation and migration, some of which are relevant to inflammation and oncogenesis. Further, serine protease inhibitors can reduce inflammation and the incidence of some tumors (Roy et al., 2010). The molecular mechanisms of these effects, however, have remained unclear but the recent discovery that major, common, mammalian serine proteases such as chymotrypsin and the related bacterial chymotryptic enzyme subtilisin, are able to deplete cells of tumor suppressor proteins (Forrest et al., 2016) has triggered renewed interest in these enzymes and their molecular targets.
The tumor suppressor proteins that were studied included Deleted in Colorectal Cancer (DCC), neogenin (a related protein with 49% structural homology with DCC) and uncoordinated-5 (unc5), all of which are receptors for the extracellular protein family of netrins (Sun et al., 2011a). In the absence of netrins, DCC and neogenin inhibit proliferation and induce apoptosis, resulting in their classification as ‘tumor suppressors’. This effect is restrained by netrin binding so that cellular survival and proliferation is dependent on the presence of the netrin-receptor interaction, giving rise to the alternative description of DCC, neogenin and unc5 as ‘dependence receptors’ (Mehlen and Guenebeaud, 2010). Conversely, in the absence of DCC and neogenin, netrin itself drives proliferation which is normally restrained by the dependence proteins. The consequence of the interplay between these proteins is that loss or removal of DCC or neogenin will allow increased cell proliferation or migration which will be further enhanced by the unopposed activity of netrin. Hence, there will be a greater probability that the cells affected by the loss of DCC and neogenin will progress to a cancerous state (Fig. 3).
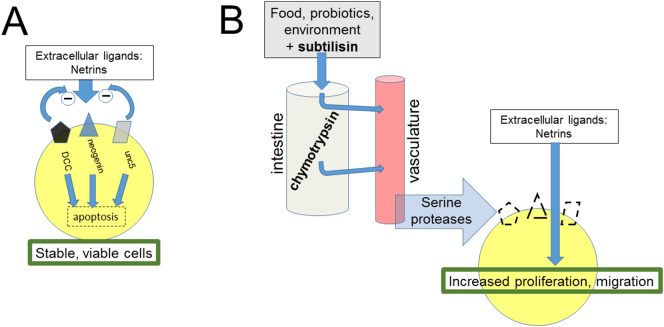
Fig. 3.
A. The transmembrane Dependence Receptors Deleted in Colorectal Cancer (DCC), neogenin and unc5 are receptors for the group of netrin ligands. The balance between intrinsic activity of these receptors which induces apoptosis, and the inhibitory effect of netrin binding which also directly promotes cell proliferation, maintains cells in a state of optimum viability. Serine proteases such as chymotrypsin in the digestive tract and systemic circulation, or exogenous chymotryptic enzymes such as subtilisin deplete cells of their Dependence Receptors, allowing netrin to drive proliferation unopposed, potentially leading to carcinogenesis.
Among the major mammalian serine proteases are the pancreatic digestive enzymes such as trypsin and chymotrypsin. Other serine proteases are produced by the liver (pro-protein convertases), leucocytes (neutrophil elastase) and prostate glands (prostate specific antigen). Chymotrypsin is of special interest since it is extraordinarily stable, being resistant to most other proteases. The concentration of chymotrypsin remains almost unchanged in transit from the pancreas to the feces. Indeed, chymotrypsin is largely responsible for the destruction of another major serine protease, trypsin, whose levels decline progressively between the upper and lower intestines while chymotrypsin is unchanged. In addition, chymotrypsin is absorbed from the intestine into the circulation of humans (Miller et al., 1960; Kabacoff et al., 1963) and laboratory animals (Megel et al., 1964) even after oral administration (Avakian, 1964), giving access of the enzyme to all vascularized tissues. An increased food intake leads to a higher secretion of chymotrypsin (Piccione et al., 2004) to handle the increased digestive demand and which persists into the feces. Over-eating will increase levels of chymotrypsin in the circulation secondary to intestinal absorption. Piccione et al. (2004) reported a correlation in dogs between the concentration of chymotrypsin in the intestinal chyme or feces and body weight. Chymotrypsin levels therefore correlate with body weight (Piccione et al., 2004; Hashimoto and Nara, 2003) whereas elevated serum levels of anti-chymotrypsin are inversely related to body weight (Friis et al., 2002). Conversely, elevated serum levels of anti-chymotrypsin are inversely related to body weight (Friis et al., 2002), consistent with the view that it is increased chymotryptic activity - whether caused by raised chymotrypsin or lowered anti-chymotrypsin - which may be responsible for increased cancer susceptibility. The concentration of chymotrypsin in the human intestine is between 1 and 10 μM, the same concentration range that has been shown to remove the tumor suppressors DCC and neogenin from cellular membranes (Forrest et al., 2016). The concentration of trypsin, in contrast, falls substantially during its intestinal transit.
Over-eating will therefore result in supra-normal levels of chymotrypsin in the intestinal contents (Hashimoto and Nara, 2003). In addition, it was established years ago that chymotrypsin is absorbed from the intestine into the circulation of humans (Miller et al., 1960) and laboratory animals (Kabacoff et al., 1963; Megel et al., 1964) even after oral administration (Avakian, 1964). Plasma levels will then be increased, giving access of the enzyme to all vascularised tissues. While not identifying the proteins directly, other groups have reported increased circulating chymotryptic activity after increasing intestinal levels (Colman, 1965; Sherry and Fletcher, 1960). In relation to the composition of diet discussed above it is highly relevant that trypsin levels were found to be four times greater in the feces of dogs given a meat-based diet compared with a cereal diet (Merritt et al., 1979).
It is interesting to note that chymotrypsin is able to metabolise insulin (Kono, 1969) raising the possibility that increased levels of chymotrypsin in the plasma and tissues of obese individuals could be at least partly responsible for both the type-2 diabetes and the cancer susceptibility resulting from over-eating.
Finally, it remains unclear whether there is any functional relationship between the chymotryptic activity of the 20S proteasomal subunits - which are targeted by several anti-cancer drugs (Neilsen et al., 2013) - and the chymotryptic activity of subtilisin and chymotrypsin. While proteasomal inhibitors often have little effect against chymotrypsin activity, there is certainly a degree of structural overlap since all these enzymes are inhibited by chymostatin.
3.3.1. Subtilisin
Similar considerations apply to the bacterial chymotryptic serine protease, subtilisin, which is able to deplete DCC and neogenin at nanomolar concentrations in cultured cells, at least an order of magnitude more potent than chymotrypsin (Forrest et al., 2016).
Indeed, just as chymotrypsin concentrations in the blood, determined by its intestinal secretion in proportion to protein intake, may contribute substantially to the obesity-cancer association, the efficacy of subtilisin may represent a major link between environmental factors and cancer. Subtilisin is abundant in the environment and several species of Bacillus which secrete subtilisin, including B. subtilis, colonise the intestine and, together with their spores, can survive gastric and intestinal digestion (Hoa et al., 2000, Hoa et al., 2001; Hong et al., 2008). Subtilisin is also among the proteases used in the preparation of animal feedstuffs and probiotics administered as alternatives to antibiotics (Hoa et al., 2000) to increase meat production in farm animals (Alexopoulos et al., 2004; Kowalski et al., 2009; Sun et al., 2010, Sun et al., 2011b; Ripamonti and Stella, 2009; Hong et al., 2008; Kampf, 2012; Te Giffel et al., 1996) from where the enzyme might enter the human food chain. Numerous studies have revealed the presence of Bacillus species in popular food items (Cachaldora et al., 2014; Matarante et al., 2004; Te Giffel et al., 1996) and subtilisin-like enzymes are secreted by many other microbes (Bonifait et al., 2011).
This risk is increased by the use of subtilisin in food processing, especially of red meats in which it tenderises the meat and increases its flavor as well as facilitating handling during processing (Piazza and Garcia, 2014; see Stone and Darlington, 2017). These various commercial used may lead to subtilisin entering the food chain, with tissue levels becoming higher in individuals consuming a high proportion of red meats or processed food. This would be consistent with epidemiological data suggesting that dietary processed red meat is more carcinogenic than fresh produce. A significantly higher risk of several forms of human cancer is associated with regular meat consumption (McCullough et al., 2013; Rohrmann et al., 2013; Key et al., 2002, Key et al., 2014; Song et al., 2014; Wie et al., 2014; Xu et al., 2014; Xue et al., 2014; Mourouti et al., 2015). Similarly carcinogenesis is increased by beef consumption in experimental animals (Alexander et al., 2011; Aune et al., 2013; Chan et al., 2011; Larsson et al., 2006; Larsson and Wolk, 2006; Norat et al., 2002; Mrkonjic et al., 2009).
Subtilisin is also one of the biological additives included in some domestic cleaning products such as biological washing powders and fluids, with which skin contact and inhalation should be carefully avoided (Gupta et al., 2002; Maurer, 2004) . In addition to the innate risks of overeating and obesity described above, it is probable that increased food intake will increase the total burden of B. subtilis and of subtilisin in the gastrointestinal tract, an effect that might be mitigated by increasing transit time. The possibility also requires consideration that increased levels of these serine proteases from different sources, mammalian, environmental and dietary, may result in synergistic effects, further increasing cancer risk. From all these sources, subtilisin and related proteases may represent a significant environmental threat of carcinogenesis.
Conversely, bacteria which generate acidic environments, such as the lactobacilli in some probiotic preparations, should counteract the effects of alkaline proteases such as subtilisin, reducing the latter's ability to deplete cells of their dependence receptors. Such an interaction might contribute to their ability to reduce the incidence of bladder, colorectal and other cancers in humans (Zhong et al., 2014; Davis and Milner, 2009).
3.3.2. Overall Dietary Consideration
Plant-based foodstuffs are generally considered to be protective against cancer (Orlich et al., 2015). Even an early review of 156 studies concluded that cancer risk in people consuming low amounts of fruits and vegetables was approximately double that of individuals with a high intake of these products, even after controlling for potentially confounding factors (Block et al., 1992) and the more recent work referred to above has repeated confirmed these findings. The presence in some dietary plant species of the family of Bowman-Birk inhibitors (BBI) provide scientifically credible reasons why diets rich in fruits and vegetables may protect against the development of many cancers.
Bowman-Birk inhibitors are relatively small proteins, highly stable within the intestine and generally resistant to heating and cooking, which are known to be absorbed from the intestine into the blood. Many BBIs are efficient inhibitors of cancer cell growth, giving them both preventative and potentially curative properties even against cancer resistant to conventional anti-cancer medication (Wan et al., 1998, Wan et al., 2002; Kennedy, 1998; Aggarwal and Shishodia, 2006). Their inhibition of serine protease-induced down-regulation of tumor suppressors (Forrest et al., 2016; Stone and Darlington, 2017) could be a key element in this anti-cancer activity.
4. Discussion
Despite the growing awareness of potential mechanisms which could contribute to a causal association between obesity and cancer, their relative importance and details of their molecular basis remain unclear. Lifestyle factors such as poor diet and low physical activity might facilitate the development of obesity and cancer independently (Norat et al., 2005; Wang and Beydoun, 2009). Simple physical factors such as intestinal transit time, which is known to be relevant to oncogenesis and is reduced with poor diets or low exercise levels, are also likely to be relevant. It is also clear that there are methodological problems associated with the study of these concepts in humans, including doubts about the validity of some forms of measurement. Obesity-associated cancer risk is commonly determined using the measurement of BMI but previous studies imply that more suitable measurements include waist circumference and visceral adiposity (Moore et al., 2004; Pischon et al., 2006). Studies limited to measurements of BMI may be inappropriate, incomplete or even misleading.
Molecular considerations underlying the link between obesity and cancer such as an abnormal insulin/IGF-1 axis, dysregulated hormonal signaling, fatty acid metabolism and chronic inflammation, or a combination of these, could be among the factors involved in carcinogenesis. In addition, we have summarised new information that serine proteases such as chymotrypsin in intestinal secretions and subtilisin in the diet and environment can deplete cells of key tumor suppressors, findings which lead to novel and exciting potential avenues for exploration. These include simple public health measures such as (a) eliminating subtilisin from food processing procedures (b) removing B. subtilis and other serine protease generators from farm animal probiotics and, thus, from the human food chain (c) increasing the consumption of fruit and vegetables containing Bowman-Birk and related serine protease inhibitors (d) promoting the thorough cleaning of root vegetables to remove B. subtilis-containing soil. There are also non-dietary sources of subtilisin, such as cleaning materials (see 37) and it may be rational to encourage the thorough rinsing of cutlery, crockery and other items used in food preparation and consumption and the thorough rinsing of clothing to remove traces of commercially added subtilisin-like enzymes in biological detergents. Together, these actions might prevent countless incidences of cancer easily and cost-effectively.
What remains unclear is the nature of the relationship between any one of the mechanisms discussed above and the etiology of cancer. The deletion of dependence receptors, for example, does increase proliferation in some cell types and does increase cell migration as expected of an aggressive, potentially metastatic cell (see Stone and Darlington, 2017). That not all cells respond in this way and that the effects are usually small rather than dramatic has been interpreted by some to indicate that these proteins are less important in cancer than might be expected. However, it has been proposed that around six independent steps are required to convert a normal cell into a rapidly proliferating, actively migrating cancer cell (Hanahan and Weinberg, 2011). That would seem to require an extremely rare combination of events. If, however, there are errors of cellular function being induced frequently throughout life, for example by repeated episodes of over-eating or consuming meat and processed products, then a partial loss of DCC or neogenin could lower the threshold for carcinogenesis to occur when one or two of the other factors strikes (e.g. radiation exposure, low vitamin levels, contact with an oncogenic stimulus such as smoking or air pollution). Tomasetti et al. (2017) have recently proposed a closely similar concept in which a single necessary but avoidable cellular disturbance could promote cancer development if it occurs on a background of a wide range of spontaneous and essentially unavoidable factors. If that is the case, the possible role of individual factors such as an obesity-related product, exposure to a toxin or radiation, or contact with a dietary or environmental serine protease, as discussed here, could become critical. Preventing such avoidable concerns might be far more important to cancer prevention than has been recognized.
5. Outstanding Questions
Most of the current ideas have little in common and important questions remain to be answered. Does chronic manipulation of carbohydrate or lipid metabolism, by dietary or genetic means, affect the incidence of cancer in experimental animals, or can a clearcut answer be obtained by a comparison of human populations in which these factors are already established as a result of local climatic (and therefore agricultural) conditions, habitual consumption, spiritual preferences or other environmental or lifestyle drivers? Is there robust evidence for genetic abnormalities associated with the production, destruction or receptor-mediated actions of compounds such as adipokines, kynurenines, serine proteases or dependence receptors? Also, given the increasing recognition of the prominent role of the microbiome in health and disease, to what extent do the microbiota produce, catabolise, transport or secrete compounds which include, or influence, the endogenous metabolites? And does the answer to that question have implications for a dietary-modulated dysbiosis with the potential for therapeutic intervention by microbial manipulation (antibiotics, biochemical interference, probiotics, etc.) (Li et al., 2009)?
Does pharmacological or genetic manipulation of the kynurenine pathway alter the susceptibility to carcinogenesis? Are different manipulations based on the hypotheses discussed related to specific forms of cancer or their stage and rate of progression?
One important objective should be to differentiate between factors which independently promote obesity or cancer, and those whose primary effect is obesogenic leading to secondary carcinogenesis. Making that differentiation would greatly assist in the clarification of the underlying mechanisms. Of the more recent hypotheses, the kynurenine hypothesis is more likely to fall into the former category, producing a degree of obesity and oncogenesis independently, whereas the serine protease and dependence receptor concept is more likely to reflect the changes in enzyme activity accompanying obesity and leading to the initiation of cancer local and systemic cancers as a result.
6. Search Strategy and Selection
The World of Knowledge and PubMed databases were used for searching. Initial searches were based on general terms intended to provide a wide-ranging overview of the subject. Also word stems were used to capture related items (obes*, overeating, BMI) and (cancer, carcinoma*, tumor*). Selection at this stage eliminated purely data-gathering, statistical and epidemiological studies which contributed little to the generation of conceptual explanations or which did not materially affect our assessment of the acceptance or rejection of potential hypotheses. Titles were trawled for publications with relevance to a wide range of potential molecular mechanisms and key words identified from this process (such as adiponectin, IGF, kynurenine, serine protease, DCC etc.) were then used for further rounds of more focussed searching including abstracts and then full papers. This left a range of studies with relatively clear relevance to major hypotheses and for each of those hypotheses a further search was focused on two or three key-words central to the hypothesis (adiponectin, IRE1, diabet*, etc., combined with kynuren*, quinolinic, chymotryp*, DCC, neogenin, netrin etc.) and with the general terms used initially. The final selection of references to be cited was based on the amount of information contained, the originality of the findings (as opposed to their confirmation or extension), or their direct relevance to the link between obesity and cancer. Since the nature of much of this review is to draw together a range of data which have not been considered related in the past, including some very fundamental aspects of physiology of the serine proteases, it has been necessary to cite some earlier papers. We consider it important to acknowledge such fundamental reports whose potentially critical importance to disease is only now being recognized.
Author Contributions
MM and TWS performed the initial literature searches and prepared the original draft manuscript, which was subsequently read and substantially modified, with the addition of much additional material, by TWS and LGD.
Funding Sources
Not applicable in the preparation of this review. The authors' experimental work cited in the review was supported by Epsom Medical Research (EMR2014/7) and The Peacock Charitable Trust (PT-LD3).
Declaration of Interest
All authors declare that they have no conflicts of interest with this review.
References
- Aggarwal B.B., Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Alexander D.D., Weed D.L., Cushing C.A., Lowe K.A. Meta-analysis of prospective studies of red meat consumption and colorectal cancer. Eur. J. Cancer Prev. 2011;20:293–307. doi: 10.1097/CEJ.0b013e328345f985. [DOI] [PubMed] [Google Scholar]
- Alexopoulos C., Georgoulakis I.E., Tzivara A., Kyriakis C.S., Govaris A., Kyriakis S.C. Field evaluation of the effect of a probiotic-containing Bacillus licheniformis and Bacillus subtilis spores on the health status, performance, and carcass quality of grower and finisher pigs. J. Vet. Med. 2004;51:306–312. doi: 10.1111/j.1439-0442.2004.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh D., Katsanis E., Larmonier N. The multifaceted role of Th17 lymphocytes and their associated cytokines in cancer. Clin. Dev. Immunol. 2013 doi: 10.1155/2013/957878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita Y., Kihara S., Ouchi N., Takahashi M., Maeda K., Miyagawa J. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- Arner E., Forrest A.R.R., Ehrlund A., Mejhert N., Itoh M., Kawaji H. Ceruloplasmin is a novel adipokine which is overexpressed in adipose tissue of obese subjects and in obesity-associated cancer cells. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0080274. (AR e80274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenescu V., Arsenescu R.I., King V., Swanson H., Cassis L.A. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ. Health Perspect. 2008;116:761–768. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan E., Atilgan H., Yavasoglu I. The prevalence of Helicobacter pylori in obese subjects. Eur. J. Int. Med. 2009;20:695–697. doi: 10.1016/j.ejim.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Attoub S., Noe V., Pirola L., Bruyneel E., Chastre E., Mareel M. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. FASEB J. 2000;14:2329–2338. doi: 10.1096/fj.00-0162. [DOI] [PubMed] [Google Scholar]
- Aune D., Chan D.S.M., Vieira A.R., Rosenblatt D.A.N., Vieira R., Greenwood D.C., Kampman E., Norat T. Red and processed meat intake and risk of colorectal adenomas: a systematic review and meta-analysis of epidemiological studies. Cancer Causes Control. 2013;24:611–627. doi: 10.1007/s10552-012-0139-z. [DOI] [PubMed] [Google Scholar]
- Avakian S. Further studies on absorption of chymotrypsin. Clin. Pharmacol. Ther. 1964;5:712–713. doi: 10.1002/cpt196456part1712. [DOI] [PubMed] [Google Scholar]
- Badawy A.A.B. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int. J. Trp. Res. 2017;10 doi: 10.1177/1178646917691938. (Art.1178646917691938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban S., Shearer R.F., Lee L.S., van Geldermalsen M., Schreuder M., Shtein H.C. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017;5 doi: 10.1186/s40170-016-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A.H., Combs T.P., Du X., Brownlee M., Scherer P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Bernstein C.N., Blanchard J.F., Kliewer E., Wajda A. Cancer risk in patients with inflammatory bowel disease. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Bhaskaran K., Douglas I., Forbes H., dos-Santos-Silva I., Leon D.A., Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block G., Patterson B., Subar A. Fruit, vegetables and cancer prevention - a review of the epidemiologic evidence. Nutr. Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- Bonifait L., Vaillancourt K., Gottschalk M., Frenette M., Grenier D. Purification and characterization of the subtilisin-like protease of Streptococcus that contributes to its virulence. Vet. Microbiol. 2011;148:333–340. doi: 10.1016/j.vetmic.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Boyd N.F., Guo H., Martin L.J., Sun L.M., Stone J., Fisher L. Mammographic density and the risk and detection of breast cancer. New Engl. J. Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- Brakenhielm E., Veitonmaki N., Cao R.H., Kihara S., Matsuzawa Y.J., Zhivotovsky B. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc. Nat. Acad. Sci. USA. 2004;101:2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito O., Rodrigo B., Malta C.S. 1-Methyl-d-tryptophan potentiates TGF-beta-induced epithelial-mesenchymal transition in T24 human bladder cancer cells. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0134858. (AR e0134858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruun J.M., Lihn A.S., Verdich C., Pedersen S.B., Toubro S., Astrup A. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am. J. Physiol. Endocrinol. Metab. 2003;285:E527–E533. doi: 10.1152/ajpendo.00110.2003. [DOI] [PubMed] [Google Scholar]
- Bujisic B., Martinon F. IRE1 gives weight to obesity-associated inflammation. Nat. Immunol. 2017;18:479–480. doi: 10.1038/ni.3725. [DOI] [PubMed] [Google Scholar]
- Byeon J.S., Jeong J.Y., Kim M.J., Lee S.M., Nam W.H., Myung S.J. Adiponectin and adiponectin receptor in relation to colorectal cancer progression. Int. J. Cancer. 2010;127:2758–2767. doi: 10.1002/ijc.25301. [DOI] [PubMed] [Google Scholar]
- Cachaldora A., Fonseca S., Gomez M., Franco I., Carballo J. Metabolic Characterization of Bacillus subtilis and Bacillus amyloliquefaciens strains isolated from traditional dry-cured sausages. J. Food Prot. 2014;77:1605–1611. doi: 10.4315/0362-028X.JFP-14-145. [DOI] [PubMed] [Google Scholar]
- Calle E.E., Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. New Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Cameron A.J., Welborn T.A., Zimmet P.Z., Dunstan D.W., Owen N., Salmon J. Overweight and obesity in Australia: the 1999-2000 Australian diabetes, obesity and lifestyle study (AusDiab) Med. J. Aust. 2003;178:427–432. doi: 10.5694/j.1326-5377.2004.tb05998.x. [DOI] [PubMed] [Google Scholar]
- Catalano S., Marsico S., Giordano C., Mauro L., Rizza P., Panno M.L. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J. Biol. Chem. 2003;278:28668–28676. doi: 10.1074/jbc.M301695200. [DOI] [PubMed] [Google Scholar]
- Catalano S., Mauro L., Marsico S., Giordano C., Rizza P., Rago V. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor α in MCF-7 cells. J. Biol. Chem. 2004;279:19908–19915. doi: 10.1074/jbc.M313191200. [DOI] [PubMed] [Google Scholar]
- Chan D.S.M., Lau R., Aune D., Vieira R., Greenwood D.C., Kampman E., Norat T. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0020456. (6AR e20456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Guillemin G.J. Kynurenine pathway metabolites in humans: disease and healthy states. Int. J. Tryptophan. Res. 2009;2:1–19. doi: 10.4137/ijtr.s2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Chang Y.C., Lan M.S., Breslin M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl-1 expression via the activation of the MEK/ERK1/2 and PI3K/Akt signaling pathways. Int. J. Oncol. 2013;42:1113–1119. doi: 10.3892/ijo.2013.1789. [DOI] [PubMed] [Google Scholar]
- Choi S.M., Tucker D.F., Gross D.N., Easton R.M., DiPilato L.M., Dean A.S. Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol. Cell. Biol. 2010;30:5009–5020. doi: 10.1128/MCB.00797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D.H., LeRoith D. Obesity, type 2 diabetes, and cancer: the insulin and IGF connection. Endocr. Relat. Cancer. 2012;19:F27–F45. doi: 10.1530/ERC-11-0374. [DOI] [PubMed] [Google Scholar]
- Colman R.W. Proteolytic enzymes in clinical medicine. Clin. Pharmacol. Ther. 1965;6:598–599. doi: 10.1002/cpt196565598. [DOI] [PubMed] [Google Scholar]
- Cottam D., Fisher B., Ziemba A., Atkinson J., Grace B., Ward D.C. Tumor growth factor expression in obesity and changes in expression with weight loss: another cause of increased virulence and incidence of cancer in obesity. Surg. Obes. Relat. Dis. 2010;6:538–541. doi: 10.1016/j.soard.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Criado G., Simelyte E., Inglis J.J., Essex D., Williams R.O. Indoleamine 2,3 dioxygenase-mediated tryptophan catabolism regulates accumulation of Th1/Th17 cells in the joint in collagen-induced arthritis. Arthritis Rheum. 2009;60:1342–1351. doi: 10.1002/art.24446. [DOI] [PubMed] [Google Scholar]
- Cui B.-P., Li P., Sun H.-J., Ding L., Zhou Y.-B., Wang J.-J. Ionotropic glutamate receptors in paraventricular nucleus mediate adipose afferent reflex and regulate sympathetic outflow in rats. Acta Physiol. 2013;209:45–54. doi: 10.1111/apha.12125. [DOI] [PubMed] [Google Scholar]
- Dal Maso L., Augustin L.S., Karalis A., Talamini R., Franceschi S., Trichopoulos D. Circulating adiponectin and endometrial cancer risk. J. Clin. Endocrinol. Metab. 2004;89:1160–1163. doi: 10.1210/jc.2003-031716. [DOI] [PubMed] [Google Scholar]
- Dalkner N., Platzer M., Bengesser S.A., Birner A., Fellendorf F.T., Queissner R. The role of tryptophan metabolism and food craving in the relationship between obesity and bipolar disorder. Clin. Nutr. (Edinburgh) 2017 doi: 10.1016/j.clnu.2017.06.024. [DOI] [PubMed] [Google Scholar]
- D'Amato N.C., Rogers T.J., Gordon M.A., Greene L.I., Cochrane D.R., Spoelstra N.S. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 2015;75:4651–4664. doi: 10.1158/0008-5472.CAN-15-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S.R., Dudek H., Tao X., Masters S., Fu H., Gotoh Y., Greenberg M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Davis C.D., Milner J.A. Gastrointestinal microflora, food components and colon cancer prevention. J. Nutr. Biochem. 2009;20:743–752. doi: 10.1016/j.jnutbio.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone V., Pallone F., Monteleone G., Stolfi C. Role of TH17 cytokines in the control of colorectal cancer. Oncoimmunology. 2013;2 doi: 10.4161/onci.26617. (AR-UNSPe26617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilippis R.A., Chang H., Dumont N., Rabban J.T., Chen Y.Y., Fontenay G.V. CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. Cancer Discov. 2012;2:826–839. doi: 10.1158/2159-8290.CD-12-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudonne M.N., Machinal-Quelin F., Serazin-Leroy V., Leneveu M.C., Pecquery R., Giudicelli Y. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem. Biophys. Res. Commun. 2002;293:622–628. doi: 10.1016/S0006-291X(02)00205-X. [DOI] [PubMed] [Google Scholar]
- DiNatale B.C., Schroeder J.C., Francey L.J., Kusnadi A., Perdew G.H. Mechanistic insights into the events that lead to synergistic induction of interleukin-6 transcription upon activation of the aryl hydrocarbon receptor and inflammatory signalling. J. Biol. Chem. 2010;285:24388–24397. doi: 10.1074/jbc.M110.118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNatale B.C., Murray I.A., Schroeder J.C. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signalling. Toxicol. Sci. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftheriadis T., Pissas G., Antoniadi G., Liakopoulos V., Stefanidis I. Indoleamine 2,3-dioxygenase depletes tryptophan, activates general control non-derepressible 2 kinase and down-regulates key enzymes involved in fatty acid synthesis in primary human CD4(+) T cells. Immunology. 2015;146:292–300. doi: 10.1111/imm.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizei S.S., Poormasjedi-Meibod M.-S., Li Y., Jalili R.B., Ghahary A. Effects of kynurenine on CD3+and macrophages in wound healing. Wound Repair Regen. 2015;23:90–97. doi: 10.1111/wrr.12252. [DOI] [PubMed] [Google Scholar]
- Engin A.B., Engin A. The interactions between kynurenine, folate, methionine and pteridine pathways in obesity. Adv. Exp. Med. Biol. 2017;960:511–527. doi: 10.1007/978-3-319-48382-5_22. [DOI] [PubMed] [Google Scholar]
- Facchini F.S., Hua N., Abbasi F., Reaven G.M. Insulin resistance as a predictor of age-related diseases. J. Clin. Endocrinol. Metab. 2001;86:3574–3578. doi: 10.1210/jcem.86.8.7763. [DOI] [PubMed] [Google Scholar]
- Fain J.N., Madan A.K., Hiler M.L., Cheema P., Bahouth S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- Fallarino I., Grohmann U., Vacca C., Bianchi R., Orabona C., Spreca A. T cell apoptosis by tryptophan metabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- Favennec M., Hennart B., Caiazzo R., Leloire A., Yengo L., Verbanck M. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity. 2015;23:2066–2074. doi: 10.1002/oby.21199. [DOI] [PubMed] [Google Scholar]
- Filho A.J.M.C., Lima C.N.C., Vasconcelos S.M.M., de Lucena D.F., Maes M., Macedo D. IDO chronic immune activation and tryptophan metabolic pathway: a potential pathophysiological link between depression and obesity. Prog. Neuropsychopharm. Biol. Psychiatry. 2018;80:234–249. doi: 10.1016/j.pnpbp.2017.04.035. [DOI] [PubMed] [Google Scholar]
- Finkelstein E.A., Khavjou O.A., Thompson H., Trogdon J.G., Pan L., Sherry B., Dietz W. Obesity and severe obesity forecasts through 2030. Am. J. Prev. Med. 2012;42:563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Fischer K.R., Durrans A., Lee S. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–479. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest C.M., Mackay G.M., Stoy N., Spiden S.L., Taylor R., Stone T.W., Darlington L.G. Blood levels of kynurenines, interleukin IL-23 and sHLA-G at different stages of Huntington's disease. J. Neurochem. 2010;112:112–122. doi: 10.1111/j.1471-4159.2009.06442.x. [DOI] [PubMed] [Google Scholar]
- Forrest C.M., Khalil O.S., Pisar M., Darlington L.G., Stone T.W. Prenatal inhibition of the tryptophan - kynurenine pathway alters synaptic plasticity and protein expression in the rat hippocampus. Brain Res. 2013;1504:1–15. doi: 10.1016/j.brainres.2013.01.031. [DOI] [PubMed] [Google Scholar]
- Forrest C.M., Khalil O.S., Pisar M., McNair K., Kornisiuk E., Snitcofsky M., Gonzalez M., Jerusalinsky D., Darlington L.G., Stone T.W. Changes in synaptic transmission and protein expression in the brains of adult offspring after prenatal inhibition of the kynurenine pathway. Neuroscience. 2013;254:241–259. doi: 10.1016/j.neuroscience.2013.09.034. [DOI] [PubMed] [Google Scholar]
- Forrest C.M., McNair K., Vincenten M.C., Darlington L.G., Stone T.W. Selective depletion of tumor suppressors Deleted in Colorectal Cancer (DCC) and neogenin by environmental and endogenous serine proteases: linking diet and cancer. BMC Cancer. 2016;16 doi: 10.1186/s12885-016-2795-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragua V., Gonzalez-Ortiz G., Villaverde C., Hervera M., Mariotti V.M., Manteca X., Baucells M.D. Preliminary study: voluntary food intake in dogs during tryptophan supplementation. Br. J. Nutr. 2011;106:S162–S165. doi: 10.1017/S0007114511000535. [DOI] [PubMed] [Google Scholar]
- Frasca D., Diaz A., Romero M., Vazquez T., Blomberg B.B. Obesity induces pro-inflammatory B cells and impairs B cell function in old mice. Mech. Ageing Dev. 2017;162:91–99. doi: 10.1016/j.mad.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis H., Gomo E., Nyazema N., Ndhlovu P., Kaestel P., Krarup H. HIV-1 viral load and elevated serum alpha(1)-antichymotrypsin are independent predictors of body composition in pregnant Zimbabwean women. J. Nutr. 2002;132:3747–3753. doi: 10.1093/jn/132.12.3747. [DOI] [PubMed] [Google Scholar]
- Frystyk J., Vestbo E., Skjaerbaek C., Mogensen C.E., Ørskov H. Free insulin-like growth factors in human obesity. Metabolism. 1995;44:37–44. doi: 10.1016/0026-0495(95)90219-8. [DOI] [PubMed] [Google Scholar]
- Gansler T.S., Hardman W., Hunt D.A., Schaffel S., Hennigar R.A. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum. Pathol. 1997;28:686–692. doi: 10.1016/s0046-8177(97)90177-5. [DOI] [PubMed] [Google Scholar]
- Gao Q., Zheng J., Yao X., Peng B. Adiponectin inhibits VEGF-A in prostate cancer cells. Tumor Biol. 2015;36:4287–4292. doi: 10.1007/s13277-015-3067-1. [DOI] [PubMed] [Google Scholar]
- George M.D., Giles J.T., Katz P.P., England B.R., Mikuls T.R., Michaud K. Impact of obesity and adiposity on inflammatory markers in patients with rheumatoid arthritis. Arthritis Care Res. 2017;69:1789–1798. doi: 10.1002/acr.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard C., Brown K.A. Obesity and breast cancer - role of estrogens and the molecular underpinnings of aromatase regulation in breast adipose tissue. Mol. Cell Endocrinol. 2017;2017 doi: 10.1016/j.mce.2017.09.014. [DOI] [PubMed] [Google Scholar]
- Ghuman S., Van Hemelrijck M., Garmo H., Holmberg L., Malmström H., Lambe M. Serum inflammatory markers and colorectal cancer risk and survival. Br. J. Cancer. 2017;116:1358–1365. doi: 10.1038/bjc.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gialamas S.P., Petridou E.T., Tseleni-Balafouta S., Spyridopoulos T.N., Matsoukis I.L., Kondi-Pafiti A. Serum adiponectin levels and tissue expression of adiponectin receptors are associated with risk, stage, and grade of colorectal cancer. Metabolism. 2011;60:1530–1538. doi: 10.1016/j.metabol.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Gobatto C.A., Mello M.A.R., Souza C.T., Ribeiro I.A. The monosodium glutamate (MSG) obese rat as a model for the study of exercise in obesity. Res. Commun. Mol. Pathol. Pharmacol. 2002;111:89–102. [PubMed] [Google Scholar]
- Goktas S., Yilmaz M.I., Caglar K., Sonmez A., Kilic S., Bedir S. Prostate cancer and adiponectin. Urology. 2005;65:1168–1172. doi: 10.1016/j.urology.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Gonzalez R.R., Cherfils S., Escobar M., Yoo J.H., Carino C., Styer A.K. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) J. Biol. Chem. 2006;281:26320–26328. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- Gonzalez R.R., Watters A., Xu Y., Singh U.P., Mann D.R., Rueda B.R. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11:R36. doi: 10.1186/bcr2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin P.J., Ennis M., Pritchard K.I., Trudeau M.E., Koo J., Madarnas Y. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J. Clin. Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- Grivennikov S., Karin E., Terzic J., Mucida D., Yu G.Y., Vallabhapurapu S. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter M.J., Hoover D.R., Yu H., Wassertheil-Smoller S., Rohan T.E., Manson J.E. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Beg Q.K., Lorenz P. Bacterial alkaline proteases: molecular approaches and industrial applications. Appl. Microbiol. Biotechnol. 2002;59:15–32. doi: 10.1007/s00253-002-0975-y. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Vazquez C., Quintana F.J. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. 2018;48:19–33. doi: 10.1016/j.immuni.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas J.L., Gajiwala K.S., Maffei M., Cohen S.L., Chait B.T., Rabinowitz D. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Han C., Zhang H.T., Du L., Liu X., Jing J., Zhao X. Serum levels of leptin, insulin, and lipids in relation to breast cancer in china. Endocrine. 2005;26:19–24. doi: 10.1385/ENDO:26:1:019. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hara E. Relationship between obesity, gut microbiome and hepatocellular carcinoma development. Dig. Dis. 2015;33:346–350. doi: 10.1159/000371679. [DOI] [PubMed] [Google Scholar]
- Hardwick J.C., Van Den Brink G.R., Offerhaus G.J., Van Deventer S.J., Peppelenbosch M.P. Leptin is a growth factor for colonic epithelial cells. Gastroenterology. 2001;121:79–90. doi: 10.1053/gast.2001.25490. [DOI] [PubMed] [Google Scholar]
- Hashimoto N., Nara H. Dietary amino acids promote pancreatic protease synthesis at the translation stage in rats. J. Nutr. 2003;133:3052–3057. doi: 10.1093/jn/133.10.3052. [DOI] [PubMed] [Google Scholar]
- Hellawell G.O., Turner G.D., Davies D.R., Poulsom R., Brewster S.F., Macaulay V.M. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res. 2002;62:2942–2950. [PubMed] [Google Scholar]
- Hermanussen M., Tresguerres J.A.F. A new anti-obesity drug treatment: first clinical evidence that, antagonising glutamate-gated Ca2+ ion channels with memantine normalises binge-eating disorders. Econ. Hum. Biol. 2005;3:329–337. doi: 10.1016/j.ehb.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hoa N.T., Baccigalupi L., Huxham A., Smertenko A., Van P.H., Ammendola S., Ricca E., Cutting S.M. Characterization of Bacillus species used for oral bacteriotherapy and bacterioprophylaxis of gastrointestinal disorders. Appl. Environ. Microbiol. 2000;66:241e5247. doi: 10.1128/aem.66.12.5241-5247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoa T.T., Duc L.H., Isticato R., Baccigalupi L., Ricca E., Van P.H., Cutting S.M. Fate and dissemination of Bacillus subtilis spores in a murine model. Appl. Environ. Microbiol. 2001;67:3819–3823. doi: 10.1128/AEM.67.9.3819-3823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H.A., Khaneja R., Tam N.M.K., Cazzato A., Tan S., Urdaci M. Bacillus subtilis isolated from the human gastrointestinal tract. Res. Microbiol. 2008;160:134–143. doi: 10.1016/j.resmic.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S., Arner P., Caro J.F., Atkinson R.L., Spiegelman B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe L.R., Subbaramaiah K., Hudis C.A., Dannenberg A.J. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin. Cancer Res. 2013;19:6074–6083. doi: 10.1158/1078-0432.CCR-12-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsing A.W., Chua S., Gao Y.T., Gentzschein E., Chang L., Deng J. Prostate cancer risk and serum levels of insulin and leptin: a population-based study. J. Natl. Cancer Inst. 2001;93:783–789. doi: 10.1093/jnci/93.10.783. [DOI] [PubMed] [Google Scholar]
- Huxley R.R., Ansary-Moghaddam A., Clifton P., Czernichow S., Parr C.L., Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int. J. Cancer. 2009;125:171–180. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- Il'yasova D., Colbert L.H., Harris T.B., Newman A.B., Bauer D.C., Satterfield S., Kritchevsky S.B. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol. Biomark. Prev. 2005;14:2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- Ip B., Cilfone N.A., Belkina A.C., DeFuria J., Jagannathan-Bogdan M., Zhu M. Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote TNF production. Obesity. 2016;24:102–112. doi: 10.1002/oby.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Ando T., Ogiso H., Arioka Y., Saito K., Seishima M. Inhibition of indoleamine 2,3-dioxygenase activity accelerates skin wound healing. Biomaterials. 2015;53:221–228. doi: 10.1016/j.biomaterials.2015.02.098. [DOI] [PubMed] [Google Scholar]
- Iyengar N.M., Hudis C.A., Dannenberg A.J. Obesity and cancer: local and systemic mechanisms. Annu. Rev. Med. 2015;66:297–309. doi: 10.1146/annurev-med-050913-022228. [DOI] [PubMed] [Google Scholar]
- Jin H., Zhang Y., You H., Tao X., Wang C., Jin G. Prognostic significance of kynurenine 3-monooxygenase and effects on proliferation, migration, and invasion of human hepatocellular carcinoma. Sci. Rep. 2015;5 doi: 10.1038/srep10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabacoff B.L., Avarian S., Wohlman A., Umhey M. Absorption of chymotrypsin from intestinal tract. Nature. 1963;199:815–816. doi: 10.1038/199815a0. [DOI] [PubMed] [Google Scholar]
- Kampf D. Mode of action of Bacillus subtilis and efficiency in piglet feeding. Feed Comp. 2012;32:30–31. [Google Scholar]
- Kennedy A.R. Chemopreventive agents: protease inhibitors. Pharmacol. Ther. 1998;78:167–209. doi: 10.1016/s0163-7258(98)00010-2. [DOI] [PubMed] [Google Scholar]
- Kennedy B.E., Sharif T., Martell E., Dai C., Kim Y., Lee P.W.K., Gujar S.A. NAD(+) salvage pathway in cancer metabolism and therapy. Pharmacol. Res. 2016;114:274–283. doi: 10.1016/j.phrs.2016.10.027. [DOI] [PubMed] [Google Scholar]
- Kern P.A., Saghizadeh M., Ong J.M., Bosch R.J., Deem R., Simsolo R.B. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J. Clin. Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshk W.A., Zineldeen D.H., El-Khadrawy O.H. Fatty acid synthase/oxidized low-density lipoprotein as metabolic oncogenes linking obesity to colon cancer via NF-kappa B in Egyptians. Med. Oncol. 2014;31:1–10. doi: 10.1007/s12032-014-0192-4. [DOI] [PubMed] [Google Scholar]
- Key T.J., Allen N.E., Spencer E.A., Travis R.C. The effect of diet on risk of cancer. Lancet. 2002;360:861–868. doi: 10.1016/S0140-6736(02)09958-0. [DOI] [PubMed] [Google Scholar]
- Key T.J., Appleby P.N., Crowe F.L., Bradbury K.E., Schmidt J.A., Travis R.C. Cancer in British vegetarians: updated analyses of 4998 incident cancers in a cohort of 32,491 meat eaters, 8612 fish eaters, 18,298 vegetarians, and 2246 vegans. Am. J. Clin. Nutr. 2014;100:378S–385S. doi: 10.3945/ajcn.113.071266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil O.S., Pisar M., Forrest C.M., Vincenten M.C.J., Darlington L.G., Stone T.W. Prenatal inhibition of the kynurenine pathway leads to structural changes in the hippocampus of adult rat offspring. Eur. J. Neurosci. 2014;39:1558–1571. doi: 10.1111/ejn.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.S. Leptin and leptin receptor expression in breast cancer. Cancer Res. Treat. 2009;41:155–163. doi: 10.4143/crt.2009.41.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Abbasi F., Reaven G.M. Impact of degree of obesity on surrogate estimates of insulin resistance. Diabetes Care. 2004;27:1998–2002. doi: 10.2337/diacare.27.8.1998. [DOI] [PubMed] [Google Scholar]
- Kim S., Keku T.O., Martin C., Galanko J., Woosley J.T., Schroeder J.C. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68:323–328. doi: 10.1158/0008-5472.CAN-07-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler J.A., Kain T., Drucker D.J. Glucagon-like peptide-1 receptor activation inhibits growth and augments apoptosis in murine CT26 colon cancer cells. Endocrinology. 2011;152:3362–3372. doi: 10.1210/en.2011-1201. [DOI] [PubMed] [Google Scholar]
- Kono T. Destruction of insulin effector system of adipose tissue cells by proteolytic enzymes. J. Biol. Chem. 1969;244:1772–1777. [PubMed] [Google Scholar]
- Kowalski Z.M., Gorka P., Schlagheck A., Jagusiak W., Micek P., Strzetelski J. Performance of Holstein calves fed milk-replacer and starter mixture supplemented with probiotic feed additive. J. Anim. Feed Sci. 2009;18:399–411. [Google Scholar]
- Kridel S.J., Axelrod F., Rozenkrantz N., Smith J.W. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64:2070–2075. doi: 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]
- Krogh-Madsen R., Plomgaard P., Keller P., Keller C., Pedersen B.K. Insulin stimulates interleukin-6 and tumor necrosis factor-α gene expression in human subcutaneous adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2004;286:E234–E238. doi: 10.1152/ajpendo.00274.2003. [DOI] [PubMed] [Google Scholar]
- Kuchiba A., Morikawa T., Yamauchi M., Imamura Y., Liao X., Chan A.T. Body mass index and risk of colorectal cancer according to fatty acid synthase expression in the nurses' health study. J. Natl. Cancer Inst. 2012;104:415–420. doi: 10.1093/jnci/djr542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik G., Klippel A., Weber M.J. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase and Akt. Mol. Cell. Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson S.C., Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int. J. Cancer. 2006;119:2657–2664. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- Larsson S.C., Orsini N., Wolk A. Processed meat consumption and stomach cancer risk: a meta-analysis. J. Nat. Cancer Inst. 2006;98:1078–1087. doi: 10.1093/jnci/djj301. [DOI] [PubMed] [Google Scholar]
- Lee B.C., Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim. Biophys. Acta. 2014;1842:446–462. doi: 10.1016/j.bbadis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.S., Park M.-S., Choung J.-S., Kim S.-S., Oh H.-H., Choi C.-S. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia. 2012;55:2456–2468. doi: 10.1007/s00125-012-2592-3. [DOI] [PubMed] [Google Scholar]
- Lee J., Lee J., Farquhar K.S., Yun J., Frankenberger C.A., Bevilacqua E. Network of mutually repressive metastasis regulators can promote cell heterogeneity and metastatic transitions. Proc. Nat. Acad. Sci. USA. 2014;111:E364–E373. doi: 10.1073/pnas.1304840111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Hullar M.A.J., Schwarz Y., Lampe J.W. Human gut bacterial communities are altered by addition of Cruciferous vegetables to a controlled fruit- and vegetable-free diet. J. Nutr. 2009;139:1685–1691. doi: 10.3945/jn.109.108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Kilani R.T., Rahmani-Neishaboor E., Jalili R.B., Ghahary A. Kynurenine increases matrix metalloproteinase-1 and-3 expression in cultured dermal fibroblasts and improves scarring in vivo. J. Invest. Dermatol. 2014;134:643–650. doi: 10.1038/jid.2013.303. [DOI] [PubMed] [Google Scholar]
- Liu B., Yu H.C., Sun G.Y., Sun X., Jin H., Zhang C.P. OX40 promotes obesity-induced adipose inflammation and insulin resistance. Cell. Molec. Life Sci. 2017;74:3827–3840. doi: 10.1007/s00018-017-2552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler D., Brocke-Heidrich K., Pfeifer G., Stocsits C., Hackermüller J., Kretzschmar A.K. Interleukin-6–dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood. 2007;110:1330–1333. doi: 10.1182/blood-2007-03-081133. [DOI] [PubMed] [Google Scholar]
- Lucarelli G., Rutigliano M., Ferro M., Giglio A., Intini A., Triggiano F. Activation of the kynurenine pathway predicts poor outcome in patients with clear cell renal cell carcinoma. Urol. Oncol. 2017;35:461e15–461e27. doi: 10.1016/j.urolonc.2017.02.011. [DOI] [PubMed] [Google Scholar]
- Ma J., Pollak M.N., Giovannucci E., Chan J.M., Tao Y., Hennekens C.H., Stampfer M.J. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J. Natl. Cancer Inst. 1999;91:620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- Magalhaes B., Peleteiro B., Lunet N. Dietary patterns and colorectal cancer: systematic review and meta-analysis. Eur. J. Cancer Prev. 2012;21:15–23. doi: 10.1097/CEJ.0b013e3283472241. [DOI] [PubMed] [Google Scholar]
- Malvi P., Chaube B., Pandey V., Vijayakumar M.V., Boreddy P.R., Mohammad N. Obesity induced rapid melanoma progression is reversed by orlistat treatment and dietary intervention: role of adipokines. Mol. Oncol. 2015;9:689–703. doi: 10.1016/j.molonc.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangge H., Summers K.L., Meinitzer A., Zelzer S., Almer G., Prassl R. Obesity-related dysregulation of the Tryptophan-Kynurenine metabolism: role of age and parameters of the metabolic syndrome. Obesity. 2014;22:195–201. doi: 10.1002/oby.20491. [DOI] [PubMed] [Google Scholar]
- Mantzoros C.S., Bolhke K., Moschos S., Cramer D.W. Leptin in relation to carcinoma in situ of the breast: a study of pre-menopausal cases and controls. Int. J. Cancer. 1999;80:523–526. doi: 10.1002/(sici)1097-0215(19990209)80:4<523::aid-ijc7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Matarante A., Baruzzi F., Cocconcelli P.S., Morea M. Genotyping and toxigenic potential of Bacillus subtilis and Bacillus pumilus strains occurring in industrial and artisanal cured sausages. Appl. Environ. Microbiol. 2004;70:5168–5176. doi: 10.1128/AEM.70.9.5168-5176.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer K.H. Detergent proteases. Curr. Opin. Biotechnol. 2004;15:330–334. doi: 10.1016/j.copbio.2004.06.005. [DOI] [PubMed] [Google Scholar]
- McCullough M.L., Gapstur S.M., Shah R., Jacobs E.J., Campbell P.T. Association between red and processed meat intake and mortality among colorectal cancer survivors. J. Clin. Oncol. 2013;31:2773–2780. doi: 10.1200/JCO.2013.49.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina E.A., Oberheu K., Polusani S.R., Ortega V., Velagaleti G.V.N., Oyajobi B.O. PKA/AMPK signaling in relation to adiponectin's antiproliferative effect on multiple myeloma cells. Leukemia. 2014;28:2080–2089. doi: 10.1038/leu.2014.112. [DOI] [PubMed] [Google Scholar]
- Megel H., Beiler M., Ho R., Strauss R. Detection of trypsin-like activity in plasma of rats after oral administration of trypsin. Arch. Biochem. Biophys. 1964;108:193–194. doi: 10.1016/0003-9861(64)90375-3. [DOI] [PubMed] [Google Scholar]
- Mehlen P., Guenebeaud C. Netrin-1 and its dependence receptors as original targets for cancer therapy. Curr. Opin. Oncol. 2010;22:46–54. doi: 10.1097/CCO.0b013e328333dcd1. [DOI] [PubMed] [Google Scholar]
- Menetrier-Caux C., Montmain G., Dieu M.C., Bain C., Favrot M.C., Caux C., Blay J.Y. Inhibition of the differentiation of dendritic cells from CD34+ progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–4791. [PubMed] [Google Scholar]
- Menu E., Kooijman R., Van Valckenborgh E., Asosingh K., Bakkus M., Van Camp B. Specific roles for the PI3K and the MEK–ERK pathway in IGF-1-stimulated chemotaxis, VEGF secretion and proliferation of multiple myeloma cells: study in the 5T33MM model. Br. J. Cancer. 2004;90:1076–1083. doi: 10.1038/sj.bjc.6601613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt A.M., Burrows C.F., Cowgill L., Streett W. Fecal fat and trypsin in dogs fed a meat-base or cereal-base diet. J. Am. Vet. Med. Assoc. 1979;174:59–61. [PubMed] [Google Scholar]
- Miller J.M., Williard R.F., Polachek A.A. An investigation of trypsin I-131 in patients. Exp. Med. Surg. 1960;18:352–370. [PubMed] [Google Scholar]
- Miyoshi Y., Funahashi T., Kihara S., Taguchi T., Tamaki Y., Matsuzawa Y. Association of serum adiponectin levels with breast cancer risk. Clin. Cancer Res. 2003;9:5699–5704. [PubMed] [Google Scholar]
- Mohamed-Ali V., Goodrick S., Rawesh A., Katz D.R., Miles J.M., Yudkin J.S. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in Vivo 1. J. Clin. Endocrinol. Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Moore L.L., Bradlee M.L., Singer M.R., Splansky G.L., Proctor M.H., Ellison R.C. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. Int. J. Obes. 2004;28:559–567. doi: 10.1038/sj.ijo.0802606. [DOI] [PubMed] [Google Scholar]
- Moore L.E., Wilson R.T., Campleman S.L. Lifestyle factors, exposures, genetic susceptibility, and renal cell cancer risk: a review. Cancer Investig. 2005;23:240–255. doi: 10.1081/cnv-200055962. [DOI] [PubMed] [Google Scholar]
- Morris P.G., Zhou X.K., Milne G.L., Goldstein D., Hawks L.C., Dang C.T. Increased levels of urinary PGE-M, a biomarker of inflammation, occur in association with obesity, aging, and lung metastases in patients with breast cancer. Cancer Prev. Res. 2013;6:428–436. doi: 10.1158/1940-6207.CAPR-12-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourouti N., Kontogianni M.D., Papavagelis C., Plytzanopoulou P., Vassilakou T., Psaltopoulou T., Malamos N., Linos A., Panagiotakos D.B. Meat consumption and breast cancer: a case-control study in women. Meat Sci. 2015;100:195–201. doi: 10.1016/j.meatsci.2014.10.019. [DOI] [PubMed] [Google Scholar]
- Moyer B.J., Rojas I.Y., Kerley-Hamilton J.S., Hazlett H.F., Nemani K.V., Trask H.W. Inhibition of the aryl hydrocarbon receptor prevents Western diet-induced obesity. Model for AHR activation by kynurenine via oxidized-LDL, TLR2/4, TGF beta, and IDO1. Toxicol. Appl. Pharmacol. 2016;300:13–24. doi: 10.1016/j.taap.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer B.J., Rojas I.Y., Kerley-Hamilton J.S., Nemani K.V., Trask H.W., Ringelberg C.S. Obesity and fatty liver are prevented by inhibition of the aryl hydrocarbon receptor. Nutr. Res. 2017;44:38–50. doi: 10.1016/j.nutres.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrkonjic M., Chappell E., Pethe V.V., Manno M., Daftar D., Greenwood C.M. Association of apolipoprotein E polymorphisms and dietary factors in colorectal cancer. Br. J. Cancer. 2009;100:1966–1974. doi: 10.1038/sj.bjc.6605097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murfitt S.A., Zaccone P., Wang X., Acharjee A., Sawyer Y., Koulman A. A metabolomics and lipidomics study of mouse models of type 1 diabetes highlights divergent metabolism in purine and tryptophan metabolism prior to disease on-set. J. Proteome Res. 2017 doi: 10.1021/acs.jproteome.7b00489. [DOI] [PubMed] [Google Scholar]
- Musso G., Gambino R., Cassader M. Obesity, diabetes, and gut microbiota the hygiene hypothesis expanded? Diabetes Care. 2010;33:2277–2284. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M.G., Grammer T.C., Wang L.M., Sun X.J., Pierce J.H., Blenis J. Insulin receptor substrate-1 mediates phosphatidylinositol 3′-kinase and p70S6k signaling during insulin, insulin-like growth factor-1, and interleukin-4 stimulation. J. Biol. Chem. 1994;269:28783–28789. [PubMed] [Google Scholar]
- Nakayama S., Miyoshi Y., Ishihara H., Noguchi S. Growth-inhibitory effect of adiponectin via adiponectin receptor 1 on human breast cancer cells through inhibition of S-phase entry without inducing apoptosis. Breast Cancer Res. Treat. 2008;112:405–410. doi: 10.1007/s10549-007-9874-3. [DOI] [PubMed] [Google Scholar]
- Nam S.Y., Lee E.J., Kim K.R., Cha B.S., Song Y.D., Lim S.K. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int. J. Obes. Relat. Metab. Disord. 1997;21:355–359. doi: 10.1038/sj.ijo.0800412. [DOI] [PubMed] [Google Scholar]
- Neilsen P.M., Pehere A.D., Pishas K., Callen D.F., Abell A.D. New 26S proteasome inhibitors with high selectivity for chymotrypsin-like activity and p53-dependent cytotoxicity. ACS Chem. Biol. 2013;8:353–359. doi: 10.1021/cb300549d. [DOI] [PubMed] [Google Scholar]
- Nguyen P.L., Ma J., Chavarro J.E., Freedman M.L., Lis R., Fedele G., Fiore C. Fatty acid synthase polymorphisms, tumor expression, body mass index, prostate cancer risk, and survival. J. Clin. Oncol. 2010;28:3958–3964. doi: 10.1200/JCO.2009.27.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira L.M., Lavigne J.A., Chandramouli G.V.R., Lui H., Barrett J.C., Hursting S.D. Dose-dependent effects of calorie restriction on gene expression, metabolism, and tumor progression are partially mediated by insulin-like growth factor-1. Cancer Med. 2012;1:275–288. doi: 10.1002/cam4.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira L.M., Dunlap S.M., Ford N.A., Hursting S.D. Calorie restriction and rapamycin inhibit MMTV-Wnt-1 mammary tumor growth in a mouse model of postmenopausal obesity. Endocrin. Rel. Cancer. 2012;19:57–68. doi: 10.1530/ERC-11-0213. [DOI] [PubMed] [Google Scholar]
- Nomiyama T., Yanase T. GLP-1 receptor agonist as treatment for cancer as well as diabetes: beyond blood glucose control. Expert. Rev. Endocrinol. Metab. 2016;11:357–364. doi: 10.1080/17446651.2016.1191349. [DOI] [PubMed] [Google Scholar]
- Norat T., Lukanova A., Ferrari P., Riboli E. Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. Int. J. Cancer. 2002;98:241–256. doi: 10.1002/ijc.10126. [DOI] [PubMed] [Google Scholar]
- Norat T., Bingham S., Ferrari P., Slimani N., Jenab M., Mazuir M. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. J. Natl. Cancer Inst. 2005;97:906–916. doi: 10.1093/jnci/dji164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarangelo F.M., Pocivavsek A. Elevated kynurenine pathway metabolism during neurodevelopment: implications for brain and behaviour. Neuropharmacology. 2017;112(S1):275–285. doi: 10.1016/j.neuropharm.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. J. Am. Med. Assoc. 2012;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino S., Nosho K., Meyerhardt J.A., Kirkner G.J., Chan A.T., Kawasaki T. Cohort study of fatty acid synthase expression and patient survival in colon cancer. J. Clin. Oncol. 2008;26:5713–5720. doi: 10.1200/JCO.2008.18.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.S., Seo H.S., Kim K.H., Pyo H., Chung B.C., Lee J. Urinary profiling of tryptophan and its related metabolites in patients with metabolic syndrome by liquid chromatography-electrospray ionization/mass spectrometry. Anal. Bioanal. Chem. 2017;409:5501–5512. doi: 10.1007/s00216-017-0486-4. [DOI] [PubMed] [Google Scholar]
- Opitz C.A., Litzenburger U.M., Sahm F., Ott M., Tritschler I., Trump S. An endogenous tumor-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- Opitz C.A., Litzenburger U.M., Opitz U., Sahm F., Ochs K., Lutz C., Wick W., Platten M. The indoleamine-2,3-dioxygenase (IDO) inhibitor 1-methyl-d-tryptophan upregulates IDO1 in human cancer cells. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0019823. (AR e19823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlich M., Singh P., Sabaté J., Fan J., Sveen L., Bennett H. Vegetarian dietary patterns and the risk of colorectal cancers. J. Am. Med. Assoc. 2015;175:767–776. doi: 10.1001/jamainternmed.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenkrug G., van der Hart M., Roeser J., Summergrad P. Peripheral tryptophan - kynurenine metabolism associated with metabolic syndrome is different in Parkinson's and Alzheimer's Diseases. Endocrinol. Diabet. Metab. J. 2017;1(11):2017. [PMC free article] [PubMed] [Google Scholar]
- Pallotta M.T., Fallarino F., Matino D., Macchiarula A., Orabona C. AhR-mediated, non-genomic modulation of IDO1 function. Front. Immunol. 2014;5:1–6. doi: 10.3389/fimmu.2014.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta M.T., Orabona C., Bianchi R., Vacca C., Fallarino F., Belladonna M.L. Forced IDO1 expression in dendritic cells restores immunoregulatory signalling in autoimmune diabetes. J. Cell. Mol. Med. 2014;18:2082–2091. doi: 10.1111/jcmm.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa V., Pezzino V., Costantino A., Belfiore A., Giuffrida D., Frittitta L. Elevated insulin receptor content in human breast cancer. J. Clin. Invest. 1990;86:1503–1510. doi: 10.1172/JCI114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W.H., Jun D.W., Kim J.T., Jeong J.H., Park H., Chang Y.S. Novel cell-based assay reveals associations of circulating serum AhR-ligands with metabolic syndrome and mitochondrial dysfunction. Biofactors. 2013;39:494–504. doi: 10.1002/biof.1092. [DOI] [PubMed] [Google Scholar]
- Parkin D.M., Boyd L. Cancers attributable to overweight and obesity in the UK in 2010. Br. J. Cancer. 2011;105:S34–S37. doi: 10.1038/bjc.2011.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrizas M., Saltiel A.R., LeRoith D. Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J. Biol. Chem. 1997;272:154–161. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- Perkins M.N., Stone T.W. An iontophoretic investigation of the action of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- Petridou E., Papadiamantis Y., Markopoulos C., Spanos E., Dessypris N., Trichopoulos D. Leptin and insulin growth factor I in relation to breast cancer (Greece) Cancer Causes Control. 2000;11:383–388. doi: 10.1023/a:1008903727238. [DOI] [PubMed] [Google Scholar]
- Pfeiler G., Hudelist G., Wülfing P., Mattsson B., Königsberg R., Kubista E. Impact of AdipoR1 expression on breast cancer development. Gynecol. Oncol. 2010;117:134–138. doi: 10.1016/j.ygyno.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Piazza G.J., Garcia R.A. Proteolysis of meat and bone meal to increase utilisation. Anim. Prod. 2014;54:200–206. [Google Scholar]
- Piccione G., Fazio F., Giudice E., Grasso F., Caola G. Blood lipids, fecal fat and chymotrypsin excretion in the dog: Influence of age, body weight and sex. J. Vet. Med. Sci. 2004;66:59–62. doi: 10.1292/jvms.66.59. [DOI] [PubMed] [Google Scholar]
- Pischon T., Lahmann P.H., Boeing H., Tjonneland A., Halkjaer J., Overvad K. Body size and risk of renal cell carcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC) Int. J. Cancer. 2006;118:728–738. doi: 10.1002/ijc.21398. [DOI] [PubMed] [Google Scholar]
- Pischon T., Nothlings U., Boeing H. Obesity and cancer. Proc. Nutr. Soc. 2008;67:128–145. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- Poormasjedi-Meibod M.S., Hartwell R., Kilani R.T., Ghahary A. Anti-scarring properties of different tryptophan derivatives. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0091955. (AR e91955) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta C., De Amici M., Quaglini S., Paglino C., Tagliani F., Boncimino A. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Ann. Oncol. 2008;19:353–358. doi: 10.1093/annonc/mdm448. [DOI] [PubMed] [Google Scholar]
- Prendergast H.C., Metz R., Muller A.J. Towards a genetic definition of cancer-associated inflammation: role of the IDO pathway. Am. J. Pathol. 2010;176:2082–2087. doi: 10.2353/ajpath.2010.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast G.C., Smith C., Thomas S., Mandik-Nayak L., Laury-Kleintop L., Metz R. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol. Immunother. 2014;63:721–735. doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A.J., Allen N.E., Appleby P.N., Crowe F.L., Travis R.C., Tipper S.J. Insulin-like growth factor-I concentration and risk of prostate cancer: results from the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomark. Prev. 2012;21:1531–1541. doi: 10.1158/1055-9965.EPI-12-0481-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L.J.H., Schultz M., Gaardsting A., Ladelund S., Garred P., Iversen K. Inflammatory biomarkers and cancer: CRP and suPAR as markers of incident cancer in patients with serious nonspecific symptoms and signs of cancer. Int. J. Cancer. 2017;141:191–199. doi: 10.1002/ijc.30732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebnord E.W., Strand E., Midttun O., Svingen G.F.T., Christensen M.H.E., Ueland P.M. The kynurenine: tryptophan ratio as a predictor of incident type 2 diabetes mellitus in individuals with coronary artery disease. Diabetologia. 2017;60:1712–1721. doi: 10.1007/s00125-017-4329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renehan A.G., Tyson M., Egger M., Heller R.F., Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- Ricart W., Fernández-Real J.M. No decrease in free IGF-I with increasing insulin in obesity-related insulin resistance. Obes. Res. 2001;9:631–636. doi: 10.1038/oby.2001.83. [DOI] [PubMed] [Google Scholar]
- Ripamonti B., Stella S. Bacterial spore formers as probiotics for animal nutrition. Large Anim. Rev. 2009;15:7–12. [Google Scholar]
- Rohrmann S., Overvad K., Bueno-de-Mesquita H.B., Jakobsen M.U., Egeberg R., Tjonneland A. Meat consumption and mortality - results from the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2013;11:AR 63. doi: 10.1186/1741-7015-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S., Ou W., Tang D., Bhattacharya N., Dei T.A.P., Fletcher J.A. Gastrointestinal stromal tumors overexpress fatty acid synthase. J. Pathol. 2006;209:369–375. doi: 10.1002/path.1983. [DOI] [PubMed] [Google Scholar]
- Roy F., Boye J.I., Simpson B. Bioactive proteins and peptides in pulse crops: pea, chickpea and lentil. Food Res. Int. 2010;43:432–442. [Google Scholar]
- Rubio M.F., Werbajh S., Cafferata E.G.A., Quaglino A., Colo G.P., Nojek I.M. TNF-α enhances estrogen-induced cell proliferation of estrogen-dependent breast tumor cells through a complex containing nuclear factor-kappa B. Oncogene. 2006;25:1367–1377. doi: 10.1038/sj.onc.1209176. [DOI] [PubMed] [Google Scholar]
- Sagan D., Kocki T., Patel S., Kocki J. Utility of kynurenic acid for non-invasive detection of metastatic spread to lymph nodes in non-small cell lung cancer. Int. J. Med. Sci. 2015;12:146–153. doi: 10.7150/ijms.7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad N., Yasmin F., Naheed S., Bari A.Z., Ayaz M.M., Zaman A. Serum levels of leptin, zinc and tryptophan in obese subjects with sleep deficits. Pakistan. J. Pharm. Sci. 2017;30:1431–1438. [PubMed] [Google Scholar]
- Schwarcz R., Stone T.W. The kynurenine pathway and the brain: challenges, controversies and promises. Neuropharmacology. 2017;112:237–247. doi: 10.1016/j.neuropharm.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin F., Carvalho M.A., Bastos D.C., Agostini M., Zecchin K.G., Alvarez-Flores M.P. The fatty acid synthase inhibitor orlistat reduces experimental metastases and angiogenesis in B16-F10 melanomas. Br. J. Cancer. 2012;107:977–987. doi: 10.1038/bjc.2012.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan B., Wang X., Wu Y., Xu C., Xia Z., Dai J. The metabolic ER stress sensor IRE1 alpha suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat. Immunol. 2017;18:519–529. doi: 10.1038/ni.3709. [DOI] [PubMed] [Google Scholar]
- Sherry S., Fletcher A.P. Proteolytic enzymes - a therapeutic evaluation. Clin. Pharmacol. Ther. 1960;1:202–226. [Google Scholar]
- Song P., Lu M., Yin Q., Wu L., Zhang D., Fu B. Red meat consumption and stomach cancer risk: a meta-analysis. J. Cancer Res. Clin. Oncol. 2014;140:979–992. doi: 10.1007/s00432-014-1637-z. [DOI] [PubMed] [Google Scholar]
- Song P., Ramprasath T., Wang H., Zou M.H. Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell. Mol. Life Sci. 2017;74:2899–2916. doi: 10.1007/s00018-017-2504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopasakis V.R., Nagaev I., Smith U. Cytokine release from adipose tissue of nonobese individuals. Int. J. Obes. 2005;29:1144–1147. doi: 10.1038/sj.ijo.0803002. [DOI] [PubMed] [Google Scholar]
- Stattin P., Lukanova A., Biessy C., Söderberg S., Palmqvist R., Kaaks R. Obesity and colon cancer: does leptin provide a link? Int. J. Cancer. 2004;109:149–152. doi: 10.1002/ijc.11668. [DOI] [PubMed] [Google Scholar]
- Stephens G.L., Wang Q., Swerdlow B., Bhat G., Kolbeck R., Fung M. Kynurenine 3-monooxygenase mediates inhibition of Th17 differentiation via catabolism of endogenous aryl hydrocarbon receptor ligands. Eur. J. Immunol. 2013;43:1727–1734. doi: 10.1002/eji.201242779. [DOI] [PubMed] [Google Scholar]
- Stienstra R., Joosten L.A., Koenen T., van Tits B., van Diepen J.A., van den Berg S.A. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T.W., Darlington L.G. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug Dis. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- Stone T.W., Darlington L.G. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br. J. Pharmacol. 2013;169:1211–1227. doi: 10.1111/bph.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T.W., Darlington L.G. Microbial carcinogenic toxins and dietary anti-cancer protectants. Cell. Mol. Life Sci. 2017;74:2627–2643. doi: 10.1007/s00018-017-2487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T.W., Perkins M.N. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur. J. Pharmacol. 1981;72:411–412. doi: 10.1016/0014-2999(81)90587-2. [DOI] [PubMed] [Google Scholar]
- Stone T.W., Forrest C.M., Darlington L.G. Kynurenine pathway inhibition as a therapeutic strategy for neuroprotection. FEBS J. 2012;279:1386–1397. doi: 10.1111/j.1742-4658.2012.08487.x. [DOI] [PubMed] [Google Scholar]
- Stone T.W., Forrest C.M., Stoy N., Darlington L.G. Involvement of kynurenines in Huntington's disease and stroke-induced brain damage. J. Neural Transm. 2012;119:261–274. doi: 10.1007/s00702-011-0676-8. [DOI] [PubMed] [Google Scholar]
- Stone T.W., Stoy N., Darlington L.G. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol. Sci. 2013;34:136–143. doi: 10.1016/j.tips.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Sugiyama M., Takahashi H., Hosono K., Endo H., Kato S., Yoneda K. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int. J Oncol. 2009;34:339–344. [PubMed] [Google Scholar]
- Sun P., Wang J.Q., Zhang H.T. Effects of Bacillus subtilis natto on performance and immune function of preweaning calves. J. Dairy Sci. 2010;93:5851–5855. doi: 10.3168/jds.2010-3263. [DOI] [PubMed] [Google Scholar]
- Sun K.L.W., Correia J.P., Kennedy T.E. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138:2153–2169. doi: 10.1242/dev.044529. [DOI] [PubMed] [Google Scholar]
- Sun P., Wang J.Q., Zhang H.T. Effects of supplementation of Bacillus subtilis natto Na and N1 strains on rumen development in dairy calves. Anim. Feed Sci. Technol. 2011;164:154–160. [Google Scholar]
- Swinburn B., Sacks G., Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am. J. Clin. Nutr. 2009;90:1453–1456. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Okimura Y., Mizuno I., Iida K., Takahashi T., Kaji H. Leptin induces mitogen-activated protein kinase-dependent proliferation of C3H10T1/2 cells. J. Biol. Chem. 1997;272:12897–12900. doi: 10.1074/jbc.272.20.12897. [DOI] [PubMed] [Google Scholar]
- Te Giffel M.C., Beumer R.R., Leijendekkers S., Rombouts F.M. Incidence of Bacillus cereus and Bacillus subtilis in foods in the Netherlands. Food Microbiol. 1996;13:53–58. [Google Scholar]
- Thaker A.I., Rao M.S., Bishnupuri K.S., Kerr T.A., Foster L., Marinshaw J.M. IDO1 metabolites activate beta-catenin signalling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology. 2013;145:416–425. doi: 10.1053/j.gastro.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorleifsson G., Walters G.B., Gudbjartsson D.F., Steinthorsdottir V., Sulem P., Helgadottir A. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat. Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- Tomasetti C., Li L., Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science. 2017;355:1330–1334. doi: 10.1126/science.aaf9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson B., Hu M., Zhang Y.Z., Chan P., Liu Z.M. Investigational glucagon-like peptide-1 agonists for the treatment of obesity. Expert Opin. Investig. Drugs. 2016;25:1167–1179. doi: 10.1080/13543784.2016.1221925. [DOI] [PubMed] [Google Scholar]
- Vandanmagsar B., Youm Y.H., Ravussin A., Galgani J.E., Stadler K., Mynatt R.L. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrell J., Broch M., Vilarrasa N., Molina A., Gómez J.M., Gutiérrez C. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes. Res. 2004;12:962–971. doi: 10.1038/oby.2004.118. [DOI] [PubMed] [Google Scholar]
- Wakil S.J. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry. 1989;28:4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- Walczak K., Dabrowski W., Langner E., Zgrajka W., Pilat J., Kocki T., Rzeski W., Turski W.A. Kynurenic acid synthesis and kynurenine aminotransferases expression in colon derived normal and cancer cells. Scand. J. Gastroenterol. 2011;46:903–912. doi: 10.3109/00365521.2011.579159. [DOI] [PubMed] [Google Scholar]
- Walczak K., Zurawska M., Kis J., Starownik R., Zgrajka W., Bar K., Turski W.A., Rzeski W. Kynurenic acid in human renal cell carcinoma: its antiproliferative and antimigrative action on Caki-2 cells. Amino Acids. 2012;43:1663–1670. doi: 10.1007/s00726-012-1247-5. [DOI] [PubMed] [Google Scholar]
- Walczak K., Deneker-Hannemann S., Jarosz B. Kynurenic acid inhibits proliferation and migration of human glioblastoma T98G cells. Pharmacol. Rep. 2014;66:130–136. doi: 10.1016/j.pharep.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Walczak K., Turski W.A., Rajtar G. Kynurenic acid inhibits colon cancer proliferation in vitro: effects on signaling pathways. Amino Acids. 2014;46:2393–2401. doi: 10.1007/s00726-014-1790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X.S., Hamilton T.C., Ware J.H., Donahue J.J., Kennedy A.R. Growth inhibition and cytotoxicity induced by Bowman-Birk inhibitor concentrate in cisplatin-resistant human ovarian cancer cells. Nutr. Cancer. 1998;31:8–17. doi: 10.1080/01635589809514672. [DOI] [PubMed] [Google Scholar]
- Wan X.S., Serota D.G., Ware J.H., Crowell J.A., Kennedy A.R. Detection of Bowman-Birk inhibitor and anti-Bowman-Birk inhibitor antibodies in sera of humans and animals treated with Bowman-Birk inhibitor concentrate. Nutr. Cancer. 2002;43:167–173. doi: 10.1207/S15327914NC432_7. [DOI] [PubMed] [Google Scholar]
- Wang Y., Beydoun M.A. Meat consumption is associated with obesity and central obesity among US adults. Int. J. Obes. 2009;33:621–628. doi: 10.1038/ijo.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., van Boxel-Dezaire A.H., Cheon H., Yang J., Stark G.R. STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proc. Nat. Acad. Sci. USA. 2013;110:16975–16980. doi: 10.1073/pnas.1315862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel B., Bjartell A., Culig Z., Persson J.L. Interleukin-6 activates PI3K/Akt pathway and regulates cyclin A1 to promote prostate cancer cell survival. Int. J. Cancer. 2008;122:1521–1529. doi: 10.1002/ijc.23261. [DOI] [PubMed] [Google Scholar]
- Wei E.K., Giovannucci E., Fuchs C.S., Willett W.C., Mantzoros C.S. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J. Natl. Cancer Inst. 2005;97:1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO World Health Organisation: Obesity and overweight factsheet. 2016. http://www.who.int/mediacentre/factsheets/fs311/en/ (updated June 2016, accessed 14-09-2017)
- Wie G.A., Cho Y.A., Kang H.H., Ryu K.A., Yoo M.K., Kim Y.A. Red meat consumption is associated with an increased overall cancer risk: a prospective cohort study in Korea. Br. J Nutr. 2014;112:238–247. doi: 10.1017/S0007114514000683. [DOI] [PubMed] [Google Scholar]
- Wolpin B.M., Meyerhardt J.A., Chan A.T., Ng K., Chan J.A., Wu K. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J. Clin. Oncol. 2009;27:176–185. doi: 10.1200/JCO.2008.17.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.H., Chou Y.C., Chou W.Y., Hsu G.C., Chu C.H., Yu C.P. Circulating levels of leptin, adiposity and breast cancer risk. Br. J. Cancer. 2009;100:578–582. doi: 10.1038/sj.bjc.6604913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Barnes G.T., Yang Q., Tan G., Yang D., Chou C.J. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Yang X.-X., Wu Y.-G., Li X.-Y., Bai B. Meat consumption and risk of oral cavity and oropharynx cancer: a meta-analysis of observational studies. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0095048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X.-J., Gao Q., Qiao J.-H., Zhang J., Xu C.-P., Liu J. Red and processed meat consumption and the risk of lung cancer: a dose-response meta-analysis of 33 published studies. Int. J. Clin. Exp. Med. 2014;7:1542–1553. [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T., Kamon J., Waki H., Terauchi Y., Kubota N., Hara K. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Kamon J., Minokoshi Y.A., Ito Y., Waki H., Uchida S. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yorifuji N., Inouse T., Iguchi M., Fujiwara K., Kakimoto K., Nouda S. The dipeptidyl peptidase-4 inhibitor sitagliptin suppresses mouse colon tumorigenesis in type 2 diabetic mice. Oncol. Rep. 2016;35:676–682. doi: 10.3892/or.2015.4429. [DOI] [PubMed] [Google Scholar]
- Yoshimoto S., Loo T.M., Atarashi K., Kanda H., Sato S., Oyadomari S. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- Yudkin J.S., Stehouwer C., Emeis J., Coppack S. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction a potential role for cytokines originating from adipose tissue? Arterioscler. Thromb. Vasc. Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- Zeng H., Lazarova D.L. Obesity-related colon cancer: Dietary factors and their mechanisms of anticancer action. Clin. Exp. Pharmacol. Physiol. 2012;39:161–167. doi: 10.1111/j.1440-1681.2011.05518.x. [DOI] [PubMed] [Google Scholar]
- Zeng H., Lazarova D.L., Bordonaro M. Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J. Gastrointest. Oncol. 2014;6:41–51. doi: 10.4251/wjgo.v6.i2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Carstens J.L., Kim J., Scheible M., Kaye J., Sugimoto H. Epithelial-to-mesenchymal transition dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L., Zhang X., Covasa M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J. Gastroenterol. 2014;20:7878–7886. doi: 10.3748/wjg.v20.i24.7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong F., Xu M., Bruno R.S., Ballard K.D., Zhu J. Targeted high performance liquid chromatography tandem mass spectrometry-based metabolomics differentiates metabolic syndrome from obesity. Exp. Biol. Med. 2017;242:773–780. doi: 10.1177/1535370217694098. [DOI] [PMC free article] [PubMed] [Google Scholar]