Abstract
Variations in nucleotide excision repair pathway genes may predispose to initiation of cancers. However, polymorphisms of ERCC1/XPF genes and neuroblastoma risk have not been investigated before. To evaluate the relevance of polymorphisms of ERCC1/XPF genes in influencing neuroblastoma susceptibility, we genotyped four polymorphisms in ERCC1/XPF genes using a Chinese population of 393 cases and 812 controls. The results showed that ERCC1 rs2298881 and rs11615 predisposed to enhanced neuroblastoma risk [CA vs. AA: adjusted odds ratio (OR) = 1.94, 95% confidence interval (CI) = 1.30–2.89, P = 0.0012; CC vs. AA: adjusted OR = 2.18, 95% CI = 1.45–3.26, P = 0.0002 for rs2298881, and AG vs. GG: adjusted OR = 1.31, 95% CI = 1.02–1.69, P = 0.038 for rs11615]. Moreover, XPF rs2276466 was also associated with increased neuroblastoma risk (GG vs. CC: adjusted OR = 1.66, 95% CI = 1.02–2.71, P = 0.043). In the combined analysis of ERCC1, we found that carriers with 2–3 risk genotypes were more likely to get risk of neuroblastoma, when compared to those with 0–1 risk genotype (adjusted OR = 1.75; 95% CI = 1.25–2.45, P = 0.0012). Our study indicates that common genetic variations in ERCC1/XPF genes predispose to neuroblastoma risk, which needs to be further validated by ongoing efforts.
Keywords: Neuroblastoma, Susceptibility, ERCC1, XPF, Polymorphism
Abbreviations: GWAS, genome-wide association study; SNP, single nucleotide polymorphism; NER, nucleotide excision repair; HWE, Hardy-Weinberg equilibrium; OR, odds ratio; CI, confidence interval; eQTL, expression quantitative trait loci
Highlights
-
•
ERCC1 rs2298881, rs11615 and XPF rs2276466 were associated with increased neuroblastoma risk.
-
•
ERCC1 rs2298881 CC and AC/CC genotypes carriers were associated with increased ERCC1 mRNA expression.
-
•
This is the most comprehensive study for ERCC1/XPF genes polymorphisms and neuroblastoma risk.
In the current study with a Chinese population of 393 neuroblastoma cases and 812 controls, we found that ERCC1 rs2298881, rs11615 and XPF rs2276466 were associated with increased neuroblastoma risk. We also confirmed that ERCC1 rs2298881 CC and AC/CC genotypes carriers were associated with increased ERCC1 mRNA expression. This is by far the most comprehensive study investigating the association between the ERCC1/XPF genes polymorphisms and neuroblastoma risk.
1. Introduction
Neuroblastoma, a heterogeneous tumor developed from neural crest progenitor cells, is the most common solid neoplasm of childhood (Matthay et al., 2016). Neuroblastoma takes up nearly 10% of all childhood cancers, yet its proportion of all pediatric oncology deaths is up to 15% (Cheung and Dyer, 2013). Neuroblastoma is characterized by wide clinical course, with some patients having spontaneous regression without chemotherapy or some having poor prognosis despite intense multi-modal therapy (Maris et al., 2007; Maris, 2010). In general, neuroblastoma cases can be classified into low-, intermediate-, and high-risk groups (Shimada et al., 1999). Nearly 50% of all the neuroblastoma patients are classified into high-risk group, and their survival rates are less than 40% despite intense multi-modal therapy (Matthay et al., 2016). Such unfavorable prognosis was mainly attributed to the extensive metastasis of tumor at the time of diagnosis (Matthay et al., 2016; Esposito et al., 2017).
According to the germline mutations, neuroblastoma is divided into familial and sporadic types. Familial neuroblastoma is rare, with approximately 1–2% of all neuroblastoma cases. The genetic etiology of familial neuroblastoma is relatively elucidated, that is the highly mutations in PHOX2B (Mosse et al., 2004; Bourdeaut et al., 2005) or ALK gene (Devoto et al., 2011). However, the genetic events predisposing individuals to sporadic neuroblastoma, the most common neuroblastoma, remains unclear. Previous studies indicated that environmental factors such as pregnancy exposures, dwelling condition, and dietary habit are potential risks of sporadic neuroblastoma (Cook et al., 2004; Menegaux et al., 2004; Muller-Schulte et al., 2017), yet there still lacks direct linkage evidence. Mounting evidence has suggested that genetic factors also influence the occurrence of neuroblastoma (Yang et al., 2017; Zhang et al., 2017). For example, common variants of NEFL and CNKN1B could influence neuroblastoma susceptibility (Capasso et al., 2014; Capasso et al., 2017).
Recent genome-wide association studies (GWASs) have identified genetic variants located in several genes (HACE1, LIN28B, BARD1, CASC15, TP53, and LMO1) associated with neuroblastoma risk by comparing neuroblastoma patients to healthy controls (Maris et al., 2008; Capasso et al., 2009; Nguyen le et al., 2011; Wang et al., 2011; Diskin et al., 2012; Diskin et al., 2014). Moreover, the role of most of these GWAS-identified single nucleotide polymorphisms (SNPs) in neuroblastoma risk have been confirmed in replication case-control studies (He et al., 2016b; He et al., 2016c; Zhang et al., 2016; He et al., 2017; Zhang et al., 2017). However, these identified genetic variations still account for only a small proportion in predisposing to neuroblastoma.
Therefore, additional gene polymorphisms associated with neuroblastoma susceptibility are needed to be identified. Due to the adoption of the multiple testing correction in the GWAS analysis, some potential SNPs might only have modest risk effects or just be omitted (Stadler et al., 2010). Thus, other research strategies were developed, which include: replication of GWAS-identified SNPs, meta-analysis of GWAS datasets, imputation and epistasis analysis, gene- or pathway-based approaches (Gao, 2011).
In human, DNA repair systems play critical roles in maintaining the stability of cellular functions and genomic integrity (Wood et al., 2001). The nucleotide excision repair (NER) pathway, one of the DNA repair systems, is responsible for excising bulky DNA lesions (Gillet and Scharer, 2006). The NER pathway includes four steps: damage recognition, DNA unwinding, damage excision, and ligation (Friedberg, 2001; Christmann et al., 2003). The eight main members of the NER process, XPA-XPG and XP-V, are all implicated in maintaining genomic integrity (Cleaver, 2000). The ERCC1 and XPF (also known as ERCC4) genes encode proteins that participate in the DNA repair pathways. These two proteins, ERCC1 and XPF, form a heterodimeric complex to cleave the DNA damage on the 5′ side of bubble structures (Sijbers et al., 1996; Evans et al., 1997). Moreover, this complex also functions in the inter-strand crosslink repair (Wood, 2010). Owing to the critical role of ERCC1/XPF complex in maintaining genomic stability, it remains a hot spot of research to explore the role of ERCC1/XPF genes variations in cancer risks. To date, epidemiological studies declared that ERCC1/XPF genes polymorphisms were associated with cancer risk at different sites, including colorectal cancer (Yang et al., 2015), breast cancer (Yang et al., 2013), gastric cancer (He et al., 2012b), and endometrial cancer (Doherty et al., 2011).
However, the genetic variants driving the ERCC1/XPF genetic association with the risk of neuroblastoma has been evaluated in few instances. To determine whether ERCC1/XPF genes variations could predispose to neuroblastoma risk or not, we conducted a case-control study in Chinese population.
2. Materials and Methods
2.1. Study Population
This study encompassed 393 cases with neuroblastoma and 812 healthy controls of Chinese origin (He et al., 2018; Zhang et al., 2018). Among them, 275 cases were from Guangzhou Women and Children's Medical Center and 118 were from The First Affiliated Hospital of Zhengzhou University (Supplemental Table 1). At the same time, 531 and 281 controls were recruited from the same district, respectively. Additional details and eligibility criteria for subject selection were reported previously (He et al., 2017). All participants or their guardians provided informed consent before the research. The details of the included subjects have been described in our previous publications (He et al., 2016a; Zhang et al., 2017). The study protocols were approved by the Institutional Review Board of Guangzhou Women and Children's Medical Center, and The First Affiliated Hospital of Zhengzhou University.
2.2. SNP Selection and Genotyping
We identified potentially functional SNPs of ERCC1/XPF genes from dbSNP database (http://www.ncbi.nlm.nih.gov/) and an online tool, SNPinfo (http://snpinfo.niehs.nih.gov/). Briefly, we searched the potentially functional candidate SNPs located in the 5′- flanking region, 5′ untranslated region, 3′ untranslated region, and exon of ERCC1/XPF genes. Additional selection criteria were reported in our previous study (He et al., 2012a). In final, three SNPs (rs2298881, rs3212986, rs11615) with low linkage disequilibrium in the ERCC1 gene (Supplemental Fig. 1, Supplemental Table 2) and one SNP (rs2276466) in the XPF gene (Supplemental Table 3) met the selection criteria. We used TIANamp Blood DNA Kit (TianGen Biotech Co. Ltd., Beijing, China) to extract genomic DNA from peripheral blood donated by subjects. All the selected SNPs were genotyped on a standard commercial TaqMan real-time PCR, with details reported elsewhere (Gong et al., 2017; Li et al., 2017; Lou et al., 2017). As a quality control, eight negative controls with water and eight replicate samples were included in each 384-well plate. Moreover, we randomly selected 10% of the samples to a second run. All duplicate sets had a concordance rate of 100%.
2.3. Statistical Analysis
First, we applied goodness-of-fit χ2 test to determine whether the selected SNPs among controls were deviated from Hardy-Weinberg equilibrium (HWE). Then we adopted two-sided chi-square test to measure the difference of the demographic variables and allele frequencies between all cases and controls. We also calculated odds ratios (ORs) and 95% confidence intervals (CIs) using logistic regression analysis. All statistical analyses were performed using the version 9.4 SAS software (SAS Institute, Cary, NC). All the P values were two sided, and P values less than 0.05 considered as significant.
2.4. SNP-gene Expression Correlation Analysis
We performed genotype and mRNA expression correlation analysis, using genotyping data from the HapMap phase II release 23 data set and mRNA expression data by genotypes from EBV-transformed B lymphoblastoid cell lines from the same 270 HapMap individuals (He et al., 2012a). We also performed the expression quantitative trait loci (eQTL) analysis using GTEx portal web site (http://www.gtexportal.org/home/) to predict potential associations between the SNPs and gene expression levels (Consortium, 2013).
3. Results
3.1. ERCC1 and XPF Genes Polymorphisms With Neuroblastoma Susceptibility
The detailed characteristics of all the subjects were presented in Supplemental Table 1 and in our previously published articles (He et al., 2018; Zhang et al., 2018). The distribution of ERCC1/XPF genes polymorphisms between all cases and controls were listed in Table 1. In analysis of neuroblastoma patients and controls, three SNPs (two in ERCC1 and one in XPF) were associated with neuroblastoma risk: rs2298881 in ERCC1 (CA vs. AA: adjusted OR = 1.94, 95% CI = 1.30–2.89, P = 0.0012; CC vs. AA: adjusted OR = 2.18, 95% CI = 1.45–3.26, P = 0.0002); rs11615 in ERCC1 (AG vs. GG: adjusted OR = 1.31, 95% CI = 1.02–1.69, P = 0.038); and rs2276466 in XPF (GG vs. CC: adjusted OR = 1.66, 95% CI = 1.02–2.71, P = 0.043). However, we failed to detect a statistically significant relationship between rs3212986 in ERCC1 and neuroblastoma risk. Higher risk of neuroblastoma was found in individuals with 2–3 combined risk genotypes of ERCC1, compared with those with 0–1 risk genotypes (adjusted OR = 1.75; 95% CI = 1.25–2.45, P = 0.0012).
Table 1.
Logistic regression analysis for the correlation of ERCC1 and XPF polymorphisms with neuroblastoma risk.
| Genotype | Cases (N = 393) | Controls (N = 812) | Pa | Crude OR (95% CI) | P | Adjusted OR (95% CI) b | Pb |
|---|---|---|---|---|---|---|---|
| rs2298881 (HWE = 0.060) | |||||||
| AA | 38 (9.67) | 145 (17.86) | 1.00 | 1.00 | |||
| CA | 184 (46.82) | 365 (44.95) | 1.92 (1.29–2.87) | 0.0013 | 1.94 (1.30–2.89) | 0.0012 | |
| CC | 171 (43.51) | 302 (37.19) | 2.16 (1.44–3.23) | 0.0002 | 2.18 (1.45–3.26) | 0.0002 | |
| Additive | 0.0007 | 1.36 (1.14–1.62) | 0.0007 | 1.36 (1.14–1.62) | 0.0007 | ||
| Dominant | 355 (90.33) | 667 (82.14) | 0.0002 | 2.03 (1.39–2.97) | 0.0003 | 2.05 (1.40–2.99) | 0.0002 |
| Recessive | 222 (56.49) | 510 (62.81) | 0.035 | 1.30 (1.02–1.66) | 0.035 | 1.30 (1.02–1.66) | 0.035 |
| rs3212986 (HWE = 0.193) | |||||||
| CC | 166 (42.24) | 372 (45.81) | 1.00 | 1.00 | |||
| CA | 180 (45.80) | 343 (42.24) | 1.18 (0.91–1.52) | 0.216 | 1.18 (0.91–1.52) | 0.210 | |
| AA | 47 (11.96) | 97 (11.95) | 1.09 (0.73–1.61) | 0.682 | 1.09 (0.73–1.61) | 0.676 | |
| Additive | 0.465 | 1.08 (0.91–1.29) | 0.389 | 1.08 (0.91–1.29) | 0.382 | ||
| Dominant | 227 (57.76) | 440 (54.19) | 0.242 | 1.16 (0.91–1.47) | 0.242 | 1.16 (0.91–1.48) | 0.236 |
| Recessive | 346 (88.04) | 715 (88.05) | 0.995 | 1.00 (0.69–1.45) | 0.995 | 1.00 (0.69–1.45) | 0.992 |
| rs11615 (HWE = 0.035) | |||||||
| GG | 209 (53.18) | 482 (59.36) | 1.00 | 1.00 | |||
| GA | 155 (39.44) | 273 (33.62) | 1.31 (1.01–1.69) | 0.039 | 1.31 (1.02–1.69) | 0.038 | |
| AA | 29 (7.38) | 57 (7.02) | 1.17 (0.73–1.89) | 0.510 | 1.18 (0.73–1.89) | 0.502 | |
| Additive | 0.114 | 1.18 (0.98–1.43) | 0.090 | 1.18 (0.98–1.43) | 0.088 | ||
| Dominant | 184 (46.82) | 330 (40.64) | 0.042 | 1.29 (1.01–1.64) | 0.042 | 1.29 (1.01–1.64) | 0.042 |
| Recessive | 364 (92.62) | 755 (92.98) | 0.820 | 1.06 (0.66–1.68) | 0.819 | 1.06 (0.67–1.68) | 0.812 |
| rs2276466 (HWE = 0.544) | |||||||
| CC | 230 (59.43) | 478 (58.87) | 1.00 | 1.00 | |||
| CG | 125 (32.30) | 294 (36.21) | 0.88 (0.68–1.14) | 0.337 | 0.88 (0.68–1.15) | 0.345 | |
| GG | 32 (8.27) | 40 (4.93) | 1.66 (1.01–2.70) | 0.044 | 1.66 (1.02–2.71) | 0.043 | |
| Additive | 0.049 | 1.08 (0.88–1.31) | 0.459 | 1.08 (0.89–1.32) | 0.452 | ||
| Dominant | 157 (40.57) | 334 (41.13) | 0.853 | 0.98 (0.76–1.25) | 0.853 | 0.98 (0.77–1.25) | 0.862 |
| Recessive | 355 (91.73) | 772 (95.07) | 0.023 | 1.74 (1.08–2.82) | 0.024 | 1.74 (1.08–2.82) | 0.024 |
| Combined effect of risk genotypes for ERCC1c | |||||||
| 0 | 38 (9.67) | 142 (17.49) | 0.005d | 1.00 | 1.00 | ||
| 1 | 14 (3.56) | 28 (3.45) | 1.87 (0.90–3.90) | 0.095 | 1.88 (0.90–3.91) | 0.093 | |
| 2 | 271 (68.96) | 517 (63.67) | 1.96 (1.33–2.88) | 0.0007 | 1.97 (1.34–2.91) | 0.0006 | |
| 3 | 70 (17.81) | 125 (15.39) | 2.09 (1.32–3.32) | 0.0017 | 2.11 (1.33–3.35) | 0.0016 | |
| 0–1 | 52 (13.23) | 170 (20.94) | 1.00 | 1.00 | |||
| 2–3 | 341 (86.77) | 642 (79.06) | 0.0012 | 1.74 (1.24–2.43) | 0.0013 | 1.75 (1.25–2.45) | 0.0012 |
The results were in bold if the 95% CI excluded 1 or P < 0.05.
χ2 test for genotype distributions between neuroblastoma cases and controls.
Adjusted for age and gender.
Risk genotypes were rs2298881 CA/CC, rs3212986 CA/AA and rs11615 GA/AA.
For additive model.
3.2. Stratification Analysis
We further evaluated the effects of the selected polymorphisms on the neuroblastoma risk among different strata including age, gender, tumor sites of origin and clinical stages. The conferring increased neuroblastoma risk of rs2298881 variant AC/CC genotypes was more evident in subgroups of age > 18 months (adjusted OR = 2.26, 95% CI = 1.44–3.56, P = 0.0004), female (adjusted OR = 2.15, 95% CI = 1.18–3.92, P = 0.012), male (adjusted OR = 1.98, 95% CI = 1.21–3.24, P = 0.007), tumor in retroperitoneal (adjusted OR = 4.47, 95% CI = 1.61–12.40, P = 0.004), tumor in mediastinum (adjusted OR = 2.37, 95% CI = 1.17–4.81, P = 0.017) and clinical stage III + IV (adjusted OR = 2.45, 95% CI = 1.46–4.11, P = 0.0007). The rs11615 GA/AA was associated with an increased risk of neuroblastoma, particularly in subgroups of age ≤ 18 (adjusted OR = 1.58, 95% CI = 1.04–2.39, P = 0.033), tumor in retroperitoneal (adjusted OR = 1.99, 95% CI = 1.27–3.12, P = 0.003), compared with the homozygous wild-type genotype. After combining risk genotypes, we observed that the patients carrying 2–3 risk genotypes had a more evident risk in age > 18 (adjusted OR = 2.05, 95% CI = 1.36–3.09, P = 0.0006), males (adjusted OR = 1.86, 95% CI = 1.19–2.90, P = 0.006), tumor in retroperitoneal (adjusted OR = 5.49, 95% CI = 1.98–15.20, P = 0.001), clinical stage I + II + 4 s (adjusted OR = 1.65, 95% CI = 1.02–2.68, P = 0.041) and clinical stage III + IV (adjusted OR =1.73, 95% CI = 1.13–2.65, P = 0.013) (Table 2).
Table 2.
Stratification analysis for the association between ERCC1 gene genotypes and neuroblastoma susceptibility.
| Variables | rs2298881 (case/control) |
Adjusted ORa (95% CI) | Pa | rs11615 (case/control) |
Adjusted ORa (95% CI) | Pa | Risk genotypes (case/control) |
Adjusted ORa (95% CI) | Pa | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | CA/CC | GG | GA/AA | 0–1 | 2–3 | |||||||
| Age, month | ||||||||||||
| ≤18 | 15/42 | 115/263 | 1.67 (0.83–3.36) | 0.151 | 61/182 | 65/123 | 1.58 (1.04–2.39) | 0.033 | 17/50 | 109/255 | 1.26 (0.69–2.28) | 0.450 |
| >18 | 27/103 | 240/404 | 2.26 (1.44–3.56) | 0.0004 | 148/300 | 119/207 | 1.16 (0.86–1.57) | 0.327 | 35/120 | 232/387 | 2.05 (1.36–3.09) | 0.0006 |
| Gender | ||||||||||||
| Female | 15/60 | 153/282 | 2.15 (1.18–3.92) | 0.012 | 86/196 | 82/146 | 1.28 (0.88–1.86) | 0.192 | 22/67 | 146/275 | 1.61 (0.95–2.71) | 0.076 |
| Male | 23/85 | 202/385 | 1.98 (1.21–3.24) | 0.007 | 123/286 | 102/184 | 1.30 (0.94–1.79) | 0.111 | 30/103 | 195/367 | 1.86 (1.19–2.90) | 0.006 |
| Sites of origin | ||||||||||||
| Adrenal gland | 19/145 | 134/667 | 1.60 (0.96–2.68) | 0.074 | 90/482 | 63/330 | 1.03 (0.73–1.47) | 0.854 | 26/170 | 127/642 | 1.35 (0.85–2.13) | 0.203 |
| Retroperitoneal | 4/145 | 83/667 | 4.47 (1.61–12.40) | 0.004 | 37/482 | 50/330 | 1.99 (1.27–3.12) | 0.003 | 4/170 | 83/642 | 5.49 (1.98–15.20) | 0.001 |
| Mediastinum | 9/145 | 100/667 | 2.37 (1.17–4.81) | 0.017 | 57/482 | 52/330 | 1.32 (0.88–1.97) | 0.176 | 15/170 | 94/642 | 1.63 (0.92–2.88) | 0.096 |
| Others | 5/145 | 31/667 | 1.31 (0.50–3.43) | 0.587 | 21/482 | 15/330 | 1.04 (0.53–2.05) | 0.910 | 5/170 | 31/642 | 1.60 (0.61–4.19) | 0.340 |
| Clinical stage | ||||||||||||
| I + II + 4 s | 19/145 | 143/667 | 1.61 (0.97–2.69) | 0.067 | 90/482 | 72/330 | 1.16 (0.82–1.63) | 0.398 | 22/170 | 140/642 | 1.65 (1.02–2.68) | 0.041 |
| III + IV | 18/145 | 193/667 | 2.45 (1.46–4.11) | 0.0007 | 110/482 | 101/330 | 1.35 (1.00–1.84) | 0.053 | 29/170 | 182/642 | 1.73 (1.13–2.65) | 0.013 |
The results were in bold if the 95% CI excluded 1 or P < 0.05.
Adjusted for age and gender, omitting the corresponding stratification factor.
XPF rs2276466 GG was associated with an increased risk of neuroblastoma, particularly in subgroups of age > 18 (adjusted OR = 2.21, 95% CI = 1.23–3.97, P = 0.008) and females (adjusted OR = 2.51, 95% CI = 1.22–5.16, P = 0.013), compared with the CC/CG genotype. However, we failed to observe significant association between XPF rs2276466 and neuroblastoma risk under the rest of the evaluated subgroups (Table 3).
Table 3.
Stratification analysis for the association between XPF rs2276466 C>G polymorphism and neuroblastoma susceptibility.
| Variables | CC/CG |
GG |
Crude OR (95% CI) | P | Adjusted ORa (95% CI) | Pa |
|---|---|---|---|---|---|---|
| (Cases/controls) | ||||||
| Age, month | ||||||
| ≤18 | 117/288 | 7/17 | 1.01 (0.41–2.51) | 0.977 | 1.01 (0.41–2.51) | 0.978 |
| >18 | 238/484 | 25/23 | 2.21 (1.23–3.98) | 0.008 | 2.21 (1.23–3.97) | 0.008 |
| Gender | ||||||
| Females | 149/327 | 17/15 | 2.49 (1.21–5.11) | 0.013 | 2.51 (1.22–5.16) | 0.013 |
| Males | 206/445 | 15/25 | 1.30 (0.67–2.51) | 0.442 | 1.32 (0.68–2.56) | 0.410 |
| Sites of origin | ||||||
| Adrenal gland | 143/772 | 9/40 | 1.22 (0.58–2.56) | 0.609 | 1.25 (0.59–2.64) | 0.558 |
| Retroperitoneal | 75/772 | 7/40 | 1.80 (0.78–4.16) | 0.168 | 1.79 (0.78–4.15) | 0.172 |
| Mediastinum | 99/772 | 10/40 | 1.95 (0.95–4.02) | 0.071 | 1.97 (0.95–4.06) | 0.068 |
| Others | 33/772 | 3/40 | 1.76 (0.52–5.97) | 0.368 | 1.71 (0.50–5.82) | 0.392 |
| Clinical stages | ||||||
| I + II + 4 s | 149/772 | 13/40 | 1.68 (0.88–3.23) | 0.116 | 1.68 (0.88–3.22) | 0.118 |
| III + IV | 190/772 | 17/40 | 1.73 (0.96–3.11) | 0.069 | 1.74 (0.96–3.14) | 0.067 |
The results were in bold if the 95% CI excluded 1 or P < 0.05.
Adjusted for age and gender, omitting the corresponding stratification factor.
3.3. Genotype and mRNA Expression Correlation Analysis
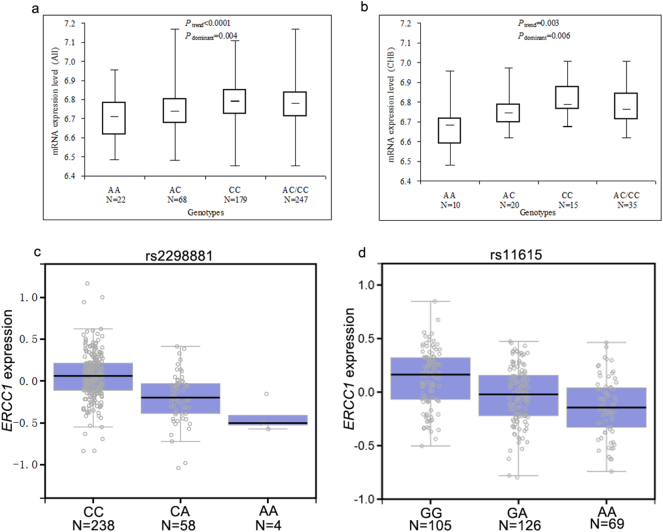
We observed that ERCC1 mRNA expression levels in rs2298881 CC and AC/CC genotypes carriers were significantly enhanced when compared to the AA genotype carriers in Chinese, Africans, and the overall population (Table 4). We also detected a higher ERCC1 mRNA expression level for rs3212986 AA genotypes for Europeans (P = 0.026) and rs3212986 AC genotype for African (P = 0.046). As to the rs2276466 polymorphism in ERCC4, the mRNA expression level was upregulated in GG genotype in Europeans (P = 0.035) and the overall populations (P = 0.021) (Table 4). More specifically, the mRNA expression level of 45 cell lines from Chinese (Fig. 1a) was similar to that of the overall populations (Fig. 1b). As a further assessment of the putative functional relevance of ERCC1 rs2298881 and rs11615, alteration in ERCC1 expression was seen in transformed fibroblasts tissues of individuals who carry polymorphic allele of ERCC1 rs2298881 (Fig. 1c) and rs11615 (Fig. 1d) based on the public database GTEx portal.
Table 4.
ERCC1 and XPF mRNA expression by the genotypes of SNPs, using data from the HapMapa.
| Race | mRNA expression (rs2298881) |
mRNA expression (rs3212986) |
mRNA expression (rs11615) |
mRNA expression (rs2276466) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotypes | No. | Mean ± SD | Pb | Ptrendc | Genotypes | No. | Mean ± SD | Pb | Ptrendc | Genotypes | No. | Mean ± SD | Pb | Ptrendc | Genotypes | No. | Mean ± SD | Pb | Ptrendc | |
| CHB | AA | 10 | 6.68 ± 0.13 | 0.003 | CC | 20 | 6.74 ± 0.13 | 0.442d | GG | 29 | 6.73 ± 0.11 | 0.044 | CC | 28 | 6.27 ± 0.09 | 0.583d | ||||
| AC | 20 | 6.76 ± 0.09 | 0.053 | AC | 19 | 6.77 ± 0.09 | 0.416 | AG | 12 | 6.79 ± 0.10 | 0.144 | CG | 13 | 6.23 ± 0.05 | 0.126 | |||||
| CC | 15 | 6.81 ± 0.08 | 0.006 | AA | 5 | 6.77 ± 0.07 | 0.664 | AA | 4 | 6.83 ± 0.07 | 0.111 | GG | 3 | 6.29 ± 0.06 | 0.619 | |||||
| AC/CC | 35 | 6.78 ± 0.09 | 0.006 | AC/AA | 24 | 6.77 ± 0.08 | 0.377 | AG/AA | 16 | 6.80 ± 0.09 | 0.054 | CG/GG | 16 | 6.24 ± 0.06 | 0.254 | |||||
| JPT | AA | 10 | 6.76 ± 0.08 | 0.242 | CC | 31 | 6.75 ± 0.09 | 0.442d | GG | 21 | 6.75 ± 0.10 | 0.872 | CC | 21 | 6.23 ± 0.07 | 0.541 | ||||
| AC | 26 | 6.74 ± 0.11 | 0.647 | AC | 13 | 6.77 ± 0.12 | 0.442 | AG | 22 | 6.76 ± 0.10 | 0.846 | CG | 19 | 6.24 ± 0.07 | 0.927 | |||||
| CC | 9 | 6.81 ± 0.07 | 0.118 | AA | 0 | – | – | AA | 2 | 6.76 ± 0.06 | 0.976 | GG | 5 | 6.26 ± 0.12 | 0.473 | |||||
| AC/CC | 35 | 6.76 ± 0.10 | 0.968 | AC/AA | 13 | 6.77 ± 0.12 | 0.442 | AG/AA | 24 | 6.76 ± 0.10 | 0.848 | CG/GG | 24 | 6.24 ± 0.08 | 0.738 | |||||
| CEU | AA | 0 | – | 0.370 | CC | 52 | 6.77 ± 0.13 | 0.725 | GG | 6 | 6.85 ± 0.13 | 0.447 | CC | 54 | 6.34 ± 0.08 | 0.062d | ||||
| AC | 11 | 6.74 ± 0.18 | – | AC | 35 | 6.74 ± 0.12 | 0.279 | AG | 49 | 6.76 ± 0.14 | 0.168 | CG | 28 | 6.36 ± 0.11 | 0.419 | |||||
| CC | 79 | 6.77 ± 0.12 | – | AA | 3 | 6.95 ± 0.04 | 0.026 | AA | 35 | 6.77 ± 0.11 | 0.111 | GG | 7 | 6.42 ± 0.10 | 0.035 | |||||
| AC/CC | 90 | 6.77 ± 0.13 | – | AC/AA | 38 | 6.76 ± 0.13 | 0.620 | AG/AA | 84 | 6.76 ± 0.13 | 0.129 | CG/GG | 35 | 6.37 ± 0.11 | 0.173 | |||||
| YRI | AA | 2 | 6.61 ± 0.003 | <.0001d | CC | 39 | 6.77 ± 0.10 | 0.208 | GG | 87 | 6.79 ± 0.10 | 0.137 | CC | 72 | 6.25 ± 0.08 | 0.220 | ||||
| AC | 11 | 6.71 ± 0.07 | 0.066 | AC | 45 | 6.81 ± 0.09 | 0.046 | AG | 3 | 6.71 ± 0.05 | 0.137 | CG | 17 | 6.28 ± 0.09 | 0.194 | |||||
| CC | 76 | 6.80 ± 0.09 | 0.004 | AA | 6 | 6.76 ± 0.05 | 0.976 | AA | 0 | – | – | GG | 1 | 6.26 | 0.850 | |||||
| AC/CC | 87 | 6.79 ± 0.09 | 0.007 | AC/AA | 51 | 6.80 ± 0.09 | 0.065 | AG/AA | 3 | 6.71 ± 0.05 | 0.137 | CG/GG | 18 | 6.28 ± 0.09 | 0.193 | |||||
| All | AA | 22 | 6.71 ± 0.11 | <.0001d | CC | 142 | 6.76 ± 0.11 | 0.095d | GG | 143 | 6.78 ± 0.10 | 0.599 | CC | 175 | 6.28 ± 0.09 | 0.046d | ||||
| AC | 68 | 6.74 ± 0.11 | 0.230 | AC | 112 | 6.78 ± 0.11 | 0.243 | AG | 86 | 6.76 ± 0.12 | 0.385 | CG | 77 | 6.29 ± 0.11 | 0.425 | |||||
| CC | 179 | 6.79 ± 0.10 | 0.001 | AA | 14 | 6.80 ± 0.09 | 0.162 | AA | 41 | 6.77 ± 0.10 | 0.793 | GG | 16 | 6.34 ± 0.12 | 0.021 | |||||
| AC/CC | 247 | 6.78 ± 0.11 | 0.004 | AC/AA | 126 | 6.78 ± 0.10 | 0.149 | AG/AA | 127 | 6.77 ± 0.12 | 0.435 | CG/GG | 93 | 6.30 ± 0.11 | 0.169 | |||||
The results were in bold if the P < 0.05.
ERCC1 and XPF genotyping data and mRNA expression levels for ERCC1 and XPF by genotypes were obtained from the HapMap phase II release 23 data from EBV-transformed lymphoblastoid cell lines from 270 individuals, including 45 unrelated Han Chinese in Beijing (CHB).
Two-side Student's t-test within the stratum.
P values for the trend test of mRNA expression among 3 genotypes for each SNP from a general linear model.
There were missing data because genotyping data not available.
Fig. 1.
Functional implication of ERCC1 gene rs2298881 and rs11615 polymorphisms. Effect of ERCC1 gene rs2298881 on mRNA expression in (a) 269 HapMap cell lines of all population and (b) 45 HapMap cell lines of unrelated CHB. The genotype of (c) rs2298881 and (d) rs11615 and expression of ERCC1 gene in transformed fibroblasts tissues were searched based on the public database GTEx Portal.
4. Discussion
To determine whether SNPs in ERCC1/XPF genes can predispose to neuroblastoma risk, we conducted the first case-control, hospital-based study using Chinese children. Our data revealed that the rs2298881 and rs11615 in ERCC1 as well as rs2276466 in XPF exhibited significant positive associations with neuroblastoma risk.
ERCC1 gene is located to chromosome 19q13.32 and comprises 10 exons. XPF is mapped to chromosome 16p13.12 and consists of 11 exons. Their encoded proteins, ERCC1 and XPF, function as a structure-specific endonuclease in a heterodimeric manner (Tsodikov et al., 2005). This heterodimer catalyzes the 5′ incision during the course of NER (Houtsmuller et al., 1999). In the ERCC1/XPF heterodimer, ERCC1 serves as a critical DNA binding subunit without endonuclease activity, whereas XPF is catalytically active (Enzlin and Scharer, 2002). It is elucidated that mutations in the ERCC1 and ERCC4 genes are associated with several human inherited disorders (Niedernhofer et al., 2006). The association of the ERCC1/XPF genes SNPs and cancer risk has been previously reported. For example, individuals carrying the ERCC1 rs3212986 or rs11615 genotype had a marginally increased risk of colorectal cancer (Hou et al., 2014). However, in a case-control study conducted in USA, Jennifer et al. failed to provide evidences between the relationship of ERCC1/XPF genes polymorphisms and endometrial cancer risk (Doherty et al., 2011). The discrepancy results suggested that the same polymorphism might function differently in cancer susceptibility in different ethnicities or different cancer sites. As certain gene SNPs have different roles in certain cancer risk, it is necessary to determine the role of ERCC1/XPF genes SNPs in neuroblastoma risk.
Herein, we are the first to explore whether ERCC1/XPF genes SNPs could contribute to the susceptibility of neuroblastoma in Chinese children. The results showed that two SNPs in ERCC1 (rs2298881 and rs11615) and rs2276466 in XPF predisposed to enhanced neuroblastoma risk. These results were quite similar to our previous study, which showed that ERCC1 rs2298881 and rs11615 variant genotypes were associated with increased gastric cancer risk (He et al., 2012b). Such relationships were also observed in many other kinds of cancers in other studies (Zhang et al., 2012).
A myriad of evidence has documented that single SNP in individual gene might not have enough power to impact the risk of overall cancer (Pan et al., 2009). Somehow, the combination of several SNPs might bring about more significant effects. Therefore, we further performed combination analysis of the effect of risk genotypes. We found an increased risk for neuroblastoma in individuals with 2–3 variant ERCC1 alleles, compared with those with 0–1 variant alleles, indicating that combinations of variant alleles within NER pathway can exhibit much stronger effect on neuroblastoma risk than the single variant. In agreement with our results, Tse et al., also found that individuals were more likely to develop esophageal adenocarcinoma, if they present with the combined four NER SNPs but not only one variant allele (Tse et al., 2008). In our previous epidemiological study conducted in other NER genes, we also observed such similar variant-dosage effect (He et al., 2016a). In the stratified analysis, we found that the increased neuroblastoma risk of rs2298881 variant AC/CC genotypes was more evident in subgroups of age > 18 months, female, male, tumor in retroperitoneal, tumor in mediastinum and clinical stage III + IV. Similar results were obtained in rs11615 GA/AA among subgroups of age ≤ 18, tumor in retroperitoneal. We also found that the patients carrying 2–3 risk genotypes had a more evident risk in age > 18, males, tumor in retroperitoneal, clinical stage I + II + 4 s and clinical stage III + IV. The conflicting results of relationship in subgroups might be attributed to limited statistical power caused by relatively small sample size. The stratification analysis of combined genotypes indicated that the contributing role of the 2–3 risk genotypes in neuroblastoma risk was similar in different clinical stages. We further adopted bioinformatic tools to explore the possible mechanisms for the SNPs showing the most significant associations. The results from HapMap data as well as eQTL analysis suggested that the increased neuroblastoma risk be associated with the upregulated expression levels of ERCC1 and XPF genes. The aberrant expression of ERCC1 and XPF genes might cause decreased NER repair ability, thus increased neuroblastoma risk.
Several limitations accompany with this study. First, the sample size is relative small, especially for the stratification analysis, which will impair the strength of the statistical power. Second, the risk of neuroblastoma cannot be explained only by the SNPs in ERCC1/XPF genes, other environmental factors also contribute to the risk of neuroblastoma. However, we cannot obtain these factors due to the nature of retrospective investigations. Third, only four SNPs in ERCC1/XPF genes were chosen for investigation, additional ERCC1/XPF genes variants contributing to neuroblastoma risk are needed to reveal. Fourth, the results should be interpreted with caution in other populations, as the population source of this study was restricted to unrelated Chinese Han ethnicity.
In summary, here we firstly provide evidence that polymorphisms in ERCC1/XPF genes could influence neuroblastoma risk. Ongoing epidemiological studies with additional functional analysis as well as with larger samples are needed to further elucidate how genetic variants at NER pathway influence predisposition to neuroblastoma tumorigenesis. Ultimately, our study may provide insight to the role of genetic variations in NER pathway in this aggressive pediatric tumor.
Funding Sources
This study was supported by grants from the Pearl River S&T Nova Programme of Guangzhou (No: 201710010086), the National Natural Science Foundation of China (No: 81502046), and the State Clinical Key Specialty Construction Project (Paediatric Surgery) 2013 (No: GJLCZD1301). The findings had no role in study design, data collection, data analysis, interpretation, writing of the report.
Conflict of Interest Disclosures
The authors declare no competing financial interests.
Authors' Contributions
JH and HX designed and supervised the study. ZZ, JZ, and JH performed the experiments, analyzed the data, and wrote the paper. WL, JZ, RZ, JT, TY, YZ collected the samples and information. All authors reviewed the manuscript. In addition, all authors have read and approved the manuscript.
Acknowledgments
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.03.003.
Contributor Information
Jing He, Email: hejing@gwcmc.org.
Huimin Xia, Email: xia-huimin@foxmail.com.
Appendix A. Supplementary data
Supplementary material
References
- Bourdeaut F., Trochet D., Janoueix-Lerosey I., Ribeiro A., Deville A., Coz C., Michiels J.F., Lyonnet S., Amiel J., Delattre O. Germline mutations of the paired-like homeobox 2B (PHOX2B) gene in neuroblastoma. Cancer Lett. 2005;228:51–58. doi: 10.1016/j.canlet.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Capasso M., Devoto M., Hou C., Asgharzadeh S., Glessner J.T., Attiyeh E.F., Mosse Y.P., Kim C., Diskin S.J., Cole K.A., Bosse K., Diamond M., Laudenslager M., Winter C., Bradfield J.P., Scott R.H., Jagannathan J., Garris M., McConville C., London W.B., Seeger R.C., Grant S.F., Li H., Rahman N., Rappaport E., Hakonarson H., Maris J.M. Common variations in BARD1 influence susceptibility to high-risk neuroblastoma. Nat. Genet. 2009;41:718–723. doi: 10.1038/ng.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso M., Diskin S., Cimmino F., Acierno G., Totaro F., Petrosino G., Pezone L., Diamond M., McDaniel L., Hakonarson H., Iolascon A., Devoto M., Maris J.M. Common genetic variants in NEFL influence gene expression and neuroblastoma risk. Cancer Res. 2014;74:6913–6924. doi: 10.1158/0008-5472.CAN-14-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso M., McDaniel L.D., Cimmino F., Cirino A., Formicola D., Russell M.R., Raman P., Cole K.A., Diskin S.J. The functional variant rs34330 of CDKN1B is associated with risk of neuroblastoma. J. Cell. Mol. Med. 2017;21:3224–3230. doi: 10.1111/jcmm.13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N.K., Dyer M.A. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat. Rev. Cancer. 2013;13:397–411. doi: 10.1038/nrc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmann M., Tomicic M.T., Roos W.P., Kaina B. Mechanisms of human DNA repair: an update. Toxicology. 2003;193:3–34. doi: 10.1016/s0300-483x(03)00287-7. [DOI] [PubMed] [Google Scholar]
- Cleaver J.E. Common pathways for ultraviolet skin carcinogenesis in the repair and replication defective groups of xeroderma pigmentosum. J. Dermatol. Sci. 2000;23:1–11. doi: 10.1016/s0923-1811(99)00088-2. [DOI] [PubMed] [Google Scholar]
- Consortium G.T. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M.N., Olshan A.F., Guess H.A., Savitz D.A., Poole C., Blatt J., Bondy M.L., Pollock B.H. Maternal medication use and neuroblastoma in offspring. Am. J. Epidemiol. 2004;159:721–731. doi: 10.1093/aje/kwh108. [DOI] [PubMed] [Google Scholar]
- Devoto M., Specchia C., Laudenslager M., Longo L., Hakonarson H., Maris J., Mosse Y. Genome-wide linkage analysis to identify genetic modifiers of ALK mutation penetrance in familial neuroblastoma. Hum. Hered. 2011;71:135–139. doi: 10.1159/000324843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diskin S.J., Capasso M., Schnepp R.W., Cole K.A., Attiyeh E.F., Hou C., Diamond M., Carpenter E.L., Winter C., Lee H., Jagannathan J., Latorre V., Iolascon A., Hakonarson H., Devoto M., Maris J.M. Common variation at 6q16 within HACE1 and LIN28B influences susceptibility to neuroblastoma. Nat. Genet. 2012;44:1126–1130. doi: 10.1038/ng.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diskin S.J., Capasso M., Diamond M., Oldridge D.A., Conkrite K., Bosse K.R., Russell M.R., Iolascon A., Hakonarson H., Devoto M., Maris J.M. Rare variants in TP53 and susceptibility to neuroblastoma. J. Natl. Cancer Inst. 2014;106 doi: 10.1093/jnci/dju047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J.A., Weiss N.S., Fish S., Fan W., Loomis M.M., Sakoda L.C., Rossing M.A., Zhao L.P., Chen C. Polymorphisms in nucleotide excision repair genes and endometrial cancer risk. Cancer Epidemiol. Biomark. Prev. 2011;20:1873–1882. doi: 10.1158/1055-9965.EPI-11-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzlin J.H., Scharer O.D. The active site of the DNA repair endonuclease XPF-ERCC1 forms a highly conserved nuclease motif. EMBO J. 2002;21:2045–2053. doi: 10.1093/emboj/21.8.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M.R., Aveic S., Seydel A., Tonini G.P. Neuroblastoma treatment in the post-genomic era. J. Biomed. Sci. 2017;24:14. doi: 10.1186/s12929-017-0319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Moggs J.G., Hwang J.R., Egly J.M., Wood R.D. Mechanism of open complex and dual incision formation by human nucleotide excision repair factors. EMBO J. 1997;16:6559–6573. doi: 10.1093/emboj/16.21.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E.C. How nucleotide excision repair protects against cancer. Nat. Rev. Cancer. 2001;1:22–33. doi: 10.1038/35094000. [DOI] [PubMed] [Google Scholar]
- Gao X. Multiple testing corrections for imputed SNPs. Genet. Epidemiol. 2011;35:154–158. doi: 10.1002/gepi.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet L.C., Scharer O.D. Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem. Rev. 2006;106:253–276. doi: 10.1021/cr040483f. [DOI] [PubMed] [Google Scholar]
- Gong J., Tian J., Lou J., Wang X., Ke J., Li J., Yang Y., Gong Y., Zhu Y., Zou D., Peng X., Yang N., Mei S., Zhong R., Chang J., Miao X. A polymorphic MYC response element in KBTBD11 influences colorectal cancer risk, especially in interaction with a MYC regulated SNP rs6983267. Ann. Oncol. 2017 doi: 10.1093/annonc/mdx789. [DOI] [PubMed] [Google Scholar]
- He J., Qiu L.X., Wang M.Y., Hua R.X., Zhang R.X., Yu H.P., Wang Y.N., Sun M.H., Zhou X.Y., Yang Y.J., Wang J.C., Jin L., Wei Q.Y., Li J. Polymorphisms in the XPG gene and risk of gastric cancer in Chinese populations. Hum. Genet. 2012;131:1235–1244. doi: 10.1007/s00439-012-1152-8. [DOI] [PubMed] [Google Scholar]
- He J., Xu Y., Qiu L.X., Li J., Zhou X.Y., Sun M.H., Wang J.C., Yang Y.J., Jin L., Wei Q.Y., Wang Y. Polymorphisms in ERCC1 and XPF genes and risk of gastric cancer in an eastern Chinese population. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Wang F., Zhu J., Zhang R., Yang T., Zou Y., Xia H. Association of potentially functional variants in the XPG gene with neuroblastoma risk in a Chinese population. J. Cell. Mol. Med. 2016;20:1481–1490. doi: 10.1111/jcmm.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Yang T., Zhang R., Zhu J., Wang F., Zou Y., Xia H. Potentially functional polymorphisms in the LIN28B gene contribute to neuroblastoma susceptibility in Chinese children. J. Cell. Mol. Med. 2016;20:1534–1541. doi: 10.1111/jcmm.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Zhong W., Zeng J., Zhu J., Zhang R., Wang F., Yang T., Zou Y., Xia H. LMO1 gene polymorphisms contribute to decreased neuroblastoma susceptibility in a Southern Chinese population. Oncotarget. 2016;7:22770–22778. doi: 10.18632/oncotarget.8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Wang F., Zhu J., Zhang Z., Zou Y., Zhang R., Yang T., Xia H. The TP53 gene rs1042522 C>G polymorphism and neuroblastoma risk in Chinese children. Aging (Albany NY) 2017;9:852–859. doi: 10.18632/aging.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Zou Y., Liu X., Zhu J., Zhang J., Zhang R., Yang T., Xia H. Association of common genetic variants in pre-microRNAs and neuroblastoma susceptibility: a two-center study in Chinese children. Mol. Ther. Nucleic Acids. 2018;11:1–8. doi: 10.1016/j.omtn.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou R., Liu Y., Feng Y., Sun L., Shu Z., Zhao J., Yang S. Association of single nucleotide polymorphisms of ERCC1 and XPF with colorectal cancer risk and interaction with tobacco use. Gene. 2014;548:1–5. doi: 10.1016/j.gene.2014.05.025. [DOI] [PubMed] [Google Scholar]
- Houtsmuller A.B., Rademakers S., Nigg A.L., Hoogstraten D., Hoeijmakers J.H., Vermeulen W. Action of DNA repair endonuclease ERCC1/XPF in living cells. Science. 1999;284:958–961. doi: 10.1126/science.284.5416.958. [DOI] [PubMed] [Google Scholar]
- Li J., Zou L., Zhou Y., Li L., Zhu Y., Yang Y., Gong Y., Lou J., Ke J., Zhang Y., Tian J., Zou D., Peng X., Chang J., Gong J., Zhong R., Zhou X., Miao X. A low-frequency variant in SMAD7 modulates TGF-beta signaling and confers risk for colorectal cancer in Chinese population. Mol. Carcinog. 2017;56:1798–1807. doi: 10.1002/mc.22637. [DOI] [PubMed] [Google Scholar]
- Lou J., Gong J., Ke J., Tian J., Zhang Y., Li J., Yang Y., Zhu Y., Gong Y., Li L., Chang J., Zhong R., Miao X. A functional polymorphism located at transcription factor binding sites, rs6695837 near LAMC1 gene, confers risk of colorectal cancer in Chinese populations. Carcinogenesis. 2017;38:177–183. doi: 10.1093/carcin/bgw204. [DOI] [PubMed] [Google Scholar]
- Maris J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris J.M., Hogarty M.D., Bagatell R., Cohn S.L. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- Maris J.M., Mosse Y.P., Bradfield J.P., Hou C., Monni S., Scott R.H., Asgharzadeh S., Attiyeh E.F., Diskin S.J., Laudenslager M., Winter C., Cole K.A., Glessner J.T., Kim C., Frackelton E.C., Casalunovo T., Eckert A.W., Capasso M., Rappaport E.F., McConville C., London W.B., Seeger R.C., Rahman N., Devoto M., Grant S.F., Li H., Hakonarson H. Chromosome 6p22 locus associated with clinically aggressive neuroblastoma. N. Engl. J. Med. 2008;358:2585–2593. doi: 10.1056/NEJMoa0708698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay K.K., Maris J.M., Schleiermacher G., Nakagawara A., Mackall C.L., Diller L., Weiss W.A. Neuroblastoma. Nat. Rev. Dis. Prim. 2016;2 doi: 10.1038/nrdp.2016.78. [DOI] [PubMed] [Google Scholar]
- Menegaux F., Olshan A.F., Neglia J.P., Pollock B.H., Bondy M.L. Day care, childhood infections, and risk of neuroblastoma. Am. J. Epidemiol. 2004;159:843–851. doi: 10.1093/aje/kwh111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosse Y.P., Laudenslager M., Khazi D., Carlisle A.J., Winter C.L., Rappaport E., Maris J.M. Germline PHOX2B mutation in hereditary neuroblastoma. Am. J. Hum. Genet. 2004;75:727–730. doi: 10.1086/424530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Schulte E., Kurlemann G., Harder A. Tobacco, alcohol and illicit drugs during pregnancy and risk of neuroblastoma: systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2017 doi: 10.1136/archdischild-2017-313615. [DOI] [PubMed] [Google Scholar]
- Nguyen le B., Diskin S.J., Capasso M., Wang K., Diamond M.A., Glessner J., Kim C., Attiyeh E.F., Mosse Y.P., Cole K., Iolascon A., Devoto M., Hakonarson H., Li H.K., Maris J.M. Phenotype restricted genome-wide association study using a gene-centric approach identifies three low-risk neuroblastoma susceptibility Loci. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer L.J., Garinis G.A., Raams A., Lalai A.S., Robinson A.R., Appeldoorn E., Odijk H., Oostendorp R., Ahmad A., van Leeuwen W., Theil A.F., Vermeulen W., van der Horst G.T., Meinecke P., Kleijer W.J., Vijg J., Jaspers N.G., Hoeijmakers J.H. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- Pan J., Lin J., Izzo J.G., Liu Y., Xing J., Huang M., Ajani J.A., Wu X. Genetic susceptibility to esophageal cancer: the role of the nucleotide excision repair pathway. Carcinogenesis. 2009;30:785–792. doi: 10.1093/carcin/bgp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada H., Ambros I.M., Dehner L.P., Hata J., Joshi V.V., Roald B., Stram D.O., Gerbing R.B., Lukens J.N., Matthay K.K., Castleberry R.P. The international neuroblastoma pathology classification (the Shimada system) Cancer. 1999;86:364–372. [PubMed] [Google Scholar]
- Sijbers A.M., de Laat W.L., Ariza R.R., Biggerstaff M., Wei Y.F., Moggs J.G., Carter K.C., Shell B.K., Evans E., de Jong M.C., Rademakers S., de Rooij J., Jaspers N.G., Hoeijmakers J.H., Wood R.D. Xeroderma pigmentosum group F caused by a defect in a structure-specific DNA repair endonuclease. Cell. 1996;86:811–822. doi: 10.1016/s0092-8674(00)80155-5. [DOI] [PubMed] [Google Scholar]
- Stadler Z.K., Thom P., Robson M.E., Weitzel J.N., Kauff N.D., Hurley K.E., Devlin V., Gold B., Klein R.J., Offit K. Genome-wide association studies of cancer. J. Clin. Oncol. 2010;28:4255–4267. doi: 10.1200/JCO.2009.25.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse D., Zhai R., Zhou W., Heist R.S., Asomaning K., Su L., Lynch T.J., Wain J.C., Christiani D.C., Liu G. Polymorphisms of the NER pathway genes, ERCC1 and XPD are associated with esophageal adenocarcinoma risk. Cancer Causes Control. 2008;19:1077–1083. doi: 10.1007/s10552-008-9171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsodikov O.V., Enzlin J.H., Scharer O.D., Ellenberger T. Crystal structure and DNA binding functions of ERCC1, a subunit of the DNA structure-specific endonuclease XPF-ERCC1. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11236–11241. doi: 10.1073/pnas.0504341102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Diskin S.J., Zhang H., Attiyeh E.F., Winter C., Hou C., Schnepp R.W., Diamond M., Bosse K., Mayes P.A., Glessner J., Kim C., Frackelton E., Garris M., Wang Q., Glaberson W., Chiavacci R., Nguyen L., Jagannathan J., Saeki N., Sasaki H., Grant S.F., Iolascon A., Mosse Y.P., Cole K.A., Li H., Devoto M., McGrady P.W., London W.B., Capasso M., Rahman N., Hakonarson H., Maris J.M. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature. 2011;469:216–220. doi: 10.1038/nature09609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R.D. Mammalian nucleotide excision repair proteins and interstrand crosslink repair. Environ. Mol. Mutagen. 2010;51:520–526. doi: 10.1002/em.20569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R.D., Mitchell M., Sgouros J., Lindahl T. Human DNA repair genes. Science. 2001;291:1284–1289. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- Yang Z., Fang X., Pei X., Li H. Polymorphisms in the ERCC1 and XPF genes and risk of breast cancer in a Chinese population. Genet. Test Mol. Biomark. 2013;17:700–706. doi: 10.1089/gtmb.2013.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Li G., Li W.F. Association between ERCC1 and XPF polymorphisms and risk of colorectal cancer. Genet. Mol. Res. 2015;14:700–705. doi: 10.4238/2015.January.30.13. [DOI] [PubMed] [Google Scholar]
- Yang X.H., Tang F., Shin J., Cunningham J.M. Incorporating genomic, transcriptomic and clinical data: a prognostic and stem cell-like MYC and PRC imbalance in high-risk neuroblastoma. BMC Syst. Biol. 2017;11:92. doi: 10.1186/s12918-017-0466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang J., Xu L., Zhou J., Guan X., Jiang F., Wu Y., Fan W. Nucleotide excision repair gene ERCC1 polymorphisms contribute to cancer susceptibility: a meta-analysis. Mutagenesis. 2012;27:67–76. doi: 10.1093/mutage/ger062. [DOI] [PubMed] [Google Scholar]
- Zhang R., Zou Y., Zhu J., Zeng X., Yang T., Wang F., He J., Xia H. The association between GWAS-identified BARD1 gene SNPs and neuroblastoma susceptibility in a Southern Chinese population. Int. J. Med. Sci. 2016;13:133–138. doi: 10.7150/ijms.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Lin H., Wang J., He J., Zhang D., Qin P., Yang L., Yan L. LMO1 polymorphisms reduce neuroblastoma risk in Chinese children: a two-center case-control study. Oncotarget. 2017;8:65620–65626. doi: 10.18632/oncotarget.20018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Chang Y., Jia W., Zhang J., Zhang R., Zhu J., Yang T., Xia H., Zou Y., He J. LINC00673 rs11655237 C>T confers neuroblastoma susceptibility in Chinese population. Biosci. Rep. 2018;38 doi: 10.1042/BSR20171667. (BSR20171667) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material